Abstract
The role of dyslipidemia in the development of retinal dysfunction remains poorly understood. Using an animal model of diet-induced obesity/pre-type 2 diabetes, we investigated molecular defects in the retina arising from consumption of a diet high in saturated fats and sugars (i.e., a Western diet). We found that feeding mice a Western diet increased the abundance of retinal sphingolipids, attenuated protein kinase B (Akt) phosphorylation, enhanced JNK activation, and increased retinal cell death. When we used palmitate or C6-ceramide (Cer) to assess sphingolipid-mediated signaling in cultured murine and human cells, we observed similar effects on Akt, JNK, and cell death. Furthermore, both Western diet and C6-Cer exposure enhanced expression of the stress-response protein regulated in development and DNA damage response 1 (REDD1) and loss of REDD1 increased C6-Cer-induced JNK activation and cell death. Exogenous REDD1 expression repressed JNK-mediated phosphorylation in cultured cells. We found that thioredoxin-interacting protein (TXNIP) expression was elevated in REDD1–deficient cell lines and C6-Cer promoted TXNIP expression in both wild-type and REDD1–deficient cells. Likewise, TXNIP knockdown attenuated JNK activation and caspase 3 cleavage after either C6-Cer exposure or REDD1 deletion. The results support a model wherein Cer-induced REDD1 expression attenuates TXNIP-dependent JNK activation and retinal cell death.—Dai, W., Miller, W. P., Toro, A. L., Black, A. J., Dierschke, S. K., Feehan, R. P., Kimball, S. R., Dennis, M. D. Deletion of the stress-response protein REDD1 promotes ceramide-induced retinal cell death and JNK activation.
Keywords: high-fat diet, apoptosis, retinopathy, sphingolipids, type 2 diabetes
In Western populations, the development of pre- and type 2 diabetes is associated with consumption of a diet with high fat and sucrose content (1). Accumulation of bioactive lipid products in tissues in response to consumption of high-fat diet (HFD) has been linked to complications of type 2 diabetes (2). However, it is not known whether a similar phenomenon occurs in the retina. Diabetic retinopathy, a neurovascular complication of diabetes mellitus, is the leading cause of vision impairment and blindness among working-age adults (3). Recent studies demonstrate HFD-induced retinal degeneration (4) and impaired function (5, 6) in rodents. However, the mechanism whereby diet causes retinal dysfunction remains poorly understood (7, 8). Thus, the present study was designed to evaluate molecular defects in the retina arising from consumption of a diet high in saturated fats and sugars (i.e., a Western diet).
Mice consuming an HFD exhibit retinal dysfunction and attenuation of protein kinase B (Akt) phosphorylation in the synaptic layers of the retina (4, 5). Akt signaling in retinal neurons provides a critical neurotrophic stimulus (9). Thus, diet-induced Akt inhibition may lead to neuroretinal cell death and dysfunction. Indeed, TUNEL+ nuclei are present in the ganglion cell layer, as well as the inner and outer nuclear layers of the retina of rats after 12 wk of an HFD (4). Retinas from mice fed an HFD also manifest a striking increase in JNK phosphorylation and neural inflammasome activation before the development of systemic glucose intolerance, electroretinographic defects, or microvascular disease (6). This evidence suggests that the retina progresses through HFD-induced inflammatory and neuroretinal degenerative stages, leading to functional deficits (6, 10).
HFDs promote tissue sphingolipid accumulation via the supply of fatty acid substrates for de novo synthesis (11). Sphingolipids, such as ceramide (Cer), sphingosine (So), sphingosine-1-phosphate (S1P), Cer-1-phosphate, and sphingomyelin (SM), are both structural lipids and signaling molecules that are involved in the regulation of cell survival, inflammation, insulin resistance, and angiogenesis (2, 11, 12). Cers are a key hub of sphingolipid metabolism and putative casual factors in the pathogenesis of diabetes and neurodegenerative diseases (12, 13). They promote apoptosis through regulation of various pathways, including inhibition of Akt and activation of JNK (13). They have been observed to suppress Akt signaling through activation of the protein phosphatase (PP)-2A, PKCζ, and a mammalian target of rapamycin complex (mTORC)-1/S6 protein kinase-1–induced feedback loop (14–16). Alternatively, Cers promote JNK activation through a mechanism involving thioredoxin-interacting protein (TXNIP) (17). Both repressed Akt activity and enhanced JNK activation can lead to mitochondrial outer membrane permeabilization and activation of caspases and proapoptotic B-cell lymphoma (BCL)-2 family members, thereby promoting apoptosis (13).
The objective of the current study was to investigate the early molecular changes in the retina associated with consumption of a Western diet. Using mass spectrometry, we observed increased content of sphingolipid subclasses in retinas of mice fed a Western diet, concomitant with increased retinal cell death. In support of results in our previous study (10), Akt phosphorylation was attenuated and JNK phosphorylation was enhanced in the retina of mice fed a Western diet. We initially investigated the role of the stress response protein regulated in development and DNA damage response 1 (REDD1), also known as DDIT4 or RTP801, suspecting that it might contribute to the inhibitory effect on Akt phosphorylation. Our laboratory has previously demonstrated a role for REDD1 in PP2A-dependent dephosphorylation of Akt (18), and other studies support increased REDD1 expression in the muscle of rodents fed a diet high in saturated fats (19, 20). Moreover, we recently demonstrated a critical role for hyperglycemia-induced REDD1 in streptozotocin-induced retinal cell death and visual dysfunction (21). Indeed, REDD1 expression was elevated in the retina of mice fed a Western diet and in cells exposed to Cer. Nevertheless, the effect of Cer on Akt phosphorylation was not different in wild-type (WT) as compared to REDD1–deficient cells. In fact, REDD1–deficient cells exhibited increased Cer-induced cell death and JNK activation related to increased expression of TXNIP.
MATERIALS AND METHODS
Animals
Six-week-old C57BL/6 male mice were maintained on a 12:12 h reverse light–dark cycle and fed ad libitum for up to 18 wk with either a Teklad control (TD.08485) diet containing 13% kcal from fat, 63.3% from carbohydrates, and 19.1% from protein or a Western (TD.88137) diet containing 42% kcal from fat, 42% kcal from carbohydrates, and 15.2% from protein (Envigo, Huntingdon, United Kingdom). Mice were not fed for 6 h before retinal extraction. The retinas were isolated and flash-frozen in liquid nitrogen, and lysates were prepared as described by Dennis et al. (22). All procedures were approved by the Penn State College of Medicine Institutional Animal Care and Use Committee. Protein concentrations were assessed by the DC Protein Assay (Bio-Rad, Hercules, CA, USA), and supernatants were combined with 2× Laemmli buffer, boiled for 5 min, and analyzed via Western blot (n = 7–8 mice/group).
Extraction of sphingolipids
Retinal lipidomic analysis (n = 5 mice/group) was performed by the Lipidomics Core at Virginia Commonwealth School of Medicine (Richmond, VA, USA). Internal standards were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Standards for sphingoid bases and sphingoid base 1-phosphates were 17-carbon chain length analogs: C17-So, (2S,3R,4E)-2-aminoheptadec-4-ene-1,3-diol (d17:1-So); C17-sphinganine, (2S,3R)-2-aminoheptadecane-1,3-diol (d17:0-Sa); C17-So 1-phosphate, heptadecasphing-4-enine-1-phosphate (d17:1-So1P); and C17-sphinganine 1-phosphate, heptadecasphinganine-1-phosphate (d17:0-Sa1P). Standards for N-acyl sphingolipids were C12-fatty acid analogs: C12-Cer, N-(dodecanoyl)-sphing-4-enine (d18:1/C12:0); C12-Cer 1-phosphate, N-(dodecanoyl)-sphing-4-enine-1-phosphate (d18:1/C12:0-Cer1P); C12- SM, N-(dodecanoyl)-sphing-4-enine-1-phosphocholine (d18:1/C12:0-SM); and C12-glucosylceremide, N-(dodecanoyl)-1-β-glucosyl-sphing-4-eine. Retinal lysates were collected into 13 × 100-mm borosilicate tubes with a Teflon-lined cap (60827-453; VWR, West Chester, PA, USA). Then 2 ml CH3OH and 1 ml CHCl3 were added, along with the internal standard cocktail (500 pmol of each species dissolved in a final total volume of 20 μl of ethanol:methanol:water 7:2:1). The contents were dispersed by using an ultra sonicator at room temperature for 30 s. This single-phase mixture was incubated at 48°C overnight. After cooling, 150 µl of 1 M KOH in CH3OH was added and, after brief sonication, incubated in a shaking water bath for 2 h at 37°C to cleave potentially interfering glycerolipids. The extract was brought to neutral pH with 12 µl of glacial acetic acid and briefly centrifuged with a table-top centrifuge, and the supernatant was reduced to dryness with a SpeedVac concentrator (Thermo Fisher Scientific, Waltham, MA, USA). The dried residue was reconstituted in 0.5 ml of the starting mobile phase solvent for liquid chromatography tandem mass spectrometry (LC-MS/MS).
LC-MS/MS of sphingoid bases, sphingoid base 1-phosphates, and complex sphingolipids
For LC-MS/MS analyses, a Shimadzu Nexera LC-30 AD binary pump system coupled to an SIL-30AC autoinjector and DGU20A5R degasser coupled to an Ab Sciex 5500 quadrupole/linear ion trap (QTrap) (Sciex, Framingham, MA, USA) operating in a triple quadrupole mode was used. Q1 and Q3 was set to pass molecularly distinctive precursor and product ions (or a scan across multiple m/z in Q1 or Q3), using N2 to collisionally induce dissociations in Q2 (which was offset from Q1 by 30–120 eV); the ion source temperature was set to 500°C. These compounds were separated by reverse phase LC with a Supelco 2.1 (inner diameter) × 50 mm Ascentis Express C18 Column (MilliporeSigma, Burlington, MA, USA) and a binary solvent system at a flow rate of 0.5 ml/min with a column oven set to 35°C. Before injection of the sample, the column was equilibrated for 0.5 min with a solvent mixture of 95% mobile phase A1 (CH3OH/H2O/HCOOH, 58/41/1 (v/v/v) with 5 mM ammonium formate) and 5% mobile phase B1 (CH3OH/HCOOH, 99/1 (v/v), with 5 mM ammonium formate), and after sample injection (typically 40 μl), the A1:B1 ratio was maintained at 95:5 for 2.25 min, followed by a linear gradient to 100% B1 over 1.5 min, which was held at 100% B1 for 5.5 min, followed by a 0.5 min gradient return to 95:5 A1:B1. The column was re-equilibrated with 95:5 A1:B1 for 0.5 min before the next run.
