Abstract
Schisantherin A (SinA), one of the most abundant active ingredients of Schisandra chinensis, was reported to protect and benefit the liver, however, its effect on alcohol-induced liver injury (ALI) was still not clear. In the present study, an ALI mice model was induced by feeding mice an alcohol-containing liquid diet for four weeks. Then, 100 mg/kg or 200 mg/kg SinA was administered to mice every day by gavage for the last two weeks. Histopathological analysis showed that alcohol-induced liver lipid vacuoles were reduced by SinA. The activities of aspartate aminotransferase (AST, 61.90 ± 14.65 vs. 93.65 ± 20.50, 50.46 ± 13.21 vs. 93.65 ± 20.50) and alanine transaminase (ALT, 41.29 ± 9.20 vs. 64.04 ± 18.13, 36.52 ± 7.71 vs. 64.04 ± 18.13) in the serum of ALI mice were significantly reduced by 100 mg/kg or 200 mg/kg SinA when compared with control mice. Alcohol-induced oxidative stress and the inflammatory response in the liver were suppressed by SinA in a dose-dependent manner. Meanwhile, treatment with SinA decreased alcohol dehydrogenase (ADH) activity and increased acetaldehyde dehydrogenase (ALDH) activity in ALI mice. Alcohol-induced upregulation of CYP2E1 and CYP1A2 in the liver was inhibited by SinA. Further, SinA suppressed activation of the NF-kB pathway in ALI mice. In conclusion, our findings demonstrate that SinA is able to protect against ALI, and this may be, at least in part, caused by regulation of alcohol metabolism and the NF-kB pathway. Our data suggest a therapeutic potential of SinA in the treatment of ALI.
Keywords: alcohol-induced liver injury, alcohol metabolism, NF-kB signaling pathway, oxidative stress, schisantherin A
Introduction
Alcoholic liver disease (ALD) caused by long-term excessive drinking is an increasing health burden worldwide [16]. The clinical progression of ALD encompasses a range of liver disorders such as intrahepatic steatosis, steatohepatitis, and liver fibrosis, finally leading to cirrhosis or even development into hepatocellular carcinoma [3]. Currently, the main therapeutic strategies for ALD include temperance, corticosteroids, biologics, nutritional therapy, and ultimately liver transplantation [14]. However, these therapeutic effects are all limited due to infection or complications [24]. Therefore, there is still an urgent need to investigate novel therapies for ALD.
Although the pathogenesis of ALD is a complex process, the oxidative stress induced by ethanol oxidative metabolism plays a noticeable role in the pathological course of liver injury [6]. The liver is the major organ that responsible for alcohol metabolism, and it oxidizes ethanol mainly through the dehydrogenase system and microsomal ethanol oxidizing system (MEOS) [5, 43]. During the metabolism processes through these two systems, massive amounts of NADH or NADP+ will be generated and result in excessive reactive oxygen species (ROS), which triggers hepatic damage such as irreversible alteration of lipids, proteins, or deoxyribonucleic acid contents [26,48]. On the other hand, chronic ethanol consumption also increases the gut-derived lipopolysaccharide (LPS) concentration in portal blood, which activates the NF-kB signaling pathway, leading to the release of proinflammatory cytokines such as TNF-α, IL-1β, and IL-6 [32]. This process further promotes the generation of ROS and exacerbates liver damage.
Schisandra chinensis is a traditional nourishing medicine in China. Schisantherin A is a dibenzocyclooctadiene lignan that considered to be the most abundant and key effective constituent in the fruit of Schisandra chinensis [42]. Studies have found that schisantherin A has beneficial effects on the brain, heart, and bone due to the anti-inflammatory, antioxidant, and detoxification pharmacological activities [8, 10, 18, 28, 35, 39, 44]. Moreover, Zheng et al. [45], reported that administration with schisantherin A ameliorated ischemia-reperfusion-induced dysfunction, histological damage, the inflammatory state and oxidative stress in the liver. Here, we investigated the protective effects of schisantherin A against liver pathologic changes, liver functions, oxidative stress, and inflammatory responses in ALI mice. The changes in the dehydrogenase system, MEOS, and NF-kB signaling pathway were studied as well.
Materials and Methods
Animals
Eight-week-old male C57BL/6 mice were obtained from Liaoning Changsheng Biotechnology Co., Ltd. (Benxi, China; license number: SCXK [Liao] 2015-0001). Mice were fed adaptively for one week with a 12 h/ 12 h light/dark cycle at a constant temperature (22 ± 1°C) and humidity (45–55%). Food and water were available ad libitum. The procedures for animal handling were in compliance with international guidelines of laboratory animal care, and all animal experiments in the present study were approved by the Institutional Animal Care and Use Committee of Shenyang Agricultural University.
Treatment and experimental groups
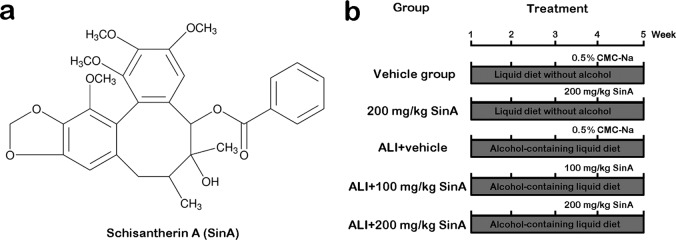
The alcohol-induced liver injury (ALI) mouse model was established as previously described [22]. In brief, mice were fed with alcohol-containing Lieber-DeCarli formulation-based liquid diet (alcohol provided 27.5% of total calories, purchased from Trophic Animal Feed High-Tech Co., Ltd., Nantong, China) for four weeks. Schisantherin A (SinA, CAS No., 58546-56-8, Fig. 1a) was obtained from Sigma-Aldrich (St. Louis, MO, USA) and solubilized with 0.5% sodium carboxymethylcellulose (CMC-Na). The animals were randomly divided into five groups (18 mice in each group): the vehicle group, 200 mg/kg SinA group, ALI+ vehicle group, ALI+ 100 mg/kg SinA group, and ALI+ 200 mg/kg SinA group. The treatment procedures for each group are presented in Fig. 1b. In short, mice were fed a liquid diet with or without alcohol, and then 100 mg/kg SinA, 200 mg/kg SinA, or the same volume of 0.5% CMC-Na as the vehicle control was administered by gavage every day from the third week on. At the end of treatment, the mice were anesthetized with 50 mg/kg pentobarbital sodium, and then the serum and liver tissues were collected for further study.
