Abstract
Schwann cells actively interact with axons of dorsal root ganglia (DRG) neurons. Exosomes mediate intercellular communication by transferring their biomaterials, including microRNAs (miRs) into recipient cells. We hypothesized that exosomes derived from Schwann cells stimulated by high glucose (HG) exosomes accelerate development of diabetic peripheral neuropathy and that exosomal cargo miRs contribute to this process. We found that HG exosomes contained high levels of miR-28, -31a, and -130a compared to exosomes derived from non–HG-stimulated Schwann cells. In vitro, treatment of distal axons with HG exosomes resulted in reduction of axonal growth, which was associated with elevation of miR-28, -31a, and -130a and reduction of their target proteins of DNA methyltransferase-3α, NUMB (an endocytic adaptor protein), synaptosome associated protein 25, and growth-associated protein-43 in axons. In vivo, administration of HG exosomes to sciatic nerves of diabetic db/db mice at 7 wk of age promoted occurrence of peripheral neuropathy characterized by impairment of nerve conduction velocity and induction of mechanic and thermal hypoesthesia, which was associated with substantial decreases in intraepidermal nerve fibers. Our findings demonstrate a functional role of exosomes derived from HG-stimulated Schwann cells in mediating development of diabetic peripheral neuropathy.—Jia, L., Chopp, M., Wang, L., Lu, X., Szalad, A., Zhang, Z. G. Exosomes derived from high-glucose–stimulated Schwann cells promote development of diabetic peripheral neuropathy.
Keywords: microRNA, neuron, axonal growth
Diabetic peripheral neuropathy (DPN) is a common complication of diabetes starting from distal nerves with sensory loss (1). Although DPN has been extensively studied, molecular mechanisms that lead to distal sensory nerve fiber damage remain poorly understood (2).
Schwann cells are the most abundant cells that myelinate axons in the peripheral nervous system. In addition to myelinating peripheral axons, Schwann cells actively interact with axons by secreting neurotrophic factors and other proteins to support axons of dorsal root ganglia (DRG) neurons (3). Hyperglycemia damages Schwann cells and dysfunctional Schwann cells are involved in DPN. For example, nerve biopsy samples from patients with DPN exhibit damaged Schwann cells that are closely associated with nerve damage (4). Cellular and molecular mechanisms underlying communication between Schwann cell DRG neurons remain to be investigated. Exosomes are endosome-derived membranous nanovesicles released by all living cells. They mediate intercellular communication by transferring cargo, including microRNAs (miRs) between source and recipient cells (5). We previously demonstrated that exosomes deliver their cargo miRs to distal axons of cortical neurons and consequently lead to augmenting axonal growth (6). Emerging data show that exosomes derived from healthy Schwann cells enhance regeneration of injured peripheral axons (7). However, it is unknown whether exosomes released by hyperglycemia-stressed Schwann cells affect axons of DRG neurons in the diabetic condition.
miRs are enriched in the axon (8) and can locally regulate axonal protein levels, leading to changes of axonal function (9). In vitro studies have demonstrated that alteration of axonal miR levels by high glucose (HG) in axons of DRG neurons inhibits axonal growth through changes of miR target proteins (10, 11). Accordingly, using in vitro and in vivo approaches, the present study tested the hypothesis that exosomes derived from hyperglycemic Schwann cells communicate with axons of DRG neurons to promote development of DPN via alteration of axonal miRs and their putative target proteins.
MATERIALS AND METHODS
All experimental procedures were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA) and approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital.
Animals
BKS.Cg-m+/+Leprdb/J (db/db) and heterozygous (db/m) mice The Jackson Laboratory (Bar Harbor, ME, USA) were used. The db/db mouse has a point mutation in the leptin receptor gene causing severe depletion of the insulin-producing β-cells of the pancreatic islets and hyperglycemia, eventually leading to diabetes (12). Db/db mice first exhibit hyperinsulinemia and hyperglycemia at 5 wk of age, and DPN in db/db mice is evident at ∼8–12 wk of age (13). Thus, to examine the effect of exosomes on promoting DPN, db/db mice at age 7 wk were used, whereas db/db mice at age 12 wk were used as positive DPN controls. Age-matched db/m mice were used as negative controls.
Culture and transfection of Schwann cells
Primary Schwann cells were isolated from neonatal mouse sciatic nerves (SNs), and cultured in HG (30 mM) or regular glucose (5 mM) (14) Schwann cell medium (ScienCell, Carlsbad, CA, USA) with 5% fetal bovine serum (FBS). When subcultured cells reached 60% confluence, the medium was changed to HG Schwann cell medium with 5% exosome-free FBS (SBI, Palo Alto, CA, USA). The cells were allowed to grow for 3 d, and then the supernatant was collected to extract exosomes. For transfection, Schwann cells were isolated and cultured in HG Schwann cell medium with 5% FBS, when subcultured cells reach 90% confluence, the Schwann cells were transfected by mimics or inhibitors of miR-28, -31a, -130a and their corresponding controls and then cultured in HG Schwann cell medium with 5% exosome-free FBS. Using an electroporation approach, we mixed miR mimics, inhibitors, or negative control at 200 pmol/well with 100 μl Nucleofector solution (Lonza, Basel, Switzerland). Schwann cells were added to transfection solution and then transferred into a cuvette and electroporated with program O-01 on the Nucleofector device (Lonza) (15). The quantitative RT-PCR (qRT-PCR) analysis was performed to assay the transfection efficiency.
Culture of primary DRG neurons
DRG neurons were cultured according to a published protocol (16). Newborn C57L/J mice (The Jackson Laboratory) at postnatal d 0–1 were used. The DRG was collected and then transferred into Neurobasal Medium (Thermo Fisher Scientific, Waltham, MA, USA) containing 0.25% trypsin (Thermo Fisher Scientific) digestion at 37°C for 30 min. DRG neurons were mechanically triturated with a Pasteur pipette 15 times and then the cells were passed through a 70-µm cell strainer (Thermo Fisher Scientific) and counted to obtain a concentration of 1 × 107 cells/ml.
DRG neurons were cultured in a microfluidic chamber (Xona Microfluidics, Temecula, CA, USA). Microgrooves embedded in the chamber allow only axons to pass and reach the axonal compartment (17). Sterilized chambers were affixed to poly-d-lysine–coated (MilliporeSigma, Burlington, MA, USA) dishes (35 mm; Corning, Corning, NY, USA). DRG neurons were cultured at a density of 1 × 106 cells/chamber in DMEM (Thermo Fisher Scientific) with 5% FBS (Corning) for 4 h. Then the medium was replaced with Neurobasal Medium (Thermo Fisher Scientific), 50 ng/ml nerve growth factor (MilliporeSigma), 2% B-27 (Thermo Fisher Scientific), 2 mM GlutaMax (Thermo Fisher Scientific), and 1% antibiotic–antimycotic (Thermo Fisher Scientific). 5-Fluorodeoxyuridine (Abcam, Cambridge, United Kingdom) was added to purify the neurons. The medium was replaced with non-5-fluorodeoxyuridine Neurobasal Medium on d in vitro (DIV) 3. Subsequently, the medium was replaced every other day. DRG neurons were cultured under HG (60 mM) or normal glucose (30 mM) condition (18).
Transfection of DRG neurons
Using a published protocol (15), the local axons were transfected for miR studies by mimics or inhibitors of miR-28, -31, and -130 and their corresponding controls or for protein knockdown studies by siRNAs against DNA methyltransferase (DNMT)-3A, an endocytic adaptor protein (NUMB), synaptosome-associated protein (SNAP)-25, and growth-associated protein (GAP)-43 and their controls. The N-TER Nanoparticle siRNA Transfection System (MilliporeSigma) was used to perform the local transfection in microfluidic chambers. On DIV3, when all the microgrooves were entirely filled with axons, which indicates that the soma and axonal sides of the chamber were isolated, the mimics, inhibitors, or their controls (20 nM), along with nanoparticles packed in N-TER peptide in Neurobasal Medium, were added into the axonal side for 72 h. Then, total RNAs and proteins were collected from the neuronal bodies and axons. Neurons for axonal measurement were cultured in additional chambers.
