Abstract
We previously developed a tissue-engineered vascular graft (TEVG) made by seeding autologous cells onto a biodegradable tubular scaffold, in an attempt to create a living vascular graft with growth potential for use in children undergoing congenital heart surgery. Results of our clinical trial showed that the TEVG possesses growth capacity but that its widespread clinical use is not yet advisable due to the high incidence of TEVG stenosis. In animal models, TEVG stenosis is caused by increased monocytic cell recruitment and its classic (“M1”) activation. Here, we report on the source and regulation of these monocytes. TEVGs were implanted in wild-type, CCR2 knockout (Ccr2−/−), splenectomized, and spleen graft recipient mice. We found that bone marrow–derived Ly6C+hi monocytes released from sequestration by the spleen are the source of mononuclear cells infiltrating the TEVG during the acute phase of neovessel formation. Furthermore, short-term administration of losartan (0.6 g/L, 2 wk), an angiotensin II type 1 receptor antagonist, significantly reduced the macrophage populations (Ly6C+/−/F480+) in the scaffolds and improved long-term patency in TEVGs. Notably, the combined effect of bone marrow–derived mononuclear cell seeding with short-term losartan treatment completely prevented the development of TEVG stenosis. Our results provide support for pharmacologic treatment with losartan as a strategy to modulate monocyte infiltration into the grafts and thus prevent TEVG stenosis.—Ruiz-Rosado, J. D. D., Lee, Y.-U., Mahler, N., Yi, T., Robledo-Avila, F., Martinez-Saucedo, D., Lee, A. Y., Shoji, T., Heuer, E., Yates, A. R., Pober, J. S., Shinoka, T., Partida-Sanchez, S., Breuer, C. K. Angiotensin II receptor I blockade prevents stenosis of tissue engineered vascular grafts.
Keywords: losartan, monocytes, macrophages
Surgical repair of congenital heart defects often requires use of vascular grafts. In many instances, there is insufficient native vessel, and synthetic vascular conduits are needed. However, these materials cannot expand as children grow, which may result in complications. In contrast, a living tissue–engineered vascular graft (TEVG) could avoid this problem by growing with the patient. We developed the first TEVG to be used in humans (1). We also performed the first clinical trial evaluating the use of TEVGs in children with single-ventricle anomalies undergoing a modified Fontan operation in which a vascular conduit is needed to connect the inferior vena cava (IVC) to the pulmonary artery (2–4). Our TEVG is made by seeding autologous bone marrow–derived mononuclear cells (BM-MNCs) onto a biodegradable tubular scaffold fabricated from a polyglycolic fiber–based mesh coated with a 50:50 copolymer of polycaprolactone and polylactic acid (3). The results of our studies confirmed the growth capacity of the TEVG, eliminating the need for subsequent surgical revisions as children grow, and showed an excellent safety profile with no graft-related deaths or graft failures (4). The most common graft-related complication was stenosis, which required angioplasty in 7 of 25 patients at a mean of 11.1 yr after implantation.
In an effort to improve TEVG performance, we developed a mouse model to analyze the cellular and molecular mechanisms underlying neovessel formation and development of TEVG stenosis (5). We showed that BM-MNC seeding promotes neovessel formation in the tissue-engineered scaffolds. Seeded cells do not directly contribute to vascular neotissue formation but instead function to recruit host-derived monocytes in the TEVG (6–8). However, we further showed that conversion of infiltrating monocytes to classically activated (M1-type) macrophages in the TEVG, mediated by TGF-β signaling, contributes to TEVG stenosis (9). Hence, regulating monocyte infiltration into the TEVGs and their subsequent activation could promote neovessel formation and reduce stenosis.
Here, we demonstrate that splenic lymphocyte antigen 6 complex (Ly6C)+hi monocytes infiltrate the TEVGs during early neotissue formation. This monocyte subset originates from the BM and is sequestered in the spleen, returning to the circulation upon TEVG implantation. Furthermore, oral administration of losartan, a U.S. Food and Drug Administration (FDA)-approved angiotensin II type 1 (AT-1) receptor inhibitor, reduced the numbers of macrophages in the implanted scaffolds and the incidence of TEVG stenosis. Finally, we report that the combination of BM-MNC seeding and short-term losartan treatment fully prevented TEVG stenosis, suggesting a novel potential therapy to improve the performance of TEVGs.
MATERIALS AND METHODS
Animals
Adult 8–12-wk-old C57BL/6J, B6.129P(Cg)-Ptprca Cx3cr1tm1Litt/LittJ (Cx3cr1gfp/gfp) and B6.129S4-Ccr2tm1Ifc/J (Ccr2−/−) female mice (all from The Jackson Laboratory, Bar Harbor, ME, USA) were used in this study. All animal experiments were approved by the Nationwide Children’s Hospital Institutional Guidelines for the Care and Use of Animals and were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA].
Scaffold fabrication
TEVG scaffolds were constructed from a nonwoven polyglycolic acid mesh (Concordia Fibers, Coventry, RI, USA) and a copolymer sealant solution of poly-l-lactide and –ε-caprolactone [P(CL/LA)] as previously described (10). Briefly, a stainless-steel needle (19 gauge) was introduced into the opposing end of a tapered cylinder with a 1 mm inner diameter. Polyglycolic acid felt (5 × 8 mm2) was inserted around the needle. A 50:50 copolymer sealant solution of ε-caprolactone and l-lactide [P(CL/LA)] (Gunze Ltd., Kyoto, Japan) was created by dissolving the copolymer at 5% (w/v) in 1,4-dioxane. The P(CL/LA) sealant was injected into the inlet of the cylinder and allowed to penetrate the felt and fuse the open seam. The hybrid polyglycolic acid–P(CL/LA) scaffolds were snap-frozen at −20°C for 30 min, then lyophilized for 24 h to eliminate the solvents. Before graft implantation for the unseeded group, or for cell seeding, the scaffolds were sterilized under UV light overnight. Because untreated/unseeded groups historically showed higher incidence of stenosis, we therefore used untreated/unseeded TEVGs as a model for TEVG stenosis. The cell seeded groups serve as a positive control, with a low incidence of stenosis.
BM-MNC isolation
BM was collected with RPMI-1640 (Thermo Fisher Scientific, Waltham, MA, USA) from the femurs and tibias of syngeneic C57BL/6 mice (n = 5 BM harvests for each 25 TEVG implantations) after euthanasia with 200 mg/kg ketamine and 20 mg/kg xylazine injected intraperitoneally). The BM cells were suspended in RPMI and added to 5 ml of Ficoll 1083 (1:1; MilliporeSigma, Burlington, MA, USA) in a 15 ml tube. A sample was taken (5–10 µl) for the pre-Ficoll cell count. The tube was centrifuged at 528 g for 30 min with “no brake” at 24°C. The upper pink layer was removed, and the clear middle MNC layer was collected and diluted with 1:1 PBS. The solution was then centrifuged at 528 g for 30 min at 24°C. Afterward, the supernatant was removed, the pellet was washed with 5 ml of PBS, and centrifugation was performed at 528 g for 30 min at 24°C. Finally, the supernatant was removed again, and the pellet was diluted with Rosewell Park Memorial Institute (RPMI)-1640 medium (∼200 µl). Cells were adjusted to 1 × 106 cells per 10 µl of RPMI-1640.
Cell seeding
The scaffold was prewet with 5 µl RPMI-1640 luminally for 5 min, and RPMI-1640 was then replaced with 10 µl of BM-MNCs (1 × 106 cells). After 10 min, a 19 gauge needle was inserted into the grafts to keep its shape; the sample was then placed in a 24-well plate with 1 ml of RPMI-1640 on each well and incubated overnight.