Cell culture
MIO-M1 WT (23) and REDD1 clustered regularly interspaced short palindromic repeats (CRISPR) human Müller cells (MIO-M1; obtained from the UCL Institute of Ophthalmology, London, United Kingdom) were maintained in DMEM (11885-084; Thermo Fisher Scientific) containing 5.6 mM glucose, and supplemented with 10% heat inactivated (55°C, 30 min) fetal bovine serum (FBS) and 1% penicillin-streptomycin. REDD1+/+ and REDD1−/− mouse embryonic fibroblasts (MEFs) were kindly provided by Dr. Leif Ellison (Department of Medicine, Harvard Medical School, Boston, MA, USA). REDD1+/+ and REDD1−/− MEFs were maintained in DMEM (11965-084; Thermo Fisher Scientific) containing 25 mM glucose and supplemented with 10% FBS and 1% penicillin-streptomycin. R28 rat retinal precursor cells were kindly provided by Dr. Gail Seigel (Ross Eye Institute, State University of New York, Buffalo, NY, USA). R28 cells were maintained in DMEM (D5523-1L; MilliporeSigma) containing 5.6 mM glucose, and supplemented with 10% FBS, 1 MEM nonessential amino acids, MEM vitamin solution, and 0.14% gentamycin. The human embryonic kidney (HEK) 293E Tet-ON HA-REDD1 advanced stable cell line (TaKaRa Bio, Mountain View, CA, USA) was grown in DMEM containing 25 mM glucose and supplemented with Tet system approved FBS and placed under selective pressure with G418 and hygromycin B as described by Dennis et al. (18). Where indicated, cell culture medium was supplemented with 1 μg/μl doxycycline (Dox; TaKaRa) to induce HA-REDD1 protein expression. All cells were maintained at 37°C and 5% CO2. Transfections were performed with Lipofectamine 2000 (Thermo Fisher Scientific), according to the manufacturer’s instructions. Plasmids for expression of FLAG-MKK7-JNK1 (active JNK) and the FLAG-MKK7-JNK1 APF variant (inactive JNK) were obtained from Dr. Rodger Davis (University of Massachusetts Medical School, Boston, MA, USA) via Addgene (Cambridge, MA, USA). Plasmid for expression of Myc-FLAG-TXNIP (MR206284) was obtained from Origene (Rockville, MD, USA). Silencer predesigned TXNIP siRNA (AM16708) was obtained from Thermo Fisher Scientific. pTREtight vector and pTREtight-HA-REDD1 plasmids were cotransfected with pRevTet-On (TaKaRa), which expresses the reverse tetracycline-controlled transactivator. Cell culture medium was supplemented with Dox (1 μg/ml; TaKaRa) to induce HA-REDD1 protein expression from the pTREtight-HA-REDD1 plasmid. For cell culture experiments, sodium palmitate (P9767-5G; MilliporeSigma) was conjugated to 10% bovine serum albumin (BSA) (A7511-25G; MilliporeSigma) and added to cell culture medium at a final concentration of 0.5 mM. Solutions of N-hexanoyl-d-sphingosine (C6-Cer) (H6524-5MG; MilliporeSigma) were prepared in DMSO at 50 mM and added to cells at a final concentration of 60 μM. At the end of the stimulation period, cells were carefully washed twice with cold PBS and harvested in 100 μl of lysis buffer containing 150 mM NaCl, 10 mM Tris, 1 mM EGTA, 1 mM EDTA (pH 7.4), 100 mM NaF, 4 mM sodium pyrophosphate, 2 mM sodium orthovanadate, 1% Triton X-100, 0·5% NP-40-Igepal, and a protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany). Lysates were centrifuged at 12,000 g for 15 min at 4°C. The resulting supernatant fractions were recovered and stored at −80°C. Protein concentration was determined via DC Protein Assay and the lysates were combined with 2× Laemmli buffer, boiled for 5 min, and analyzed via Western blot analysis.
CRISPR/Cas9 genome editing to knock out REDD1 in MIO-M1 cells
Lentiviral production was performed according to the manufacturer’s protocol (ViraPower Lentiviral Expression Systems; Thermo Fisher Scientific) using a pLentiCRISPR v.2 construct containing a REDD1 (DDIT4) guide RNA (GenScript, Piscataway, NJ, USA) genomic RNA sequence (5′-GTTTGACCGCTCCACGAGCC-3′). MIO-M1 cells were incubated with viral particles for 48 h and then selected with 3 μg/ml puromycin for 72 h. Single clones were isolated, amplified, and subjected to puromycin selection 2 additional times before validating the cell line by DNA sequencing and Western blot analysis.
Western blot analysis
Lysates were fractionated with Criterion precast 4–15% gels (Bio-Rad). Proteins were transferred to PVDF, blocked in 5% milk in Tris-buffered saline–Tween 20, washed, and incubated overnight at 4°C with appropriate antibodies found in Table 1. Protein loading was assessed by Ponceau staining or MemCode Reversible Protein Staining. The antigen–antibody interaction was visualized with ECL (Clarity Reagent; Bio-Rad) using a Fluorochem E imaging system (ProteinSimple, Santa Clara, CA, USA). Blots were quantified with ImageJ software (National Institutes of Health, Bethesda, MD, USA).
TABLE 1.
Antibodies used for Western blot analysis
| Antibody | Catalog no. | Lot no. | Molecular mass (kDa) | Species | Specificity |
|---|---|---|---|---|---|
| Cell Signaling Technology (Danvers, MA, USA) | |||||
| AKT-p (S473) | 4060 | 19 | 60 | Rabbit | H, M, R |
| AkT-p (T308) | 9275 | 20 | 60 | Rabbit | H, M, R |
| AKT | 9272 | 27 | 60 | Rabbit | H, M, R |
| JNK-p (T183/Y185) | 9251 | 24 | 46, 54 | Rabbit | H, M, R |
| JNK | 9252 | 15 | 46, 54 | Rabbit | H, M, R |
| c-Jun-p (S63) | 9261 | 14 | 48 | Rabbit | H, M, R |
| c-Jun | 9165 | 9 | 43, 48 | Rabbit | H, M, R |
| p38 MAPK-p (Thr180/Tyr182) | 4511 | 13 | 43 | Rabbit | H, M, R |
| p38 MAPK | 8690 | 6 | 40 | Rabbit | H, M, R |
| Cleaved caspase 3 Asp175 | 9664 | 21 | 17, 19 | Rabbit | H, M, R |
| TXNIP | 14715 | 1 | 55 | Rabbit | H, M, R |
| Actin | 3700 | 14 | 46 | Mouse | H, M, R |
| Santa Cruz Biotechnology (Dallas, TX, USA) | |||||
| GAPDH | sc-32233 | K0315 | 37 | Mouse | H, M, R |
| α-Tublin | sc-32293 | C0112 | 55 | Mouse | H, M, R |
| Protein Tech (Manchester, United Kingdom) | |||||
| REDD1 | 10638-1-AP | 51272 | 35 | Rabbit | H, M, R |
| Origene Technologies (Rockville, MD, USA) | |||||
| DDK (FLAG tag) | TA5011-100 | A043 | - | Mouse | M |
| Bethyl Laboratories (Montgomery, TX, USA) | |||||
| Secondary antibody | A120-101P | 40 | - | Goat | R |
| Secondary antibody | A90-116P | 38 | - | Goat | M |
Primary and secondary antibodies used for Western blot analysis are listed. Antibody specificity to human (H), mouse (M), and rat (R) is also indicated.
Cell death ELISA
Two retinas per sample were homogenized in 100 μl lysis buffer from a Cell Death Detection ELISA Plus Kit (11774425001; Roche Diagnostics), with a plastic homogenizer and pellet pestle motor (Eppendorf, Hamburg, Germany). Samples (n = 4–6 mice per group) were incubated by rocking at room temperature for 30 min and centrifuged at 200 g for 10 min at 4°C. Apoptotic cell death was then assessed according to the manufacturer’s instructions.
RNA isolation and quantitative real-time PCR
Total RNA was extracted with Trizol reagent according to the manufacturer’s protocol (Thermo Fisher Scientific). RNA (1 μg) was reverse transcribed with the High Capacity cDNA Reverse Transcription Kit (Thermo Fisher Scientific) and subjected to real-time quantitative (q)PCR (QuantStudio 12K Flex Real-Time PCR System) using QuantiTect SYBR Green master mix (Qiagen, Germantown, MD, USA). Mouse and rat primers used for amplification of REDD1 and GAPDH mRNA have been published (24,25). Mean cycle threshold (Ct) values for REDD1 and GAPDH were determined for control and experimental samples. Changes in REDD1 mRNA expression levels were normalized to GAPDH mRNA expression using the 2−∆∆Ct calculations, as described by Livak and Schmittgen (26).
Flow cytometry
Cell apoptosis was characterized with the PE Annexin V Apoptosis Detection Kit I (559763, 6179925; BD Biosciences, Franklin Lakes, NJ, USA) according to the manufacturer’s instructions. The percentage of live, early apoptotic [Annexin V positive/7-aminoactinomycin D (7-AAD) negative], late-dual apoptotic [Annexin V positive/7-AAD positive] and dead/necrotic [Annexin V negative/7-AAD positive] were determined with a 10-color FACSCanto flow cytometer and FACSDiva (BD Biosciences).
Statistical analysis
Data are expressed as means ± sem. Student’s t test or 1-way ANOVA was used to analyze signaling data. Fisher’s least-significant difference test was used to identify specific differences if a significant overall F value was observed after a 1-way ANOVA. Relationships were determined by Pearson product moment correlation analysis. Significance was set at P < 0.05 for all analyses.
