Abstract
Measures of the adipokine chemerin are elevated in multiple cardiovascular diseases, including hypertension, but little mechanistic work has been done to implicate chemerin as being causative in such diseases. The chemerin knockout (KO) rat was created to test the hypothesis that removal of chemerin would reduce pressure in the normal and hypertensive state. Western analyses confirmed loss of chemerin in the plasma and tissues of the KO vs. wild-type (WT) rats. Chemerin concentration in plasma and tissues was lower in WT females than in WT males, as determined by Western analysis. Conscious male and female KO rats had modest differences in baseline measures vs. the WT that included systolic, diastolic, mean arterial and pulse pressures, and heart rate, all measured telemetrically. The mineralocorticoid deoxycorticosterone acetate (DOCA) and salt water, combined with uninephrectomy as a hypertensive stimulus, elevated mean and systolic blood pressures of the male KO higher than the male WT. By contrast, all pressures in the female KO were lower than their WT throughout DOCA-salt treatment. These results revealed an unexpected sex difference in chemerin expression and the ability of chemerin to modify blood pressure in response to a hypertensive challenge.—Watts, S. W., Darios, E. S., Mullick, A. E., Garver, H., Saunders, T. L., Hughes, E. D., Filipiak, W. E., Zeidler, M. G., McMullen, N., Sinal, C. J., Kumar, R. K., Ferland, D. J., Fink, G. D. The chemerin knockout rat reveals chemerin dependence in female, but not male, experimental hypertension.
Keywords: blood pressure, adipokines, vascular contraction
Chemerin was originally described as a peptide found in inflammatory fluid (1). It has since been described as an adipokine (2–4) and has gained attention as a result of findings of elevated blood levels in multiple human diseases. These include obesity (5–9), overweight children (10), metabolic syndrome (2, 11–16), rheumatoid arthritis (17), type 2 diabetes (18–20), polycystic ovarian syndrome (21–23), coronary artery disease (24), obstructive sleep apnea (25), renal dysfunction with and without type 2 diabetes (26–28), acute ischemic stroke (29), nonalcoholic fatty liver disease (30), atrial fibrillation (31), psoriasis with subclinical cardiac involvement (32), and preeclampsia (33–35). The intent of this nonexhaustive list is to make the point that the epidemiologic and clinical measures of chemerin (36) are strong in implicating chemerin in disease, particularly those involving cardiovascular dysfunction. However, our understanding of the mechanisms by which chemerin participates in these diseases, such that chemerin could be identified as causal or participatory in the disease—as opposed to being associative—is poor. Presently, we focus on the endpoint of blood pressure as it is regulated by chemerin.
Chemerin has prohypertensive actions in the vasculature. First, the biologically active peptide chemerin-9 (37) constricts isolated human and rat arteries (38, 39); the efficacy of chemerin-9 increases in arteries from a rat made deoxycorticosterone acetate (DOCA)-salt hypertensive or in normal rats with the endothelial layer removed (38). Chemerin-induced contraction is mediated by the Chemerin1 receptor (formerly known as ChemR23) (40, 41) and uses signaling pathways well established as mediating smooth muscle contraction (42–44). Second, chemerin reduces endothelium-dependent relaxation and augments arterial contraction to other agonists (43, 45). Third, stimulation of the Chemerin1 receptor by the agonist chemerin-9 amplifies electrical field-stimulated sympathetic nerve-mediated arterial contraction (46). These 3 events would promote an elevation in blood pressure by increasing total peripheral resistance. Recently, Kunimoto et al. (47) showed that chronic chemerin infusion (>6 wk) in the mouse elevates blood pressure. Importantly, chemerin expression is observed in the perivascular adipose tissue (PVAT), immediately adjacent to an artery, in the media of which the Chemerin1 receptor is expressed in the rat and human (38, 48). Finally, independent groups have confirmed that chemerin is a constrictor in human arteries (38, 39). Thus, the components of the vasculature are arranged in the human and rat in such a way that the agonist chemerin (in PVAT) is expected to be prohypertensive through stimulation of the medial Chemerin1 receptor. The vascular studies cited support this idea and support the use of the rat as a model to test the physiologic importance of chemerin to blood pressure regulation.
We aimed to study the contributions made by chemerin to blood pressure in two ways. First, we asked whether chemerin played a role in normal blood pressure regulation. Second, we wanted to determine whether the prohypertensive actions of chemerin would be revealed in a hypertension model. To move these studies into the whole animal and perform experiments that permit more than associative links to be made between chemerin and blood pressure, we created the chemerin knockout (KO) rat. This approach, rather than using a specific receptor antagonist, was done to understand the totality of chemerin’s contributions to the cardiovascular system. The chemerin KO rat was created using clustered regularly interspaced short palindromic repeats/CRISPR-associated system (CRISPR/Cas) technology and single-guide RNAs (sgRNAs) against the chemerin gene, retinoic acid receptor responder protein 2 (Rarres2; also known as tazarotene-induced gene 1 protein). The mineralocorticoid-dependent hypertension model—the DOCA-salt rat—was used because chemerin-9-induced maximal contraction of arteries isolated from the DOCA-salt rat was elevated compared with arteries from sham normotensive rats (38). The hypothesis tested, in both male and female rats, was that removal of chemerin would reduce blood pressure under normal conditions and in DOCA-salt hypertension.
MATERIALS AND METHODS
Construction of chemerin WT and KO rat
This original work was done at the Transgenic Animal Model Facility at the University of Michigan. CRISPR/Cas9 technology (49, 50) was used to generate a genetically modified rat strain with a chemerin (Rarres2) gene KO. Five sgRNA targets were identified with the algorithm described by Hsu et al. (51) and cloned into plasmid pX330 (plasmid 42230; Addgene, Cambridge, MA, USA; a kind gift of Feng Zhang, Massachusetts Institute of Technology, Cambridge, MA, USA), as described in Ran et al. (52). Each circular pX330 plasmid was coelectroporated into primary rat embryonic fibroblasts with a PGKpuro plasmid (53). Genomic DNA was prepared from the cells after transient selection with puromycin. A 599 bp DNA fragment spanning the expected Cas9 cut site was PCR amplified with forward primer 5′-CCCCCAACCCCTTAACTTAG-3′ and reverse primer 5′-GACTTGGGACATGGAGGAAA-3′. Amplicons were subjected to CEL 1 endonuclease digestion, essentially as described (54). In brief, amplicons were melted and annealed and then subjected to CEL 1 digestion. The digests were analyzed by agarose gel electrophoresis. Gels were stained with SYBR Gold (S11494; Thermo Fisher Scientific, Waltham, MA, USA) to achieve greater sensitivity in the detection of insertion/deletion mutations (indels) in the amplicons. The presence of indels produced by nonhomologous-endjoining repair of Cas9-induced double-strand breaks resulted in the presence of lower MW DNA fragments following CEL 1 endonuclease digestion for the sgRNA target. One sgRNA and protospacer adjacent motif showed chromosome cleavage activity in exon 2. The active guide target was 5′-GTCCGCTGTGCCCAGCCATA-3′ protospacer adjacent motif: GGG with a cut site in codon 11.
Rat zygote microinjection
Rat zygote microinjection was carried out as described (55). Animals were housed in an Association for Assessment and Accreditation of Laboratory Animal Care-accredited facility in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA). Procedures were approved by the University of Michigan’s Institutional Animal Care and Use Committee. pX330 plasmid DNA expressing the active sgRNA was purified with an Endotoxin-Free Kit (Qiagen, Germantown, MD, USA). DNA concentration was adjusted to 5 ng/μl for pronuclear microinjection (56). Fertilized mouse eggs (30) were microinjected with purified DNA to detect embryo toxicity before large-scale rat zygote microinjection. These mouse eggs survived microinjection and developed to the blastocyst stage in cell culture, indicating that the purified pX330 DNA was nontoxic. Rat zygotes for microinjection were obtained by mating superovulated Sprague-Dawley females with males of the same strain [Crl:CD(SD), strain code 1; Charles River Laboratory, Wilmington, MA, USA]. Four hundred forty-nine rat zygotes were microinjected; 349 survived injection and were transferred to pseudopregnant females. Genomic DNA was isolated from tail-tip biopsies of 86 potential founders that were born and analyzed for CRISPR/Cas9-induced indels with a CEL 1 endonuclease. A total of 49 G0 rat pups (60%) were identified as carrying mutations in Rarres2. Five G0 founder rats were identified for breeding to obtain germline transmission of the mutant Rarres2 gene. Cloning and DNA sequencing (57) of 65 G1 pups showed that 3 G1 offspring (2 males and 1 female) were heterozygous for the same mutation in the Rarres2 gene. The mutant allele had a 240 bp deletion around the expected Cas 9 cut site. Nonhomologous endjoining removed 181 bp from the 3′ side of intron 1 and the first 59 bp of exon 2, including the splice acceptor and the first 36 coding bases of Rarres2. One G1 male and G1 female were interbred. Thirteen G2 pups from this first heterozygous breeding were born, 7 of which were wild type (WT) and 6 of which were heterozygous. The original founders and these pups were transferred to Michigan State University and regenotyped for confirmation using the PCR conditions described later. Heterozygous G2 pups were bred to result in the first G3 homozygous WT and KO animals.
Validation of KO rat
Ear or tail tissue was taken for every rat before experimentation and in some rats following experimentation to validate/confirm the genotype of the rat. PCR was used with the following parameters: forward primer: 5′-GACTTGGGACATGGAGGAAA-3′; reverse primer: 5′-CCCCCAACCCCTTAACTTAG-3′. GoTaq Green (M7128; Promega, Madison, WI, USA) was used, according to the manufacturer’s protocol on a real-time cycler. Cycling conditions were 1× 95°C, 1 min; 35 cycles of 95°C, 15 s, then 58°C for 15 s, and then 72°C for 40 s; and 1× 72°C, 4 min and then 4°C after. Expected WT product size is 600 bp, and the mutant allele product is 360 bp, run on a standard agarose gel.
Breeding
Heterozygous pairs of animals continued to be bred, with breeding pairs retired after 6 mo, and animals crossed outside of brother-sister breedings. Ear punches from the male and female KO were sent back to the University of Michigan for validation of the KO and determination of the damage in both alleles (see Supplemental Fig. 2). As anticipated, both alleles lacked the splice site and the first 12 aa of exon 2. Once genotyped, WT and KO rats were implanted with an IMI-500 transponder under the neck skin for unique identification. The transponder was read by the BMDS Smart probe (BioMedic Data Systems, Seaford, DE, USA). This allowed us to house animals together in a social nature where possible, while being confident in individual rat identity.
Animal use and husbandry
All animals were housed in temperature-controlled rooms and given free access to food and water. Rooms were maintained on a 12-h light/dark cycle. All animals received enrichment in their cages (shelter, tubes, Nylabones), and breeding/weaning females also had nesting materials placed in their cage. Cages were checked daily. When pups were born, each pup was visually checked for milk in its belly as a sign of successful feeding by its mother (e.g., adequate maternal care). All animals were randomized to the experiment in which they participated. Age-matched WT littermates were always used as controls.
