Abstract
Peptidylarginine deiminase 4 (PAD4) is a nuclear citrullinating enzyme that is critically involved in the release of decondensed chromatin from neutrophils as neutrophil extracellular traps (NETs). NETs, together with fibrin, are implicated in host defense against pathogens; however, the formation of NETs (NETosis) has injurious effects that may outweigh their protective role. For example, PAD4 activity produces citrullinated neoantigens that promote autoimmune diseases, such as rheumatoid arthritis, to which PAD4 is genetically linked and where NETosis is prominent. NETs are also generated in basic sterile inflammatory responses that are induced by many inflammatory stimuli, including cytokines, hypoxia, and activated platelets. Mice that lack PAD4—deficient in NETosis—serve as an excellent tool with which to study the importance of NETs in disease models. In recent years, animal and human studies have demonstrated that NETs contribute to the etiology and propagation of many common noninfectious diseases, the focus of our review. We will discuss the role of NETs in thrombotic and cardiovascular disease, the induction of NETs by cancers and its implications for cancer progression and cancer-associated thrombosis, and elevated NETosis in diabetes and its negative impact on wound healing, and will propose a link between PAD4/NETs and age-related organ fibrosis. We identify unresolved issues and new research directions.—Wong, S. L., Wagner, D. D. Peptidylarginine deiminase 4: a nuclear button triggering neutrophil extracellular traps in inflammatory diseases and aging.
Keywords: NETs, PAD4, thrombosis, cancer, diabetes
Neutrophils are crucial for protecting us from infection in injury. Like an army, they arrive to the site of microbial invasion or inflammation. Although indispensable for our survival, their passage leaves behind significant collateral damage. The most recently recognized—and perhaps the most destructive weapon of neutrophils—is the release of nuclear chromatin lined with toxic proteins, called neutrophil extracellular traps (NETs). Peptidylarginine deiminase (PAD) 4 is the key enzyme that orchestrates the nuclear explosion of NETs. By reducing the charge of histones, it breaks up nucleosomes, thus unwinding chromatin (1). NET production was first described in 2004 to be induced by bacteria at sites of infection (2). Pathogens were thus documented to be excellent stimulants of NET release (NETosis) (3); however, as soon as the following year (2005), the production of NETs was observed in the sterile pathologic process of preeclampsia, a disease of human pregnancy (4). In preeclampsia, levels of plasma cell-free DNA were already known to correlate with disorder severity (5). The discovery of NETs explained its origin.
The presence of extracellular DNA in many other inflammatory and thrombotic conditions has since been identified to be a result, at least in part, of NETosis. The important role that DNases play in breaking up this life-threatening product of NETosis was recently made evident (6).
In this review, we briefly introduce PAD4 and NETosis, and focus on examples of major noninfectious diseases for which targeting NETs or their generation would likely be highly beneficial to the patient. The role of NETs in infection was recently reviewed by others (7, 8).
INTRODUCTION TO NETosis AND PAD4
NETosis, distinct from necrosis and apoptosis (9), involves chromatin decondensation before its release (1, 10). PAD4, which is expressed mainly in granulocytes and cancer cells (11, 12), is a crucial enzyme in NETosis (13, 14). Among the 5 PADs in the family, PAD4 is the only isoform that contains a nuclear localization sequence located at the N terminus of the protein (15), thus allowing its translocation into the nucleus. PAD4 changes positively charged arginine to neutral citrulline on histones, thereby loosening the interaction among histones and between histones and DNA, which allows tightly packed chromatin to unravel (1). Ammonia is generated as a byproduct of the citrullination reaction (16). Citrullinated histone H3 (H3Cit) and H4 (H4Cit) are useful biomarkers for the presence of NETs in plasma and tissue sections. Neutrophil elastase (17, 18), together with other proteases (19), contributes to NETosis by cleaving histones to unfold chromatin. Amulic et al. (20) recently demonstrated that neutrophils can hijack cell-cycle machineries for NETosis, including the activation of cyclin-dependent kinases 4/6 and phosphorylation of lamins, that could be involved in the breakdown of the nuclear envelope. Other signaling molecules, such as Rac small GTPases and the p21-activated kinases (21, 22), also modulate NETosis, but many more players are yet to be discovered. NETosis typically occurs within 1–4 h after neutrophil stimulation in vitro. Neutrophils delobulate their multilobular nuclei, decondense their chromatin, and expel nuclear contents into the cytosol, where nuclear, granular, and cytosolic proteins are mixed before plasma membrane rupture and NET release (Fig. 1) (9). Not all neutrophils release NETs with the same propensity. The proinflammatory activity of neutrophils increases while they age in the circulation, as does their capacity to release NETs (23). Under inflammatory conditions, such as lupus (24–26) and infection (27, 28), a subset of neutrophils—termed low-density granulocytes—cosegregates with peripheral blood mononuclear cells after density-gradient separation. These cells are also highly susceptible to NETosis (25, 26).
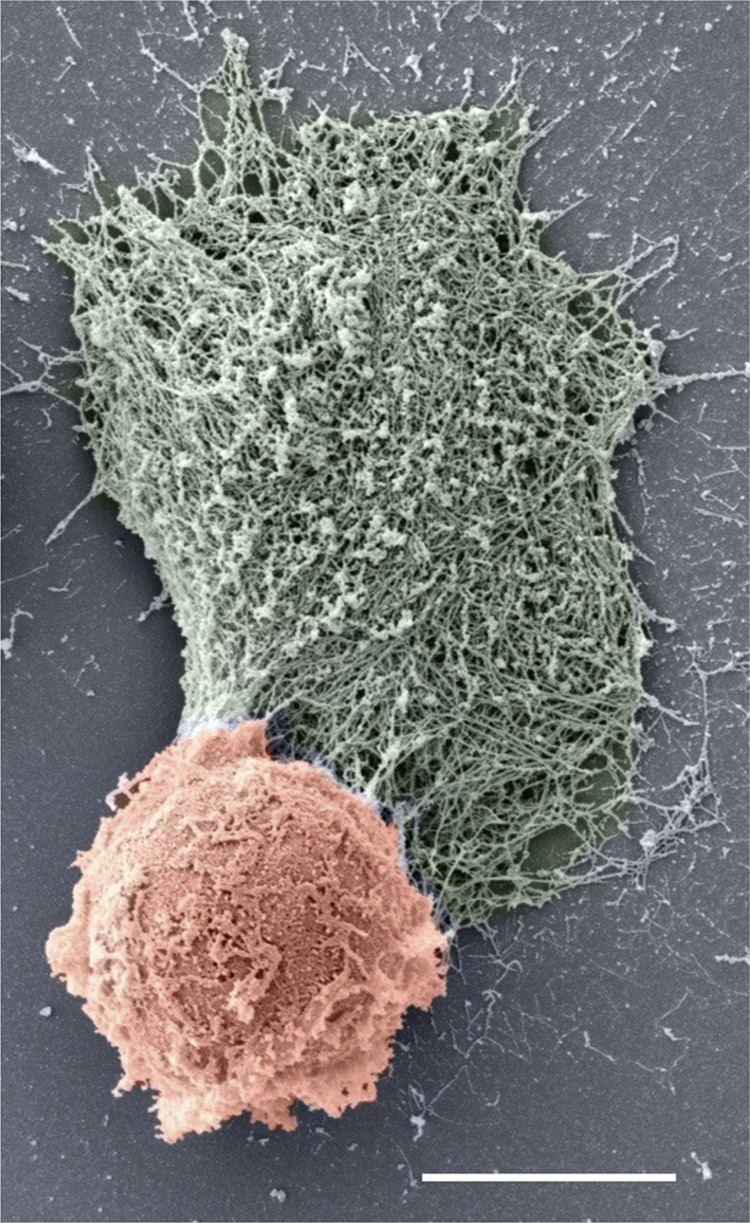
Figure 1.
Spontaneous NETosis in a neutrophil that was isolated from a patient with RA. Scanning electron micrograph shows a NETting neutrophil (pseudocolored in red). The NET (pseudocolored in green) appears as a large web-like structure emanating from a break in the plasma membrane. Scale bar, 5 μm. Image courtesy of Chanchal Sur Chowdhury (Center for Microscopy, University of Basel, Basel, Switzerland).
An alternative mechanism of NET formation, called vital NETosis, has been more recently observed in both mice and humans during infection (29). Vital NETosis happens in vivo within minutes postneutrophil activation through decondensed chromatin release via vesicles (29). The remaining neutrophil cytoplasts stay viable and are able to phagocytose and kill microbes (29).
NETosis can be induced by various stimuli, including pathogens, cytokines, and hypoxia (30–32); however, a combination of sterile and/or infectious stimulants may produce the most drastic NETosis response. For example, in transfusion-related acute lung injury—the leading cause of transfusion-related death—poor outcomes are associated with preexisting neutrophil-priming risk factors, such as exposure to bacterial products, high levels of cytokines, or activated platelets, before the activation of neutrophils and NET induction by specific anti-neutrophil antibodies in the transfused product (33–36). Thus, 2 hits or multiple hits on neutrophils can result in a damaging and even deadly outcome.
PAD4 is genetically linked to rheumatoid arthritis (RA), as individuals with polymorphisms in the enzyme are predisposed to develop the disease (37, 38). Neutrophils in patients with RA are highly susceptible to NETosis (Fig. 1), which is possibly explained by the prominent translocation of PAD4 to the nucleus even without stimulation (39). Recently, Carmona-Rivera and colleagues (40) demonstrated that NETs and associated citrullinated proteins, after internalization by fibroblast-like synoviocytes, are presented as NET peptides to antigen-specific T cells to activate arthritogenic adaptive immunity, which suggests NETs as a source for arthritogenic citrullinated autoantigens in RA. PAD4/NETs are therefore strong inducers and propagators of autoimmune diseases (41), as well as many other important diseases.
THROMBOSIS AND CARDIOVASCULAR DISEASE
Fuchs et al. (42) provided a surprising observation that NETs promote thrombosis. This now seems to be a well-established finding, and our group reviewed the subject in depth in 2014 (43). Fuchs and colleagues first showed in a flow chamber that NETs provide a scaffold for platelet and erythrocyte binding. NETs activate the bound platelets, which causes their aggregation. NETs also promote fibrin deposition (42) by activating coagulation (44). The presence of NETs is likely the reason for the resistance of venous thrombi to thrombolysis by tissue plasminogen activator, as plasmin cannot degrade DNA (42). NETs form in experimental deep vein thrombosis (DVT) in baboons (42) and mice (14, 45, 46). In addition to neutrophils and monocytes, platelets play an active role in DVT (46). Activated platelet P-selectin (47) and platelet-derived high-mobility group box 1 (48) promote neutrophil recruitment and NETosis. PAD4-deficient mice that are defective in NETosis or mice that have been treated with DNase 1 that cleaves NETs are protected from the inferior vena cava stenosis-induced DVT (14, 45, 46). Two types of DNases—DNase 1 and DNase 1–like 3 (DNase 1L3) —have recently been reported to safeguard vascular patency by digesting extracellular DNA present in blood (6). Under chronic neutrophilia, neutrophils are stimulated and mice that lack both DNases die from vascular occlusions formed by NET-rich clots (6). Patients with acute thrombotic microangiopathies were also often demonstrated to have low plasma DNase activity due to a reduction in circulating DNase 1 levels, which resulted in defective NET degradation and excessive microthrombosis (49). In specimens obtained from humans, NETs are observed primarily in DVTs and pulmonary emboli in their organizing stage, where layers of neutrophils and platelets are present (50). Plasma DNA and biomarkers of NETs are elevated in patients with DVT (51–53). PAD4 is released with NETs, where it remains active (54), thus enabling it to citrullinate plasma proteins and change their behavior. A preliminary study from our lab recently showed that PAD4, in vitro, citrullinates ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type-1 motif, member 13), an important enzyme that cleaves the prothrombotic protein, von Willebrand factor, to reduce its biologic activity. ADAMTS13 loses enzymatic activity after citrullination, which suggests that NETosis could have additional ways by which to promote thrombosis (55). Tilvawala et al. (56) recently reported that extracellular PADs citrullinate a variety of serine protease inhibitors (serpins) in RA. Cirullinated serpins lose the ability to inhibit their cognate proteases, including antithrombin and C1 inhibitor. The resulting enhanced activity of the serine proteases could lead to dysregulated blood coagulation, fibrinolysis, and complement activation, thus exacerbating inflammation and increasing the risk of thrombosis (56).