RESULTS
Retinal sphingolipid abundances are increased by consumption of a Western diet
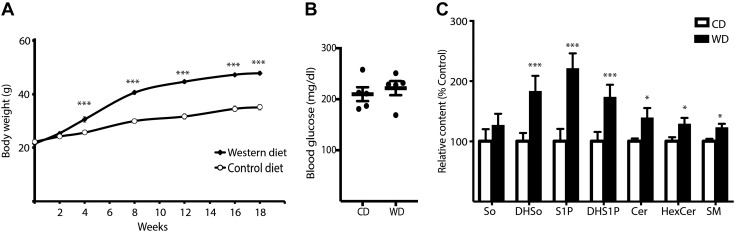
Mice fed a Western diet for 4–18 wk exhibited elevated body weight as compared to mice fed a control diet (Fig. 1A). Postprandial blood glucose concentrations were not different after 18 wk of Western diet as compared to the control chow group (Fig. 1B). To assess whether a Western diet affects retinal sphingolipid levels, we performed lipidomic analysis on whole retinas from mice fed either a Western diet or a control chow diet for 18 wk. LC-MS/MS was used to evaluate So, dihydrosphingosine (DHSo), sphingosine 1-phosphate (S1P), dihydro-sphingosine 1-phosphate (DHS1P), as well as Cer, hexosylceramide (HexCer), and SM of various N-acyl chain lengths in the retina of mice fed a Western diet for 18 wk as compared to mice fed a control diet (Supplemental Table S1). Except for observing no change in So content, we detected a Western diet-induced increase in the abundance of other sphingolipid subclasses including DHSo, S1P, DHS1P, and the combined total of Cer, HexCer, and SM species of various chain lengths (Fig. 1C). A Western diet did not alter the relative ratios of these major sphingolipid species, as there was a trend toward an increase in the total amount of all sphingolipids detected in the retina of mice fed a Western diet (2.45 ± 0.11 vs. 3.02 ± 0.26 nmol/mg of retina; P = 0.08). Consistent with a previous report (27), ∼70% of retinal Cers contained long-chain saturated C16:0 or C18:0 fatty acids, whereas only ∼20% contained very-long-chain (C20:0 or longer) fatty acyl species (Supplemental Table S1). These relative percentages were also not altered by the Western diet. The diet increased several individual sphingolipid species of various chain lengths in the retinas of mice fed the diet, including Cers containing C24:0, C24:1, and C26:0 fatty acids (Supplemental Table S1).
Figure 1.
Retinal sphingolipid levels are enhanced by consumption of a Western diet before the onset of hyperglycemia. C57BL/6 mice were fed either a Western diet (WD) or control diet (CD) for up to 18 wk before retinal harvest. A) Body weight was monitored at the time points indicated. B, C) Fasting blood glucose (B) and retinal sphingolipid content (C) were assessed after 18 wk of consuming the diets. Total retinal content for each sphingolipid subclass was normalized to retinal weight and is expressed as a percentage of the mean value observed in the retina of control chow fed mice. Results are means ± sem (n = 5). *P < 0.05, ***P < 0.01 vs. control diet.
Western diet consumption promotes retinal signaling defects in Akt/JNK and increases cell death
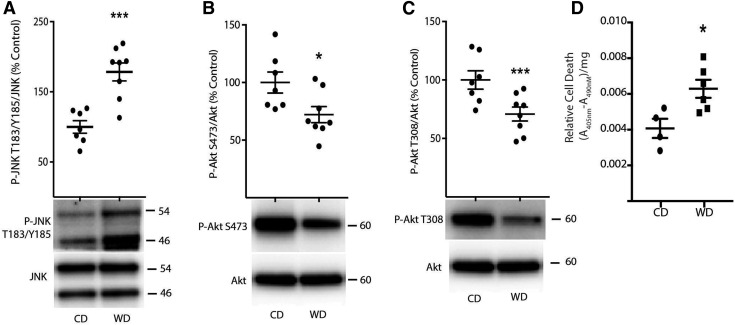
Elevated sphingolipids can regulate various signaling pathways, including promoting activation of the JNK signaling pathway and inhibition of the Akt signaling pathway (13, 28). Therefore, we assessed JNK and Akt phosphorylation in the retina of mice fed either a control or Western diet. Phosphorylation of JNK on Thr183/Tyr185 was enhanced in the retina of mice fed a Western diet, as compared to mice receiving a control chow diet (Fig. 2A). Moreover, phosphorylation of Akt on Ser473 (Fig. 2B) and Thr308 (Fig. 2C) was attenuated in the retina of mice fed a Western diet vs. mice fed a control chow diet. Akt signaling provides a critical prosurvival stimulus in retinal neurons (9). Previous studies demonstrate an increase in apoptotic nuclei in the retina of mice fed an HFD (4). Thus, we also evaluated the impact of a Western diet on the presence of cytoplasmic nucleosomes, a hallmark of cell death, in retinal lysates. In the retina of mice fed a Western diet, apoptotic cell death was elevated as compared to those receiving a control diet (Fig. 2D).
Figure 2.
JNK and Akt phosphorylation are altered concomitant with increased cell death in the retina of mice fed a Western diet. C57BL/6 mice were fed either a Western diet (WD) or control diet (CD) diet. A–C) JNK phosphorylation on Thr183/Tyr185 (A) and phosphorylation of Akt on Ser473 (B) and Thr308 (C) were assessed in retinal lysates obtained after 14 wk of diet by Western blot analysis. Representative blots are shown. Protein molecular mass (kDa) is indicated at the right of the blots. D) Apoptotic cell death was assessed by ELISA in retinal lysates after 18 wk of diet for the presence of nucleosomal fragments in the cytoplasm. Results are means ± sem (n = 4–7). *P < 0.05, ***P < 0.01 vs. control diet.
Palmitate and C6-Cer attenuate Akt phosphorylation, enhance JNK activity, and promote cell death
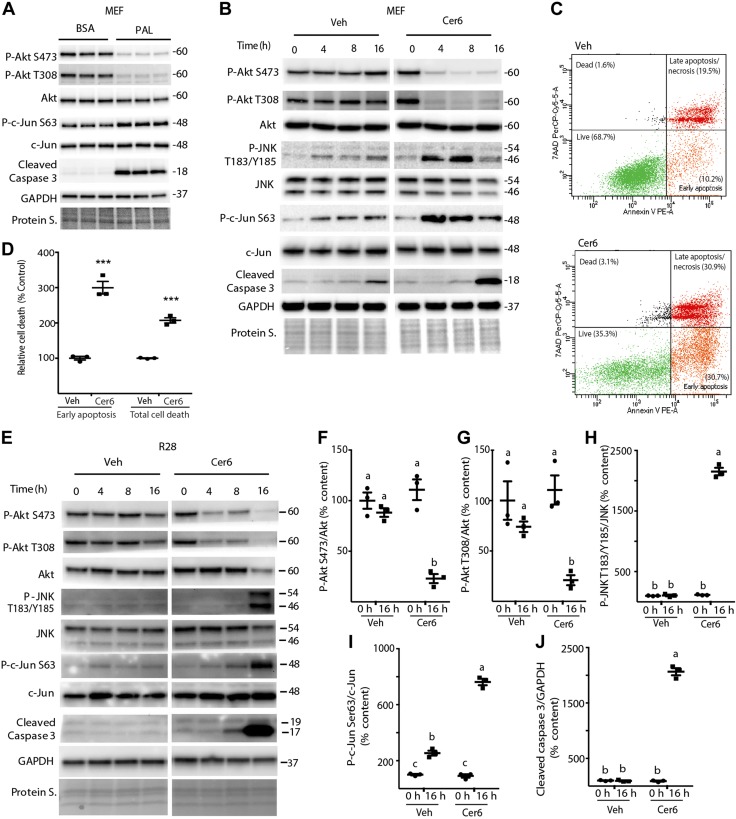
In the Western diet, palmitate is the predominant saturated fatty acid and a key substrate for de novo sphingolipid synthesis. The enzyme serine palmitoyl transferase condenses palmitoyl-CoA and serine in the first and rate-limiting step of this pathway (29). Previous studies demonstrate that supplementation of cell culture medium with palmitate results in increased Cer synthesis (for example, ref. 30). Consistent with the retina of mice fed an HFD, addition of palmitate to cell culture medium attenuated Akt phosphorylation at Ser473 and Thr308 in MEFs (Fig. 3A). Moreover, palmitate promoted phosphorylation of the well-established JNK substrate c-Jun on Ser63 and increased cleavage of caspase 3, a cysteine protease that becomes active during the late stages of apoptosis (31). Cer is considered to be the central hub of sphingolipid metabolism because it can be converted to other interconnected bioactive lipid species, including DHSo, S1P, DHS1P, HexCer, and SM (2). However, poor solubility in aqueous solution makes delivery of native Cer difficult. Thus, cells were treated with the cell-permeable and biologically active Cer analog C6-Cer, to further evaluate the signaling pathways involved in diet-induced retinal dysfunction. As with palmitate, phosphorylation of Akt at both Ser473 and Thr308 was repressed after C6-Cer addition to the culture medium, compared with vehicle (Fig. 3B). Phosphorylation of JNK and c-Jun were also enhanced by C6-Cer exposure. Consistent with the effect of palmitate, Cer6 increased caspase 3 cleavage in MEFs (Fig. 3B). To further examine C6-Cer-induced apoptosis, we used Annexin V/7-AAD staining. Analysis by flow cytometry demonstrated that early apoptosis and total cell death were markedly elevated by C6-Cer (Fig. 3C, D). Similarly, in R28 retinal cells in culture, phosphorylation of Akt at both Ser473 and Thr308 was attenuated by C6-Cer (Fig. 3E–G), whereas phosphorylation of JNK on Thr183/Tyr185 and c-Jun on Ser63 were enhanced by C6-Cer vs. vehicle alone (Fig. 3E, H, I). As expected, caspase 3 cleavage was also increased by C6-Cer in R28 cells. Overall, the results demonstrate that palmitate and C6-Cer addition to culture medium attenuate Akt phosphorylation, enhance JNK activity, and promote cell death.
Figure 3.
Palmitate and C6-Cer attenuate Akt phosphorylation, enhance JNK activity, and promote cell death. MEFs (A–D) and R28 retinal cells (E–J) were maintained in culture medium. A) Cells were exposed to 0.5 mM BSA-conjugated palmitate (PAL) or BSA alone for 8 h. B–J) Cells were exposed to 1.2 μl/ml DMSO vehicle (Veh) or 60 μM C6-Cer (Cer6), as indicated. Total Akt, JNK, c-Jun, cleaved caspase 3, GAPDH, and phosphorylation of Akt on Ser473 and Thr308, JNK on Thr183/Tyr185, and c-Jun on Ser63 were assessed by Western blot analysis (B, E). Protein loading was assessed by protein staining (Protein S). Protein molecular mass (kDa) is indicated at the right of the blots. Annexin V/7AAD staining, followed by fluorescence-activated cell sorting, was performed to measure apoptosis in MEFs 24 h after Cer6 addition to the culture medium (C, D). Representative flow cytometry results are shown (C). Quantification of early apoptosis and total cell death (D). ***P < 0.01 vs. vehicle. Quantification of phosphorylation of Akt (Ser473) (F), Akt (Thr308) (G), JNK (T183/Y185) (H), c-Jun (Ser63) (I), and cleaved caspase 3 levels (J) at 0 and 16 h in R28 cells. Bands (B, E) are from the same blot, but are not contiguous. Results are expressed as means ± sem (n = 3) and are representative of 2 independent experiments; within each experiment, 3 independent samples were analyzed. Different letters (a–c) above scatter plots (F–J) denote significantly different results. P < 0.05.