TEVG implantation
TEVG scaffolds with or without cell seeding were implanted into the IVC of 8–10-wk-old female mice as previously described (5, 10). Briefly, ketoprofen (100 mg/kg) was used as a preanesthesia analgesic followed by anesthetizing with an intraperitoneal injection consisting of ketamine (100 mg/kg) and xylazine (10 mg/kg). A midline laparotomy incision was made, and a self-retaining retractor was inserted. The intestines were wrapped with gauze that was moistened in sterile saline. The aorta and IVC were separated, 2 microclamps were placed on both sides of the aorta and the IVC, and the IVC was then transected. The graft was implanted as an IVC interposition graft with proximal and distal end-to-end anastomosis using a sterile 10-0 suture. The skin was then closed in 2 layers by using a 6-0 black polyamide monofilament suture, and animals were moved to a recovery cage with a warming pad until the mouse became fully mobile. Upon recovery, the mouse was returned to a new cage, and pain medication (ibuprofen, 30 mg/kg, drinking water) was given for 48 h.
Splenectomy
Mice were anesthetized by using a ketamine xylazine cocktail with ketoprofen as preanesthesia analgesic. Hair in the surgical area was shaved with a razor and then disinfected by using PDI-Povidone-Iodine (Express Medical Supplies, Fountain Inn, SC, USA) and alcohol prep pads (Thermo Fisher Scientific). A midline laparotomy incision from below the xyphoid to the suprapubic region was performed, and a self-retaining retractor inserted. The intestines were wrapped in saline-moistened gauze and retracted. The spleen was exposed. Vessels supplying the spleen were ligated, and the spleen was removed. The splenectomy was followed with either graft implantation or spleen transplantation plus graft implantation. After all procedures were performed, the intestines were returned to the abdominal cavity. The abdominal musculature and skin were closed in 2 layers by using 6-0 sterile micro suture (AROSurgical, Newport Beach, CA, USA). The estimated time of the splenectomy was 5–10 min.
Spleen transplantation
Spleen transplantation procedures were performed as previously described (11). In the donor spleen harvest, Cx3cr1gfp/gfp mice were anesthetized by using a ketamine/xylazine cocktail. The hair in the surgical area was removed by using a shaver and then disinfected by using PDI-Povidone-iodine (Express Medical Supplies, Fountain Inn, SC, USA) and alcohol pads (Thermo Fisher Scientific). A midline laparotomy incision from the xyphoid to the suprapubic region was made. The chest was opened, and the heart was exposed. The right atrium was cut open, 15 ml ice-cold heparin saline (100-U heparin/1 ml saline) was perfused through a left ventricle puncture using a 25 gauge needle to remove blood and prevent coagulation. Microsurgery was performed by using an operating microscope with zoom magnification. The donor spleens were exposed and carefully dissected. All aortic and venous branches were ligated except the splenic arteries and veins. The harvested spleens were left in ice-cold heparin saline (100-U heparin/1 ml saline) for further transplantation.
For the spleen transplantation to a recipient mouse, wild-type (WT) mice were anesthetized by using a ketamine/xylazine cocktail with ketoprofen as preanesthesia analgesic. Hair was removed, and a midline laparotomy incision was performed as described in the donor mice. A self-retaining retractor was inserted, and the intestines were wrapped in saline-moistened gauze and retracted. The infrarenal aorta and IVC were bluntly defined. The vascular control was obtained with microvascular clamps. The harvested spleen was transplanted into the intraperitoneal cavity of the recipient mouse, and arterial and venous anastomoses were performed by using a sterile 10-0 suture. End-to-side anastomoses of the aortic cuff to the recipient abdominal aorta, and of the portal vein to the recipient IVC, were established. The spleen transplantation was preceded by self-splenectomy and followed with graft implantation. The abdominal musculature and skin were closed in 2 layers by using 6-0 sterile micro suture (AROSurgical). after all the procedures were done.
Losartan treatment
Immediately after TEVG implantation, the mice were treated orally with losartan in drinking water. The high and low doses of losartan were 0.6 and 0.1 g/L, respectively. These doses were determined based on the literature, which indicates effective losartan doses in mouse models (12–15). Mice were treated with losartan for 6 mo for the long-term treatment group and 2 wk for the short-term treatment group. In the short-term treatment group, losartan water was replaced with regular drinking water after 2 wk. The mice were euthanized after 2 wk, 6 mo, or 1 yr according to the study cohort.
Losartan high-performance liquid chromatography
The blood from losartan-treated mice was drawn to measure the concentration of losartan in plasma at 1, 3, 5, 7, and 10 d, using a retro-orbital blood collection method; whole blood samples were then collected at the time of euthanasia at 6 mo. Plasma was separated from the blood, and levels of losartan in the blood were analyzed at the Analytical Chemistry/Small Molecule Core at the Research Institute at Nationwide Children’s Hospital.
Blood pressure monitoring
Blood pressure from the 3 mouse groups [high-dose losartan (0.6 g/L), low-dose losartan (0.1 g/L), and control (n = 5 each)] were monitored by using the CODA 2 Channel High Throughput Non-Invasive Blood Pressure System (Kent Scientific, Torrington, CT, USA) (16).
Echocardiography
To determine the effect of losartan on cardiac function, the cardiac output was measured by using a high-frequency ultrasound system with a pulsed-wave Doppler mode (30 MHz; VisualSonics, Inc., Toronto, ON, Canada). Mice were anesthetized by using 1.5% isoflurane (Baxter, Deerfield, IL, USA), vaporized with oxygen at a flow rate of 1 L/min, and transthoracic echocardiography was performed. Cardiac output was measured by using the built-in software and converted to a cardiac index by dividing the cardiac output by mouse body weight.
Ultrasonography
To determine the graft stenosis rate, mice were imaged at 2, 8, and 24 wk after implantation with a high-frequency Doppler ultrasound system (Vevo 2100; VisualSonics). Mice were anesthetized by using isoflurane (1.5%; Baxter) with 99% oxygen. After clipping the abdominal hair of the mice, ultrasound gel (Aquasonic Clear, Fairfield, NJ, USA) was applied to the abdomen. Long-axis images were acquired in both B-mode and color Doppler. Stenosis was defined a priori as >50% narrowing of the lumen compared with the original scaffold lumen size on B-mode imaging.
TEVG harvest
Mice were euthanized with a cocktail overdose of ketamine (200 mg/kg) and xylazine (20 mg/kg). Subsequently, the chest was cut open, and a small cut was made on the right atrium; mice were systemically perfused from the left ventricle with 20 ml of 10% formalin. After perfusion, TEVGs were harvested and prepared for histologic analysis. For fluorescence-activated cell sorting (FACS) analysis, mice were perfused with 20 ml of PBS, and the harvested TEVGs were then immediately placed in ice-cold cell culture medium.
Histology/immunohistochemistry morphometry
Explanted grafts were fixed with 10% formalin overnight and then embedded in paraffin as previously described (6–8). The embedded sections were stained with hematoxylin and eosin for luminal diameter and patency measurement. Luminal graft diameters were measured by using ImageJ software (NIH). Stenosis was defined as a >75% decrease in the luminal diameter. Graft occlusion was defined as a complete obliteration of the lumen.
Cytofluorometric analysis
Upon harvesting, TEVGs and spleens were minced by using scissors and then digested in HBSS solution with 1 mg/ml collagenase type IV, 20 µg/ml DNase I, 200 U/ml hyaluronidase, and 1 mg/ml bovine serum albumin/fraction V (Thermo Fisher Scientific). After enzymatic dissociation, single-cell suspensions were stained for flow cytometry. Harvested cells were incubated in 1 μg/ml of anti-mouse FcR antibody in 100 ml PBS containing 0.5% BSA plus 0.02% NaN3 (FACS buffer) for 15 min on ice. After washing, 1–3 × 106 cells were stained in FACS buffer for 15 min at 4°C with various fluorescent mAb combinations and further collected on an LSR II cytofluorometer (BD, Franklin Lakes, NJ, USA). Stained cells were gated according to size scatter (SSC-A) and forward scatter (FSC-A) to eliminate debris. Doublets were eliminated by using FSC height (FSC-H) and FSC-A. Blue-fluorescent reactive dye L23105 (Thermo Fisher Scientific) was used to exclude dead cells. Absolute cell numbers were calculated, with the total cell count multiplied successively by the percentages for the appropriate gates obtained through analysis in FlowJo Software (FlowJo, Ashland, OR, USA). Antibody conjugates were used for extracellular staining and purchased from BioLegend (San Diego, CA, USA): anti-CD11b (Alexa Fluor 700); anti-F4/80 (Brilliant Violet 605), anti-Ly6G (PER-CP), anti-CD45 (Brilliant Violet 510), anti-Ly6C (Brilliant Violet 421), and anti-CD68 (APC).
