Abstract
Neutrophil extracellular trap (NET) formation constitutes an important extracellular antimicrobial function of neutrophils that plays a protective role in bacterial pneumonia. Formation of reactive oxygen species (ROS) such as highly diffusible hydrogen peroxide (H2O2) is a hallmark of oxidative stress during inflammatory lung conditions including pneumonia. However, the impact of exogenous ROS on NET formation and the signaling pathway involved in the process is not completely understood. Here we demonstrate that the ROS-sensing, nonselective, calcium-permeable channel transient receptor potential melastatin 2 (TRPM2) is required for NET formation in response to exogenous H2O2. This TRPM2-dependent H2O2-mediated NET formation involved components of autophagy and activation of AMPK and p38 MAPK, but not PI3K and AKT. Primary neutrophils from Trpm2−/− mice fail to activate this pathway with a block in NET release and a concomitant decrease in their antimicrobial capacity. Consequently, Trpm2−/− mice were highly susceptible to pneumonic infection with Klebsiella pneumoniae owing to an impaired NET formation and high bacterial burden despite increased neutrophil infiltration in their lungs. These results identify a key role of TRPM2 in regulating NET formation by exogenous ROS via AMPK/p38 activation and autophagy machinery, as well as a protective antimicrobial role of TRPM2 in pneumonic bacterial infection.—Tripathi, J. K., Sharma, A., Sukumaran, P., Sun, Y., Mishra, B. B., Singh, B. B., Sharma, J. Oxidant sensor cation channel TRPM2 regulates neutrophil extracellular trap formation and protects against pneumoseptic bacterial infection.
Keywords: reactive oxygen species, cell signaling, pneumonic sepsis
Neutrophils readily infiltrate mucosal surfaces to combat microbial infections (1–3). The traditional antimicrobial behavior of neutrophils mainly constitutes phagocytosis followed by intracellular destruction of internalized microbes. A novel paradigm in neutrophil antimicrobial program is the formation of neutrophil extracellular traps (NETs), which are DNA fibrils expelled by these cells. NETs are decorated with granular contents, including various proteases, and have been reported to play protective roles in infectious diseases by trapping, neutralizing, and destroying microbes extracellularly without the need for phagocytizing them (4–6). By contrast, an exuberant NET formation has been linked to development of many inflammatory diseases or disorders by promoting a hyperactive immune response (7, 8). To harness the beneficial outcome of NETs while avoiding their potentially harmful effects, a clear understanding of the molecular mechanism behind NET formation is essential yet thus far remains elusive.
During pneumonic infection, such as that with Klebsiella pneumoniae (KPn), an opportunistic pathogen and causative agent of 5–20% of all Gram-negative sepsis cases (9, 10), the mucosal lung surface constitutes an oxidant-rich environment (11). During inflammation, neutrophils alone, which abundantly infiltrate the site of injury, can produce ∼10 nmol/min O2− per million neutrophils during the oxidative burst (12). These cells, together with other phagocytes as well as epithelial and endothelial cells, produce abundant reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), which readily diffuses to extracellular spaces, resulting in a highly oxidative milieu where a damaging concentration of ROS can reach millimolar levels (13, 14). Although the precise concentrations of ROS in lungs during pneumonic septic conditions are not known, high metabolic rates of generation of diffusible host-derived reactive species like superoxide and H2O2 have been reported (12, 15). These host-derived ROS are now considered important signaling molecules capable of modulating various cellular processes involved in inflammation, angiogenesis and related pathologies (16). However, the factors involved in neutrophils’ ability to sense these extracellular ROS and how ROS affects NET formation machinery is not completely understood. In this regard, TRPM2 is a nonselective Ca2+-permeable membrane cation channel, which is highly expressed in myeloid cells and has been shown to regulate neutrophil influx and macrophage function by sensing ROS (17, 18). This channel is activated by ROS directly (17) or via generation of intracellular ADP ribose (ADPR) that binds to the NUDT9-H region of the channel to induce gating of this channel and mediate calcium influx (19). Although TRPM2-mediated signaling has been implicated in several physiologic and pathophysiological processes such as insulin secretion, endothelium permeability, dendritic cell maturation, and chemotaxis (20, 21); its role in NET formation is completely unknown.
In this study, using primary neutrophils and a preclinical pneumoseptic model of KPn infection in TRPM2-sufficient and -deficient mice, we examined the in vitro and in vivo roles of TRPM2 as well as the downstream signaling events in NET formation and their pathophysiological consequences during pneumoseptic bacterial infection. We identify a novel TRPM2/AMPK/MAPK pathway of NET formation with involvement of autophagy machinery that posits TRPM2 as a novel antimicrobial component of protective response against pneumoseptic bacterial infection and a putative therapeutic target for NET-related pathologies.
MATERIALS AND METHODS
Bacterial strains and mice
KPn samples (strain 43816; American Type Culture Collection, Manassas, VA, USA) were grown to log phase in Luria–Bertani (LB) medium at 37°C. All in vitro and in vivo experiments were performed using 6- to 8-wk-old wild-type (WT) C57BL/6 or Trpm2−/− mice on the same background. Trpm2−/− were developed in the laboratory of Dr. Yasuo Mori (Kyoto University, Kyoto, Japan). Breeding pairs were kindly provided under a material transfer agreement from Dr. Yasuo Mori and bred in the animal facility of the University of North Dakota. The animals were used according to institutional and federal guidelines.
Human subjects and isolation of human blood neutrophils
Blood samples (15–20 ml) were collected from healthy donors (males and females aged 18–60 yr) by venipuncture under a protocol approved by institutional review boards of the University of North Dakota (201503-298) and Altru (IRB ST151). Neutrophils were isolated and purified using an EasySep Direct Human Neutrophil Isolation Kit (Stemcell Technologies, Vancouver, BC, Canada), as per the manufacturer’s instructions. Neutrophil purity was assessed by flow cytometry using CD15 and CD16 antibodies and nuclear morphology. Stimulation of neutrophils and analysis of NET formation was performed similar to mouse neutrophils as described below.
In vitro and in vivo analysis of NETs
For in vitro studies, peritoneal neutrophils were isolated as previously described (22, 23) (80–85% pure as assessed by flow cytometry using Ly6G and CD11b antibodies) and stimulated with an optimal concentration of H2O2 (10 mM) for 4 h at 37°C, as previously reported (24, 25), with or without a 30 min pretreatment with BAPTA-AM (5 µM). Cells were fixed with 4% paraformaldehyde and NETs were stained using Sytox Green (Molecular Probes, Eugene, OR, USA) as previously described (22, 23, 26). Images were acquired by EVOS FL Cell Imaging System (Thermo Fisher Scientific, Waltham, MA, USA). The percentage of NETs was manually calculated in blinded fashion by dividing the number of NET-forming neutrophils with the total number of cells in 10 randomly selected microscopic fields and multiplying the resulting values by 100. For the in vivo NETs analysis, neutrophils isolated from bronchoalveolar lavage (BAL) of mice were cytocentrifuged on glass microscope slides followed by Sytox Green staining and NET quantification as previously reported (22, 23, 26).
Measurement of calcium influx
Phorbol 12-myristate 13-acetate–induced intracellular ROS generation was calculated by detecting hydrogen peroxide using the Fluoro H2O2 Detection Kit (Cell Technology, Fremont, CA, USA) as previously described (23). For the intracellular calcium influx measurement, fluorescence intensity of Fura-2–loaded cells was monitored as previously described (27–29). Briefly, primary neutrophils were stimulated with the indicated concentrations of H2O2 with or without pretreatment of 0.1 mM flufenamic acid (FFA; MilliporeSigma, Burlington, MA, USA) for 30 min, followed by incubation with 2 μm Fura-2 (Thermo Fisher Scientific) for 45 min. The intensity of Fura-2–loaded control cells was recorded with a CCD camera–based imaging system (Compix, Irvine, CA, USA). The images were processed with the C imaging, PCI software (Compix), to show ratios of Fura-2 fluorescence from excitation at 340 nm to that from excitation at 380 nm (F340/F380).
