Abstract
Enabling motor control by epidural electrical stimulation of the spinal cord is a promising therapeutic technique for the recovery of motor function after a spinal cord injury (SCI). Although epidural electrical stimulation has resulted in improvement in hindlimb motor function, it is unknown whether it has any therapeutic benefit for improving forelimb fine motor function after a cervical SCI. We tested whether trains of pulses delivered at spinal cord segments C6 and C8 would facilitate the recovery of forelimb fine motor control after a cervical SCI in rats. Rats were trained to reach and grasp sugar pellets. Immediately after a dorsal funiculus crush at C4, the rats showed significant deficits in forelimb fine motor control. The rats were tested to reach and grasp with and without cervical epidural stimulation for 10 weeks post-injury. To determine the best stimulation parameters to activate the cervical spinal networks involved in forelimb motor function, monopolar and bipolar currents were delivered at varying frequencies (20, 40, and 60 Hz) concomitant with the reaching and grasping task. We found that cervical epidural stimulation increased reaching and grasping success rates compared to the no stimulation condition. Bipolar stimulation (C6− C8+ and C6+ C8−) produced the largest spinal motor-evoked potentials (sMEPs) and resulted in higher reaching and grasping success rates compared with monopolar stimulation (C6− Ref+ and C8− Ref+). Forelimb performance was similar when tested at stimulation frequencies of 20, 40, and 60 Hz. We also found that the EMG activity in most forelimb muscles as well as the co-activation between flexor and extensor muscles increased post-injury. With epidural stimulation, however, this trend was reversed indicating that cervical epidural spinal cord stimulation has therapeutic potential for rehabilitation after a cervical SCI.
Keywords: Cervical spinal cord injury, corticospinal tract, epidural electrical stimulation, motor-evoked potentials, reaching and grasping
Introduction
Electrical stimulation of the spinal cord is a promising therapy for the rehabilitation of sensorimotor function after a spinal cord injury (SCI) (Edgerton and Roy, 2012; Jackson and Zimmermann, 2012; Dietz and Fouad, 2014; Alam et al., 2016). Stimulation of the lumbosacral spinal cord has resulted in successful restoration of weight-bearing standing and stepping in complete paraplegic cats and rats (Gerasimenko et al., 2003; Saigal et al., 2004; Ichiyama et al., 2005; Gerasimenko et al., 2007; Courtine et al., 2009; Musienko et al., 2009; Wenger et al., 2014). Spinal cord stimulation at the lumbosacral cord in human subjects with a clinically complete SCI has resulted in recovery of standing and of some volitional control of leg movements as well as improvement in autonomic function (Minassian et al., 2004; Harkema et al., 2011; Angeli et al., 2014).
Restoration of arm and hand function is one of the highest priorities of individuals with a cervical SCI (Anderson, 2004). Previous work has shown that intraspinal stimulation at the cervical segments of the spinal cord elicits motor responses in multiple forelimb muscles in rats (Sunshine et al., 2013) and that selected stimulation parameters can facilitate functional reaching and grasping movements in non-injured monkeys (Zimmermann et al., 2011; Sharpe and Jackson, 2014). It also has been shown that chronic intraspinal stimulation at the cervical spinal cord can improve forelimb function in rats with a cervical SCI (Kasten et al., 2013; Mondello et al., 2014). In a recent study, we have demonstrated that epidural stimulation at the cervical spinal cord acutely improves forelimb grip strength in rats with a cervical SCI (Alam et al., 2015). The effects of epidural stimulation on reaching and grasping performance, however, remain unknown.
The objectives of the present study were to 1) determine the effects of epidural electrical stimulation of the cervical spinal cord on forelimb functional recovery in rats with an incomplete cervical SCI and 2) identify the effectiveness of different stimulation parameters, i.e., electrode configuration and polarity and stimulation frequency and intensity, in successfully facilitating forelimb reaching and grasping. To accomplish these objectives, adult rats were trained and tested to reach and grasp for single sugar pellets. Intramuscular EMG electrodes were implanted in several forelimb muscles and epidural stimulation electrodes were implanted at cervical spinal cord segments C6 and C8. Reaching and grasping performance was measured without and with different epidural stimulation paradigms after a cervical SCI. The results show that bipolar stimulation (C6− C8+ and C6+ C8−) over a range of 20-60 Hz improves reaching and grasping performance compared to the no-stimulation condition. The results also indicate that cervical epidural spinal cord stimulation can facilitate the sensorimotor networks that control forelimb function similar to that observed previously in facilitating locomotor function of the hindlimbs with epidural stimulation of the lumbosacral segments (Ichiyama et al., 2005; Gerasimenko et al., 2007; Courtine et al., 2009).
Materials and methods
All experimental procedures were approved by the Animal Research Committee at the University of California Los Angeles (UCLA) and conducted in compliance with the Guide for the Care and Use of Laboratory Animals (National Institutes of Health, Publication No. 86-23, revised 1985).
Animals and surgical procedures
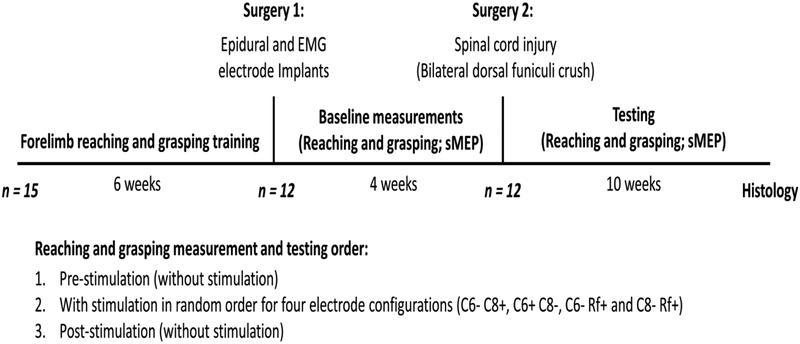
Fifteen healthy female Long-Evans rats (270–350 gm body weight) were used in this study. The rats were housed individually at a constant room temperature of 25°C and humidity of 40% and were maintained on a 12:12 hour light: dark cycle. Water was supplied ad libitum and rat chow availability was monitored carefully based on percentage weight gain. The rats were trained to reach and grasp sugar pellets using their preferred paw as described previously (Whishaw and Tomie, 1989). The 12 rats showing the best reaching and grasping performance underwent two surgeries. In the first surgery (Surgery 1), the rats were implanted with EMG and spinal epidural electrodes. Four weeks later, in a second surgery (Surgery 2), the rats received an incomplete cervical SCI (Fig. 1).
Figure 1: Experimental design.
Fifteen rats were trained to reach and grasp sugar pellets with their preferred paw. The 12 rats having the best performance were implanted with stimulating epidural electrodes at spinal cord levels C6 and C8 and recording intramuscular electrodes in several forelimb muscles (Surgery 1). Baseline reaching and grasping performance was determined and spinal motor-evoked potentials (sMEPs) were recorded for 4 weeks post-implantation. Subsequently, all 12 rats received a dorsal funiculi crush at C4 (Surgery 2). In the following 10 weeks post-injury, reaching and grasping performance and sMEPs were tested biweekly. In each testing session, reaching and grasping performance was tested pre-, during, and post-stimulation using four different electrode configurations. Three stimulation frequencies (20, 40, and 60 Hz) were tested on alternate days of each week of testing.