Fig. 1.
Chemical structure of Schisantherin A and the experimental protocol of each group. ALI, alcohol-induced liver injury; SinA, Schisantherin A.
Histopathological analysis
The liver tissues were fixed with 4% paraformaldehyde, and embedded in paraffin, and then serially cut into 5-µm sections. After that, the sections were stained with hematoxylin and eosin (H&E) following the standard procedures. Oil Red O staining was performed as previously reported [19]. Pathological changes were observed under an optical microscope (BX53, Olympus, Tokyo, Japan).
Biochemical analysis and enzyme-linked immunosorbent assay (ELISA)
Liver tissues were homogenized with saline and centrifuged for 10 min at 430 g, and then the supernatant was collected. Next, the malondialdehyde (MDA) and glutathione (GSH) levels and superoxide dismutase (SOD), myeloperoxidase (MPO), and alcohol dehydrogenase (ADH) activities in the liver were examined with the appropriate kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The levels of TNF-α, IL-1β, and IL-6 in the liver were determined by mouse TNF-α, IL-1β, and IL-6 ELISA kits (MultiSciences, Beijing, China), respectively. To detect the activity of acetaldehyde dehydrogenase (ALDH), liver tissues were homogenized with precooled ALDH detection solution and centrifuged at 10,000 g for 10 min. Then, the supernatant was collected, and the ALDH activity was measured (Abnova, Walnut, CA, USA). The activities of aspartate aminotransferase (AST) and alanine transaminase (ALT) in serum were determined by commercial kits (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). All detection procedures were strictly performed in accordance with the manufacturer’s instructions.
Quantitative real-time PCR (qPCR)
Total RNA was extracted by using the TRIzol solution (Bioteke Corporation, Beijing, China). cDNA was synthesized from total RNA with Super M-MLV Reverse Transcriptase (Bioteke Corporation). The primer sequences used in this study were as follows: 5’-ctggagatctaccgatacaca-3’, forward, and 5’-gcagcaggatggctaagaag-3’, reverse, for CYP1A2; 5’-ccaccctcctcctcgtatc-3’, forward, and 5’-ccttgacagccttgtagcc-3’, reverse, for CYP2E1; and 5’-ctgtgcccatctacgagggctat-3’, forward, and 5’-tttgatgtcacgcacgatttcc-3’, reverse, for β-actin. qPCR was performed with 2×Power Taq PCR MasterMix (Bioteke Corporation) and SYBR Green (Solarbio, Beijing, China) on an Exicycler TM 96 (Bioneer, Daejeon, Korea). Relative mRNA levels were analyzed by using the 2-ΔΔCt method [31]. β-actin was used as an internal control.
Western blot
Proteins from liver tissues were isolated with RIPA buffer (Beyotime, Haimen, China) and were quantified with a bicinchoninic acid (BCA) protein quantitation kit (Beyotime). Equal amounts of proteins were separated by 5% or 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and electrophoretically transferred onto polyvinylidene difluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After blocking with 5% nonfat milk, the membranes were incubated with primary antibodies (rabbit anti-CYP2E1 antibody, rabbit anti-CYP1A2 antibody, rabbit anti-p65 antibody, each 1: 1,000 diluted, purchased from proteintech, Wuhan, China; rabbit anti-p-p65Ser536 antibody, rabbit anti-p-IkBSer32 antibody, rabbit anti-IkB antibody, each 1: 1,000 diluted, purchased from Cell Signaling Technology, Danvers, MA, USA) at 4°C overnight. After that, the membranes were incubated with a horseradish peroxidase-conjugated goat anti-rabbit secondary antibody (1:5,000 dilution, Beyotime) at 37°C for 45 min. Finally, the specific protein bands were visualized with an enhanced chemiluminescent (ECL) reagent (Beyotime).
Electrophoretic mobility shift assay (EMSA)
Nuclear proteins from the liver were collected with a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime). After quantification by the BCA method (Beyotime), the proteins were combined into the specific oligonucleotides (sequence: 5’-agttgaggggactttcccaggc-3’) as per the users manual (Viagene, Changzhou, China). Then the binding complexes were separated by electrophoresis on 6.5% polyacrylamide gel and electrophoretically transferred onto a nylon membrane. The biotin-labeled DNA on the membrane was cross-linked under an ultraviolet lamp, and then the specific bands were finally detected by ECL assay.
Statistical analysis
All data were expressed as the mean ± SD. Differences between groups were analyzed with one-way analysis of variance (ANOVA) followed by Tukey’s multiple comparisons test by using GraphPad Prism software version 6.0 (GraphPad Software, San Diego, CA, USA). A P-value less than 0.05 was considered to be statistically significant.
Results
SinA attenuated alcohol-induced liver pathological and functional injury
The pathological changes of the liver were analyzed by H&E assay. As shown in Fig. 2a, mice in the vehicle and 200 mg/kg SinA groups showed normal structures in the liver; however, micro- and macrovesicular steatosis was obviously seen in the hepatocytes around the central vein of alcohol-fed mice. As expected, alcoholic liver mice administrated 100 or 200 mg/kg SinA exhibited reduced steatosis. These changes were also confirmed by Oil Red O staining (Fig. 2b). To evaluate the effects of SinA on liver function in mice, we further examined the activities of ALT and AST in serum. As shown in Fig. 2c and d, feeding with the alcohol-containing diet resulted in significant increases in ALT (64.04 ± 18.13 vs. 25.68 ± 5.69) and AST (93.65 ± 20.50 vs.38.49 ± 10.25) activities as compared with the mice in the vehicle group. Treatment with 100 mg/kg or 200 mg/kg SinA markedly reduced the ALT (41.29 ± 9.20 vs. 64.04 ± 18.13, 36.52 ± 7.71 vs. 64.04 ± 18.13) and AST (1.90 ± 14.65 vs. 93.65 ± 20.50, 50.46 ± 13.21 vs. 93.65 ± 20.50) activities compared with the ALI mice. These data indicate that SinA has protective potential in alcohol-induced liver injury.