Phenotyping and clinical chemistry
Animals were bled (under isoflurane anesthesia in 2% oxygen, inhalation) through a tail-vein draw or as a terminal draw through a ventricular puncture. Blood was placed in a BD Vacutainer K2 EDTA tube (Becton Dickinson, Franklin Lakes, NJ, USA) and immediately placed on ice. Blood was spun at 2000 rpm for 20 min on a Sorvall Legend XTR centrifuge (Thermo Fisher Scientific) at 4°C. Plasma was separated from clotted blood components and taken for protein measurement using a Bicinchoninic Assay Kit (BCA1-1KT; MilliporeSigma, Burlington, MA, USA). These samples were then used for either detection of plasma chemerin or for clinical measures. From this point, the operator was blinded to whether the sample came from a KO or WT rat. The operator was not blinded to whether the sample came from a male or female, as such information was required for setting up separate gels or for grouping for clinical chemistry. Plasma clinical values are listed in Table 1 and were measured on a clinical analyzer (Olympus, Center Valley, PA, USA) following the manufacturer’s instructions.
TABLE 1.
Clinical values for 12- to 14-wk-old male and female chemerin WT and KO rats
| Groups | ALB (g/dL) | ALT (IU/L) | ASTa (IU/L) | T.Bil, (mg/dL) | CHOL (mg/dL) | CRE (mg/dL) | GLUa (mg/dL) | TRIG (mg/dL) | BUN (mg/dL) | NEFA (mM) | Ketone (mM) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Male WT (n = 6) | 3.0 ± 0.1 | 40.7 ± 3.2 | 212.7 ± 43.6 | 0.2 ± 0 | 73.2 ± 4.8 | 0.3 ± 0 | 261.8 ± 23.9 | 143 ± 12.8 | 21.4 ± 0.7 | 0.4 ± 0.1 | 273.5 ± 33.3 |
| Male KO (n = 6) | 3.0 ± 0 | 35.2 ± 1.2 | 107 ± 8.6* | 0.1 ± 0.0 | 74.2 ± 5.4 | 0.3 ± 0.0 | 299.3 ± 30.0 | 143.2 ± 24 | 24.6 ± 1.3 | 0.3 ± 0 | 391.2 ± 25.9 |
| Female WT (n = 5) | 3.6 ± 0.2† | 43.3 ± 1.4 | 132.8 ± 13.9 | 0.1 ± 0.0 | 68.6 ± 2.8 | 0.4 ± 0 | 319.8 ± 69.8 | 165.8 ± 28.3 | 22.6 ± 1.1 | 0.4 ± 0.1 | 403.2 ± 39.4† |
| Female KO (n = 5) | 3.8 ± 0.1† | 44.4 ± 4.0 | 125.8 ± 12.7 | 0.2 ± 0 | 69.6 ± 3.6 | 0.4 ± 0.0 | 248.8 ± 16.8 | 198.8 ± 19.1 | 21.8 ± 1.1 | 0.6 ± 0.1 | 358.4 ± 27.2 |
Sample size indicated in parentheses. One-way ANOVA with Tukey’s post hoc test was used to compare 4 groups (male WT, male KO, female WT, female KO) among one another, as long as variances were equal. ALB, albumin; ALT, alanine aminotransferase; CHOL, total cholesterol; CRE, creatinine; GLU, glucose; T.Bil, total bilirubin; TRIG, triglycerides.
Variances in the clinical measures were unequal, and a Kruskal-Wallis test, followed by Dunn’s, was used to determine statistical differences.
P < 0.05 between same-sex WT.
P < 0.05 between different sexes of same genotype, marked in female.
Western analyses
Plasma or tissue homogenates were used to validate KO at the tissue level. All samples had 100 µg total protein loaded per lane on standard polyacrylamide gels (15%). Gel proteins were transferred to PVDF-FL membrane using wet transfer. Li-Cor Revert Kit (Lincoln, NE, USA) was used to stain total protein and scanned on the 700 channel of a Li-Cor Odyssey CLx. The membrane was then rehydrated in methanol, rinsed in water, incubated in reversal solution for 5 min, and then rinsed in wash solution twice. All blots were then blocked in 4% chick egg ovalbumin (A5378; MilliporeSigma) for 3 h on a rocker at 4°C. Chemerin antibody [112520; Abcam, Cambridge, United Kingdom; Research Resource Identifier (RRID): AB_10864055], diluted to 1:1000 in blocking buffer, was incubated for 48–72 h with constant rocking at 4°C. Blots were then rinsed with Tris-buffered saline + 0.1% Tween-20, 3 times for 10 min, followed by incubation with Li-Cor IRDye secondary antibody (926-32210, 800CW goat anti-mouse at 1:1000; RRID: AB_621842) for 1 h with rocking. Secondary solution was removed, and the blot was washed 3 × 10 min with Tris-buffered saline + Tween and imaged on a Li-Cor Odyssey CLx in the 800 channel. A transferrin antibody was used as a loading control for plasma (ab82411; Abcam; RRID: AB_1659060), while Li-Cor total protein stain was used for other proteins.
Tango assay
Bioactive chemerin was measured using a rat Chemerin1 receptor in a cell-based reporter assay, as described in Parlee et al. (58). Human embryonic kidney 293T cells, which constitutively express a fusion protein composed of a tobacco etch virus protease linked to human-arrestin2 and a transcriptional-transactivator (tTA)-dependent luciferase reporter gene, were transfected with a plasmid (Chemerin1-TL-tTA) that expresses a fusion construct of Chemerin1 joined at its C terminus to a tTA by a tobacco etch virus N1a protease cleavage site. Chemerin binding to the Chemerin1-tTA fusion protein recruits the arrestin-protease fusion construct to the receptor, resulting in tTA cleavage, allowing tTa to pass into the nucleus, where it transcribes the luciferase reporter gene. Concentration response curves were conducted for rat recombinant chemerin and rat plasma samples tested in a blinded fashion (e.g., no prior knowledge of WT, KO, or male or female status). The control of these same cells without the Chemerin1-tTA fusion protein was run, and the values reported for each plasma sample were derived by subtracting the value observed in the no-receptor sample from the receptor sample.
Proteome profiler adipokine array
An equivalent amount of total protein (1 mg) from plasma of a WT and KO rat was incubated concurrently on rat-specific adipokine arrays (ARY016; R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s protocol. IRDye 800CW streptavidin secondary antibody (926-32230; Li-Cor; RRID: AB_2722576) was used as the secondary antibody to develop the array and analyzed on the Li-Cor Odyssey CLx in the 800 channel.
Isometric contraction
The thoracic aorta and superior mesenteric artery were dissected and placed into physiologic salt solution [PSS; in mM: NaCl 130, KCl 4.7, KH2PO4 1.18, MgSO4 ⋅ 7H2O 1.7, NaHCO3 14.8, dextrose 5.5, CaNa2 EDTA 0.03, CaCl2 1.6 (pH 7.2)]. Vessels were guided onto a wire in a silastic-filled Petri dish and cleaned of PVAT under PSS. The endothelium was removed in the thoracic aortic rings for the purpose of measuring maximum chemerin-9-induced contraction, whereas the endothelium was left intact in the superior mesenteric artery for measurement of endothelial function. These two different arteries were used from the same rat and experiments done such that the WT and KO rats were always paired. We were capable of mounting 6 tissues in a day, with rat aortic rings −E from WT and KO and 2 rings of superior mesenteric artery +E of WT and KO. This allowed for direct comparisons of the effects of life-long chemerin removal in response to chemerin (aorta) and 2 important parameters of vascular function, contraction to norepinephrine (NE), and endothelial function (mesenteric artery). Cleaned vessels cut into rings (∼3 mm wide) for measurement of isometric contractile force. Rings were mounted in warmed (37°C) and aerated (95% O2, 5% CO2) tissue baths (30 ml PSS) on Grass isometric transducers (FT03; Grass Instruments, Quincy, MA, USA), connected to an ADInstruments PowerLab (ADInstruments, Colorado Springs, CO, USA). Tissues were placed under optimal resting tension (thoracic aorta 4000 mg; superior mesenteric artery 1200 mg) and allowed to equilibrate for 1 h before an initial challenge with a maximal concentration of phenylephrine (PE; 10−5 M, P6126; MilliporeSigma). After this challenge, tissues were washed until the tone returned to baseline. Tissues were contracted with a half-maximal (10−8 M) concentration of PE and the endothelium-dependent relaxant acetylcholine (ACh; 10−6 M, A9101; MilliporeSigma) added to test for removal (aorta) or intactness (mesenteric artery) of the endothelial layer. Tissues were washed again multiple times until the tone returned to baseline. For the aorta, cumulative concentration response curves to Chemerin-9 (0248; Genscript, Piscataway, NJ, USA) were constructed, with additions made only after contraction plateaued with an addition. For the superior mesenteric artery, cumulative curves to NE (A7256; MilliporeSigma) or to ACh (in a half-maximally PE-contracted tissue) were performed randomly such that time was not a factor in the tissue response.
Telemetry implantation
Radiotelemeter transmitters (model TA11PA C40 or S10; Data Sciences International, St. Paul, MN, USA), with attached catheters with pressure-sensing tips, were implanted subcutaneously through a 1–1.5 cm incision in the left inguinal area, whereas rats were under isoflurane anesthesia. Catheters were introduced into the left femoral artery, 3–5 mm distal to the level of the peritoneal wall, and the tip was advanced to the abdominal aorta. Rats were allowed 3–4 d to recover postoperatively, and then 4 d of baseline measurements were made. Mean arterial pressure, systolic pressure, diastolic pressure, heart rate, and pulse pressure were recorded throughout the duration of the study, both during this baseline period and after DOCA-salt treatment (later). Measures were recorded for 10 s every 10 min throughout the duration of the study and are presented as a 24 h average, as collected by Dataquest Art v.4.2 software (Data Sciences International).
DOCA-salt surgery and tissue procurement
All animals served as their own control. After baseline recordings, all animals underwent a uninephrectomy and implantation of a DOCA-impregnated silastic pellet at a dose of 200 mg/kg, s.c. At the same time, animals were placed on drinking water with 1.0% NaCl and 0.2% KCl. Animals were on this regimen for 4 wk and then euthanized. Under isoflurane anesthesia, blood samples were taken for plasma, and tissues were removed for weight, for preparation of a histologic sample, or for Western analyses.
Data presentation
Data are reported as means ± sem for the number of individual animals, indicated in parentheses with most graphs in scatterplot. In some instances, a box and whiskers form is used, reporting median and quartiles. For Western analyses, Li-Cor Image Studio v.5.2.5 (RRID: SCR_015795) was used for visualization of the total protein stain on the blot and visualization of chemerin. Images shown were modified in brightness/contrast only as a whole. Tango results were determined by a standard curve run in every experiment, and samples were run in duplicate. Adipokine arrays were visualized on the Li-Cor CLx instrument, and paired WT and KO were always scanned together such that comparisons can be made between the 2. The Li-Cor CLx Image Studio program was used to quantify the spots on the array. Contractility data are reported as a percentage of PE contraction. Potency values [−log EC50 value (M)] were calculated within Graph Pad Prism 7.0 (GraphPad Software, La Jolla CA, USA; RRID: SCR_015807). If maximum values were not achieved, then the potency values reported are estimated, and true values are equal to or greater than these estimated values. Telemetry-derived values (all pressures, heart rate, activity, temperature) were captured by the Dataquest ART program of Data Sciences International. Imaging of histologic samples was performed on a Nikon TE2000 inverted microscope (Tokyo, Japan) with the Cell Tools program (Molecular Machines & Industries, Zurich, Switzerland).
Statistics
When comparing 2 groups, the appropriate paired or unpaired, 1- or 2-tailed Student’s t test was used when variances were equal. When comparing 3 or more groups at the same time point, a 1-way ANOVA with Tukey’s post hoc was used to compare the groups among one another, as long as variances were equal, as validated by Bartlett’s test. When comparing 3 or more groups over time, a repeated-measures ANOVA with Tukey’s post hoc was used. For blood pressures, times were divided into week-long segments for statistical comparisons. When variances were unequal, a Kruskal-Wallis test, followed by Dunn’s test was used to determine statistical differences among 3 or more groups.