All survival surgical procedures were conducted under aseptic conditions. Rats were anesthetized with isoflurane gas (1.5 to 2.5%) administered via facemask to effect throughout the surgery. To prevent hypothermia, body temperature was maintained at 37°C using a heating pad (TP-500, Gaymar Industries Inc., Orchard Park, NY, USA). Post-surgery, the rats were given lactated ringers (5-6 cc, s.c.) and placed in an incubator maintained at 27°C until fully recovered. The rats were given an antibiotic (Enrofloxacin, 0.05 mg/kg, s.c.) and an analgesic (Buprenorphine HCl, 0.5 mg/kg, s.c.) twice daily for three days post-surgery. Rodent food pellets, fresh fruit (orange and apple slices), and cereal (fruit loops) were placed in the cage during the first week of recovery.
Surgery I: EMG and epidural electrode implantation
Intramuscular EMG electrodes were implanted unilaterally to the preferred paw in five forelimb muscles (deltoid, biceps brachii, pronator teres, flexor digitorum, and extensor digitorum) relevant for performing reaching and grasping movements. A skin incision was made along the midline of the skull. The connective tissue and the muscles covering the skull were reflected laterally. The skull was thoroughly dried and stainless steel screws were firmly inserted into the exposed bone. A miniature connector (Omnetics, Minneapolis, MN, USA) was placed between the screws and rigidly affixed to the bone using dental cement. Skin and fascial incisions were made to expose the bellies of the forelimb muscles of interest. Two multi-stranded Teflon-coated stainless steel wires (AS632, Cooner Wire, Chatsworth, CA, USA) connected to the pins of the head-connector were passed subcutaneously to each proximal muscle: deltoid and biceps brachii. Two smaller PFA-insulated multi-stranded stainless steel wires (793200, A-M Systems, Sequim, WA, USA) connected to the same head-connector were similarly passed to each distal muscle: pronator teres, flexor digitorum, and extensor digitorum. The wires were passed into each muscle belly using 27-gauge (proximal muscles) or 30-gauge (distal muscles) needles and a small notch (~0.5-1.0 mm) was made in the wire coating to form an EMG recording electrode. The electrodes then were positioned and the electrode wires were anchored at both ends with 4.0 and 5.0 Ethilon sutures. The EMG wires were coiled near each implant site for stress relief. Stimulation through the head connector was used to verify proper placement of the electrodes.
For the cervical epidural electrode implantation, a partial laminectomy was performed at the C6 and T1 vertebral levels to expose the C6 and C8 spinal cord levels. Teflon-coated stainless steel wires (AS632, Cooner Wire, Chatsworth, CA, USA) from the head-connector were passed subcutaneously to the laminectomy sites and then passed under the spinous processes of the remaining vertebrae between the partial laminectomies and above the dura mater. Stimulation electrodes were made by removing a small portion of the Teflon (~1 mm) to expose the stainless steel wire on the surface facing the spinal cord. The electrodes were secured in position by suturing the wire to the dura mater above and below the electrode using 8.0 Ethilon suture. In addition, the Teflon was pulled gently over the cut end of the wires to prevent stimulation through this site. A loop was formed near the site of insertion of the wires to provide stress relief. A common reference (Rf) wire (~1 cm of the Teflon removed at the distal end) was inserted subcutaneously near the shoulder on the dominant paw side. All exposed areas were kept moist with 0.9% saline washes. All incisions were closed using 4.0 Vicryl for the muscle and connective tissue layers and 4.0 Ethilon for the skin.
Surgery II: Cervical spinal cord injury
A longitudinal midline skin incision was made dorsal to the spinal column and the underlying back muscles were reflected laterally to provide access to the vertebrae overlying the cervical spinal cord segments. Special care was taken to not displace nor damage the previously implanted epidural electrodes. A partial laminectomy was performed at C3-C4 vertebrae to expose the spinal cord and the underlying dura was opened with a longitudinal incision (1-3 mm). To produce an incomplete cervical SCI, the C4 dorsal funiculi were crushed bilaterally by placing the tips of fine forceps 2 mm apart (1 mm on each side of the midline), inserting the tips 2 mm in depth into the spinal cord, and then squeezing the tips together and holding them closed for 20 sec. Histological evaluation of this injury has been reported previously (Alam et al., 2015). All exposed areas were kept moist with 0.9% saline washes. All incisions were closed using 4.0 Vicryl for the muscle and connective tissue layers and 4.0 Ethilon for the skin.
Reaching and grasping training and testing
The rats were acclimated to the testing environment prior to all surgeries. Each rat was identified as right- or left-handed (preferred paw) during the standard reaching and grasping task (Whishaw and Tomie, 1989; McKenna and Whishaw, 1999). The rats were placed individually inside a translucent acrylic box (18 cm × 15 cm × 31 cm) with a small slit in the front wall (3 cm × 1.5 cm). A 45 mg banana-flavored sugar pellet (Dustless Precision Pellets, Bio-Serv, Frenchtown, NJ, USA) was placed on a platform 1 cm away from the slit and the rats were trained to reach and grasp the pellet with the preferred paw. During the testing session, a total of 20 pellets were presented to each rat and the ratio of the number of pellets eaten to the total number of attempts was calculated as the success rate. In each reaching and grasping cycle, the animal was positioned facing the front wall window with both forelimb paws on the cage floor. Reaching usually started with activation of the deltoid and/or biceps muscles, i.e., the most proximal forelimb muscles evaluated. The rats were tested pre-injury (baseline testing) and up to 10 weeks post-injury. Video recordings of this task were taken at three different camera angles: front, left, and right at 100 frames/s each using a motion capture system (Simi Reality Motion Systems, Unterschleissheim, Germany). Light blinks to the videos were initiated through manual pulses to assist in the synchronization of the forelimb EMG signals with the video recordings during the reaching and grasping behavior.
Spinal epidural stimulation protocol
Epidural electrical stimulation was delivered through six electrode combinations, i.e., two bipolar (C6− C8+ and C6+ C8−) and four monopolar (C6− Rf+, C6+ Rf−, C8− Rf+, and C8+ Rf−) configurations. With the rats awake and at rest, stimulation pulses (200 μsec pulse width) were delivered at 2 Hz at different current intensities using a constant current stimulator (Grass SIU5; Grass Instruments, Warwick, RI, USA) to produce spinal motor-evoked potentials (sMEP) in each implanted forelimb muscle. The signals were filtered (band-pass; 30-1000 Hz) and amplified (1000 ×) using a multichannel analog amplifier (Differential AC amplifier Model 1700, AM-Systems Inc., Sequim, WA, USA). The amplified signals and the stimulation monitor and synchronization pulses were digitized at 10 KHz and stored on a computer using a data acquisition card (NI PCI-6052E, National Instruments Inc., Austin, TX, USA) operated with a custom designed software written in LabVIEW (National Instruments Inc., Austin, TX, USA). The initial appearance of sMEPs was used to determine the stimulation threshold for each stimulation configuration.
