Abstract
Purpose:
In this report, we characterize the timing and behavior of malignant ovarian germ cell tumors (GCTs) in pediatric patients with dysgenetic gonads compared to those with normal gonadal development.
Patients and methods:
Patients from the Children’s Oncology Group AGCT0132 with malignant ovarian GCTs were included. Within this population, we sought to identify patients with gonadoblastoma, streak ovaries, or other evidence of gonadal dysgenesis (GD). Patients with malignant GCTs containing one or more of the following histologies—yolk sac tumor, embryonal carcinoma, or choriocarcinoma—were included. Patients were compared with respect to event-free survival (EFS) and overall survival (OS).
Results:
Nine patients with GD, including seven with gonadoblastoma (mean age, 9.3 years), were compared to 100 non-GD patients (mean age, 12.1 years). The estimated 3-year EFS for patients with GD was 66.7% (95% CI 28.2–87.8%) and for non-GD patients was 88.8% (95% CI 80.2–93.8%). The estimated 3-year OS for patients with GD was 87.5% (95% CI 38.7–98.1%) and for non-GD patients was 97.6% (95% CI of 90.6–99.4%).
Conclusion:
Patients presenting with nongerminomatous malignant ovarian GCTs in the context of GD have a higher rate of events and death than counterparts with normal gonads. These findings emphasize the importance of noting a contralateral streak ovary or gonadoblastoma at histology for any ovarian GCT and support the recommendation for early bilateral gonadectomy in patients known to have GD with Y chromosome material. In contrast to those with pure dysgerminoma, these patients may represent a high-risk group that requires a more aggressive chemotherapy regimen.
Keywords: gonadal dysgenesis, malignant ovarian germ cell tumor, pediatric outcome
1. BACKGROUND
Gonadal dysgenesis (GD) results from a disorder of sexual development in which chromosomal, gonadal, or anatomic sex are atypical.1–3 This condition affects one in 4,500–5,000 live births,2 and encompasses a wide spectrum of phenotypic presentations including normally virilized males or undervirilized males, patients with ambiguous genitalia, and normal phenotypic females.3 The diagnosis of GD places the patient at an increased risk (15–60%) for developing gonadal germ cell tumors (GCT), especially gonadoblastoma.1–7 The presence of Y-chromosome material or an intraabdominal gonad position increases the risk of developing a gonadal GCT.6,8,9
Gonadoblastoma is a benign neoplasm that exclusively occurs in dysgenetic gonads of children and young adults.10 In the original series of 74 patients described by Scully, 36% were bilateral.7 Approximately 80% of cases occur in phenotypic females, with virilization observed in 60%.10 In more than 90% of reported gonadoblastoma, a Y chromosome or fragment can be identified.10 Gonadoblastoma are characterized by islands or nests of primordial germ cells, mixed with sex cord derivatives, that are capable of undergoing transformation into germinoma or other invasive malignant GCTs.10,11 Transformation of gonadoblastoma to malignant GCTs is reported in up to 60% of patients with GD.10 Most case series have small numbers of patients and describe pure germinoma as the predominant malignant histology. Additional tumor types observed with gonadoblastoma include yolk sac tumor, embryonal carcinoma, and choriocarcinoma. Benign variants of teratoma may also occur.
Endocrinology consensus on the management of intersex disorders recommends gonadectomy in all high-risk groups (patients with Y-chromosomal material [GBY region] and/or intraabdominal gonads or streak gonads) at diagnosis due to the risk of malignancy (15–60%).6 Patients with complete androgen insensitivity syndrome (CAIS) are felt to be a lower risk group with later onset of tumors that may have gonadectomy deferred until puberty to allow for potential beneficial effects of hormonal secretion to occur.1,2
Patients with unrecognized GD who present with malignant ovarian GCTs may not behave in an expected fashion. In this report, we characterize the timing and behavior of malignant ovarian GCTs in those with dysgenetic gonads compared to those patients with normal gonadal development. We examine the frequency of patients with dysgenetic gonads in the overall population of malignant ovarian GCTs, and compare the histologies, age and stage at presentation, event rates, and survival rates of patients with normal and abnormal gonads among ovarian patients enrolled on Children’s Oncology Group (COG) study AGCT0132.