Electrophysiology
The electrophysiological experiment (patch clamp) was carried out according to established protocol (27–29). In brief, patch-clamp experiments were performed in a tight-seal, whole-cell configuration at room temperature (22–25°C) using an Axopatch 200B Amplifier (Molecular Devices, San Jose, CA, USA). Voltage ramps ranging from −90 to 90 mV over a period of 1 s were imposed every 4 s from a holding potential of 0 mV and digitized at a rate of 1 kHz. A liquid junction potential of <8 mV was not corrected, and capacitive currents and series resistance were determined and minimized. For analysis, the first ramp was used for leak subtraction for the subsequent current records. Currents were normalized to the initial size of the cell to obtain current densities (pA/pF).
Western blot analysis
For the analysis of signaling molecules, neutrophils were plated in 90-mm dishes at the density of 10 × 106 cells and stimulated with H2O2 with or without pretreatment (30 min) of 5 µM poly-ADPR polymerase enzyme (PARP) inhibitor (DPQ; MilliporeSigma), 10 µM AMPK inhibitor (Compound C; MilliporeSigma) or 10 µM p38 inhibitor (SB203580; InvivoGen, San Diego, CA, USA). Cells were lysed in 1-time RIPA buffer (20–188; MilliporeSigma) supplemented with 0.05% SDS, 1% Triton X-100, 20% glycerol, 1 mM PMSF, and 1-time protease and phosphatase inhibitors (Thermo Fisher Scientific) and resolved on SDS-PAGE as previously described (30, 31). Immunoreactivity of LC3A/B, p-p38, p-AMPKα1, PI3K, p-AKT, and β-actin antibodies (all purchased from Cell Signaling Technology) were detected using super signal west Pico Chemiluminisecnt detection reagent (Thermo Fisher Scientific) and analyzed on BioRad Reader (Bio-Rad Laboratories, Hercules, CA, USA) using Chembio software (Medford, NY, USA). Densitometry of individual blots was done using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
NET-mediated killing assay
Antimicrobial activity of H2O2 induced NETs was determined by established methods (32, 33). The peritoneal neutrophils isolated from TRPM2-sufficient WT and Trpm2−/− mice were plated in 6-well tissue culture plates and incubated for 30 min at 37°C in the presence of the phagocytosis inhibitor cytochalasin D (10 μg/ml; MilliporeSigma). Neutrophils were stimulated with 10 mM H2O2 for 3 h to induce NET formation. The cells that formed NETs were then washed and replaced with fresh medium to remove residual H2O2. To ensure NET-mediated eradication of bacteria, NETs were incubated with KPn at a multiplicity of infection of 10 without and with DNase I (100 U/ml; Thermo Fisher Scientific) for 15 min before bacteria were added. After 90 min of incubation, well contents were scraped, serially diluted and plated on LB agar plates to enumerate colony forming units (CFUs).
Infection of mice, survival, and bacterial burden
WT and Trpm2−/− mice were infected intranasally with 3 × 104 cfu KPn as previously described (22, 26). Survival of the mice was recorded for up to 14 d postinfection (p.i.). Death was recorded as infection-induced mortality. In some experiments, the mice were euthanized 3 d p.i. and blood and lungs were harvested and processed for bacterial burden analysis as previously described (22, 26).
Histologic analyses
Frozen lung tissues were processed for histologic analysis as previously described (22, 23, 26, 34). Ten-micrometer-thick, sequential horizontal sections of frozen lung tissues thus obtained were stained with hematoxylin and eosin (H&E) for pathologic analysis as previously described (22, 23, 26, 34).
Flow cytometry and CBA analysis
Lungs from WT and Trpm2−/− mice were harvested from mice 3 d p.i. with KPn and processed for isolation of total lung cells as previously described (22, 23, 26, 34). Enumeration of neutrophils by flow cytometry (BD LSR II) was done by quantitating Ly6G+ CD11b+ cells stained with Pacific Blue antimouse CD11b (clone M1/70) and allophycocyanin antimouse Ly6G (clone 1A8) Abs (BioLegend, San Diego, CA, USA). FACSdiva and FlowJo software (FlowJo, Ashland, OR, USA) was used to analyze all data. For the cytokine expression profile, lung homogenates from uninfected or KPn-infected mice 3 d p.i. (22, 23, 26, 34) were analyzed using BD Cytometric Bead Array (CBA) kits as per manufacturer’s instruction (BD Biosciences, Franklin Lakes, NJ, USA). A BD LSR II (BD Biosciences) flow cytometer was used for data acquisition and BD Biosciences FCAP Array Software (v.3.0) was used for data analysis.
Statistical analysis
Statistical analysis of survival studies was performed by a Kaplan–Meier log-rank test; bacterial burdens were analyzed by a nonparametric Mann–Whitney U test. All 2-group comparisons were performed using a Student’s t test [SigmaPlot 13.0 (Systat Software, San Jose, CA, USA), GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA, USA) and Origin 9.0 (Origin Lab, Northampton, MA, USA)]. Values of P ≤ 0.05 were considered statistically significant. Nonparametric ANOVA and Dunn’s post hoc analyses were used for multiple-group comparisons.
RESULTS
Exogenous H2O2 induces robust NET formation in calcium dependent manner
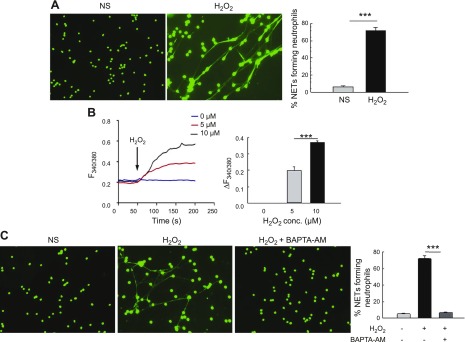
To examine the impact of exogenous ROS on NET formation, we directly stimulated neutrophils with H2O2. As shown in Fig. 1A, H2O2 induced robust NET formation in primary neutrophils and increased the relative concentration of intracellular calcium as assessed by using a fluorescent dye, Fura-2 (Fig. 1B). Importantly, a dose-dependent increase in H2O2-mediated calcium entry was observed where addition of both 5 and 10 μM concentrations of H2O2 significantly increased cytosolic calcium concentration. This H2O2-mediated increased intracellular calcium was required for NET formation as the cells treated with BAPTA-AM [1,2-bis(2-aminophenoxy)ethane-N,N,N′,N′-tetraacetic acid tetrakis(acetoxymethyl ester); a selective calcium chelator] showed significantly reduced NET formation upon H2O2 stimulation (Fig. 1C). Taken together, these data show that extracellular oxidant stimulation increases calcium influx, which is required for robust NET formation, thus indicating the involvement of a calcium channel in this process.
Figure 1.
A) Exogenous H2O2 induces robust NET formation and calcium influx in vitro in neutrophils. Representative fluorescence images of WT neutrophils unstimulated (NS) or stimulated with H2O2 for 4 h. Cells were fixed and NETs were stained with DNA dye Sytox Green. Original magnification, ×200. Percentage of NET-forming neutrophils (mean ± se) from 4 independent experiments is shown in the bar graph. B) Calcium imaging by Fura-2 acetoxymethyl ester fluorescence measurement was performed on primary neutrophils in the absence (NS) or presence of 0–10 µM H2O2. Average analog plots of the fluorescence ratio (340/380 nm) from an average of 40–50 cells are shown. The bar graph shows the average data from 3 independent experiments for changes in cytosolic calcium under these conditions in unstimulated and H2O2-stimulated primary neutrophils. C) Neutrophils were pretreated for 30 min with 5 µM BAPTA-AM before stimulation with 10 mM H2O2 for 4 h. Representative fluorescent images of neutrophils are shown (original magnification, ×200). Bar graph shows quantification (average ± se) of NET-forming neutrophils from 3 independent experiments. ***P < 0.001.