RESULTS
Chemerin gene was successfully mutated
Supplemental Fig. S1 shows the design for mutation of exon 2 of the Rarres2 gene (Supplemental Fig. S1A) and the DNA mutations (Supplement Fig. S1B; mutant vs. WT) in the first pups born after sgRNA administration to Sprague-Dawley zygotes from Charles River Laboratories. The male and female heterozygotes born from these original mutated rats were used as breeding pairs for creating the WT and KO rats that were used in this study. Validation of homozygous mutation, placed in exon 2 of the Rarres2 gene, was performed at the University of Michigan in a blinded fashion, an example of which is shown in Supplemental Fig. S2.
Circulating and tissue chemerin was abolished in the KO rat
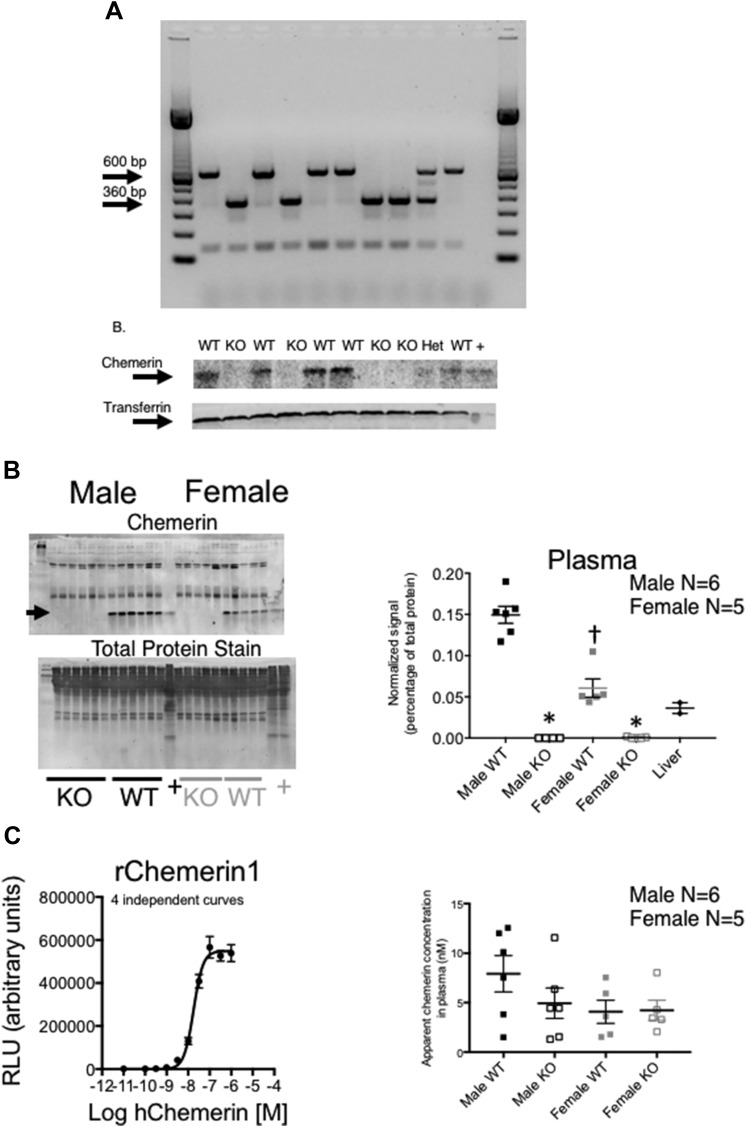
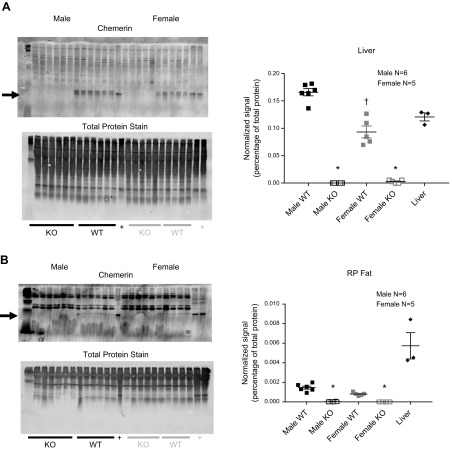
In the original second filial generation of rats, Rarres2 was successfully mutated homozygously, as verified through genotyping (Fig. 1A, upper). Chemerin protein was abolished in the plasma in those same animals with a KO genotype when compared with those with a WT genotype (Fig. 1A, lower). In other cohorts (12–14 wk olds), plasma, liver, and retroperitoneal (RP) fat were taken from littermate males and females to test whether the Rarres2 mutation reduced chemerin protein synthesis in tissues. Plasma chemerin of the KO vs. WT was abolished (Fig. 1B) in both sexes. Unexpectedly, plasma chemerin was significantly lower in WT females than WT males (Fig. 1B, left and right). A Tango GPCR assay, designed to detect active chemerin isoforms (58) (standard curve in Fig. 1C, left), was not able to detect a significantly higher level of active chemerin in the male WT vs. KO, although the male WT values were quantitatively higher (Fig. 1C, right). No differences were observed between female WT and KO samples. Reflective of plasma measures, chemerin was abolished in the liver and RP fat of the male and female KO when compared with their respective WT (Fig. 2) and was significantly lower in content in the female vs. male liver (Fig. 2A). A total protein stain of the membrane before development of the blot with antibody was performed as a loading control and is shown for each experiment, as depicted in Figs. 1 and 2.
Figure 1.
A) Validation of WT, heterozygous (Het), or KO status using PCR (upper) and Western analyses (lower) of plasma in 10 different animals (each one per lane). Transferrin was used as a loading control for protein. DNA and protein samples are from the same animal, as marked by WT, Het, or KO rats. Plus sign indicates liver homogenates from a normal male Sprague-Dawley rat. Representative of over 200 rats. B) Comparison of chemerin in male and female rats from plasma (left). +, normal Sprague-Dawley male (from outside of colony) liver homogenate. Arrow points to chemerin. Total protein stain on membranes was done before blocking and adding the primary antibody against chemerin. Chemerin signal was normalized to total protein for each respective lane in the graph (right), where horizontal bars are means ± sem for number of animals in parentheses, surrounded by individual plotted values. C) Standard curve for human (h)chemerin in the Tango rat (r)Chemerin-1 assay (left) and in plasma from WT and KO male and female rats (right), as measured as receptor-dependent signal. Asterisk denotes significantly different vs. same sex WT; dagger indicates comparison to different male WT, as determined by ANOVA, followed by Tukey’s post hoc test. RLU, relative light units.
Figure 2.
Comparison of chemerin in liver (A) and RP fat (B) from male (left blots) and female (right blots) rats. Plus sign indicates normal Sprague-Dawley male (from outside of colony) liver homogenate. Arrows point to chemerin. Total protein stains on membranes were done before blocking and adding the primary antibody against chemerin. Right panels: chemerin signal was normalized to total protein for each respective lane in the graphs where horizontal bars are means ± sem for number of animals in parentheses, surrounded by scattered values. Asterisk denotes significantly different vs. same sex WT; dagger indicates comparison to different male WT, as determined by ANOVA, followed by Tukey’s post hoc test.
Chemerin KO rat appears grossly normal vs. WT: growth, tissue weights, and clinical values
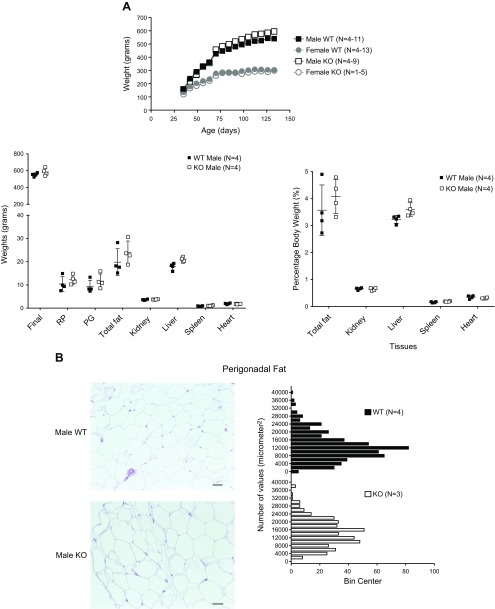
The first litters of KO and WT born were allowed to age to 20 wk to observe growth (Fig. 3A, left); this ultimately included only 1 female KO rat. At 20 wk of age, there was no statistical difference in the absolute body weight, total fat content, kidney, liver, spleen, or heart weights in the male KO vs. WT (reported as absolute values or as a percentage of body weight; Fig. 3A, right). Female rats were significantly smaller than males when age matched. No gross abnormalities in perigonadal (PG) fat, a source of chemerin, of the KO male were noted, although KO adipocytes tended to be bigger than WT (Fig. 3B). Gross histology of the liver and skin, 2 sites in addition to fat where chemerin is made, revealed no substantial differences in cellular organization or tissue structure (data not shown). In later cohorts of animals, the Adipokine Proteome Profile Arrays revealed no complete loss of any of the 27 adipokines tested on this array in the KO vs. WT (data not shown). Clinical panels on plasma from male and female KO and WT rats are shown in Table 1. Values for alanine aminotransferase, total bilirubin, total cholesterol, creatinine, glucose, triglycerides, blood urea nitrogen (BUN), and nonesterified fatty acids (NEFAs) were not different between male and female KO rats when compared with their respective WT controls. For albumin, values in the females were higher when compared with those from the male. For aspartate aminotransferase (AST), the male KO was significantly lower than male WT. Finally, ketone levels in the female WT were significantly greater than the male WT.
Figure 3.
A) Growth curves for male and female and male tissue weights (absolute, percentage body weight; right) of some of the first WT and KO progeny born. Horizontal bars represent means ± sem, surrounded by scattered values for the number of animals in parentheses. Insufficient female KO were born in these litters to include them in these tissue measures. B) Histologic section (left) and quantitation of adipocyte size (right) in PG fat in male WT (n = 4) and KO (n = 3) rats that were allowed to grow to 20 wk. Insufficient female KO were born in these litters to include them in these measures. Bars represent means ± sem for the number of animals in parentheses. Original scale bars, 50 μm. Animals included in this graph are all littermates.
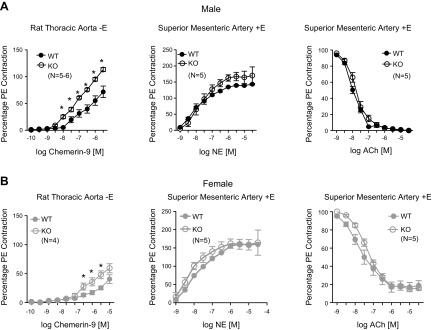
Arteries from the KO rat show enhanced contractility selectively to Chemerin-9 vs. the WT
Arteries from littermate male and female WT and KO rats (12–14 wk) were used to investigate basic arterial function, including contraction to the adrenergic agonist NE and endothelium-dependent relaxation to ACh, as well as contraction to the Chemerin-1 receptor agonist Chemerin-9. Table 2 reports the potencies [−log EC50 values (M)] and maximums that define the pharmacological curves shown in Fig. 4. In males (Fig. 4A) and females (Fig. 4B), Chemerin-9–induced maximum contraction was greater in the KO vs. WT (Fig. 4, left). Moreover, Chemerin-9–induced maximum contraction in aorta from the male was greater than their genotype-matched female (percent PE contraction; WT male: 71.9 ± 10.6; female: 40.7 ± 7.6, P < 0.05). The potency of Chemerin-9 was elevated in the KO vs. the WT, male and female, although potencies were estimated, given that a maximum was not formally achieved. These findings contrasted with no change in potency or maximum-induced contraction caused by NE in between the male WT and KO, the female WT and KO, or between genotype-matched male and female (Fig. 4, middle). Likewise, maximum PE contraction (reported in legend) was not different, using these same comparisons. Finally, there was no change in the potency or efficacy of ACh-induced relaxation in PE-contracted arteries when comparing KO and WT within a sex (Fig. 4, right). Superior mesenteric rings from the males (KO and WT) relaxed completely to ACh, whereas ∼16–19% of PE-induced contraction remained in the female. This difference was not modified by removal of chemerin.