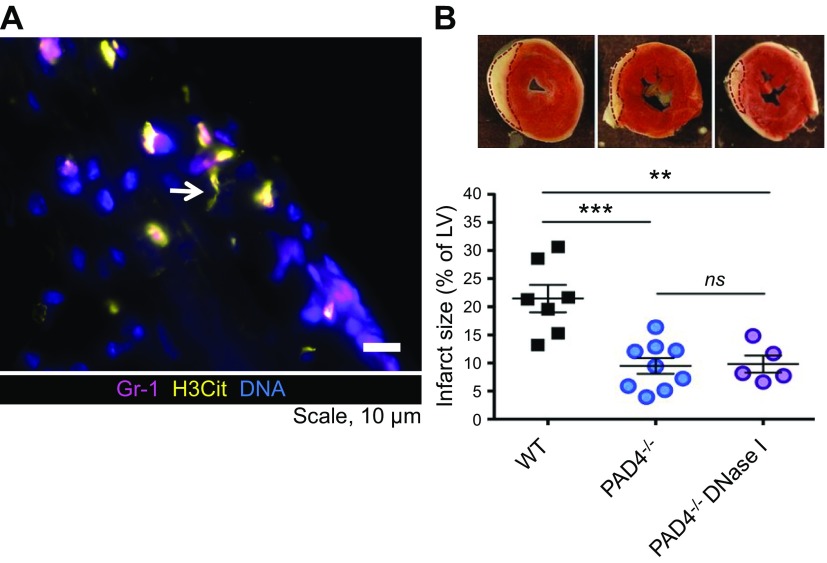
Myocardial ischemia/reperfusion (I/R) injury involves both inflammation and microthrombosis. H3Cit, a NET biomarker, is present in the infarcted myocardium of wild-type mice subjected to transient occlusion of the left anterior descending artery and 23 h of reperfusion (Fig. 2A). Simultaneously, a decline in cardiac function, indicated by a decrease in left ventricular ejection fraction, is observed. H3Cit is absent in the myocardium of PAD4-deficient mice that underwent the same procedure, and their cardiac function is well preserved. Treatment with DNase 1 at the time of reperfusion and 11 h after reduces myocardial infarct size and restores cardiac function in wild-type mice. DNase 1 treatment provides no additional benefits to PAD4-deficient mice (Fig. 2B), which suggests that PAD4-mediated NETosis is the main source of the toxic extracellular chromatin in myocardial I/R injury (57). Likewise, Cl-amidine, a PAD inhibitor, lessened myocardial infarction in wild-type mice and preserved their ejection fraction and cardiac output as determined at 7 d postsurgery (58). In humans, NET content in coronary thrombi correlates positively with myocardial infarct size, whereas lesion site DNase activity correlates negatively with infarct size (59).
Figure 2.
NETs formed in the myocardium after I/R injury contribute to myocardial infarction in mice. A) Immunofluorescence microscopy revealed that Gr-1–positive neutrophils (pink) are releasing H3Cit-positive NETs (yellow) in the infarcted myocardium of wild-type (WT) mice. Arrow indicates a NET. B) Triphenyltetrazolium chloride (TTC) staining of cross-sections of the left ventricle at 24 h after myocardial I/R injury (top). Infarcted areas appear as white tissue are outlined with dotted lines. Summarized data of left ventricle infarct size, expressed as the percentage of left ventricle (LV; bottom). The infarct size is remarkably smaller in PAD4−/− mice compared with WT mice. Systemic treatment with DNase 1 does not further reduce the infarct size of PAD4−/− mice, which indicates that the extracellular DNA in the damaged heart predominantly originates from NETs. From Savchenko et al. (57). Reprinted with permission from the American Society of Hematology.
NETs are also implicated in stroke, although experimental evidence of a causal relationship using PAD4 mutant mice is lacking. Plasma levels of DNA and nucleosomes are elevated in wild-type mice whose middle cerebral artery has been transiently occluded, and the size of the brain infarct is reduced with DNase 1 treatment—even when treatment started 1 h after the onset of reperfusion—or histone neutralization, which strongly suggests the contribution of extracellular chromatin in ischemic stroke injury (31). In humans, plasma H3Cit levels are elevated at the onset of acute ischemic stroke and are independently associated with all-cause mortality at 1 yr of follow-up (60). NET biomarkers, including H3Cit and H4Cit, are present in cerebral thrombi from humans who suffered acute ischemic stroke. Impressively, DNase 1 enhances tissue-type plasminogen activator–induced thrombolysis of these thrombi ex vivo (61, 62), which again indicates that thrombo-inflammatory clots require breakage of their nucleic acid component to achieve resolution.
CANCER PROGRESSION AND CANCER-ASSOCIATED THROMBOSIS
“Tumors, wounds that do not heal (63, 64),” have long been linked to inflammation and thrombotic events. Cancer cells often release proinflammatory cytokines—notably, granulocyte colony-stimulating factor (G-CSF) —that can systemically preactivate neutrophils. Indeed, neutrophils of mice that bear G-CSF–secreting tumors, such as chronic myelogenous leukemia–like myeloproliferative neoplasia, 4T1 mammary carcinoma, and Lewis lung carcinoma (LLC), are predisposed to form NETs when stimulated in vitro (65). At latter stages of breast cancer in mice, NETs form in vivo spontaneously, as indicated by a surge in plasma DNA and circulating H3Cit levels. This coincides with the formation of thrombi that are rich with DNA in the lungs of the tumor-bearing mice, which suggests that NETs are involved in the thrombosis process (65). Whereas a previous study reported that neutrophils from patients with myeloproliferative neoplasms (MPNs) have impaired NETosis in vitro (66), a more extensive recent study found that neutrophils from patients with MPN and an MPN mouse model—a knock-in of the disease driver mutation Jak2V617F—have increased propensity to NETosis, which is PAD4-dependent and likely mediated by increased PAD4 expression in neutrophils (67). Ruxolitinib, a JAK2 inhibitor, abrogates NET formation, implicating the active kinase in NETosis. As in human patients, MPN in mice is associated with a prothrombotic phenotype that could be reversed by the JAK2 inhibitor (67).
Cancer cells and cancer-activated platelets also induce NETosis. Pancreatic cancer cells were demonstrated to induce reactive oxygen species–independent NETosis after 30 min of incubation with neutrophils (68). Platelets that are primed by pancreatic cancer cells induce NETosis compared with control platelets (68). Similarly, platelets from patients with gastric cancer induce NETosis in neutrophils derived from controls (69). Compared with platelets from healthy individuals, platelets in patients with lung cancer express significantly higher levels of P-selectin (70), which is known to induce NETosis (47). This creates a vicious cycle of inflammation (71) in which cancer activates platelets, which induces NETosis that, in turn, activates platelets, further promoting neutrophil activation and NETosis. Histones are strong activators of platelets (42), and digestion of NETs by DNase may leave some histones in tissue, propagating injury (72) and possibly microthrombosis. A recent prospective observational cohort study (73), which observed more than 900 patients with cancer, concluded that an initially high level of the NET biomarker, H3Cit, predicts the occurrence of venous thromboembolism in these patients. Thus, PAD4/NETs are likely a driving force in the pathogenesis of cancer-associated thrombosis. The NET effect may not be limited to venous thrombosis. Disseminated arterial microthrombosis was found in stroke patients with elevated plasma troponin levels who were later diagnosed with cancer. NETs were observed in widespread arterial microthrombi in the heart, brain, and lungs in the autopsies of such patients (74). H3Cit was also evaluated for its survival prognostic value in cancer. High plasma H3Cit level (>29.8 ng/ml) was associated with a 2-fold increase in the risk of short-term mortality (75).
Tissue factor (TF) is a powerful driver of thrombosis in cancer, as cancers release TF-positive microparticles (76). Of interest, cancer-derived procoagulant microparticles and exosomes bind to NETs (77, 78). Infusion of TF-positive cancer microparticles promotes the rapid onset of DVT in mice without cancers (78). There are strong correlations between plasma DNA/nucleosome levels and microparticles-associated TF activity in patients with cancer-related disseminated intravascular coagulation. Indeed, NETosis and TF-containing cancer microparticles make for a dangerous combination that predisposes to thrombosis (79).
Cancer-induced NETosis can cause systemic organ damage. Mice that bear metastatic G-CSF–secreting mammary carcinoma exhibit NET-mediated vascular leakage and a reduction in vascular perfusion in organs, such as the heart and kidney, although these are not metastatic sites. Treatment with DNase 1 or anti–G-CSF antibody completely restores renal vascular function (80). Moreover, in humans, as discussed above, NET-mediated microthrombosis in cancer was demonstrated to cause damage to the heart and brain (74).
In addition to NETs forming in the circulation, NETosis is also prominent within tumors in humans and mice (81–83). The presence of NETs seems to favor tumor growth. In a murine lung carcinoma model (LLC), LLC tumors become packed with NETs when implanted in wild-type mice and grow faster than in PAD4-deficient mice where NETs rarely form (83). This NET effect only occurs in tumors that release G-CSF. No NETs are formed in the tumors of wild-type mice that bear non-G-CSF–releasing B16 melanoma, and tumor growth is identical in wild-type and PAD4−/− recipients (83). Neutrophils from patients with chronic lymphocytic leukemia are primed by circulating IL-8 and predisposed to form NETs (84). Coculture of NETs and leukemic B cell leads to delayed spontaneous apoptosis of the leukemic B cells and increases the expression of activation markers on the leukemic cells, which indicates a supportive role of NETs for cancer cell survival (84).
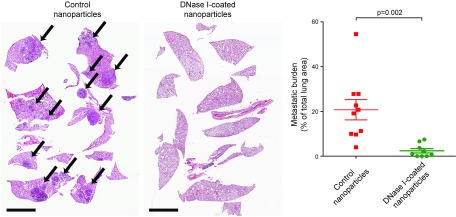
Of importance, it seems that NETs also assist the spread of cancer. The first observation came from a cecal ligation and puncture model of systemic sepsis, which induces NETosis. Here, NETs are deposited in microvessels, entrap circulating tumor cells, and promote metastasis, which is abolished by DNase 1 or a neutrophil elastase inhibitor (85). Neutrophil elastase is present on NETs where it retains activity (72). More recently, Park et al. (86) uncovered that metastatic cancer cells could stimulate rapid NETosis at sites of dissemination in the absence of infection. NETosis occurs in the lungs after intravenous injection of metastatic breast cancer cells in mice, and the NET burden in the lungs persists for days. Treatment of mice with DNase 1–coated nanoparticles significantly reduces lung metastasis of breast cancer cells (Fig. 3), which indicates that extracellular DNA (NETs) promotes metastasis (86) and perhaps also metastatic tumor growth (Fig. 3). Of note, surgical stress can also induce metastasis-supporting NETosis. Patients who underwent major liver resection surgery in an attempt to remove metastatic colorectal cancer experienced liver I/R, which resulted in elevated circulating levels of NETs. High levels of NETs were associated with a >4-fold reduction in disease-free survival (87). To confirm this observation experimentally, mice were subjected to liver I/R surgery, which induced widespread intrahepatic NET formation and deposition that were associated with an increase of hepatic metastasis of colon cancer. Again, DNase 1 treatment attenuates the surgical stress–induced prometastatic effect of NETs (87). The impact of DNase treatment on cancer metastasis observed before the discovery of NETs was reviewed by Hawes and colleagues (88).