To determine the effects of epidural spinal cord stimulation on forelimb fine motor control, monophasic stimulation pulses were delivered during the reaching and grasping task at sub-threshold current intensities (60-70% of the previously recorded sMEP threshold current). The experiments were conducted six days per week: the first three days were used to determine the sMEP thresholds and the next three days were used to determine the reaching and grasping scores (see below). Two bipolar (C6− C8+ and C6+ C8−) and two monopolar (C6− Rf+ and C8− Rf+) stimulation configurations were tested in random order between the pre- and poststimulation reaching and grasping tests (without stimulation) as described in Figure 1. The other two monopolar configurations (C6+ Rf− and C8+ Rf−) were not used due to their high sMEP threshold currents (Fig. 2A). For each of the stimulation configurations, three stimulation frequencies (20, 40, and 60 Hz) were tested separately on different days. The first three consecutive days of each week were used to determine the sMEP thresholds: current from the different electrode configurations was delivered at increasing intensities at 0.5 Hz until an evoked potential (sMEP) was obtained for any of the muscles tested. This procedure required ~50 pulses for each electrode configuration. On the subsequent three consecutive days of each week reaching and grasping scores were determined at the three stimulation frequencies. On each day the reaching and grasping scores were measured without stimulation (pre-stimulation), with the four stimulation configurations, and then again without stimulation (post-stimulation). The duration of the entire testing session was ~20-25 min for each rat during which the rat was stimulated for ~12-15 min. The data were collected for all pre-, during-, and post-stimulation sessions.
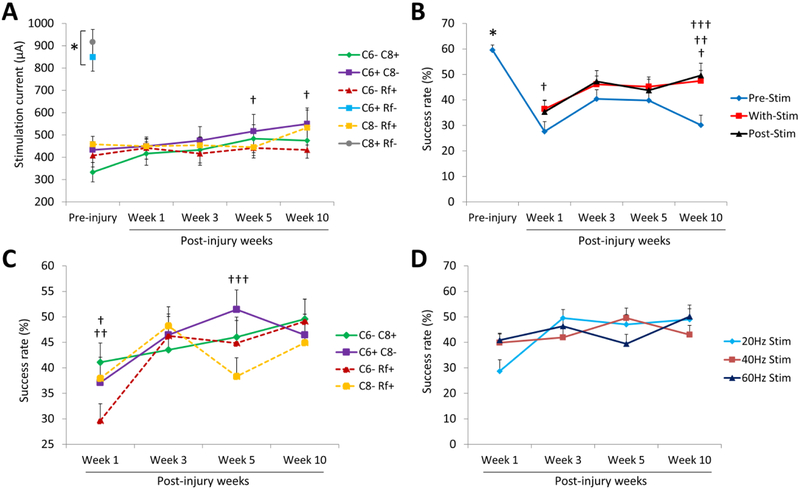
Figure 2: Cervical electrical stimulation facilitates forelimb reaching and grasping function after a cervical SCI.
(A) Mean (±SEM) threshold current required for each stimulation electrode configuration to elicit spinal motor evoked potentials (sMEPs) in any of the five forelimb muscles tested pre-injury and at different time points post-injury. C6+ Rf− and C8+ Rf− vs. other electrode configurations (*p < 0.05). C6+ C8− configuration at weeks 5 and 10 vs. week 1 (†p < 0.05). (B) Mean (±SEM) success rates for reaching and grasping when the rats were not receiving epidural stimulation (blue line) and the average of all the success rates obtained with the combination of all the stimulation parameters tested during (red line) and immediately after (black line) receiving epidural stimulation. Pre-injury vs. post-injury without stimulation (*p < 0.05). Pre-stimulation vs. with stimulation (†p < 0.05) and post-stimulation (††p < 0.05). Difference between pre-stimulation and post-stimulation at week 1 vs. other post-injury time points (†††p < 0.05). (C) Effects of the stimulation electrode configuration on reaching and grasping performance. Mean (±SEM) success rates for reaching and grasping with all four electrode configurations were obtained by combining all of the frequencies tested. C6− C8+ vs. C6− Rf+ (†p < 0.05). C6+ C8− vs. C6− Rf+ (††p < 0.05) and C8− Rf+ (†††p < 0.05). (D) Effects of stimulation frequency on reaching and grasping performance. Mean (±SEM) success rates for reaching and grasping for all three stimulation frequencies showed no differences when combining all stimulation electrode configurations tested.
Data analyses
To calculate the reaching and grasping success rate, successful attempts were scored as 1 and unsuccessful attempts as 0. For qualitative assessment (accuracy) of the reaching and grasping task, we used a three point scoring system where 0 = absent, 0.5 = impaired or ambiguity regarding the movement, and 1 = normal (Whishaw et al., 2003; García-Alías et al., 2015). A total of seven components of the movement were analyzed: limb advance, digit extension, pronation (arpeggio), digit flexion, supination I, supination II, and pellet release. The EMG signals were synchronized to the exact video frame using a custom written program in LabVIEW (National Instruments, Austin, TX, USA). This allowed us to determine the exact EMG time points when the rat initially lifted its paw, when it grasped the food pellet, and when the food was delivered to the mouth. The EMG recordings for successful movements at the different time points were rectified and integrated and then used to compare the muscle activation patterns across the time points. The amount of co-activation between the antagonistic flexor digitorum and extensor digitorum muscles was determined using joint probability density distributions (de Guzman et al., 1991).
sMEP data were analyzed offline using a custom written program in MATLAB (MathWorks Inc., Natick, MA, USA). The program allowed detection of distinct raw EMG signals that corresponded to the rat’s active (roaming) and non-active (resting) periods during normal cage activity using an algorithm (Solnik et al., 2010). sMEPs were analyzed during the non-active periods for all tested forelimb muscles. A single trial of sMEPs was defined as evoked responses during the 30 ms time window after the start of the stimulation pulse. This 30 ms time window then was divided into an early (first 10 ms) and a late (last 20 ms) component. The sMEPs subsequently were rectified and averaged for each component of the time window. The area under the rectified sMEP curves was measured to determine the total muscle activation at each stimulation current intensity and electrode configuration.
Statistics
Repeated measures analysis of variance (ANOVA) was used to determine any overall differences among the success rates for pre-, during-, and post-stimulation comparisons. Individual group differences were determined using the Bonferroni post-hoc test. Differences in pre- vs. post-injury levels of muscle activation for different paired conditions with vs. without stimulation were analyzed using two-tailed paired t-tests. Differences in the success rates for reaching and grasping between pre- vs. post-injury also were determined using two-tailed paired t-test. Differences between groups were considered statistically significant at p < 0.05 (* and †). All statistical analyses were performed using Prism (GraphPad Software Inc., La Jolla, CA, USA) and SPSS Statistics (IBM Corporation, Armonk, NY, USA).
Results
Cervical epidural spinal cord stimulation facilitates the recovery of forelimb skilled motor function after a cervical SCI
Prior to the cervical injury, we evaluated the amount of current intensity required using the different electrode configurations to evoke motor responses (sMEPs) in all muscles tested. The stimulation thresholds for the C6+ Rf− and C8+ Rf− electrode configurations (850 ± 64.01 and 916.67 ± 56.18, respectively) were higher (p < 0.05, one-way ANOVA) compared to the other stimulation configurations (333.33 ± 43.23, 433 ± 37.60, 408.33 ± 51.43 and 458.33 ± 35.80) (Fig. 2A) and these intensities also evoked robust twitches in the trunk and limb muscles that disrupted the rat’s movements. Thus, these configurations were excluded from further testing. The threshold currents for the remaining four electrode configurations were relatively low and grouped within a narrow range of intensities. During the 10 weeks of post-injury testing the threshold currents were fairly stable, except for the C6+ C8− configuration that required significantly higher currents (516.67 ± 75.71 and 550 ± 71.24, p < 0.05, two-way ANOVA) at weeks 5 and 10 post-injury.