TABLE 2.
Potency and efficacy of Chemerin-9, NE, and ACh in aortic rings from male and female WT and KO rats
| Compound |
Male WT |
Male KO |
Female WT |
Female KO |
||||
|---|---|---|---|---|---|---|---|---|
| −Log EC50 (M) | Max | −Log EC50 (M) | Max | −Log EC50 (M) | Max | −Log EC50 (M) | Max | |
| Chemerin | 6.76 ± 0.15 | 71.9 ± 10.6 | 7.11 ± 0.06* | 113.5 ± 3.6* | 6.16 ± 0.18 | 40.7 ± 7.6† | 6.79 ± 0.18* | 59.5 ± 7.8*,† |
| NE | 7.65 ± 0.16 | 143.1 ± 2.3 | 7.67 ± 0.16 | 170.1 ± 27.0 | 8.32 ± 0.16 | 159.9 ± 5.4 | 8.57 ± 0.19 | 164.6 ± 34.4 |
| ACh | 8.02 ± 0.04 | 0 ± 0 | 7.83 ± 0.04 | 0 ± 0 | 7.66 ± 0.12 | 18.91 ± 5.2† | 7.34 ± 0.04 | 16.0 ± 1.00† |
Points are means ± sem for number of animals indicated in Fig. 4. Max, maximum effect achieved, reported as a percentage of the maximum PE (Chemerin-9, NE) or as a half-maximal PE contraction remaining (ACh).
P < 0.05 between same sex value vs. WT.
P < 0.05 between different sexes of same genotype with female values marked.
Figure 4.
Arterial responses to Chemerin-9 (left), NE (middle), and ACh (against a half-maximal PE contraction; right) in rings of thoracic aorta and superior mesenteric arteries from male (A) and female (B) WT and KO rats. Points are means ± sem for the number of animals in parentheses. Asterisk denotes significant differences (P < 0.05) by a 2-way ANOVA with Bonferroni’s correction between WT and KO. Contraction to PE (y axis): male aorta: WT = 1987 ± 107 mg, KO = 1677 ± 81 mg (P > 0.05); male superior mesenteric artery: WT = 1150 ± 38 mg, KO = 944 ± 29 mg (P > 0.05); female aorta: WT = 1381 ± 70, KO = 1603 ± 66 mg (P > 0.05); female superior mesenteric artery: WT = 804 ± 26 mg, KO = 920 ± 45 mg (P > 0.05). Statistical analysis for PE contraction was done using an unpaired Student’s t test between the KO and WT values.
We investigated the possibility of an adaptive change in chemerin receptor expression by measuring mRNA expression of Chemerin-1, Chemerin-2, and CCRL2, all receptors for chemerin, in a tissue saved from the WT and KO rats used in the contractility experiments—the brown fat pad. Consistent with a loss of chemerin, Chemerin1 mRNA and Chemerin-2 mRNA were elevated in the male KO vs. WT but not the female. Expression of CCRL2 in this tissue was lower than the other 2 receptors, and differences between the WT and KO could not be detected (Supplemental Fig. S3).
Basal cardiovascular parameters of chemerin WT and KO rats are not different
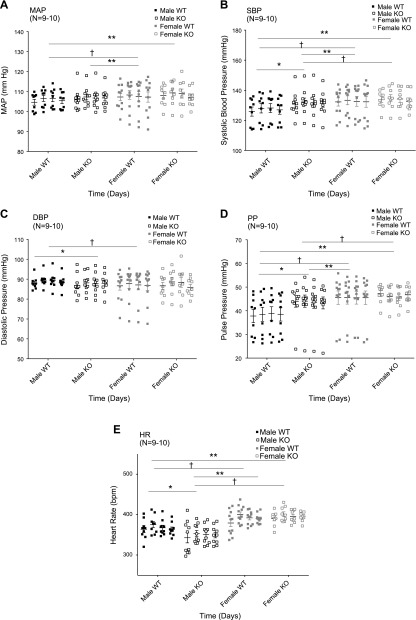
In another 3 separate cohorts of animals (born at 3 separate times, months apart; aged 10–14 wk at beginning of experiment), radiotelemeters were implanted for measurement of basal blood pressures (mean, systolic, diastolic, and pulse) and heart rate. Measures from 4 days of these parameters, gathered in the conscious state, and 5 d after telemeter implantation are shown in Fig. 5. Supplemental Fig. S4 demonstrates the circadian rhythm of these animals during the time period over which the 4 d of control measures were taken, validating that they were in normal rhythm during baseline measurements.
Figure 5.
Mean arterial pressure (MAP; A), systolic blood pressure (SBP; B), diastolic blood pressure (DBP; C), pulse pressure (PP; D), and heart rate (HR; E) in WT and KO male and female rats (number indicated in parentheses) over 4 control days. Scatterplot values for the number of animals in parentheses around horizontal bars that are means. Repeated-measures 1-way ANOVA was performed, followed by Tukey’s post hoc. Asterisk denotes statistically significant (P < 0.05) differences between WT and KO within same sex; dagger represents rats within same genotype but different sex; double asterisks signify between different sex and different genotype.
Pressures
Loss of chemerin did not change mean arterial blood pressure when comparing WT and KO within a sex (Fig. 5A). However, male KO rats had a higher systolic (Fig. 5B) and lower (Fig. 5C) diastolic pressure than their WT controls. This was reflected in the significantly lower pulse pressure in male WT compared with male KO (Fig. 5D). By contrast, female pressures—mean, systolic, diastolic, or pulse—were not changed by loss of a functional chemerin gene. The circadian rhythm of mean arterial blood pressure for a subset of animals over the course of 4 d was normal (data not shown).
Heart rate
As expected, heart rate in the females was higher than males (Fig. 5E). Male WT had modestly higher heart rates than male KO rats during this control period. Such a difference was not observed between the female WT and KO rat.
Female but not male KO rats show reduction in hypertension developed to DOCA-salt
The same animals in which basal parameters were measured all underwent a uninephrectomy and were given a DOCA-impregnated pellet (200 mg/kg) and placed on drinking water that contained 1% NaCl + 0.2% KCl. Animals were tracked for measurement of pressures and heart rate over the standard DOCA-salt treatment of 4 wk. Results are shown in Fig. 6 with DOCA-salt treatment initiated at the dotted vertical lines. Repeated-measures ANOVA for each variable was performed on all 4 groups over time. Statistical results are shown only for the genotype difference within a sex, and values for male and female are graphed separately for clarity.
Figure 6.
Mean arterial pressure (A), systolic blood pressure (B), diastolic blood pressure (C), pulse pressure (D) and heart rate (E) in WT and KO male (left) and female (right) rats given DOCA, salt water, and uninephrectomy at the dashed vertical lines on d 8/9. Points are means ± sem for the number of animals in parentheses. Repeated-measures 1-way ANOVA was performed, followed by Tukey’s post hoc on all 4 groups and only within-sex differences are shown in the graphs, grouped separately for clarity. Asterisk denotes statistically significant (P < 0.05) differences between WT and KO overall; dagger indicates difference as determined within week-to-week comparison for simplicity.
Pressures
Males responded to DOCA-salt with the expected elevation in blood pressure. Contrary to our hypothesis, the male KO had a larger increase in mean and systolic blood pressure than their WT controls (Fig. 6, left). Diastolic pressure was variable (Fig. 6C, left) over time, such that the WT and KO were not significantly different. Pulse pressure remained elevated in the male KO DOCA vs. the male WT DOCA. Females had a more modest elevation in blood pressure (∼7–8 mmHg less) compared with the male’s in response to DOCA-salt. Furthermore, in contrast to males, the increase in mean and systolic and diastolic blood pressure was lower in the female chemerin KO vs. its littermate WT controls in response to DOCA-salt (Fig. 6, right).
Heart rate
Heart rate in the male KO DOCA rat was lower than the male WT DOCA rat (Fig. 6E), whereas 2 groups of female rats given DOCA-salt continued to be statistically similar (Fig. 6E). Female heart rate remained higher than that of their male littermates.
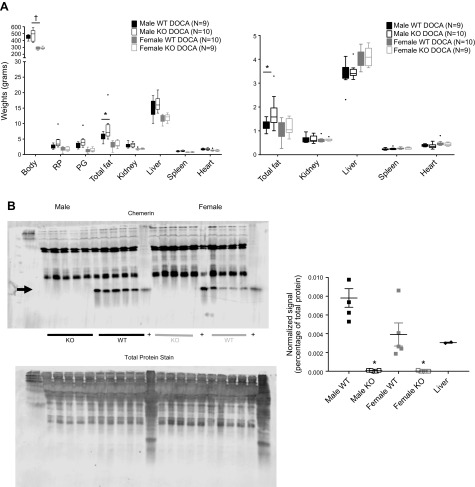
Body weights, tissues masses, and chemerin expression at end of DOCA-salt
At the end of DOCA-salt treatment, tissues were removed for measurement of body weight, fat weights, and weights of end organs affected in hypertension (kidney, liver, spleen, and heart). In Fig. 7A, left, absolute weights were compared. An elevation in the amount of RP fat and a tendency for PG fat to be elevated in the male KO DOCA compared with WT resulted in a gross measure of body fat (RP + PG) being statistically greater in the male DOCA KO vs. WT but not in the female. No such genotype-driven changes were observed in the females. The elevation in total fat in the male DOCA KO vs. WT remained true when these data were graphed as a percentage of body weight (Fig. 7A, right). When comparing WT and KO within each sex, there were no significant reductions in the weights of the kidney, heart (Fig. 7A), or arterial medial thickness/lumen ratio (data not shown), all of which are target organs/tissue of hypertension. Importantly, DOCA-salt itself did not abolish chemerin plasma expression (chemerin densitometry/total protein as arbitrary densitometry units: male WT = 0.085 ± 0.008; male WT DOCA = 0.080 ± 0.007; female WT = 0.044 ± 0.005; female WT DOCA = 0.058 ± 0.019; P > 0.05 when comparing same-sex groups by 2-tailed t test). Figure 7B shows, for a subgroup, that those animals studied in radiotelemetry in Figs. 5 and 6 showed the appropriate presence or absence of plasma chemerin in the WT and KO, respectively.
Figure 7.