Fig. 2.
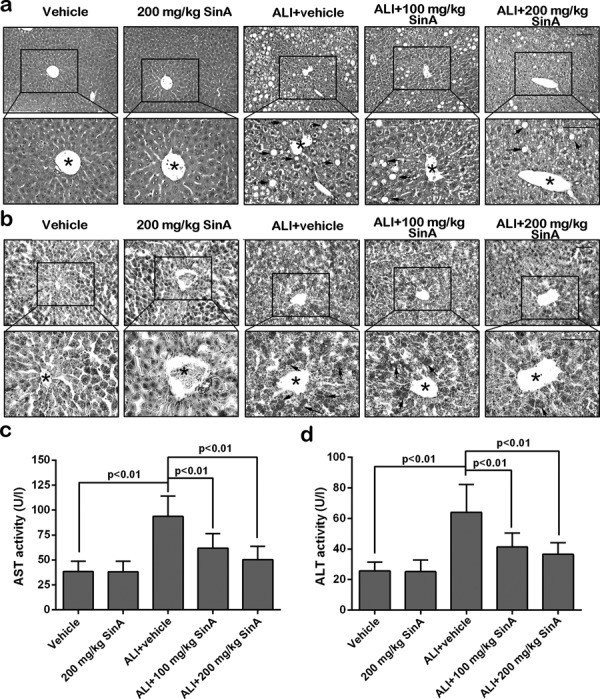
The effects of SinA on liver pathological changes and liver function. Liver pathological changes were analyzed by hematoxylin and eosin (H&E) staining (a) and Oil Red O staining (b). Scale bars = 100 µm. Typical pictures from six independent experiments are shown. Serum aspartate aminotransferase (AST) activity (c) and alanine transaminase (ALT) activity (d) were determined with commercial kits. Each experiment was repeated six times. Data are expressed as the mean ± SD. The black stars indicate the central vein, the black arrows indicate steatosis, and the green arrows indicate the inflammatory cell infiltration. ALI, alcohol-induced liver injury; SinA, Schisantherin A.
SinA alleviated alcohol-induced liver oxidative stress and inflammatory responses
Excessive ROS and the resulting oxidative stress are major causes of alcohol-induced liver injury. Thus, the levels and activities of several oxidative stress indicators were measured. As shown in Fig. 3a–d, we found that alcohol feeding strongly increased the liver MDA level and MPO activity and reduced the GSH level and SOD activity. Nevertheless, when compared with ALI mice, administration of 200 mg/kg SinA to alcoholic liver mice decreased the MDA level and MPO activity and elevated the GSH level and SOD activity. Effects of SinA on liver inflammatory response were also detected (Fig. 3e–g). Alcohol feeding led to increased inflammatory cytokine contents in the liver, such as TNF-α, IL-1β, and IL-6, which were all decreased by SinA treatment in a dose-dependent manner. Collectively, these results suggested that SinA could ameliorate alcohol-induced liver oxidative stress and inflammatory responses in a dose-dependent manner.
Fig. 3.
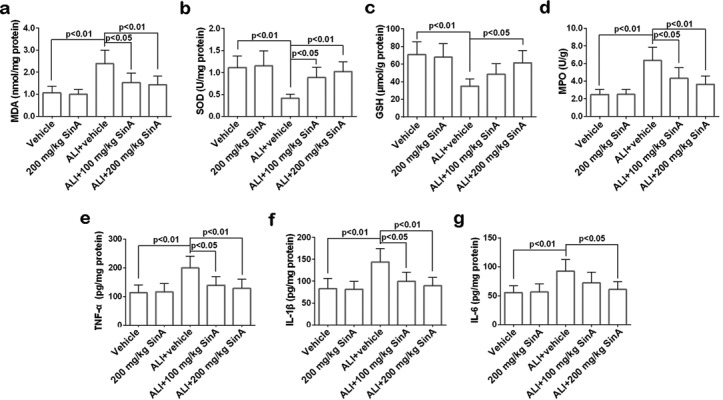
The effects of SinA on oxidative stress and inflammatory responses in the liver. The malondialdehyde (MDA) level, superoxide dismutase (SOD) activity, glutathione (GSH) level, myeloperoxidase (MPO) activity, and TNF-1α, IL-1β, and IL-6 content in the liver were measured by commercial kits. Each experiment was repeated six times. Data are expressed as the mean ± SD. ALI, alcohol-induced liver injury; SinA, Schisantherin A.
SinA regulated alcohol metabolism in the liver
To determine the effects of SinA on alcohol metabolism in the liver, the activities of the primary metabolizing enzymes ADH and ALDH were measured. Compared with the mice in the vehicle group, the activity of ADH in the liver of ALI mice was markedly enhanced, while, the ALDH activity was inhibited distinctly; however, such changes were prominently reversed by treatment with SinA (Fig. 4). Cytochrome P450 (CYP) is the main component of MEOS; hence, the expression of two major forms of CYP, CYP2E1 and CYP1A2, was measured (Fig. 5). qPCR and western blot analysis revealed that the expression levels of CYP2E1 and CYP1A2 were upregulated in alcoholic liver mice and that treatment with SinA inhibited alcohol-induced overexpression of these two proteins in a dose-dependent manner. Overall, these data indicated that SinA improved liver alcohol metabolism by regulation of the ADH/ALDH dehydrogenase system and MEOS system.
Fig. 4.