Statistical analysis
Statistical comparisons were performed by using either a Student’s t test or 1-way ANOVA followed by Tukey’s multiple comparisons for the data following normal distribution. A Kruskal-Wallis test was used when the data followed a nonnormal distribution. For post hoc analysis, a Mann-Whitney U test was used with Bonferroni-Holm correction. The stenosis rates were compared by using Fisher’s exact test. Survival proportions were compared by using a log-rank test with Bonferroni-Holm correction. Values of P < 0.05 were considered significant. Graphed data are presented as means ± sd or sem.
RESULTS
The population of Ly6C+hi monocytes infiltrate the TEVG in a CCR2-dependent manner
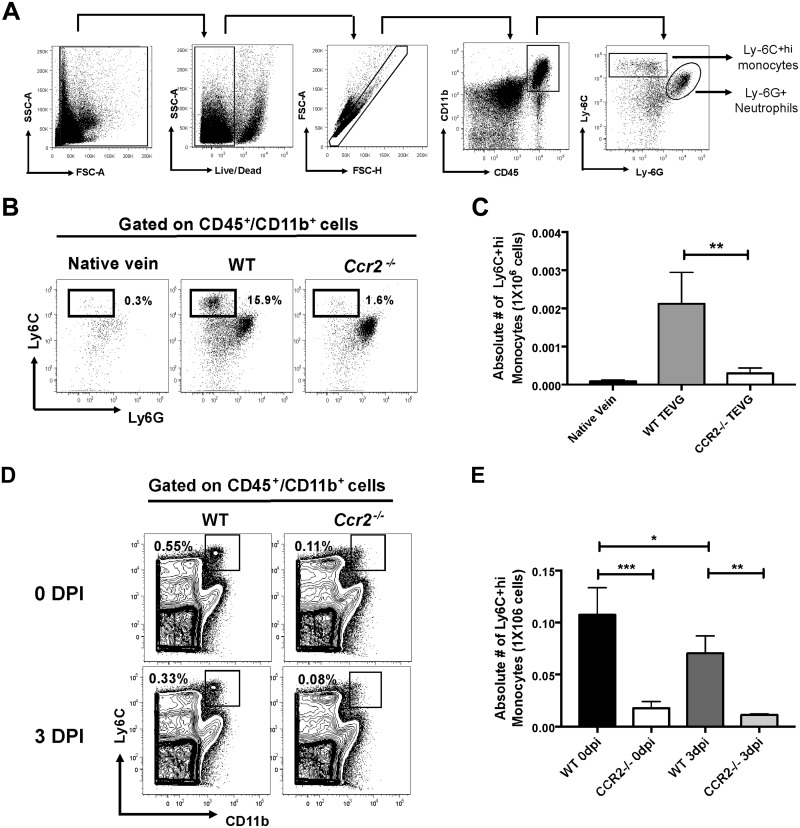
Previously, we showed that MNCs residing in TEVGs have a fundamental role in promoting neovessel formation (6–8). Thus, understanding the origin and recruitment of infiltrating monocytes in the biodegradable scaffold is crucial to modulate tissue remodeling and potential stenosis after TEVG implantation. To this end, we implanted TEVGs as IVC interposition grafts, in both WT and CC chemokine receptor 2 knockout (Ccr2−/−) mice. Because CCR2 has an essential role in monocyte egress from the BM into the blood (17, 18), this strategy allowed us to evaluate the contribution of the BM to the population of TEVG-infiltrating monocytes. Using flow cytometry, we first identified a monocyte population expressing CD45+/CD11b+/Ly6G−/Ly-6C+hi (hereafter, Ly-6C+hi) (Fig. 1A) in TEVGs from WT mice at 3 d postimplantation. The number of Ly-6C+hi monocytes was drastically reduced in TEVGs (P < 0.01) (Fig. 1B, C) and spleens (P < 0.001) (Fig. 1D, E) from Ccr2−/− mice compared with their WT counterparts. These results are agreement with a previous study indicating a role for the BM as a crucial supplier of monocytes to the spleen (19). Furthermore, the population of splenic Ly6C+hi monocytes in WT mice was significantly reduced from 0 to 3 d postimplantation (P < 0.05), suggesting the early deployment of Ly6C+hi monocytes from the spleen upon TEVG implantation.
Figure 1.
BM-derived monocytes populate the TEVGs. A) Gating strategy from flow cytometric analysis in harvested cells from TEVGs at 3 d postimplantation (DPI). Newly recruited monocytes were identified as LD−/CD45+/CD11b+/Ly-6C+hi/Ly-6G− cells. B, C) Representative dot plots (B) and absolute numbers (C) of Ly-6C+hi monocytes in TEVGs from WT and Ccr2−/− mice at 0 and 3 DPI. D, E) Representative dot plots (D) and absolute numbers (E) of Ly-6C+hi monocytes in spleen from WT and Ccr2−/− mice at 3 DPI. Measn ± sem; n = 6/group. *P < 0.05, **P < 0.01 ***P < 0.001.
The spleen is the major reservoir of TEVG-infiltrating monocytes
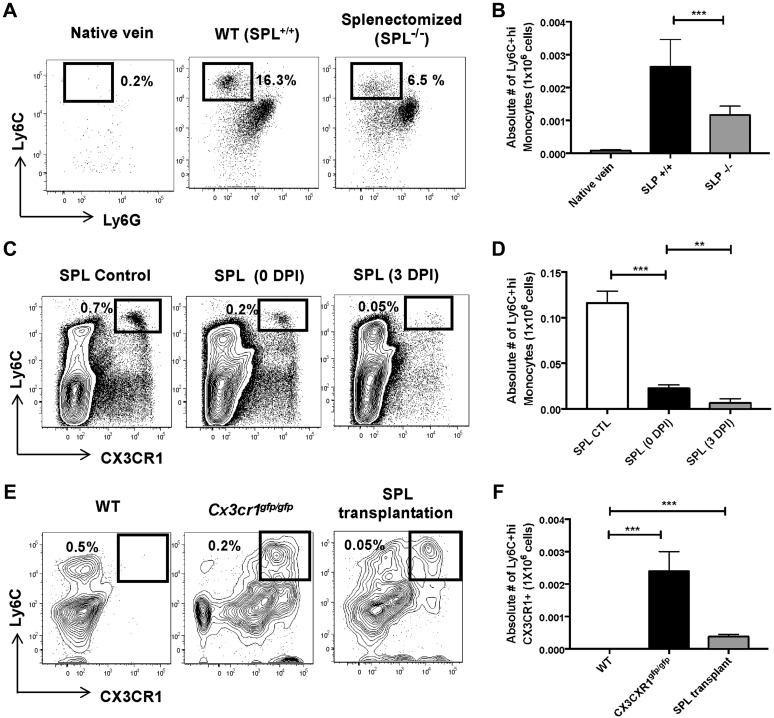
Because our data suggest that Ly6C+hi monocytes egress from the spleen into the circulation during early neotissue formation, we evaluated the contribution of the spleen to the pool of infiltrating monocytes in the biodegradable scaffolds. To achieve this goal, we first implanted TEVGs in WT and splenectomized animals. Native veins from untreated animals were used as negative controls. We found that surgical splenectomy decreased the number of Ly-6C+hi monocytes in TEVGs after 3 d postimplantation (P < 0.001) (Fig. 2A, B). To confirm the ability of splenic Ly-6C+hi monocytes to infiltrate the biodegradable scaffolds, we studied splenectomized WT (CD45.2) mice that received spleens, by transplantation, from transgenic Cx3cr1gfp/gfp (CD45.1) animals (Supplemental Fig. 1A), as previously reported (11). We observed that Ly-6C+hi/CX3CR1+ monocytes in the donor spleens were significantly diminished from 0 to 3 d postimplantation (P < 0.01) (Fig. 2C, D), indicating an early egress of these monocytes from the spleen to circulation. Furthermore, Ly-6C+hi/CX3CR1+ monocytes (Fig. 2E, F), and CD45.1+ cells from donor spleens (Supplemental Fig. 1B, C), were observed in TEVGs from recipient WT mice at 3 d postimplantation. TEVGs and spleens from WT and Cx3cr1gfp/gfp mice were used as negative and positive controls, respectively (Fig. 2E, F and Supplemental Fig. 1B). It is noteworthy that although spleen transplantation in WT mice significantly increased the number of TEVG-infiltrating monocytes compared with splenectomized animals (P < 0.05) (Supplemental Fig. 1D), it did not fully recover the population of Ly6C+hi monocytes observed in TEVGs from untreated controls (P < 0.05). This phenomenon is likely due to the transplantation procedure by itself, which as previously reported (11) and confirmed here, significantly reduces the number of reservoir monocytes from the donor spleen (SPL control vs. SPL 0 d postimplantation, P < 0.01; Fig. 2C, D); consequently, reduced splenic Ly-6C+hi monocytes in the blood (Supplemental Fig. 2A, B) are available to infiltrate the scaffolds. These data corroborate the role of the spleen as a critical source of monocytes in our TEVG model.