Isolation of exosomes from HG-treated Schwann cells
HG exosomes were isolated from supernatant of Schwann cells cultured in exosome-free HG medium according a published protocol (6). The supernatant was collected and filtered through a 0.22-μm filter (MilliporeSigma) to remove dead cells and large debris. The filtered supernatant was further subjected to centrifugation at 10,000 g for 30 min. Ultracentrifugation was then performed at 100,000 g for 1 h to collect the HG exosomes. After ultracentrifugation, the supernatant was collected as a negative control, and the pellet was resuspended with 100 μl sterilized PBS for the experiments. HG exosomes were further characterized by Western blot analysis to detect the exosome markers Alix, CD63, and heat shock protein (Hsp)-70. Numbers and sizes of HG exosomes were measured with an instrument for microparticle measurement (Izon Science, Christchurch, New Zealand).
To identify HG exosomes, exosomes were labeled according to a published protocol (6). The HG exosomes were transfected with Texas-red–labeled siRNA with the Exo-fect Exome Transfection Kit (System Biosciences, Palo Alto, CA, USA). HG exosomes at a concentration of 3 × 1011 were incubated with transfection solution at 37°C in a shaker for 10 min, and the HG exosomes were placed on ice for 30 min to stop the reaction. Transfected HG exosomes were collected by centrifugation and resuspended by 100 μl PBS.
Local injection of HG exosomes into the SN
Generally, db/db mice develop detectable DPN starting at the age of ∼10 wk (19). To examine the effect of HG exosomes on development of DPN, db/db mice at the age of 7 wk were used. HG exosomes were locally injected into the SN of db/db mice according to a protocol published by Gonzalez et al., (20) with some modification. In mice under anesthesia, a modified needle of 1 ml syringe containing 2 µl of HG exosomes (1 × 108 particles) was placed in the sciatic notch and was also used as an electrode for electromyography (EMG) measurement. When an EMG signal was detected, indicating that the syringe needle had penetrated the SNs, HG exosomes or vehicle was then injected. The injection into db/db mice was performed daily for 7 consecutive d starting at the age of 7 wk. The mice were euthanized at the age of 9 wk, and tissues were collected. To examine whether HG exosomes are localized to SNs, labeled HG exosomes were injected, and SNs were collected 24 h after the injection.
EMG
Motor and sensory nerve conduction velocity (MCV and SCV, respectively) were measured according to a published protocol (21). Mice were anesthetized with isoflurane, and the stimulating electrodes were placed at the knee or sciatic notch. Single square wave pulses were generated by a stimulator (Model 2100; A-M Systems, Everett, WA, USA). The electromyography signals were recorded by 2 electrodes placed in the dorsum of the foot with a Grass Amplifier (Model P5; Grass Instruments, Quincy, MA, USA). MCV and SCV were calculated according to the protocol.
Measurement of tactile allodynia and thermal sensitivity
Von Frey and tail flick tests were performed to examine the tactile and thermal sensitivity, respectively. A series of Von Frey filaments (Stoelting, Wood Dale, IL, USA) with force that ranged from 0.4 to 6.0 g were applied to the plantar surface of the left hind paw with pressure causing the filament to buckle (22). A paw withdrawal in response to each stimulus was recorded and a 50% paw withdrawal threshold was calculated according to a published formula. The tail flick tests were measured with a thermal stimulation meter (Model 336 TG combination tail-flick and paw algesia meter; IITC Life Science, Woodland Hills, CA, USA), according to previously published methods (21). Mice were placed within a Plexiglas chamber on a transparent glass surface and allowed to acclimate for at least 20 min. For the plantar test, the meter was activated after placing the stimulator directly beneath the plantar surface of the hind paw. The paw-withdrawal latency in response to the radiant heat (intensity, 15%; cutoff time, 30 s) was recorded. For tail-flick test, the meter was set at 40% heating intensity with a cutoff at 10 s. For both tests, at least 5 readings per animal were taken at 15-min intervals, and the average was calculated.
Isolation of total RNA and real-time RT-PCR analysis
MiRNeasy Mini Kit (Qiagen, Hilden, Germany) was used to isolate total RNA from cultured DRG neurons (DIV6), or DRG, SN, and foot pad tissues of db/db and db/m mice. qRT-PCR analysis was performed on ABI 7000 and ABI ViiA 7 PCR instrument (Thermo Fisher Scientific) according to published methods (15, 23). For the reverse transcription, 15 μl of reverse transcription reactions were used, consisting of 1–10 ng total RNA, 5U MultiScribe Reverse Transcriptase, 0.5 mM each of dNTPs, 1× reverse transcription buffer, 4U RNase inhibitor, and nuclease-free water (all from Thermo Fisher Scientific). The program was as follows: 16°C for 30 min, 42°C for 30 min, and 85°C for 5 min. Twenty microliters of qRT-PCR reaction was used, consisting of 1× TaqMan Universal PCR Master Mix No AmpErase UNG, 1× TaqMan miR assay, 1.33 μl undiluted cDNA, and nuclease-free water. The running program was 95°C for 10 min, followed by 40 cycles at 95°C for 15 s, and 60°C for 1 min. Each sample obtained from at least 3 independent experiments was tested in triplicate. Relative levels of miRs were calculated by means of the formula 2−ΔΔCt after normalizing ΔΔCt values to a reference miR U6. Cycle threshold values and melting curve were checked. The method of 2−ΔΔCt was used to calculate the relative levels (24).
Western blot analysis
Protein samples from cultured DRG neurons were isolated on DIV6 according to published protocols (15, 25). Protein samples from DRG, SN, and foot pad tissues of db/db and db/m mice were harvested with the same methods. In vitro samples from 4 individual microfluidic chambers were pooled for 1 Western blot. The protein concentration was measured with a Bicinchoninic Acid Protein Assay Kit (Thermo Fisher Scientific). Western blot analysis was performed according to previously described methods (15, 25). Equal amounts of proteins were loaded. Primary antibodies were mouse anti-Alix (1:1000; Cell Signaling Technology, Danvers, MA, USA), rabbit anti-CD63 (1:1000; Santa Cruz Biotechnology, Dallas, TX, USA), rabbit anti-DNMT3A (1:1000; Cell Signaling Technology), rabbit anti-GAP43 (1:1000; Abcam), rabbit anti-Hsp70 (1:1000; Abcam), rabbit anti-insulin like growth factor 2 mRNA binding protein 1 (IGF2BP1; 1:1000; Abcam), rabbit-myelin-associated glycoprotein (1:1000; Abcam), rabbit anti-myelin transcription factor–1 (MYT1, 1:1000; MilliporeSigma), rabbit anti-NUMB (1:1000; Abcam), rabbit anti-SNAP25 (1:1000; Santa Cruz Biotechnology), rabbit anti-sprouty–related EVH1 domain–containing-1 (1:1000; Abcam), rabbit anti-transforming growth factor β receptor-2 (TGFBR2, 1:1000; Abcam). The optical density of protein bands was measured and calculated by means of a Fluorchem E instrument (ProteinSimple, San Jose, CA, USA).
FISH
FISH in combination with fluorescent immunostaining was performed according to our published protocol (26). The cells were fixed in 4% paraformaldehyde and then hybridized with locked nucleic acid probes against miR-28, -31a, and -130a. Signals were detected with peroxidase-conjugated anti-FAM (Roche, Basel, Switzerland) followed by incubation tyramine-signal-amplification–Cy3 substrate for 10 min at room temperature. After that, immunofluorescent staining was performed with primary antibodies against βIII-Tubulin (TUJ1, a marker of neuroblasts; Abcam) and FITC-conjugated secondary antibody (Jackson ImmunoResearch, West Grove, PA, USA).