H2O2 activates cation channel TRPM2 in neutrophils, which is required for NET formation induced by exogenous H2O2
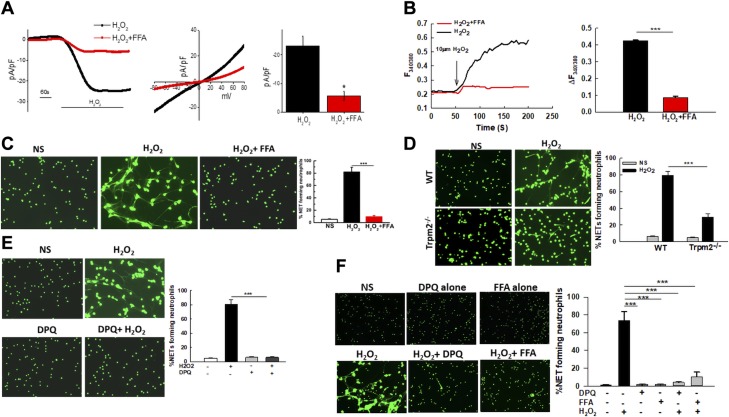
We next assessed the calcium channel most likely to be involved in H2O2-stimulated NET formation. For this current, recordings in whole cell configuration were performed. H2O2 stimulation of neutrophils evoked a nonselective inward current with current–voltage properties similar to those characteristic of TRPM2 (35, 36) (Fig. 2A). Importantly, this H2O2-induced TRPM2-like current in primary neutrophils was attenuated by treatment with FFA, a potent TRPM2 inhibitor (Fig. 2A). FFA treatment also significantly inhibited H2O2-induced calcium influx (Fig. 2B) as well as NET formation in response to H2O2 (Fig. 2C), further suggesting the involvement of TRPM2 in this process. Of import, consistent with this data, Trpm2−/− neutrophils (Fig. 2D), as well as neutrophils transfected with TRPM2 small interfering RNA (Supplemental Fig. 1) exhibited significantly impaired NET formation. An important mechanism of TRPM2 activation downstream of oxidative stress is by binding of ADPR, which is generated by PARP activation (19). To solidify the involvement of TRPM2 in NET formation pathway, neutrophils stimulated by H2O2 were treated with PARP inhibitor DPQ. As shown in Fig. 2E, inhibition of TRPM2 agonist ADPR markedly inhibited H2O2-induced NET formation strongly suggesting that TRPM2 is required for the formation of NETs in response to ROS.
Figure 2.
In vitro stimulation with exogenous H2O2 activates cation channel TRPM2 in neutrophils, which is required for NET formation. A) The TRPM2-like current in H2O2-stimulated primary neutrophils was measured by whole cell patch-clamp method in the presence or absence of 0.1 mM FFA. Respective current–voltage (I–V) curves and current intensity (average of 8–10 recordings represented by bar graph) at −80 mV are shown. Attenuation of current density was found with FFA-treated neutrophils. B) Ca2+ imaging was performed in 10 µM H2O2-stimulated neutrophils with and without 0.1 mM FFA. Analog plots of the fluorescence ratio (340/380) from an average of 40–60 cells are shown. Bar graph shows quantification (mean ± sd) of the fluorescence ratio (340/380) from 3 independent experiments.. C) Neutrophils were pretreated for 30 min)with 0.1 mM FFA and subsequently stimulated with 10 mM H2O2 for 4 h. NETs were stained using Sytox Green. Representative fluorescent images of NETs are shown (original magnification, ×200). Bar graph shows quantification of NETs as the average ± se of 3 independent experiments. D) Representative fluorescent images of WT and Trpm2−/− neutrophils unstimulated (NS) or stimulated with H2O2 for 4 h. NETs were fixed and stained with Sytox Green (original magnification, ×200). The bar graph shows quantification of NETs (average ± se) in WT and Trpm2−/− neutrophils. The results include data from 5 independent experiments. E) Neutrophils were pretreated for 30 min with 5 µM DPQ (PARP inhibitor) and subsequently stimulated with H2O2 for 4 h. NETs were visualized by staining with Sytox Green. Representative fluorescent images are shown (original magnification, ×200). Bar graph shows average NETs ± se of 3 independent experiments. F) Neutrophils isolated from peripheral blood of healthy adults were stimulated with H2O2 with or without a 30 min pretreatment with 5 µM DPQ or 0.1 mM FFA. NETs were stained using Sytox Green. Representative fluorescence images from 1 out of 3 individuals are shown (original magnification, ×200). The bar graph shows the quantification of NETs (average ± se) from 3 healthy adults (1 female and 2 males). *P < 0.05 (statistically significant), ***P < 0.001.
To ensure that the results obtained from murine neutrophils could be extrapolated to human cells, we purified human neutrophils from blood of healthy donors. The cells were then exposed to H2O2 with or without TRPM2 inhibitor FFA or PARP inhibitor DPQ. Indeed, human neutrophils generated robust NETs in response to H2O2 treatment, which was dramatically inhibited by treatment with TRPM2 pathway inhibitors (Fig. 2F). Overall, our results with both the murine and the human neutrophils showed that exogenous H2O2 activates the cation channel TRPM2 in neutrophils to induce NET formation.
TRPM2 mediates NET formation via activation through MAPK signaling pathway and autophagy machinery
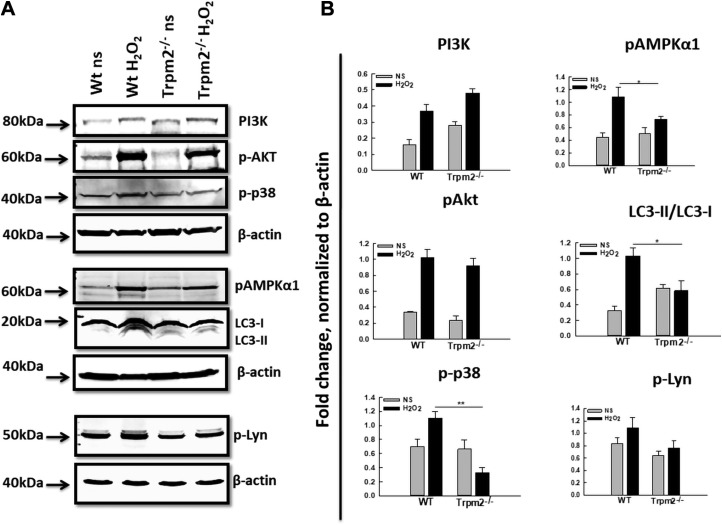
Having established the role of TRPM2 in ROS-mediated NET formation, we next examined the downstream signaling pathway involved. We have recently reported that activation of autophagy is required for NET formation (23). We examined the effect of directly stimulating neutrophils with H2O2 via the autophagy pathway in a TRPM2-dependent manner to induce NET formation. As shown in Fig. 3A, treatment of WT neutrophils with H2O2 caused increased conversion of the nascent autophagy protein LC3 (microtubule-associated protein 1 light chain 3α), also called ATG8 (LC3-I), into its processed form, LC3-II, which serves as a marker of autophagy activation and autophagosome formation. Trpm2−/− neutrophils, however, were found to exhibit impaired LC3 activation upon H2O2 stimulation, correlating the defective NET formation in the absence of TRPM2 with reduced autophagy activation. Western blot analysis of multiple key kinases reported to be involved in NET formation and the autophagy process revealed increased phosphorylation of PI3K, AKT, p38 MAPK, AMPK, and Lyn in the WT neutrophils upon H2O2 stimulation compared with the unstimulated cells (Fig. 3A). Absence of TRPM2 had no significant effect on activation of PI3K, AKT, or Lyn because the Trpm2−/− neutrophils exhibited similar levels of phosphorylation as the WT neutrophils upon H2O2 stimulation (Fig. 3A, B). By contrast, H2O2-stimulated Trpm2−/− neutrophils showed significantly reduced phosphorylation of the catalytic subunit AMPKα1 and p38 MAPK compared with their WT counterparts (Fig. 3A, B). Together, these results suggest the involvement of AMPK, p38 MAPK, and the autophagy pathway in TRPM2-mediated activation of neutrophils upon oxidant stimulation.