Figure 2.
The spleen (SPL) is the primary reservoir of infiltrating monocytes in the TEVGs. A, B) Representative dot plots (A) and absolute numbers (B) of Ly-6C+hi monocytes in TEVGs from WT (SPL+/+) and splenectomized (SPL−/−) animals. C, D) Population of Ly-6C+hi monocytes (C) and their absolute numbers (D) in spleens from CD45.1 CX3CR1gfp/gfp mice before transplantation (SPL control), and 0 or 3 d postimplantation (DPI). E, F) Dot plots (E) and total numbers (F) of Ly6C+hi/CXC3CR1+ monocytes in TEVGs from CD45.2 WT, CD45.1 CX3CR1gfp/gfp, and CD45.2 WT mice receiving a CD45.1 CX3CR1gfp/gfp spleen. Means ± sem; n = 6/group. **P < 0.01, ***P < 0.001.
Short-term administration of losartan reduced macrophage infiltration and stenosis development in TEVGs
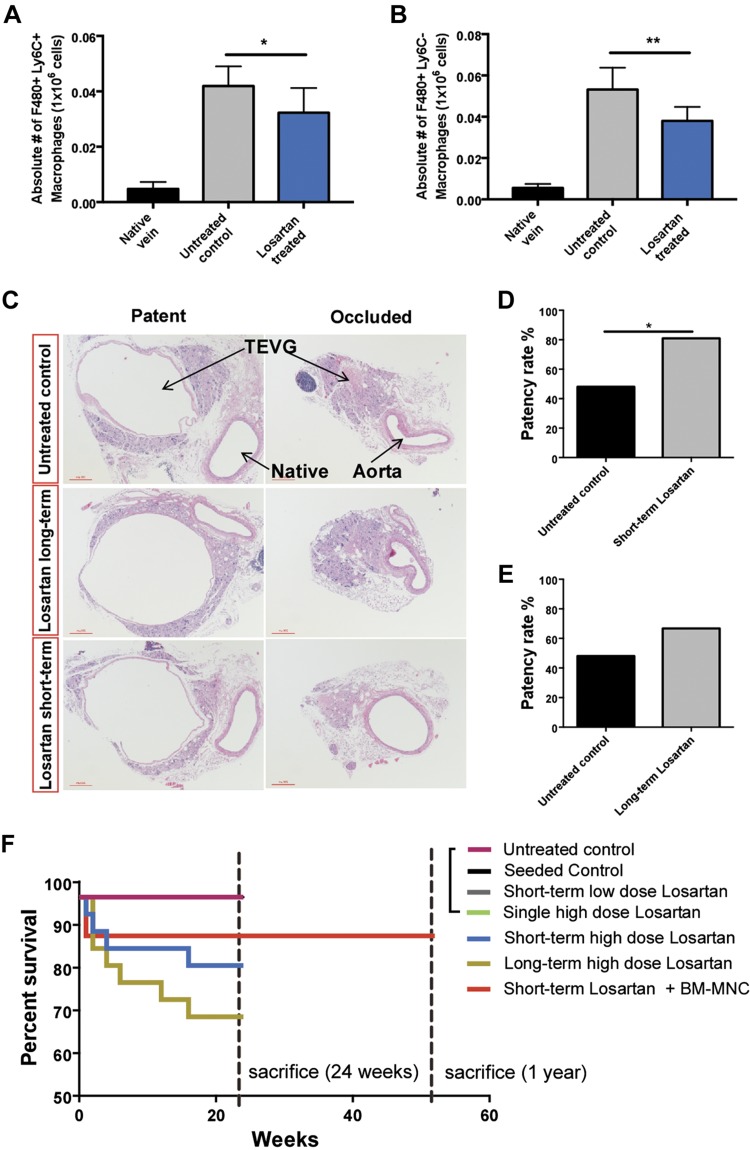
Studies indicate a critical role for angiotensin II in driving monocyte migration from the spleen into inflamed tissues, in an AT-1–receptor dependent manner (11, 20, 21). Therefore, we studied the effect of losartan, an AT-1 receptor antagonist, in the recruitment of MNCs into the TEVG and consequent long-term stenosis. TEVG recipient WT animals were treated either for the short term (2 wk) or for the long term (24-wk) with losartan (0.6 g/L). Using a flow cytometry technique, we first identified 2 macrophage populations in the scaffolds harvested from WT mice at 2 wk postimplantation (Supplemental Fig. 3A). One subset was characterized as F480+/Ly6C−/CD115+low/CD68+hi, and the other expressed F480+/Ly6C+/CD115+hi/CD68+low (referred as F480+/Ly6C− and F480+/Ly6C+) (Supplemental Fig. 3A, B). Mice treated with short-term losartan exhibited significantly reduced macrophages (F480+/Ly6C− and F480+/Ly6C+) in TEVGs compared with untreated animals (P < 0.05; P < 0.01) (Fig. 3A, B and Supplemental Fig. 3C). In addition, macroscopic and ultrasound assessments of both patent and occluded scaffolds (Supplemental Fig. 3D–I) showed that TEVG patency was significantly improved in the short-term–treated group compared with the untreated controls (81% vs. 48%; P < 0.05) (Fig. 3C, D). However, long-term treatment with losartan yielded no significant differences in the patency rate compared with the control group (P < 0.0634) (Fig. 3E) and induced lower survival rates in animals compared with short-term treated and untreated mice (72% vs. 84% and 100%; P < 0.05, P < 0.05, respectively) (Fig. 3F). Our results show that short-term administration of losartan is sufficient to significantly reduce infiltrating macrophages in the scaffold and reduce stenosis without detrimental outcomes caused by prolonged losartan treatment.
Figure 3.