Immunofluorescent staining and measurements of axon and intraepidermal nerve fiber densities
Axonal staining and measurement were performed as previously described by us (27). A monoclonal antibody against TUJ1 (1:1000) was used. The lengths of the 15 longest axons in each chamber were measured using a microscopic computer imaging device. The axonal length was recorded for 3 d from DIV3 to -5. For in vitro studies, the area of fluorescent signals from labeled HG exosomes and immunoreactive neurons were measured according to our published protocol (28). For in vivo studies, SN tissues injected with labeled HG exosomes were fixed in 4% paraformaldehyde for 1 h, and then frozen at −80°C. The sections (8 µm) were stained with TUJ1 (1:50; Abcam) and myelin basic protein (1:50; Covance, Princeton, NJ, USA) and imaged with the microscopic computer imaging device. A pAb against protein gene product 9.5 (1:1000; MilliporeSigma) was used to detect intraepidermal nerve fiber densities in plantar skin. The nerve fiber densities were calculated according a published protocol (29).
Bioinformatics and statistical analyses
Bioinformatics were evaluated by using Ingenuity Pathways Analysis (IPA) (Qiagen, Germantown, MD, USA) and www.targetscan.org (30). For IPA, Fisher’s exact test was used to calculate the P value. Other data were analyzed by using SPSS 11.5 (Armonk, NY, USA). For functional tests, data were evaluated for normality. Ranked data or a nonparametric approach was considered if the data were not normally distributed. The repeated-measures ANOVA was considered with a dependent factor of time and an independent factor of groups. A 1-way ANOVA with the post hoc Bonferroni test was used for multiple group experiment analysis. A Student’s t test was used for 2-group comparisons. A value of P < 0.05 was considered significant. Values are presented as means ± sd.
RESULTS
Exosomes secreted from HG-treated Schwann cells suppress axonal growth
To examine the effect of exosomes derived from HG-treated Schwann cells on axonal growth, we first isolated and characterized exosomes from Schwann cells cultured under the HG condition (Fig. 1A, B). The HG exosomes had an average diameter of 108 ± 12 nm (Fig. 1A) and exhibited exosomal markers of Alix, CD63, and Hsp70 (Fig. 1B). To examine the effect of HG exosomes on distal axons, DRG neurons were cultured in a microfluidic device, which permits distal axons to grow into the axonal compartment (Fig. 1C). DRG neurons were cultured under regular glucose condition. Our preliminary experiments showed that HG exosomes at concentrations of 1 × 107 or 1 × 108 reduced axonal growth, but did not induce the cell death of DRG neurons, whereas HG exosomes at 1 × 109 particles/ml induced cell death and axonal damage. Therefore, HG exosomes at concentrations of 1 × 107 and 1 × 108 particles/ml were used in the present study. We first treated whole DRG neurons with HG exosomes and found that the exosomes reduced axonal growth (Fig. 1D). Then, we performed local axonal treatment and found that application of HG exosomes into the distal axon compartment on DIV2 reduced axonal lengths from DIV3 to -5 (Fig. 1E, F). Texas-red–labeled HG exosomes were detected in distal axons, suggesting that axons internalize HG exosomes (Fig. 2). These data indicate that HG exosomes locally suppress axonal growth of DRG neurons.
Figure 1.
Exosomes secreted from HG-treated Schwann cells suppress axonal growth. A, B) Exosomes isolated by differential ultracentrifugation from the supernatant of HG-treated Schwann cells had an average particle size of ∼100 nm (A) and exhibited exosomal marker proteins of Alix, CD63, and Hsp70 measured by Western blot analysis (B). C) Microfluidic device showing the cell body and axonal compartments. When they were cultured in the cell body compartment, DRG neurons extend their axons into the axonal compartment by crossing microgrooves. D) Quantitative data of axonal length when HG exosomes at concentrations of 1 × 107 and 1 × 108 particles/ml were added into both cell body and axonal compartments. E, F) Representative immunofluorescent images (E) and quantitative data (F) of axonal length when HG exosomes at concentrations of 1 × 107 and 1 × 108 particles/ml were added to the axonal compartment alone show that HG exosomes reduced axonal lengths (n = 6 chambers/3 individual experiments/group). Scale bars, 100 μm. *P < 0.05.
Figure 2.
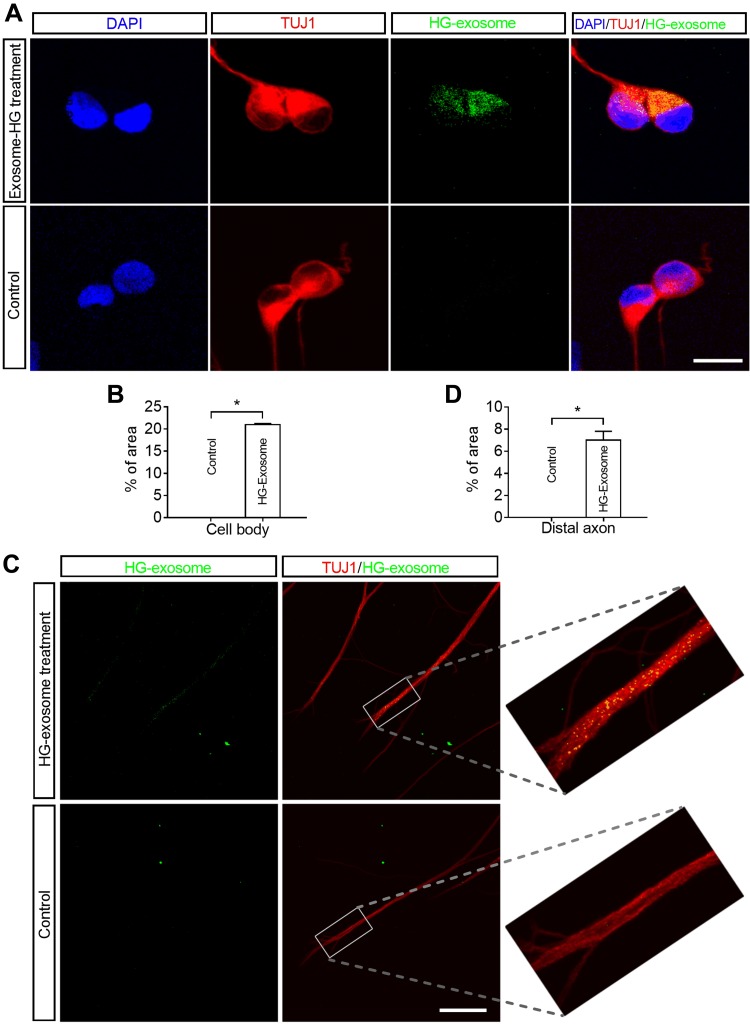
Cell bodies and distal axons of DRG neurons internalize HG exosomes. A, C) Representative immunofluorescent images acquired from the cell body compartment show the presence of HG exosomes (green) within the cytoplasm of TUJ1+ DRG neurons (A, red) or TUJ1+ distal axon (C, green), when Exo-fect labeled HG exosomes were applied to the cell body compartment or the axonal compartment only, respectively. The nuclei were labeled with DAPI (blue). B, D) Quantitative data of green fluorescent signals of labeled HG exosomes within cell bodies and distal axons (n = 3 chambers/group). Scale bars: 10 μm (A), 5 μm (C). *P < 0.05.
