Abstract
The gut microbiota regulates key hepatic functions, notably through the production of bacterial metabolites that are transported via the portal circulation. We evaluated the effects of metabolites produced by the gut microbiota from aromatic amino acids (phenylacetate, benzoate, p-cresol, and indole) on liver inflammation induced by bacterial endotoxin. Precision-cut liver slices prepared from control mice, Kupffer cell (KC)-depleted mice, and obese mice (ob/ob) were treated with or without LPS and bacterial metabolites. We observed beneficial effects of indole that dose-dependently reduced the LPS-induced up-regulation of proinflammatory mediators at both mRNA and protein levels in precision-cut liver slices prepared from control or ob/ob mice. KC depletion partly prevented the antiinflammatory effects of indole, notably through a reduction of nucleotide-binding domain and leucine-rich repeat containing (NLR) family pyrin domain-containing 3 (NLRP3) pathway activation. In vivo, the oral administration of indole before an LPS injection reduced the expression of key proteins of the NF-κB pathway and downstream proinflammatory gene up-regulation. Indole also prevented LPS-induced alterations of cholesterol metabolism through a transcriptional regulation associated with increased 4β-hydroxycholesterol hepatic levels. In summary, indole appears as a bacterial metabolite produced from tryptophan that is able to counteract the detrimental effects of LPS in the liver. Indole could be a new target to develop innovative strategies to decrease hepatic inflammation.—Beaumont, M., Neyrinck, A. M., Olivares, M., Rodriguez, J., de Rocca Serra, A., Roumain, M., Bindels, L. B., Cani, P. D., Evenepoel, P., Muccioli, G. G., Demoulin, J.-B., Delzenne, N. M. The gut microbiota metabolite indole alleviates liver inflammation in mice.
Keywords: gut–liver axis, LPS, Kupffer cells, cholesterol metabolism, PCLS
As a result of its anatomic position in the digestive system, the liver is constantly exposed to bacterial compounds coming from the gut (1). LPS, also known as endotoxins (components of gram-negative bacteria outer membrane), can translocate to the liver via the portal circulation, especially when the gut barrier is impaired, as shown, for example, during diet-induced obesity or in genetic obese (ob/ob) mice (2–4). Although the hepatic immune system is tolerogenic in physiologic conditions, exposure to high levels of LPS induces the release of large amounts of proinflammatory mediators, including upon activation of the resident macrophages–Kupffer cells (KC) (5). Endotoxemia and inflammation are common features of most chronic liver diseases such as nonalcoholic fatty liver disease (NAFLD), nonalcoholic steatohepatitis, and alcoholic hepatitis, and they contribute to their progression (6–8). Targeting liver inflammation therefore appears to be a promising strategy to support or restore hepatic homeostasis.
In the last decade, the gut microbiota has emerged as a key regulator of liver physiology through the gut–liver axis (4, 9). Liver inflammation has been associated with an alteration of the gut microbiota composition in patients with nonalcoholic steatohepatitis and alcoholic hepatitis (10, 11). The causal role of this dysbiosis has been demonstrated by transferring the microbiota of patients with severe alcoholic hepatitis into mice (10). Among the potential mechanisms involved, regulation of the gut barrier function by intestinal bacteria has been shown to play a central role in microbiota–liver interactions (12). In addition, microbiota-derived bioactive molecules are considered to be key regulators of immunity in organs at a distance from the gut, notably the liver (13). Indeed, except for the gut itself, the liver is the most exposed organ to metabolites produced by the gut microbiota (4). Bacterial products such as short-chain fatty acids, trimethylamine, ethanol, and secondary bile acids have been shown to regulate liver homeostasis (12, 14). However, only a few of the wide variety of microbiota-derived metabolites have been tested for their effects on liver inflammation.
Comparison of the metabolome of conventional and germ-free or antibiotic-treated mice revealed that the microbiota largely contributes to the peripheral plasma or urine metabolome, which are downstream the liver (15, 16). One of the most striking findings of these studies is that the liver is exposed to microbial metabolites produced from aromatic amino acids (AAA) such as phenylacetate, benzoate, p-cresol, and indole (17). Indeed, the concentrations of the corresponding host–microbiota cometabolites (i.e., bacterial metabolites modified by host enzymes), namely phenylacetylglycine, hippurate, p-cresyl sulfate, and indoxyl-3-sulfate (I3S), have been strongly linked to the presence of the gut microbiota (15, 16). However, the effects of these microbiota-derived compounds on liver inflammation have not been studied so far.
In this study, we first tested the effects of AAA-derived bacterial metabolites on liver inflammation induced by LPS using the model of precision-cut liver slices (PCLS). This tissue culture system preserves the interactions among the different types of hepatic cells in their original tissue matrix, providing a complex ex vivo model to study microbiota-derived metabolite–liver interactions (18). We identified the tryptophan-derived bacterial metabolite indole as a potent antiinflammatory molecule in the liver, and we confirmed this effect in PCLS prepared from genetically obese ob/ob mice, which we used as a model of NAFLD associated with chronic liver inflammation. KC depletion experiments allowed us to highlight the partial contribution of these cells to the antiinflammatory effects of indole. Finally, oral administration of indole in mice reduced the LPS-induced liver inflammation in association with a regulation of hepatic cholesterol metabolism, as revealed by transcriptome profiling.
MATERIALS AND METHODS
Animals
All mice were purchased from Janvier Labs (Le Genest-Saint-Isle, France) and were maintained in a specific pathogen-free environment. Animals were housed in groups of 3 mice per cage in a controlled environment (12-h day/night light cycle) with free access to water and food (AIN-93M; Research Diets, New Brunswick, NJ, USA) for 1 wk before experiments. Housing conditions were as specified by the Belgian law of May 29, 2013, on Protection of Laboratory Animals (Agreement LA 1230314). Approval of the animal experiments performed in this study was provided by the local ethical committee (2017/UCL/MD/005).
Experiments
Experiment 1
Male C57BL/6JRj mice (12 wk old) were anesthetized with ketamine (100 mg/kg of body weight; Nimatek; Eurovet Animal Health, Bladel, The Netherlands) and xylazine (10 mg/kg of body weight; Rompun; Bayer, Leverkusen, Germany). The liver was collected and immediately processed for PCLS preparation or KC isolation. Mice were humanely killed by cervical dislocation. All PCLS experiments were performed with n ≥ 4 mice in each condition.
Experiment 2
Male C57BL/6JRj mice (12 wk old) anesthetized with ketamine (50 mg/kg of body weight) and xylazine (5 mg/kg of body weight) and received a retroorbital intravenous injection of NaCl 0.9% (control, n = 9) or clodronate liposomes (CL; 10 mg/kg of body weight, n = 8 http://www.clodronateliposomes.org). Two days later, mice were anesthetized with ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg of body weight). The liver was collected and immediately processed for PCLS preparation as described below. Mice were humanely killed by cervical dislocation.
Experiment 3
Male B6.V-Lep ob/+ JRj (n = 4) and B6.V-Lep ob/ob JRj (n = 6) (5–6 wk old) were anesthetized with ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg of body weight). The liver was collected and immediately processed for PCLS preparation as described below. Mice were humanely killed by cervical dislocation.
Experiment 4
Male C57BL/6J mice (12 wk old) that had had food withheld for 3 h received by gavage sterile ultrapure water (vehicle) or indole (MilliporeSigma, Burlington, MA, USA) dissolved in sterile ultrapure water warmed at 55°C to improve its solubility (3 µmol/20 g of body weight in a volume of 200 µl/20 g of body weight), based on a procedure previously described (19). Thirty minutes later, mice received an intraperitoneal injection of NaCl 0.9% (vehicle) or LPS (10 mg/kg of body weight, Escherichia coli O127:B8; MilliporeSigma). Mice were assigned to 1 of 3 groups: water + NaCl (n = 4), water + LPS (n = 6), or indole + LPS (n = 6). Four hours later, mice were anesthetized with ketamine (100 mg/kg of body weight) and xylazine (10 mg/kg of body weight). After removal of the gallbladder, the liver was freeze-clamped in liquid nitrogen. All samples were stored at −80°C until analysis. Mice were humanely killed by cervical dislocation.