Figure 3.
Targeting NETs with DNase 1 reduces metastasis. Mice were treated with DNase 1–coated nanoparticles or control nanoparticles i.p. 2 h before intravenous injection of 4T1 breast cancer cells. Mice continued to receive nanoparticle treatment daily for 2 wk, and were euthanized when weight loss occurred. Hematoxylin and eosin–stained lung sections (left), and quantitative analysis shows that the metastatic burden was reduced by the DNase 1 treatment (right). Arrows indicate metastases. Scale bars, 4 mm. From Park et al. (86). Reprinted with permission from the American Association for the Advancement of Science.
What are the mechanisms by which NETosis impacts cancer cells? NETs may promote cancer cell adhesion (89, 90). Human gastric cancer cells were demonstrated to adhere to NETs that were generated by low-density neutrophils obtained from human postoperative peritoneal lavage in vitro. When cancer cells were coinjected with low-density neutrophils into the abdominal cavity of nude mice, peritoneal metastasis was enhanced (90). One intriguing but unexplored facet is the effect of the NETosis byproduct, ammonia, on tumors. To this point, a recent study determined that breast cancer cells can recycle ammonia—their own metabolic waste—to maximally use nitrogen to support tumor growth and proliferation (91). It is plausible that the ammonia generated in NETosis inside the tumor could be similarly used, which perhaps explains, in part, the tumor-promoting effect of NETosis.
DIABETES AND ITS COMPLICATIONS
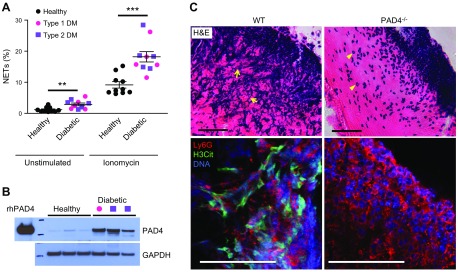
Patients with diabetes have elevated circulating levels of proinflammatory cytokines, such as TNF-α and IL-6 (92, 93). Their neutrophils also produce higher levels of reactive oxygen species (94). All of these present a chronic low-grade inflammatory milieu that could activate/prime neutrophils. Indeed, neutrophils that are isolated from patients with diabetes are prone to produce NETs spontaneously (Fig. 4A) (93, 95, 96). Plasma nucleosome levels are increased in patients with type 2 diabetes mellitus (T2DM) (93, 97). In parallel with increased NETosis, neutrophil PAD4 protein expression is elevated approximately 4-fold in patients with type 1 diabetes mellitus (T1DM) and T2DM (Fig. 4B), which perhaps explains their pro-NETotic phenotype (95). The molecular basis of PAD4 up-regulation is not known and will be important to determine.
Figure 4.
Enhanced NETosis in neutrophils from patients with diabetes and deposition of NETs in mouse skin wounds. A) Both T1DM and T2DM prime human neutrophils to produce NETs. B) PAD4 protein expression is strikingly up-regulated in the neutrophils of patients with diabetes. T1DM, pink circle; T2DM, purple square. C) Hematoxylin and eosin staining revealed prominent extracellular DNA structures, appearing as blue streaks (arrows), in the scab of WT mice 3 d postinjury (top). Neutrophils remain intact (arrowheads) and NET structures are absent in the scab of PAD4−/− mice. Confocal immunofluorescence microscopy showed that H3Cit (green) colocalizes with externalized DNA (blue) in neutrophil (Ly6G) -rich area (red) in WT, but not PAD4−/− scab, which indicates the crucial role of PAD4 in NETosis in the mouse skin wounds (bottom). Scale bars, 50 μm. Adapted from Wong et al. (95).
A common denominator between T1DM and T2DM is elevated blood glucose. Incubation of neutrophils in the presence of high glucose, as in hyperglycemia, enhances NET release by neutrophils from healthy individuals (95, 97–99). Although hyperglycemia induces NETosis ex vivo, patients with T2DM who are treated with metformin, which normalizes glucose levels in 6 mo, have NETosis reduced only after 12 mo when circulating levels of proinflammatory cytokines are also normalized (93), which indicates that cytokines likely exert a major effect in the basal diabetes-associated NETosis in vivo. Recently, spontaneous NETosis was also reported in gestational diabetes mellitus (GDM), a unique form of glucose intolerance that occurs transiently during pregnancy (99). The pro-NETotic effect of the GDM patients’ plasma was attenuated with a TNF-α antagonist (99). As PAD4/NETs are implicated in animal models of pregnancy loss (100, 101), the gravity of GDM should not be underestimated (102).
Increased spontaneous NETosis in diabetes contributes to diabetic complications. Impaired wound healing is one of the most prominent problems in diabetes. NETs are formed in skin wounds in mice (Fig. 4C), which hinder wound repair (95). In diabetic mice, the NET burden in wounds is even higher, which leads to additional delay in wound closure (95). PAD4 deficiency, or treatments with DNase 1 or Cl-amidine, significantly ameliorates healing in mice (95, 103). Patients with diabetes with nonhealing diabetic foot ulcers have elevated circulating NET biomarkers compared with those patients without the ulcers, and the wounds of diabetic foot ulcers contain excessive cytotoxic NET-associated proteins, such as neutrophil proteinase-3, neutrophil elastase, and myeloperoxidase (103).
Recent studies in nondiabetic mice provide some insight into the induction of NETosis in wounds. Stavrou et al. (104) reported that coagulation factor XII (FXII) is secreted from neutrophils after activation. FXII acts on the urokinase plasminogen activator receptor on the neutrophil plasma membrane, independent of its enzymatic activity, which up-regulates αMβ2 integrin and increases intracellular calcium concentration that leads to histone citrullination and NET formation. Mice that are FXII deficient have reduced neutrophil recruitment to wounds, less NET burden, and faster wound healing (104). Altogether, the negative impact of NETosis on wound healing is now well established; however, the cellular mechanism of how NETs hinder healing is not understood. Hypotheses on whether NETs are toxic to keratinocytes or physically obstruct their migration or dysregulate angiogenesis warrant investigation. PAD4 inhibition and NET disruption by DNases should now be considered for the treatment of diabetic wounds (95).
As previously discussed, NETs promote many inflammatory and thrombotic diseases. Thus, elevated NETs could contribute to known diabetic complications, such as increased incidences of heart disease and thrombosis (105, 106). A recent case-control study demonstrated that elevated levels of circulating DNA-histone complexes are a risk factor for diabetic retinopathy (107), the most common microvascular diabetic complication, which affects approximately 35% of patients with diabetes (108).
T1DM is an autoimmune disease. As with RA, it is plausible that NETs could be implicated in the initiation of T1DM. Although definitive evidence of the causal relationship between NET formation and the onset of T1DM is lacking, there are reports that implicate NETs in T1DM etiology. Neutrophil elastase, proteinase 3, and NETosis are markedly increased in patients with T1DM, in particular during the first yr of disease onset (109). Neutrophil infiltration and NETs were found in the pancreatic islets of nonobese diabetic mice as young as age 3 wk—that is, before the onset of diabetes (110). NET-binding cathelicidin-related antimicrobial peptide stimulates plasmacytoid dendritic cells to generate IFN-α, thereby initiating diabetogenic T-cell response, and the subsequent T1DM development (110). Of interest, 4-wk-old nonobese diabetic mice that were orally treated daily during the first wk and then once a week until age 20 wk with Lactococcus lactis engineered to express stable Staphylococcal nuclease that digests NETs have notably reduced circulating levels of NETs and proinflammatory cytokines, which results in a significant reduction of T1DM incidence (111). Such NET-degrading probiotic bacteria could be useful in ameliorating many NET-driven conditions beyond diabetes and diabetic complications.
AGING AND AGE-RELATED ORGAN FIBROSIS
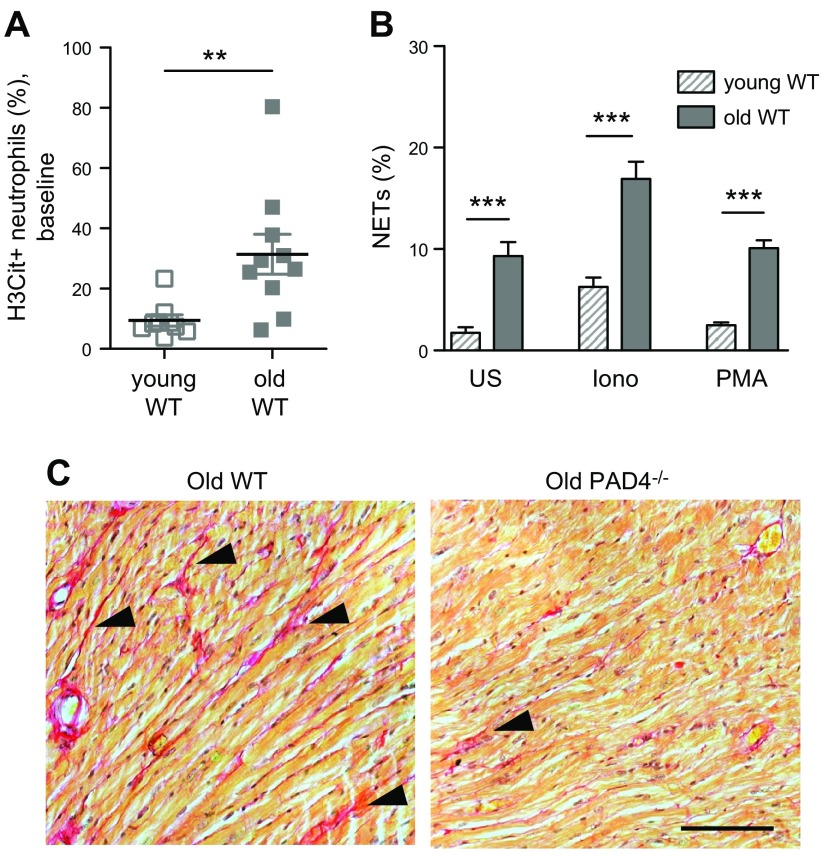
In aged humans and mice, there is an imbalance between innate and adaptive immunity. Leukocyte production is skewed toward myeloid cells over the lymphoid lineage, with an increase in neutrophil and platelet counts (112, 113). Aging also results in redox imbalance and the accumulation of oxidative stress (114). How, then, is NETosis modulated in aging? Martinod et al. (113) investigated this question by comparing the neutrophil behavior of aged (14–24 mo old) and young (2 mo old) mice. The authors observed that more circulating neutrophils from aged mice are H3Cit positive (Fig. 5A), which indicates a higher nuclear activity of PAD4 at baseline compared with neutrophils from young mice. Furthermore, when neutrophils are isolated from aged mice and plated, they generate NETs spontaneously, and their NETosis is exacerbated after stimulation (Fig. 5B). There are significantly more circulating platelet-neutrophil complexes in aged mice, which possibly explains why their neutrophils are pro-NETotic, as activated platelets can induce NET formation via platelet-neutrophil interactions mediated by P-selectin/P-selectin glycoprotein ligand-1, glycoprotein Ibα/Mac-1, and TLR4 (47, 115, 116).