A) Endpoint body and tissue weights as absolute measures (left) or as a percentage body weight (right) in WT and KO male and female rats given DOCA, salt water, and uninephrectomy for 4 wk. This box and whisker plot used the Tukey method, where the center line is the median, and the whiskers only extend to the farthest point within 1.5× the interquartile range from the edge of the box (box edges are always 25th and 75th percentiles). Points outside box are outliers. Asterisk denotes statistically significant (P < 0.05) differences between same sex WT. Dagger indicates between genders, as performed by 1-way ANOVA with Tukey’s post hoc correction. B) Comparison of plasma chemerin in male (left side of blots) and female (right side of blots) rats after 4 wk of DOCA-salt. Arrow points to chemerin. Plus sign indicates normal Sprague Dawley male (from outside of colony) liver homogenate. Total protein stains on membranes were done before blocking and adding the primary antibody against chemerin. Chemerin signal was normalized to total protein for each respective lane in the graph (right), where vertical bars are means ± sem with scattered points for number of animals in parentheses. Asterisk denotes statistically significant (P < 0.05) difference between same-sex WT.
DISCUSSION
The work presented in this manuscript is the first to describe a total body KO of the adipokine chemerin in the rat. We created this animal to test how chemerin is involved in blood pressure regulation. We hypothesized that removal of the chemerin gene/protein would reduce normal blood pressure and would attenuate the development hypertension. Our data, as collected in Table 3, falsify this hypothesis for the male but are consistent with the hypothesis for the female.
TABLE 3.
Comparison of the general outcome of the KO vs. WT during DOCA-salt hypertension
| Parameter | Male | Female |
|---|---|---|
| MAP | ↑ | ↓ |
| SBP | ↑ | ↓ |
| DBP | — | ↓ |
| PP | ↑ | — |
| HR | ↓ | — |
| Activity | ↓ | — |
| Body fat | ↑ | — |
↑, increase; ↓, decrease; —, no change; DBP, diastolic blood pressure; HR, heart rate; MAP, mean arterial pressure; SBP, systolic blood pressure.
Confidence in the KO rat
Confidence in KO of chemerin was supported by consistent and repeatable genotyping, the observation that phenotyping was consistent with genotyping, and a specific and expected upregulation of Chemerin-9-induced contraction of arteries isolated from a KO vs. WT rat (male and female). Enhanced contraction could be because of upregulated Chemerin-1 receptor expression. At least in the male brown fat pad, Chemerin-1 and Chemerin-2 mRNA was elevated, consistent with this idea. The lack of elevation in the female with chemerin KO is interesting and needs further exploration.
Westerns were used to phenotype samples, as no commercially available ELISA kits reliably measure rat chemerin. The Tango assay was an additional measure of chemerin, one that detects active forms of chemerin (58). Several limitations of this assay are important to mention. First, in performing this assay for the first time in rat plasma, we discovered that circulating rat chemerin, at least in nondiseased rats, is close to the detection level of the assay. The lower limit of detection with rat serum/plasma is ∼1 nM, and this is where the samples from the females measure, making it difficult to demonstrate a statistical difference. This assay typically has a large signal/noise ratio and dynamic range but is subject to a low background signal with biologic samples. This can be corrected, to some extent, with nonreceptor controls, which were run, and this correction has the greatest impact on samples with chemerin bioactivity, at or near the lower limit of detection, exactly the spot where our samples are. Additionally, this assay measures activity relative to a single-most active form of chemerin (Chemerin-157), and biologic samples are likely to contain multiple forms of chemerin protein. As such, the chemerin measured in this assay is a fraction of what would be observed in the Westerns. Finally, as the Tango assay relies on detection of a single mode of receptor signaling through β-arrestin recruitment, this signal may be dampened by forms of chemerin that exhibit biased agonism and do not effectively promote β-arrestin recruitment but preferentially signal through other modes. This is an area just beginning to be investigated. Overall, the approach of using the Tango assay was useful in understanding its limitations and providing at least a partial confirmatory measure that the KO (male) lacked bioactive chemerin and that males have higher levels of plasma chemerin than do females.
Chemerin KO rat is similar to the chemerin KO mouse
Creation of a rat chemerin KO was important because of the reliance of the hypertension field on the rat as a model. We have something with which to compare this new animal, as the chemerin KO mouse was created in 2011 (59). Like the chemerin KO mouse, KO rats were grossly normal, females weighed less than males, and the KO gained weight with aging, similar to their respective WT mouse. Likewise, plasma levels of cholesterol and triglycerides were not modified between KO and WT. Our studies also agree in that NEFAs were lower in the KO (female) vs. the WT. Whereas a reduction in NEFA in the KO vs. WT mice was observed, it is unclear if this was observed in both sexes. Their goals for removing chemerin in the mouse were different from ours, with their focus understanding the glucose regulation. Their KO mice were glucose intolerant compared with the WT. Whereas we did not perform a glucose challenge, it is notable that the resting glucose and triglycerides levels of all rats—KO and WT, male and female—were higher than is reported for normal male and female Sprague-Dawley rats of a similar age (60). Our animals were not denied access to food before taking their blood, and this likely is the source of the elevated glucose and triglycerides. AST (normal = 72–116 IU/L for male/female) and BUN (10–17 mg/dl for male/female) were elevated above normal in our rats compared with normal Sprague-Dawley rats (60). There was, however, a reduction (normal) AST in the male KO vs. WT, and the female KO AST was not different from WT. The same was true for BUN. It is thus unlikely that removal of chemerin causes general liver or kidney damage, which is important to know with the consideration of blood pressure regulation. Overall, there were more similarities than differences between the chemerin KO mouse and the chemerin KO rat.
Chemerin regulates blood pressure differently in the male and female rat
We neither expected to find that chemerin in the female plasma and tissues would be lower than males nor do we understand why this would be so. There has been no direct comparison of chemerin in sexes, such as we report here in the rat. This sex difference was observed in all the cohorts of animals we tested, supporting the reproducibility of this finding. A possible explanation is that at least in the DOCA females, the fat depots, as one source of chemerin, were a modestly smaller percentage of total body weight when compared with the males. Interestingly, chemerin was modestly less potent and significantly less efficacious in causing contraction of the isolated aorta from the female vs. the male rat. This was also unexpected, given the lower chemerin levels observed in the female vs. the male, where one might expect a greater magnitude of contraction in the female vessels that are exposed endogenously to a lower concentration of chemerin. This raises the idea of sex differences in chemerin receptor expression in the vasculature, as well as consideration for what long-term removal of chemerin does to receptor function. These animals were born without chemerin, an approach that is necessarily different in potential outcomes than acute removal of chemerin. These ideas will be explored in future studies.
Even with these differences in chemerin expression, loss of chemerin did not modify basal mean arterial blood pressure in either sex. The systolic and diastolic blood pressures of the male KO were significantly raised and lowered vs. the WT, respectively, such that mean arterial pressures were not modified, but pulse pressure was significantly elevated in the KO; these measures were unchanged in the female KO vs. WT. An unexpected finding was reduction of heart rate in the male KO vs. WT. There is no support for chemerin directly affecting heart rate, but a few studies investigate the interaction of chemerin and the heart. Specifically, peptidase inhibitor 16 is a regulator of chemerin processing in the myocardium (61), serum chemerin concentration is associated with atrial fibrillation and remodeling and dilated cardiomyopathy with heart failure (31, 62), chemerin induces apoptosis in murine cardiomyocytes (63), and the chemerin gene is present in baboon and chimpanzee hearts (64). Investigation into the potential for chemerin-mediated changes in cardiac function has merit.
Upon observing no differences in basal cardiovascular parameters, we moved to test the animals with a challenge. We used an approach that took the greatest care in not relying on sets of particular parents for WT and KO progeny, not selecting the time of year in which the experiment is done, and being able to show that we can reproduce our findings in-house. Our use of the DOCA-salt model in the males and females was consistent with the published finding that males typically achieve a higher magnitude of blood pressure to DOCA-salt than the WT females (by ∼7–8 mmHg) (65–70). Contrary to our hypothesis, removal of chemerin elevated in the male but reduced in the female the magnitude of blood pressure elevation achieved with DOCA-salt. These data suggest that chemerin could be anti-hypertensive in the male and pro-hypertensive in the female. We did not investigate arterial contractility (response to Chemerin-9, NE, and ACh) in the DOCA rats, and this would be interesting to do from the perspective of knowing whether the male exhibited vascular hyper-reactivity, whereas the female did not.
Sex differences in chemerin’s biologic actions have not been investigated. The possibility that chemerin could act in such opposing manners is supported by a literature that poses chemerin as being both inflammatory and anti-inflammatory. Generally, studies support chemerin being prohypertensive, proinflammatory, and propathogenic; these studies are the premise for the original hypothesis of this work (5, 9, 71–77). By contrast, chemerin has been described as exerting anti-inflammatory actions (78–81). For example, Cash et al. (82) report that a synthetic, chemerin-derived peptide, Chemerin-15, suppressed inflammation, as mediated by the macrophage. Likewise, chemerin suppressed murine allergic asthma (83) in the mouse and exerted anti-inflammatory roles in human vascular endothelial cells (81). The Chemerin-1 receptor mediated both proinflammatory and anti-inflammatory actions in diesel exhaust particulate-induced acute lung inflammation in the mouse (84) and reduced lung inflammation in a mouse model of acute viral pneumonia (85). Others, though, have not found Chemerin-15 to be effective in ameliorating inflammatory disease (nonalcoholic steatohepatitis) (86) or for causing direct arterial relaxation (38). The complexity of the biologic effects of chemerin is just beginning to be unraveled.
A possibility is that loss of chemerin unmasks a difference in response of males and females to other endogenous Chemerin-1 receptor agonists, such as resolvin E1, a proposed activator of the Chemerin-1 receptor that provides resolution to inflammation (87). For this to be true, resolvin E1 levels would have to be higher in the females than the males, and these data are not available in the rat or human. This, though, assumes that it is only actions mediated by the Chemerin-1 receptor that would be influenced by chemerin removal. Two other receptors for chemerin exist: Chemerin-2 (formerly G protein-coupled receptor 1) and CCRL2 (73, 88, 89). Chemerin-2 can mediate biologic signals activated by chemerin, whereas CCRL2 appears to be more of a chaperone of chemerin. Chemerin-1 and Chemerin-2 mRNA levels were higher in the male KO vs. WT (brown fat pad), suggesting that there are adaptations to loss of chemerin. It will be our job to understand whether this occurs throughout the body, including the vasculature, and why this does not occur in the female. It stands to reason that this difference in adaption could be why males and females are different; other receptors and agonists come to “rescue” the lack of chemerin in the male but not the female. We cannot exclude that cardiovascular processes mediated by either of these receptors or that the combination of all 3 are what mediates, in the chemerin KO, the rise in arterial pressure in males but falls in the females when given DOCA-salt. One might expect that the male KO, and less so the female KO, would be protected from hypertension because of loss of the more highly expressed chemerin. How chemerin functions at different sites that control blood pressure, as well as interacts with these different receptors, is something we simply do not know. We could not test some of these ideas, as specific agonists/antagonists for Chemerin-2 and CCRL2 do not exist. Likewise, we have not been able to use the Chemerin-1 receptor antagonist CCX832 (38) in in vivo experiments. It would be ideal to have an antagonist at each chemerin receptor to, in an acute manner, understand the involvement of each chemerin receptor in the regulation of blood pressure. Chemerin removal could also cause changes in the male and female complement of active immune cells, creating functional differences between the sexes, such that final blood pressures are modified. This is reasonable to consider given the well-known actions of chemerin on immune cell movement and function (1, 5, 37, 71) and the role of immune cells, such as the macrophage in DOCA-salt hypertension (90).