2. METHODS
The patients included in this report are all patients with an ovarian GCT enrolled on the COG protocol AGCT0132. Details of AGCT0132 are described in detail elsewhere.12 The patient was required to have a malignant histology defined as yolk sac tumor, embryonal carcinoma, or choriocarcinoma. Mixed GCTs could also have additional histologies including teratoma, gonadoblastoma, and dysgerminoma/seminoma. Patients with pure mature teratoma/immature teratoma or pure dysgerminoma/seminoma were excluded. All patients underwent central pathologic review. Briefly, patients with stage I ovarian and testicular malignant GCTs underwent active surveillance after surgical resection and if a recurrence were noted, they were transferred to the intermediate-risk arm to undergo chemotherapy. Also included in the intermediate-risk arm were patients with ovarian stage II or III tumors; patients with ovarian stage IV tumors were excluded from this protocol. Details for COG staging criteria have been reported previously.13 The chemotherapy consisted of three cycles of compressed pediatric PEb (cisplatin 100 mg/m2/cycle and etoposide 500 mg/m2/cycle, delivered days 1–3 and bleomycin 15,000 units/m2 per cycle on day 1).12
For this study, we identified patients with gonadoblastoma, streak ovaries, or other evidence of GD. This GD group was compared to all primary non-GD ovarian tumors. Demographics including primary site, phenotypic gender, and chromosomal abnormality if known, age at diagnosis, stage at diagnosis, and additional histology in the primary specimen were evaluated.
Patients were compared with respect to event-free survival (EFS) and overall survival (OS). EFS was defined as the time from the start of chemotherapy until disease progression, diagnosis of a second malignant neoplasm, death, or last patient contact, whichever occurred first. Patients who did not experience an event were censored at last contact. OS was defined as the time from enrolment until death or last patient contact, whichever occurred first. The survivor function for EFS and OS were estimated by the method of Kaplan and Meier.14 Con fidence intervals (CIs) for the survivor function were calculated using the complementary log-log transformation. A P-value of 0.05 or less was considered evidence to contradict the null hypothesis. No adjustment was made for the number of statistical tests performed.
3. RESULTS
The COG protocol AGCT0132 was opened to all member institutions in November 2003 and closed in July 2011. Data current to March 2015 were used in this analysis. Overall results of the trial are reported elsewhere.15 One hundred thirty-eight females with ovarian GCTs were enrolled; there were no patients with GD in the stage I; hence this report is limited to the 109 patients in the intermediate-risk group. Characteristics of this population are summarized in Table 1. The patients ranged in age from 2 to 12 years (mean 9.3 years) in the GD group, compared to 0 to 20 years (mean 12.1 years) in the non-GD group. Among the GD group, 78% of the tumors were mixed histology compared to 66% in the non-GD group. Among the 109 intermediate-risk ovarian patients enrolled, there were 47 stage II and 62 stage III patients. Within the GD group, two (22%) were stage II and seven (78%) were stage III patients. In contrast, in the non-GD group, 45 (45%) were stage II and 55 (55%) were stage III patients. The median and ranges of b-HCG and AFP levels at diagnosis are presented in Table 1. GD was identified in association with an ovarian primary malignant GCT in nine patients representing 8% of the intermediate-risk ovarian tumors. This group was compared to all primary non-GD ovarian tumors (n = 100).
TABLE 1.
Baseline characteristics of the GD group relative to the non-GD group
| GD group (n = 9) | Non-GD group (n = 100) | P-value | |
|---|---|---|---|
| Age (years) | 0.018d | ||
| 0–10 | 6 (67%) | 26(26%) | |
| 11+ | 3 (33%) | 74 (74%) | |
| Median age (range), years | 10 (2–12) | 12 (0–20) | 0.01e |
| Ovary stage | 0.295d | ||
| II | 2 (22%) | 45 (45%) | |
| III | 7 (78%) | 55 (55%) | |
| Histology | 1.000d | ||
| Pure yolk sac | 2 (22%) | 23 (23%) | |
| cMixed tumor | 7a (78%) | 66b (66%) | |
| Missing | 11(11%) | ||
| Median initial b-HCG (range) | 3.5 (0–11,540) | 1 (0–999,140) | 0.36e |
| Median initial AFP (range) | 9,598 (2–280,003) | 3,487.5 (2–4,233,000) | 0.59e |
Three tumors contained elements of choriocarcinoma.
Sixteen tumors contained elements of choriocarcinoma.