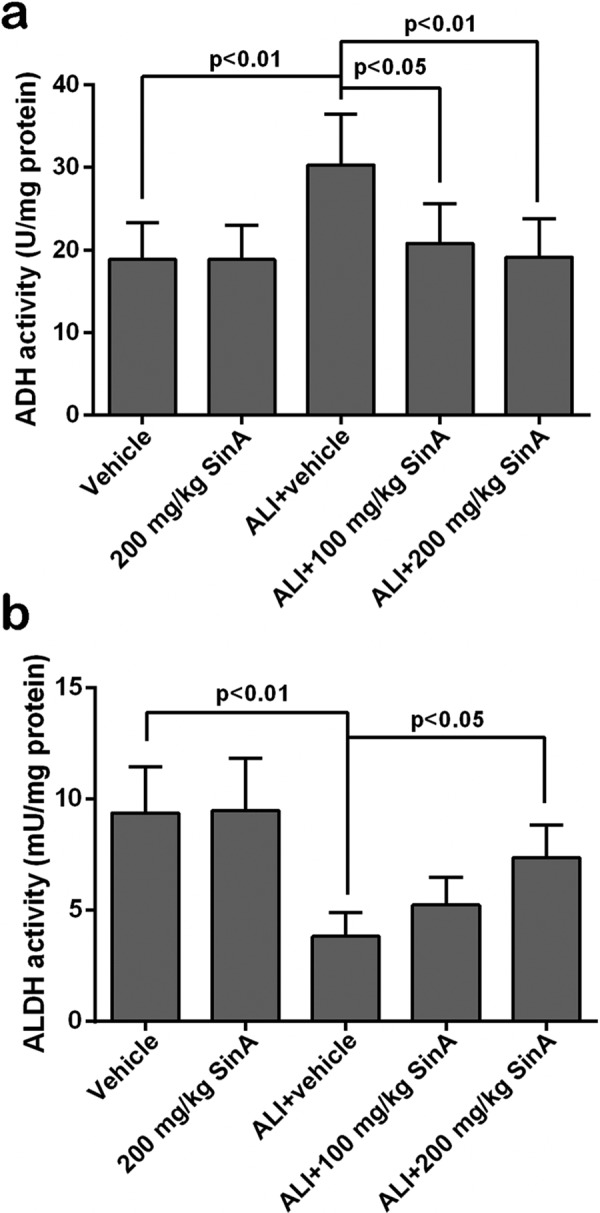
The effects of SinA on the ADH/ALDH dehydrogenase system. The activities of alcohol dehydrogenase (ADH) and acetaldehyde dehydrogenase (ALDH) were detected by commercial kits. Each experiment was repeated six times. Data are expressed as the mean ± SD. ALI, alcohol-induced liver injury; SinA, Schisantherin A.
Fig. 5.
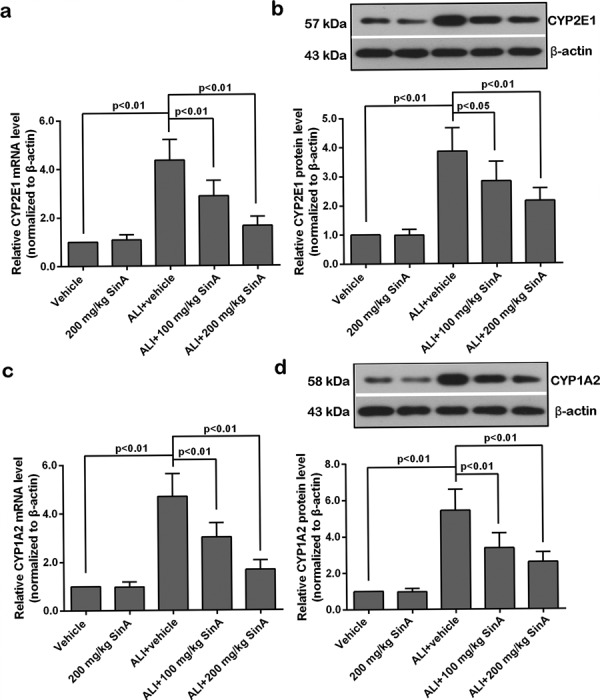
The effects of SinA on the expression of CYP2E1 and CYP1A2. The mRNA levels of CYP2E1 (a) and CYP1A2 (c) were measured by qPCR. The protein levels of CYP2E1 (b) and CYP1A2 (d) were determined by western blot. Representative images from six repeats are shown. Data are expressed as the mean ± SD. ALI, alcohol-induced liver injury; SinA, Schisantherin A.
SinA suppressed alcohol-induced activation of the NF-kB pathway
NF-kB is a nuclear transcription factor that acts as an important mediator of oxidative stress and the inflammatory response [49]. To investigate the mechanism of the liver-protective effect of SinA, we further examined the activation of the NF-kB signaling pathway. As illustrated in Fig. 6a and b, as compared with the vehicle group, the expression levels of p-p65 and p-IkB in the liver of ALI mice were upregulated in conjunction with a decrease in IkB level, and these changes were reverted by SinA in a dose-dependent manner. Consistent with these data, EMSA results showed that the NF-kB binding activity was significantly enhanced in alcoholic liver mice and was reduced by treatment with SinA (Fig. 6c). Taken together, SinA treatment suppressed activation of the NF-kB pathway in the liver of ALI mice.
Fig. 6.
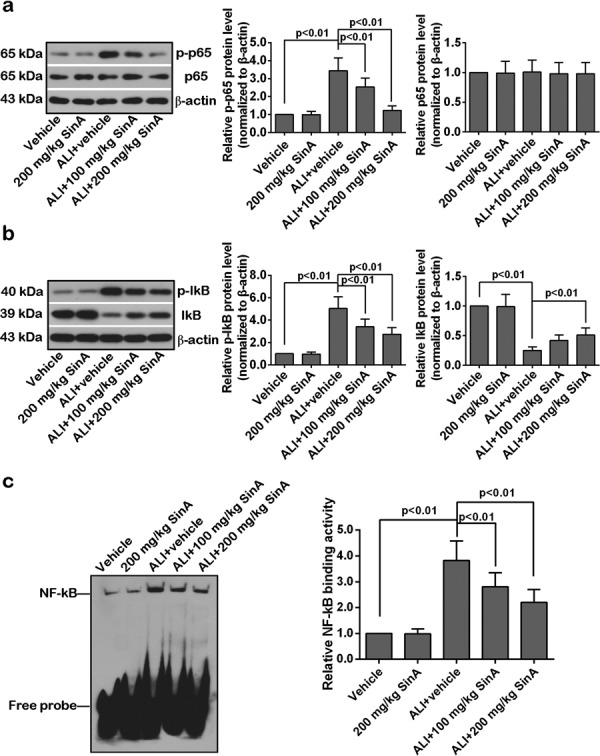
The effects of SinA on NF-kB activity. The protein levels of p-p65 and p65 (a) and p-IkB and IkB (b) were determined by western blot. The binding activity of NF-kB was detected by electrophoretic mobility shift assay (EMSA). The typical bands from six repeats are shown. Data are expressed as the mean ± SD. ALI, alcohol-induced liver injury; SinA, Schisantherin A.