Precision-cut liver slices
Immediately after liver collection, PCLS (∼250 µm thick) were prepared from liver tissue cores (5 mm diameter) in oxygenated ice-cold Krebs-Ringer solution (NaCl 144 mM, KCl 5.8 mM, KH2PO4 1.4 mM, MgSO4 1.4 mM, NaHCO3 0.2%, CaCl2 2.6 mM, glucose 5.5 mM) using a Krumdieck slicer. PCLS were incubated in oxygenated Waymouth medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with insulin (100 U/ml; Actrapid), antibiotics (penicillin/streptomycin 100 U/ml; Thermo Fisher Scientific), and fatty acid–free bovine serum albumin (0.3%) at 4°C during 30 min and then at 37°C under agitation during 1 h. For treatment, PCLS were incubated with water (control) or LPS (100 µg/ml, Escherichia coli O127:B8; MilliporeSigma) and DMSO 0.1% (vehicle) or bacterial metabolites (phenylacetate, benzoate, p-cresol, indole, and I3S; MilliporeSigma) in oxygenated Waymouth medium supplemented with insulin (100 U/ml), antibiotics (penicillin/streptomycin, 100 U/ml) and fatty acid–free bovine serum albumin (0.1%) at 37°C during 4 h under agitation (2 PCLS/flask containing 1 ml of incubation medium). After incubation, PCLS were rinsed with ice-cold NaCl 0.9% and frozen in dry ice. Incubation media were collected and frozen in dry ice. All samples were stored at −80°C until analysis.
KC isolation
KC were isolated from 4 mice using a previously described procedure (20). Briefly, liver tissue was minced before digestion in collagenase P (Roche, Basel, Switzerland) for 30 min at 37°C. After filtration (70 µM), homogenates were centrifuged (50 g, 5 min, 4°C) to remove parenchymal cells. The supernatant was centrifuged (400 g, 10 min, 4°C), and the pellet containing nonparenchymal cells was washed in PBS before centrifugation (400 g, 10 min, 4°C). Nonparenchymal cells were resuspended in DMEM (Thermo Fisher Scientific) containing FCS 10% and penicillin/streptomycin 1% (Thermo Fisher Scientific). Cells (107 cells per well) in 6-well plates were incubated for 2 h (CO2 5%, 37°C). After elimination of nonadherent cells, the adherent cells (KC) were washed twice with warm PBS. After an overnight incubation (CO2 5%, 37°C), isolated KC were treated with water (control) or LPS (100 µg/ml) and DMSO 0.1% (vehicle) or indole (100 µM) for 4 h.
Tissue mRNA analyses
Total RNA was isolated from liver tissues using the TriPure Isolation Reagent (Roche Diagnostics). cDNA was prepared by reverse transcription of 1 µg of total RNA using the Reverse Transcription System (Promega, Madison, WI, USA). Real-time PCR was performed with a StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) using Mastermix Plus for SYBR Assay (Eurogentec, Liège, Belgium). Data were analyzed according to the 2−ΔΔCt method. The purity of the amplified product was verified by analyzing the melt curve performed at the end of the amplification step. The ribosomal protein L19 (Rpl19) gene was chosen as a reference gene. The primer sequences of the targeted genes are listed in Supplemental Table 1.
Transcriptome analysis
For the in vivo experiment (experiment 4), hepatic transcriptome profiling was performed using the Clariom D mouse assay with the GeneChip WT Plus Reagent Kit (Thermo Fisher Scientific) according to the manufacturer’s instructions (n = 5 in each group). All samples had a RNA integrity number of >8, as determined using 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Western blot analysis
Liver samples (50 mg) were homogenized in 700 µl ice-cold lysis buffer [20 mM Tris, 270 mM sucrose, 5 mM EGTA, 1 mM EDTA, 1% Triton X-100, 1 mM sodium orthovanadate, 50 mM B-glycerophosphate, 5 mM sodium pyrophosphate, 50 mM sodium fluoride, 1 mM DTT, 10% protease inhibitor cocktail (Roche)] using a TissueLyzer (Qiagen, Germantown, MD, USA). After centrifugation (10,000 g, 10 min, 4°C), protein concentration was measured in the supernatant using the Pierce BCA Protein Assay Kit (Thermo Fisher Scientific). Liver protein (40 µg) were separated by 12% SDS-PAGE and transferred to nitrocellulose membrane using the Trans-Blot Turbo Transfer System (Bio-Rad, Hercules, CA, USA) before blocking in Tris-buffered saline–Tween 20 (TBS-T) containing 5% nonfat dry milk (Bio-Rad) for 1 h at room temperature. Membranes were incubated overnight at 4°C with primary antibodies (dilution 1/1000 in 1% BSA TBS-T; Cell Signaling Technology, Danvers, MA, USA) detecting NF-κB p65 (8242), phospho–NF-κB p65 (Ser536) (3033), IκBα (4814), and phospho-IκBα (Ser32) (2859) or the loading control protein β-actin (ab6276, 1/10,000 in 1% nonfat milk TBS-T; Abcam, Cambridge, MA, USA). After membrane washing, horseradish peroxidase–linked secondary antibodies (7074 and 7076; 1/1000 in 1% nonfat milk TBS-T; Cell Signaling Technology) were incubated for 1 h at room temperature. Signals were revealed using the SuperSignal West Pico and Femto Chemiluminescent substrates (Thermo Fisher Scientific) and analyzed with the ImageQuant TL instrument and software v.8.1 (GE Healthcare, Waukesha, WI, USA).
Biochemical analysis
Alanine transaminase (ALAT) and aspartate transaminase (ASAT) activities were measured in the culture medium of PCLS using kits according to the manufacturer’s instructions (Diasys, Waterbury, CT, USA). IL-1B, chemokine ligand 2 (CCL2), and TNF were quantified in PCLS incubation medium (undiluted) using a Multiplex Immunoassay Kit (Bioplex; Bio-Rad) and measured using Luminex technology (Bioplex; Bio-Rad). Hepatic cholesterol was measured using commercial kits (Diasys) after chloroform–methanol extraction as previously described (21). Hepatic oxysterols were quantified by HPLC-MS as previously described (22). Reactive oxygen species (ROS and thiobarbituric acid reactive substances (TBARS) were measured in the liver tissue as previously described (23).
Statistical analyses and graphical representations
Statistical analyses were performed by R 3.4.3 software (2017; R Foundation for Statistical Computing, Vienna, Austria; http://www.r-project.org/) with the packages lme4, car, and lsmeans. For PCLS experiments, to take into account that several slices were prepared from the same mouse, a linear mixed model was used, followed by ANOVA (lmer and Anova functions), with the use of mouse as a random effect. The fixed effects used are indicated in the figure captions. Mean values were compared pairwise with the use of the Tukey correction (lsmeans function). For the in vivo experiment, a linear model was used, followed by ANOVA (lm and Anova functions), and mean values were compared pairwise with the use of the Tukey correction (lsmeans function). For correlation analysis, Spearman correlation coefficient (r) was calculated (cor.test function). For microarray experiments, raw data were normalized with the robust multiarray average method, and mean expression values were compared using multiple t tests in the Transcriptome Analysis Console software (Affymetrix, Santa Clara, CA, USA). Differentially expressed genes (DEG) were defined with P < 0.05 and a fold change of ±1.2 or more between the 2 groups (LPS and LPS + indole). Functional analysis was performed on the list of DEG with the Ingenuity Pathway Analysis software (Qiagen). Significantly enriched biologic functions were defined with enrichment P < 0.05 and at least implicating 4 DEG. For all statistical tests, P < 0.05 was considered to be significant. All plots were generated by GraphPad Prism 5 and 7 software (GraphPad Software, La Jolla, CA, USA). Heat maps were generated in R with the package made4 (heatplot function).