Axonal application of HG exosomes increases levels of miR-28, -31a, and -130a in distal axons of DRG neurons
Exosomes communicate with recipient cells by transferring their cargo materials, including miRs (5, 31). Accordingly, we first examined the effect of HG on changes of miR profiles in Schwann cells and their exosomes by means of a Taqman PCR-based miR array that contains 835 miRs. Based on Ct values, the top 20 highly expressed miRs within HG exosomes were selected compared to exosomes derived from Schwann cells cultured under regular glucose (Fig. 3A). At least a 2-fold change was considered an increase. Among these 20 miRs, levels of miR-28, -31a, 34a (data not shown), -130a, and -138 were increased in an HG condition, both in HG exosomes and in Schwann cells. qRT-PCR analysis confirmed elevation of miR-28, -31a, 34a (data not shown), and -130a both in HG-treated Schwann cells and their exosomes, whereas miR-138 was reduced in HG-treated Schwann cells and their exosomes (Fig. 3B, C). These data suggest that HG-increased miRs in Schwann cells are sorted into their exosomes. Indeed, when Schwann cells were transfected with siRNAs against these individual miRs in an HG condition, levels of miR-28, -31a, and -130a in Schwann cells and their exosomes were substantially reduced.
Figure 3.
HG exosomes increase levels of miR-28, -31a, and -130a in DRG neurons. A) Heat maps of miRs acquired from Taqman miR array analysis show the top 20 highly expressed miRs ranked by Ct values in HG exosomes. Levels of these miRs were compared between HG exosomes and control exosomes (left column), and between HG-Schwann cells and control-Schwann cells (right column). B, C) Asterisks indicate 2-fold change of miRs. qRT-PCR analysis showed that transfection of Schwann cells with siRNAs against miR-28, -31a, and -130a abolished HG-elevated these 3 miRs in HG exosomes (B) and in Schwann cells (C). D) Treatment of distal axons with HG exomes derived from Schwann cells transfected with siRNA against these 3 miRs did not increase levels of these 3 miRs within the axons. E, F) Application of HG exosomes into the distal axons elevated levels of miR-28, -31a, and -130a in distal axons (E), but not in cell bodies of DRG neurons (F). F–I) qRT-PCR (F) and FISH (G–I) analysis showed that application of HG exosomes into cell bodies and distal axons increased miR-28, -31a, and -130a in entire DRG neurons (n = 6 chambers/3 individual experiments /group). Scale bars, 10 μm. Asterisks denote fold change ≥2 (A) or P < 0.05 (B–F).
We then examined whether treatment of DRG neurons with HG exosomes affects miRs in DRG neurons. First, we measured miR levels in untreated DRG neurons. qRT-PCR analysis showed that miR-28, -31a, and -130a were not changed under HG conditions. Application of HG exosomes into distal axons increased levels of miR-28, -31a, and -130a in axons of DRG neurons, whereas the treatment of distal axons with HG exosomes derived from Schwann cells transfected with individual miR inhibitors did not significantly elevate miR-28, -31a, and -130a in axons (Fig. 3D). Axonal application of HG exosomes did not affect these miR levels in samples isolated from the cell body compartment (Fig. 3E). These data suggest that HG exosomes affect miRs in distal axons of DRG neurons. However, when they were applied into the cell body and distal axon compartments, HG exosomes increased levels of miR-28, -31, and -130 in cell bodies and axons, as measured by means of qRT-PCR and FISH (Fig. 3F–I).
MiR-28, -31a, and -130a locally regulate axonal growth
To examine whether alteration of miR-28, -31a, and -130a in axons of DRG neurons affects axonal growth, we endogenously attenuated these 3 miRs or elevated them by locally transfecting axons with inhibitors or mimics of miR-28, -31a, and -130a. Local transfection was performed on DIV2, when all of the microgrooves were entirely filled with axons in an HG condition. Inhibition of miR-28, -31a, and -130a increased axonal length, whereas mimics of these miRs decreased axonal length in an HG condition compared with the controls (Fig. 4). These data suggest that elevation of axonal miR-28, -31a, and -130a levels suppresses axonal growth.
Figure 4.
MiR-28, -31a and -130a regulate axonal growth. qRT-PCR analysis (A, D, G) and axonal length measurements (B, C, E, F, H, I) show that axonal local transfection of siRNAs or mimics of miR-28 (A–C), -31a (D–F), and -130a (G–I) significantly reduced and increased, respectively, these 3 miR levels in axons (A, D, G), whereas siRNAs and mimics of these miRs significantly augmented and decreased, respectively, axonal growth, as shown by representative images (B, E, H) and quantitative measurements (C, F, I) (n = 6 chambers/3 individual experiments /group). Scale bars, 10 μm. *P < 0.05.
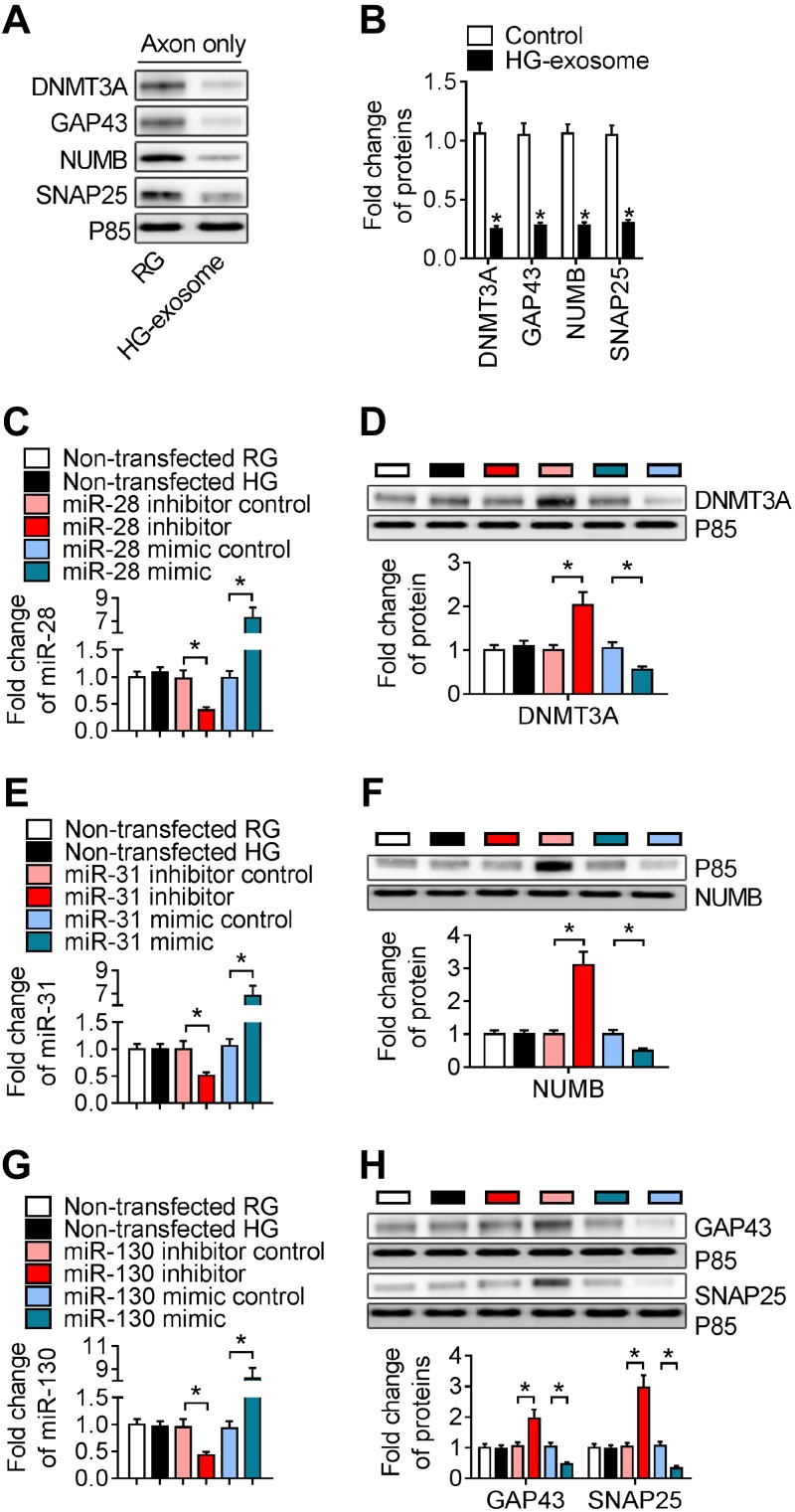