Discussion
SinA (molecular formula, C30H32O9; formula weight, 536.57) is one of the native lignans of Schisandra chinensis that formed by the polymerization of two phenylpropanoid derivatives. Studies have reported that SinA could reduce the ROS level [44], inhibit the P-glycoprotein expression [34], multidrug resistance-associated protein 1 [25], TLR4/CD88 [39], mitogen-activate protein kinase [45], and NF-kB pathways [10, 28, 46], and possesses antioxidant effects, anti-carcinogenic effects, and anti-hepatotoxic effects; and have protective effects on the respiratory system, nervous system, and bone tissues [10, 25, 28, 34, 39, 44,45,46]. In the present study, the effects of SinA on the alcohol-induced liver injury were investigated, the underlying mechanisms were also explored. Treatment with SinA improved liver function and alleviated alcohol-induced liver pathologic changes, oxidative stress, and inflammatory responses. SinA also promoted liver alcohol metabolic status by the regulation of the ADH/ALDH dehydrogenase and MEOS systems. In addition, alcohol-induced activation of the NF-kB pathway was suppressed by the administration of SinA. Our study disclosed a liver-protective effect of SinA, and this effect seemed to depend on its regulation of alcohol metabolism and the NF-kB pathway.
Alcohol metabolism in the liver produces ROS, which enhance peroxidation of lipids, protein, and DNA, ultimately leading to abnormal liver function and lipid accumulation [5]. In this study, we demonstrated that SinA decreased vesicular steatosis and the activities of ALT and AST in alcohol-fed mice. These results indicat a liver-protective property of SinA. The beneficial effects of SinA on liver function were also confirmed in ischemia/reperfusion-induced liver injury mice [45].
During the processes of alcohol-induced liver injury, oxidative stress and the resulting inflammatory response play a central role [1, 16, 20]. MDA is an indicator of lipid peroxidation that has often been used to evaluate the ROS level [11]. MPO, the marker of oxidative stress, is capable of producing free radicals with the oxidizing ability [23]. SOD and GSH are antioxidant enzymes that can scavenge ROS [4]. In the current study, the alcohol-induced rise in MDA level and MPO activity, and decrease in SOD and GSH activities were strongly reversed by the administration of SinA, suggesting an anti-oxidation effect of SinA. These results were in agreement with previous studies showing that SinA is a potent antioxidant in 6-OHDA-induced dopaminergic neuron damage [44] and Aβ-induced neurodegeneration [27]. Additionally, the levels of inflammatory cytokines, TNF-α, IL-1β, and IL-6, in the livers of the ALI mice were all decreased by treatment with SinA, indicating an anti-inflammatory action of SinA. In fact, SinA has been reported to exhibit anti-inflammatory properties in lipopolysaccharide-induced acute respiratory distress syndrome [46], ischemia/reperfusion-induced neuronal injury [39] and IL-1β-induced chondrocytes inflammation [28]. Altogether, our data imply that SinA limits alcohol-induced liver injury by reducing oxidative stress and inflammatory responses.
Ethanol metabolism in the liver is a two-step process; it is first converted to acetaldehyde by ADH, and then, acetaldehyde is rapidly oxidized into acetate by ALDH [33]. Acetaldehyde, the intermediate metabolite, is a highly reactive compound that is strongly toxic to hepatocytes [13, 37]. We found that the activity of ADH was increased in the livers of the ALI mice, while the ALDH activity was decreased; however, this metabolic imbalance were dramatically reversed by the treatment with SinA. These findings suggest that SinA could promote the elimination of acetaldehyde by regulation of the ADH/ALDH dehydrogenase system.
The MEOS system, including CYP2E1, CYP1A2, and CYP3A4, is another metabolic system that participates in alcohol metabolism [2, 17]. Initiation of the MEOS system by intake of excessive alcohol will lead to the production of free radicals that cause oxidative stress in liver [7, 9, 12]. CYP2E1 plays a major role in liver microsomes-mediated ethanol oxidation; however, ethanol is oxidized also by CYP1A2 in the livers of human and rat [29, 36]. Whether CYP1A2 is involved in alcohol metabolism in the livers of mice has not yet been reported, but it is clear that CYP1A2 could accept electrons from CYP2E1 and decrease the microsomal ROS production [38]. Here, the expression levels of CYP2E1 and CYP1A2 were upregulated in ALI mice, indicating that the MEOS was activated by long-term alcohol intake. In addition, the upregulation of CYP2E1 and CYP1A2 induced by alcohol was significantly decreased by the treatment with SinA, and these results demonstrated that SinA could protect the liver from alcohol-induced injury by the deactivation of MEOS. The MEOS suppression effect of SinA in the liver was also confirmed in acetaminophen-induced liver injury mice [21] and normal rat liver microsomal incubations in vitro [41].
In agreement with previous observations in alcohol-induced liver injury [30, 40], we found that the NF-kB pathway in the liver was activated by alcohol feeding. Activation of NF-kB has been reported to contribute to liver injury through production of pro-inflammatory cytokines [15, 47]. It is reported that treatment with SinA could alleviate inflammatory reactions and inhibit the NF-kB pathway in LPS-treated RAW 264.7 cells [10], IL-1β-treated human chondrocytes [28] and LPS-induced acute respiratory distress syndrome mice [46]. Consistent with previous studies [10, 28, 46], treatment with SinA abrogated the activation of the NF-kB pathway, and this could be another explanation for the protective effect of SinA against alcohol-induced liver injury.