RESULTS
Tryptophan-derived bacterial metabolite indole prevents LPS-induced inflammation in PCLS
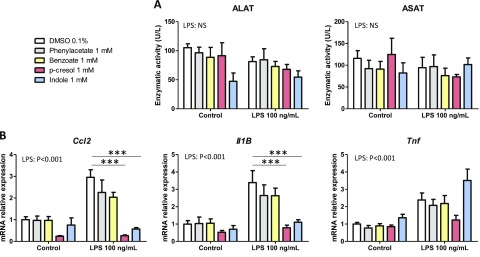
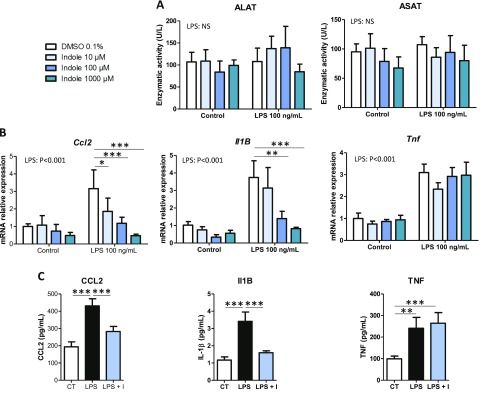
As a first screening step on PCLS, we tested the effects of bacterial metabolites (1 mM) produced from AAA (phenylacetate, benzoate, p-cresol, and indole) and known to reach the liver via the portal circulation (15). None of the metabolites significantly altered the activity of ALAT and ASAT in the incubation medium, suggesting the absence of cytotoxicity (Fig. 1A). Moreover, no significant effect of the metabolites was observed at the mRNA level for the key proinflammatory genes Ccl2, Il-1B, and Tnf coding for the proteins chemokine ligand 2, IL-1β, and TNF, respectively (Fig. 1B). Next, we evaluated potential interactions between the AAA-derived bacterial metabolites and LPS that can also be transported from the gut to the liver via the portal circulation. LPS (100 ng/µl) did not alter ALAT and ASAT activities in the incubation medium, while it induced a 2- to 3-fold increase in the mRNA levels of Ccl2, Il-1B, and Tnf (Fig. 1A, B). p-Cresol and indole totally prevented the LPS-induced up-regulation of Ccl2 and Il-1B, whereas phenylacetate and benzoate had no effect on proinflammatory gene expression (Fig. 1B). In contrast, p-cresol and indole did not significantly decrease Tnf mRNA levels. Because p-cresol has been previously shown to be toxic for intestinal epithelial cells (24) while indole has beneficial effects on gut barrier function (25, 26), we decided to further explore the hepatic effects of this potentially protective bacterial metabolite derived from tryptophan. In order to evaluate whether the effects of indole were dose dependent, PCLS exposed or not to LPS were treated with 10 to 1000 µM indole. None of the treatments was cytotoxic (Fig. 2A). Indole dose dependently decreased the LPS-induced up-regulation of Ccl2 and Il-1B, with these effects being statistically significant from 10 to 100 µM, respectively (Fig. 2B). Moreover, the LPS-induced secretion of CCL2 and IL-1B proteins in the culture medium was strongly inhibited by indole (100 µM) (Fig. 2C). Of note, the LPS-induced secretion of IL-1B by PCLS was low in our short-term experiment. In contrast, indole had no effect on the LPS-induced TNF protein secretion, in agreement with the mRNA profiles. Overall, our results showed that the bacterial metabolite indole has antiinflammatory effects in the PCLS model.
Figure 1.
Effects of bacterial metabolites produced from AAA on PCLS. PCLS were treated with or without LPS (100 ng/ml) and bacterial metabolites (1 mM). A) ALAT and ASAT activities were measured in culture medium. B) mRNA levels were quantified in PCLS using real-time quantitative PCR. Data are presented as means ± sem, n ≥ 4 mice in each condition (experiment 1). Mixed-model ANOVA was used with LPS and metabolite treatments as fixed effects and mouse as random effect. For pairwise comparison, mean value of each metabolite-treated group was compared to mean of respective vehicle-treated group in water or LPS conditions. NS, not significant. ***P < 0.001 (adjusted by Tukey method).
Figure 2.
Dose effects of indole on PCLS. PCLS were treated with or without LPS (100 ng/ml) and indole (10–1000 µM). A) ALAT and ASAT were measured in culture medium. B) mRNA levels were quantified in PCLS using real-time quantitative PCR. C) Cytokines were quantified in culture medium using Luminex. For this experiment, intermediate concentration of indole was used (100 µM). For IL-1B, concentration in control and LPS + indole group were extrapolated beyond standard range, but fluorescent signal was always higher than signal measured in blank. Data are presented as means ± sem, n ≥ 5 mice in each condition (experiment 1). Mixed-model ANOVA was used with LPS and indole (I) treatments as fixed effects and mouse as random effect. For pairwise comparison, mean value of each indole-treated group was compared to mean of respective vehicle group in water- or LPS-treated conditions. NS, not significant. ***P < 0.001, **P < 0.01 *P < 0.05 (adjusted by Tukey method).
Antiinflammatory effects of indole are partly dependent on KC in PCLS
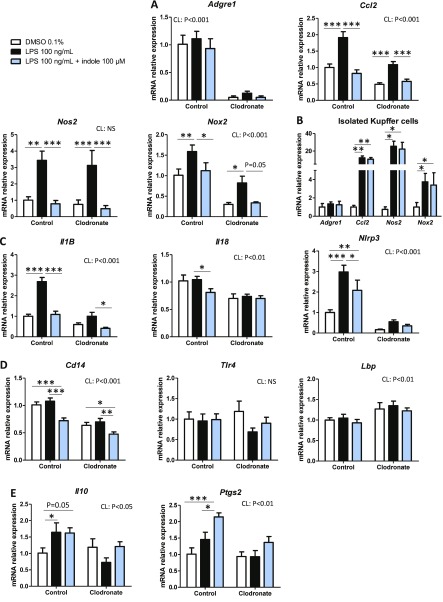
KC play a key role in the response to LPS in the liver (5, 27). To investigate the potential implication of these cells in the antiinflammatory effects of indole, we depleted KC with CL (28). Then PCLS were prepared from mice with or without KC. As expected, the mRNA level of the Adgre1 gene (coding for the mature macrophage marker protein F4/80) was decreased in PCLS prepared from CL-treated mice compared to control mice (Fig. 3A). The basal expression level of Ccl2 was reduced by 2-fold in PCLS prepared from CL-treated mice (Fig. 3A), showing a significant contribution of KC to the expression of this gene. However, LPS was still able to increase its expression, suggesting that KC are not solely responsible for Ccl2 induction by LPS. Indole (100 µM) also prevented LPS effect after CL treatment, suggesting that it may target pathways in other cell types activated by LPS. Interestingly, the expression profile of Nos2 (coding for the key oxidative stress inducer protein NOS2) was similar in PCLS prepared from control and CL-treated mice, suggesting that KC did not contribute to the regulation of the expression of this gene by LPS and indole. The LPS-induced up-regulation of Nox2 (coding for NADPH oxidase 2, another protein implicated in oxidative stress) was also prevented by indole in a KC-independent manner, despite a lower basal expression level after KC depletion. To confirm these results, isolated KC were treated with LPS (100 ng/ml) and indole (100 µM) (Fig. 3B). The expression of Adgre1 was similar in all experimental conditions, suggesting similar purity of the KC preparation. In isolated KC, indole did not prevent the LPS-induced up-regulation of Ccl2, Nos2, and Nox2. Collectively, these result show that the presence of other cells than KC is required for the indole-induced down-regulation of Ccl2, Nos2, and Nox2.
Figure 3.