Effect of losartan in MNC infiltration and patency in the TEVG. A, B) Absolute numbers of macrophages Ly6C−/F480+ (A) and Ly6C+/F480+ (B) in native vein and TEVGs from untreated and short-term losartan-treated mice at 2 wk postimplantation (n = 8/group). C) Representative histologic images of TEVGs from untreated control, long-term losartan-treated (6 mo, 0.6 g/L), and short-term losartan-treated (2 wk, 0.6 g/L) mice. D, E) Comparison of the patency rate between untreated, short-term, and long-term losartan-treated groups. F) Percent survival at either 6 mo or 1 yr post–TEVG implantation; n = 25/group. *P < 0.05, **P < 0.01.
Low doses of losartan improve TEVG patency and prevent detrimental side effects in mice
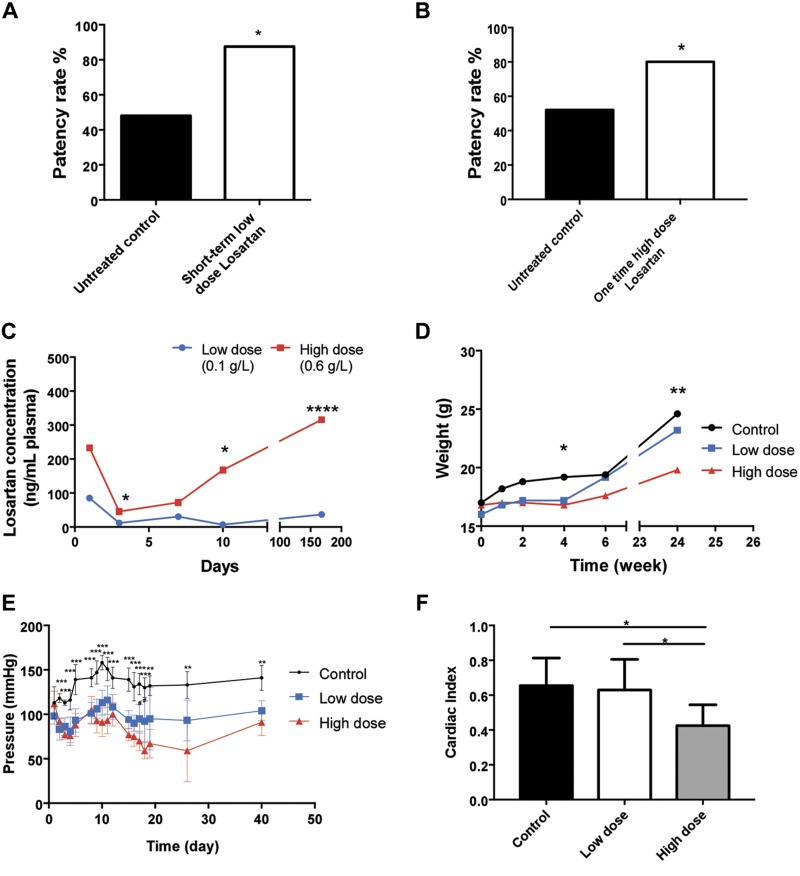
We next evaluated the effect of 2 additional losartan treatment schemas in biodegradable scaffolds. Treated mice received either low doses of losartan (0.1 g/L) for 2 wk or a single high dose of losartan (0.6 g/L) after TEVG implantation. The scaffolds were collected and compared with untreated controls at 6 mo postimplantation. Low-dose–treated mice exhibited a significantly higher patency rate than that of high-dose–treated or untreated mice (87.5% vs. 80% and 48%; P < 0.05, P < 0.05) (Fig. 4A, B). Because we observed adverse effects in mice treated with prolonged high doses of losartan (Fig. 3F), we monitored the physiologic impact of low doses and a single high dose of losartan in TEVG recipient mice. The plasma levels of losartan in the high-dose group initially decreased at 3 d postimplantation and then steadily increased over the 6-mo study period. Losartan concentration in blood from mice treated with low doses remained consistent throughout the study (Fig. 4C). Although high-dose–treated mice exhibited reduced body weight compared with low-dose–treated and untreated animals (P < 0.05, P < 0.05) (Fig. 4D), these 3 cohorts showed comparable survival rates during the course of the study (100% survival rate; Fig. 3F). Both the low-dose and high-dose groups displayed a significant reduction in blood pressure compared with the control group (P < 0.05, P < 0.05) (Fig. 4E). In addition, the high-dose–treated group had a significantly lower cardiac index than the low-dose–treated and untreated cohorts (P < 0.05, P < 0.05; Fig. 4F). These data suggest that low doses of losartan can be used to prevent TEVG stenosis and minimize detrimental outcomes due to drug toxicities.
Figure 4.
Low-dose and 1-time high-dose losartan treatments on TEVG patency and their physiologic effects in mice. A, B) Comparison of TEVG patency between the untreated control and either low-dose losartan treatment (2–wk, 0.1 g/L) (A) or 1-time high-dose losartan (0.6 g/L) (B). C) Comparison of losartan concentration in plasma between the low-dose and high-dose losartan groups. D) Weight change comparison among the control, low-dose, and high-dose losartan groups. E) Mean arterial pressure comparison among the control, low-dose, and high-dose losartan groups. F) Cardiac output comparison among the control, low-dose, and high-dose losartan groups. Untreated control and 1-time high-dose losartan groups (n = 25); short-term low-dose losartan group (n = 8). *P < 0.05, **P < 0.01, ***P < 0.001.
The combination of BM-MNC seeding and losartan treatment completely prevents TEVG stenosis
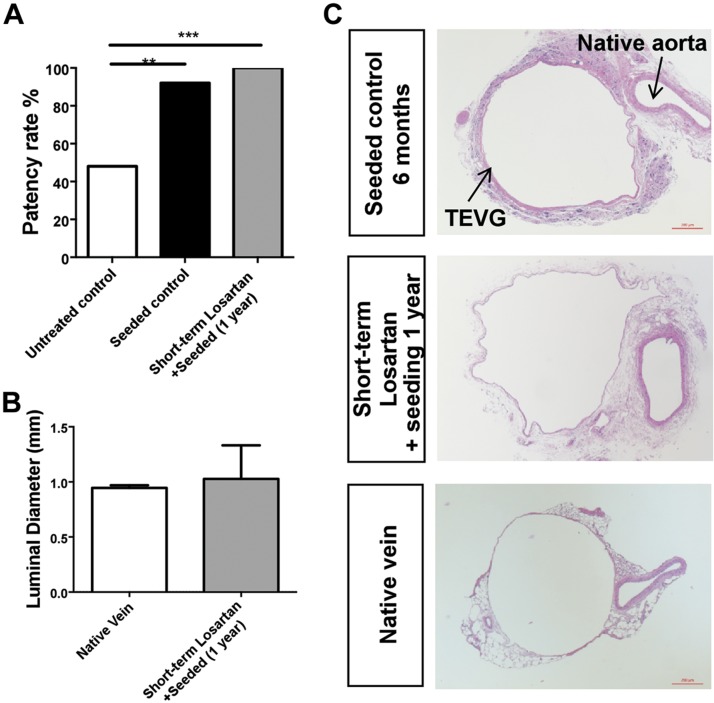
BM-MNC seeding is currently used in our laboratory to reduce the incidence of TEVG stenosis. However, alternative strategies are required to further reduce the incidence of TEVG stenosis. To evaluate whether the combination of losartan treatment and BM-MNC seeding can prevent stenosis in biodegradable scaffolds, we administered losartan for a short-term period (0.6 g/L, for 2 wk) to animals with implanted BM-MNC seeded grafts. Implanted TEVGs were collected after 1-yr postimplantation to investigate late-term patency and stenosis. Both seeded and unseeded scaffold groups were harvested at 6 mo and used as positive and negative controls, respectively (n = 25). Although seeded and unseeded scaffolds showed 92 and 48% patency, respectively, the combination of both losartan treatment and cell seeding resulted in a 100% patency rate (Fig. 5A). The luminal diameter in the group of combined treatments is similar to that of the native vein (Fig. 5B, C). In addition, seeded scaffolds obtained from losartan-treated mice display a thin wall composed of a monolayer of endothelial cells, concentric layers of smooth muscle cells, and an outer layer of fibroblasts (Fig. 5C). This evidence indicates that the combined effect of short-term losartan treatment and BM-MNC seeding can be an effective method to prevent TEVG stenosis.