Taken together, our study demonstrated that treatment with SinA reduced liver vesicular steatosis, oxidative stress, and inflammatory responses and improved liver function of ALI mice. The liver-protection effects of SinA seem to be at least partly depend on its regulation of alcohol metabolism and the NF-kB pathway. Our study provides the preliminary evidence for the therapeutic potential of SinA for ALI.
Conflict of Interests
The authors declare that they have no competing interests.
Acknowledgments
This study was supported by grants from Innovative Talent Support Program for Institution of Higher Learning of Liaoning Province (No. LR2017038), and the Tianzhushan Scholars of Shenyang Agricultural University (No. 2018), and the National Natural Science Foundation of China (No. 31201325), and Liaoning BaiQianWan Talents Program.
The authors are grateful to staff from the High-tech Zone Laboratory of Public Test and Analysis Service (Shenyang, China) for their assistance in the experiments and English editing.
References
- 1.Ambade A., Catalano D., Lim A., Kopoyan A., Shaffer S.A., Mandrekar P.2014. Inhibition of heat shock protein 90 alleviates steatosis and macrophage activation in murine alcoholic liver injury. J. Hepatol. 61: 903–911. doi: 10.1016/j.jhep.2014.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bansal S., Srinivasan S., Anandasadagopan S., Chowdhury A.R., Selvaraj V., Kalyanaraman B., Joseph J., Avadhani N.G.2012. Additive effects of mitochondrion-targeted cytochrome CYP2E1 and alcohol toxicity on cytochrome c oxidase function and stability of respirosome complexes. J. Biol. Chem. 287: 15284–15297. doi: 10.1074/jbc.M111.314062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitkopf K., Nagy L.E., Beier J.I., Mueller S., Weng H., Dooley S.2009. Current experimental perspectives on the clinical progression of alcoholic liver disease. Alcohol. Clin. Exp. Res. 33: 1647–1655. doi: 10.1111/j.1530-0277.2009.01015.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cao Y.W., Jiang Y., Zhang D.Y., Zhang X.J., Hu Y.J., Li P., Su H., Wan J.B.2015. The hepatoprotective effect of aqueous extracts of Penthorum chinense Pursh against acute alcohol-induced liver injury is associated with ameliorating hepatic steatosis and reducing oxidative stress. Food Funct. 6: 1510–1517. doi: 10.1039/C5FO00098J [DOI] [PubMed] [Google Scholar]
- 5.Cederbaum A.I., Lu Y., Wu D.2009. Role of oxidative stress in alcohol-induced liver injury. Arch. Toxicol. 83: 519–548. doi: 10.1007/s00204-009-0432-0 [DOI] [PubMed] [Google Scholar]
- 6.Ceni E., Mello T., Galli A.2014. Pathogenesis of alcoholic liver disease: role of oxidative metabolism. World J. Gastroenterol. 20: 17756–17772. doi: 10.3748/wjg.v20.i47.17756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cha J.Y., Ahn H.Y., Cho Y.S., Je J.Y.2013. Protective effect of cordycepin-enriched Cordyceps militaris on alcoholic hepatotoxicity in Sprague-Dawley rats. Food Chem. Toxicol. 60: 52–57. doi: 10.1016/j.fct.2013.07.033 [DOI] [PubMed] [Google Scholar]
- 8.Chang R., Li Y., Yang X., Yue Y., Dou L., Wang Y., Zhang W., Li X.2013. Protective role of deoxyschizandrin and schisantherin A against myocardial ischemia-reperfusion injury in rats. PLoS One 8: e61590. doi: 10.1371/journal.pone.0061590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen X., Li R., Liang T., Zhang K., Gao Y., Xu L.2013. Puerarin improves metabolic function leading to hepatoprotective effects in chronic alcohol-induced liver injury in rats. Phytomedicine 20: 849–852. doi: 10.1016/j.phymed.2013.04.001 [DOI] [PubMed] [Google Scholar]
- 10.Ci X., Ren R., Xu K., Li H., Yu Q., Song Y., Wang D., Li R., Deng X.2010. Schisantherin A exhibits anti-inflammatory properties by down-regulating NF-kappaB and MAPK signaling pathways in lipopolysaccharide-treated RAW 264.7 cells. Inflammation 33: 126–136. doi: 10.1007/s10753-009-9166-7 [DOI] [PubMed] [Google Scholar]
- 11.Ding R.B., Tian K., Cao Y.W., Bao J.L., Wang M., He C., Hu Y., Su H., Wan J.B.2015. Protective effect of panax notoginseng saponins on acute ethanol-induced liver injury is associated with ameliorating hepatic lipid accumulation and reducing ethanol-mediated oxidative stress. J. Agric. Food Chem. 63: 2413–2422. doi: 10.1021/jf502990n [DOI] [PubMed] [Google Scholar]
- 12.Ellefson W.M., Lakner A.M., Hamilton A., McKillop I.H., Bonkovsky H.L., Steuerwald N.M., Huet Y.M., Schrum L.W.2011. Neonatal androgenization exacerbates alcohol-induced liver injury in adult rats, an effect abrogated by estrogen. PLoS One 6: e29463. doi: 10.1371/journal.pone.0029463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farfán Labonne B.E., Gutiérrez M., Gómez-Quiroz L.E., Konigsberg Fainstein M., Bucio L., Souza V., Flores O., Ortíz V., Hernández E., Kershenobich D., Gutiérrez-Ruíz M.C.2009. Acetaldehyde-induced mitochondrial dysfunction sensitizes hepatocytes to oxidative damage. Cell Biol. Toxicol. 25: 599–609. doi: 10.1007/s10565-008-9115-5 [DOI] [PubMed] [Google Scholar]