After baseline testing of reaching and grasping (as described in the Methods and materials section), the rats received a dorsal funiculi crush at C4 that selectively damaged the dorsal corticospinal tract and dorsal columns (Alam et al., 2015). The injury did not affect gross arm and hand function and the rats were able to use their forelimbs to hold food pellets and to maintain posture and locomote. Skilled hand movements, however, were impaired and the success rates for reaching and grasping during pre-stimulation testing were lower than pre-injury (59.58 ± 2) over the 10-week post-injury period (27.62 ± 3.89, 40.42 ± 3.60, 39.80 ± 4.03, and 30.12 ± 3.93, at week 1, 3, 5, and 10 post-injury) (p < 0.05, one-way ANOVA) (Fig. 2B). Reaching and grasping success rates were higher with stimulation compared to pre-stimulation at 1 (36.45 ± 3.54 vs. 27.62 ± 3.89) and 10 (47.52 ± 4.01 vs. 30.12 ± 3.93) weeks post-injury (p < 0.05, two-way ANOVA) (Fig. 2B). We analyzed the effects of electrode configuration (Fig. 2C) and stimulation frequency (Fig. 2D) on the success rates to reach and grasp. For all electrode configurations tested, there was a general trend for the animals to improve their reaching and grasping abilities over time. However, at different time points, at least one bipolar stimulation (C6+ C8− or C6− C8+) produced significantly higher (p < 0.05, two-way ANOVA) success rate than the scores achieved during one of two monopolar stimulations (C6− Rf+ or C8− Rf+) (Fig. 2C). The success rates for reaching and grasping were not different among the different stimulation frequencies at any time point post-injury (Fig. 2D).
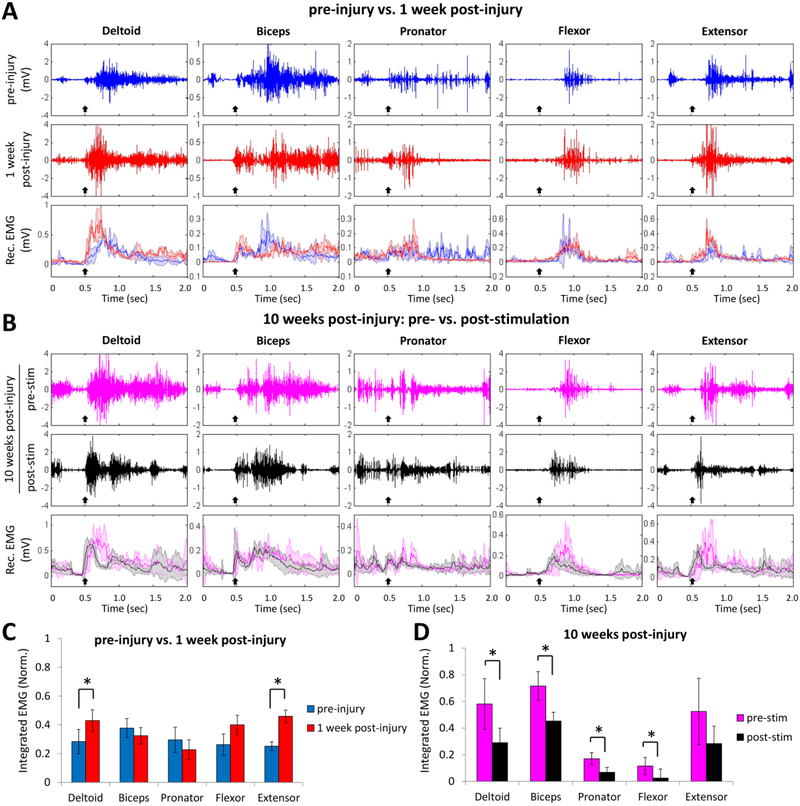
EMG activity was monitored in the forelimb muscles during reaching and grasping pre-injury and post-injury. Pre-injury the EMG recordings showed a specific temporal pattern of muscle recruitment that was initiated with the lifting of the paw (Fig. 3A, black arrow) and in Supplementary Video 1. The more proximal muscles (deltoid, biceps, and pronator) were activated as soon as the paw was lifted, whereas the more distal muscles that were involved with finger extension and flexion were activated during the later stages of the reaching and grasping movement, i.e., the arpeggio, grasping, and pellet release components of the movement. At 1 week post-injury there were muscle-specific EMG changes in amplitude and pattern of activity throughout the reaching and grasping task. For instance, the amplitude of the initial EMG burst of the deltoid and extensor (0.43 ± 0.07 and 0.46 ± 0.0.4, respectively) were significantly higher (p < 0.05, paired t-test) compared to pre-injury (0.28 ± 0.08 and 0.25 ± 0.03, respectively) (Fig. 3C). The activity pattern in the biceps was characterized by an initial burst of low amplitude with a burst of higher amplitude sustained during the later part of the task. The duration of the pronator activity tended to be consistently low in the later part of the task. The most consistent change in the distal muscles observed was higher burst amplitudes in the extensor than flexor muscle. At 10 weeks post-injury, the EMG amplitude of all the muscles remained higher compared to pre-injury values. The EMG amplitudes, however, were significantly lower (p < 0.05, paired t-test) in most muscles post-stimulation compared to pre-stimulation (deltoid: 0.29 ± 0.11 vs. 0.58 ± 0.19; biceps: 0.45 ± 0.06 vs. 0.72 ± 0.11; pronator: 0.07 ± 0.04 vs. 0.17 ± 0.04; flexor: 0.02 ± 0.06 vs. 0.11 ± 0.06) (Fig. 3D).
Figure 3: Effects of cervical electrical stimulation on forelimb muscle synergies post-injury.