Effects of indole on PCLS prepared from mice without KC. PCLS were prepared after injection of CL or NaCl (control) before treatment with or without LPS (100 ng/ml) and indole (100 µM). A, C–E) mRNA levels were quantified in PCLS using real-time quantitative PCR, n ≥ 8 mice in each condition (experiment 2). Mixed-model ANOVA was used with CL, LPS, and indole treatments as fixed effects and mouse as random effect. Mean values were compared pairwise in control or CL conditions. B) mRNA levels were quantified by real-time quantitative PCR in isolated KC treated with or without LPS (100 ng/ml) and indole (100 µM), n = 4 mice in each condition (experiment 1). Mixed-model ANOVA was used with LPS and indole treatments as fixed effects and mouse as random effect. Mean values were compared pairwise. Data are presented as means ± sem. NS, not significant. ***P < 0.001, **P < 0.01, *P < 0.05 (adjusted by Tukey method).
LPS failed to significantly up-regulate Il-1B mRNA levels in PCLS prepared from CL-treated mice, and the effects of indole were strongly attenuated (Fig. 3C), showing that KC play a pivotal role in the regulation of Il-1B expression in our model. In agreement with this finding, Il-18 and nucleotide-binding domain and leucine-rich repeat containing (NLR) family pyrin domain-containing 3 (Nlrp3) mRNA levels (coding for the protein IL-18 and the inflammasome NLR family pyrin domain-containing 3) were reduced by indole in PCLS prepared from control mice but not in PCLS prepared from CL-treated mice, suggesting that indole down-regulates the NLRP3–IL-1B–IL-18 pathway in a KC-dependent manner. Regarding the sensing of LPS at the cell surface, indole down-regulated the expression of Cd14 independently of the presence of KC, while no effect was observed for Tlr4 and Lbp coding for the Toll-like receptor 4 (TLR4) and lipopolysaccharide binding protein (Fig. 3D). Last, we found that indole did not regulate the expression of the regulatory cytokine Il-10 but induced a 2-fold up-regulation of Ptgs2 [coding for the prostaglandin–endoperoxide synthase 2 (Fig. 3E), implicated in the release of tolerogenic bioactive lipids (5)] in a KC-dependent manner. Our results showed that the antiinflammatory effects of indole were partly mediated by KC but that other liver cell types (e.g., hepatocytes, or stellate or endothelial cells) are required for some of the beneficial effects of indole.
Indole interacts with liver xenobiotic metabolism in PCLS
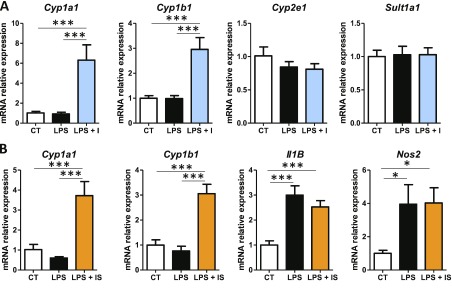
Indole, as a xenobiotic, can be metabolized in the liver by the cytochrome P450, family 2, subfamily E, member 1 (Cyp2e1), as well as through sulfate-transfer implicating sulfotransferase family 1A, member 1 (Sult1a1) (29, 30). We tested whether the antiinflammatory effects of indole were associated with an alteration of xenobiotic metabolism. Indole (100 µM) had no effect on Cyp2e1 and Sult1a1 mRNA levels in PCLS (Fig. 4A). In contrast, indole induced a strong up-regulation of cytochrome P450, family 1, subfamily A, polypeptide 1 (Cyp1a1) and cytochrome P450, family 1, subfamily B, member 1 (Cyp1b1) (Fig. 4A), which are key target genes of the aryl hydrocarbon receptor (AhR), a pivotal transcription factor in xenobiotic metabolism (31). As a next step, we wanted to explore whether indole metabolization by the host into I3S could alter its biologic activity. I3S (100 µM) failed to prevent the LPS-induced up-regulation of Il-1B and Nos2, even if it induced a strong up-regulation of Cyp1a1 and Cyp1b1 in PCLS (Fig. 4B). These data suggest that indole itself—and not its metabolite, I3S—was antiinflammatory and that up-regulation of Cyp1a1 and Cyp1b1 by indolic compounds may occur independently of their effects on inflammation. In order to confirm this hypothesis, we repeated the experiments with a simultaneous incubation with the AhR inhibitor CH-223191 (10 µM). However, these experiments were inconclusive because CH-223191 down-regulated Cyp1a1 expression in the control condition but failed to prevent its induction by indole in the PCLS model.
Figure 4.
Interactions between indole and xenobiotic metabolism in PCLS. PCLS were treated with or without LPS (100 ng/ml) and indole or I3S (100 µM). mRNA levels were quantified by real-time quantitative PCR in PCLS treated with LPS and indole (A) or I3S (B) (experiment 1). Data are presented as means ± sem, n ≥ 4 mice in each condition. CT, control; I, indole; IS, I3S. Mixed-model ANOVA was used with LPS and metabolite treatments as fixed effects and mouse as random effect. Mean values were compared pairwise. ***P < 0.001, *P < 0.05 (adjusted by Tukey method).
Indole down-regulates proinflammatory gene expression in PCLS prepared from genetically obese mice
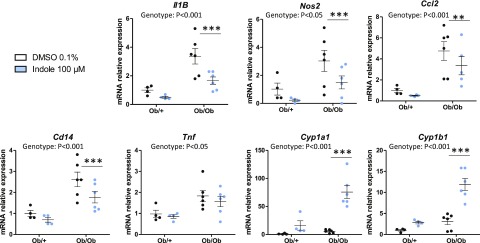
In order to test whether the effects of indole could be observed in a model of NAFLD associated with chronic liver inflammation, PCLS were prepared from ob/+ (control) or ob/ob (leptin deficient) mice. As expected, PCLS prepared from ob/ob mice overexpressed the proinflammatory genes Il-1B, Nos2, Ccl2, Tnf, and Cd14 (Fig. 5). Interestingly, treating PCLS with indole (100 µM) reduced the expression of all these genes, except Tnf, a finding in good agreement with the results obtained in the acute LPS exposure model. Interestingly, ob/ob mice overexpressed the AhR target genes Cyp1a1 and Cyp1b1 in basal conditions, and their expression was strongly induced by indole. In summary, our results show that the antiinflammatory effects of indole, as well as its ability to induce the expression of AhR target genes, are observed also in a mouse model characterized by metabolic endotoxemia and hepatic inflammation.
Figure 5.
Effects of indole on PCLS prepared from genetically obese (ob/ob) mice. PCLS were treated with or without indole (100 µM). mRNA levels were quantified in PCLS using real-time quantitative PCR. Data are presented as means ± sem, n ≥ 4 mice in each group (experiment 3). Mixed-model ANOVA was used with genotype and indole treatment as fixed effects and mouse as random effect. Mean values were compared pairwise in ob/+ or ob/ob groups. ***P < 0.001, **P < 0.01 (adjusted by Tukey method).
Oral administration of indole reduces LPS-induced liver inflammation in vivo
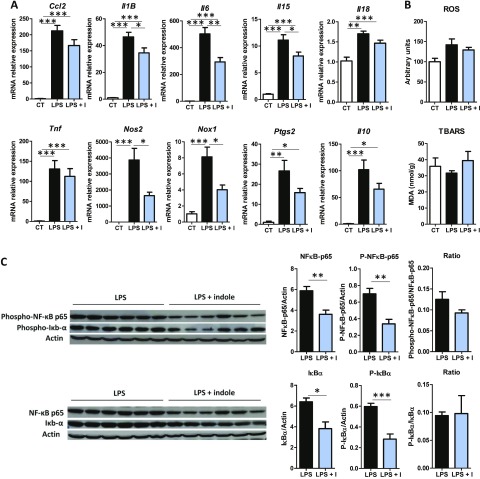
In order to test if our findings on PCLS were relevant in vivo, indole was administered by gavage to mice 30 min before intraperitoneal injection of LPS. As expected, LPS strongly induced proinflammatory gene expression (Fig. 6A). Indole oral administration reduced the LPS-induced up-regulation of the cytokines Il-1B, Il-6, and Il-15. Indole also down-regulated the gene expression of Nos2 and Nox2, suggesting a decrease in the induction of oxidative stress. However, LPS did not significantly increase the hepatic levels of ROS and TBARS, maybe because of the short time period between LPS injection and the animal’s death (Fig. 6B). Of note, some differences were observed compared to the PCLS model because the amplitude of the effects of indole was lower in vivo and indole did not significantly regulate in vivo the expression of Ccl2, Il-18, and Ptgs2 (Fig. 6A). These results might be related to the very important induction of proinflammatory gene expression by LPS in vivo, and the concentration of indole reaching the liver might be lower than the concentration tested ex vivo. Because the transcription of most of the genes down-regulated by indole are controlled by the NF-κB pathway (6), we quantified by Western blot NF-κB p65 and IκBα. Indole markedly reduced the protein level of the total and phosphorylated forms of NF-κB p65 and IκBα, resulting in a similar ratio between the 2 forms (Fig. 6C). In agreement with the results obtained in PCLS, our results obtained in vivo show that orally administered indole has antiinflammatory effects in the liver and that these effects are associated with a down-regulation of the level of key proteins of the NF-κB pathway.