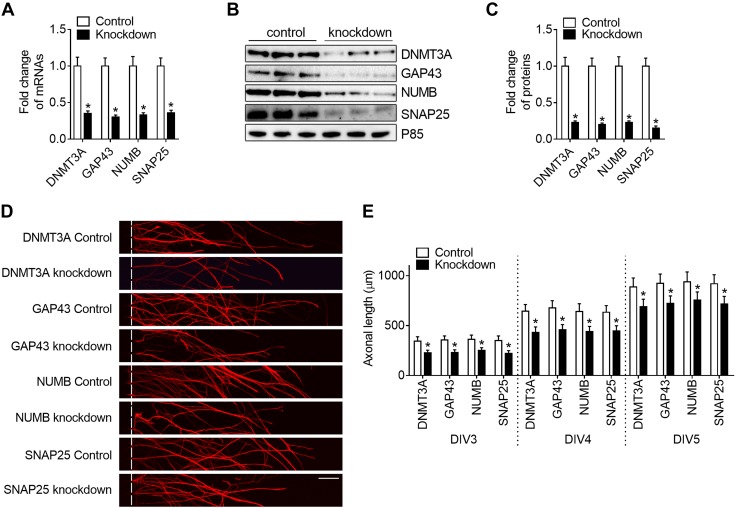
Moreover, we found that application of HG exosomes into distal axons significantly reduced protein levels of DNMT3A, NUMB, GAP43, and SNAP25 in axons compared with axons treated with vehicle (Fig. 5A, B). Axonal inhibition or elevation of miR-28, -31a, or -130a increased or decreased protein levels of DNMT3A, NUMB, GAP43, and SNAP25 in axons compared to their controls (Fig. 5C–H). Based on analysis of Targetscan and IPA, miR-28 putatively targets 3′UTR of DNMT3A gene, miR-31a for NUMB, and miR-130a for GAP43, and SNAP25. To examine the effect of these proteins on axonal growth, we locally knocked down these genes in axons of DRG neurons in regular glucose conditions. Individual knockdown of these genes resulted in ∼70% reduction of their mRNAs and 80% reduction of their proteins (Fig. 6A–C) and significantly reduced axonal growth (Fig. 6D, E). Collectively, these data suggest that miR-28, -31, and -130 potentially target these genes to regulate axonal growth, which may contribute to the inhibitory effect of HG exosomes on axonal growth.
Figure 5.
HG exosomes reduce protein levels of DNMT3A, NUMB, SNAP25, and GAP43 in distal axons of DRG neurons. Inhibition and elevation of miR-28, -31a, and -130a in distal axons were inversely related to these protein levels. A, B) Representative Western blot images of DNMT3A, NUMB, SNAP25, and GAP43 (A) and quantitative data of these proteins (B) in distal axons treated with HG exosomes or left untreated under regular glucose condition. C–H) Transfection of axons with siRNA against miR-28 (C), -31a (E), and -130a (G) or with mimics of miR-28 (C), -31a (E), and -130a (G) increased or decreased the protein levels of DNMT3A (D), NUMB (F), and GAP43 and SNAP25 (H) (n = 6 chambers/3 individual experiments/group). *P < 0.05.
Figure 6.
A–C) Downregulation of DNMT3A, NUMB, SNAP25, and GAP43 decreases axonal growth. qRT-PCR (A) and Western blot (B) analyses show that transfection of axons with siRNAs against DNMT3A, NUMB, SNAP25, and GAP43 significantly reduced mRNA (A) and protein (C) levels of DNMT3A, NUMB, SNAP25, and GAP43 in axons of DRG neurons under regular glucose condition. D, E) Representative images of distal axons (D) and quantitative data of axonal length over DIV3 to -5 (E) show that reduction of these proteins resulted in decreases of axonal growth compared to axons transfected by control siRNAs (n = 6 chambers/3 individual experiments/group). Scale bar, 10 μm. *P < 0.05.
HG exosomes promote development of DPN in diabetic mice
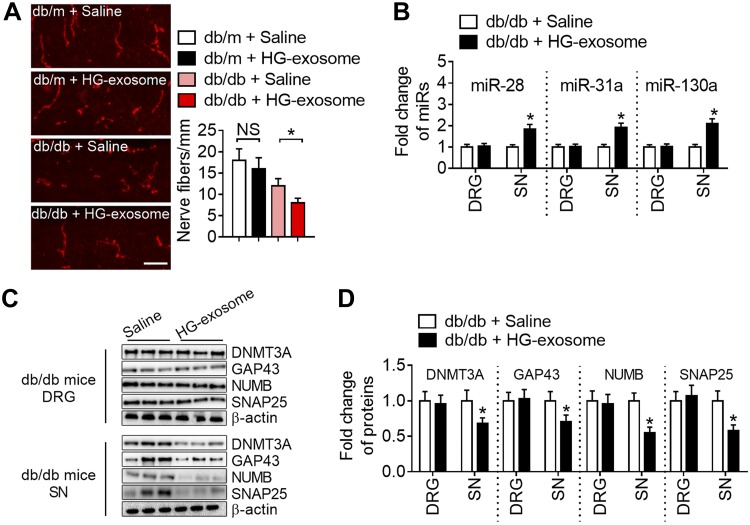
To examine whether HG exosomes promote development of DPN, the exosomes were administered daily for 7 consecutive d into the SNs of db/db mice (Fig. 7A) at the age of 7 wk when these mice did not show peripheral neuropathy (Fig. 7B–E). Db/db mice treated with HG exosomes exhibited reduction of nerve conduction velocity and mechanical and thermal hypoesthesia at the age of 8 wk, and the neuropathy persisted at least for another week after the termination of HG exosome treatment compared to age-matched db/db mice treated with saline (Fig. 7B–E). Administration of HG exosomes to age-matched db/m mice did not induce any peripheral neuropathy. Immunofluorescent staining revealed that labeled HG exosome fluorescent signals were detected in the SN after local SN injection of labeled HG exosomes (Fig. 7F). HG exosome treatment reduced PGP 9.5–positive intraepidermal nerve fibers (Fig. 8A). qRT-PCR analysis of SN and DRG tissues harvested from db/db mice at age of 9 wk showed that HG exosome treatment increased levels of miR-28, -31, and -130 in the SN, but not DGR, tissue (Fig. 8B), which was associated with increases of DNMT3A, GAP43, NUMB, and SNAP25 proteins in SN tissue (Fig. 8C, D). These data suggest that HG exosomes promote development of peripheral neuropathy in diabetic mice and that HG exosomes alter miR-28, -31, and -130 and their target proteins in the SN tissue.
Figure 7.
HG exosomes promote development of peripheral neuropathy. A) Local injection of HG exosomes to the SNs of db/m or db/db mice at age 7 wk. In mice under anesthesia, a modified needle of a 1-ml syringe containing 2 µl of HG exosomes (1 × 108 particles) advanced to the position of the sciatic notch, used as an electrode with an EMG system, and then the EMG was measured. When an EMG signal was detected, indicating that the syringe needle had penetrated into the SNs, HG exosomes or vehicle was injected. B–E) Quantitative data of MCV (B), SCV (C), Von Frey test (D), and tail flick test (E) in db/m and db/db mice treated with saline or HG exosomes. F) Representative immunofluorescent images show that labeled HG exosome signals (red) were localized to TUJ1+ (green) and MBP+ (blue) axons and myelin in the SNs (n = 10/group. Scale bars, 10 μm. *P < 0.05.