Figure 5.
Combined effect of BM-MNC seeding and losartan treatment. A) Comparison of the patency rate among the untreated control (6 mo), seeded control (6 mo), and short-term losartan with BM-MNC seeding (1 yr) groups. B) Comparison of luminal diameter between the native vein and short-term losartan with BM-MNC seeding. C) Representative histologic images of the seeded control, short-term losartan treatment with BM-MNC seeding, and native vein. Untreated control and seeded control (n = 25); short-term losartan with BM-MNC seeding (n = 21). **P < 0.01, ***P < 0.001.
DISCUSSION
Although different materials have been implemented to develop functional biologic vessels, many of these approaches have been limited by various complications such as infection, calcification, foreign body reaction, stenosis, and lack of growth potential [reviewed in Palumbo et al. (22)]. Our long-term goal is to improve outcomes for children requiring congenital heart surgery by utilizing tissue engineering methods to create vascular grafts, patches, and replacement heart valves with a capacity to grow, thus eliminating the risk of somatic overgrowth (the process by which a patient outgrows his or her prosthetic graft). We have previously shown that the TEVG was the first human-made vascular conduit, with growth potential, in patients with congenital heart defects. However, their long-term efficacy is limited by stenosis (3, 4). We therefore focused our efforts on elucidating the mechanisms of TEVG neotissue formation and stenosis development in the hopes of rationally designing a translational strategy for inhibiting the formation of TEVG stenosis in clinical applications. We have shown that the TEVG neovessel formation process is an inflammatory mediated regenerative event, in which host monocyte-derived macrophages are critical in tissue remodeling. By partially inhibiting monocyte infiltration into the TEVG scaffold, neovessel formation was impeded (8). It is thus critical to regulate MNC infiltration into the TEVG to have sufficient neotissue formation without the development of stenosis.
The present study sought to prevent TEVG stenosis by regulating monocyte cell infiltration into the biodegradable scaffold. We first identified the monocyte population residing in the implanted grafts during early (3 d postimplantation) neotissue formation. We observed that inflammatory Ly6C+hi monocytes infiltrate the TEVG in a CCR2-dependent manner. Because CCR2 is not involved in the migration of Ly6C+hi monocytes from the blood into tissues but is required for Ly6C+hi monocytes to exit from the BM into the blood (17, 18), these data suggest that TEVG-infiltrating monocytes originate from the BM. In addition, spleens from Ccr2−/− mice showed reduced Ly6C+hi monocytes compared with their WT counterparts. This phenomenon is in agreement with previous reports highlighting the importance of BM as a source of Ly6C+hi monocytes for the spleen (19). Furthermore, the population of splenic monocytes in WT mice decreased upon TEVG implantation, suggesting the mobilization of Ly6C+hi monocytes from the spleen into the TEVGs. This hypothesis was shown by using WT and splenectomized animals. Surgical splenectomy markedly reduced the pool of TEVG-infiltrating monocytes, indicating a role for the spleen as the primary monocyte reservoir in our TEVG model. The ability of splenic monocytes to migrate into implanted scaffolds was corroborated by using splenectomized WT (CD45.2) animals that were given, by transplantation, spleens from CD45.1 Cx3cr1gfp/gfp mice, in which nearly all green fluorescent protein-positive splenocytes are monocytes or their lineage descendants, as previously reported (11). We observed that Ly6C+hi/Cx3cr1gf/gfp monocytes from donor spleens infiltrated TEVGs from recipient WT mice as soon as 3 d postimplantation. Thus, these findings indicate that splenic monocytes are able to migrate from the spleen into biodegradable scaffolds during early neovessel formation.
Compelling studies have indicated an important contribution of spleen-derived monocytes to the pathology observed in experimental models of atherosclerosis (23, 24), stroke (25, 26), and myocardial infarction (24, 26, 27). The angiotensin II/AT-1 signaling pathway has been shown to participate in the rapid deployment of Ly6C+hi monocytes from the spleen (11, 20, 21). We therefore evaluated the effect of an AT-1 receptor blocker, losartan, on MNC infiltration and development of TEVG stenosis. Both short- and long-term treatments with high doses of losartan (0.6 g/L) significantly reduced the populations of F480+/Ly6C− and F480+/Ly6C+ macrophages in TEVGs at 2 wk postimplantation. The expression of both Ly6C and CD115 in F480+/Ly6C+ macrophages suggest that these phagocytes derive from newly recruited monocytes whereas the expression of CD68 and down-regulation of Ly6C in the subset of F480+/Ly6C−macrophages indicate that these are fully differentiated macrophages in the tissue. Interestingly, the short-term treatment with losartan improved TEVG patency compared with the untreated group. In contrast, mice treated with long-term losartan exhibited comparable TEVG patency and significantly reduced survival rates vs. untreated controls. The high dose of losartan (0.6 g/L, equivalent to 50–60 mg/kg) was initially selected because this concentration has been shown to be effective in reversing aortic root dilation in rodent models of Marfan syndrome (12, 13, 28, 29). In addition, a recent clinical trial explored the safety and efficacy of losartan in patients aged 6 mo to 6 yr (30). They used a dosage scheme of 0.1 mg/kg/d (low), 0.3 mg/kg/d (medium), or 0.7 mg/kg/d (high). Importantly, across all dosage ranges, there was a very low incidence of adverse drug–related events (1/99 with hypotension, 1/99 with a decrease in the glomerular filtration rate, and 2/99 with laboratory derangements). These findings suggest that losartan is better tolerated in humans than in mice. Thus, we subsequently treated mice for a short term with low doses of losartan (0.1 g/L, 2-wk), or a single high dose (0.6 g/L), to evaluated their side effects and influence in TEVG patency. Although administration of a single high dose of losartan lowered blood pressure and the cardiac index compared with low-dose treatment, both treatments showed 100% survival rates and significantly improved TEVG patency compared with untreated controls 6 mo postimplantation.
It is noteworthy that although a positive effect for low and high doses of losartan in preventing TEVG stenosis is associated with reduced infiltration of inflammatory monocytes into the scaffold, we cannot disregard the contribution of other mechanisms that have been reported for losartan. An antifibrotic role has been shown for losartan in reducing the TGF-β signaling pathway (31), collagen deposition (32, 33), and production of metalloproteinases (34). In addition, losartan decreases vasoconstriction (35, 36) and favors vasodilation via direct abrogation of the angiotensin II/AT-1 signaling pathway in smooth muscle cells, a critical pathway in smooth muscle cell constriction (37, 38). The contribution of these mechanisms in preventing TEVG stenosis is currently under investigation in our laboratory.
Interestingly, we observed that the combined effect of BM-MNC seeding and short-term losartan treatment fully prevented TEVG stenosis at 1 yr after TEVG implantation. We understand that although there are many similarities between the IVC and extracardiac Fontan circuit, there are also important anatomic and hemodynamic differences between human and mouse, which could confound our results. Thus, larger animal models such as sheep, currently under investigation in our laboratory, or pigs, such as the one used by Buscemi et al. (39), can be an important tool to test new materials and therapies that may lead to improved vascular grafts. Despite these limitations, the FDA accepts the mouse model for preclinical evaluation of safety and efficacy of Fontan conduits. We believe these findings can support clinical investigation, which will help us in the design of an improved TEVG to address asymptomatic stenosis in larger animal models and potentially in humans. Based on these results, we contend that it would be reasonable to translate this discovery from the bench to the bedside.