Figure 6.
Effects of indole on liver inflammation in vivo. Mice orally received water or indole (3 µmol/20 g of body weight) before intraperitoneal injection of vehicle or LPS (10 mg/kg of body weight). A) mRNA levels were quantified in liver using real-time quantitative PCR. Results are expressed relative to mRNA level measured in control group (set at 1). B) ROS and TBARS were quantified in liver. C) Left: Western blot of β-actin, and total and phosphorylated forms of NF-κB p65 and IκBα in liver lysate. right: band intensities were quantified relative to band intensity of β-actin, used as loading control. Data are presented as means ± sem, n = 6 mice in LPS and LPS + indole (LPS + I) groups, n = 4 in control group (CT) (experiment 4). ANOVA was used to test treatment effect, and mean values were compared pairwise. ***P < 0.001, **P < 0.01, *P < 0.05 (adjusted by Tukey method).
Indole regulates hepatic cholesterol metabolism in mice treated with LPS
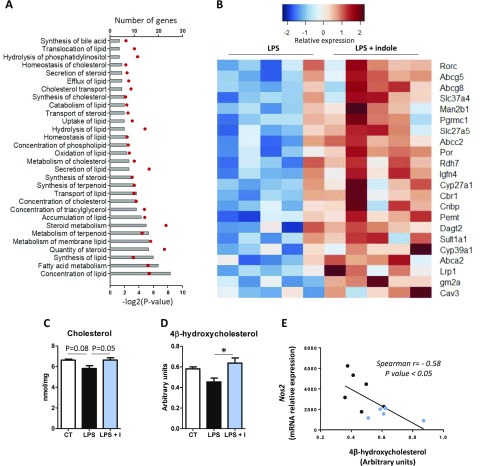
In order to further characterize the effects of indole, we performed transcriptome profiling in the liver of the mice treated with LPS after oral administration of indole or vehicle. We identified 217 genes that were differentially expressed between the LPS- and LPS + indole–treated mice (Supplemental Table 2). Functional analysis revealed that there was an overrepresentation of genes implicated in lipid metabolism, and more specifically in cholesterol metabolism (Fig. 7A). In total, 52 DEG (24% of the total number of DEG) were implicated in lipid metabolism (Supplemental Table 3). Interestingly, most of these genes (92%) were up-regulated in mice treated with LPS + indole compared to LPS alone (Fig. 7B). In order to confirm the physiologic relevance of these findings, we measured hepatic cholesterol levels. LPS tended to decrease hepatic cholesterol levels, while indole prevented this effect (Fig. 7C). Because several DEG are targets of the liver X receptor (LXR) (32), we sought to evaluate whether indole regulated the hepatic concentration of oxysterols that are key ligands for LXR. Interestingly, we found that among the panel of 10 oxysterols analyzed (Supplemental Table 4), the concentration of 4β-hydroxycholesterol was higher in the liver of mice treated with indole and LPS compared to mice treated with LPS alone (Fig. 7D). Finally, we observed that 4β-hydroxycholesterol concentration was negatively correlated with the expression of genes related to inflammation, as exemplified for Nos2 mRNA levels (Fig. 7E), suggesting a potential link between the antiinflammatory effects of indole and its effects on cholesterol metabolism.
Figure 7.
Effects of indole on liver sterols metabolism in vivo. Mice orally received water or indole (3 µmol per 20 g of body weight) before intraperitoneal injection of vehicle or LPS (10 mg/kg of body weight). Liver transcriptome was compared between LPS and LPS + indole (LPS + I) groups using microarrays (n = 5 mice in each group) (experiment 4). A) Significantly enriched functions implicated in biologic category “Lipid metabolism” identified by Ingenuity Pathway Analysis software. Bars show number of DEG implicated in each function. Red dots represent −log2 (P value) of enrichment. B) Heat map representing mRNA levels (log2) of selected DEG participating to functions enriched in biologic category, “Lipid metabolism.” C, D) Cholesterol (C) and 4β-hydroxycholesterol (D) were quantified in liver. Data are presented as means ± sem, n = 6 mice in LPS and LPS + I groups, n = 4 in control group. ANOVA was used to test treatment effect, and mean values were compared pairwise. *P < 0.05 (adjusted by Tukey method). E) Spearman correlation between 4β-hydroxycholesterol hepatic concentration and Nos2 mRNA levels in liver of mice treated with LPS (black dots, n = 6) or LPS + I (blue dots, n = 6).
DISCUSSION
Indole is produced from l-tryptophan by the bacteria-specific enzyme tryptophanase (encoded by TnaA in more than 80 gram-positive and gram-negative species) and can be detected in human and mouse feces at low millimolar concentration (33, 34). This metabolite and related bacterial compounds (indole-3-aldehyde, indole-3-lactate, and indole-3-propionate) were previously shown to reduce intestinal inflammation and to prevent gut barrier dysfunction (19, 25, 26, 35–38). In the present study, we show for the first time that indole alleviates inflammation in the liver, away from the gut. In the PCLS ex vivo model, indole dose-dependently reduced acute hepatic inflammation induced by LPS both at mRNA and protein levels. Similarly, indole decreased proinflammatory gene expression in PCLS prepared from ob/ob mice that are characterized by metabolic endotoxemia and liver inflammation (2, 3). Last, in vivo oral administration of indole partly prevented LPS-induced liver inflammation and associated alterations of cholesterol metabolism.
Indole is able to cross the intestinal epithelium and reach the liver, probably via its capacity to freely diffuse through lipid membranes (39). On the basis of the results obtained in the present study, several mechanisms underlying the antiinflammatory effects of indole in the liver can be proposed. The first step of endotoxin-induced inflammation is LPS recognition at cell surface, a process involving 3 key proteins: CD14, lipopolysaccharide binding protein (LBP), and TLR4 (40). In PCLS, indole reduced the mRNA levels of Cd14, potentially leading to a reduction of LPS signaling that could partly contribute to its antiinflammatory effects. After LPS binding to TLR4 at the cell membrane, the NF-κB pathway is activated and up-regulates proinflammatory gene expression (6). Interestingly, indole down-regulated the expression of several NF-κB–responsive genes (e.g., Nos2, Il-1B, Ccl2). Moreover, we found that indole reduced in vivo the expression of 2 key proteins of the NF-κB pathway in the liver, potentially contributing to the immunoregulatory effects of this bacterial metabolite. In our model, indole regulated the protein level of NF-κB p65 and IκBα rather than their phosphorylation. Our results are in agreement with a previous study demonstrating that indole modulated the NF-κB pathway in intestinal epithelial cells in vitro (25). Then, downstream of NF-κB signaling, LPS induces activation of the NLRP3 inflammasome pathway that initiates the release of IL-1B and IL-18 (8). Interestingly, indole down-regulated the expression of Nlrp3, Il-1B, and Il-18 in PCLS, suggesting an inhibition of this pathway. These effects were not observed (or strongly attenuated) in PCLS prepared from CL-treated mice, in agreement with previous results showing that NLRP3 activation is specific to KC in the liver (41). Last, indole up-regulated the Ptgs2 gene expression in PCLS, potentially leading to an increased production of prostaglandin E2 that has immunoregulatory properties in the liver and is protective against liver injury (5, 42, 43). These effects were lost in PCLS prepared from CL-treated mice, consistent with the major role of KC in the expression of PTGS2 in the liver (44).