Figure 3.
Signaling pathway of TRPM2-mediated in vitro NET formation in response to H2O2 stimulation. A) Western blot analysis was performed on equal amounts of protein from cell lysates of WT and Trpm2−/− neutrophils unstimulated (NS) or stimulated with H2O2 using antibodies against various signaling proteins. The experiments were performed 3–4 times and representative blots for individual proteins are shown. B) Densitometry was performed for respective protein bands using ImageJ software. For the calculation of fold change, average pixel intensities of specific bands were normalized to the housekeeping protein β-actin. For LC3, the fold change depicts the ratio of normalized LC3-II to LC3-I. The average of data from 3 independent experiments is shown. *P < 0.05, **P < 0.01.
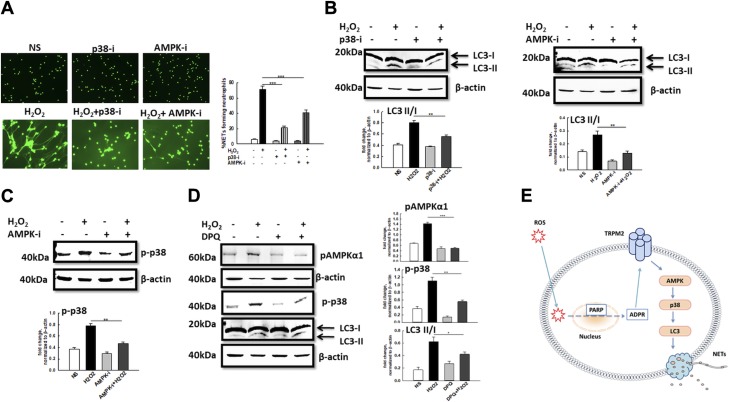
In order to determine whether these signaling kinases were involved in NET formation in response to H2O2 stimulation, neutrophils were pretreated with specific kinase inhibitors and NET formation upon H2O2 stimulation was quantified. In line with the increased phosphorylation of AMPK and p38 MAPK, treatment with Compound C (AMPK inhibitor) and SB203580 (p38 inhibitor) significantly impaired NET formation in response to H2O2 (Fig. 4A). Inhibition of AMPK as well as p38 phosphorylation impaired H2O2-induced activation of autophagy in WT neutrophils (Fig. 4B), implicating these signaling molecules in the H2O2/autophagy/NET formation axis. Furthermore, treatment with AMPK inhibitor significantly reduced the phosphorylation of p38 (Fig. 4C), suggesting that AMPK is acting upstream of p38 to induce autophagy activation and NET formation in response to H2O2. Treating WT neutrophils with the ADPR inhibitor DPQ impaired phosphorylation of both AMPK and p-38 as well as autophagy activation (Fig. 4D), thus connecting the AMPK/p38/autophagy/NET formation pathway with TRPM2 activation. Taken together, our data strongly suggest a putative signaling pathway downstream of TRPM2 in neutrophils where H2O2 stimulates ADPR-mediated TRPM2 activation in neutrophils, which in turn engages AMPK, p38 MAPK, and autophagy machinery leading to NET formation (Fig. 4E).
Figure 4.
The AMPK/p38/autophagy axis regulates in vitro NET formation in response to H2O2 stimulation. Inhibition of phosphorylation of AMPK and p38 impairs NET formation. A) Representative fluorescent images of WT neutrophils pretreated for 30 min with 10 µM of phospho-p38 inhibitor SB203580 (p38-i) or 10 µM of phospho-AMPK inhibitor Compound C (AMPK-i) followed by stimulation with H2O2. NETs were visualized using Sytox Green staining. Original magnification, ×200. Bar graph shows quantification of NETs (average ± se) from 3 independent experiments. ***P < 0.001. B) Western blot analysis of autophagy activation indicated by level of LC3-II expression in WT neutrophils stimulated with 10 mM H2O2 in the presence or absence of p38-i and AMPK-i. Bar graph depicts densitometry analysis of protein bands showing the ratio of LC3-II over LC3-I after normalization of band intensities using that of β-actin. Data shown is the average of 3 to 4 independent experiments. C) Western blot analysis of phospho-p38 expression in H2O2-stimulated neutrophils in the presence or absence of AMPK-i. Bar graph depicts densitometry analysis protein band intensities normalized to β-actin. D) Western blot analysis of phospho-AMPKα1, phospho-p38, and LC3-I and -II expression in H2O2-stimulated neutrophils in the presence or absence of DPQ. Bar graph shows densitometry of specific band intensities normalized to β-actin. E) A model depicting signaling pathway of TRPM2-mediated NET formation in response to ROS.
Trpm2−/− neutrophils are defective in NET-mediated destruction of bacteria
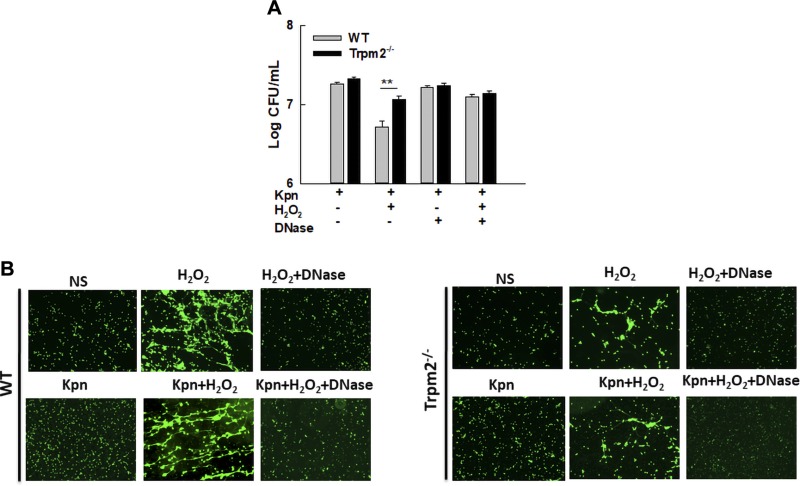
We next examined the physiologic relevance of TRPM2-mediated NET formation to the eradication of bacteria, a well-established antimicrobial function of NETs. H2O2-induced NETs in WT neutrophils were capable of killing KPn, a causative agent of nosocomial pneumonia and sepsis as shown by reduced recovery of viable bacteria from WT neutrophils that produced NETs in vitro (Fig. 5A). Destruction of this bacteria was specific to NETs because treatment with DNAse, which causes the disintegration of NETs (Fig. 5B), increased the recovery of viable bacteria (Fig. 5A). By contrast, Trpm2−/− neutrophils exhibited drastically reduced capacity for destroying bacteria, as evidenced by significantly higher numbers of bacteria recovered from Trpm2−/− neutrophils stimulated with H2O2 (Fig. 5A). TRPM2 deficiency did not impair other antimicrobial functions of phagocytic cells because both the WT and Trpm2−/− neutrophils (Supplemental Fig. 2A) and macrophages (Supplemental Fig. 2B) were found to be fully competent in phagocytosis and intracellular clearance of KPn. Taken together, these data suggest a specific protective function of TRPM2 in controlling the NET-mediated antibacterial function of neutrophils.
Figure 5.
Trpm2−/− neutrophils are defective in NET-mediated destruction of bacteria in vitro. A) WT and Trpm2−/− neutrophils were stimulated with 10 mM H2O2 to induce NET formation and subsequently infected with KPn (multiplicity of infection = 10) in the presence of cytochalasin D with or without DNase I for 90 min. Data presented in the bar graph is the average ± se of 4 independent experiments. **P < 0.01. B) Representative fluorescent images of various treatments described in A are shown (original magnification, ×100).