Figure 5.
PAD4 activity/NETosis increase with age, and PAD4/NETs are implicated in age-related organ fibrosis. A) More circulating neutrophils from old (age 14–17 mo) wild-type (WT) mice were H3Cit positive compared with those of young (age 1.5–3.5 mo) mice. B) Compared with young WT mice, a higher percentage of neutrophils from old WT mice produced NETs spontaneously, as well as in the presence of stimulants, after incubation ex vivo. C) Sirius red staining revealed more interstitial collagen deposition (red, arrowheads) in the heart of old WT mice than in age-matched PAD4−/− mice. Scale bar, 100 μm. Iono, ionomycin; PMA, phorbol 12-myristate 13-acetate; US, unstimulated. From Martinod et al. (113).
What are the consequences of increased NETosis in aging mice? PAD4/NETs seem to contribute to organ fibrosis, which results in organ function decline (113). Whereas the hearts and lungs of aged wild-type mice exhibit significant interstitial fibrosis, the fibrosis phenotype is significantly less severe in aged PAD4-deficient mice (Fig. 5C). In particular, myocardial fibrosis is minimal, and by consequence, cardiac function—both systolic and diastolic—of aged PAD4-deficient mice is preserved and comparable to that of young mice (113). As PAD4 is not only expressed in neutrophils, it is important to demonstrate directly that the PAD4-mediated chromatin release indeed contributes to organ fibrosis, rather than PAD4-regulated gene expression in the organs. For this, young wild-type and PAD4-deficient mice were subjected to ascending aortic constriction, a pressure-overload model of cardiac fibrosis in which DNase treatment is feasible (113). Indeed, NETs and platelet aggregates are observed in the myocardium of wild-type mice at 1 d postsurgery, with subsequent cardiac dysfunction that occurs as early as 3 d and significant fibrosis in 4 wk. All of these are greatly diminished in PAD4 deficiency or by 7 d of DNase 1 treatment postconstriction (113). Thus, it seems that NETs contribute to experimental fibrosis and PAD4/NETs play a key role in the functional decline of organs. There are currently limited treatment options for fibrotic diseases (117), and targeting NETs/PAD4 could be a new approach by which to curtail age-related organ fibrosis or dysfunction. As PAD4 is a culprit that mediates organ fibrosis, interesting questions that are worth additional investigation include whether PAD4 deficiency increases the longevity of mice and whether these mice are healthier overall. Implications of NET-mediated organ injury may explain why severe childhood infections—that cause NETosis—may years later negatively impact the longevity of the individual (118, 119). In addition, whether and how PAD4/NETs-mediated fibrosis involves TGF-β, a known mediator of fibrosis whose release from platelets increases with aging (113), remains to be examined.
UNRESOLVED ISSUES AND FUTURE DIRECTIONS
How important are PAD4 and NETosis in pathologies of the brain?
In addition to age-related organ fibrosis, NETs have been observed in age-related neurodegenerative diseases. NETs are present in the brain parenchyma of murine transgenic models of Alzheimer’s disease (AD), as well as in the brains of humans with AD (120). Neutrophil depletion or inhibition of neutrophil trafficking alleviates AD-like pathology and cognitive deficits in mice (120), which suggests an important role for neutrophils and possibly NETs in AD pathogenesis. Citrullinated proteins accumulate in PAD4-expressing neurons in the hippocampus and cerebral cortex, brain regions where neurodegenerative changes that are typical for AD occur (121). It is plausible that the release of intracellular citrullinated proteins into the brain interstitium and circulation when the neurons die stimulates autoantibody production, which contributes to neurodegenerative disease (121). In addition, non-neuronal cells in the CNS also express PAD2 and PAD4 [e.g., astrocytes (121, 122), microglial cells (121, 123), and oligodendrocytes (124, 125)]. TNF-α can actually stimulate nuclear translocation of PAD4 in oligodendrocytes and augment their histone H3 citruillination (125). As experimental PAD4 overexpression in cancer cells causes chromatin release (126), one relevant question is whether PAD4-expressing neurons in diseased or inflamed brains are able to actively extrude their chromatin as extracellular traps. Tissue-specific PAD4 knockout mice will be important for verification of the origin of extracellular DNA in the brain or the source of PAD4 or PAD(s) that may continue to citrullinate extracellular proteins upon release to the parenchyma.
Why is regulated chromatin release highly conserved?
Not limited to vertebrates, regulated chromatin release also occurs in the root border cells of plants (127). The mechanism of the chromatin release is not known, as plants do not have PAD enzymes. DNase 1 abrogates the root tip resistance to microbial infection, which indicates that the border cell extracellular DNA serves as a means of host defense, like a plant analog to NETs (128). Of interest, bacterial pathogens can also release extracellular DNA to form biofilm in plant xylem vessels that conduct water and nutrients upwards. After infection of the plant, these bacteria modulate the biofilm structure using their extracellular nucleases to facilitate spreading of colonies (129). Fine-tuning between extracellular DNA and nuclease release could be a strategy that bacteria employ to enhance their survival and spread in mammals as well. By inducing other cells to release extracellular DNA, including NETs from neutrophils (2), bacteria could use that extracellular DNA as building blocks to form biofilms. Biofilms that are formed from NETs can provide protection for bacteria in diseases, such as otitis media in humans (130). The role of human DNases in the regulation of NETs/bacterial symbiosis in diseases, such as periodontitis, and in the dissemination of neoantigens that promote autoimmunity (131) needs to be addressed.
There have been controversies on whether the role of PAD4 is as important in human NETosis as it is in murine NETosis. Whereas Wang et al. and Lewis et al. demonstrated that inhibitors of PAD or PAD4 significantly reduce human NETosis, Kenny et al. reported that these inhibitors are ineffective (1, 132, 133). Clearly, the precise contribution of PAD4 in human NETosis should be better clarified. Perhaps there is a delay in NETosis under PAD4 inhibition, which could explain the discrepant results; however, as discussed earlier, in diabetes, MPNs, and RA, in which NETosis is elevated, human neutrophils present up-regulated expression and/or increased nuclear translocation of PAD4 (39, 67, 95). Compared with the many publications on NETs, only a few reports suggest that other blood cells, such as macrophages and eosinophils, release chromatin (134–137). The mechanism of chromatin release by these blood cells and its associated pathologic contributions, such as that reported in acute kidney injury (137), remain to be established.
What are the positive functions of NETs in humans?
NETs, together with fibrin, are a means of host defense (2, 138); however, other aspects of the positive functions of NETs are rarely investigated. NETs are highly prothrombotic and procoagulant (43). Could they be critically important in enhancing hemostasis of large wounds after serious injuries to prevent excessive blood loss? By promoting fibrin deposition (42), they likely protect an individual from invading pathogens after physical injuries. In addition to protein citrullination, the byproduct generated in NETosis is ammonia. What is the function of the massive release of ammonia during NETosis? Does it contribute to the bactericidal effects of NETs? Recently, it was also found that NETs are generated at the site of immunization and drive the activity of aluminum-based adjuvant, thereby enhancing adjuvant-induced adaptive immune response (139). It is possible that NET generation during an infection may improve natural immunization for the pathogen and optimize Ab production in future encounters. It is clear that the precise temporal regulation of NETosis is critical for balancing the positive functions of NETs and the collateral damage that they can cause.
How are cytoplasts generated?
Vital NETosis, which results in the generation of cytoplasts, is underexplored. Currently, there is only one study that has demonstrated neutrophil cytoplast formation in NETosis in vivo (29), and, to this day, cytoplast generation has not been observed in vitro. What are the cellular and molecular mechanisms of cytoplast formation? Depending on stimulants, not all NETs have the same protein composition (140). Are cytoplast membrane components also different depending on the stimulus, as microparticles generated from monocytes are (141)? Are cytoplasts generated as a large microparticle before the cell bursts in NETosis or by resealing the plasma membrane after NET release? Do cytoplasts only form in infections or also in sterile inflammation? The observation of cytoplasts certainly opens up a new avenue for NETosis investigation.
CONCLUSIONS
The discovery of NETs refined our understanding of inflammation. Originally found in infectious settings, NETs were soon implicated in various autoimmune conditions and major sterile inflammatory settings, including cardiovascular disease, thrombosis, cancer, and diabetes, as well as in aging-associated organ fibrosis. These new findings provide an important basis for NETs/PAD4 to serve as therapeutic targets directed to limit the detrimental NET effects. The field has made great progress since the discovery of NETs 14 yr ago, but many open questions remain. The cell biology of NETosis, including signaling pathways, the process to cytoplast formation, the evolutionary roles of actively released chromatin, and the impact of NETosis on adaptive immunity are some of the emerging areas inspiring future exploration.
ACKNOWLEDGMENTS
The authors thank Deya Cherpokova and Elise DeRoo for critical reading of the manuscript, Caleb Staudinger and Sarah Walker (all from Boston Children’s Hospital) for assistance in manuscript preparation. Some of the research described here was supported by the U.S. National Institutes of Health (NIH) National Heart, Lung, and Blood Institute Grant R35HL135765 (to D.D.W.). The authors declare no conflicts of interest.
Glossary
- AD
Alzheimer disease
- DVT
deep vein thrombosis
- FXII
factor XII
- G-CSF
granulocyte colony-stimulating factor
- GDM
gestational diabetes mellitus
- H3Cit
citrullinated histone H3
- H4Cit
citrullinated histone H4
- I/R
ischemia/reperfusion
- LLC
Lewis lung carcinoma
- MPN
myeloproliferative neoplasm
- NET
neutrophil extracellular trap
- PAD
peptidylarginine deiminase
- RA
rheumatoid arthritis
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TF
tissue factor
AUTHOR CONTRIBUTIONS
S.L.W. and D.D.W wrote the paper.