Mixed Tumor refers to tumors with multiple germ cell elements at least one of which was malignant. In the GD group, all seven of nine patients had elements of gonadoblastoma. See Table 2 for further histology.
Two-sided Fisher’s exact test.
Two-sided Wilcoxon test.
In Table 2, we provide further detail on the nine patients with GD. Of the patients with GD, seven patients had gonadoblastoma identified from review of the pathologic records. Of the two patients without gonadoblastoma, one patient had CAIS. This patient’s primary tumor was mixed histology containing malignant yolk sac and peripheral neuroendocrine tumor (PNET) like stromal elements. The other patient had streak gonads with calcification likely representing burned out gonadoblastoma as described by Scully.7
TABLE 2.
Ovarian germ cell tumors in patients with gonadal dysgenesis identified from AGCT 0132
| Patient | Age (years) | Stage | Histology | Event | Death | Details |
|---|---|---|---|---|---|---|
| 1 | 2 | III | YST PNET-like areas | No | No | CAIS |
| 2 | 8 | III | YST, Ca2+ | No | No | Streak ovary |
| GB | ||||||
| 3 | 9 | II | Left: EC, CC, G, IT Right: GB | Yes | Yes | Death 7.4 years from lymphangiosarcoma due to massive hemorrhage of pleural and peritoneal metastasis |
| 4 | 12 | III | GB, CC, G,T | Yes | Yes | Death 1.6 years from diagnosis |
| 5 | 12 | III | GB, YST, IT, G, EC, CC, sarcoma | No | No | |
| 6 | 10 | III | Right: YST, G,GB Left: GB | No | No | |
| 7 | 10 | III | G, YST, Ca2+ | Yes | No | Pelvic recurrence at 18 months from diagnosis |
| 8 | 11 | II | YST, G, GB | Yes | No | Recurrence at site of primary tumor |
| 9 | 10 | III | YST, EC, GB | No | No | |
YST, yolk sac tumor; PNET, primitive neuroectodermal tumor; GB, gonadoblastoma; G, germinoma; CC, choriocarcinoma; T, teratoma; EC, embryonal carcinoma; CAIS, complete androgen insensitivity syndrome; Ca2+, contains areas of calcification within primary tumor.
Within the GD group, there were four events—two recurrences and two deaths. Patient 3 died 7.4 years following diagnosis from a second malignancy (lymphangiosarcoma). This patient died of massive hemorrhage from pleural and peritoneal metastasis. Patient 4 died1.6 years following recurrence at the site of the primary tumor. This patient had a mixed tumor containing choriocarcinoma that was unresponsive to therapy. Patient 7 developed a recurrent tumor at 18 months from diagnosis. This was identified with imaging outside the primary tumor bed. Patient 8 developed a recurrence in the primary tumor bed and was detected via elevated AFP at scheduled follow-up. This patient was alive as of March 2015. There were no treatment-related deaths in this cohort.
Information regarding the contralateral gonad was incomplete as secondary operative procedures and pathology reports were not always available. One patient had bilateral gonadectomy at initial procedure and the contralateral gonad had gonadoblastoma. The patient with CAIS had a second procedure soon after completing chemotherapy for residual nodal disease and the contralateral gonad removed had normal testicular tissue. One patient with biopsy only at diagnosis due to extensive disease had bilateral gonadectomy soon after completing chemotherapy with bilateral gonadoblastoma and still had viable choriocarcinoma, germinoma, and teratoma in the primary ovary. Two patients are known to have had later contralateral gonadectomy but pathology reports are unavailable. In four patients, no information was available.