Trpm2−/− mice are more susceptible to pneumoseptic infection with KPn
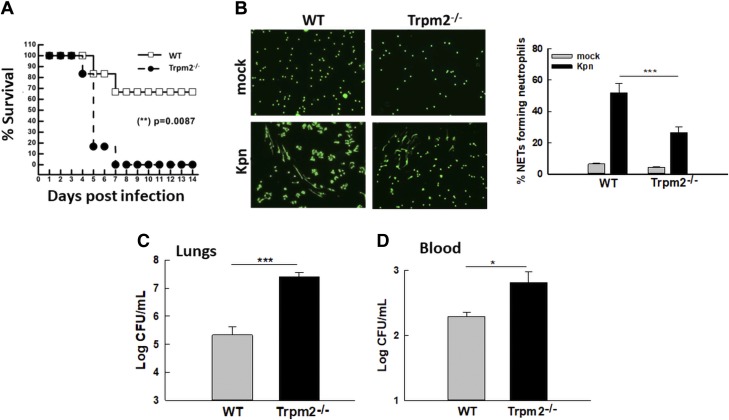
To examine the implication of TRPM2-mediated NET formation and bacterial clearance in KPn pathogenesis, overall disease severity and survival were compared in WT and Trpm2−/− mice infected with KPn. Infection of WT mice with the sublethal doses of KPn used in our previous studies (22, 26, 34) resulted in a 70–75% survival rate, with only transient signs of disease (ruffled fur, lethargy) in early stages of infection (Fig. 6A). By contrast, Trpm2−/− mice were significantly more susceptible to this dose; the majority of mice had succumbed to infection 4 d p.i. and all mice were dead 7 d p.i. (Fig. 6A). These mice exhibited progressive development of disease and overt signs of infection (weight loss, piloerection, hunched gait, lethargy, and increased respiratory rate). The increased susceptibility of Trpm2−/− mice further supported a protective role played by this channel during pneumonic KPn infection.
Figure 6.
Increased susceptibility of Trpm2−/− mice to pulmonary infection with KPn correlates with impaired NET formation in vivo. A) WT and Trpm2−/− mice were intranasally infected with 3.0 × 104 CFUs of KPn in 20 µl sterile PBS and disease progression was assessed daily for 14 d. Statistical comparison of susceptibility was made using the Kaplan–Meier survival curve. **P = 0.0087. The data shown are from 3 independent experiments (n = 18/group). B) WT and Trpm2−/− mice were mock infected (PBS only) or intranasally infected with 3.0 × 104 CFUs of KPn in 20 µl sterile PBS. Cells from BAL were isolated 3 d p.i. and cytocentrifuged on glass microscope slides. NETs were fixed and stained with Sytox Green as described in the Materials and Methods. Representative fluorescent images from 4 or 5 independent experiments are shown (original magnification, ×200). The bar graph shows the average ± se of NETs from 4 independent experiments. ***P < 0.001. C) WT and Trpm2−/− mice were intranasally infected with KP; 3 d p.i., the mice were euthanized and lungs were isolated, homogenized, and plated. Means ± se from 3 independent experiments are displayed (n = 9/group). ***P < 0.001. D) Blood samples collected 3 d p.i. from KPn-infected WT and Trpm2−/− mice were serially diluted and plated on LB plates. Bacterial burden was enumerated after incubating the plates overnight at 37°C. Means ± se from 3 independent experiments are displayed (n = 9/group). *P < 0.05.
To correlate the increased susceptibility of Trpm2−/− mice with our in vitro observation of NET-mediated bacterial clearance, lungs of the infected WT and KO mice were lavaged and NET formation in cytocentrifuged BAL cells was assessed. As shown in Fig. 6B, Trpm2−/− mice exhibited drastically reduced NET formation in their BAL neutrophils compared with their WT counterparts. This impaired NET formation correlated with a significantly higher bacterial load both in the lungs (Fig. 6C) and in the blood (Fig. 6D) of these animals than that found in the WT mice. Together with the results of our in vitro NET-mediated killing assays, these data showed that Trpm2−/− mice are highly susceptible to pulmonary infection with KPn due to an impaired NET formation and a concomitant bacterial clearance capacity in the absence of TRPM2.
Trpm2−/− mice exhibit severe neutrophilic lung pathology and hyperinflammatory response during pneumonic KPn infection
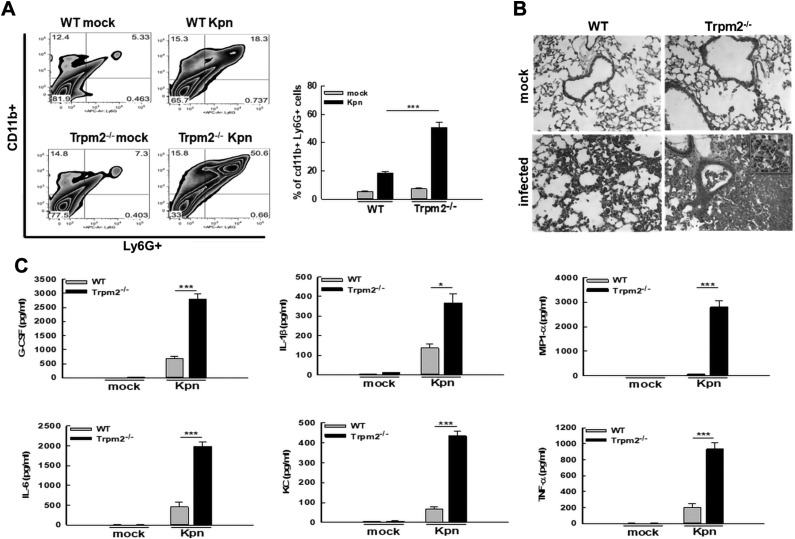
The TRPM2 channel has been reported to regulate neutrophil influx (17, 18). To examine whether the differences in neutrophils infiltrating the lungs of WT vs. Trpm2−/− mice could account for the reduction in NETs observed in Trpm2−/− mice during KPn infection, flow cytometry analysis of total lung cells was performed. This data revealed that both the percentage and the total numbers of Ly6G+ CD11b+ neutrophils were significantly higher in the lungs of Trpm2−/− mice upon infection than in the WT mice (Fig. 7A). This indicated that the reduction in NETs observed in lungs of infected Trpm2−/− mice did not result from an impairment of neutrophil influx in the absence of TRPM2. The increased accumulation of neutrophils contributed to the increased lung pathology (Fig. 7B), with lung consolidation and neutrophilia (Fig. 7B, inset) observed in the comparative analysis of H&E-stained cryosections of KPn infected WT and Trpm2−/− mice. Cytometric bead array (CBA) analysis showed significantly higher levels of neutrophil-associated mediators G-CSF (granulocyte-colony stimulating factor), CXCL1 (C-X-C motif chemokine ligand 1; KC in mice), and MIP-1α (mitogen-activated protein kinase associated protein 1), as well as inflammatory cytokines IL-1β, IL-6, and TNF-α in the lungs of KPn-infected Trpm2−/− mice (Fig. 7C). Taken together, our data suggest a protective function of TRPM2 in pneumoseptic infection with KPn, where absence of this protein results in reduced NET formation, increased bacterial burden, and a concomitant severe lung pathology and hyperinflammatory response that likely contributes to the increased susceptibility of Trpm2−/− mice to KPn infection.
Figure 7.