Figure 8.
The effects of HG exosomes on intraepidermal nerve fibers miR-28, -31a, and -130a and their target proteins. A) Representative immunofluorescent images of intraepidermal nerve fibers in plantar skin of mice at age of 9 wk and quantitative data of the nerve fiber density of db/m and db/db mice treated with saline or HG exosomes. B) qRT-PCR analysis shows levels of miR-28, -31a and -130a in SN and DRG tissues of db/db mice at the age of 9 wk after treatment with saline or HG exosomes. C, D) Representative Western blot images of DNMT3A, NUMB, SNAP25, and GAP43 proteins and quantitative data of these proteins in DRG tissues and SN of db/db mice treated with saline and HG exosomes (n = 10 /group). Scale bar, 50 μm. *P < 0.05.
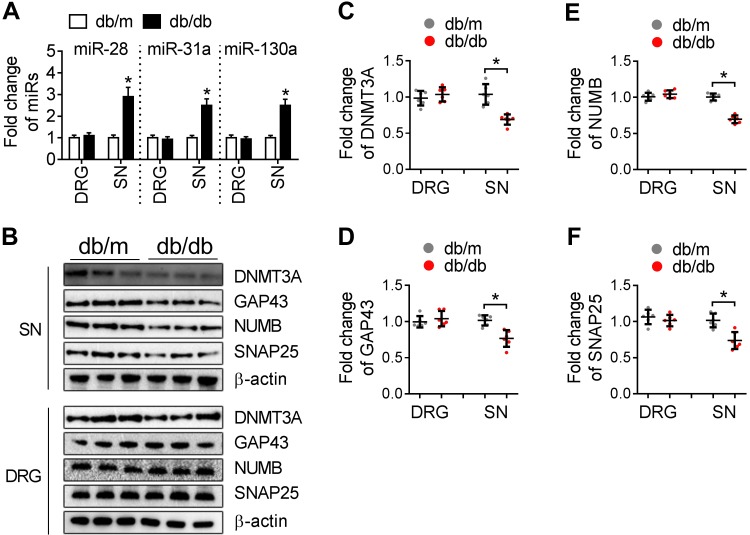
To examine whether miR-28, -31 and -130 and their putative target genes are involved in DPN, we measured levels of these miRs and proteins in DRG and SN tissues of db/db mice at the age of 12 wk, when these mice exhibited typical DPN. qRT-PCR analysis showed that levels of these miRs were increased in SN tissue, but not in DRG tissue of db/db mice compared to age-matched db/m mice (Fig. 9A). Moreover, Western blot analysis revealed substantial reduction of protein levels of DNMT3A, NUMB, GAP43, and SNAP25 in SN, but not in DRG tissue of db/db mice (Fig. 9B–F). SN tissue of db/db mice did not show reduction of other proteins encoded by genes that could putatively be targeted by miR-28, -31, and -130, such as IGF2BP1 and MYT1 (data not shown). These data suggest that miR-28, -31, and -130 and their putative target proteins are involved in DPN.
Figure 9.
Diabetic db/db mice at age of 12 wk exhibit elevation of miR-28, -31a, and -130a and reduction of DNMT3A, NUMB, SNAP25, and GAP43 proteins in the SN tissues. A) qRT-PCR analysis shows levels of miR-28, -31a, and -130a in the SN and DRG tissues of db/db or db/m mice at the age of 12 wk. B) Representative Western blots showed DNMT3A, NUMB, SNAP25, and GAP43 proteins in the SN and DRG tissues. C–F) Quantitative data showed proteins of DNMT3A (C), NUMB (D), SNAP25 (E), and GAP43 (F) in the SN and DRG tissues of db/m or db/db mice (n = 4 mice/group). *P < 0.05.
DISCUSSION
Dysfunction of Schwann cells impairs axonal function of DRG neurons. The present study provides evidence that local administration of exosomes derived from Schwann cells cultured under HG condition to SNs induces early onset of peripheral neuropathy in diabetic db/db mice. Transferring of miRs, such as miR-28, -31a, and -130a by HG exosomes and therefore reduction of their putative target proteins in the SN tissue may contribute to the development of DPN. These novel findings suggest that HG-stimulated Schwann cell-derived exosomes play a functional role in mediating development of DPN.
Multiple mechanisms underlying DPN have been proposed, including polyol pathway, hexosamine, PKC, advanced glycation end products, insulin, and inflammation. However, clinical trials targeting these mechanisms have been unsuccessful (2), suggesting that other means of pathogenesis are involved in development of DPN. Exosomes derived from myelinating cells, such as oligodendrocytes and Schwann cells, communicate with cortical and DRG neurons, respectively, to regulate axonal function (32). For example, Schwann cell-secreted exosomes can be internalized by axons of the DRG neuron and promotes their regeneration after SN injuries (7). However, the effect of exosome-derived Schwann cells in a condition of hyperglycemia on peripheral neuropathy is unknown. Using in vitro and in vivo approaches, the present study now provides evidence to demonstrate that exosomes derived from HG-stimulated Schwann cells can be internalized by distal axons of DRG neurons, suppress axonal growth, and promote development of peripheral neuropathy in diabetic mice. The present study, together with other published studies, indicates that Schwann cell–derived exosomes play a functional role in the peripheral nerve system.
Our data revealed that HG exosomes were enriched with miR-28, -31a, and -130. Although previous studies have shown that diabetes induces these 3 miRs (33, 34), the effect of these miRs within HG exosomes on neurons has not been investigated. DPN initially affects distal nerve fibers (2). We thus applied HG exosomes to distal axons of DRG neurons cultured in the microfluidic device and demonstrated that HG exosomes were internalized by distal axons. Using gain and loss of function, the present study demonstrated that 3 miRs, miR-28, -31a, and -130, elevated in HG exosomes regulated distal axonal growth. These results are consistent with findings reported by us and others showing that exosomal cargo miRs primarily contribute to the exosomal effect on recipient cell function and that exosomal miRs in distal axons locally regulate axonal growth (35).
Several genes that are involved in the nervous system were found among the bioinformatics list of the 3 miR target genes including DNMT3A, GAP43, IGF2BP1, MYT1, NUMB, SNAP25, sprouty-related EVH1 domain–containing-1, and TGFBR2. We functionally validated NDNMT3A as an miR-28 target, NUMB as an miR-31a target and SNAP25 and GAP43 as miR-130 targets in mediating axonal growth. However, other predicted target genes were not validated in sensory neurons, highlighting the context dependence of functionality of miRs in regulating target genes (36). DNMT3A is a major DNMT that plays an important role in regulating neuronal function. DNMT3A is essential for synaptic plasticity in the CNS (37) and ablation of DNMT3A results in motor defects (38). NUMB mediates neurogenesis (39). By binding to NOTCH1, NUMB modulates neuronal differentiation (40) and inactivation of NUMB results in abnormal neurogenesis and cortical morphogenesis (41). SNAP25 is a member of SNARE proteins, which consists of a family of related proteins that play a role in vesicle targeting and fusion (42). SNAP25 regulates neurite outgrowth in the nervous system (43). GAP43 is an intrinsic determinant of neuronal development and plasticity (44) and supports axons to grow over long distances (45). Ablation of GAP43 leads to abnormal neuronal pathfinding (46), whereas overexpression of GAP43 promotes nerve sprouting (47). The present study indicates that additional studies are warranted to investigate the roles of these genes in mediating distal axonal damage under diabetic conditions, although these genes have been indicated to be involved in axonal growth.