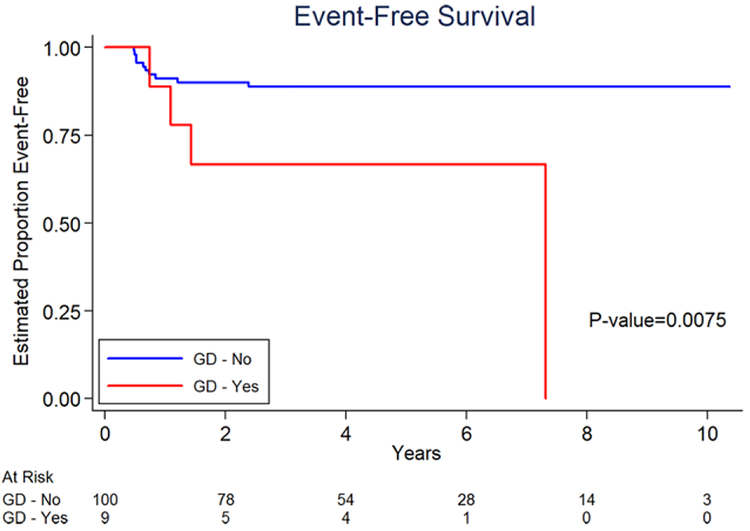
The estimated 3-year EFS for patients with GD was 66.7% (95% CI 28.2–87.8%) and for non-GD patients was 88.8% (95% CI of 80.2–93.8%) (Figure 1). Patients who did not have GD in this analysis were at significantly reduced risk of EFS event (P = 0.0075). The estimated 3-year OS for patients with GD was 87.5% (95% CI 38.7–98.1%)and for non-GD patients was 97.6% (95% CI of 90.6–99.4%) (Figure 2). Patients who did not have GD in this analysis were at significantly reduced risk of death (P = 0.0012).
FIGURE 1.
Event-free survival of GD versus non-GD occurring within primary ovarian germ cell tumors
FIGURE 2.
Overall survival of GD versus non-GD within primary ovarian germ cell tumors
4. DISCUSSION
The primary analytic cohort consisted of patients with a nongerminomatous malignant ovarian GCT enrolled on AGCT0132 as intermediate-risk patients. GD was noted in 8% of the intermediate-risk patients and is comparable to the 14% incidence of GD in a series of ovarian GCTs in adolescent and adult patients by Lin at al., which also included germinoma and immature teratoma.16 In our series, patients with GD are apparently at higher risk of treatment failure with standard chemotherapy (EFS 66.7% [95% CI 28.2–87.8%] vs. 88.8% [95% CI 80.2–93.8%] and OS 87.5% [95% CI 38.7–98.1%] vs. 97.6% [95% CI 90.6–99.4%]). The mean age at diagnosis for patients with abnormal gonads was younger than the comparison group (9.3 years vs.12.1 years) and all were less than 13 years. No patient with GD presented in the low risk group (stage I).
Our results suggest that patients presenting with nongerminomatous malignant ovarian GCTs within dysgenetic gonads are at a significantly decreased EFS and OS when compared to non-GD ovarian tumors. Only one of the seven patients was known to have GD prior to surgery. This 2-year-old female child with CAIS presented with yolk sac tumor and PNET-like areas in an intraabdominal testis. She was in a low-risk group where gonadectomy is typically delayed until puberty. There is only one previous case report of germ cell malignancy in a patient less than 10 years with CAIS.2,17 Although there is a recognized association of CAIS with the development of gonadoblastoma (30%), there is no reported association of CAIS and PNET-like tumors in the context of GD.18 The other patients were discovered due to the finding of a streak gonad at operation or by the finding of gonadoblastoma at histology. This observation reinforces the importance of careful inspection and documentation of the contralateral ovary at initial operation as well as thorough histological examination of the specimen. The finding of bilateral tumors at diagnosis would preclude the ability to detect a streak ovary on the contralateral side. In the previously reported intergroup study of ovarian GCTs by Billmire et al.,13 11 of 131 patients had bilateral disease at diagnosis and two of those also had gonadoblastoma. Chromosomal studies were not available in that report. An interesting finding was reported by Hennes et al.19 in their review of the Kiel German Childhood Tumor Registry. They chose to undertake molecular genetic analysis of their ovarian GCT patients with bilateral tumors at diagnosis. All of their patients were phenotypic females with no history of genetic abnormality and included both benign and malignant GCTs. Y chromosomal DNA was found in six patients. Five of the six patients had bilateral germinoma with three showing gonadoblastoma. The sixth patient had immature teratoma bilaterally with elevated AFP but no yolk sac component seen at histology. One patient with dysgerminoma had a tumor recurrence but responded to therapy.
Two patients had somatic components in their tumor at diagnosis (PNET and sarcoma). Previous studies have reported similar sarcomatous elements within malignant GCTs, either within the primary or metastatic sites.20 Guo et al.21 suggested that sarcomatous elements confined to the primary GCTs might not have a higher risk of mortality than stage-matched controls without sarcoma elements. In our series, patient 1 (PNET like) had a diagnosis of CAIS, and to date has had no disease-related events. Patient 5 (stromal sarcoma) similarly was disease free at last follow-up. This is in contrast to data reported by Terenziana et al. that reported a worse prognosis for GCTs containing malignant somatic components in the pediatric population.22 This group suggested that surgery played an essential role in localized disease, with complete resection associated with desirable outcomes.22 This may be reflected in the outcome in our series, where both patients had complete resection, and are alive and well at last follow-up.