Losartan is FDA approved and has an excellent safety profile in our target patient population, pediatric Fontan patients. Although the FDA initially only approved losartan in 1995 for use in adult patients, losartan has gained FDA approval for patients aged >6 yr with hypertension (40). In addition, the pharmacology of losartan leads to minimal interaction with other drugs; specifically, there is no interaction with digoxin, warfarin, furosemide, and hydrochlorothiazide, all of which may be used for patients after completion of Fontan surgery. Although our data suggest that short-term, low-dose treatment with losartan inhibits the formation of TEVG stenosis, the dose and duration of therapy needed clinically must be determined. Because our previous studies in our mouse model showed that the initial 2 wk after TEVG implantation is a critical period for the development TEVG stenosis, we suggest beginning with a 2-wk course of treatment at an initial dose of 0.7 mg/kg/d coupled with careful screening for drug-related toxicity and early discontinuation of therapy in the event of a drug-related complication. We believe these data support clinical investigation at this juncture.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors acknowledge Brendan Radel, Mellissa Mauntel, and Ekene Onwuka (all from the Tissue Engineering Center, Nationwide Children’s Hospital) for their contributions on weight change, blood pressure, plasma level of losartan measurements, and preparation of the histologic samples and surgical procedures. This research was supported by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (Grants R01-HL098228 and R01-HL128847 to C.K.B.). S.P.-S. was supported by NIH National Institute of Allergy and Infectious Diseases Grants R01AI092117 and R21AI120013. J.D.D.R.-R. and D.M.-S. received support from Consejo Nacional de Ciencia y Tecnología (CONACYT). C.K.B. and T.S. have received grant support from the Pall Corp. and Gunze Ltd. None of the work presented was funded by Gunze Ltd. or Pall Corp. The authors declare no conflicts of interest.
Glossary
- AT-1
angiotensin II type 1
- BM
bone marrow
- BM-MNC
bone marrow–derived mononuclear cell
- CCR2
C-C motif chemokine receptor type 2
- CX3CR1
C-X3-C motif chemokine receptor 1
- IVC
inferior vena cava
- FACS
fluorescence-activated cell sorting
- FDA
U.S. Food and Drug Administration
- MNC
mononuclear cell
- RPMI
Rosewell Park Memorial Institute
- TEVG
tissue-engineered vascular graft
- WT
wild type
- Ly6C
lymphocyte antigen 6 complex
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. S. Pober, S. Partida-Sanchez, and C. K. Breuer designed the research; J. D. D. Ruiz-Rosado, Y.-U. Lee, N. Mahler, T. Yi, F. Robledo-Avila, D. Martinez-Saucedo, T. Shoji, E. Heuer, and A. R. Yates performed the research; J. D. D. Ruiz-Rosado, Y.-U. Lee, and A. Y. Lee analyzed the data; J. D. D. Ruiz-Rosado and Y.-U. Lee wrote the paper; and T. Shinoka and S. Partida-Sanchez contributed new reagents or analytic tools.
REFERENCES
- 1.Shin’oka T., Imai Y., Ikada Y. (2001) Transplantation of a tissue-engineered pulmonary artery. N. Engl. J. Med. 344, 532–533 [DOI] [PubMed] [Google Scholar]
- 2.Naito Y., Imai Y., Shin’oka T., Kashiwagi J., Aoki M., Watanabe M., Matsumura G., Kosaka Y., Konuma T., Hibino N., Murata A., Miyake T., Kurosawa H. (2003) Successful clinical application of tissue-engineered graft for extracardiac Fontan operation. J. Thorac. Cardiovasc. Surg. 125, 419–420 [DOI] [PubMed] [Google Scholar]
- 3.Shin’oka T., Matsumura G., Hibino N., Naito Y., Watanabe M., Konuma T., Sakamoto T., Nagatsu M., Kurosawa H. (2005) Midterm clinical result of tissue-engineered vascular autografts seeded with autologous bone marrow cells. J. Thorac. Cardiovasc. Surg. 129, 1330–1338 [DOI] [PubMed] [Google Scholar]
- 4.Hibino N., McGillicuddy E., Matsumura G., Ichihara Y., Naito Y., Breuer C., Shinoka T. (2010) Late-term results of tissue-engineered vascular grafts in humans. J. Thorac. Cardiovasc. Surg. 139, 431–436, 436.e1–436.e2 [DOI] [PubMed] [Google Scholar]
- 5.Roh J. D., Nelson G. N., Brennan M. P., Mirensky T. L., Yi T., Hazlett T. F., Tellides G., Sinusas A. J., Pober J. S., Saltzman W. M., Kyriakides T. R., Breuer C. K. (2008) Small-diameter biodegradable scaffolds for functional vascular tissue engineering in the mouse model. Biomaterials 29, 1454–1463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roh J. D., Sawh-Martinez R., Brennan M. P., Jay S. M., Devine L., Rao D. A., Yi T., Mirensky T. L., Nalbandian A., Udelsman B., Hibino N., Shinoka T., Saltzman W. M., Snyder E., Kyriakides T. R., Pober J. S., Breuer C. K. (2010) Tissue-engineered vascular grafts transform into mature blood vessels via an inflammation-mediated process of vascular remodeling. Proc. Natl. Acad. Sci. USA 107, 4669–4674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hibino N., Villalona G., Pietris N., Duncan D. R., Schoffner A., Roh J. D., Yi T., Dobrucki L. W., Mejias D., Sawh-Martinez R., Harrington J. K., Sinusas A., Krause D. S., Kyriakides T., Saltzman W. M., Pober J. S., Shin’oka T., Breuer C. K. (2011) Tissue-engineered vascular grafts form neovessels that arise from regeneration of the adjacent blood vessel. FASEB J. 25, 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hibino N., Yi T., Duncan D. R., Rathore A., Dean E., Naito Y., Dardik A., Kyriakides T., Madri J., Pober J. S., Shinoka T., Breuer C. K. (2011) A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. FASEB J. 25, 4253–4263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y. U., de Dios Ruiz-Rosado J., Mahler N., Best C. A., Tara S., Yi T., Shoji T., Sugiura T., Lee A. Y., Robledo-Avila F., Hibino N., Pober J. S., Shinoka T., Partida-Sanchez S., Breuer C. K. (2016) TGF-β receptor 1 inhibition prevents stenosis of tissue-engineered vascular grafts by reducing host mononuclear phagocyte activation. FASEB J. 30, 2627–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y. U., Yi T., Tara S., Lee A. Y., Hibino N., Shinoka T., Breuer C. K. (2014) Implantation of inferior vena cava interposition graft in mouse model. J. Vis. Exp. 86, 1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Swirski F. K., Nahrendorf M., Etzrodt M., Wildgruber M., Cortez-Retamozo V., Panizzi P., Figueiredo J. L., Kohler R. H., Chudnovskiy A., Waterman P., Aikawa E., Mempel T. R., Libby P., Weissleder R., Pittet M. J. (2009) Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science 325, 612–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Habashi J. P., Judge D. P., Holm T. M., Cohn R. D., Loeys B. L., Cooper T. K., Myers L., Klein E. C., Liu G., Calvi C., Podowski M., Neptune E. R., Halushka M. K., Bedja D., Gabrielson K., Rifkin D. B., Carta L., Ramirez F., Huso D. L., Dietz H. C. (2006) Losartan, an AT1 antagonist, prevents aortic aneurysm in a mouse model of Marfan syndrome. Science 312, 117–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holm T. M., Habashi J. P., Doyle J. J., Bedja D., Chen Y., van Erp C., Lindsay M. E., Kim D., Schoenhoff F., Cohn R. D., Loeys B. L., Thomas C. J., Patnaik S., Marugan J. J., Judge D. P., Dietz H. C. (2011) Noncanonical TGFβ signaling contributes to aortic aneurysm progression in Marfan syndrome mice. Science 332, 358–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lim D. S., Lutucuta S., Bachireddy P., Youker K., Evans A., Entman M., Roberts R., Marian A. J. (2001) Angiotensin II blockade reverses myocardial fibrosis in a transgenic mouse model of human hypertrophic cardiomyopathy. Circulation 103, 789–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chu K. Y., Lau T., Carlsson P. O., Leung P. S. (2006) Angiotensin II type 1 receptor blockade improves beta-cell function and glucose tolerance in a mouse model of type 2 diabetes. Diabetes 55, 367–374 [DOI] [PubMed] [Google Scholar]