We have also studied only one model of experimental hypertension. The DOCA-salt model was used with purpose. Arteries isolated from the DOCA-salt rat showed a profoundly increased contraction to chemerin-9 when compared with arteries from sham rats (38). This model is also known to depend on activation of the sympathetic nervous system, a system that chemerin amplified at the level of the arterial neuroeffector junction (46). We hypothesized that we would have the best chance of observing an effect on blood pressure in the DOCA-salt model. Interestingly, the DOCA-salt model showed a significant loss in fat (∼50%) compared with animals that are close in age (compare Fig. 3B with 7B). As such, the contributions of chemerin could have been underestimated by using a model that has loss of fat, 1 source of chemerin.
Is chemerin expression different in male and female humans?
Are these findings meaningful to the human? The literature is considerably diverse about sex differences in chemerin expression. Some literature supports different chemerin levels in the female vs. male and different responses in a pathologic state, although caution must be taken, as chemerin was measured from different body sources. For example, nocturnal serum chemerin levels were higher in the obese female vs. normal weight controls but not in males (91). Likewise, chemerin mRNA expression in subcutaneous fat was significantly higher in women vs. men (92), but plasma levels were not different. A similar finding of higher s.c. chemerin mRNA expression in female vs. male (normal) was observed by Martínez-García et al. (93). This raises the question of whether and how subcutaneous fat contributes to circulating chemerin levels. Schmid et al. (94) demonstrated a significantly higher basal chemerin serum concentration in the female vs. the male in unfed, healthy volunteers. By contrast to these reports that females have higher plasma levels or subcutaneous mRNA of chemerin than males, Luque-Ramírez et al. (95) found no difference in male vs. female. In a control group from a Japanese population diagnosed with metabolic syndrome or type 2 diabetes mellitus, chemerin levels in control males were higher than females (96). In our model, chemerin levels in the plasma in multiple cohorts were lower in the female vs. the male WT rats. The diversity in outcome of this collective literature points to the importance of recognizing the source of chemerin and the potential that local actions of chemerin may be more biologically important than that reflected by plasma measures.
CONCLUSIONS
Creation of the chemerin KO rat by CRISPR/Cas9 technology revealed an unexpected sex difference in the ability of chemerin to modify blood pressure during challenge with a hypertensive stimulus of mineralocorticoid and salt. These intriguing data elevate the importance of studying chemerin in blood pressure regulation with an understanding that the chemerin–chemerin receptor axis responds to absence of chemerin somewhat differently in the male and female rat. Likewise, the finding of a significantly different level of chemerin in the female vs. male begs the question as to why this is so, whether this occurs at other levels of the chemerin–chemerin receptor axis, and whether this could be confirmed in independent labs in the human. Such a finding would elevate chemerin and their receptors as potential biologic targets that may be sex dependent.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors are grateful to Bridget Seitz (Department of Pharmacology and Toxicology, Michigan State University) for helping with blood procurement for making plasma samples. This work was supported by the U.S. National Institutes of Health/National Heart, Lung, and Blood Institute, (Grant HL 117847). The authors declare no conflicts of interest.
Glossary
- ACh
acetylcholine
- AST
aspartate aminotransferase
- BUN
blood urea nitrogen
- CRISPR/Cas
clustered regularly interspaced short palindromic repeats/clustered regularly interspaced short palindromic repeats-associated system
- DOCA
deoxycorticosterone acetate
- indel
insertion/deletion mutation
- KO
knockout
- NE
norepinephrine
- NEFA
nonesterified fatty acid
- PE
phenylephrine
- PG
perigonadal
- PSS
physiologic salt solution
- PVAT
perivascular adipose tissue
- Rarres2
retinoic acid receptor responder protein 2
- RP
retroperitoneal
- RRID
Research Resource Identifier
- sgRNA
single-guide RNA
- tTA
transcriptional-transactivator
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. W. Watts conceived of the idea, designed research, conducted experiments, acquired data, analyzed data, and wrote the manuscript; E. S. Darios designed research, conducted experiments, acquired data, analyzed data, and modified the manuscript; A. E. Mullick conducted experiments, acquired data, analyzed data, and modified the manuscript; H. Garver conducted experiments, acquired data, and modified the manuscript; T. L. Saunders provided the reagent (creation of original rats), conducted experiments, acquired data, analyzed experiments, and modified the manuscript; E. D. Hughes identified the CRISPR reagent that caused chromosome breaks in Rarres2 and modified the manuscript; W. E. Filipiak performed rat zygote microinjection to produce chemerin KO rats and modified the manuscript; M. G. Zeidler developed the genotyping methods to identify mutant animals, performed DNA cloning to define the Rarres2 mutation, and modified the manuscript; N. McMullen performed Tango experiments, acquired data, analyzed data, and modified the manuscript; C. J. Sinal developed the Tango assay and performed these analyses and modified the manuscript; R. K. Kumar performed the adipokine array experiments and analyses and modified the manuscript; D. J. Ferland helped with Tango analyses, performed the RT-PCR for the chemerin receptors, and modified the manuscript; and G. D. Fink helped with experimental design for in vivo experiments, acquired data, and modified the manuscript.
REFERENCES
- 1.Wittamer V., Franssen J.-D., Vulcano M., Mirjolet J.-F., Le Poul E., Migeotte I., Brézillon S., Tyldesley R., Blanpain C., Detheux M., Mantovani A., Sozzani S., Vassart G., Parmentier M., Communi D. (2003) Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 198, 977–985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bozaoglu K., Bolton K., McMillan J., Zimmet P., Jowett J., Collier G., Walder K., Segal D. (2007) Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 148, 4687–4694 [DOI] [PubMed] [Google Scholar]
- 3.Goralski K. B., McCarthy T. C., Hanniman E. A., Zabel B. A., Butcher E. C., Parlee S. D., Muruganandan S., Sinal C. J. (2007) Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 282, 28175–28188 [DOI] [PubMed] [Google Scholar]
- 4.Roh S. G., Song S. H., Choi K. C., Katoh K., Wittamer V., Parmentier M., Sasaki S. (2007) Chemerin--a new adipokine that modulates adipogenesis via its own receptor. Biochem. Biophys. Res. Commun. 362, 1013–1018 [DOI] [PubMed] [Google Scholar]
- 5.Ernst M. C., Sinal C. J. (2010) Chemerin: at the crossroads of inflammation and obesity. Trends Endocrinol. Metab. 21, 660–667 [DOI] [PubMed] [Google Scholar]
- 6.Sell H., Divoux A., Poitou C., Basdevant A., Bouillot J.-L., Bedossa P., Tordjman J., Eckel J., Clément K. (2010) Chemerin correlates with markers for fatty liver in morbidly obese patients and strongly decreases after weight loss induced by bariatric surgery. J. Clin. Endocrinol. Metab. 95, 2892–2896 [DOI] [PubMed] [Google Scholar]
- 7.Shin H. Y., Lee D. C., Chu S. H., Jeon J. Y., Lee M. K., Im J. A., Lee J. W. (2012) Chemerin levels are positively correlated with abdominal visceral fat accumulation. Clin. Endocrinol. (Oxf.) 77, 47–50 [DOI] [PubMed] [Google Scholar]
- 8.Arica P. C., Aydin S., Zengin U., Kocael A., Orhan A., Zengin K., Gelisgen R., Taskin M., Uzun H. (2018) The effects on obesity related peptides of laparoscopic gastric band applications in morbidly obese patients. J. Invest. Surg. 31, 89–95 [DOI] [PubMed] [Google Scholar]
- 9.Chang S. S., Eisenberg D., Zhao L., Adams C., Leib R., Morser J., Leung L. (2016) Chemerin activation in human obesity. Obesity (Silver Spring) 24, 1522–1529 [DOI] [PubMed] [Google Scholar]
- 10.Sledzińska M., Szlagatys-Sidorkiewicz A., Brzezinski M., Kaźmierska K., Sledziński T., Kamińska B. (2017) Serum chemerin in children with excess body weight may be associated with ongoing metabolic complications—a pilot study. Adv. Med. Sci. 62, 383–386 [DOI] [PubMed] [Google Scholar]
- 11.Stejskal D., Karpisek M., Hanulova Z., Svestak M. (2008) Chemerin is an independent marker of the metabolic syndrome in a Caucasian population--a pilot study. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc Czech Repub. 152, 217–221 [DOI] [PubMed] [Google Scholar]
- 12.Ebert T., Gebhardt C., Scholz M., Wohland T., Schleinitz D., Fasshauer M., Bluher M., Stumvoll M., Kovacs P., Tonjes A. (2018) Relationship between twelve adipocytokinesa and distinct components of the metabolic syndrome. J. Clin. Endocrinol. Metab. 103, 1015–1023 [DOI] [PubMed] [Google Scholar]
- 13.Wolk K., Sabat R. (2016) Adipokines in psoriasis: an important link between skin inflammation and metabolic alterations. Rev. Endocr. Metab. Disord. 17, 305–317 [DOI] [PubMed] [Google Scholar]
- 14.Zylla S., Pietzner M., Kühn J. P., Völzke H., Dörr M., Nauck M., Friedrich N. (2017) Serum chemerin is associated with inflammatory and metabolic parameters—results of a population-based study. Obesity (Silver Spring) 25, 468–475 [DOI] [PubMed] [Google Scholar]
- 15.Li Y., Shi B., Li S. (2014) Association between serum chemerin concentrations and clinical indices in obesity or metabolic syndrome: a meta-analysis. PLoS One 9, e113915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang D., Yuan G. Y., Wang X. Z., Jia J., Di L. L., Yang L., Chen X., Qian F. F., Chen J. J. (2013) Plasma chemerin level in metabolic syndrome. Genet. Mol. Res. 12, 5986–5991 [DOI] [PubMed] [Google Scholar]
- 17.Dessein P. H., Tsang L., Woodiwiss A. J., Norton G. R., Solomon A. (2014) Circulating concentrations of the novel adipokine chemerin are associated with cardiovascular disease risk in rheumatoid arthritis. J. Rheumatol. 41, 1746–1754 [DOI] [PubMed] [Google Scholar]
- 18.Yang M., Yang G., Dong J., Liu Y., Zong H., Liu H., Boden G., Li L. (2010) Elevated plasma levels of chemerin in newly diagnosed type 2 diabetes mellitus with hypertension. J. Investig. Med. 58, 883–886 [DOI] [PubMed] [Google Scholar]