Most reports of germ cell malignancy arising in gonadoblastoma are from unselected series of either ovarian germ cell malignancies or patients with known abnormal gonads. Prior series note germinoma to be the most common malignancy in patients with abnormal gonads and describe good success with treatment. In Lin’s series16 of 50 patients age 13–49 years, 7 patients with GD were found with five containing gonadoblastoma. Six of those patients had germinoma as the only malignant histology and one had a mixed tumor with teratoma and embryonal carcinoma (i.e., teratocarcinoma). Clinical outcome was similar to those with normal karyotype.16
Controversy exists with respect to timing of gonadectomy with most favoring gonadectomy at diagnosis in those patients known to have elements of Y chromosome. Patients felt to be at lower risk often have gonadectomy delayed until puberty to allow maintenance of hormonal factors for secondary sexual development. The younger age at diagnosis of malignancy in our patients is of concern as all were less than 13 years of age.
The concept of surveillance with tumor markers and imaging has been considered as an aid in early detection. Tumor markers are only available for yolk sac and choriocarcinoma but are lacking for the more common germinoma in this population. Therefore, we would suggest that monitoring with tumor markers is insufficient for early diagnosis. There also is insufficient evidence to recommend safe surveillance of intraabdominal gonads with imaging modalities. Alaniz et al.8 evaluated 39 patients with GD who underwent imaging prior to prophylactic gonadectomy. They were able to identify only 40–50% of gonads with ultrasound or magnetic resonance imaging, with no significant difference between modalities.8 In addition, 28% of these patients had gonadoblastoma and one had malignancy and the imaging identified none of these findings.
The limited number of patients with GD limited this study. Because chromosomal analysis of all patients was not required, there may be some misclassification of patients in whom the GD was either not recognized or reported to COG. In addition, because this study did not include patients with pure dysgerminoma, we cannot comment on the outcome of patients with GD who develop dysgerminoma versus patients without GD who develop dysgerminoma. Additionally, because this was a clinical trial of patients with proven malignant GCTs, we cannot comment on the likelihood of developing a GCT in a patient with GD.
Our results suggest that patients with nondysgerminomatous malignant ovarian GCTs that arise in the context of GD have a higher rate of events and death than counterparts with normal gonads. These tumors occurred at a younger mean age than their comparison group and had more advanced stage tumors at diagnosis. The discovery of streak gonads at operation for an ovarian tumor or the finding of gonadoblastoma at histology should prompt early determination of chromosomal analysis and mandates early contralateral gonadectomy when GD is confirmed. These findings support the recommendation for early bilateral gonadectomy in patients known to have GD with Y chromosome material and raise the issue as to whether they should be managed as poor-risk patients with more aggressive chemotherapy protocols.
Abbreviations:
- CAIS
complete androgen insensitivity syndrome
- COG
Children’s Oncology Group
- EFS
event-free survival
- GCT
germ cell tumor
- GD
gonadal dysgenesis
- OS
overall survival
- PNET
peripheral neuroendocrine tumor
Footnotes
CONFLICT OF INTEREST
The authors declare that there is no conflict of interest.