Western diet consumption and C6-Cer promote REDD1 expression
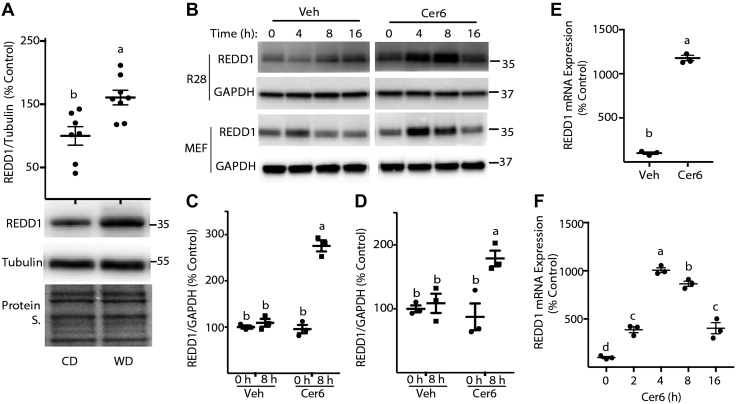
One mechanism whereby Western diet and palmitate/Cer exposure may act to repress Akt phosphorylation is by promoting expression of REDD1. REDD1 protein expression was significantly enhanced in retinas of mice fed a Western diet (Fig. 4A) vs. the retinas of mice fed a control diet. Similarly, in both R28 cells and MEFs in culture, a transient increase in REDD1 protein expression was observed after C6-Cer addition to culture medium (Fig. 4B). Eight hours after exposure to C6-Cer, REDD1 protein expression was markedly enhanced in R28 cells (Fig. 4B, C) and MEFs (Fig. 4B, D), as compared to DMSO alone. We also assessed REDD1 mRNA expression in R28 cells and MEFs after C6-Cer addition to culture medium. Consistent with the increase in protein, REDD1 mRNA expression was increased after C6-Cer exposure in R28 cells (Fig. 4E) and MEFs (Fig. 4F). Notably, the C6-Cer–induced increase in REDD1 protein (Fig. 4B) and mRNA (Fig. 4F) expression was transient and was not maintained with more prolonged exposure times.
Figure 4.
REDD1 expression is enhanced by consumption of a Western diet and C6-Cer exposure of cells in culture. A) C57BL/6 mice were fed either a Western diet (WD) or control diet (CD) for 14 wk before retinal extraction. REDD1 protein expression was assessed relative to tubulin by Western blot analysis (n = 7). Protein loading was assessed by protein staining (Protein S.). B) R28 cells and MEFs were maintained in culture medium and treated with 1.2 μl/ml DMSO vehicle (Veh) or 60 μM C6-Cer (Cer6). Bands are from the same blot, but are not contiguous. C–F) Quantification of REDD1 protein expression in R28 (C) and MEFs (D) at either 0 or 8 h after addition of Cer6 to the culture medium. REDD1 mRNA expression in R28 cells (E) or in MEFs (F) was assessed by RT-PCR 12 h after addition of either Cer6 or a vehicle to culture medium. Representative blots are shown. Protein molecular mass (kDa) is indicated at the right of the blots. Results are representative of 2 independent experiments; within each experiment, 3 independent samples were analyzed and are means ± sem (n = 3). Different letters (a–c) above scatter plots (A, C–F) denote significantly different results. P < 0.05.
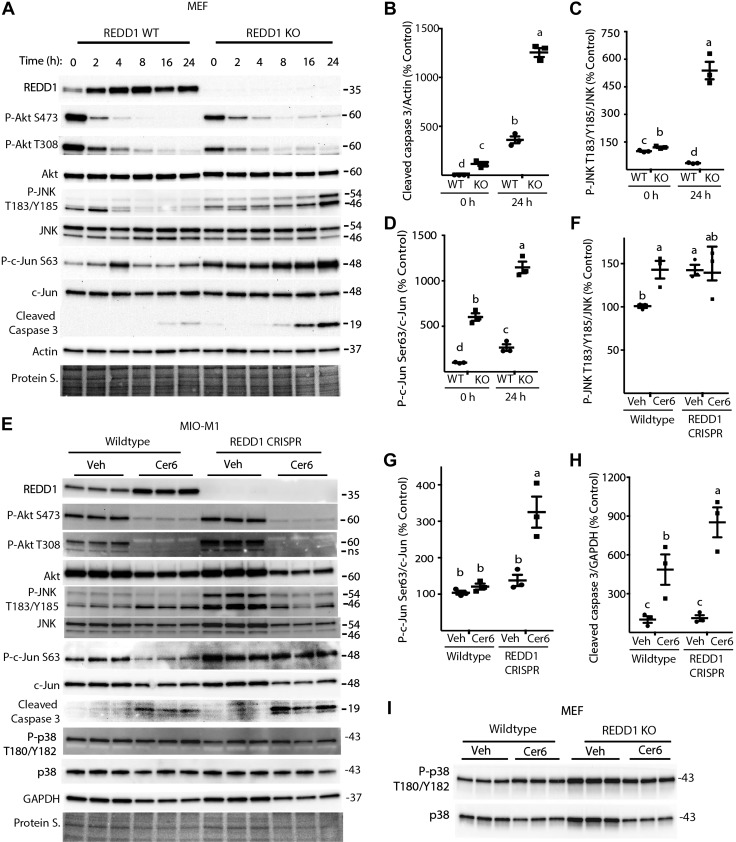
REDD1 deletion enhances C6-Cer–induced JNK activity and cleaved caspase 3 levels
To evaluate the role of REDD1 in Cer-induced cell death, WT and REDD1-deficient MEFs were exposed to C6-Cer. Consistent with the previous observations, C6-Cer enhanced REDD1 protein expression, repressed Akt phosphorylation, and promoted caspase 3 cleavage in WT MEFs (Fig. 5A, B). As expected, in REDD1-deficient MEFs, no REDD1 protein expression was detected, regardless of C6-Cer addition. REDD1 deletion had no effect on C6-Cer–induced Akt dephosphorylation (Fig. 5A). However, in REDD1-deficient MEFs, phosphorylation of JNK on Thr183/Tyr185 and c-Jun on Ser63 were enhanced compared to WT cells before C6-Cer exposure (Fig. 5A, C, D). Moreover, unlike WT cells in which C6-Cer transiently enhanced JNK and c-Jun phosphorylation, REDD1-deficient MEFs exhibited enhanced phosphorylation of these proteins 24 h after Cer addition to culture medium (Fig. 5A). In support of the findings in MEFs, phosphorylation of Akt at Ser473 and Thr308 was suppressed similarly after C6-Cer exposure in both WT and REDD1–deficient retinal MIO-M1 cells (Fig. 5E). JNK phosphorylation on Thr183/Tyr185 was also increased in vehicle treated REDD1-deficient MIO-M1 cells (Fig. 5E, F). Moreover, REDD1-deficient MIO-M1 cells exhibited enhanced c-Jun phosphorylation in addition to increased caspase 3 cleavage after C6-Cer addition as compared to WT cells (Fig. 5E–H). A previous report demonstrates that Cer causes p38 and JNK activation and siRNA-mediated knockdown of either pathway partially reduces Cer-induced apoptosis (17). Unlike c-Jun phosphorylation, a similar change in p38 phosphorylation was not observed concomitant with the increased Cer-induced caspase 3 cleavage in REDD1-deficient MIO-M1 cells (Fig. 5E) or MEFs (Fig. 5I). Thus, increased caspase 3 cleavage in REDD1-deficient cells after C6 Cer exposure was associated with increased JNK activation, but not an increase in p38 phosphorylation.
Figure 5.
Deletion of REDD1 enhances C6-Cer–induced JNK activity and cleaved caspase 3 expression. A) WT and REDD-knockout (KO) MEFs were treated with C6-Cer (Cer6, 60 μM) for the indicated times. E) WT and REDD1 CRISPR human Müller cells (MIO-M1) were treated with 60 μM Cer6 or DMSO vehicle (Veh) for 24 h. I) WT and REDD1 KO MEFs were treated with C6-Cer as previously described for 16 h. Total REDD1, Akt, JNK, c-Jun, cleaved caspase 3, p38, actin, GAPDH, and phosphorylation of Akt on Ser473 and Thr308, JNK on Thr183/Tyr185, c-Jun on Ser63, and p38 on Thr180/Tyr182 were assessed in whole-cell lysates by Western blot analysis. B–D) Quantification of cleaved caspase 3 expression relative to actin (B), JNK phosphorylation at Thr183/Tyr185 relative to total JNK expression (C), and c-Jun phosphorylation at Ser63 relative to total c-Jun expression (D) at 0 and 24 h after addition of Cer6 to culture medium in A. F–H) Quantification of JNK phosphorylation at T183/Y185 relative to total JNK expression (F), c-Jun phosphorylation at Ser63 relative to total c-Jun expression (G), and cleaved caspase 3 expression relative to GAPDH (H) in E. Protein loading was assessed by protein staining (Protein S). Protein molecular mass (kDa) is indicated at the right of the blots. ns, not specific. Blots shown are representative of results for 2 experiments; within each experiment, 3 independent samples were analyzed. Results are means ± sem (n = 3). Different letters (a–c) above scatter plots (B–D, F–I) denote significantly different results. P < 0.05.