Trpm2−/− mice exhibit severe neutrophilic lung pathology and hyperinflammatory response during pneumonic KPn infection. A) Flow cytometry analysis of lung cells isolated from mock-infected control and KPn-infected WT and Trpm2−/− mice. Total lung cells were isolated from mice by collagenase treatment 3 d p.i. The cells were stained with anti–CD11b-PB and anti–Ly6G-APC antibodies and analyzed using flow cytometry. Data presented in the bar graph depict means ± se from 3 independent experiments. ***P < 0.001. B) Histopathological assessment by H&E staining of lung cryosections obtained from mock-infected control and KPn-infected WT and Trpm2−/− mice 3 d p.i. (original magnification, ×200). Inset shows a magnified area depicting neutrophils. C) Trpm2−/− mice exhibit hyperinflammatory response during KPn-induced pneumonia. Lungs isolated 3 d p.i. from mock-infected control or KPn-infected WT and Trpm2−/− mice were analyzed for cytokines using a BD Cytometric Bead Array Kit. Means ± se from 2 independent experiments are shown (n = 4–5/group).
DISCUSSION
We and others have shown that ROS and autophagy play an intricate role in NET formation (23, 37, 38). However, the means by which neutrophils sense exogenous ROS and by which downstream signaling is activated to trigger NET formation are unclear. Here we show that TRPM2, a cation channel, senses ROS to drive NET formation by activating the AMPK/p38 MAPK pathway and autophagy machinery. Inhibition of this pathway impairs the capacity of neutrophils to form NETs in response to ROS. We also show that TRPM2-dependent NET formation is required to activate the antimicrobial function of neutrophils and thus protect against a pneumoseptic infection caused by KPn. Extensive formation of ROS in inflammatory conditions such as mucosal infection is a well-known phenomenon. Our studies provide insight into how this highly oxidative environment can trigger NETosis, an antimicrobial function of neutrophils, by TRPM2-mediated activation of the AMPK/p38 signaling pathway and autophagy machinery.
Many studies have captured the role of intracellular ROS in the process of NET formation (39). Upon activation, the primary ROS produced by the neutrophils are superoxide anion and H2O2. Myeloperoxidase-mediated oxidation of chloride by H2O2 produces hypochlorous acid. Among these oxidant species, H2O2 is highly diffusible, making it a highly relevant oxidant species for exogenous activation of neutrophils during such inflammatory conditions as acute lung infection and sepsis. These present a highly oxidative environment where the activated cells release ROS in the milieu, causing the extracellular concentration of ROS to reach supraphysiological levels (11, 13, 14, 40). Although several mechanisms of ROS scavenging exist in biologic systems, in infectious inflammatory lung conditions these detoxifying mechanisms are likely overwhelmed by enormous amounts of H2O2 generated by various cells. Traditionally considered to be noxious molecules that cause nonspecific cell and tissue damage, ROS are now regarded as signal-transduction factors capable of activating a number of signaling pathways (41). Our results showing NET formation in response to in vitro monostimulation with exogenous H2O2 in the absence of any pathogen or pathogen-associated molecular patterns support the direct activating capacity of ROS. In this regard, TRPM2—present on membranes of a variety of cell types but most abundantly expressed on myeloid cells—acts as a sensor for ROS, converting the oxidative stress into Ca2+ signaling (20, 42, 43). We show that external H2O2 stimulation of neutrophils induces a TRPM2-like current and intracellular calcium influx, which is required for H2O2-induced NET formation because calcium chelation and treatment with FFA, an antipyretic agent and a known TRPM2 inhibitor, blocked this process. Oxidant sensing by TRPM2 occurs primarily through the generation of ADPR via activation of PARP1. We found that blockage of ADPR by treatment with PARP1 inhibitor DPQ resulted in impaired NET formation in response to H2O2. Importantly, our findings of TRPM2-dependent H2O2-induced NET formation were replicated in human neutrophils, strongly supporting the translational potential of our study. Subsequent use of various pharmacologic inhibitors of downstream signaling components led us to discover that TRPM2 activation by H2O2 causes AMPK phosphorylation upstream of p38 MAPK, resulting in autophagy activation and NET formation. Activation of p38 MAPK by TRPM2 channel activity reportedly contributes to LPS/IFN-γ–induced activation of microglia (44) and increases intracellular bactericidal activity of neutrophils upon lysophosphatidylcholine stimulation (45). The activation of PI3K and AKT but not p38 in Trpm2−/− neutrophils suggests that phosphorylation of this specific MAPK is tightly coupled with TRPM2 activation—a prerequisite for NET formation. This was further supported by our data showing NET impairment upon treating neutrophils with the phospho-p38 inhibitor SB203580. To the best of our knowledge, AMPK activation downstream of TRPM2-mediated oxidant sensing has not been reported previously. A significant reduction of AMPK phosphorylation in response to H2O2 in Trpm2−/− neutrophils as well as blockage of p38 phosphorylation and NET formation upon the inhibition of AMPK suggests that AMPK downstream of TRPM2 activation by ROS is involved in p38 phosphorylation. Emerging evidence, including that from our group, increasingly supports a fundamental role of autophagy in NET formation (23, 46, 47). Our data showing inhibition of autophagy activation in the absence of TRPM2 as well as by blockage of the AMPK/p38 axis downstream of this channel adds new components to this process by identifying a new pathway of TRPM2-mediated activation of AMPK/p38/autophagy signaling, which is required for NET formation in response to oxidant sensing.
We found TRPM2-mediated NET formation to be an essential antimicrobial mechanism for protection against infection with KPn, a prominent causative agent of nosocomial pneumonia and sepsis (48). Antimicrobial activity of NETs has been reported for several bacterial, viral, and even parasitic infections (49), but the involvement of TRPM2 in the process has not been reported until now. In inflammatory disease conditions with prominent neutrophil and monocyte or macrophage components, TRPM2 is shown to regulate neutrophil migration and influx; however, conflicting findings regarding the protective and pathologic roles of TRPM2 have been reported. In sterile inflammatory conditions such as mouse models of dextran sodium sulfate–induced colitis, the carrageenan-induced pain model, and bleomycin-induced lung injury, Trpm2−/− mice were largely protected from the disease owing to reduced production of neutrophil chemotactic factor CXCL2 and hence a markedly suppressed neutrophil infiltration (18, 50, 51). By contrast, a protective role of TRPM2 in bacterial infections is reported similar to our study, although the underlying mechanisms have varied. In Francisella tularensis and Listeria monocytogenes infections, TRPM2 was found to contribute to innate immunity via production of inflammatory cytokines (52, 53). More recently, TRPM2 was implicated in the regulation of phagosomal maturation and the eradication of Staphylococcus aureus in macrophages, as well as the restriction of neutrophil migration (17, 54). Although all of these studies provided important insights into the diverse functions of TRPM2 in a variety of disease settings, TRPM2’s contribution to NET formation remained obscure. Our results show that the absence of TRPM2 impaired the NET-mediated antibacterial capacity of neutrophils despite being fully able to perform phagocytosis and destroy pathogens. These findings provide evidence for a novel role of TRPM2 in regulating NET formation with an important protective role in pneumoseptic bacterial infection owing to the antimicrobial activity of NETs. Although speculative at this point, reduced inflammation and improved disease outcome reported in absence of TRPM2 in sterile inflammatory diseases may be the result of impaired NET formation because exuberant NET formation is associated with exacerbation of inflammation in autoimmune diseases (8, 55, 56). Our studies thus open up a new avenue to research in this area and introduce the potential of TRPM2 modulation as a promising immunomodulatory strategy for NET-associated pathologies.