REFERENCES
- 1.Wang Y., Li M., Stadler S., Correll S., Li P., Wang D., Hayama R., Leonelli L., Han H., Grigoryev S. A., Allis C. D., Coonrod S. A. (2009) Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J. Cell Biol. 184, 205–213 10.1083/jcb.200806072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkmann V., Reichard U., Goosmann C., Fauler B., Uhlemann Y., Weiss D. S., Weinrauch Y., Zychlinsky A. (2004) Neutrophil extracellular traps kill bacteria. Science 303, 1532–1535 10.1126/science.1092385 [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V., Zychlinsky A. (2012) Neutrophil extracellular traps: is immunity the second function of chromatin? J. Cell Biol. 198, 773–783 10.1083/jcb.201203170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta A. K., Hasler P., Holzgreve W., Gebhardt S., Hahn S. (2005) Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum. Immunol. 66, 1146–1154 10.1016/j.humimm.2005.11.003 [DOI] [PubMed] [Google Scholar]
- 5.Zhong X. Y., Laivuori H., Livingston J. C., Ylikorkala O., Sibai B. M., Holzgreve W., Hahn S. (2001) Elevation of both maternal and fetal extracellular circulating deoxyribonucleic acid concentrations in the plasma of pregnant women with preeclampsia. Am. J. Obstet. Gynecol. 184, 414–419 10.1067/mob.2001.109594 [DOI] [PubMed] [Google Scholar]
- 6.Jiménez-Alcázar M., Rangaswamy C., Panda R., Bitterling J., Simsek Y. J., Long A. T., Bilyy R., Krenn V., Renné C., Renné T., Kluge S., Panzer U., Mizuta R., Mannherz H. G., Kitamura D., Herrmann M., Napirei M., Fuchs T. A. (2017) Host DNases prevent vascular occlusion by neutrophil extracellular traps. Science 358, 1202–1206 10.1126/science.aam8897 [DOI] [PubMed] [Google Scholar]
- 7.Sollberger G., Tilley D. O., Zychlinsky A. (2018) Neutrophil extracellular traps: the biology of chromatin externalization. Dev. Cell 44, 542–553 10.1016/j.devcel.2018.01.019 [DOI] [PubMed] [Google Scholar]
- 8.Honda M., Kubes P. (2018) Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat. Rev. Gastroenterol. Hepatol. 15, 206–221 10.1038/nrgastro.2017.183 [DOI] [PubMed] [Google Scholar]
- 9.Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. (2007) Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 10.1083/jcb.200606027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neeli I., Khan S. N., Radic M. (2008) Histone deimination as a response to inflammatory stimuli in neutrophils. J. Immunol. 180, 1895–1902 10.4049/jimmunol.180.3.1895 [DOI] [PubMed] [Google Scholar]
- 11.Asaga H., Nakashima K., Senshu T., Ishigami A., Yamada M. (2001) Immunocytochemical localization of peptidylarginine deiminase in human eosinophils and neutrophils. J. Leukoc. Biol. 70, 46–51 [PubMed] [Google Scholar]
- 12.Chang X., Han J. (2006) Expression of peptidylarginine deiminase type 4 (PAD4) in various tumors. Mol. Carcinog. 45, 183–196 10.1002/mc.20169 [DOI] [PubMed] [Google Scholar]
- 13.Li P., Li M., Lindberg M. R., Kennett M. J., Xiong N., Wang Y. (2010) PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J. Exp. Med. 207, 1853–1862 10.1084/jem.20100239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinod K., Demers M., Fuchs T. A., Wong S. L., Brill A., Gallant M., Hu J., Wang Y., Wagner D. D. (2013) Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc. Natl. Acad. Sci. USA 110, 8674–8679 10.1073/pnas.1301059110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakashima K., Hagiwara T., Yamada M. (2002) Nuclear localization of peptidylarginine deiminase V and histone deimination in granulocytes. J. Biol. Chem. 277, 49562–49568 10.1074/jbc.M208795200 [DOI] [PubMed] [Google Scholar]
- 16.Smith B. C., Denu J. M. (2009) Chemical mechanisms of histone lysine and arginine modifications. Biochim. Biophys. Acta 1789, 45–57 10.1016/j.bbagrm.2008.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Papayannopoulos V., Metzler K. D., Hakkim A., Zychlinsky A. (2010) Neutrophil elastase and myeloperoxidase regulate the formation of neutrophil extracellular traps. J. Cell Biol. 191, 677–691 10.1083/jcb.201006052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Metzler K. D., Goosmann C., Lubojemska A., Zychlinsky A., Papayannopoulos V. (2014) A myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Reports 8, 883–896 10.1016/j.celrep.2014.06.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Martinod K., Witsch T., Farley K., Gallant M., Remold-O’Donnell E., Wagner D. D. (2016) Neutrophil elastase-deficient mice form neutrophil extracellular traps in an experimental model of deep vein thrombosis. J. Thromb. Haemost. 14, 551–558 10.1111/jth.13239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amulic B., Knackstedt S. L., Abu Abed U., Deigendesch N., Harbort C. J., Caffrey B. E., Brinkmann V., Heppner F. L., Hinds P. W., Zychlinsky A. (2017) Cell-cycle proteins control production of neutrophil extracellular traps. Dev. Cell 43, 449–462.e5 [DOI] [PubMed] [Google Scholar]
- 21.Lim M. B., Kuiper J. W., Katchky A., Goldberg H., Glogauer M. (2011) Rac2 is required for the formation of neutrophil extracellular traps. J. Leukoc. Biol. 90, 771–776 10.1189/jlb.1010549 [DOI] [PubMed] [Google Scholar]
- 22.Gavillet M., Martinod K., Renella R., Wagner D. D., Williams D. A. (2018) A key role for Rac and Pak signaling in neutrophil extracellular traps (NETs) formation defines a new potential therapeutic target. Am. J. Hematol. 93, 269–276 10.1002/ajh.24970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang D., Chen G., Manwani D., Mortha A., Xu C., Faith J. J., Burk R. D., Kunisaki Y., Jang J. E., Scheiermann C., Merad M., Frenette P. S. (2015) Neutrophil ageing is regulated by the microbiome. Nature 525, 528–532 10.1038/nature15367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hacbarth E., Kajdacsy-Balla A. (1986) Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 29, 1334–1342 10.1002/art.1780291105 [DOI] [PubMed] [Google Scholar]
- 25.Denny M. F., Yalavarthi S., Zhao W., Thacker S. G., Anderson M., Sandy A. R., McCune W. J., Kaplan M. J. (2010) A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 184, 3284–3297 10.4049/jimmunol.0902199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Villanueva E., Yalavarthi S., Berthier C. C., Hodgin J. B., Khandpur R., Lin A. M., Rubin C. J., Zhao W., Olsen S. H., Klinker M., Shealy D., Denny M. F., Plumas J., Chaperot L., Kretzler M., Bruce A. T., Kaplan M. J. (2011) Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 187, 538–552 10.4049/jimmunol.1100450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deng Y., Ye J., Luo Q., Huang Z., Peng Y., Xiong G., Guo Y., Jiang H., Li J. (2016) Low-density granulocytes are elevated in mycobacterial infection and associated with the severity of tuberculosis. PLoS One 11, e0153567 10.1371/journal.pone.0153567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rocha B. C., Marques P. E., Leoratti F. M. S., Junqueira C., Pereira D. B., Antonelli L. R. D. V., Menezes G. B., Golenbock D. T., Gazzinelli R. T. (2015) Type I interferon transcriptional signature in neutrophils and low-density granulocytes are associated with tissue damage in malaria. Cell Reports 13, 2829–2841 10.1016/j.celrep.2015.11.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yipp B. G., Petri B., Salina D., Jenne C. N., Scott B. N., Zbytnuik L. D., Pittman K., Asaduzzaman M., Wu K., Meijndert H. C., Malawista S. E., de Boisfleury Chevance A., Zhang K., Conly J., Kubes P. (2012) Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat. Med. 18, 1386–1393 10.1038/nm.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brinkmann V., Zychlinsky A. (2007) Beneficial suicide: why neutrophils die to make NETs. Nat. Rev. Microbiol. 5, 577–582 10.1038/nrmicro1710 [DOI] [PubMed] [Google Scholar]
- 31.De Meyer S. F., Suidan G. L., Fuchs T. A., Monestier M., Wagner D. D. (2012) Extracellular chromatin is an important mediator of ischemic stroke in mice. Arterioscler. Thromb. Vasc. Biol. 32, 1884–1891 10.1161/ATVBAHA.112.250993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yipp B. G., Kubes P. (2013) NETosis: how vital is it? Blood 122, 2784–2794 10.1182/blood-2013-04-457671 [DOI] [PubMed] [Google Scholar]
- 33.Mulder H. D., Augustijn Q. J., van Woensel J. B., Bos A. P., Juffermans N. P., Wösten-van Asperen R. M. (2015) Incidence, risk factors, and outcome of transfusion-related acute lung injury in critically ill children: a retrospective study. J. Crit. Care 30, 55–59 10.1016/j.jcrc.2014.10.005 [DOI] [PubMed] [Google Scholar]
- 34.Tariket S., Sut C., Hamzeh-Cognasse H., Laradi S., Pozzetto B., Garraud O., Cognasse F. (2016) Transfusion-related acute lung injury: transfusion, platelets and biological response modifiers. Expert Rev. Hematol. 9, 497–508 10.1586/17474086.2016.1152177 [DOI] [PubMed] [Google Scholar]
- 35.Thomas G. M., Carbo C., Curtis B. R., Martinod K., Mazo I. B., Schatzberg D., Cifuni S. M., Fuchs T. A., von Andrian U. H., Hartwig J. H., Aster R. H., Wagner D. D. (2012) Extracellular DNA traps are associated with the pathogenesis of TRALI in humans and mice. Blood 119, 6335–6343 10.1182/blood-2012-01-405183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Caudrillier A., Kessenbrock K., Gilliss B. M., Nguyen J. X., Marques M. B., Monestier M., Toy P., Werb Z., Looney M. R. (2012) Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J. Clin. Invest. 122, 2661–2671 10.1172/JCI61303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du Y., Liu X., Guo J. P., Liu X., Li R., Zhao Y., Liu X., Li M. H., Li Z. G. (2014) Association between PADI4 gene polymorphisms and anti-cyclic citrullinated peptide antibody positive rheumatoid arthritis in a large Chinese Han cohort. Clin. Exp. Rheumatol. 32, 377–382 [PubMed] [Google Scholar]
