Abstract
This study aimed to evaluate the protective effect of everolimus, a mechanistic target of rapamycin (mTOR) inhibitor, on cisplatin chemotherapy-induced ovarian toxicity. Eighty sexually mature, virgin, female, 7-week-old C57BL/6J mice were divided into four groups: control, cisplatin (Cis), everolimus (mTORi), and everolimus plus cisplatin (mTORi+Cis). Mice in the Cis and mTORi+Cis groups were intraperitoneally injected with 2 mg/kg of cisplatin for 15 d. Mice in the mTORi and mTORi+Cis groups were orally administered 2.5 mg/kg of everolimus for 29 d, from one week before the first cisplatin injection to one week after the last cisplatin injection. Histological examinations were performed 24 h after the last everolimus administration. The primordial, primary, and antral follicles were significantly depleted in the Cis group compared with that in the control group, confirming the gonadotoxicity of cisplatin. The number of primordial, secondary, and antral follicles was significantly higher in the mTORi+Cis group than in the Cis group, thereby displaying the effect of mTORi-treatment on ovarian protection. Primordial, secondary, and antral follicle counts were similar in the mTORi+Cis and the control groups. The results of this study indicate a protective effect of an mTOR inhibitor against cisplatin chemotherapy-induced gonadotoxicity in the ovarian reserve in an in vivo mouse model.
Keywords: chemotherapeutic agent-induced gonadotoxicity, cisplatin, fertility preservation, mechanistic target of rapamycin inhibitor, ovarian protection
Introduction
With advancements in biomedical technology, an increasing number of patients are surviving cancer. Preserving fertility in adolescent and young adult (AYA) cancer survivors is a socially important issue [25] because anticancer chemotherapy and radiotherapy markedly decrease gonadal function. Cisplatin is a platinum-based type of cytotoxic anti-cancer agent, and the American Society of Clinical Oncology stated that cisplatin is an example of anticancer drugs with gonadal toxicity [18]. Despite its high gonadal toxicity, cisplatin is often used to treat cancers in AYAs, especially for ovarian germ cell cancer [21], breast cancer [1], locally advanced cervical cancer [3, 8, 22], and other malignant tumours [10]. The aforementioned malignant tumours are common among AYAs [17].
There are three alternatives for the protection of female fertility from chemotherapy-induced toxicity: cryopreservation of ovaries, cryopreservation of oocytes or embryos, and administration of ovarian-protective drugs [18]. “Ferto-protective adjuvant therapy” refers to the administration of adjuvant therapy during or prior to chemotherapy, which can prevent the loss of the ovarian reserves; this term was defined by Woodruff in 2009 [30]. The process for cryopreservation of ovaries is currently under development, and the cryopreservation of oocytes or embryos has limitations including the risk of delay in initiating anti-tumour treatment, a risk of obtaining an insufficient number of cells, and the requirement of assisted reproductive technology. Development of ferto-protective adjuvant therapies is clinically significant; a natural pregnancy after chemotherapy is possible if the ferto-protective adjuvant drug does not attenuate the antitumor effect while also preventing chemotherapy-induced ovarian toxicity.
Until now, no drug had displayed robust ferto-protective activity in human clinical trials; hence, new drugs were needed. Recently, activation of the PI3K/PTEN/AKT pathway was detected in cisplatin chemotherapy-induced ovarian toxicity, and the underlying mechanisms cause the loss of follicles [5]. Therefore, we focused on mechanistic target of rapamycin (mTOR), a downstream protein in the PI3K/PTEN/AKT pathway, and hypothesised that an mTOR inhibitor (mTORi) would effectively combat cisplatin-induced ovarian toxicity.
This study, to our knowledge, is the first to investigate the role of mTOR inhibitors in preventing ovarian toxicity of cisplatin.
Materials and Methods
Animals
Eighty sexually mature, virgin, female C57BL/6J mice (7-week-old) were purchased from CLEA Japan, Inc. (Tokyo, Japan). The animals were housed in the Animal Center of the National Defense Medical Center under a 12:12-h light/dark cycle with ad libitum access to food and water. The animals were acclimatised for 1 week before the experiment. All procedures were carried out in accordance with the animal care and welfare guidelines of Shiga University of Medical Science. All experimental procedures were approved by the Shiga University of Medical Science Animal Experimentation Center Committee.
Materials
Everolimus (dry powder) was purchased from Selleck (USA). Microemulsion vehicle-formulated everolimus (propylene glycol and Tween 80) were purchased from Sigma-Aldrich (USA). Cisplatin was purchased from Nichiiko, Japan. Everolimus and an everolimus-free vehicle were administered orally. Cisplatin was diluted in 0.9% saline. Cisplatin in saline (control vehicle) were administered through intraperitoneal injection.