REFERENCES
- 1.Huang H, Wang C, Tian Q. Gonadal tumour risk in 292 phenotypic female patients with disorders of sex development containing Y chro mosome or Y-derived sequence. Clin Endocrinol (Oxf) 2017;86:621–627. [DOI] [PubMed] [Google Scholar]
- 2.Kathrins M, Kolon TF. Malignancy in disorders of sex development. Transl Androl Urol 2016;5:794–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCann-Crosby B, Mansouri R, Dietrich JE, et al. State of the art review in gonadal dysgenesis: challenges in diagnosis and management. Int J Pediatr Endocrinol 2014;2014:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Abacı A, Çatlı G, Berberoğlu M. Gonadal malignancy risk and prophylactic gonadectomy in disorders of sexual development. J Pediatr Endocrinol Metab 2015;28:1019–1027. [DOI] [PubMed] [Google Scholar]
- 5.Jiang JF, Xue W, Deng Y, Tian QJ, Sun AJ. Gonadal malignancy in 202 female patients with disorders of sex development containing Y-chromosome material. Gynecol Endocrinol 2016;32:338–341. [DOI] [PubMed] [Google Scholar]
- 6.Lee PA, Houk CP, Ahmed SF, Hughes IA, International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics.2006;118:e488–e500. [DOI] [PubMed] [Google Scholar]
- 7.Scully RE. Gonadoblastoma: a review of 74 cases. Cancer.1970;25:1340–1356. [DOI] [PubMed] [Google Scholar]
- 8.Alaniz VI, Kobernik EK, Dillman J, Quint EH. Utility of ultrasound and magnetic resonance imaging in patients with disorders of sex development who undergo prophylactic gonadectomy. J Pediatr Adolesc Gynecol 2016;29:577–581. [DOI] [PubMed] [Google Scholar]
- 9.Oliveira RM, Verreschi IT, Lipay MV, Eça LP, Guedes AD, Bianco B. Y chromosome in Turner syndrome: review of the literature. Sao Paulo Med J. 2009;127:373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talerman A, Roth LM. Recent advances in the pathology and classification of gonadal neoplasms composed of germ cells and sex cord derivatives. Int J Gynecol Pathol 2007;26:313–321. [DOI] [PubMed] [Google Scholar]
- 11.Ulbright TM, Young RH. Gonadoblastoma and selected other aspects of gonadal pathology in young patients with disorders of sex development. Semin Diagn Pathol 2014;31:427–440. [DOI] [PubMed] [Google Scholar]
- 12.Billmire DF, Cullen JW, Rescorla FJ, et al. Surveillance after initial surgery for pediatric and adolescent girls with stage I ovarian germ cell tumors: report from the Children’s Oncology Group. J Clin Oncol 2014;32:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Billmire D, Vinocur C, Rescorla F, et al. Outcome and staging evaluation in malignant germ cell tumors of the ovary in children and adolescents: an intergroup study. J Pediatr Surg 2004;39:424–429. discussion 424. [DOI] [PubMed] [Google Scholar]
- 14.Kaplan EL. Nonparametric Estimation from Incomplete Observations. J Am Statist Assoc 1958;53:457–481. [Google Scholar]
- 15.Shaikh F, Cullen JW, Olson TA, et al. Reduced and compressed cisplatin-based chemotherapy in children and adolescents with intermediate-risk extracranial malignant germ cell tumors: a report from the Children’s Oncology Group. J Clin Oncol 2017;35:1203–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin KY, Bryant S, Miller DS, Kehoe SM, Richardson DL, Lea JS. Malignant ovarian germ cell tumor—role of surgical staging and gonadal dys-genesis. Gynecol Oncol 2014;134:84–89. [DOI] [PubMed] [Google Scholar]
- 17.Handa N, Nagasaki A, Tsunoda M, et al. Yolk sac tumor in a case of testicular feminization syndrome. J Pediatr Surg 1995;30:1366–1367; discussion 1367. [DOI] [PubMed] [Google Scholar]
- 18.Liu AX, Shi HY, Cai ZJ, et al. Increased risk of gonadal malignancy and prophylactic gonadectomy: a study of 102 phenotypic female patients with Y chromosome or Y-derived sequences. Hum Reprod 2014;29:1413–1419. [DOI] [PubMed] [Google Scholar]
- 19.Hennes E, Zahn S, Lopes LF, et al. Molecular genetic analysis of bilateral ovarian germ cell tumors. Klin Padiatr 2012;224:359–365. [DOI] [PubMed] [Google Scholar]
- 20.Malagón HD, Valdez AM, Moran CA, Suster S. Germ cell tumors with sarcomatous components: a clinicopathologic and immunohistochemical study of 46 cases. Am J Surg Pathol 2007;31:1356–1362. [DOI] [PubMed] [Google Scholar]
- 21.Guo CC, Punar M, Contreras AL, et al. Testicular germ cell tumors with sarcomatous components: an analysis of 33 cases. Am J Surg Pathol 2009;33:1173–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Terenziani M, D’Angelo P, Bisogno G, et al. Teratoma with a malignant somatic component in pediatric patients: the Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP) experience. Pediatr Blood Cancer. 2010;54:532–537. [DOI] [PubMed] [Google Scholar]