- 16.Daugherty A., Rateri D., Hong L., Balakrishnan A. (2009) Measuring blood pressure in mice using volume pressure recording, a tail-cuff method. J. Vis. Exp. 27, 1–2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Serbina N. V., Pamer E. G. (2006) Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 [DOI] [PubMed] [Google Scholar]
- 18.Tsou C. L., Peters W., Si Y., Slaymaker S., Aslanian A. M., Weisberg S. P., Mack M., Charo I. F. (2007) Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J. Clin. Invest. 117, 902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shand F. H., Ueha S., Otsuji M., Koid S. S., Shichino S., Tsukui T., Kosugi-Kanaya M., Abe J., Tomura M., Ziogas J., Matsushima K. (2014) Tracking of intertissue migration reveals the origins of tumor-infiltrating monocytes. Proc. Natl. Acad. Sci. USA 111, 7771–7776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi C., Pamer E. G. (2011) Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 11, 762–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leuschner F., Panizzi P., Chico-Calero I., Lee W. W., Ueno T., Cortez-Retamozo V., Waterman P., Gorbatov R., Marinelli B., Iwamoto Y., Chudnovskiy A., Figueiredo J. L., Sosnovik D. E., Pittet M. J., Swirski F. K., Weissleder R., Nahrendorf M. (2010) Angiotensin-converting enzyme inhibition prevents the release of monocytes from their splenic reservoir in mice with myocardial infarction. Circ. Res. 107, 1364–1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palumbo V. D., Bruno A., Tomasello G., Damiano G., Lo Monte A. I. (2014) Bioengineered vascular scaffolds: the state of the art. Int. J. Artif. Organs 37, 503–512 [DOI] [PubMed] [Google Scholar]
- 23.Potteaux S., Ait-Oufella H., Mallat Z. (2015) Role of splenic monocytes in atherosclerosis. Curr. Opin. Lipidol. 26, 457–463 [DOI] [PubMed] [Google Scholar]
- 24.Dutta P., Courties G., Wei Y., Leuschner F., Gorbatov R., Robbins C. S., Iwamoto Y., Thompson B., Carlson A. L., Heidt T., Majmudar M. D., Lasitschka F., Etzrodt M., Waterman P., Waring M. T., Chicoine A. T., van der Laan A. M., Niessen H. W., Piek J. J., Rubin B. B., Butany J., Stone J. R., Katus H. A., Murphy S. A., Morrow D. A., Sabatine M. S., Vinegoni C., Moskowitz M. A., Pittet M. J., Libby P., Lin C. P., Swirski F. K., Weissleder R., Nahrendorf M. (2012) Myocardial infarction accelerates atherosclerosis. Nature 487, 325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim E., Yang J., Beltran C. D., Cho S. (2014) Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J. Cereb. Blood Flow Metab. 34, 1411–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leuschner F., Rauch P. J., Ueno T., Gorbatov R., Marinelli B., Lee W. W., Dutta P., Wei Y., Robbins C., Iwamoto Y., Sena B., Chudnovskiy A., Panizzi P., Keliher E., Higgins J. M., Libby P., Moskowitz M. A., Pittet M. J., Swirski F. K., Weissleder R., Nahrendorf M. (2012) Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J. Exp. Med. 209, 123–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Van der Laan A. M., Ter Horst E. N., Delewi R., Begieneman M. P., Krijnen P. A., Hirsch A., Lavaei M., Nahrendorf M., Horrevoets A. J., Niessen H. W., Piek J. J. (2014) Monocyte subset accumulation in the human heart following acute myocardial infarction and the role of the spleen as monocyte reservoir. Eur. Heart J. 35, 376–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brooke B. S., Habashi J. P., Judge D. P., Patel N., Loeys B., Dietz H. C., III (2008) Angiotensin II blockade and aortic-root dilation in Marfan’s syndrome. N. Engl. J. Med. 358, 2787–2795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Habashi J. P., Doyle J. J., Holm T. M., Aziz H., Schoenhoff F., Bedja D., Chen Y., Modiri A. N., Judge D. P., Dietz H. C. (2011) Angiotensin II type 2 receptor signaling attenuates aortic aneurysm in mice through ERK antagonism. Science 332, 361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Webb N. J., Wells T. G., Shahinfar S., Massaad R., Dankner W. M., Lam C., Santoro E. P., McCrary Sisk C., Blaustein R. O. (2014) A randomized, open-label, dose-response study of losartan in hypertensive children. Clin. J. Am. Soc. Nephrol. 9, 1441–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nataatmadja M., West J., Prabowo S., West M. (2013) Angiotensin II receptor antagonism reduces transforming growth factor beta and Smad signaling in thoracic aortic aneurysm. Ochsner J. 13, 42–48 [PMC free article] [PubMed] [Google Scholar]
- 32.Ju H., Zhao S., Jassal D. S., Dixon I. M. (1997) Effect of AT1 receptor blockade on cardiac collagen remodeling after myocardial infarction. Cardiovasc. Res. 35, 223–232 [DOI] [PubMed] [Google Scholar]
- 33.Varo N., Etayo J. C., Zalba G., Beaumont J., Iraburu M. J., Montiel C., Gil M. J., Monreal I., Díez J. (1999) Losartan inhibits the post-transcriptional synthesis of collagen type I and reverses left ventricular fibrosis in spontaneously hypertensive rats. J. Hypertens. 17, 107–114 [DOI] [PubMed] [Google Scholar]
- 34.Guo Y. S., Wu Z. G., Yang J. K., Chen X. J. (2015) Impact of losartan and angiotensin II on the expression of matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 in rat vascular smooth muscle cells. Mol. Med. Rep. 11, 1587–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Baan J., Jr., Chang P. C., Vermeij P., Pfaffendorf M., van Zwieten P. A. (1996) Effects of losartan on vasoconstrictor responses to angiotensin II in the forearm vascular bed of healthy volunteers. Cardiovasc. Res. 32, 973–979 [PubMed] [Google Scholar]
- 36.Gottlieb S. S., Dickstein K., Fleck E., Kostis J., Levine T. B., LeJemtel T., DeKock M. (1993) Hemodynamic and neurohormonal effects of the angiotensin II antagonist losartan in patients with congestive heart failure. Circulation 88, 1602–1609 [DOI] [PubMed] [Google Scholar]
- 37.Sayeski P. P., Bernstein K. E. (2001) Signal transduction mechanisms of the angiotensin II type AT(1)-receptor: looking beyond the heterotrimeric G protein paradigm. J. Renin Angiotensin Aldosterone Syst. 2, 4–10 [DOI] [PubMed] [Google Scholar]
- 38.Xu F., Mao C., Hu Y., Rui C., Xu Z., Zhang L. (2009) Cardiovascular effects of losartan and its relevant clinical application. Curr. Med. Chem. 16, 3841–3857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Buscemi S., Palumbo V. D., Maffongelli A., Fazzotta S., Palumbo F. S., Licciardi M., Fiorica C., Puleio R., Cassata G., Fiorello L., Buscemi G., Lo Monte A. I. (2017) Electrospun PHEA-PLA/PCL scaffold for vascular regeneration: a preliminary in vivo evaluation. Transplant. Proc. 49, 716–721 [DOI] [PubMed] [Google Scholar]
- 40.(2015) Cozaar package insert, Merck & Co., Inc., Whitehouse Station, New Jersey [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.