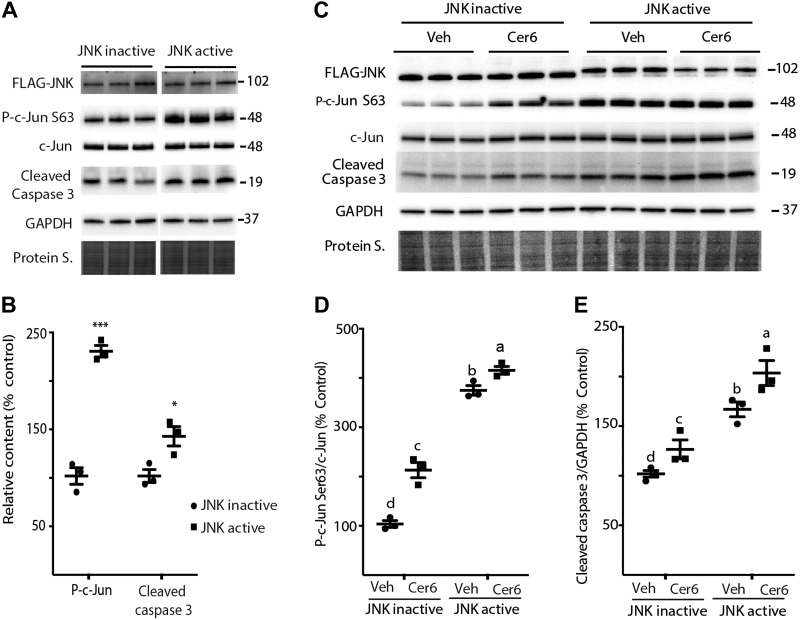
Active JNK promotes apoptosis in retinal cells
To test the hypothesis that increased JNK activity is sufficient to promote C6-Cer–induced apoptosis, we expressed either FLAG-MKK7-JNK1 (active JNK) or FLAG-MKK7-JNK1 APF variant (inactive JNK) in WT MEFs and R28 cells. Following C6-Cer exposure, phosphorylation of c-Jun on Ser63 was enhanced in MEFs by active JNK as compared with inactive JNK (Fig. 6A, B). Moreover, active JNK increased caspase 3 cleavage as compared to inactive JNK (Fig. 6A, B). Active JNK was also sufficient to increase c-Jun phosphorylation and caspase 3 cleavage in DMSO-treated R28 cells (Fig. 6C–E). Consistent with the findings in MEFs, active JNK promoted c-Jun phosphorylation at Ser63 and increased cleaved caspase 3 levels in R28 cells after addition of C6-Cer to cell culture medium (Fig. 6C–E).
Figure 6.
Activated JNK enhances c-Jun phosphorylation and promotes caspase 3 cleavage. A) MEFs were transfected with either FLAG-MKK7-JNK1 (JNK active) or FLAG-MKK7-JNK1 APF variant (JNK inactive) for 24 h before exposure to C6-Cer (Cer6, 60 μM) for 10 h. B) Quantification of phosphorylation of c-Jun (Ser63) and cleaved caspase 3 levels. *P < 0.05, ***P < 0.01. C) R28 cells were transfected with FLAG-MKK7-JNK1 (JNK active) or FLAG-MKK7-JNK1 APF variant (JNK inactive) for 16 h before exposure to 60 μM Cer6 or 1.2 μl/ml DMSO vehicle (Veh) for 8 h. D, E) Quantification of phosphorylation of c-Jun (Ser63) (D) and cleaved caspase 3 (E) levels. FLAG-MKK7-JNK1 was observed at 102 kDa. Bands are from the same blot, but are not contiguous (A). Protein loading was assessed by protein staining (Protein S). Protein molecular mass (kDa) is indicated at the right of the blots. Blots shown are representative of results for 2 experiments; within each experiment, 3 independent samples were analyzed. Results are means ± sem (n = 3). Different letters (a–c) above the scatter plots (D, E) denote significantly different results. P < 0.05.
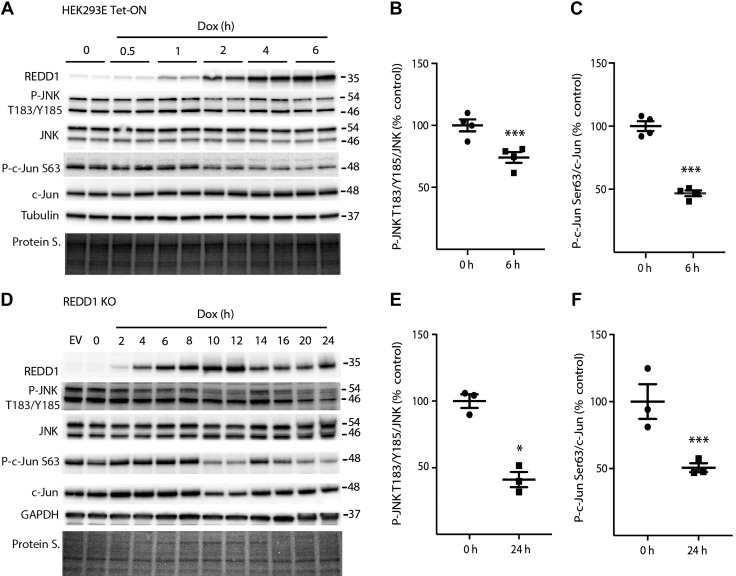
Induced REDD1 expression attenuates JNK activation
To further evaluate the impact of REDD1 on JNK/c-Jun phosphorylation in the absence of Cer, we utilized a HEK293 cell line with tetracycline-inducible hemagglutinin–tagged REDD1 expression (18). Upon Dox administration, REDD1 protein abundance was increased, reaching a maximum by 4 h, which was maintained for at least 6 h (Fig. 7A). Increased REDD1 protein expression was sufficient to attenuate JNK phosphorylation on Thr183/Tyr185 and c-Jun phosphorylation on Ser63 (Fig. 7A–C). In REDD1-deficient MEFs, REDD1 expression was restored with a similar Dox-inducible system (Fig. 7D). Consistent with the observations in HEK293E Tet-ON cells, REDD1 expression repressed JNK and c-Jun phosphorylation in REDD1-knockout MEFs (Fig. 7D–F).
Figure 7.
REDD1 expression attenuates JNK activity. A) HEK293E Tet-On HA-REDD1 cells were treated with Dox for the times indicated. Total REDD1, JNK, c-Jun, α-tubulin, GAPDH, and phosphorylation of JNK on Thr183/Tyr185 and c-Jun on Ser63 were assessed in whole-cell lysates by Western blot analysis. B, C) Quantification of phosphorylation of JNK (T183/Y185) (B) and c-Jun (Ser63) (C) at 0 and 6 h. D) REDD1-knockout (KO) MEFs were either dual transfected with p-TRE HA-REDD1 and pREV Tet-ON plasmids or transfected with an empty vector control plasmid (EV). Transfected MEFs were exposed to Dox for 24 h or the indicated time. E, F) Quantification of phosphorylation of JNK (T183/Y185) (E) and c-Jun (Ser63) (F) at 0 and 24 h. Protein loading was assessed by protein stain (Protein S). Blots shown are representative of results for 2 experiments; within each experiment, 2 independent samples were analyzed. Protein molecular mass (kDa) is indicated at the right of the blots. Results are expressed as means ± sem (n = 3). *P < 0.05, ***P < 0.01 vs. time 0 h.
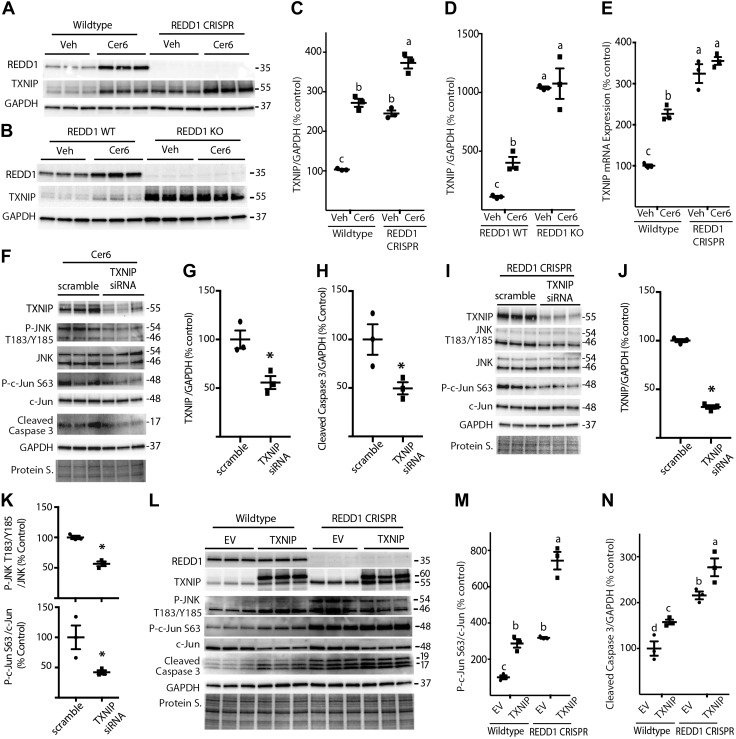
REDD1 represses JNK activity through a TXNIP-dependent mechanism
Cer promotes JNK activation at least in part through increased expression of TXNIP (17). TXNIP down-regulates thioredoxin, an inhibitor of the MAPKKK ASK1, which acts upstream of JNK. TXNIP and REDD1 are obligatory partners in a pro-oxidant complex (32, 33). Thus, TXNIP-dependent JNK activation may be enhanced in REDD1-deficient cells. C6-Cer addition to culture medium increased TXNIP protein (Fig. 8A–D) and mRNA abundance (Fig. 8E) in WT cells. TXNIP expression in REDD1-deficient cells was also enhanced as compared to WT cells at both the protein (Fig. 8A–D) and mRNA (Fig. 8E) levels. To evaluate the role of TXNIP in the effects of C6-Cer and REDD1 deletion on JNK and caspase 3, TXNIP expression was knocked down with siRNA. In WT MIO-M1 cells treated with C6-Cer, TXNIP abundance was repressed by siRNA treatment (Fig. 8F, G). Associated with TXNIP knockdown, phosphorylation of JNK and c-Jun, as well as cleaved caspase 3 levels were reduced in cells exposed to C6-Cer (Fig. 8F, H). In REDD1 CRISPR MIO-M1 cells, TXNIP knockdown attenuated phosphorylation of JNK and c-Jun (Fig. 8I–K). Alternatively, exogenous expression of TXNIP in WT MIO-M1 cells enhanced phosphorylation of JNK and c-Jun and increased cleaved caspase 3 levels (Fig. 8L). Notably, c-Jun phosphorylation and cleaved caspase 3 levels were increased in REDD1-deficient MIO-M1 cells vs. WT, and exogenous TXNIP expression further promoted c-Jun phosphorylation and caspase 3 cleavage (Fig. 8L–N). Overall, the results support a model whereby REDD1 deletion promotes TXNIP-dependent JNK activation and caspase 3 cleavage.
Figure 8.