- 19.Han J., Kim S. H., Suh Y. J., Lim H. A., Shin H., Cho S. G., Kim C. W., Lee S. Y., Lee D. H., Hong S., Kim Y. S., Nam M. S. (2016) Serum chemerin levels are associated with abdominal visceral fat in type 2 diabetes. J. Korean Med. Sci. 31, 924–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu B., Zhao M., Jiang W., Ma J., Yang C., Shao J., Gu P. (2015) Independent association of circulating level of chemerin with functional and early morphological vascular changes in newly diagnosed type 2 diabetic patients. Medicine (Baltimore) 94, e1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang R., Yue J., Sun Y., Zheng J., Tao T., Li S., Liu W. (2015) Increased serum chemerin concentrations in patients with polycystic ovary syndrome: relationship between insulin resistance and ovarian volume. Clin. Chim. Acta 450, 366–369 [DOI] [PubMed] [Google Scholar]
- 22.Kort D. H., Kostolias A., Sullivan C., Lobo R. A. (2015) Chemerin as a marker of body fat and insulin resistance in women with polycystic ovary syndrome. Gynecol. Endocrinol. 31, 152–155 [DOI] [PubMed] [Google Scholar]
- 23.Yang S., Wang Q., Huang W., Song Y., Feng G., Zhou L., Tan J. (2016) Are serum chemerin levels different between obese and non-obese polycystic ovary syndrome women? Gynecol. Endocrinol. 32, 38–41 [DOI] [PubMed] [Google Scholar]
- 24.Dong B., Ji W., Zhang Y. (2011) Elevated serum chemerin levels are associated with the presence of coronary artery disease in patients with metabolic syndrome. Intern. Med. 50, 1093–1097 [DOI] [PubMed] [Google Scholar]
- 25.Feng X., Li P., Zhou C., Jia X., Kang J. (2012) Elevated levels of serum chemerin in patients with obstructive sleep apnea syndrome. Biomarkers 17, 248–253 [DOI] [PubMed] [Google Scholar]
- 26.Hu W., Feng P. (2011) Elevated serum chemerin concentrations are associated with renal dysfunction in type 2 diabetic patients. Diabetes Res. Clin. Pract. 91, 159–163 [DOI] [PubMed] [Google Scholar]
- 27.Leiherer A., Muendlein A., Kinz E., Vonbank A., Rein P., Fraunberger P., Malin C., Saely C.H., Drexel H. (2016) High plasma chemerin is associated with renal dysfunction and predictive for cardiovascular events: insights from phenotype and genotype characterization. Vascul. Pharmacol. 77, 60–68 [DOI] [PubMed] [Google Scholar]
- 28.Blaszak J., Szolkiewicz M., Sucajtys-Szulc E., Konarzewski M., Lizakowski S., Swierczynski J., Rutkowski B. (2015) High serum chemerin level in CKD patients is related to kidney function, but not to its adipose tissue overproduction. Ren. Fail. 37, 1033–1038 [DOI] [PubMed] [Google Scholar]
- 29.Zhao D., Bi G., Feng J., Huang R., Chen X. (2015) Association of serum chemerin levels with acute ischemic stroke and carotid artery atherosclerosis in a Chinese population. Med. Sci. Monit. 21, 3121–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kukla M., Zwirska-Korczala K., Hartleb M., Waluga M., Chwist A., Kajor M., Ciupinska-Kajor M., Berdowska A., Wozniak-Grygiel E., Buldak R. (2010) Serum chemerin and vaspin in non-alcoholic fatty liver disease. Scand. J. Gastroenterol. 45, 235–242 [DOI] [PubMed] [Google Scholar]
- 31.Zhang G., Xiao M., Zhang L., Zhao Y., Yang Q. (2017) Association of serum chemerin concentrations with the presence of atrial fibrillation. Ann. Clin. Biochem. 54, 342–347 [DOI] [PubMed] [Google Scholar]
- 32.Aksu F., Caliskan M., Keles N., Erek Toprak A., Uzuncakmak T. K., Kostek O., Yilmaz Y., Demircioglu K., Cekin E., Ozturk I., Karadag A. S. (2017) Chemerin as a marker of subclinical cardiac involvement in psoriatic patients. Cardiol. J. 24, 276–283 [DOI] [PubMed] [Google Scholar]
- 33.Stepan H., Philipp A., Roth I., Kralisch S., Jank A., Schaarschmidt W., Lössner U., Kratzsch J., Blüher M., Stumvoll M., Fasshauer M. (2011) Serum levels of the adipokine chemerin are increased in preeclampsia during and 6 months after pregnancy. Regul. Pept. 168, 69–72 [DOI] [PubMed] [Google Scholar]
- 34.Cetin O., Kurdoglu Z., Kurdoglu M., Sahin H. G. (2017) Chemerin level in pregnancies complicated by preeclampsia and its relation with disease severity and neonatal outcomes. J. Obstet. Gynaecol. 37, 195–199 [DOI] [PubMed] [Google Scholar]
- 35.Duan D. M., Niu J. M., Lei Q., Lin X. H., Chen X. (2011) Serum levels of the adipokine chemerin in preeclampsia. J. Perinat. Med. 40, 121–127 [DOI] [PubMed] [Google Scholar]
- 36.Eichelmann F., Weikert C., di Giuseppe R., Biemann R., Isermann B., Schulze M. B., Boeing H., Aleksandrova K. (2017) Methodological utility of chemerin as a novel biomarker of immunity and metabolism. Endocr. Connect. 6, 340–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wittamer V., Grégoire F., Robberecht P., Vassart G., Communi D., Parmentier M. (2004) The C-terminal nonapeptide of mature chemerin activates the chemerin receptor with low nanomolar potency. J. Biol. Chem. 279, 9956–9962 [DOI] [PubMed] [Google Scholar]
- 38.Watts S. W., Dorrance A. M., Penfold M. E., Rourke J. L., Sinal C. J., Seitz B., Sullivan T. J., Charvat T. T., Thompson J. M., Burnett R., Fink G. D. (2013) Chemerin connects fat to arterial contraction. Arterioscler. Thromb. Vasc. Biol. 33, 1320–1328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kennedy A. J., Yang P., Read C., Kuc R. E., Yang L., Taylor E. J., Taylor C. W., Maguire J. J., Davenport A. P. (2016) Chemerin eleicts potent constrictor actions via chemokine-like receptor 1 (CMKLR1), not G-protein coupled receptor 1 (GPR1)—in human and rat vasculature. J. Am. Heart Assoc. 5, e004421 https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27742615&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davenport A. P., Alexander S. P., Sharman J. L., Pawson A. J., Benson H. E., Monaghan A. E., Liew W. C., Mpamhanga C. P., Bonner T. I., Neubig R. R., Pin J. P., Spedding M., Harmar A. J. (2013) International Union of Basic and Clinical Pharmacology. LXXXVIII. G Protein-coupled receptor list: recommendations for new pairings with cognate ligands. Pharmacol. Rev. 65, 967–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy A. J., Davenport A. P. (2018) International Union of Basic and Clinical Pharmacology CIII: chemerin receptors CMKLR1 (chemerin1) and GPR1 (chemerin2) nomenclature, pharmacology and function. Pharmacol. Rev. 70, 174–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferland D. J., Darios E. S., Neubig R. R., Sjögren B., Truong N., Torres R., Dexheimer T. S., Thompson J. M., Watts S. W. (2017) Chemerin-induced arterial contraction is Gi- and calcium-dependent. Vascul. Pharmacol. 88, 30–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lobato N. S., Neves K. B., Filgueira F. P., Fortes Z. B., Carvalho M. H., Webb R. C., Oliveira A. M., Tostes R. C. (2012) The adipokine chemerin augments vascular reactivity to contractile stimuli via activation of the MEK-ERK1/2 pathway. Life Sci. 91, 600–606 [DOI] [PubMed] [Google Scholar]
- 44.Neves K. B., Nguyen Dinh Cat A., Lopes R. A., Rios F. J., Anagnostopoulou A., Lobato N. S., de Oliveira A. M., Tostes R. C., Montezano A. C., Touyz R. M. (2015) Chemerin regulates crosstalk between adipocytes and vascular cells through Nox. Hypertension 66, 657–666 [DOI] [PubMed] [Google Scholar]
- 45.Neves K. B., Lobato N. S., Lopes R. A., Filgueira F. P., Zanotto C. Z., Oliveira A. M., Tostes R. C. (2014) Chemerin reduces vascular nitric oxide/cGMP signalling in rat aorta: a link to vascular dysfunction in obesity? Clin. Sci. (Lond.) 127, 111–122 [DOI] [PubMed] [Google Scholar]
- 46.Darios E. S., Winner B. M., Charvat T., Krasinksi A., Punna S., Watts S. W. (2016) The adipokine chemerin amplifies electrical field-stimulated contraction in the isolated rat superior mesenteric artery. Am. J. Physiol. Heart Circ. Physiol. 311, H498–H507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kunimoto H., Kazama K., Takai M., Oda M., Okada M., Yamawaki H. (2015) Chemerin promotes the proliferation and migration of vascular smooth muscle and increases mouse blood pressure. Am. J. Physiol. Heart Circ. Physiol. 309, H1017–H1028 [DOI] [PubMed] [Google Scholar]
- 48.Kostopoulos C. G., Spiroglou S. G., Varakis J. N., Apostolakis E., Papadaki H. H. (2014) Chemerin and CMKLR1 expression in human arteries and periadventitial fat: a possible role for local chemerin in atherosclerosis? BMC Cardiovasc. Disord. 14, 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cong L., Ran F. A., Cox D., Lin S., Barretto R., Habib N., Hsu P. D., Wu X., Jiang W., Marraffini L. A., Zhang F. (2013) Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mali P., Yang L., Esvelt K. M., Aach J., Guell M., DiCarlo J. E., Norville J. E., Church G. M. (2013) RNA-guided human genome engineering via Cas9. Science 339, 823–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hsu P. D., Scott D. A., Weinstein J. A., Ran F. A., Konermann S., Agarwala V., Li Y., Fine E. J., Wu X., Shalem O., Cradick T. J., Marraffini L. A., Bao G., Zhang F. (2013) DNA targeting specificity of RNA-guided Cas9 nucleases. Nat. Biotechnol. 31, 827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ran F. A., Hsu P. D., Wright J., Agarwala V., Scott D. A., Zhang F. (2013) Genome engineering using the CRISPR-Cas9 system. Nat. Protoc. 8, 2281–2308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McBurney M. W., Fournier S., Jardine K., Sutherland L. (1994) Intragenic regions of the murine Pgk-1 locus enhance integration of transfected DNAs into genomes of embryonal carcinoma cells. Somat. Cell Mol. Genet. 20, 515–528 [DOI] [PubMed] [Google Scholar]
- 54.Otto E. A., Helou J., Allen S. J., O’Toole J. F., Wise E. L., Ashraf S., Attanasio M., Zhou W., Wolf M. T., Hildebrandt F. (2008) Mutation analysis in nephronophthisis using a combined approach of homozygosity mapping, CEL I endonuclease cleavage, and direct sequencing. Hum. Mutat. 29, 418–426 [DOI] [PubMed] [Google Scholar]
- 55.Filipiak W. E., Saunders T. L. (2006) Advances in transgenic rat production. Transgenic Res. 15, 673–686 [DOI] [PubMed] [Google Scholar]
- 56.Mashiko D., Fujihara Y., Satouh Y., Miyata H., Isotani A., Ikawa M. (2013) Generation of mutant mice by pronuclear injection of circular plasmid expressing Cas9 and single guided RNA. Sci. Rep. 3, 3355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Geng L., Xin W., Huang D. W., Feng G. (2006) A universal cloning vector using vaccinia topoisomerase I. Mol. Biotechnol. 33, 23–28 [DOI] [PubMed] [Google Scholar]
- 58.Parlee S. D., Ernst M. C., Muruganandan S., Sinal C. J., Goralski K. B. (2010) Serum chemerin levels vary with time of day and are modified by obesity and tumor necrosis factor-alpha. Endocrinology 151, 2590–2602 [DOI] [PubMed] [Google Scholar]