Our in vivo data demonstrated that elevation of miR-28, -31a, and -130 by HG exosomes was inversely associated with protein levels of these 4 proteins in the SNs of diabetic mice that exhibited DPN. Moreover, an inverse relationship between these miRs and their target proteins was detected in the SNs, but not in DRG neurons of diabetic mice with DPN, suggesting that this network of miRs and proteins are selectively involved in distal nerve fiber damaged by hyperglycemia. Collectively, our data suggest that in addition to its direct effect on axonal damage, hyperglycemia could impair distal nerve fibers by Schwann cell exosome-delivered miRs.
In summary, the present study provides valuable new insights into the role of Schwann cell–derived exosomes in mediating DPN.
ACKNOWLEDGMENTS
This work was supported by U.S. National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke Grants R01 NS075084 (to L.W.) and R01 NS075156 (to Z.G.Z.), and NIH National Institute of Diabetes and Digestive and Kidney Diseases Grant R01 DK097519 (to L.W.). The authors declare no conflicts of interest.
Glossary
- DIV
day in vitro
- DNMT
DNA methyltransferase
- DPN
diabetic peripheral neuropathy
- DRG
dorsal root ganglia
- EMG
electromyography
- FBS
fetal bovine serum
- GAP
growth-associated protein
- HG
high glucose
- IGF2BP
insulin like growth factor 2 mRNA binding protein
- IPA
Ingenuity Pathway Analysis
- MCV
motor nerve conduction velocity
- miR
microRNA
- MYT
myelin transcription factor
- qRT-PCR
quantitative RT-PCR
- SCV
sensory nerve conduction velocity
- SN
sciatic nerve
- SNAP
synaptosome-associated protein
- TUJ1
βIII-Tubulin
AUTHOR CONTRIBUTIONS
L. Jia was responsible for the conception and design of the study, the performance of most of the experiments, data analysis and interpretation, and writing the manuscript; X. Lu and A. Szalad collected the tissues; and M. Chopp, L. Wang, and Z. G. Zhang were responsible for the conception and design of the study, data analysis and interpretation, writing the manuscript, and had full access to all the data.
REFERENCES
- 1.Peltier A., Goutman S. A., Callaghan B. C. (2014) Painful diabetic neuropathy [published correction appears in BMJ 2014;348:g3440]. BMJ 348, g1799 10.1136/bmj.g1799 [DOI] [PubMed] [Google Scholar]
- 2.Feldman E. L., Nave K. A., Jensen T. S., Bennett D. L. H. (2017) New horizons in diabetic neuropathy: mechanisms, bioenergetics, and pain. Neuron 93, 1296–1313 10.1016/j.neuron.2017.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherman D. L., Brophy P. J. (2005) Mechanisms of axon ensheathment and myelin growth. Nat. Rev. Neurosci. 6, 683–690 10.1038/nrn1743 [DOI] [PubMed] [Google Scholar]
- 4.Said G. (2007) Diabetic neuropathy: a review. Nat. Clin. Pract. Neurol. 3, 331–340 10.1038/ncpneuro0504 [DOI] [PubMed] [Google Scholar]
- 5.Pegtel D. M., Cosmopoulos K., Thorley-Lawson D. A., van Eijndhoven M. A., Hopmans E. S., Lindenberg J. L., de Gruijl T. D., Würdinger T., Middeldorp J. M. (2010) Functional delivery of viral miRs via exosomes. Proc. Natl. Acad. Sci. USA 107, 6328–6333 10.1073/pnas.0914843107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang Y., Chopp M., Liu X. S., Katakowski M., Wang X., Tian X., Wu D., Zhang Z. G. (2017) Exosomes derived from mesenchymal stromal cells promote axonal growth of cortical neurons. Mol. Neurobiol. 54, 2659–2673 10.1007/s12035-016-9851-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Verrilli M. A., Picou F., Court F. A. (2013) Schwann cell-derived exosomes enhance axonal regeneration in the peripheral nervous system. Glia 61, 1795–1806 10.1002/glia.22558 [DOI] [PubMed] [Google Scholar]
- 8.Natera-Naranjo O., Aschrafi A., Gioio A. E., Kaplan B. B. (2010) Identification and quantitative analyses of microRNAs located in the distal axons of sympathetic neurons. RNA 16, 1516–1529 10.1261/rna.1833310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan B. B., Kar A. N., Gioio A. E., Aschrafi A. (2013) MicroRNAs in the axon and presynaptic nerve terminal. Front. Cell. Neurosci. 7, 126 10.3389/fncel.2013.00126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jia L., Wang L., Chopp M., Li C., Zhang Y., Szalad A., Zhang Z. G. (2017) MiR-29c/PRKCI regulates axonal growth of dorsal root ganglia neurons under hyperglycemia. Mol. Neurobiol. 55, 851–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jia L., Chopp M., Wang L., Lu X., Zhang Y., Szalad A., Zhang Z. G. (2018) MiR-34a regulates axonal growth of dorsal root ganglia neurons by targeting FOXP2 and VAT1 in postnatal and adult mouse. [E-pub ahead of print] Mol. Neurobiol. 10.1007/s12035-018-1047-3 PubMed [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen H., Charlat O., Tartaglia L. A., Woolf E. A., Weng X., Ellis S. J., Lakey N. D., Culpepper J., Moore K. J., Breitbart R. E., Duyk G. M., Tepper R. I., Morgenstern J. P. (1996) Evidence that the diabetes gene encodes the leptin receptor: identification of a mutation in the leptin receptor gene in db/db mice. Cell 84, 491–495 10.1016/S0092-8674(00)81294-5 [DOI] [PubMed] [Google Scholar]
- 13.O’Brien P. D., Sakowski S. A., Feldman E. L. (2014) Mouse models of diabetic neuropathy. ILAR J. 54, 259–272 10.1093/ilar/ilt052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suzuki T., Sekido H., Kato N., Nakayama Y., Yabe-Nishimura C. (2004) Neurotrophin-3-induced production of nerve growth factor is suppressed in Schwann cells exposed to high glucose: involvement of the polyol pathway. J. Neurochem. 91, 1430–1438 10.1111/j.1471-4159.2004.02824.x [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y., Ueno Y., Liu X. S., Buller B., Wang X., Chopp M., Zhang Z. G. (2013) The MicroRNA-17-92 cluster enhances axonal outgrowth in embryonic cortical neurons. J. Neurosci. 33, 6885–6894 10.1523/JNEUROSCI.5180-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Molliver D. C., Wright D. E., Leitner M. L., Parsadanian A. S., Doster K., Wen D., Yan Q., Snider W. D. (1997) IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron 19, 849–861 10.1016/S0896-6273(00)80966-6 [DOI] [PubMed] [Google Scholar]
- 17.Taylor A. M., Blurton-Jones M., Rhee S. W., Cribbs D. H., Cotman C. W., Jeon N. L. (2005) A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods 2, 599–605 10.1038/nmeth777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Russell J. W., Sullivan K. A., Windebank A. J., Herrmann D. N., Feldman E. L. (1999) Neurons undergo apoptosis in animal and cell culture models of diabetes. Neurobiol. Dis. 6, 347–363 10.1006/nbdi.1999.0254 [DOI] [PubMed] [Google Scholar]