- 38.Reyes-Castillo Z., Palafox-Sánchez C. A., Parra-Rojas I., Martínez-Bonilla G. E., del Toro-Arreola S., Ramírez-Dueñas M. G., Ocampo-Bermudes G., Muñoz-Valle J. F. (2015) Comparative analysis of autoantibodies targeting peptidylarginine deiminase type 4, mutated citrullinated vimentin and cyclic citrullinated peptides in rheumatoid arthritis: associations with cytokine profiles, clinical and genetic features. Clin. Exp. Immunol. 182, 119–131 10.1111/cei.12677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sur Chowdhury C., Giaglis S., Walker U. A., Buser A., Hahn S., Hasler P. (2014) Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 16, R122 10.1186/ar4579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Carmona-Rivera C., Carlucci P. M., Moore E., Lingampalli N., Uchtenhagen H., James E., Liu Y., Bicker K. L., Wahamaa H., Hoffmann V., Catrina A. I., Thompson P., Buckner J. H., Robinson W. H., Fox D. A., Kaplan M. J. (2017) Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2, eaag3358 10.1126/sciimmunol.aag3358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta S., Kaplan M. J. (2016) The role of neutrophils and NETosis in autoimmune and renal diseases. Nat. Rev. Nephrol. 12, 402–413 10.1038/nrneph.2016.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fuchs T. A., Brill A., Duerschmied D., Schatzberg D., Monestier M., Myers D. D., Jr., Wrobleski S. K., Wakefield T. W., Hartwig J. H., Wagner D. D. (2010) Extracellular DNA traps promote thrombosis. Proc. Natl. Acad. Sci. USA 107, 15880–15885 10.1073/pnas.1005743107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinod K., Wagner D. D. (2014) Thrombosis: tangled up in NETs. Blood 123, 2768–2776 10.1182/blood-2013-10-463646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Massberg S., Grahl L., von Bruehl M. L., Manukyan D., Pfeiler S., Goosmann C., Brinkmann V., Lorenz M., Bidzhekov K., Khandagale A. B., Konrad I., Kennerknecht E., Reges K., Holdenrieder S., Braun S., Reinhardt C., Spannagl M., Preissner K. T., Engelmann B. (2010) Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat. Med. 16, 887–896 10.1038/nm.2184 [DOI] [PubMed] [Google Scholar]
- 45.Brill A., Fuchs T. A., Savchenko A. S., Thomas G. M., Martinod K., De Meyer S. F., Bhandari A. A., Wagner D. D. (2012) Neutrophil extracellular traps promote deep vein thrombosis in mice. J. Thromb. Haemost. 10, 136–144 10.1111/j.1538-7836.2011.04544.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.von Brühl M. L., Stark K., Steinhart A., Chandraratne S., Konrad I., Lorenz M., Khandoga A., Tirniceriu A., Coletti R., Köllnberger M., Byrne R. A., Laitinen I., Walch A., Brill A., Pfeiler S., Manukyan D., Braun S., Lange P., Riegger J., Ware J., Eckart A., Haidari S., Rudelius M., Schulz C., Echtler K., Brinkmann V., Schwaiger M., Preissner K. T., Wagner D. D., Mackman N., Engelmann B., Massberg S. (2012) Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J. Exp. Med. 209, 819–835 10.1084/jem.20112322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Etulain J., Martinod K., Wong S. L., Cifuni S. M., Schattner M., Wagner D. D. (2015) P-selectin promotes neutrophil extracellular trap formation in mice. Blood 126, 242–246 10.1182/blood-2015-01-624023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dyer M. R., Chen Q., Haldeman S., Yazdani H., Hoffman R., Loughran P., Tsung A., Zuckerbraun B. S., Simmons R. L., Neal M. D. (2018) Deep vein thrombosis in mice is regulated by platelet HMGB1 through release of neutrophil-extracellular traps and DNA. Sci. Rep. 8, 2068 10.1038/s41598-018-20479-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiménez-Alcázar M., Napirei M., Panda R., Köhler E. C., Kremer Hovinga J. A., Mannherz H. G., Peine S., Renné T., Lämmle B., Fuchs T. A. (2015) Impaired DNase1-mediated degradation of neutrophil extracellular traps is associated with acute thrombotic microangiopathies. J. Thromb. Haemost. 13, 732–742 10.1111/jth.12796 [DOI] [PubMed] [Google Scholar]
- 50.Savchenko A. S., Martinod K., Seidman M. A., Wong S. L., Borissoff J. I., Piazza G., Libby P., Goldhaber S. Z., Mitchell R. N., Wagner D. D. (2014) Neutrophil extracellular traps form predominantly during the organizing stage of human venous thromboembolism development. J. Thromb. Haemost. 12, 860–870 10.1111/jth.12571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Diaz J. A., Fuchs T. A., Jackson T. O., Kremer Hovinga J. A., Lämmle B., Henke P. K., Myers D. D., Jr., Wagner D. D., Wakefield T. W.; Michigan Research Venous Group (2013) Plasma DNA is elevated in patients with deep vein thrombosis. J. Vasc. Surg. Venous Lymphat. Disord. 1, 341–348.e1 10.1016/j.jvsv.2012.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Montfoort M. L., Stephan F., Lauw M. N., Hutten B. A., Van Mierlo G. J., Solati S., Middeldorp S., Meijers J. C., Zeerleder S. (2013) Circulating nucleosomes and neutrophil activation as risk factors for deep vein thrombosis. Arterioscler. Thromb. Vasc. Biol. 33, 147–151 10.1161/ATVBAHA.112.300498 [DOI] [PubMed] [Google Scholar]
- 53.Jiménez-Alcázar M., Limacher A., Panda R., Méan M., Bitterling J., Peine S., Renné T., Beer J. H., Aujesky D., Lämmle B., Fuchs T. A. (2018) Circulating extracellular DNA is an independent predictor of mortality in elderly patients with venous thromboembolism. PLoS One 13, e0191150 10.1371/journal.pone.0191150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spengler J., Lugonja B., Ytterberg A. J., Zubarev R. A., Creese A. J., Pearson M. J., Grant M. M., Milward M., Lundberg K., Buckley C. D., Filer A., Raza K., Cooper P. R., Chapple I. L., Scheel-Toellner D. (2015) Release of active peptidyl arginine deiminases by neutrophils can explain production of extracellular citrullinated autoantigens in rheumatoid arthritis synovial fluid. Arthritis. Rheumatol. 67, 3135–3145 10.1002/art.39313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sorvillo N., Martinod K., Staudinger C., Wagner D. (2017) PAD4 citrullination of ADAMTS13: a new link between NETosis and thrombosis. Res. Pract. Thromb. Haemost. 1, 254 [Google Scholar]
- 56.Tilvawala R., Nguyen S. H., Maurais A. J., Nemmara V. V., Nagar M., Salinger A. J., Nagpal S., Weerapana E., Thompson P. R. (2018) The rheumatoid arthritis-associated citrullinome. [E-pub ahead of print] Cell Chem. Biol. DOI: 10.1016/j.chembiol.2018.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Savchenko A. S., Borissoff J. I., Martinod K., De Meyer S. F., Gallant M., Erpenbeck L., Brill A., Wang Y., Wagner D. D. (2014) VWF-mediated leukocyte recruitment with chromatin decondensation by PAD4 increases myocardial ischemia/reperfusion injury in mice. Blood 123, 141–148 10.1182/blood-2013-07-514992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novotny J., Chandraratne S., Weinberger T., Philippi V., Stark K., Ehrlich A., Pircher J., Konrad I., Oberdieck P., Titova A., Hoti Q., Schubert I., Legate K. R., Urtz N., Lorenz M., Pelisek J., Massberg S., von Brühl M. L., Schulz C. (2018) Histological comparison of arterial thrombi in mice and men and the influence of Cl-amidine on thrombus formation. PLoS One 13, e0190728 10.1371/journal.pone.0190728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mangold A., Alias S., Scherz T., Hofbauer T., Jakowitsch J., Panzenböck A., Simon D., Laimer D., Bangert C., Kammerlander A., Mascherbauer J., Winter M. P., Distelmaier K., Adlbrecht C., Preissner K. T., Lang I. M. (2015) Coronary neutrophil extracellular trap burden and deoxyribonuclease activity in ST-elevation acute coronary syndrome are predictors of ST-segment resolution and infarct size. Circ. Res. 116, 1182–1192 10.1161/CIRCRESAHA.116.304944 [DOI] [PubMed] [Google Scholar]
- 60.Vallés J., Lago A., Santos M. T., Latorre A. M., Tembl J. I., Salom J. B., Nieves C., Moscardó A. (2017) Neutrophil extracellular traps are increased in patients with acute ischemic stroke: prognostic significance. Thromb. Haemost. 117, 1919–1929 10.1160/TH17-02-0130 [DOI] [PubMed] [Google Scholar]
- 61.Ducroux C., Di Meglio L., Loyau S., Delbosc S., Boisseau W., Deschildre C., Ben Maacha M., Blanc R., Redjem H., Ciccio G., Smajda S., Fahed R., Michel J. B., Piotin M., Salomon L., Mazighi M., Ho-Tin-Noe B., Desilles J. P. (2018) Thrombus neutrophil extracellular traps content impair tPA-induced thrombolysis in acute ischemic stroke. Stroke 49, 754–757 10.1161/STROKEAHA.117.019896 [DOI] [PubMed] [Google Scholar]
- 62.Laridan E., Denorme F., Desender L., François O., Andersson T., Deckmyn H., Vanhoorelbeke K., De Meyer S. F. (2017) Neutrophil extracellular traps in ischemic stroke thrombi. Ann. Neurol. 82, 223–232 10.1002/ana.24993 [DOI] [PubMed] [Google Scholar]
- 63.Dvorak H. F. (1986) Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 315, 1650–1659 10.1056/NEJM198612253152606 [DOI] [PubMed] [Google Scholar]
- 64.Dvorak H. F. (2015) Tumors: wounds that do not heal-redux. Cancer Immunol. Res. 3, 1–11 10.1158/2326-6066.CIR-14-0209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demers M., Krause D. S., Schatzberg D., Martinod K., Voorhees J. R., Fuchs T. A., Scadden D. T., Wagner D. D. (2012) Cancers predispose neutrophils to release extracellular DNA traps that contribute to cancer-associated thrombosis. Proc. Natl. Acad. Sci. USA 109, 13076–13081 10.1073/pnas.1200419109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marin Oyarzún C. P., Carestia A., Lev P. R., Glembotsky A. C., Castro Ríos M. A., Moiraghi B., Molinas F. C., Marta R. F., Schattner M., Heller P. G. (2016) Neutrophil extracellular trap formation and circulating nucleosomes in patients with chronic myeloproliferative neoplasms. Sci. Rep. 6, 38738 10.1038/srep38738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wolach O., Sellar R. S., Martinod K., Cherpokova D., McConkey M., Chappell R. J., Silver A. J., Adams D., Castellano C. A., Schneider R. K., Padera R. F., DeAngelo D. J., Wadleigh M., Steensma D. P., Galinsky I., Stone R. M., Genovese G., McCarroll S. A., Iliadou B., Hultman C., Neuberg D., Mullally A., Wagner D. D., Ebert B. L. (2018) Increased neutrophil extracellular trap formation promotes thrombosis in myeloproliferative neoplasms. Sci. Transl. Med. 10, eaan8292 10.1126/scitranslmed.aan8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Abdol Razak N., Elaskalani O., Metharom P. (2017) Pancreatic cancer-induced neutrophil extracellular traps: a potential contributor to cancer-associated thrombosis. Int. J. Mol. Sci. 18, 487 10.3390/ijms18030487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang C., Sun W., Cui W., Li X., Yao J., Jia X., Li C., Wu H., Hu Z., Zou X. (2015) Procoagulant role of neutrophil extracellular traps in patients with gastric cancer. Int. J. Clin. Exp. Pathol. 8, 14075–14086 [PMC free article] [PubMed] [Google Scholar]
- 70.Gong L., Cai Y., Zhou X., Yang H. (2012) Activated platelets interact with lung cancer cells through P-selectin glycoprotein ligand-1. Pathol. Oncol. Res. 18, 989–996 10.1007/s12253-012-9531-y [DOI] [PubMed] [Google Scholar]