(A) Raw EMG signals from the forelimb muscles during reaching and grasping pre-injury (blue trace) and 1 week post-injury (red trace), and the mean (±SD, color shading) rectified (Rec.) EMG signals (n = 20 trials each) from the same rat. Black arrow indicates the initiation of lifting the forelimb paw. (B) Raw EMG signals from the forelimb muscles during reaching and grasping at 10 weeks post-injury pre-stimulation (pink traces) and post-stimulation (black traces), and the mean (±SD, color shading) rectified EMG signals (n = 20 trials each) from the same rat. Black arrow indicates the initiation of lifting the forelimb paw. (C) Comparison of the mean (±SEM) integrated EMG values (n = 40 trials, 5 rats) obtained pre-injury and 1 week post-injury during reaching and grasping without stimulation. Two muscles, i.e., the deltoid and extensor, had significantly higher EMG activity levels 1-week post-injury compared to pre-injury when performing the task. (D) Comparison of the mean (±SEM) integrated EMG values (n = 40 trials, 5 rats) obtained prior (pre-stim) and immediate after (post-stim) receiving epidural stimulation at 10 weeks post-injury. *: significantly different at p < 0.05
Cervical epidural electrical stimulation improves distal muscle coordination post-injury
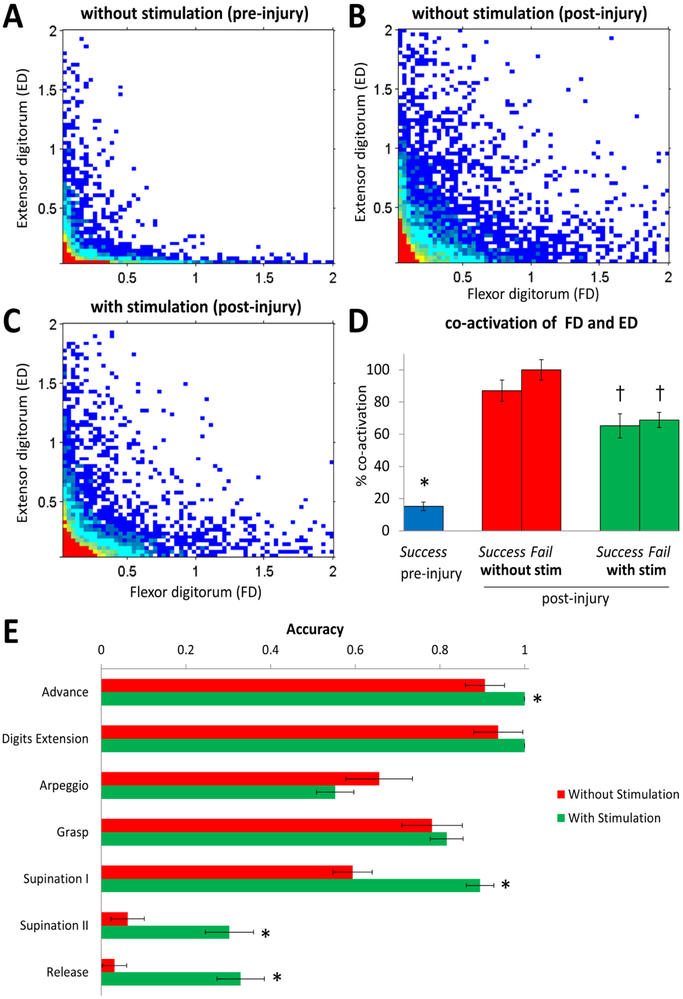
Figure 4A–C shows the joint probability density distributions between the flexor digitorum and extensor digitorum muscles during a single pellet grasp under different conditions. Pre-injury, these distal antagonist muscles showed a distinguishable reciprocal activation pattern (Fig. 4A). Ten weeks post-injury there was a greater level of simultaneous activation (co-activation) when testing without stimulation (Fig. 4B) compared to pre-stimulation, whereas the activity patterns were more similar, but not returned, to pre-injury levels with stimulation (Fig. 4C). The mean percent co-activation levels were lower (p < 0.05, one-way ANOVA) in pre- than post-injury with and without stimulation during both successful and failed grasping attempts (15.22 ± 2.67 vs. 86.96 ± 6.66, 100 ± 6.31, 65.22 ± 7.50 or 68.84 ± 4.74) (Fig. 4D). In addition, the co-activation values during stimulation were significantly lower compared to the co-activation during failed attempts without stimulation (100 ± 6.31 vs. 65.22 ± 7.50 or 68.84 ± 4.74, p < 0.05, one-way ANOVA).
Figure 4: Effects of cervical electrical stimulation on antagonistic distal forelimb muscle activation during reaching and grasping.
Joint probability density distributions for the flexor digitorum (FD) and extensor digitorum (ED) in a rat pre-injury without stimulation (A), and 10 weeks post-injury without (B) and with (C) C6+ C8− stimulation are shown. (D) Mean (±SEM) percentage of co-activation during reaching and grasping (normalized to maximum observed for any of the three experimental conditions). *: lower than all conditions post-injury (p < 0.05). †: lower than the co-activation during failed forelimb reaching attempts post-injury without stimulation (p < 0.05). (E) Qualitative scores of accuracy (mean ±SEM; n = 60 trials) for the seven components of reaching and grasping at 10 weeks post-injury with and without epidural stimulation. *: higher than without stimulation (p < 0.05).
The reaching and grasping task was segmented into seven components based on the modified Whishaw scale (García-Alías et al., 2015). The accuracy during each component of the task, except for the arpeggio, trended to be greater with than without stimulation at 10 weeks post-injury with significant improvements during the advance, supination I and II, and pellet release components (0.91 ± 0.05 vs. 0.55 ± 0.04, 0.59 ± 0.05 vs. 0.89 ± 0.03, 0.06 ± 0.04 vs. 0.30 ± 0.06, and 0.03 ± 0.03 vs. 0.31 ± 0.06, p < 0.05, one-way ANOVA) (Fig. 4E). These improvements resulted in more successful reaching and grasping during epidural stimulation (Supplementary Video 2).
Spinal network excitability is increased post-injury
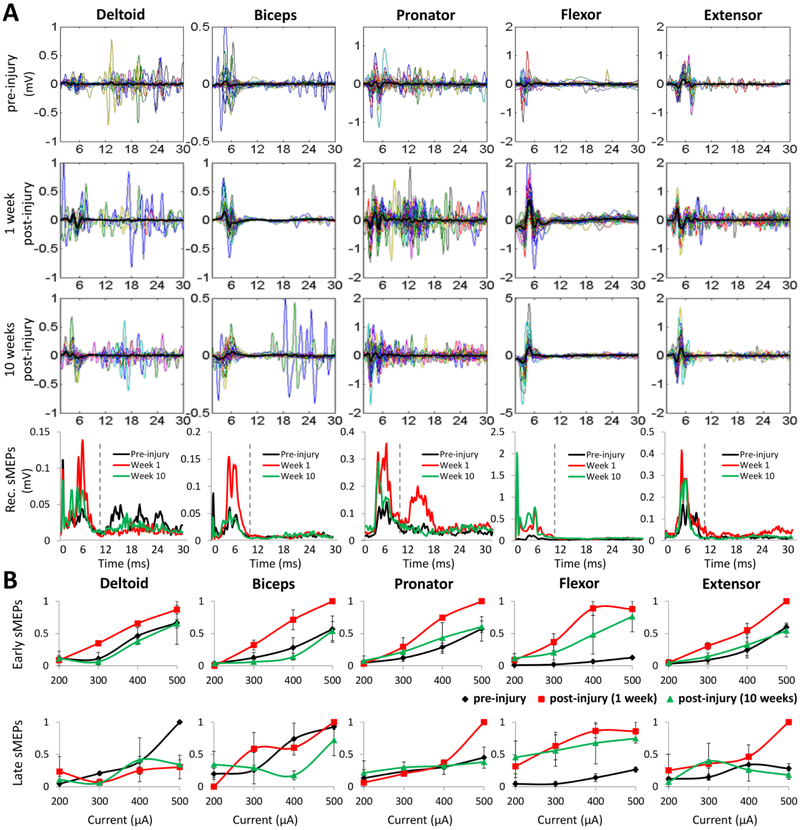
Spinally evoked potentials (sMEPs) were elicited by stimulating the spinal cord via the epidural implanted electrodes when the rats were awake and resting in their cage as described previously (Alam et al., 2015). C6− C8+ bipolar stimulation produced robust sMEPs in all five forelimb muscles at a 400-¼A current intensity (Fig. 5A). Similar to the changes in EMG amplitudes, the sMEP amplitudes increased immediately post-injury (1 week post-injury) and then returned to values similar to pre-injury at 10 weeks post-injury in most muscles (bottom row of Fig. 5A). The amplitudes of the early component of the sMEPs generally increased with increasing current intensities and were consistently higher over the range of currents for each muscle at 1 week post-injury than pre-injury or at 10 weeks post-injury (Fig. 5B). While the amplitude of the late component also generally increased in all muscles with increasing stimulation current, there were no significant differences in the amplitudes for any of the time points pre- or post-injury (Fig. 5B). Comparisons of the early and late recruitment curves for each muscle pre-injury, at 1 week post-injury, and at 10 weeks post-injury suggest an immediate increase in spinal excitability post-injury and then a return towards pre-injury levels.