In total, our findings presented here show that oxidant sensing by TRPM2 is crucial for NET formation by engaging a novel AMPK/p38/autophagy pathway, which plays a protective antimicrobial function in pneumonic bacterial infection. Our results thus posit TRPM2 and its downstream signaling pathway as important therapeutic targets for pharmacological modulation of NET formation in a variety of inflammatory and infectious disease conditions.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Sarah Abrahamson (Research Specialist, Imaging Core Facility, Biomedical Sciences) for the technical support for the microscopy. The Flow Cytometry Core Facility at the University of North Dakota is supported by grants from IDeA Networks of Biomedical Research Excellence (P20GM103442) and the Centers of Biomedical Research Excellence (COBRE; P20GM113123). The Imaging Core Facility is supported by COBRE Grant P20GM113123 from the U.S. National Institutes of Health (NIH)/National Institute of General Medical Sciences (NIGMS). This work was supported by NIH/National Institute of Allergy and Infectious Diseases Grants R21AI107457 and R01AI121804 (to J.S.), NIH National Institute of Dental and Craniofacial Research Grant R21DE024300 (to B.B.S. and B.B.M.) and R01DE017102 (to B.B.S), and NIH/NIGMS Grant P20GM113123 (to J.S. and B.B.M.). A.S. is partially supported by a postdoctoral award from the Office of the Vice President of Research, University of North Dakota. The authors declare no conflicts of interest.
Glossary
- ADPR
ADP ribose
- BAL
bronchoalveolar lavage
- CFU
colony-forming unit
- H&E
hematoxylin and eosin
- KPn
Klebsiella pneumoniae
- LB
Luria–Bertani
- NET
neutrophil extracellular traps
- PARP
poly-ADP ribose polymerase enzyme
- p.i.
postinfection
- ROS
reactive oxygen species
- TRPM2
transient receptor potential melastatin 2
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. K. Tripathi and A. Sharma performed the experiments, collected data, analyzed the data, discussed the data, and contributed to manuscript writing; P. Sukumaran and Y. Sun performed calcium imaging and patch-clamp experiments and consolidated data; B. B. Mishra and B. B. Singh codesigned some experiments, discussed the data, and critically read the manuscript; and J. Sharma designed and supervised the study and wrote the manuscript.
REFERENCES
- 1.Balamayooran G., Batra S., Theivanthiran B., Cai S., Pacher P., Jeyaseelan S. (2012) Intrapulmonary G-CSF rescues neutrophil recruitment to the lung and neutrophil release to blood in Gram-negative bacterial infection in MCP-1−/− mice. J. Immunol. 189, 5849–5859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovach M. A., Standiford T. J. (2012) The function of neutrophils in sepsis. Curr. Opin. Infect. Dis. 25, 321–327 [DOI] [PubMed] [Google Scholar]
- 3.Mócsai A. (2013) Diverse novel functions of neutrophils in immunity, inflammation, and beyond. J. Exp. Med. 210, 1283–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 [DOI] [PubMed] [Google Scholar]
- 5.Brinkmann V., Zychlinsky A. (2012) Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198, 773–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yipp B. G., Kubes P. (2013) NETosis: how vital is it? Blood 122, 2784–2794 [DOI] [PubMed] [Google Scholar]
- 7.Grayson P. C., Kaplan M. J. (2016) At the bench: neutrophil extracellular traps (NETs) highlight novel aspects of innate immune system involvement in autoimmune diseases. J. Leukoc. Biol. 99, 253–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jorch S. K., Kubes P. (2017) An emerging role for neutrophil extracellular traps in noninfectious disease. Nat. Med. 23, 279–287 [DOI] [PubMed] [Google Scholar]
- 9.Eddens T., Kolls J. K. (2012) Host defenses against bacterial lower respiratory tract infection. Curr. Opin. Immunol. 24, 424–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nordmann P., Cuzon G., Naas T. (2009) The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect. Dis. 9, 228–236 [DOI] [PubMed] [Google Scholar]
- 11.Nathan C., Cunningham-Bussel A. (2013) Beyond oxidative stress: an immunologist’s guide to reactive oxygen species. Nat. Rev. Immunol. 13, 349–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mittal M., Siddiqui M. R., Tran K., Reddy S. P., Malik A. B. (2014) Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 20, 1126–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sies H. (2017) Hydrogen peroxide as a central redox signaling molecule in physiological oxidative stress: oxidative eustress. Redox Biol. 11, 613–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sies H., Berndt C., Jones D. P. (2017) Oxidative Stress. Annu. Rev. Biochem. 86, 715–748 [DOI] [PubMed] [Google Scholar]
- 15.Forman H. J., Bernardo A., Davies K. J. (2016) What is the concentration of hydrogen peroxide in blood and plasma? Arch. Biochem. Biophys. 603, 48–53 [DOI] [PubMed] [Google Scholar]
- 16.Roy J., Galano J. M., Durand T., Le Guennec J. Y., Lee J. C. (2017) Physiological role of reactive oxygen species as promoters of natural defenses. FASEB J. 31, 3729–3745 [DOI] [PubMed] [Google Scholar]
- 17.Wang G., Cao L., Liu X., Sieracki N. A., Di A., Wen X., Chen Y., Taylor S., Huang X., Tiruppathi C., Zhao Y. Y., Song Y., Gao X., Jin T., Bai C., Malik A. B., Xu J. (2016) Oxidant sensing by TRPM2 inhibits neutrophil migration and mitigates inflammation. Dev. Cell 38, 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yamamoto S., Shimizu S., Kiyonaka S., Takahashi N., Wajima T., Hara Y., Negoro T., Hiroi T., Kiuchi Y., Okada T., Kaneko S., Lange I., Fleig A., Penner R., Nishi M., Takeshima H., Mori Y. (2008) TRPM2-mediated Ca2+ influx induces chemokine production in monocytes that aggravates inflammatory neutrophil infiltration. Nat. Med. 14, 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Perraud A. L., Fleig A., Dunn C. A., Bagley L. A., Launay P., Schmitz C., Stokes A. J., Zhu Q., Bessman M. J., Penner R., Kinet J. P., Scharenberg A. M. (2001) ADP-ribose gating of the calcium-permeable LTRPC2 channel revealed by Nudix motif homology. Nature 411, 595–599 [DOI] [PubMed] [Google Scholar]
- 20.Ogawa N., Kurokawa T., Mori Y. (2016) Sensing of redox status by TRP channels. Cell Calcium 60, 115–122 [DOI] [PubMed] [Google Scholar]
- 21.Syed Mortadza S. A., Wang L., Li D., Jiang L. H. (2015) TRPM2 channel–mediated ROS-sensitive Ca2+ signaling mechanisms in immune cells. Front. Immunol. 6, 407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jondle C. N., Sharma A., Simonson T. J., Larson B., Mishra B. B., Sharma J. (2016) Macrophage galactose-type lectin-1 deficiency is associated with increased neutrophilia and hyperinflammation in Gram-negative pneumonia. J. Immunol. 196, 3088–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sharma A., Simonson T. J., Jondle C. N., Mishra B. B., Sharma J. (2017) Mincle-mediated neutrophil extracellular trap formation by regulation of autophagy. J. Infect. Dis. 215, 1040–1048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Akong-Moore K., Chow O. A., von Köckritz-Blickwede M., Nizet V. (2012) Influences of chloride and hypochlorite on neutrophil extracellular trap formation. PLoS One 7, e42984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohbuchi A., Kono M., Kitagawa K., Takenokuchi M., Imoto S., Saigo K. (2017) Quantitative analysis of hemin-induced neutrophil extracellular trap formation and effects of hydrogen peroxide on this phenomenon. Biochem. Biophys. Rep. 11, 147–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharma A., Steichen A. L., Jondle C. N., Mishra B. B., Sharma J. (2014) Protective role of Mincle in bacterial pneumonia by regulation of neutrophil mediated phagocytosis and extracellular trap formation. J. Infect. Dis. 209, 1837–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sukumaran P., Sun Y., Antonson N., Singh B. B. (2017) Dopaminergic neurotoxins induce cell death by attenuating NF-kB-mediated regulation of TRPC1 expression and autophagy. FASEB J. 32, 1640–1652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun Y., Chauhan A., Sukumaran P., Sharma J., Singh B. B., Mishra B. B. (2014) Inhibition of store-operated calcium entry in microglia by helminth factors: implications for immune suppression in neurocysticercosis. J. Neuroinflammation 11, 210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y., Zhang H., Selvaraj S., Sukumaran P., Lei S., Birnbaumer L., Singh B. B. (2017) Inhibition of L-type Ca2+ channels by TRPC1-STIM1 complex is essential for the protection of dopaminergic neurons. J. Neurosci. 