- 19.Shi T. J., Zhang M. D., Zeberg H., Nilsson J., Grünler J., Liu S. X., Xiang Q., Persson J., Fried K. J., Catrina S. B., Watanabe M., Arhem P., Brismar K., Hökfelt T. G. (2013) Coenzyme Q10 prevents peripheral neuropathy and attenuates neuron loss in the db−/db− mouse, a type 2 diabetes model. Proc. Natl. Acad. Sci. USA 110, 690–695 10.1073/pnas.1220794110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gonzalez S., Fernando R. N., Perrin-Tricaud C., Tricaud N. (2014) In vivo introduction of transgenes into mouse sciatic nerve cells in situ using viral vectors. Nat. Protoc. 9, 1160–1169 10.1038/nprot.2014.073 [DOI] [PubMed] [Google Scholar]
- 21.Wang L., Chopp M., Szalad A., Liu Z., Lu M., Zhang L., Zhang J., Zhang R. L., Morris D., Zhang Z. G. (2012) Thymosin β4 promotes the recovery of peripheral neuropathy in type II diabetic mice. Neurobiol. Dis. 48, 546–555 10.1016/j.nbd.2012.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaplan S. R., Bach F. W., Pogrel J. W., Chung J. M., Yaksh T. L. (1994) Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods 53, 55–63 10.1016/0165-0270(94)90144-9 [DOI] [PubMed] [Google Scholar]
- 23.Wang L., Chopp M., Szalad A., Zhang Y., Wang X., Zhang R. L., Liu X. S., Jia L., Zhang Z. G. (2014) The role of miR-146a in dorsal root ganglia neurons of experimental diabetic peripheral neuropathy. Neuroscience 259, 155–163 10.1016/j.neuroscience.2013.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y., Chopp M., Liu X. S., Kassis H., Wang X., Li C., An G., Zhang Z. G. (2015) MicroRNAs in the axon locally mediate the effects of chondroitin sulfate proteoglycans and cGMP on axonal growth. Dev. Neurobiol. 75, 1402–1419 10.1002/dneu.22292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu X. S., Chopp M., Pan W. L., Wang X. L., Fan B. Y., Zhang Y., Kassis H., Zhang R. L., Zhang X. M., Zhang Z. G. (2017) MicroRNA-146a promotes oligodendrogenesis in stroke. Mol. Neurobiol. 54, 227–237 10.1007/s12035-015-9655-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jia L., Wang L., Chopp M., Zhang Y., Szalad A., Zhang Z. G. (2016) MicroRNA 146a locally mediates distal axonal growth of dorsal root ganglia neurons under high glucose and sildenafil conditions. Neuroscience 329, 43–53 10.1016/j.neuroscience.2016.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang L., Chopp M., Zhang Y., Xiong Y., Li C., Sadry N., Rhaleb I., Lu M., Zhang Z. G. (2016) Diabetes mellitus impairs cognitive function in middle-aged rats and neurological recovery in middle-aged rats after stroke. Stroke 47, 2112–2118 10.1161/STROKEAHA.115.012578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lupachyk S., Watcho P., Shevalye H., Vareniuk I., Obrosov A., Obrosova I. G., Yorek M. A. (2013) Na+/H+ exchanger 1 inhibition reverses manifestation of peripheral diabetic neuropathy in type 1 diabetic rats. Am. J. Physiol. Endocrinol. Metab. 305, E396–E404 10.1152/ajpendo.00186.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lewis B. P., Shih I. H., Jones-Rhoades M. W., Bartel D. P., Burge C. B. (2003) Prediction of mammalian microRNA targets. Cell 115, 787–798 10.1016/S0092-8674(03)01018-3 [DOI] [PubMed] [Google Scholar]
- 31.Simons M., Raposo G. (2009) Exosomes: vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 21, 575–581 10.1016/j.ceb.2009.03.007 [DOI] [PubMed] [Google Scholar]
- 32.Fields R. D., Stevens-Graham B. (2002) New insights into neuron-glia communication. Science 298, 556–562 10.1126/science.298.5593.556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vienberg S., Geiger J., Madsen S., Dalgaard L. T. (2017) MicroRNAs in metabolism. Acta Physiol. (Oxf.) 219, 346–361 10.1111/apha.12681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guay C., Regazzi R. (2013) Circulating microRNAs as novel biomarkers for diabetes mellitus. Nat. Rev. Endocrinol. 9, 513–521 10.1038/nrendo.2013.86 [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z. G., Chopp M. (2016) Exosomes in stroke pathogenesis and therapy. J. Clin. Invest. 126, 1190–1197 10.1172/JCI81133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Erhard F., Haas J., Lieber D., Malterer G., Jaskiewicz L., Zavolan M., Dölken L., Zimmer R. (2014) Widespread context dependency of microRNA-mediated regulation. Genome Res. 24, 906–919 10.1101/gr.166702.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Feng J., Zhou Y., Campbell S. L., Le T., Li E., Sweatt J. D., Silva A. J., Fan G. (2010) Dnmt1 and Dnmt3a maintain DNA methylation and regulate synaptic function in adult forebrain neurons. Nat. Neurosci. 13, 423–430 10.1038/nn.2514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nguyen S., Meletis K., Fu D., Jhaveri S., Jaenisch R. (2007) Ablation of de novo DNA methyltransferase Dnmt3a in the nervous system leads to neuromuscular defects and shortened lifespan. Dev. Dyn. 236, 1663–1676 10.1002/dvdy.21176 [DOI] [PubMed] [Google Scholar]
- 39.Johnson J. E. (2003) Numb and Numblike control cell number during vertebrate neurogenesis. Trends Neurosci. 26, 395–396 10.1016/S0166-2236(03)00166-8 [DOI] [PubMed] [Google Scholar]
- 40.Wakamatsu Y., Maynard T. M., Jones S. U., Weston J. A. (1999) NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron 23, 71–81 10.1016/S0896-6273(00)80754-0 [DOI] [PubMed] [Google Scholar]
- 41.Li H. S., Wang D., Shen Q., Schonemann M. D., Gorski J. A., Jones K. R., Temple S., Jan L. Y., Jan Y. N. (2003) Inactivation of Numb and Numblike in embryonic dorsal forebrain impairs neurogenesis and disrupts cortical morphogenesis. Neuron 40, 1105–1118 10.1016/S0896-6273(03)00755-4 [DOI] [PubMed] [Google Scholar]
- 42.Bennett M. K. (1995) SNAREs and the specificity of transport vesicle targeting. Curr. Opin. Cell Biol. 7, 581–586 10.1016/0955-0674(95)80016-6 [DOI] [PubMed] [Google Scholar]
- 43.Frassoni C., Inverardi F., Coco S., Ortino B., Grumelli C., Pozzi D., Verderio C., Matteoli M. (2005) Analysis of SNAP-25 immunoreactivity in hippocampal inhibitory neurons during development in culture and in situ. Neuroscience 131, 813–823 10.1016/j.neuroscience.2004.11.042 [DOI] [PubMed] [Google Scholar]
- 44.Benowitz L. I., Routtenberg A. (1997) GAP-43: an intrinsic determinant of neuronal development and plasticity. Trends Neurosci. 20, 84–91 10.1016/S0166-2236(96)10072-2 [DOI] [PubMed] [Google Scholar]
- 45.Goslin K., Schreyer D. J., Skene J. H., Banker G. (1988) Development of neuronal polarity: GAP-43 distinguishes axonal from dendritic growth cones. Nature 336, 672–674 10.1038/336672a0 [DOI] [PubMed] [Google Scholar]
- 46.Strittmatter S. M., Fankhauser C., Huang P. L., Mashimo H., Fishman M. C. (1995) Neuronal pathfinding is abnormal in mice lacking the neuronal growth cone protein GAP-43. Cell 80, 445–452 10.1016/0092-8674(95)90495-6 [DOI] [PubMed] [Google Scholar]
- 47.Aigner L., Arber S., Kapfhammer J. P., Laux T., Schneider C., Botteri F., Brenner H. R., Caroni P. (1995) Overexpression of the neural growth-associated protein GAP-43 induces nerve sprouting in the adult nervous system of transgenic mice. Cell 83, 269–278 10.1016/0092-8674(95)90168-X [DOI] [PubMed] [Google Scholar]