Figure 5: Changes in spinal excitability after SCI.
(A) Examples of sMEPs evoked in each forelimb muscle during C6− C8+ stimulation (400 μA current intensity) of the same rats preinjury and at 1 and 10 weeks post-injury. Each panel shows 30 superimposed sMEPs (different colors) with the mean shown in black. sMEPs were rectified (Rec.) and the means for each time point for each muscle are shown in the bottom row. The vertical dashed lines in the bottom row separate the early (<10 ms) and late (10-30 ms) sMEP responses. (B) Changes in the mean (±SEM) sMEP magnitudes normalized to the maximum value (area under the rectified curve) of the early and late responses for each muscle pre-injury (black trace) and at 1 (red trace) and 10 (green trace) weeks post-injury are shown as a function of increasing stimulation current intensities.
Discussion
In the present study we have evaluated the effectiveness of different stimulation parameters on the performance of a skilled forelimb motor task, acutely and chronically, after an incomplete cervical SCI. Stimulation between the C6 and C8 spinal cord segments produced the most consistently improved performance. In addition, we have compared skilled performance during stimulation as well as immediately after stimulation using multiple stimulation parameters. The rats with an incomplete SCI at C4 showed a greater ability to reach and grasp with their forelimbs with the cervical epidural stimulation distal to the injury. This functional enhancement persisted for several minutes to hours, but the decay time was not measured systematically during this study. The magnitude of the improvement in forelimb function in response to stimulation was greatest at 10 weeks post-injury, largely due to decline in performance that occurred in absence of stimulation from 5 to 10 weeks post-injury. Pre-stimulation reaching score at week 10 was lower from week 5, probably due to the 4 weeks (week 6 to 9) of no-testing i.e., no exposure to the reaching environment for 4 weeks may have contributed to this drop in performance. With stimulation, however, the performance improved even better than previous weeks.
Effects of epidural spinal cord stimulation after a SCI
It has been demonstrated previously that epidural electrical stimulation of the lumbar spinal cord can activate the spinal networks controlling standing and stepping in spinalized rats and cats (Ichiyama et al., 2005; Musienko et al., 2009; Lavrov et al., 2015) and generate full weight-bearing standing and some volitional control of the lower-limb muscles in human patients (Harkema et al., 2011; Angeli et al., 2014). Overall, these results indicate that in the absence of or severely reduced supraspinal innervation, the spinal networks are able to process and execute coordinated movements when facilitated with epidural spinal cord stimulation and appropriate sensory inputs. The present study provides evidence that epidural spinal cord stimulation can be used to modulate the excitability of the cervical circuitry associated with forelimb function.
Whereas locomotion depends on the activation of spinal networks related to the central pattern generation (Grillner and Wallén, 1985), the necessity and location of a similar cervical spinal network for improving forearm reaching and grasping movements is unknown (Alstermark and Isa, 2012). We have previously reported effective epidural stimulation parameters of the cervical spinal cord for enhancing grip strength after an incomplete SCI (Alam et al., 2015). In two tetraplegic subjects with a severe spinal injury for more than a year that were implanted with epidural electrode arrays, it was demonstrated that the cervical cord could be modulated to improve hand grip strength (Lu et al., 2016).
In paralyzed rats, the activation of the spinal locomotor networks was achieved initially by delivering supra-threshold epidural electrical stimulation (Ichiyama et al., 2005; Courtine et al., 2009). A subsequent study of neuromodulation of the hindlimbs in complete mid-thoracic spinal rats has shown similar effects of neuromodulation when stimulating at 20% below motor threshold (Gad et al., 2013) In the present study we also have delivered sub-threshold electrical current to incomplete spinal cord injured animals. We hypothesize that under these conditions epidural stimulation modulates the cervical spinal networks by elevating the net baseline excitability level (readjusting the balance between excitatory and inhibitory drive) of the spinal neural networks involved in the motor task.
After a corticospinal tract injury, the animals partially recovered the ability to reach and grasp by attempting a range of forelimb and body postures adjustments. Once the pellet was grasped, the retraction of the paw was the most affected movement which did not recover to the pre-injury state. Besides the initial postural position of the forelimbs, major differences between the stimulation and non-stimulation conditions were observed during the supination I and II stages of the reaching cycle.
The EMG data show that following the injury, the energy and co-activation of the forelimb distal muscles were increased when reaching and grasping compared to pre-injury, indicating the hyper-excited state of spinal neural networks (Fig. 4). However, when the animals received cervical epidural stimulation, both the level of muscles activity and the co-activation decreased towards but not to the pre-injury physiological state. Nevertheless, other factors must influence the animals’ abilities to perform forelimb fine motor tasks, as the degree of muscle co-activation was very similar in the successful and failed reaching attempts. Overall, the regained physiological state increased the potential for the supraspinal commands to reach the spinal motor networks that define the level and motor pools to be recruited. Regarding co-activation of cervical muscle groups, our best interpretation is that during the period immediately post-injury, there is a rapid facilitation of connectivity among spinal neuronal networks, resulting in a loss of selectivity in activating muscle groups to produce a normal level of coordination. We consistently observed the phenomenon as illustrated in Figure 4 where there is an initial increase in co-activation, but this level of co-activation declines over time. This observation is consistent with the trend of there being reduced excitability at 10 weeks compared to one-week post-injury as shown in Figure 5. Our interpretation is that there are multiple mechanisms involved during different stages of the post-injury adaptations.
We focused our comparisons of the effectiveness of a range of stimulation parameters based on what we had previously demonstrated in facilitating grip strength in a single treatment session (Alam et al., 2015). Dorsal funiculus injuries mainly affect the animals’ fine motor control ability to precisely grasp pellets but not their ability to grossly move their forelimbs (García-Alías et al., 2008). The results shows that the stimulation between C6 and C8 spinal segments facilitated the best motor recovery compared to the other electrode configurations, suggesting that wider electrical fields, and presumably, activation of larger spinal networks, can improve motor recovery. Thus, we placed epidural stimulation electrodes on C6 and C8 spinal segments and a third electrode subcutaneously on the back, with the intention of modulating the spinal networks, or part of them, located in the vicinity of the segmental interneurons and motoneuron pools controlling the forelimb muscles (McKenna et al., 2000)
We further studied the effects of the stimulation frequencies delivered at 20, 40 and 60 Hz. Given previous results from spinalized animals (Shah et al., 2016) and humans (Lu et al., 2016) demonstrating that the frequency of stimulation can be used as a way of modulating motor performance, we were surprised with the lack of robust differences between these frequencies. Differences in frequency responses need to be examined further. For example, transcranial electromagnetic stimulation at <1 Hz, has been reported to suppress motor excitability in humans (Di Lazzaro et al., 2008). Furthermore, it is important to consider that the intensity of stimulation may affect the modulatory impact of any frequency tested (Gad et al., 2013).