REDD1 deletion promotes JNK activity in a TXNIP-dependent manner. A) WT and REDD1 CRISPR MIO-M1 cells were treated with 1.2 μl/ml DMSO vehicle (Veh) or 60 μM C6-Cer (Cer6) for 8 h. B) WT and REDD1-knockout (KO) MEFs were treated similarly with Veh or Cer6 for 4 h. C, D) Quantification of TXNIP protein expression in R28 (C) and MEFs (D), respectively. E, F) WT and REDD1 CRISPR MIO-M1 cells (E) were treated as described in A, and TXNIP mRNA was assessed by RT-PCR (F). WT MIO-M1 cells were transfected with either scrambled RNA or TXNIP siRNA for 18 h and then treated with Cer6 for 12 h. G, H) Quantification of TXNIP (G) and cleaved caspase 3 (H) levels in F. I) REDD1 CRISPR MIO-M1 cells were transfected with scrambled RNA or TXNIP siRNA for 48 h. J, K) Quantification of TXNIP (J) and phosphorylation of JNK (Thr183/Tyr185)/c-Jun (Ser63) (K) in I. Graphs in K share x-axis labels. L) WT and REDD1 CRISPR MIO-M1 cells were transfected with myc-FLAG-TXNIP plasmid (TXNIP) or an empty vector control plasmid (EV) for 24 h. Abundance of total REDD1, TXNIP, JNK, c-Jun, cleaved caspase 3, GAPDH, and phosphorylation of JNK on Thr183/Tyr185 and c-Jun on Ser63 were assessed in whole-cell lysates by Western blot analysis. M, N) Quantification of phosphorylation of c-Jun (Ser63) (M) and cleaved caspase 3 levels (N) in K. Protein loading was assessed by protein staining (Protein S.). Blots are representative of 2–3 experiments; within each experiment, 3 independent samples were analyzed. Protein molecular mass (kDa) is indicated at the right of the blots. Results are means ± sem (n = 3). Different letters (a–c) above the scatter plots (C–E, M, N) denote significantly different results. P < 0.05, *P < 0.05 vs. scrambled RNA.
DISCUSSION
In the present study, we sought to evaluate signaling defects in retina associated with consumption of a Western diet. Using LC-MS/MS, we found DHSo, S1P, DHS1P, Cer, HexCer, and SM to be increased in the retinas of mice fed a Western diet, when compared with mice fed control chow. Concomitant with the increase in sphingolipid subclasses, the presence of cytoplasmic nucleosomes, an indicator of cell death, was also elevated in the retina of mice fed a Western diet. Western diet increased several individual sphingolipid species of various chain lengths in the retina, including Cers containing extremely long-chain C24:0, C24:1, and C26:0 fatty acids. Very long chain Cers were recently found to stabilize tight junctions and prevent retinal vascular permeability (34). Sphingolipid species have diverse biologic roles with differing chain lengths, often having opposite functions. Because of this complexity, the observed changes must be interpreted cautiously. However, an increase in the total amount of various sphingolipid classes in retina is consistent with observations in other tissues including liver, adipose, skeleton, muscle, and heart, where increased sphingolipid content is observed in rodents fed a diet high in saturated fat content (12, 35). To our knowledge, this is the first report that retinal sphingolipid content is affected by diet. On the other hand, altered sphingolipid metabolism in retina has been observed in models of type 1 diabetes (27, 36). In the retina of streptozotocin-treated rats, total Cer content is reduced, but glucosylceramide content is increased. Notably, in the present study the increase in retinal sphingolipid content occurred before the presence of hyperglycemia, as fasting blood glucose concentrations were not different between mice fed a Western diet or control chow.
Dyslipidemia is traditionally characterized by high levels of plasma cholesterol, triglycerides, and LDL cholesterol, and reduced levels of HDL cholesterol (8). Patients with type 1 or 2 diabetes have dyslipidemia, but a greater proportion of those with type 2 diabetes have dyslipidemia because of selective insulin resistance and associated hyperinsulinemia (12, 37). Thus, dyslipidemia in pre- and type 2 diabetes potentially has a more profound role in the development of retinal dysfunction than in previous observations based on models of type 1 diabetes. Consistent with this assumption, no association between total cholesterol, triglycerides, HDL cholesterol, incident diabetic retinopathy (DR), and macular edema was reported in several studies of patients with type 1 diabetes. However, some clinical studies have positively associated high cholesterol, LDL cholesterol, and triglyceride levels with retinopathy progression in type 2 diabetes (7). Patients with type 2 diabetes treated with the lipid-lowering drug fenofibrate exhibit reduced progression of DR and other microvascular complications (38). It is also worth noting some nontraditional but diabetes-altered lipids, such as sphingolipids and phospholipids, are more harmful lipotoxic agents and stronger risk markers for microvascular disease than total cholesterol and triglyceride levels (12, 35, 39). Nevertheless, clinically obesity and diet have not consistently been associated with retinal defects independent of diabetes. However, clinical diagnosis of DR relies heavily on ophthalmoscopic detection of microaneurysms, hemorrhages, and cotton-wool spots, and functional deficits in the neural retina often precede these visible signs of disease.
In the current study, retinal cells cultured in the presence of cell-permeable Cer exhibited attenuated Akt phosphorylation, enhanced JNK activation, and increased apoptosis. Similarly, the retina of mice fed a Western diet also exhibited impaired Akt phosphorylation, enhanced JNK activation, and increased cell death when compared to mice receiving control chow. These observations are consistent with previous studies showing that mice fed an HFD exhibit dramatic activation of JNK concomitant with attenuation of Akt signaling, retinal degeneration, and impaired retinal function (4–6, 10). Both Akt signaling and JNK activity are established regulators of retinal neuronal apoptosis (40–42). Hyperglycemia and dysregulated sphingolipid metabolism can lead to impaired insulin signaling, enhanced JNK activation, and increased cell death (12, 13, 43–45). However, in the current study, hyperglycemia was not observed with Western diet–induced signaling abnormities and retinal apoptotic cell death. Thus, dysregulated sphingolipid mechanism may contribute to the early development of diet-induced signaling abnormalities that lead to retinal inflammation and neurodegeneration independent of hyperglycemia.
In response to growth factor signaling, Akt is recruited to the plasma membrane via interaction of its pleckstrin homology domain with phosphatidyl inositol triphosphate (PIP3). There, the activation loop of Akt is phosphorylated at Thr308 by phosphoinositide-dependent kinase 1 (46), whereas the C-terminal hydrophobic motif is phosphorylated at Ser473 by mTORC2 (47). Phosphorylation of Akt at Thr308 occurs before Ser473 (48), and phosphorylation of both residues is associated with higher in vitro activity of the kinase (49). Cer has long been known to activate PP2A (50) and in a number of cell types PP2A inhibition is sufficient to prevent the inhibitory effect of Cer on Akt phosphorylation (51). REDD1 mediates PP2A-dependent dephosphorylation of Akt at Thr308 (18), and in retinal cells exposed to hyperglycemic conditions, REDD1 deletion is sufficient to prevent Akt dephosphorylation (21). Thus, we initially suspected that Cer-induced REDD1 expression may be necessary for inhibition of Akt phosphorylation. However, in both REDD1-deficient MEFs and MIO-M1 cells, Cer exposure still effectively repressed Akt phosphorylation at both Ser473 and Thr308, potentially because, in many cell types, Cers function through a dual mechanism of action, whereby translocation of Akt to the plasma membrane becomes impaired and PP2A-mediated dephosphorylation of the kinase is enhanced (52). Cer has no effect on generation of PIP3 by PI3K (53), but rather induces atypical PKCζ-mediated phosphorylation of Akt at Thr34 and suppresses binding of PIP3 to the pleckstrin homology–domain of Akt (54). Thus, Cers may act to inhibit Akt in retina by both preventing phosphorylation and promoting dephosphorylation of the kinase. Regardless, there appear to be key differences in the role of REDD1 in the response to Cer addition to culture medium as compared to hyperglycemia; and the present study does not provide evidence of a role for Cer-induced REDD1 in Akt dephosphorylation.
REDD1 is a highly conserved stress response protein positively regulated by hypoxia, DNA damage, energy stress, endoplasmic reticular stress, and nutrient deprivation (55). Herein, we demonstrated that REDD1 protein expression is enhanced in retinas of mice fed a Western diet vs. those fed a control diet. Similarly, in retinal cells in culture, C6-Cer induced REDD1 mRNA and protein expression. In the present study, loss of REDD1 promoted the phosphorylation of JNK and its downstream target c-Jun. Conversely, inducible REDD1 expression was inversely associated with JNK and c-Jun phosphorylation. This observation is consistent with a role for REDD1, whereby the protein represses activation of inflammatory signaling pathways. Deletion of REDD1 increased both Cer-induced JNK activation and cell death. In agreement of previous studies demonstrating that overexpression of JNK is sufficient to promote apoptosis (56–58), we found that in MEFs and R28 retinal precursor cells expressing a constitutively active JNK variant, Cer-induced caspase 3 cleavage was elevated. Similar to its effect on Akt (18), REDD1 may be acting to repress JNK rather than promote its activation. Whether this occurs through a mechanism involving PP2A is unknown. However, unlike our findings with Akt, we have been unable to demonstrate JNK coimmunoprecipitation with REDD1. The present study also supports a model in which increased Cer-induced cell death and JNK activation are due to increased TXNIP expression in REDD1-deficient cell lines. If this model is correct, it would explain the apparent discrepancy between the results showing that in the retina a Western diet leads to both increased REDD1 expression and cell death, whereas in cells in culture REDD1 deficiency exacerbates Cer-induced cell death. In other words, Western diet–induced retinal cell death may be increased in mice deficient in REDD1 vs. WT mice because of increased TXNIP expression in the former.
TXNIP is a pro-oxidative stress, proinflammatory, and proapoptotic protein that is induced by diabetes and hyperglycemia in retina (59, 60). TXNIP binds to thioredoxin and inhibits its thiol-reducing and oxidant-scavenging activity, which in turn promotes JNK and p38MAPK activation, thereby triggering cellular oxidative stress and apoptosis (17). REDD1 is a binding partner of TXNIP in the formation of a pro-oxidant complex (32). In agreement with a previous report (17), TXNIP protein expression was elevated in cells upon C6-Cer addition to culture medium. Moreover, inhibition of TXNIP expression with siRNA reduced both JNK and c-Jun phosphorylation in response to C6-Cer. We extended the results of the previous report by demonstrating that TXNIP mRNA and protein expression were also enhanced by REDD1 deletion. In REDD1-deficient retinal cells, TXNIP knockdown attenuated JNK/c-Jun phosphorylation; whereas expression of TXNIP enhanced c-Jun phosphorylation and cleaved caspase 3 levels. Overall, our findings support a model wherein increased JNK activation and cell death in REDD1-deficient cells are the result of elevated TXNIP expression. Protein–protein interaction between REDD1 and TXNIP has been reported in 3 independent studies (32, 33, 61). Yet, a detailed mechanism by which REDD1 and TXNIP interact remains to be defined. Park and colleagues (33) demonstrated that TXNIP plays a crucial role in the stabilization of ATF4-induced REDD1 protein. However, this mechanism does not account for transcriptional induction of TXNIP in REDD1-deficient cell lines. It is tempting to speculate that increased TXNIP expression in REDD1-deficient cells is the result of enhanced FOXO1 phosphorylation (18), as FOXO1 is known to negatively regulate TXNIP transcription (60). In both REDD1-deficient MEFs and MIO-M1 cells, FoxO1/3 phosphorylation was enhanced; however, the mechanism involved in increased TXNIP expression remains to be fully explored.