- 71.Wagner D. D., Frenette P. S. (2008) The vessel wall and its interactions. Blood 111, 5271–5281 10.1182/blood-2008-01-078204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kolaczkowska E., Jenne C. N., Surewaard B. G., Thanabalasuriar A., Lee W. Y., Sanz M. J., Mowen K., Opdenakker G., Kubes P. (2015) Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat. Commun. 6, 6673 10.1038/ncomms7673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mauracher L. M., Posch F., Martinod K., Grilz E., Däullary T., Hell L., Brostjan C., Zielinski C., Ay C., Wagner D. D., Pabinger I., Thaler J. (2018) Citrullinated histone H3, a biomarker of neutrophil extracellular trap formation, predicts the risk of venous thromboembolism in cancer patients. J. Thromb. Haemost. 16, 508–518 10.1111/jth.13951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thålin C., Demers M., Blomgren B., Wong S. L., von Arbin M., von Heijne A., Laska A. C., Wallén H., Wagner D. D., Aspberg S. (2016) NETosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb. Res. 139, 56–64 10.1016/j.thromres.2016.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Thålin C., Lundström S., Seignez C., Daleskog M., Lundström A., Henriksson P., Helleday T., Phillipson M., Wallén H., Demers M. (2018) Citrullinated histone H3 as a novel prognostic blood marker in patients with advanced cancer. PLoS One 13, e0191231 10.1371/journal.pone.0191231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Falanga A., Russo L., Milesi V., Vignoli A. (2017) Mechanisms and risk factors of thrombosis in cancer. Crit. Rev. Oncol. Hematol. 118, 79–83 10.1016/j.critrevonc.2017.08.003 [DOI] [PubMed] [Google Scholar]
- 77.Leal A. C., Mizurini D. M., Gomes T., Rochael N. C., Saraiva E. M., Dias M. S., Werneck C. C., Sielski M. S., Vicente C. P., Monteiro R. Q. (2017) Tumor-derived exosomes induce the formation of neutrophil extracellular traps: implications for the establishment of cancer-associated thrombosis. Sci. Rep. 7, 6438 10.1038/s41598-017-06893-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thomas G. M., Brill A., Mezouar S., Crescence L., Gallant M., Dubois C., Wagner D. D. (2015) Tissue factor expressed by circulating cancer cell-derived microparticles drastically increases the incidence of deep vein thrombosis in mice. J. Thromb. Haemost. 13, 1310–1319 10.1111/jth.13002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hell L., Thaler J., Martinod K., Ay C., Posch F., Wagner D. D., Pabinger I. (2016) OC-16 - neutrophil extracellular traps and tissue factor-bearing microvesicles: a liaison dangereuse causing overt DIC in cancer patients? Thromb. Res. 140 (Suppl 1), S174–S175 10.1016/S0049-3848(16)30133-5 [DOI] [PubMed] [Google Scholar]
- 80.Cedervall J., Zhang Y., Huang H., Zhang L., Femel J., Dimberg A., Olsson A. K. (2015) Neutrophil extracellular traps accumulate in peripheral blood vessels and compromise organ function in tumor-bearing animals. Cancer Res. 75, 2653–2662 10.1158/0008-5472.CAN-14-3299 [DOI] [PubMed] [Google Scholar]
- 81.Ho-Tin-Noé B., Carbo C., Demers M., Cifuni S. M., Goerge T., Wagner D. D. (2009) Innate immune cells induce hemorrhage in tumors during thrombocytopenia. Am. J. Pathol. 175, 1699–1708 10.2353/ajpath.2009.090460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Berger-Achituv S., Brinkmann V., Abed U. A., Kühn L. I., Ben-Ezra J., Elhasid R., Zychlinsky A. (2013) A proposed role for neutrophil extracellular traps in cancer immunoediting. Front. Immunol. 4, 48 10.3389/fimmu.2013.00048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Demers M., Wong S. L., Martinod K., Gallant M., Cabral J. E., Wang Y., Wagner D. D. (2016) Priming of neutrophils toward NETosis promotes tumor growth. OncoImmunology 5, e1134073 10.1080/2162402X.2015.1134073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Podaza E., Sabbione F., Risnik D., Borge M., Almejún M. B., Colado A., Fernández-Grecco H., Cabrejo M., Bezares R. F., Trevani A., Gamberale R., Giordano M. (2017) Neutrophils from chronic lymphocytic leukemia patients exhibit an increased capacity to release extracellular traps (NETs). Cancer Immunol. Immunother. 66, 77–89 10.1007/s00262-016-1921-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cools-Lartigue J., Spicer J., McDonald B., Gowing S., Chow S., Giannias B., Bourdeau F., Kubes P., Ferri L. (2013) Neutrophil extracellular traps sequester circulating tumor cells and promote metastasis. J. Clin. Invest. 123, 3446–3458 10.1172/JCI67484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Park J., Wysocki R. W., Amoozgar Z., Maiorino L., Fein M. R., Jorns J., Schott A. F., Kinugasa-Katayama Y., Lee Y., Won N. H., Nakasone E. S., Hearn S. A., Küttner V., Qiu J., Almeida A. S., Perurena N., Kessenbrock K., Goldberg M. S., Egeblad M. (2016) Cancer cells induce metastasis-supporting neutrophil extracellular DNA traps. Sci. Transl. Med. 8, 361ra138 10.1126/scitranslmed.aag1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tohme S., Yazdani H. O., Al-Khafaji A. B., Chidi A. P., Loughran P., Mowen K., Wang Y., Simmons R. L., Huang H., Tsung A. (2016) Neutrophil extracellular traps promote the development and progression of liver metastases after surgical stress. Cancer Res. 76, 1367–1380 10.1158/0008-5472.CAN-15-1591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hawes M. C., Wen F., Elquza E. (2015) Extracellular DNA: a bridge to cancer. Cancer Res. 75, 4260–4264 10.1158/0008-5472.CAN-15-1546 [DOI] [PubMed] [Google Scholar]
- 89.Najmeh S., Cools-Lartigue J., Rayes R. F., Gowing S., Vourtzoumis P., Bourdeau F., Giannias B., Berube J., Rousseau S., Ferri L. E., Spicer J. D. (2017) Neutrophil extracellular traps sequester circulating tumor cells via β1-integrin mediated interactions. Int. J. Cancer 140, 2321–2330 10.1002/ijc.30635 [DOI] [PubMed] [Google Scholar]
- 90.Kanamaru R., Ohzawa H., Miyato H., Matsumoto S., Haruta H., Kurashina K., Saito S., Hosoya Y., Yamaguchi H., Yamashita H., Seto Y., Lefor A. K., Sata N., Kitayama J. (2018) Low density neutrophils (LDN) in postoperative abdominal cavity assist the peritoneal recurrence through the production of neutrophil extracellular traps (NETs). Sci. Rep. 8, 632 10.1038/s41598-017-19091-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Spinelli J. B., Yoon H., Ringel A. E., Jeanfavre S., Clish C. B., Haigis M. C. (2017) Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science 358, 941–946 10.1126/science.aam9305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alexandraki K. I., Piperi C., Ziakas P. D., Apostolopoulos N. V., Makrilakis K., Syriou V., Diamanti-Kandarakis E., Kaltsas G., Kalofoutis A. (2008) Cytokine secretion in long-standing diabetes mellitus type 1 and 2: associations with low-grade systemic inflammation. J. Clin. Immunol. 28, 314–321 10.1007/s10875-007-9164-1 [DOI] [PubMed] [Google Scholar]
- 93.Carestia A., Frechtel G., Cerrone G., Linari M. A., Gonzalez C. D., Casais P., Schattner M. (2016) NETosis before and after hyperglycemic control in type 2 diabetes mellitus patients. PLoS One 11, e0168647 10.1371/journal.pone.0168647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Karima M., Kantarci A., Ohira T., Hasturk H., Jones V. L., Nam B. H., Malabanan A., Trackman P. C., Badwey J. A., Van Dyke T. E. (2005) Enhanced superoxide release and elevated protein kinase C activity in neutrophils from diabetic patients: association with periodontitis. J. Leukoc. Biol. 78, 862–870 10.1189/jlb.1004583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wong S. L., Demers M., Martinod K., Gallant M., Wang Y., Goldfine A. B., Kahn C. R., Wagner D. D. (2015) Diabetes primes neutrophils to undergo NETosis, which impairs wound healing. Nat. Med. 21, 815–819 10.1038/nm.3887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Joshi M. B., Baipadithaya G., Balakrishnan A., Hegde M., Vohra M., Ahamed R., Nagri S. K., Ramachandra L., Satyamoorthy K. (2016) Elevated homocysteine levels in type 2 diabetes induce constitutive neutrophil extracellular traps. Sci. Rep. 6, 36362 10.1038/srep36362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Menegazzo L., Ciciliot S., Poncina N., Mazzucato M., Persano M., Bonora B., Albiero M., Vigili de Kreutzenberg S., Avogaro A., Fadini G. P. (2015) NETosis is induced by high glucose and associated with type 2 diabetes. Acta Diabetol. 52, 497–503 10.1007/s00592-014-0676-x [DOI] [PubMed] [Google Scholar]
- 98.Miyoshi A., Yamada M., Shida H., Nakazawa D., Kusunoki Y., Nakamura A., Miyoshi H., Tomaru U., Atsumi T., Ishizu A. (2016) Circulating neutrophil extracellular trap levels in well-controlled type 2 diabetes and pathway involved in their formation induced by high-dose glucose. Pathobiology 83, 243–251 10.1159/000444881 [DOI] [PubMed] [Google Scholar]
- 99.Stoikou M., Grimolizzi F., Giaglis S., Schäfer G., van Breda S. V., Hoesli I. M., Lapaire O., Huhn E. A., Hasler P., Rossi S. W., Hahn S. (2017) Gestational diabetes mellitus is associated with altered neutrophil activity. Front. Immunol. 8, 702 10.3389/fimmu.2017.00702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Erpenbeck L., Chowdhury C. S., Zsengellér Z. K., Gallant M., Burke S. D., Cifuni S., Hahn S., Wagner D. D., Karumanchi S. A. (2016) PAD4 deficiency decreases inflammation and susceptibility to pregnancy loss in a mouse model. Biol. Reprod. 95, 132 10.1095/biolreprod.116.140293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mizugishi K., Yamashita K. (2017) Neutrophil extracellular traps are critical for pregnancy loss in sphingosine kinase-deficient mice on 129Sv/C57BL/6 background. FASEB J. 31, 5577–5591 10.1096/fj.201700399RR [DOI] [PubMed] [Google Scholar]
- 102.Rosenstein M. G., Cheng Y. W., Snowden J. M., Nicholson J. M., Doss A. E., Caughey A. B. (2012) The risk of stillbirth and infant death stratified by gestational age in women with gestational diabetes. Am. J. Obstet. Gynecol. 206, 309.e1–309.e7 10.1016/j.ajog.2012.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fadini G. P., Menegazzo L., Rigato M., Scattolini V., Poncina N., Bruttocao A., Ciciliot S., Mammano F., Ciubotaru C. D., Brocco E., Marescotti M. C., Cappellari R., Arrigoni G., Millioni R., Vigili de Kreutzenberg S., Albiero M., Avogaro A. (2016) NETosis delays diabetic wound healing in mice and humans. Diabetes 65, 1061–1071 10.2337/db15-0863 [DOI] [PubMed] [Google Scholar]
- 104.Stavrou E. X., Fang C., Bane K. L., Long A. T., Naudin C., Kucukal E., Gandhi A., Brett-Morris A., Mumaw M. M., Izadmehr S., Merkulova A., Reynolds C. C., Alhalabi O., Nayak L., Yu W. M., Qu C. K., Meyerson H. J., Dubyak G. R., Gurkan U. A., Nieman M. T., Sen Gupta A., Renné T., Schmaier A. H. (2018) Factor XII and uPAR upregulate neutrophil functions to influence wound healing. J. Clin. Invest. 128, 944–959 10.1172/JCI92880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Laakso M., Kuusisto J. (2014) Insulin resistance and hyperglycaemia in cardiovascular disease development. Nat. Rev. Endocrinol. 10, 293–302 10.1038/nrendo.2014.29 [DOI] [PubMed] [Google Scholar]
- 106.Morel O., Jesel L., Abbas M., Morel N. (2013) Prothrombotic changes in diabetes mellitus. Semin. Thromb. Hemost. 39, 477–488 10.1055/s-0033-1343888 [DOI] [PubMed] [Google Scholar]