Neural behavioral strategies in the recovery of reaching and grasping after a cervical SCI
After a dorsal funiculi crush, the major impairment for reaching and grasping task was at the last component of the task cycle, i.e., once the pellet is grasped and held the paw is retrieved for releasing the pellet into the mouth. In uninjured animals, once the pellet is grasped, the shoulder and elbow are flexed bringing the pellet towards the mouth and then the paw is rotated, the digits extended, and the pellet released into the mouth (Whishaw and Pellis, 1990). After SCI, however, the animals employed a different motor strategy, i.e., they moved the body towards the pellets rather than bringing the pellets towards the mouth. The loss of fine motor control of the distal muscles is reflected in multiple motor alternatives in the recruitment of motor pools involved in the release of the pellet to the mouth. We suggest that under these circumstances, a major factor in regaining fine control of the forepaw is a reduction in the prominence of co-contraction among antagonistic motor pools of the distal muscles.
The changes in the EMG patterns also reflected a novel strategy for reaching and grasping. For example, in the initial preparation of performing the task, the activation of the proximal muscles not involved in the grasping itself was different post-injury compared to pre-injury. The most dramatic change, however, was apparent in the final stages of the reaching and grasping when the stimulation had the greatest effect in improving the accuracy of the performance. This phase-dependent effect might be expected based on the recent observations by Azim (Azim et al., 2014) where inactivation of specific V2a interneurons had a specific effect on the later phases of reaching and grasping. Postural and locomotion impairment also was obtained when depleting the animals of proprioceptive inputs (Takeoka et al., 2014). Combined these results demonstrate that after a significant SCI the cervical neuromotor system developed novel neural strategies for accomplishing the same end result, emphasizing the importance of the high level of redundancy within these neural networks.
Reaching and grasping improvement was not only achieved acutely during the stimulation, but remained during a short stage post-stimulation. A recent study by McPherson and colleagues (McPherson et al., 2015) reported that reaching and grasping improved in animals receiving closed-loop intraspinal stimulation triggered by increased activity of the biceps brachii. In our paradigm, epidural stimulation was continuously delivered while the animals performed the task and showed improvement during and post-stimulation. Moreover, the animals showed recovery only when the current was delivered, suggesting that some type of biochemical and anatomical reorganization must drive this recovery, only when the stimulation is applied. In the present study, we have not attempted to dissect the enhancement of sensory vs. motor function after spinal stimulation on reaching and grasping. Therefore, we cannot exclude that spinal stimulation influences sensory processing at spinal and supraspinal levels (Fuentes et al., 2009), nor the possibility that electrical stimulation acts on peripheral proprioception system (Wegner et al., 2016), or activates spinal interneurons (Courtine et al., 2009). It is probable that the neuromodulation effects are produced by the combination of all these mechanisms. However, the strong effects observed on spinalized animals that were able to step on treadmill, indicate that the electrical current must at least act locally, facilitating or activating spinal networks.
Conclusions
Epidural spinal cord stimulation can effectively neuromodulate the cervical spinal cord in a way that improves reaching and grasping in rats after an incomplete cervical injury, both in the acute and chronic stages post-injury. Changes in the EMG patterns and evoked potentials immediately post-injury indicate a possible loss of supraspinal inhibition to the spinal cord networks and/or a general increase of synaptic connectivity among spinal interneuronal networks. In addition to improving forelimb fine motor control, cervical epidural stimulation significantly changed the muscle synergies of these spinal injured rats toward those observed pre-injury, suggesting a physiological recovery with this intervention.
Supplementary Material
A representative animal performs reaching and grasping before injury, and 1-week post-injury.
A representative animal performs reaching and grasping at 10-weeks post-injury in two conditions in the same testing session: (1) without stimulation, and (2) with stimulation. Note less over-reaching with stimulation at 10-weeks post-injury.
Highlights:
Epidural stimulation electrodes were implanted chronically at C6 and C8
Dorsal funiculi were injured at C4 spinal cord in adult female rats
Sub-threshold C6-C8 stimulation improved forelimb fine motor control
Muscle synergies during stimulation followed pre-injury synergies
Evoked potentials at 10-weeks post-injury were more similar to pre-injury than at 1-week post-injury
Acknowledgements
This research project was supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB) NIH 1U01EB015521, the Christopher & Dana Reeve Foundation, and the RFBR No. 13-04-12030, and by Russian Scientific Fund project No. 14-45-00024. The authors thank Maynor Herrera for his assistance in surgeries, Jin Lee for his assistance during the experiments, and Sharon Zdunowski for her technical support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
V. Reggie Edgerton, Roland R. Roy and Yury Gerasimenko – researchers on the study team hold shareholder interest in NeuroRecovery Technologies. Drs. Edgerton, Roy, Lu and Gerasimenko also hold certain inventorship rights on intellectual property licensed by The Regents of the University of California to NeuroRecovery Technologies and its subsidiaries.
References
- Alam M, Rodrigues W, Pham BN, Thakor NV (2016) Brain-machine interface facilitated neurorehabilitation via spinal stimulation after spinal cord injury: Recent progress and future perspectives. Brain Research 1646:25–33. [DOI] [PubMed] [Google Scholar]
- Alam M, Garcia-Alias G, Shah PK, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR (2015) Evaluation of optimal electrode configurations for epidural spinal cord stimulation in cervical spinal cord injured rats. Journal of Neuroscience Methods 247:50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alstermark B, Isa T (2012) Circuits for Skilled Reaching and Grasping. Annual Review of Neuroscience 35:559–578. [DOI] [PubMed] [Google Scholar]
- Anderson KD (2004) Targeting recovery: priorities of the spinal cord-injured population. J Neurotrauma 21:1371–1383. [DOI] [PubMed] [Google Scholar]
- Angeli CA, Edgerton VR, Gerasimenko YP, Harkema SJ (2014) Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 137:1394–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azim E, Jiang J, Alstermark B, Jessell TM (2014) Skilled reaching relies on a V2a propriospinal internal copy circuit. Nature 508:357–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtine G, Gerasimenko Y, van den Brand R, Yew A, Musienko P, Zhong H, Song B, Ao Y, Ichiyama RM, Lavrov I, Roy RR, Sofroniew MV, Edgerton VR (2009) Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat Neurosci 12:1333–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Guzman CP, Roy RR, Hodgson JA, Edgerton VR (1991) Coordination of motor pools controlling the ankle musculature in adult spinal cats during treadmill walking. Brain Res 555:202–214. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Pilato F, Dileone M, Profice P, Oliviero A, Mazzone P, Insola A, Ranieri F, Tonali PA, Rothwell JC (2008) Low-frequency repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. The Journal of Physiology 586:4481–4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz V, Fouad K (2014) Restoration of sensorimotor functions after spinal cord injury. Brain 137:654–667. [DOI] [PubMed] [Google Scholar]