- 59.Takahashi M., Okimura Y., Iguchi G., Nishizawa H., Yamamoto M., Suda K., Kitazawa R., Fujimoto W., Takahashi K., Zolotaryov F. N., Hong K. S., Kiyonari H., Abe T., Kaji H., Kitazawa S., Kasuga M., Chihara K., Takahashi Y. (2011) Chemerin regulates β-cell function in mice. Sci. Rep. 1, 123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Charles River Laboratories. (2016) Resources: Clinical laboratory parameters for Crl: CD (SD) rats. Retrieved November 9, 2017, from http://www.criver.com/files/pdfs/rms/cd/rm_rm_r_clinical_parameters_cd_rat_06.aspx
- 61.Regn M., Laggerbauer B., Jentzsch C., Ramanujam D., Ahles A., Sichler S., Calzada-Wack J., Koenen R. R., Braun A., Nieswandt B., Engelhardt S. (2016) Peptidase inhibitor 16 is a membrane-tethered regulator of chemerin processing in the myocardium. J. Mol. Cell. Cardiol. 99, 57–64 [DOI] [PubMed] [Google Scholar]
- 62.Zhang O., Ji Q., Lin Y., Wang Z., Huang Y., Lu W., Liu X., Zhang J., Liu Y., Zhou Y. J. (2015) Circulating chemerin levels elevated in dilated cardiomyopathy patients with overt heart failure. Clin. Chim. Acta 448, 27–32 [DOI] [PubMed] [Google Scholar]
- 63.Rodríguez-Penas D., Feijóo-Bandín S., García-Rúa V., Mosquera-Leal A., Durán D., Varela A., Portolés M., Roselló-Lletí E., Rivera M., Diéguez C., Gualillo O., González-Juanatey J. R., Lago F. (2015) The adipokine chemerin induces apoptosis in cardiomyocytes. Cell. Physiol. Biochem. 37, 176–192 [DOI] [PubMed] [Google Scholar]
- 64.González-Alvarez R., Garza-Rodríguez Mde L., Delgado-Enciso I., Treviño-Alvarado V. M., Canales-Del-Castillo R., Martínez-De-Villarreal L. E., Lugo-Trampe Á., Tejero M. E., Schlabritz-Loutsevitch N. E., Rocha-Pizaña M. R., Cole S. A., Reséndez-Pérez D., Moises-Alvarez M., Comuzzie A. G., Barrera-Saldaña H. A., Garza-Guajardo R., Barboza-Quintana O., Rodríguez-Sánchez I. P. (2015) Molecular evolution and expression profile of the chemerine encoding gene RARRES2 in baboon and chimpanzee. Biol. Res. 48, 31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dai S. Y., Peng W., Zhang Y. P., Li J. D., Shen Y., Sun X. F. (2015) Brain endogenous angiotensin II receptor type 2 (AT2-R) protects against DOCA/salt-induced hypertension in female rats. J. Neuroinflammation 12, 47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Giachini F. R., Sullivan J. C., Lima V. V., Carneiro F. S., Fortes Z. B., Pollock D. M., Carvalho M. H., Webb R. C., Tostes R. C. (2010) Extracellular signal-regulated kinase 1/2 activation, via downregulation of mitogen-activated protein kinase phosphatase 1, mediates sex differences in desoxycorticosterone acetate-salt hypertension vascular reactivity. Hypertension 55, 172–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kawanishi H., Hasegawa Y., Nakano D., Ohkita M., Takaoka M., Ohno Y., Matsumura Y. (2007) Involvement of the endothelin ET(B) receptor in gender differences in deoxycorticosterone acetate-salt-induced hypertension. Clin. Exp. Pharmacol. Physiol. 34, 280–285 [DOI] [PubMed] [Google Scholar]
- 68.Tostes R. C., David F. L., Carvalho M. H., Nigro D., Scivoletto R., Fortes Z. B. (2000) Gender differences in vascular reactivity to endothelin-1 in deoxycorticosterone-salt hypertensive rats. J. Cardiovasc. Pharmacol. 36(5 Suppl 1), S99–S101 [DOI] [PubMed] [Google Scholar]
- 69.Lange D. L., Haywood J. R., Hinojosa-Laborde C. (1998) Role of the adrenal medullae in male and female DOCA-salt hypertensive rats. Hypertension 31, 403–408 [DOI] [PubMed] [Google Scholar]
- 70.Stallone J. N. (1995) Mesenteric vascular responses to vasopressin during development of DOCA-salt hypertension in male and female rats. Am. J. Physiol. 268, R40–R49 [DOI] [PubMed] [Google Scholar]
- 71.Hart R., Greaves D. R. (2010) Chemerin contributes to inflammation by promoting macrophage adhesion to VCAM-1 and fibronectin through clustering of VLA-4 and VLA-5. J. Immunol. 185, 3728–3739 [DOI] [PubMed] [Google Scholar]
- 72.Ferland D. J., Watts S. W. (2015) Chemerin: a comprehensive review elucidating the need for cardiovascular research. Pharmacol. Res. 99, 351–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rourke J. L., Dranse H. J., Sinal C. J. (2013) Towards an integrative approach to understanding the role of chemerin in human health and disease. Obes. Rev. 14, 245–262 [DOI] [PubMed] [Google Scholar]
- 74.Xiong W., Luo Y., Wu L., Liu F., Liu H., Li J., Liao B., Dong S. (2016) Chemerin stimulates vascular smooth muscle cell proliferation and carotid neointimal hyperplasia by activating mitogen-activated protein kinase signaling. PLoS One 11, e0165305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meric M., Soylu K., Avci B., Yuksel S., Gulel O., Yenercag M., Coksevim M., Uzun A. (2014) Evaluation of plasma chemerin levels in patients with non-dipper blood pressure patterns. Med. Sci. Monit. 20, 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Gu P., Cheng M., Hui X., Lu B., Jiang W., Shi Z. (2015) Elevating circulation chemerin level is associated with endothelial dysfunction and early atherosclerotic changes in essential hypertensive patients. J. Hypertens. 33, 1624–1632 [DOI] [PubMed] [Google Scholar]
- 77.Gu P., Jiang W., Lu B., Shi Z. (2014) Chemerin is associated with inflammatory markers and metabolic syndrome phenotypes in hypertension patients. Clin. Exp. Hypertens. 36, 326–332 [DOI] [PubMed] [Google Scholar]
- 78.Godlewska U., Brzoza P., Sroka A., Majewski P., Jentsch H., Eckert M., Eick S., Potempa J., Zabel B. A., Cichy J. (2017) Antimicrobial and attractant roles for chemerin in the oral cavity during inflammatory gum disease. Front. Immunol. 8, 353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wargent E. T., Zaibi M. S., O’Dowd J. F., Cawthorne M. A., Wang S. J., Arch J. R., Stocker C. J. (2015) Evidence from studies in rodents and in isolated adipocytes that agonists of the chemerin receptor CMKLR1 may be beneficial in the treatment of type 2 diabetes. PeerJ 3, e753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wang L., Yang T., Ding Y., Zhong Y., Yu L., Peng M. (2015) Chemerin plays a protective role by regulating human umbilical vein endothelial cell-induced nitric oxide signaling in preeclampsia. Endocrine 48, 299–308 [DOI] [PubMed] [Google Scholar]
- 81.Yamawaki H., Kameshima S., Usui T., Okada M., Hara Y. (2012) A novel adipocytokine, chemerin exerts anti-inflammatory roles in human vascular endothelial cells. Biochem. Biophys. Res. Commun. 423, 152–157 [DOI] [PubMed] [Google Scholar]
- 82.Cash J. L., Hart R., Russ A., Dixon J. P., Colledge W. H., Doran J., Hendrick A. G., Carlton M. B., Greaves D. R. (2008) Synthetic chemerin-derived peptides suppress inflammation through ChemR23. J. Exp. Med. 205, 767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zhao L., Yang W., Yang X., Lin Y., Lv J., Dou X., Luo Q., Dong J., Chen Z., Chu Y., He R. (2014) Chemerin suppresses murine allergic asthma by inhibiting CCL2 production and subsequent airway recruitment of inflammatory dendritic cells. Allergy 69, 763–774 [DOI] [PubMed] [Google Scholar]
- 84.Provoost S., De Grove K. C., Fraser G. L., Lannoy V. J., Tournoy K. G., Brusselle G. G., Maes T., Joos G. F. (2016) Pro- and anti-inflammatory role of ChemR23 signaling in pollutant-induced inflammatory lung responses. J. Immunol. 196, 1882–1890 [DOI] [PubMed] [Google Scholar]
- 85.Bondue B., Vosters O., de Nadai P., Glineur S., De Henau O., Luangsay S., Van Gool F., Communi D., De Vuyst P., Desmecht D., Parmentier M. (2011) ChemR23 dampens lung inflammation and enhances anti-viral immunity in a mouse model of acute viral pneumonia. PLoS Pathog. 7, e1002358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Pohl R., Rein-Fischboeck L., Meier E. M., Eisinger K., Krautbauer S., Buechler C. (2015) Resolvin E1 and chemerin C15 peptide do not improve rodent non-alcoholic steatohepatitis. Exp. Mol. Pathol. 98, 295–299 [DOI] [PubMed] [Google Scholar]
- 87.Arita M., Ohira T., Sun Y. P., Elangovan S., Chiang N., Serhan C. N. (2007) Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J. Immunol. 178, 3912–3917 [DOI] [PubMed] [Google Scholar]
- 88.Mattern A., Zellmann T., Beck-Sickinger A. G. (2014) Processing, signaling, and physiological function of chemerin. IUBMB Life 66, 19–26 [DOI] [PubMed] [Google Scholar]
- 89.Bondue B., Wittamer V., Parmentier M. (2011) Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 22, 331–338 [DOI] [PubMed] [Google Scholar]
- 90.Mui R. K., Fernandes R., Garver H. G., van Rooijen N., Galligan J. J. (2018) Macrophage-dependent impairment of α2-adrenergic autoreceptor function in sympathetic neurons from DOCA-salt but not high fat diet-induced hypertensive rats., Am. J. Physiol. Heart Circ. 314, H863–H877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Daxer J., Herttrich T., Zhao Y. Y., Vogel M., Hiemisch A., Scheuermann K., Körner A., Kratzsch J., Kiess W., Quante M. (2017) Nocturnal levels of chemerin and progranulin in adolescents: influence of sex, body mass index, glucose metabolism and sleep. J. Pediatr. Endocrinol. Metab. 30, 57–61 [DOI] [PubMed] [Google Scholar]
- 92.Alfadda A. A., Sallam R. M., Chishti M. A., Moustafa A. S., Fatma S., Alomaim W. S., Al-Naami M. Y., Bassas A. F., Chrousos G. P., Jo H. (2012) Differential patterns of serum concentration and adipose tissue expression of chemerin in obesity: adipose depot specificity and gender dimorphism. Mol. Cells 33, 591–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Martínez-García M. A., Montes-Nieto R., Fernández-Durán E., Insenser M., Luque-Ramírez M., Escobar-Morreale H. F. (2013) Evidence for masculinization of adipokine gene expression in visceral and subcutaneous adipose tissue of obese women with polycystic ovary syndrome (PCOS). J. Clin. Endocrinol. Metab. 98, E388–E396 [DOI] [PubMed] [Google Scholar]
- 94.Schmid A., Bala M., Leszczak S., Ober I., Buechler C., Karrasch T. (2016) Pro-inflammatory chemokines CCL2, chemerin, IP-10 and RANTES in human serum during an oral lipid tolerance test. Cytokine 80, 56–63 [DOI] [PubMed] [Google Scholar]
- 95.Luque-Ramírez M., Martínez-García M. A., Montes-Nieto R., Fernández-Durán E., Insenser M., Alpañés M., Escobar-Morreale H. F. (2013) Sexual dimorphism in adipose tissue function as evidenced by circulating adipokine concentrations in the fasting state and after an oral glucose challenge. Hum. Reprod. 28, 1908–1918 [DOI] [PubMed] [Google Scholar]
- 96.Takahashi M., Inomata S., Okimura Y., Iguchi G., Fukuoka H., Miyake K., Koga D., Akamatsu S., Kasuga M., Takahashi Y. (2013) Decreased serum chemerin levels in male Japanese patients with type 2 diabetes: sex dimorphism. Endocr. J. 60, 37–44 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.