Tryptophan-derived bacterial metabolites (indole, tyramine, indole-3-actetate, and skatole) have been shown to be (weak) AhR agonists (34, 45–47). Because this receptor tunes inflammatory responses (31), its activation by indole could contribute to its antiinflammatory effects. In PCLS, indole strongly up-regulated Cyp1a1 and Cyp1b1 gene expression, confirming that this bacterial metabolite is an AhR agonist in the liver. Interestingly, these effects were amplified in PCLS prepared from ob/ob mice, in agreement with the previously reported higher expression of Cyp1a1 in ob/ob mice (48). I3S was previously shown to be also an AhR agonist (49), so we explored whether this major metabolite of indole could have similar antiinflammatory effects than its precursor. However, I3S was not able to reduce LPS-induced up-regulation of proinflammatory genes in PCLS, suggesting that the metabolization of indole by the liver might reduce its beneficial effects. Divergent effects of indole and its derivative, I3S, were already observed regarding their ability to induce the expression of tight junction proteins in intestinal epithelial cells in vitro (26). In contrast, similar to indole, I3S strongly induced the expression of the AhR target genes Cyp1a1 and Cyp1b1, confirming a previous observation that I3S is also an AhR agonist (49). Because both indole and I3S activated AhR while only indole reduced proinflammatory gene expression, we concluded that the antiinflammatory effects of indole are likely to involve other mechanisms than AhR signaling.
Transcriptome profiling revealed that oral administration of indole up-regulated the expression of genes implicated in cholesterol transport in the liver of mice treated with LPS. Endotoxins are known to impair cholesterol homeostasis through a decreased expression of LXR target genes implicated in cholesterol efflux, such as ATP-binding cassette transporters (50, 51). Conversely, LXR activation attenuates inflammatory responses through antagonism of NF-κB signaling (50). Interestingly, indole prevented the LPS-induced decrease in the concentration of hepatic cholesterol and 4β-hydroxycholesterol that is an LXR agonist (52). Moreover, the negative correlation between Nos2 mRNA levels and 4β-hydroxycholesterol concentration suggest potential links between inflammation and cholesterol metabolism in our model. Further experiments will be required to determine whether the regulation of cholesterol metabolism by indole is a cause or a consequence of its antiinflammatory effects in the liver, but LXR could be the link between both events.
Our data provide strong evidence that indole has antiinflammatory effects in the liver. However, more experiments are needed to prove the physiologic relevance of this finding, notably in the context of chronic liver diseases. For instance, long-term administration of indole to ob/ob mice might reveal beneficial effects of this bacterial metabolite that could be relevant for NAFLD or nonalcoholic steatohepatitis. Another important perspective of this work would be to determine if indole produced in vivo by the gut microbiota is able to regulate hepatic inflammation. Indeed, a limitation of our study is that the beneficial effects of indole were observed at a relatively high concentration (100 µM). The estimation of liver exposition to indole in vivo is difficult because this bacterial metabolite is continuously produced in the gut and absorbed through the mucosa before being metabolized into I3S in the liver (29, 30). An interesting experiment would be to up-regulate the production of indole by the microbiota and evaluate the consequences for hepatic inflammation. Increasing tryptophan availability for the microbiota would be the most straightforward strategy to promote indole production in vivo. Interestingly, tryptophan supplementation in mice was shown to modulate the progression of NAFLD, although conflicting results were obtained and the potential link with the production of indole was not explored (53, 54). In a preliminary experiment, we found that free dietary tryptophan supplementation (0.5% w/v in drinking water) in mice failed to increase indole production, as evaluated by excretion of I3S in the urine, a commonly used proxy for intestinal production of indole (55, 56). Because free amino acids are highly absorbed in the small intestine, they might not reach the distal part of the gut, where the bacterial density is higher (17). In contrast, some dietary proteins escape the small intestine (<10%) (57) and might be an important source of exogenous tryptophan for the microbiota (17). Indeed, in humans, high-protein diets increase indole production by the microbiota, as shown by an increase in I3S urinary excretion (58). However, increasing dietary protein intake lacks specificity because it also increases the production of many other amino acid–derived bacterial metabolites. Alternatively, increased exposure of the liver to indole could be obtained through administration of indole-producing bacteria (probiotic approach) (34) or through direct administration of indole (postbiotic approach) (59).
In summary, our results show that the tryptophan-derived bacterial metabolite indole has antiinflammatory effects in the liver. We propose that targeting indole might be a promising strategy for the management of liver inflammation. Our work emphasizes the interest of studying microbiota-derived metabolites to discover new regulators of hepatic functions.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank V. Allaeys and B. Es Saadi (Metabolism and Nutrition Research Group, Louvain Drug Research Institute) for their skillful technical assistance. N.M.D. is a recipient of an European Union (EU) grant (613979 MyNewGut Project) and Belgium National Scientific Research Fund (FRS-FNRS) (Belgium) grants. M.O. is a beneficiary of a “MOVE-IN Louvain” Incoming Postdoctoral Fellowship cofunded by the Marie Curie Actions of the European Commission. P.D.C., a senior research associate at the FRS-FNRS, is a recipient of a 2013 European Research Council Starting grant (336452-ENIGMO) and a 2015 Baillet Latour grant for medical research, and is supported by the FRS-FNRS via the Fund for Strategic Fundamental Research (FRFS)–Walloon Excellence in Life Sciences and Biotechnology (WELBIO) under Grant WELBIO-CGR-2017. A.D.R.S. is a recipient of a postdoctoral fellowship from FRS-FNRS. The authors declare no conflicts of interest.
Glossary
- AAA
aromatic amino acids
- AhR
aryl hydrocarbon receptor
- ALAT
alanine transaminase
- ASAT
aspartate transaminase
- CCL2
chemokine ligand 2
- CL
clodronate liposome
- Cyp1a1
cytochrome P450, family 1, subfamily A, polypeptide 1
- Cyp1b1
cytochrome P450, family 1, subfamily B, member 1
- Cyp2e1
cytochrome P450, family 2, subfamily E, member 1
- DEG
differentially expressed genes
- I3S
indoxyl-3-sulfate
- KC
Kupffer cells
- LXR
liver X receptor
- NAFLD
nonalcoholic fatty liver disease
- NLR
nucleotide-binding domain and leucine-rich repeat containing
- NLRP3
nucleotide-binding domain and leucine-rich repeat containing family pyrin domain-containing 3
- NOX2
NAPDH oxidase 2
- PCLS
precision-cut liver slices
- PTGS2
prostaglandin–endoperoxide synthase 2
- ROS
reactive oxygen species
- Sult1a1
sulfate-transfer implicating sulfotransferase family 1A, member 1
- TBARS
thiobarbituric acid reactive substances
- TBS-T
Tris-buffered saline–Tween 20
- TLR4
Toll-like receptor 4
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Beaumont, A. M. Neyrinck, and N. M. Delzenne conceived the experiments; M. Beaumont, A. M. Neyrinck, M. Olivares, J. Rodriguez, A. de Rocca Serra, and M. Roumain conducted the experiments; G. G. Muccioli supervised oxysterols measurements; J.-B. Demoulin supervised microarray experiments; P. D. Cani provided ob/ob mice; M. Beaumont, A. M. Neyrinck, and N. M. Delzenne wrote the report; M. Olivares, J. Rodriguez, A. d. R. Serra, M. Roumain, G. G. Muccioli, L. B. Bindels, P. D. Cani, P. Evenepoel, and J.-B. Demoulin provided intellectual input and contributed to the writing of the report; and N. M. Delzenne planned and supervised all experiments.