37, 3364–3377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pani B., Ong H. L., Brazer S. C., Liu X., Rauser K., Singh B. B., Ambudkar I. S. (2009) Activation of TRPC1 by STIM1 in ER-PM microdomains involves release of the channel from its scaffold caveolin-1. Proc. Natl. Acad. Sci. USA 106, 20087–20092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Selvaraj S., Sun Y., Watt J. A., Wang S., Lei S., Birnbaumer L., Singh B. B. (2012) Neurotoxin-induced ER stress in mouse dopaminergic neurons involves downregulation of TRPC1 and inhibition of AKT/mTOR signaling. J. Clin. Invest. 122, 1354–1367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jin L., Batra S., Jeyaseelan S. (2017) Diminished neutrophil extracellular trap (NET) formation is a novel innate immune deficiency induced by acute ethanol exposure in polymicrobial sepsis, which can be rescued by CXCL1. PLoS Pathog. 13, e1006637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Juneau R. A., Stevens J. S., Apicella M. A., Criss A. K. (2015) A thermonuclease of Neisseria gonorrhoeae enhances bacterial escape from killing by neutrophil extracellular traps. J. Infect. Dis. 212, 316–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steichen A. L., Binstock B. J., Mishra B. B., Sharma J. (2013) C-type lectin receptor Clec4d plays a protective role in resolution of Gram-negative pneumonia. J. Leukoc. Biol. 94, 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu X., Cotrim A., Teos L., Zheng C., Swaim W., Mitchell J., Mori Y., Ambudkar I. (2013) Loss of TRPM2 function protects against irradiation-induced salivary gland dysfunction. Nat. Commun. 4, 1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun Y., Sukumaran P., Selvaraj S., Cilz N. I., Schaar A., Lei S., Singh B. B. (2018) TRPM2 promotes neurotoxin MPP+/MPTP-induced cell death. Mol. Neurobiol. 55, 409–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kenno S., Perito S., Mosci P., Vecchiarelli A., Monari C. (2016) Autophagy and reactive oxygen species are involved in neutrophil extracellular traps release induced by C. albicans morphotypes. Front. Microbiol. 7, 879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Remijsen Q., Kuijpers T. W., Wirawan E., Lippens S., Vandenabeele P., Vanden Berghe T. (2011) Dying for a cause: NETosis, mechanisms behind an antimicrobial cell death modality. Cell Death Differ. 18, 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sollberger G., Tilley D. O., Zychlinsky A. (2018) Neutrophil extracellular traps: the biology of chromatin externalization. Dev. Cell 44, 542–553 [DOI] [PubMed] [Google Scholar]
- 40.Nguyen G. T., Green E. R., Mecsas J. (2017) Neutrophils to the ROScue: mechanisms of NADPH oxidase activation and bacterial resistance. Front. Cell. Infect. Microbiol. 7, 373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gonzalez-Gonzalez F. J., Chandel N. S., Jain M., Budinger G. R. S. (2017) Reactive oxygen species as signaling molecules in the development of lung fibrosis. Transl. Res. 190, 61–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hecquet C. M., Malik A. B. (2009) Role of H2O2-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb. Haemost. 101, 619–625 [PMC free article] [PubMed] [Google Scholar]
- 43.Takahashi N., Kozai D., Kobayashi R., Ebert M., Mori Y. (2011) Roles of TRPM2 in oxidative stress. Cell Calcium 50, 279–287 [DOI] [PubMed] [Google Scholar]
- 44.Miyake T., Shirakawa H., Kusano A., Sakimoto S., Konno M., Nakagawa T., Mori Y., Kaneko S. (2014) TRPM2 contributes to LPS/IFNγ-induced production of nitric oxide via the p38/JNK pathway in microglia. Biochem. Biophys. Res. Commun. 444, 212–217 [DOI] [PubMed] [Google Scholar]
- 45.Hong C. W., Kim T. K., Ham H. Y., Nam J. S., Kim Y. H., Zheng H., Pang B., Min T. K., Jung J. S., Lee S. N., Cho H. J., Kim E. J., Hong I. H., Kang T. C., Lee J., Oh S. B., Jung S. J., Kim S. J., Song D. K. (2010) Lysophosphatidylcholine increases neutrophil bactericidal activity by enhancement of azurophil granule-phagosome fusion via glycine.GlyR alpha 2/TRPM2/p38 MAPK signaling. J. Immunol. 184, 4401–4413 [DOI] [PubMed] [Google Scholar]
- 46.Maugeri N., Campana L., Gavina M., Covino C., De Metrio M., Panciroli C., Maiuri L., Maseri A., D’Angelo A., Bianchi M. E., Rovere-Querini P., Manfredi A. A. (2014) Activated platelets present high mobility group box 1 to neutrophils, inducing autophagy and promoting the extrusion of neutrophil extracellular traps. J. Thromb. Haemost. 12, 2074–2088 [DOI] [PubMed] [Google Scholar]
- 47.Remijsen Q., Vanden Berghe T., Wirawan E., Asselbergh B., Parthoens E., De Rycke R., Noppen S., Delforge M., Willems J., Vandenabeele P. (2011) Neutrophil extracellular trap cell death requires both autophagy and superoxide generation. Cell Res. 21, 290–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Metan G., Akova M. (2016) Reducing the impact of carbapenem-resistant Enterobacteriaceae on vulnerable patient groups: what can be done? Curr. Opin. Infect. Dis. 29, 555–560 [DOI] [PubMed] [Google Scholar]
- 49.Papayannopoulos V. (2017) Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 [DOI] [PubMed] [Google Scholar]
- 50.Haraguchi K., Kawamoto A., Isami K., Maeda S., Kusano A., Asakura K., Shirakawa H., Mori Y., Nakagawa T., Kaneko S. (2012) TRPM2 contributes to inflammatory and neuropathic pain through the aggravation of pronociceptive inflammatory responses in mice. J. Neurosci. 32, 3931–3941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yonezawa R., Yamamoto S., Takenaka M., Kage Y., Negoro T., Toda T., Ohbayashi M., Numata T., Nakano Y., Yamamoto T., Mori Y., Ishii M., Shimizu S. (2016) TRPM2 channels in alveolar epithelial cells mediate bleomycin-induced lung inflammation. Free Radic. Biol. Med. 90, 101–113 [DOI] [PubMed] [Google Scholar]
- 52.Knowles H., Heizer J. W., Li Y., Chapman K., Ogden C. A., Andreasen K., Shapland E., Kucera G., Mogan J., Humann J., Lenz L. L., Morrison A. D., Perraud A. L. (2011) Transient Receptor Potential Melastatin 2 (TRPM2) ion channel is required for innate immunity against Listeria monocytogenes. Proc. Natl. Acad. Sci. USA 108, 11578–11583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shakerley N. L., Chandrasekaran A., Trebak M., Miller B. A., Melendez J. A. (2016) Francisella tularensis catalase restricts immune function by impairing TRPM2 channel activity. J. Biol. Chem. 291, 3871–3881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di A., Kiya T., Gong H., Gao X., Malik A. B. (2017) Role of the phagosomal redox-sensitive TRP channel TRPM2 in regulating bactericidal activity of macrophages. J. Cell Sci. 130, 735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bonaventura A., Liberale L., Carbone F., Vecchié A., Diaz-Cañestro C., Camici G. G., Montecucco F., Dallegri F. (2018) The pathophysiological role of neutrophil extracellular traps in inflammatory diseases. Thromb. Haemost. 118, 6–27 [DOI] [PubMed] [Google Scholar]
- 56.Lightfoot Y. L., Kaplan M. J. (2017) Disentangling the role of neutrophil extracellular traps in rheumatic diseases. Curr. Opin. Rheumatol. 29, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.