- 107.Park J. H., Kim J. E., Gu J. Y., Yoo H. J., Park S. H., Kim Y. I., Nam-Goong I. S., Kim E. S., Kim H. K. (2016) Evaluation of circulating markers of neutrophil extracellular trap (NET) formation as risk factors for diabetic retinopathy in a case-control association study. Exp. Clin. Endocrinol. Diabetes 124, 557–561 10.1055/s-0042-101792 [DOI] [PubMed] [Google Scholar]
- 108.Yau J. W., Rogers S. L., Kawasaki R., Lamoureux E. L., Kowalski J. W., Bek T., Chen S. J., Dekker J. M., Fletcher A., Grauslund J., Haffner S., Hamman R. F., Ikram M. K., Kayama T., Klein B. E., Klein R., Krishnaiah S., Mayurasakorn K., O’Hare J. P., Orchard T. J., Porta M., Rema M., Roy M. S., Sharma T., Shaw J., Taylor H., Tielsch J. M., Varma R., Wang J. J., Wang N., West S., Xu L., Yasuda M., Zhang X., Mitchell P., Wong T. Y.; Meta-Analysis for Eye Disease (META-EYE) Study Group (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35, 556–564 10.2337/dc11-1909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Y., Xiao Y., Zhong L., Ye D., Zhang J., Tu Y., Bornstein S. R., Zhou Z., Lam K. S., Xu A. (2014) Increased neutrophil elastase and proteinase 3 and augmented NETosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes 63, 4239–4248 10.2337/db14-0480 [DOI] [PubMed] [Google Scholar]
- 110.Diana J., Simoni Y., Furio L., Beaudoin L., Agerberth B., Barrat F., Lehuen A. (2013) Crosstalk between neutrophils, B-1a cells and plasmacytoid dendritic cells initiates autoimmune diabetes. Nat. Med. 19, 65–73 10.1038/nm.3042 [DOI] [PubMed] [Google Scholar]
- 111.Lang J., Wang X., Liu K., He D., Niu P., Cao R., Jin L., Wu J. (2017) Oral delivery of staphylococcal nuclease by Lactococcus lactis prevents type 1 diabetes mellitus in NOD mice. Appl. Microbiol. Biotechnol. 101, 7653–7662 10.1007/s00253-017-8480-5 [DOI] [PubMed] [Google Scholar]
- 112.Cakman I., Kirchner H., Rink L. (1997) Zinc supplementation reconstitutes the production of interferon-alpha by leukocytes from elderly persons. J. Interferon Cytokine Res. 17, 469–472 10.1089/jir.1997.17.469 [DOI] [PubMed] [Google Scholar]
- 113.Martinod K., Witsch T., Erpenbeck L., Savchenko A., Hayashi H., Cherpokova D., Gallant M., Mauler M., Cifuni S. M., Wagner D. D. (2017) Peptidylarginine deiminase 4 promotes age-related organ fibrosis. J. Exp. Med. 214, 439–458 10.1084/jem.20160530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Cui H., Kong Y., Zhang H. (2012) Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 646354 10.1155/2012/646354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Clark S. R., Ma A. C., Tavener S. A., McDonald B., Goodarzi Z., Kelly M. M., Patel K. D., Chakrabarti S., McAvoy E., Sinclair G. D., Keys E. M., Allen-Vercoe E., Devinney R., Doig C. J., Green F. H., Kubes P. (2007) Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 13, 463–469 10.1038/nm1565 [DOI] [PubMed] [Google Scholar]
- 116.Rossaint J., Herter J. M., Van Aken H., Napirei M., Döring Y., Weber C., Soehnlein O., Zarbock A. (2014) Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood 123, 2573–2584 10.1182/blood-2013-07-516484 [DOI] [PubMed] [Google Scholar]
- 117.Gourdie R. G., Dimmeler S., Kohl P. (2016) Novel therapeutic strategies targeting fibroblasts and fibrosis in heart disease. Nat. Rev. Drug Discov. 15, 620–638 10.1038/nrd.2016.89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bengtsson T., Lindström M. (2003) Airborne infectious diseases during infancy and mortality in later life in southern Sweden, 1766-1894. Int. J. Epidemiol. 32, 286–294 10.1093/ije/dyg061 [DOI] [PubMed] [Google Scholar]
- 119.Finch C. E., Crimmins E. M. (2004) Inflammatory exposure and historical changes in human life-spans. Science 305, 1736–1739 10.1126/science.1092556 [DOI] [PubMed] [Google Scholar]
- 120.Zenaro E., Pietronigro E., Della Bianca V., Piacentino G., Marongiu L., Budui S., Turano E., Rossi B., Angiari S., Dusi S., Montresor A., Carlucci T., Nanì S., Tosadori G., Calciano L., Catalucci D., Berton G., Bonetti B., Constantin G. (2015) Neutrophils promote Alzheimer’s disease-like pathology and cognitive decline via LFA-1 integrin. Nat. Med. 21, 880–886 10.1038/nm.3913 [DOI] [PubMed] [Google Scholar]
- 121.Acharya N. K., Nagele E. P., Han M., Coretti N. J., DeMarshall C., Kosciuk M. C., Boulos P. A., Nagele R. G. (2012) Neuronal PAD4 expression and protein citrullination: possible role in production of autoantibodies associated with neurodegenerative disease. J. Autoimmun. 38, 369–380 10.1016/j.jaut.2012.03.004 [DOI] [PubMed] [Google Scholar]
- 122.Asaga H., Ishigami A. (2001) Protein deimination in the rat brain after kainate administration: citrulline-containing proteins as a novel marker of neurodegeneration. Neurosci. Lett. 299, 5–8 10.1016/S0304-3940(00)01735-3 [DOI] [PubMed] [Google Scholar]
- 123.Asaga H., Akiyama K., Ohsawa T., Ishigami A. (2002) Increased and type II-specific expression of peptidylarginine deiminase in activated microglia but not hyperplastic astrocytes following kainic acid-evoked neurodegeneration in the rat brain. Neurosci. Lett. 326, 129–132 10.1016/S0304-3940(02)00334-8 [DOI] [PubMed] [Google Scholar]
- 124.Akiyama K., Sakurai Y., Asou H., Senshu T. (1999) Localization of peptidylarginine deiminase type II in a stage-specific immature oligodendrocyte from rat cerebral hemisphere. Neurosci. Lett. 274, 53–55 10.1016/S0304-3940(99)00678-3 [DOI] [PubMed] [Google Scholar]
- 125.Mastronardi F. G., Wood D. D., Mei J., Raijmakers R., Tseveleki V., Dosch H. M., Probert L., Casaccia-Bonnefil P., Moscarello M. A. (2006) Increased citrullination of histone H3 in multiple sclerosis brain and animal models of demyelination: a role for tumor necrosis factor-induced peptidylarginine deiminase 4 translocation. J. Neurosci. 26, 11387–11396 10.1523/JNEUROSCI.3349-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Leshner M., Wang S., Lewis C., Zheng H., Chen X. A., Santy L., Wang Y. (2012) PAD4 mediated histone hypercitrullination induces heterochromatin decondensation and chromatin unfolding to form neutrophil extracellular trap-like structures. Front. Immunol. 3, 307 10.3389/fimmu.2012.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wen F., White G. J., VanEtten H. D., Xiong Z., Hawes M. C. (2009) Extracellular DNA is required for root tip resistance to fungal infection. Plant Physiol. 151, 820–829 10.1104/pp.109.142067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hawes M., Allen C., Turgeon B. G., Curlango-Rivera G., Minh Tran T., Huskey D. A., Xiong Z. (2016) Root border cells and their role in plant defense. Annu. Rev. Phytopathol. 54, 143–161 10.1146/annurev-phyto-080615-100140 [DOI] [PubMed] [Google Scholar]
- 129.Minh Tran T., MacIntyre A., Khokhani D., Hawes M., Allen C. (2016) Extracellular DNases of Ralstonia solanacearum modulate biofilms and facilitate bacterial wilt virulence. Environ. Microbiol. 18, 4103–4117 10.1111/1462-2920.13446 [DOI] [PubMed] [Google Scholar]
- 130.Hong W., Juneau R. A., Pang B., Swords W. E. (2009) Survival of bacterial biofilms within neutrophil extracellular traps promotes nontypeable Haemophilus influenzae persistence in the chinchilla model for otitis media. J. Innate Immun. 1, 215–224 10.1159/000205937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.White P. C., Chicca I. J., Cooper P. R., Milward M. R., Chapple I. L. (2016) Neutrophil extracellular traps in periodontitis: a web of intrigue. J. Dent. Res. 95, 26–34 10.1177/0022034515609097 [DOI] [PubMed] [Google Scholar]
- 132.Kenny E. F., Herzig A., Krüger R., Muth A., Mondal S., Thompson P. R., Brinkmann V., Bernuth H. V., Zychlinsky A. (2017) Diverse stimuli engage different neutrophil extracellular trap pathways. eLife 6, e24437 10.7554/eLife.24437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lewis H. D., Liddle J., Coote J. E., Atkinson S. J., Barker M. D., Bax B. D., Bicker K. L., Bingham R. P., Campbell M., Chen Y. H., Chung C. W., Craggs P. D., Davis R. P., Eberhard D., Joberty G., Lind K. E., Locke K., Maller C., Martinod K., Patten C., Polyakova O., Rise C. E., Rüdiger M., Sheppard R. J., Slade D. J., Thomas P., Thorpe J., Yao G., Drewes G., Wagner D. D., Thompson P. R., Prinjha R. K., Wilson D. M. (2015) Inhibition of PAD4 activity is sufficient to disrupt mouse and human NET formation. Nat. Chem. Biol. 11, 189–191 10.1038/nchembio.1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Goldmann O., Medina E. (2013) The expanding world of extracellular traps: not only neutrophils but much more. Front. Immunol. 3, 420 10.3389/fimmu.2012.00420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Liu P., Wu X., Liao C., Liu X., Du J., Shi H., Wang X., Bai X., Peng P., Yu L., Wang F., Zhao Y., Liu M. (2014) Escherichia coli and Candida albicans induced macrophage extracellular trap-like structures with limited microbicidal activity. PLoS One 9, e90042 10.1371/journal.pone.0090042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ueki S., Konno Y., Takeda M., Moritoki Y., Hirokawa M., Matsuwaki Y., Honda K., Ohta N., Yamamoto S., Takagi Y., Wada A., Weller P. F. (2016) Eosinophil extracellular trap cell death-derived DNA traps: their presence in secretions and functional attributes. J. Allergy Clin. Immunol. 137, 258–267 10.1016/j.jaci.2015.04.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Okubo K., Kurosawa M., Kamiya M., Urano Y., Suzuki A., Yamamoto K., Hase K., Homma K., Sasaki J., Miyauchi H., Hoshino T., Hayashi M., Mayadas T. N., Hirahashi J. (2018) Macrophage extracellular trap formation promoted by platelet activation is a key mediator of rhabdomyolysis-induced acute kidney injury. Nat. Med. 24, 232–238 10.1038/nm.4462 [DOI] [PubMed] [Google Scholar]
- 138.Gaertner F., Massberg S. (2016) Blood coagulation in immunothrombosis: at the frontline of intravascular immunity. Semin. Immunol. 28, 561–569 10.1016/j.smim.2016.10.010 [DOI] [PubMed] [Google Scholar]
- 139.Stephen J., Scales H. E., Benson R. A., Erben D., Garside P., Brewer J. M. (2017) Neutrophil swarming and extracellular trap formation play a significant role in Alum adjuvant activity. NPJ vaccines 2, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Papayannopoulos V. (2018) Neutrophil extracellular traps in immunity and disease. Nat. Rev. Immunol. 18, 134–147 10.1038/nri.2017.105 [DOI] [PubMed] [Google Scholar]
- 141.Bernimoulin M., Waters E. K., Foy M., Steele B. M., Sullivan M., Falet H., Walsh M. T., Barteneva N., Geng J. G., Hartwig J. H., Maguire P. B., Wagner D. D. (2009) Differential stimulation of monocytic cells results in distinct populations of microparticles. J. Thromb. Haemost. 7, 1019–1028 10.1111/j.1538-7836.2009.03434.x [DOI] [PMC free article] [PubMed] [Google Scholar]