REFERENCES
- 1.Strnad P., Tacke F., Koch A., Trautwein C. (2017) Liver—guardian, modifier and target of sepsis. Nat. Rev. Gastroenterol. Hepatol. 14, 55–66 [DOI] [PubMed] [Google Scholar]
- 2.Cani P. D., Bibiloni R., Knauf C., Waget A., Neyrinck A. M., Delzenne N. M., Burcelin R. (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet–induced obesity and diabetes in mice. Diabetes 57, 1470–1481 [DOI] [PubMed] [Google Scholar]
- 3.Cani P. D., Possemiers S., Van de Wiele T., Guiot Y., Everard A., Rottier O., Geurts L., Naslain D., Neyrinck A., Lambert D. M., Muccioli G. G., Delzenne N. M. (2009) Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2–driven improvement of gut permeability. Gut 58, 1091–1103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brandl K., Kumar V., Eckmann L. (2017) Gut–liver axis at the frontier of host–microbial interactions. Am. J. Physiol. Gastrointest. Liver Physiol. 312, G413–G419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heymann F., Tacke F. (2016) Immunology in the liver—from homeostasis to disease. Nat. Rev. Gastroenterol. Hepatol. 13, 88–110 [DOI] [PubMed] [Google Scholar]
- 6.Luedde T., Schwabe R. F. (2011) NF-κB in the liver—linking injury, fibrosis and hepatocellular carcinoma. Nat. Rev. Gastroenterol. Hepatol. 8, 108–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chassaing B., Etienne-Mesmin L., Gewirtz A. T. (2014) Microbiota–liver axis in hepatic disease. Hepatology 59, 328–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Szabo G., Petrasek J. (2015) Inflammasome activation and function in liver disease. Nat. Rev. Gastroenterol. Hepatol. 12, 387–400 [DOI] [PubMed] [Google Scholar]
- 9.Tilg H., Cani P. D., Mayer E. A. (2016) Gut microbiome and liver diseases. Gut 65, 2035–2044 [DOI] [PubMed] [Google Scholar]
- 10.Llopis M., Cassard A. M., Wrzosek L., Boschat L., Bruneau A., Ferrere G., Puchois V., Martin J. C., Lepage P., Le Roy T., Lefèvre L., Langelier B., Cailleux F., González-Castro A. M., Rabot S., Gaudin F., Agostini H., Prévot S., Berrebi D., Ciocan D., Jousse C., Naveau S., Gérard P., Perlemuter G. (2016) Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut 65, 830–839 [DOI] [PubMed] [Google Scholar]
- 11.Boursier J., Mueller O., Barret M., Machado M., Fizanne L., Araujo-Perez F., Guy C. D., Seed P. C., Rawls J. F., David L. A., Hunault G., Oberti F., Calès P., Diehl A. M. (2016) The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology 63, 764–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leung C., Rivera L., Furness J. B., Angus P. W. (2016) The role of the gut microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 13, 412–425 [DOI] [PubMed] [Google Scholar]
- 13.Blander J. M., Longman R. S., Iliev I. D., Sonnenberg G. F., Artis D. (2017) Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 18, 851–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schnabl B., Brenner D. A. (2014) Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wikoff W. R., Anfora A. T., Liu J., Schultz P. G., Lesley S. A., Peters E. C., Siuzdak G. (2009) Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites. Proc. Natl. Acad. Sci. USA 106, 3698–3703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng X., Xie G., Zhao A., Zhao L., Yao C., Chiu N. H. L., Zhou Z., Bao Y., Jia W., Nicholson J. K., Jia W. (2011) The footprints of gut microbial–mammalian co-metabolism. J. Proteome Res. 10, 5512–5522 [DOI] [PubMed] [Google Scholar]
- 17.Portune K. J., Beaumont M., Davila A.-M., Tomé D., Blachier F., Sanz Y. (2016) Gut microbiota role in dietary protein metabolism and health-related outcomes: the two sides of the coin. Trends Food Sci. Technol. 57, 213–232 [Google Scholar]
- 18.De Graaf I. A. M., Olinga P., de Jager M. H., Merema M. T., de Kanter R., van de Kerkhof E. G., Groothuis G. M. M. (2010) Preparation and incubation of precision-cut liver and intestinal slices for application in drug metabolism and toxicity studies. Nat. Protoc. 5, 1540–1551 [DOI] [PubMed] [Google Scholar]
- 19.Whitfield-Cargile C. M., Cohen N. D., Chapkin R. S., Weeks B. R., Davidson L. A., Goldsby J. S., Hunt C. L., Steinmeyer S. H., Menon R., Suchodolski J. S., Jayaraman A., Alaniz R. C. (2016) The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes 7, 246–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li P. Z., Li J. Z., Li M., Gong J. P., He K. (2014) An efficient method to isolate and culture mouse Kupffer cells. Immunol. Lett. 158, 52–56 [DOI] [PubMed] [Google Scholar]
- 21.Neyrinck A. M., Possemiers S., Verstraete W., De Backer F., Cani P. D., Delzenne N. M. (2012) Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J. Nutr. Biochem. 23, 51–59 [DOI] [PubMed] [Google Scholar]
- 22.Mutemberezi V., Masquelier J., Guillemot-Legris O., Muccioli G. G. (2016) Development and validation of an HPLC-MS method for the simultaneous quantification of key oxysterols, endocannabinoids, and ceramides: variations in metabolic syndrome. Anal. Bioanal. Chem. 408, 733–745 [DOI] [PubMed] [Google Scholar]
- 23.Neyrinck A. M., Etxeberria U., Taminiau B., Daube G., Van Hul M., Everard A., Cani P. D., Bindels L. B., Delzenne N. M. (In press) Rhubarb extract prevents hepatic inflammation induced by acute alcohol intake, an effect related to the modulation of the gut microbiota. Mol. Nutr. Food Res. 61 [DOI] [PubMed] [Google Scholar]
- 24.Andriamihaja M., Lan A., Beaumont M., Audebert M., Wong X., Yamada K., Yin Y., Tomé D., Carrasco-Pozo C., Gotteland M., Kong X., Blachier F. (2015) The deleterious metabolic and genotoxic effects of the bacterial metabolite p-cresol on colonic epithelial cells. Free Radic. Biol. Med. 85, 219–227 [DOI] [PubMed] [Google Scholar]
- 25.Bansal T., Alaniz R. C., Wood T. K., Jayaraman A. (2010) The bacterial signal indole increases epithelial-cell tight-junction resistance and attenuates indicators of inflammation. Proc. Natl. Acad. Sci. USA 107, 228–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada Y., Kinoshita M., Harada K., Mizutani M., Masahata K., Kayama H., Takeda K. (2013) Commensal bacteria–dependent indole production enhances epithelial barrier function in the colon. PLoS One 8, e80604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tacke F. (2017) Targeting hepatic macrophages to treat liver diseases. J. Hepatol. 66, 1300–1312 [DOI] [PubMed] [Google Scholar]
- 28.Van Rooijen N., Sanders A. (1996) Kupffer cell depletion by liposome-delivered drugs: comparative activity of intracellular clodronate, propamidine, and ethylenediaminetetraacetic acid. Hepatology 23, 1239–1243 [DOI] [PubMed] [Google Scholar]
- 29.Banoglu E., Jha G. G., King R. S. (2001) Hepatic microsomal metabolism of indole to indoxyl, a precursor of indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 26, 235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Banoglu E., King R. S. (2002) Sulfation of indoxyl by human and rat aryl (phenol) sulfotransferases to form indoxyl sulfate. Eur. J. Drug Metab. Pharmacokinet. 27, 135–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Murray I. A., Patterson A. D., Perdew G. H. (2014) Aryl hydrocarbon receptor ligands in cancer: friend and foe. Nat. Rev. Cancer 14, 801–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schulman I. G. (2017) Liver X receptors link lipid metabolism and inflammation. FEBS Lett. 591, 2978–2991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lee J.-H., Lee J. (2010) Indole as an intercellular signal in microbial communities. FEMS Microbiol. Rev. 34, 426–444 [DOI] [PubMed] [Google Scholar]