- 14.Frazier T.H., Stocker A.M., Kershner N.A., Marsano L.S., McClain C.J.2011. Treatment of alcoholic liver disease. Therap. Adv. Gastroenterol. 4: 63–81. doi: 10.1177/1756283X10378925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gan F., Liu Q., Liu Y., Huang D., Pan C., Song S., Huang K.2018. Lycium barbarum polysaccharides improve CCl4-induced liver fibrosis, inflammatory response and TLRs/NF-kB signaling pathway expression in wistar rats. Life Sci. 192: 205–212. doi: 10.1016/j.lfs.2017.11.047 [DOI] [PubMed] [Google Scholar]
- 16.Gao B., Bataller R.2011. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141: 1572–1585. doi: 10.1053/j.gastro.2011.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.García-Bañuelos J., Panduro A., Gordillo-Bastidas D., Gordillo-Bastidas E., Muñoz-Valle J.F., Gurrola-Díaz C.M., Sánchez-Enríquez S., Ruiz-Madrigal B., Bastidas-Ramírez B.E.2012. Genetic polymorphisms of genes coding to alcohol-metabolizing enzymes in western Mexicans: association of CYP2E1*c2/CYP2E1*5B allele with cirrhosis and liver function. Alcohol. Clin. Exp. Res. 36: 425–431. doi: 10.1111/j.1530-0277.2011.01617.x [DOI] [PubMed] [Google Scholar]
- 18.He Y., Zhang Q., Shen Y., Chen X., Zhou F., Peng D.2014. Schisantherin A suppresses osteoclast formation and wear particle-induced osteolysis via modulating RANKL signaling pathways. Biochem. Biophys. Res. Commun. 449: 344–350. doi: 10.1016/j.bbrc.2014.05.034 [DOI] [PubMed] [Google Scholar]
- 19.Horiguchi N., Wang L., Mukhopadhyay P., Park O., Jeong W.I., Lafdil F., Osei-Hyiaman D., Moh A., Fu X.Y., Pacher P., Kunos G., Gao B.2008. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology 134: 1148–1158. doi: 10.1053/j.gastro.2008.01.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Q., Huang R., Zhang S., Lin J., Wei L., He M., Zhuo L., Lin X.2013. Protective effect of genistein isolated from Hydrocotyle sibthorpioides on hepatic injury and fibrosis induced by chronic alcohol in rats. Toxicol. Lett. 217: 102–110. doi: 10.1016/j.toxlet.2012.12.014 [DOI] [PubMed] [Google Scholar]
- 21.Jiang Y., Fan X., Wang Y., Tan H., Chen P., Zeng H., Huang M., Bi H.2015. Hepato-protective effects of six schisandra lignans on acetaminophen-induced liver injury are partially associated with the inhibition of CYP-mediated bioactivation. Chem. Biol. Interact. 231: 83–89. doi: 10.1016/j.cbi.2015.02.022 [DOI] [PubMed] [Google Scholar]
- 22.Kim D.K., Kim Y.H., Jang H.H., Park J., Kim J.R., Koh M., Jeong W.I., Koo S.H., Park T.S., Yun C.H., Park S.B., Chiang J.Y.L., Lee C.H., Choi H.S.2013. Estrogen-related receptor γ controls hepatic CB1 receptor-mediated CYP2E1 expression and oxidative liver injury by alcohol. Gut 62: 1044–1054. doi: 10.1136/gutjnl-2012-303347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Latchoumycandane C., Nagy L.E., McIntyre T.M.2015. Myeloperoxidase formation of PAF receptor ligands induces PAF receptor-dependent kidney injury during ethanol consumption. Free Radic. Biol. Med. 86: 179–190. doi: 10.1016/j.freeradbiomed.2015.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leggio L., Kenna G.A., Ferrulli A., Zywiak W.H., Caputo F., Swift R.M., Addolorato G.2011. Preliminary findings on the use of metadoxine for the treatment of alcohol dependence and alcoholic liver disease. Hum. Psychopharmacol. 26: 554–559. doi: 10.1002/hup.1244 [DOI] [PubMed] [Google Scholar]
- 25.Li L., Pan Q., Sun M., Lu Q., Hu X.2007. Dibenzocyclooctadiene lignans: a class of novel inhibitors of multidrug resistance-associated protein 1. Life Sci. 80: 741–748. doi: 10.1016/j.lfs.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 26.Li S., Tan H.Y., Wang N., Zhang Z.J., Lao L., Wong C.W., Feng Y.2015. The Role of Oxidative Stress and Antioxidants in Liver Diseases. Int. J. Mol. Sci. 16: 26087–26124. doi: 10.3390/ijms161125942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li X., Zhao X., Xu X., Mao X., Liu Z., Li H., Guo L., Bi K., Jia Y.2014. Schisantherin A recovers Aβ-induced neurodegeneration with cognitive decline in mice. Physiol. Behav. 132: 10–16. doi: 10.1016/j.physbeh.2014.04.046 [DOI] [PubMed] [Google Scholar]
- 28.Liao S., Zhou K., Li D., Xie X., Jun F., Wang J.2016. Schisantherin A suppresses interleukin-1β-induced inflammation in human chondrocytes via inhibition of NF-κB and MAPKs activation. Eur. J. Pharmacol. 780: 65–70. doi: 10.1016/j.ejphar.2016.03.032 [DOI] [PubMed] [Google Scholar]
- 29.Lieber C.S.2004. The discovery of the microsomal ethanol oxidizing system and its physiologic and pathologic role. Drug Metab. Rev. 36: 511–529. doi: 10.1081/DMR-200033441 [DOI] [PubMed] [Google Scholar]