- Edgerton VR, Roy RR (2012) A new age for rehabilitation. Eur J Phys Rehabil Med 48:99–109. [PubMed] [Google Scholar]
- Fuentes R, Petersson P, Siesser WB, Caron MG, Nicolelis MA (2009) Spinal cord stimulation restores locomotion in animal models of Parkinson's disease. Science 323(5921):1578–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gad P, Choe J, Shah P, Garcia-Alias G, Rath M, Gerasimenko Y, Zhong H, Roy RR, Edgerton VR (2013) Sub-threshold spinal cord stimulation facilitates spontaneous motor activity in spinal rats. J Neuroeng Rehabil 10:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Alías G, Truong K, Shah PK, Roy RR, Edgerton VR (2015) Plasticity of subcortical pathways promote recovery of skilled hand function in rats after corticospinal and rubrospinal tract injuries. Experimental Neurology 266:112–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Alías G, Lin R, Akrimi SF, Story D, Bradbury EJ, Fawcett JW (2008) Therapeutic time window for the application of chondroitinase ABC after spinal cord injury. Experimental Neurology 210:331–338. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Avelev VD, Nikitin OA, Lavrov IA (2003) Initiation of locomotor activity in spinal cats by epidural stimulation of the spinal cord. Neurosci Behav Physiol 33:247–254. [DOI] [PubMed] [Google Scholar]
- Gerasimenko YP, Ichiyama RM, Lavrov IA, Courtine G, Cai L, Zhong H, Roy RR, Edgerton VR (2007) Epidural Spinal Cord Stimulation Plus Quipazine Administration Enable Stepping in Complete Spinal Adult Rats. J Neurophysiol 98:2525–2536. [DOI] [PubMed] [Google Scholar]
- Grillner S, Wallén P (1985) Central Pattern Generators for Locomotion, with Special Reference to Vertebrates. Annual Review of Neuroscience 8:233–261. [DOI] [PubMed] [Google Scholar]
- Harkema S, Gerasimenko Y, Hodes J, Burdick J, Angeli C, Chen Y, Ferreira C, Willhite A, Rejc E, Grossman RG, Edgerton VR (2011) Effect of epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: a case study. The Lancet 377:1938–1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichiyama RM, Gerasimenko YP, Zhong H, Roy RR, Edgerton VR (2005) Hindlimb stepping movements in complete spinal rats induced by epidural spinal cord stimulation. Neuroscience Letters 383:339–344. [DOI] [PubMed] [Google Scholar]
- Jackson A, Zimmermann JB (2012) Neural interfaces for the brain and spinal cord[mdash]restoring motor function. Nat Rev Neurol 8:690–699. [DOI] [PubMed] [Google Scholar]
- Kasten MR, Sunshine MD, Secrist ES, Horner PJ, Moritz CT (2013) Therapeutic intraspinal microstimulation improves forelimb function after cervical contusion injury. Journal of Neural Engineering 10:044001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavrov I, Musienko PE, Selionov VA, Zdunowski S, Roy RR, Reggie Edgerton V, Gerasimenko Y (2015) Activation of spinal locomotor circuits in the decerebrated cat by spinal epidural and/or intraspinal electrical stimulation. Brain Research 1600:84–92. [DOI] [PubMed] [Google Scholar]
- Lu DC, Edgerton VR, Modaber M, AuYong N, Morikawa E, Zdunowski S, Sarino ME, Sarrafzadeh M, Nuwer MR, Roy RR, Gerasimenko Y (2016) Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Neurorehabilitation and Neural Repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Whishaw IQ (1999) Complete compensation in skilled reaching success with associated impairments in limb synergies, after dorsal column lesion in the rat. J Neurosci 19:1885–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JE, Prusky GT, Whishaw IQ (2000) Cervical motoneuron topography reflects the proximodistal organization of muscles and movements of the rat forelimb: A retrograde carbocyanine dye analysis. The Journal of Comparative Neurology 419:286–296. [DOI] [PubMed] [Google Scholar]
- McPherson JG, Miller RR, Perlmutter SI (2015) Targeted, activity-dependent spinal stimulation produces long-lasting motor recovery in chronic cervical spinal cord injury. Proceedings of the National Academy of Sciences of the United States of America 112:12193–12198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minassian K, Jilge B, Rattay F, Pinter MM, Binder H, Gerstenbrand F, Dimitrijevic MR (2004) Stepping-like movements in humans with complete spinal cord injury induced by epidural stimulation of the lumbar cord: electromyographic study of compound muscle action potentials. Spinal Cord 42:401–416. [DOI] [PubMed] [Google Scholar]
- Mondello SE, Kasten MR, Horner PJ, Moritz CT (2014) Therapeutic intraspinal stimulation to generate activity and promote long-term recovery. Front Neurosci 8:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musienko PE, Pavlova NV, Selionov VA, Gerasimenko Iu P (2009) Locomotion induced by epidural stimulation in decerebrate cat after spinal cord injury. Biofizika 54:293–300. [PubMed] [Google Scholar]
- Saigal R, Renzi C, Mushahwar VK (2004) Intraspinal microstimulation generates functional movements after spinal-cord injury. Neural Systems and Rehabilitation Engineering, IEEE Transactions on 12:430–440. [DOI] [PubMed] [Google Scholar]
- Shah PK, Sureddi S, Alam M, Zhong H, Roy RR, Edgerton VR, Gerasimenko Y (2016) Unique Spatiotemporal Neuromodulation of the Lumbosacral Circuitry Shapes Locomotor Success after Spinal Cord Injury. Journal of Neurotrauma. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe AN, Jackson A (2014) Upper-limb muscle responses to epidural, subdural and intraspinal stimulation of the cervical spinal cord. Journal of Neural Engineering 11:016005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solnik S, Rider P, Steinweg K, DeVita P, Hortobágyi T (2010) Teager–Kaiser energy operator signal conditioning improves EMG onset detection. European Journal of Applied Physiology 110:489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine MD, Cho FS, Lockwood DR, Fechko AS, Kasten MR, Moritz CT (2013) Cervical intraspinal microstimulation evokes robust forelimb movements before and after injury. Journal of Neural Engineering 10:036001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeoka A, Vollenweider I, Courtine G, Arber S (2014) Muscle Spindle Feedback Directs Locomotor Recovery and Circuit Reorganization after Spinal Cord Injury. Cell 159:1626–1639. [DOI] [PubMed] [Google Scholar]
- Wenger N, Moraud EM, Raspopovic S, Bonizzato M, DiGiovanna J, Musienko P, Morari M, Micera S, Courtine Gg (2014) Closed-loop neuromodulation of spinal sensorimotor circuits controls refined locomotion after complete spinal cord injury. Science Translational Medicine 6:255ral33–255ral33. [DOI] [PubMed] [Google Scholar]
- Wenger N, Moraud EM, Gandar J, Musienko P, Capogrosso M, Baud L, Le Goff CG, Barraud Q, Pavlova N, Dominici N, Minev IR, Asboth L, Hirsch A, Duis S, Kreider J, Mortera A, Haverbeck O, Kraus S, Schmitz F, DiGiovanna J, van den Brand R, Bloch J, Detemple P, Lacour SP, Bézard E, Micera S, Courtine G (2016) Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat Med 22(2): 138–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whishaw IQ, Tomie JA (1989) Olfaction directs skilled forelimb reaching in the rat. Behav Brain Res 32:11–21. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Pellis SM (1990) The structure of skilled forelimb reaching in the rat: A proximally driven movement with a single distal rotatory component. Behavioural Brain Research 41:49–59. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Piecharka DM, Drever FR (2003) Complete and Partial Lesions of the Pyramidal Tract in the Rat Affect Qualitative Measures of Skilled Movements: Impairment in Fixations as a Model for Clumsy Behavior. Neural Plasticity 10:77–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann JB, Seki K, Jackson A (2011) Reanimating the arm and hand with intraspinal microstimulation. Journal of Neural Engineering 8:054001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A representative animal performs reaching and grasping before injury, and 1-week post-injury.
A representative animal performs reaching and grasping at 10-weeks post-injury in two conditions in the same testing session: (1) without stimulation, and (2) with stimulation. Note less over-reaching with stimulation at 10-weeks post-injury.