- 34.Jin U.-H., Lee S.-O., Sridharan G., Lee K., Davidson L. A., Jayaraman A., Chapkin R. S., Alaniz R., Safe S. (2014) Microbiome-derived tryptophan metabolites and their aryl hydrocarbon receptor-dependent agonist and antagonist activities. Mol. Pharmacol. 85, 777–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zelante T., Iannitti R. G., Cunha C., De Luca A., Giovannini G., Pieraccini G., Zecchi R., D’Angelo C., Massi-Benedetti C., Fallarino F., Carvalho A., Puccetti P., Romani L. (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39, 372–385 [DOI] [PubMed] [Google Scholar]
- 36.Venkatesh M., Mukherjee S., Wang H., Li H., Sun K., Benechet A. P., Qiu Z., Maher L., Redinbo M. R., Phillips R. S., Fleet J. C., Kortagere S., Mukherjee P., Fasano A., Le Ven J., Nicholson J. K., Dumas M. E., Khanna K. M., Mani S. (2014) Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 41, 296–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jennis M., Cavanaugh C. R., Leo G. C., Mabus J. R., Lenhard J., Hornby P. J. (In press) Microbiota-derived tryptophan indoles increase after gastric bypass surgery and reduce intestinal permeability in vitro and in vivo. Neurogastroenterol. Motil. 30 [DOI] [PubMed] [Google Scholar]
- 38.Cervantes-Barragan L., Chai J. N., Tianero M. D., Di Luccia B., Ahern P. P., Merriman J., Cortez V. S., Caparon M. G., Donia M. S., Gilfillan S., Cella M., Gordon J. I., Hsieh C.-S., Colonna M. (2017) Lactobacillus reuteri induces gut intraepithelial CD4+CD8αα+ T cells. Science 357, 806–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Piñero-Fernandez S., Chimerel C., Keyser U. F., Summers D. K. (2011) Indole transport across Escherichia coli membranes. J. Bacteriol. 193, 1793–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kitchens R. L., Thompson P. A. (2005) Modulatory effects of sCD14 and LBP on LPS–host cell interactions. J. Endotoxin Res. 11, 225–229 [DOI] [PubMed] [Google Scholar]
- 41.Petrasek J., Bala S., Csak T., Lippai D., Kodys K., Menashy V., Barrieau M., Min S.-Y., Kurt-Jones E. A., Szabo G. (2012) IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quiroga J., Prieto J. (1993) Liver cytoprotection by prostaglandins. Pharmacol. Ther. 58, 67–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neyrinck A. M., Alexiou H., Delzenne N. M. (2004) Kupffer cell activity is involved in the hepatoprotective effect of dietary oligofructose in rats with endotoxic shock. J. Nutr. 134, 1124–1129 [DOI] [PubMed] [Google Scholar]
- 44.Neyrinck A. M., Gomez C., Delzenne N. M. (2004) Precision-cut liver slices in culture as a tool to assess the physiological involvement of Kupffer cells in hepatic metabolism. Comp. Hepatol. 3(Suppl 1), S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rasmussen M. K., Balaguer P., Ekstrand B., Daujat-Chavanieu M., Gerbal-Chaloin S. (2016) Skatole (3-methylindole) is a partial aryl hydrocarbon receptor agonist and induces CYP1A1/2 and CYP1B1 expression in primary human hepatocytes. PLoS One 11, e0154629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng Y., Jin U.-H., Allred C. D., Jayaraman A., Chapkin R. S., Safe S. (2015) Aryl hydrocarbon receptor activity of tryptophan metabolites in young adult mouse colonocytes. Drug Metab. Dispos. 43, 1536–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hubbard T. D., Murray I. A., Bisson W. H., Lahoti T. S., Gowda K., Amin S. G., Patterson A. D., Perdew G. H. (2015) Adaptation of the human aryl hydrocarbon receptor to sense microbiota-derived indoles. Sci. Rep. 5, 12689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu J., Kulkarni S. R., Li L., Slitt A. L. (2012) UDP-glucuronosyltransferase expression in mouse liver is increased in obesity- and fasting-induced steatosis. Drug Metab. Dispos. 40, 259–266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schroeder J. C., Dinatale B. C., Murray I. A., Flaveny C. A., Liu Q., Laurenzana E. M., Lin J. M., Strom S. C., Omiecinski C. J., Amin S., Perdew G. H. (2010) The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry 49, 393–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tall A. R., Yvan-Charvet L. (2015) Cholesterol, inflammation and innate immunity. Nat. Rev. Immunol. 15, 104–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Feingold K. R., Grunfeld C. (2010) The acute phase response inhibits reverse cholesterol transport. J. Lipid Res. 51, 682–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mutemberezi V., Guillemot-Legris O., Muccioli G. G. (2016) Oxysterols: from cholesterol metabolites to key mediators. Prog. Lipid Res. 64, 152–169 [DOI] [PubMed] [Google Scholar]
- 53.Ritze Y., Bárdos G., Hubert A., Böhle M., Bischoff S. C. (2014) Effect of tryptophan supplementation on diet-induced non-alcoholic fatty liver disease in mice. Br. J. Nutr. 112, 1–7 [DOI] [PubMed] [Google Scholar]
- 54.Osawa Y., Kanamori H., Seki E., Hoshi M., Ohtaki H., Yasuda Y., Ito H., Suetsugu A., Nagaki M., Moriwaki H., Saito K., Seishima M. (2011) l-Tryptophan–mediated enhancement of susceptibility to nonalcoholic fatty liver disease is dependent on the mammalian target of rapamycin. J. Biol. Chem. 286, 34800–34808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rothhammer V., Mascanfroni I. D., Bunse L., Takenaka M. C., Kenison J. E., Mayo L., Chao C.-C., Patel B., Yan R., Blain M., Alvarez J. I., Kébir H., Anandasabapathy N., Izquierdo G., Jung S., Obholzer N., Pochet N., Clish C. B., Prinz M., Prat A., Antel J., Quintana F. J. (2016) Type I interferons and microbial metabolites of tryptophan modulate astrocyte activity and central nervous system inflammation via the aryl hydrocarbon receptor. Nat. Med. 22, 586–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sonowal R., Swimm A., Sahoo A., Luo L., Matsunaga Y., Wu Z., Bhingarde J. A., Ejzak E. A., Ranawade A., Qadota H., Powell D. N., Capaldo C. T., Flacker J. M., Jones R. M., Benian G. M., Kalman D. (2017) Indoles from commensal bacteria extend healthspan. Proc. Natl. Acad. Sci. USA 114, E7506–E7515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gaudichon C., Bos C., Morens C., Petzke K. J., Mariotti F., Everwand J., Benamouzig R., Daré S., Tomé D., Metges C. C. (2002) Ileal losses of nitrogen and amino acids in humans and their importance to the assessment of amino acid requirements. Gastroenterology 123, 50–59 [DOI] [PubMed] [Google Scholar]
- 58.Beaumont M., Portune K. J., Steuer N., Lan A., Cerrudo V., Audebert M., Dumont F., Mancano G., Khodorova N., Andriamihaja M., Airinei G., Tomé D., Benamouzig R., Davila A.-M., Claus S. P., Sanz Y., Blachier F. (2017) Quantity and source of dietary protein influence metabolite production by gut microbiota and rectal mucosa gene expression: a randomized, parallel, double-blind trial in overweight humans. Am. J. Clin. Nutr. 106, 1005–1019 [DOI] [PubMed] [Google Scholar]
- 59.Klemashevich C., Wu C., Howsmon D., Alaniz R. C., Lee K., Jayaraman A. (2014) Rational identification of diet-derived postbiotics for improving intestinal microbiota function. Curr. Opin. Biotechnol. 26, 85–90 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.