- 30.Liu Y., Wang J., Li L., Hu W., Qu Y., Ding Y., Meng L., Teng L., Wang D.2017. Hepatoprotective Effects of Antrodia cinnamomea: The Modulation of Oxidative Stress Signaling in a Mouse Model of Alcohol-Induced Acute Liver Injury. Oxid. Med. Cell. Longev. 2017: 7841823. doi: 10.1155/2017/7841823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak K.J., Schmittgen T.D.2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 32.Okiyama W., Tanaka N., Nakajima T., Tanaka E., Kiyosawa K., Gonzalez F.J., Aoyama T.2009. Polyenephosphatidylcholine prevents alcoholic liver disease in PPARalpha-null mice through attenuation of increases in oxidative stress. J. Hepatol. 50: 1236–1246. doi: 10.1016/j.jhep.2009.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oyama T., Isse T., Kagawa N., Kinaga T., Kim Y.D., Morita M., Sugio K., Weiner H., Yasumoto K., Kawamoto T.2005. Tissue-distribution of aldehyde dehydrogenase 2 and effects of the ALDH2 gene-disruption on the expression of enzymes involved in alcohol metabolism. Front. Biosci. 10: 951–960. doi: 10.2741/1589 [DOI] [PubMed] [Google Scholar]
- 34.Pan Q., Lu Q., Zhang K., Hu X.2006. Dibenzocyclooctadiene lingnans: a class of novel inhibitors of P-glycoprotein. Cancer Chemother. Pharmacol. 58: 99–106. doi: 10.1007/s00280-005-0133-1 [DOI] [PubMed] [Google Scholar]
- 35.Sa F., Zhang L.Q., Chong C.M., Guo B.J., Li S., Zhang Z.J., Zheng Y., Hoi P.M., Lee S.M.2015. Discovery of novel anti-parkinsonian effect of schisantherin A in in vitro and in vivo. Neurosci. Lett. 593: 7–12. doi: 10.1016/j.neulet.2015.03.016 [DOI] [PubMed] [Google Scholar]
- 36.Salmela K.S., Kessova I.G., Tsyrlov I.B., Lieber C.S.1998. Respective roles of human cytochrome P-4502E1, 1A2, and 3A4 in the hepatic microsomal ethanol oxidizing system. Alcohol. Clin. Exp. Res. 22: 2125–2132. doi: 10.1111/j.1530-0277.1998.tb05926.x [DOI] [PubMed] [Google Scholar]
- 37.Setshedi M., Wands J.R., Monte S.M.2010. Acetaldehyde adducts in alcoholic liver disease. Oxid. Med. Cell. Longev. 3: 178–185. doi: 10.4161/oxim.3.3.12288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shertzer H.G., Clay C.D., Genter M.B., Schneider S.N., Nebert D.W., Dalton T.P.2004. Cyp1a2 protects against reactive oxygen production in mouse liver microsomes. Free Radic. Biol. Med. 36: 605–617. doi: 10.1016/j.freeradbiomed.2003.11.013 [DOI] [PubMed] [Google Scholar]
- 39.Shi Y.W., Zhang X.C., Chen C., Tang M., Wang Z.W., Liang X.M., Ding F., Wang C.P.2017. Schisantherin A attenuates ischemia/reperfusion-induced neuronal injury in rats via regulation of TLR4 and C5aR1 signaling pathways. Brain Behav. Immun. 66: 244–256. doi: 10.1016/j.bbi.2017.07.004 [DOI] [PubMed] [Google Scholar]
- 40.Tahir M., Rehman M.U., Lateef A., Khan R., Khan A.Q., Qamar W., Ali F., O’Hamiza O., Sultana S.2013. Diosmin protects against ethanol-induced hepatic injury via alleviation of inflammation and regulation of TNF-α and NF-κB activation. Alcohol 47: 131–139. doi: 10.1016/j.alcohol.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 41.Wang B., Yang S., Hu J., Li Y.2014. Multifaceted interaction of the traditional Chinese medicinal herb Schisandra chinensis with cytochrome P450-mediated drug metabolism in rats. J. Ethnopharmacol. 155: 1473–1482. doi: 10.1016/j.jep.2014.07.026 [DOI] [PubMed] [Google Scholar]
- 42.Wei H., Tao X., Di P., Yang Y., Li J., Qian X., Feng J., Chen W.2013. Effects of traditional chinese medicine Wuzhi capsule on pharmacokinetics of tacrolimus in rats. Drug Metab. Dispos. 41: 1398–1403. doi: 10.1124/dmd.112.050302 [DOI] [PubMed] [Google Scholar]
- 43.Zakhari S.2006. Overview: how is alcohol metabolized by the body? Alcohol Res. Health 29: 245–254. [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L.Q., Sa F., Chong C.M., Wang Y., Zhou Z.Y., Chang R.C., Chan S.W., Hoi P.M., Yuen Lee S.M.2015. Schisantherin A protects against 6-OHDA-induced dopaminergic neuron damage in zebrafish and cytotoxicity in SH-SY5Y cells through the ROS/NO and AKT/GSK3β pathways. J. Ethnopharmacol. 170: 8–15. doi: 10.1016/j.jep.2015.04.040 [DOI] [PubMed] [Google Scholar]
- 45.Zheng N., Liu F., Lu H., Zhan Y., Zhang M., Guo W., Ding G.2017. Schisantherin A protects against liver ischemia-reperfusion injury via inhibition of mitogen-activated protein kinase pathway. Int. Immunopharmacol. 47: 28–37. doi: 10.1016/j.intimp.2017.03.019 [DOI] [PubMed] [Google Scholar]
- 46.Zhou E., Li Y., Wei Z., Fu Y., Lei H., Zhang N., Yang Z., Xie G.2014. Schisantherin A protects lipopolysaccharide-induced acute respiratory distress syndrome in mice through inhibiting NF-κB and MAPKs signaling pathways. Int. Immunopharmacol. 22: 133–140. doi: 10.1016/j.intimp.2014.06.004 [DOI] [PubMed] [Google Scholar]
- 47.Zhou L., Zhao D., An H., Zhang H., Jiang C., Yang B.2015. Melatonin prevents lung injury induced by hepatic ischemia-reperfusion through anti-inflammatory and anti-apoptosis effects. Int. Immunopharmacol. 29: 462–467. doi: 10.1016/j.intimp.2015.10.012 [DOI] [PubMed] [Google Scholar]
- 48.Zhou Z., Wang L., Song Z., Lambert J.C., McClain C.J., Kang Y.J.2003. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-alpha production. Am. J. Pathol. 163: 1137–1146. doi: 10.1016/S0002-9440(10)63473-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zima T., Kalousová M.2005. Oxidative stress and signal transduction pathways in alcoholic liver disease. Alcohol. Clin. Exp. Res. 29:(Suppl): 110S–115S. doi: 10.1097/01.alc.0000189288.30358.4b [DOI] [PubMed] [Google Scholar]