Overall, the present study provides new insights into the mechanisms whereby consumption of a Western diet causes retinal dysfunction. Whereas current therapeutic treatments for DR largely address the microvascular complications that characterize the later proliferative stages of disease progression, it is clear that neuroretinal function is impaired before the onset of vascular lesions. Thus, an improved understanding of the mechanisms that contribute to neuroretinal cell death and dysfunction are of the utmost clinical significance. A role for Cer signaling in retinal neurodegeneration has been demonstrated (45). However, we report for the first time that a Western diet can promote retinal sphingolipid accumulation in association with attenuation of Akt phosphorylation, enhanced JNK activation, and increased retinal cell death, before the onset of hyperglycemia. Thus, therapeutic strategies to restore normal sphingolipid metabolism in retina may represent a viable option for treating complications associated with the early stages of DR.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Leonard Jefferson (Department of Cellular and Molecular Physiology, Penn State College of Medicine) for critically evaluating the manuscript, Dr. Gail Seigel (Ross Eye Institute, State University of New York, Buffalo, NY, USA) for permission to use the R28 retinal cell line, Dr. Leif Ellisen (Department of Medicine, Harvard Medical School, Boston, MA, USA) for providing REDD1 WT and knockout MEFs, and the staff of the Flow Cytometry Core Facility (Penn State University College of Medicine, Hershey, PA, USA) for assistance. The research was supported by The American Diabetes Association Pathway to Stop Diabetes Grant 1-14-INI-04 and U.S. National Institutes of Health (NIH)/National Eye Institute Grant EY023612 (to M.D.D.) and NIH/National Institute of Diabetes and Digestive and Kidney Diseases Grants DK13499 and DK15658 (to S.R.K.). Services and products in support of the research project were generated by the Virginia Commonwealth University Massey Cancer Center (Richmond, VA, USA) Lipidomics Shared Resource, supported, in part, with funding from NIH/National Cancer Institute Grant P30 CA016059. The authors declare no conflicts of interest.
Glossary
- 7-AAD
7-aminoactinomycin D
- Akt
protein kinase B
- BCL
B-cell lymphoma
- BSA
bovine serum albumin
- Cer
ceramide
- CRISPR
clustered regularly interspaced short palindromic repeats
- DHSo
dihydrosphingosine
- DHS1P
dihydro-sphingosine 1-phosphate
- Dox
doxycycline
- DR
diabetic retinopathy
- FBS
fetal bovine serum
- HEK
human embryonic kidney
- HexCer
hexosylceramide
- HFD
high-fat diet
- LC-MS/MS
liquid chromatography tandem mass spectrometry
- MEF
mouse embryonic fibroblast
- mTORC
mammalian target of rapamycin complex
- PP
protein phosphatase
- REDD1
regulated in development and DNA damage response 1
- SM
sphingomyelin
- So
sphingosine
- S1P
sphingosine 1-phosphate
- TXNIP
thioredoxin-interacting protein
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
W. Dai performed data acquisition and data analysis and drafted the manuscript; W. P. Miller helped generate the data and wrote parts of the manuscript; A. L. Toro and A. J. Black helped generate the data; S. K. Dierschke drafted parts of the manuscript; R. P. Feehan generated the REDD1 CRISPR KO MIO-M1 cell line; S. R. Kimball designed experiments and reviewed and edited the manuscript; and M. D. Dennis designed experiments, reviewed and edited the manuscript, and is the guarantor of this study.
REFERENCES
- 1.Hu F. B. (2011) Globalization of diabetes: the role of diet, lifestyle, and genes. Diabetes Care 34, 1249–1257 10.2337/dc11-0442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hannun Y. A., Obeid L. M. (2008) Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9, 139–150 10.1038/nrm2329 [DOI] [PubMed] [Google Scholar]
- 3.Wong T. Y., Cheung C. M., Larsen M., Sharma S., Simó R. (2016) Diabetic retinopathy. Nat. Rev. Dis. Primers 2, 16012 10.1038/nrdp.2016.12 [DOI] [PubMed] [Google Scholar]
- 4.Marçal A. C., Leonelli M., Fiamoncini J., Deschamps F. C., Rodrigues M. A., Curi R., Carpinelli A. R., Britto L. R., Carvalho C. R. (2013) Diet-induced obesity impairs AKT signalling in the retina and causes retinal degeneration. Cell Biochem. Funct. 31, 65–74 10.1002/cbf.2861 [DOI] [PubMed] [Google Scholar]
- 5.Chang R. C., Shi L., Huang C. C., Kim A. J., Ko M. L., Zhou B., Ko G. Y. (2015) High-fat diet-induced retinal dysfunction. Invest. Ophthalmol. Vis. Sci. 56, 2367–2380 10.1167/iovs.14-16143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rajagopal R., Bligard G. W., Zhang S., Yin L., Lukasiewicz P., Semenkovich C. F. (2016) Functional deficits precede structural lesions in mice with high-fat diet-induced diabetic retinopathy. Diabetes 65, 1072–1084 10.2337/db15-1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Modjtahedi B. S., Bose N., Papakostas T. D., Morse L., Vavvas D. G., Kishan A. U. (2016) Lipids and diabetic retinopathy. Semin. Ophthalmol. 31, 10–18 10.3109/08820538.2015.1114869 [DOI] [PubMed] [Google Scholar]
- 8.Mbata O., Abo El-Magd N. F., El-Remessy A. B. (2017) Obesity, metabolic syndrome and diabetic retinopathy: beyond hyperglycemia. World J. Diabetes 8, 317–329 10.4239/wjd.v8.i7.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barber A. J., Nakamura M., Wolpert E. B., Reiter C. E., Seigel G. M., Antonetti D. A., Gardner T. W. (2001) Insulin rescues retinal neurons from apoptosis by a phosphatidylinositol 3-kinase/Akt-mediated mechanism that reduces the activation of caspase-3. J. Biol. Chem. 276, 32814–32821 10.1074/jbc.M104738200 [DOI] [PubMed] [Google Scholar]
- 10.Miller W. P., Ravi S., Martin T. D., Kimball S. R., Dennis M. D. (2017) Activation of the stress response kinase JNK (c-Jun N-terminal Kinase) attenuates insulin action in retina through a p70S6K1-dependent mechanism. J. Biol. Chem. 292, 1591–1602 10.1074/jbc.M116.760868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iqbal J., Walsh M. T., Hammad S. M., Hussain M. M. (2017) Sphingolipids and lipoproteins in health and metabolic disorders. Trends Endocrinol. Metab. 28, 506–518 10.1016/j.tem.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meikle P. J., Summers S. A. (2017) Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 13, 79–91 10.1038/nrendo.2016.169 [DOI] [PubMed] [Google Scholar]
- 13.Morad S. A., Cabot M. C. (2013) Ceramide-orchestrated signalling in cancer cells. Nat. Rev. Cancer 13, 51–65 10.1038/nrc3398 [DOI] [PubMed] [Google Scholar]
- 14.Hsieh C. T., Chuang J. H., Yang W. C., Yin Y., Lin Y. (2014) Ceramide inhibits insulin-stimulated Akt phosphorylation through activation of Rheb/mTORC1/S6K signaling in skeletal muscle. Cell. Signal. 26, 1400–1408 10.1016/j.cellsig.2014.03.004 [DOI] [PubMed] [Google Scholar]
- 15.Bourbon N. A., Sandirasegarane L., Kester M. (2002) Ceramide-induced inhibition of Akt is mediated through protein kinase Czeta: implications for growth arrest. J. Biol. Chem. 277, 3286–3292 10.1074/jbc.M110541200 [DOI] [PubMed] [Google Scholar]
- 16.Dobrowsky R. T., Kamibayashi C., Mumby M. C., Hannun Y. A. (1993) Ceramide activates heterotrimeric protein phosphatase 2A. J. Biol. Chem. 268, 15523–15530 [PubMed] [Google Scholar]
- 17.Chen C. L., Lin C. F., Chang W. T., Huang W. C., Teng C. F., Lin Y. S. (2008) Ceramide induces p38 MAPK and JNK activation through a mechanism involving a thioredoxin-interacting protein-mediated pathway. Blood 111, 4365–4374 10.1182/blood-2007-08-106336 [DOI] [PubMed] [Google Scholar]
- 18.Dennis M. D., Coleman C. S., Berg A., Jefferson L. S., Kimball S. R. (2014) REDD1 enhances protein phosphatase 2A-mediated dephosphorylation of Akt to repress mTORC1 signaling. Sci. Signal. 7, ra68 10.1126/scisignal.2005103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dungan C. M., Li J., Williamson D. L. (2016) Caloric restriction normalizes obesity-induced alterations on regulators of skeletal muscle growth ignaling. Lipids 51, 905–912 s 10.1007/s11745-016-4168-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williamson D. L., Li Z., Tuder R. M., Feinstein E., Kimball S. R., Dungan C. M. (2014) Altered nutrient response of mTORC1 as a result of changes in REDD1 expression: effect of obesity vs. REDD1 deficiency. J. Appl. Physiol. (1985) 117, 246–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller W. P., Yang C., Mihailescu M. L., Moore J. A., Dai W., Barber A. J., Dennis M. D. (2018) Deletion of the Akt/mTORC1 repressor REDD1 prevents visual dysfunction in a rodent model of type 1 diabetes. Diabetes 67, 110–119 10.2337/db17-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dennis M. D., Kimball S. R., Fort P. E., Jefferson L. S. (2015) Regulated in development and DNA damage 1 is necessary for hyperglycemia-induced vascular endothelial growth factor expression in the retina of diabetic rodents. J. Biol. Chem. 290, 3865–3874 10.1074/jbc.M114.623058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Limb G. A., Salt T. E., Munro P. M., Moss S. E., Khaw P. T. (2002) In vitro characterization of a spontaneously immortalized human Müller cell line (MIO-M1). Invest. Ophthalmol. Vis. Sci. 43, 864–869 [PubMed] [Google Scholar]
- 24.Kimball S. R., Do A. N., Kutzler L., Cavener D. R., Jefferson L. S. (2008) Rapid turnover of the mTOR complex 1 (mTORC1) repressor REDD1 and activation of mTORC1 signaling following inhibition of protein synthesis. J. Biol. Chem. 283, 3465–3475 10.1074/jbc.M706643200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kelleher A. R., Kimball S. R., Dennis M. D., Schilder R. J., Jefferson L. S. (2013) The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am. J. Physiol. Endocrinol. Metab. 304, E229–E236 10.1152/ajpendo.00409.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 27.Fox T. E., Han X., Kelly S., Merrill A. H., II, Martin R. E., Anderson R. E., Gardner T. W., Kester M. (2006) Diabetes alters sphingolipid metabolism in the retina: a potential mechanism of cell death in diabetic retinopathy. Diabetes 55, 3573–3580 10.2337/db06-0539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ueda N. (2015) Ceramide-induced apoptosis in renal tubular cells: a role of mitochondria and sphingosine-1-phoshate. Int. J. Mol. Sci. 16, 5076–5124 10.3390/ijms16035076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Perry D. K., Carton J., Shah A. K., Meredith F., Uhlinger D. J., Hannun Y. A. (2000) Serine palmitoyltransferase regulates de novo ceramide generation during etoposide-induced apoptosis. J. Biol. Chem. 275, 9078–9084 10.1074/jbc.275.12.9078 [DOI] [PubMed] [Google Scholar]
- 30.Listenberger L. L., Ory D. S., Schaffer J. E. (2001) Palmitate-induced apoptosis can occur through a ceramide-independent pathway. J. Biol. Chem. 276, 14890–14895 10.1074/jbc.M010286200 [DOI] [PubMed] [Google Scholar]
- 31.Porter A. G., Jänicke R. U. (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 6, 99–104 10.1038/sj.cdd.4400476 [DOI] [PubMed] [Google Scholar]
- 32.Qiao S., Dennis M., Song X., Vadysirisack D. D., Salunke D., Nash Z., Yang Z., Liesa M., Yoshioka J., Matsuzawa S., Shirihai O. S., Lee R. T., Reed J. C., Ellisen L. W. (2015) A REDD1/TXNIP pro-oxidant complex regulates ATG4B activity to control stress-induced autophagy and sustain exercise capacity. Nat. Commun. 6, 7014 10.1038/ncomms8014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin H. O., Seo S. K., Kim Y. S., Woo S. H., Lee K. H., Yi J. Y., Lee S. J., Choe T. B., Lee J. H., An S., Hong S. I., Park I. C. (2011) TXNIP potentiates Redd1-induced mTOR suppression through stabilization of Redd1. Oncogene 30, 3792–3801 10.1038/onc.2011.102 [DOI] [PubMed] [Google Scholar]
- 34.Kady N. M., Liu X., Lydic T. A., Syed M. H., Navitskaya S., Wang Q., Hammer S. S., O’Reilly S., Huang C., Seregin S. S., Amalfitano A., Chiodo V. A., Boye S. L., Hauswirth W. W., Antonetti D. A., Busik J. V. (2018) ELOVL4-mediated production of very long-chain ceramides stabilizes tight junctions and prevents diabetes-induced retinal vascular permeability. Diabetes 67, 769–781 10.2337/db17-1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi S., Snider A. J. (2015) Sphingolipids in high fat diet and obesity-related diseases. Mediators Inflamm. 2015, 520618 10.1155/2015/520618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tikhonenko M., Lydic T. A., Wang Y., Chen W., Opreanu M., Sochacki A., McSorley K. M., Renis R. L., Kern T., Jump D. B., Reid G. E., Busik J. V. (2010) Remodeling of retinal Fatty acids in an animal model of diabetes: a decrease in long-chain polyunsaturated fatty acids is associated with a decrease in fatty acid elongases Elovl2 and Elovl4. Diabetes 59, 219–227 10.2337/db09-0728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schofield J. D., Liu Y., Rao-Balakrishna P., Malik R. A., Soran H. (2016) Diabetes dyslipidemia. Diabetes Ther. 7, 203–219 10.1007/s13300-016-0167-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wright A. D., Dodson P. M. (2011) Medical management of diabetic retinopathy: fenofibrate and ACCORD Eye studies. Eye (Lond.) 25, 843–849 10.1038/eye.2011.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammer S. S., Busik J. V. (2017) The role of dyslipidemia in diabetic retinopathy. Vision Res. 139, 228–236 10.1016/j.visres.2017.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guma M., Rius J., Duong-Polk K. X., Haddad G. G., Lindsey J. D., Karin M. (2009) Genetic and pharmacological inhibition of JNK ameliorates hypoxia-induced retinopathy through interference with VEGF expression. Proc. Natl. Acad. Sci. USA 106, 8760–8765 10.1073/pnas.0902659106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura M., Barber A. J., Antonetti D. A., LaNoue K. F., Robinson K. A., Buse M. G., Gardner T. W. (2001) Excessive hexosamines block the neuroprotective effect of insulin and induce apoptosis in retinal neurons. J. Biol. Chem. 276, 43748–43755 10.1074/jbc.M108594200 [DOI] [PubMed] [Google Scholar]
- 42.Coffey E. T. (2014) Nuclear and cytosolic JNK signalling in neurons. Nat. Rev. Neurosci. 15, 285–299 10.1038/nrn3729 [DOI] [PubMed] [Google Scholar]
- 43.Cheung N., Mitchell P., Wong T. Y. (2010) Diabetic retinopathy. Lancet 376, 124–136 10.1016/S0140-6736(09)62124-3 [DOI] [PubMed] [Google Scholar]
- 44.Duh E. J., Sun J. K., Stitt A. W. (2017) Diabetic retinopathy: current understanding, mechanisms, and treatment strategies. JCI Insight 2, 93751 10.1172/jci.insight.93751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen H., Tran J. T., Brush R. S., Saadi A., Rahman A. K., Yu M., Yasumura D., Matthes M. T., Ahern K., Yang H., LaVail M. M., Mandal M. N. (2012) Ceramide signaling in retinal degeneration. Adv. Exp. Med. Biol. 723, 553–558 10.1007/978-1-4614-0631-0_70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Balpha. Curr. Biol. 7, 261–269 10.1016/S0960-9822(06)00122-9 [DOI] [PubMed] [Google Scholar]
- 47.Sarbassov D. D., Guertin D. A., Ali S. M., Sabatini D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 307, 1098–1101 10.1126/science.1106148 [DOI] [PubMed] [Google Scholar]
- 48.Humphrey S. J., Yang G., Yang P., Fazakerley D. J., Stöckli J., Yang J. Y., James D. E. (2013) Dynamic adipocyte phosphoproteome reveals that Akt directly regulates mTORC2. Cell Metab. 17, 1009–1020 10.1016/j.cmet.2013.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Alessi D. R., Andjelkovic M., Caudwell B., Cron P., Morrice N., Cohen P., Hemmings B. A. (1996) Mechanism of activation of protein kinase B by insulin and IGF-1. EMBO J. 15, 6541–6551 [PMC free article] [PubMed] [Google Scholar]
- 50.Dobrowsky R. T., Hannun Y. A. (1993) Ceramide-activated protein phosphatase: partial purification and relationship to protein phosphatase 2A. Adv. Lipid Res. 25, 91–104 [PubMed] [Google Scholar]
- 51.Teruel T., Hernandez R., Lorenzo M. (2001) Ceramide mediates insulin resistance by tumor necrosis factor-alpha in brown adipocytes by maintaining Akt in an inactive dephosphorylated state. Diabetes 50, 2563–2571 10.2337/diabetes.50.11.2563 [DOI] [PubMed] [Google Scholar]
- 52.Stratford S., Hoehn K. L., Liu F., Summers S. A. (2004) Regulation of insulin action by ceramide: dual mechanisms linking ceramide accumulation to the inhibition of Akt/protein kinase B. J. Biol. Chem. 279, 36608–36615 10.1074/jbc.M406499200 [DOI] [PubMed] [Google Scholar]
- 53.Schubert K. M., Scheid M. P., Duronio V. (2000) Ceramide inhibits protein kinase B/Akt by promoting dephosphorylation of serine 473. J. Biol. Chem. 275, 13330–13335 10.1074/jbc.275.18.13330 [DOI] [PubMed] [Google Scholar]
- 54.Powell D. J., Hajduch E., Kular G., Hundal H. S. (2003) Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCzeta-dependent mechanism. Mol. Cell. Biol. 23, 7794–7808 10.1128/MCB.23.21.7794-7808.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Reuschel E. L., Wang J., Shivers D. K., Muthumani K., Weiner D. B., Ma Z., Finkel T. H. (2015) REDD1 is essential for optimal T cell proliferation and survival. PLoS One 10, e0136323 10.1371/journal.pone.0136323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dhanasekaran D. N., Reddy E. P. (2008) JNK signaling in apoptosis. Oncogene 27, 6245–6251 10.1038/onc.2008.301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brandt B., Abou-Eladab E. F., Tiedge M., Walzel H. (2010) Role of the JNK/c-Jun/AP-1 signaling pathway in galectin-1-induced T-cell death. Cell Death Dis. 1, e23 10.1038/cddis.2010.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu J., Lin A. (2005) Role of JNK activation in apoptosis: a double-edged sword. Cell Res. 15, 36–42 10.1038/sj.cr.7290262 [DOI] [PubMed] [Google Scholar]
- 59.Devi T. S., Somayajulu M., Kowluru R. A., Singh L. P. (2017) TXNIP regulates mitophagy in retinal Müller cells under high-glucose conditions: implications for diabetic retinopathy. Cell Death Dis. 8, e2777 10.1038/cddis.2017.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh L. P. (2013) Thioredoxin interacting protein (TXNIP) and pathogenesis of diabetic retinopathy. J. Clin. Exp. Ophthalmol. 4, 297 10.4172/2155-9570.1000287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvarez-Garcia O., Olmer M., Akagi R., Akasaki Y., Fisch K. M., Shen T., Su A. I., Lotz M. K. (2016) Suppression of REDD1 in osteoarthritis cartilage, a novel mechanism for dysregulated mTOR signaling and defective autophagy. Osteoarthritis Cartilage 24, 1639–1647 10.1016/j.joca.2016.04.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.