Determination of in vivo ovarian toxicity
The eighty mice used in this study were divided equally into four groups: Control, cisplatin (Cis), everolimus (mTORi), and everolimus plus cisplatin (mTORi+Cis). Mice in the Cis and mTORi+Cis groups were intraperitoneally administered 2 mg/kg of cisplatin daily for 15 d. Mice in the Control and mTORi groups were similarly administered saline. Mice in the mTORi and mTORi+Cis groups were orally administered 2.5 mg/kg of everolimus daily 1 week before to 1 week after cisplatin administration. Mice in the Control and Cis groups were similarly administered an everolimus-free vehicle (Fig. 1). After the measurement of final mice body weight, they were euthanized on day 30, 1 week after the last cisplatin injection. During euthanasia, the ovaries were harvested and immediately fixed in Bouin’s solution following the measurement of ovarian weight.
Fig. 1.
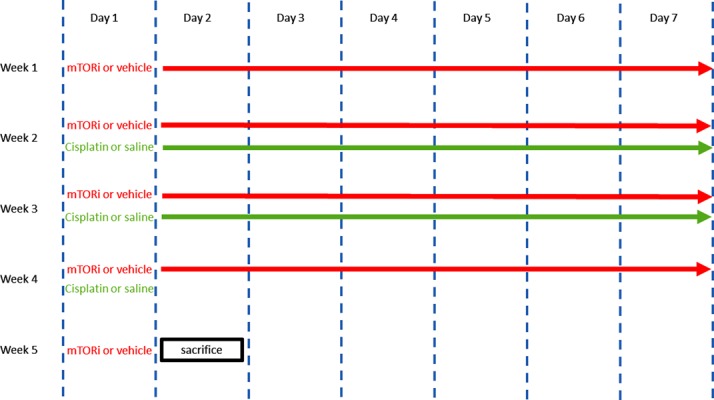
Experimental scheme: 2.5 mg/kg of everolimus or vehicle was orally administered daily from week 1 day 1 to week 5 day 1; 2 mg/kg of cisplatin or saline was administered through intraperitoneal injection daily from week 2 day 1 to week 4 day 1. Mice were euthanized on week 5 day 2.
Haematoxylin and eosin (H&E) staining and follicle classification
After fixation, the ovaries were embedded in paraffin, cut into 4 µm thick sections, and stained with H&E. Procedures for follicle identification and enumeration were conducted as previously described [9, 20, 28]. Follicles were counted in every 25th section throughout the entire ovary, and the total number was determined. Follicles were counted only when the nucleus was present in the section.
Statistical analysis
Results were analysed with Prism version 6.0 software (GraphPad, USA). All data are presented as mean ± SEM value. Differences between groups were tested by one-way analysis of variance (ANOVA). If a significant overall difference was found, the post hoc Tukey test was computed for multiple comparisons. A value of P<0.05 was considered statistically significant.
Results
The Cis group showed a significantly lower final body weight than the control group (P<0.001); the mTORi+Cis group also showed a significantly lower final body weight than the mTORi group (P<0.001). However, there was no significant difference in final body weight between the control and mTORi Cis, and mTORi+Cis groups (Fig. 2).
Fig. 2.
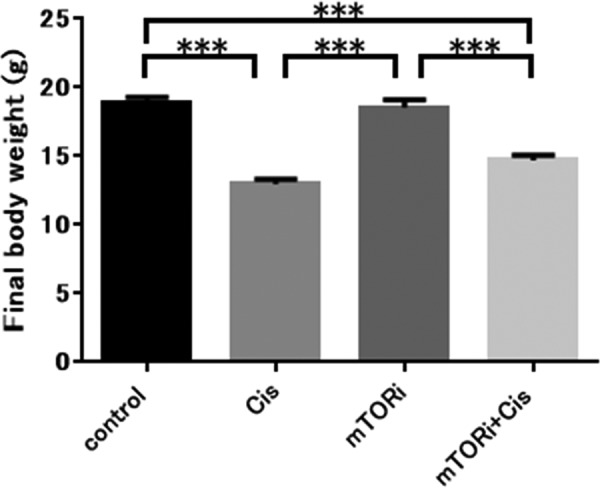
Final body weight. The final body weight of mice was significantly reduced when cisplatin was administered. Regarding the final body weight, the Cis group presented a lower value than the control group (***P<0.001) and the mTORi+Cis group presented a lower value than the mTORi group (***P<0.001). The final body weight of mice was not significantly different between the group treated with TOR inhibitor and the untreated group; control group and mTORi had equivalent final body weights (n.s., not significant; P>0.05), and the Cis group and mTORi+Cis group had equivalent final body weights (n.s., not significant; P>0.05). All data represent the mean ± SEM value.
Regarding ovarian weight, the Cis group presented a significantly lower value than the control group (P<0.01). The mTORi+Cis group presented a significantly higher ovarian weight than the mTORi group (P<0.05); however, there was no significant difference between the ovarian weight of the control group and that of the mTORi+Cis group (Fig. 3).
Fig. 3.
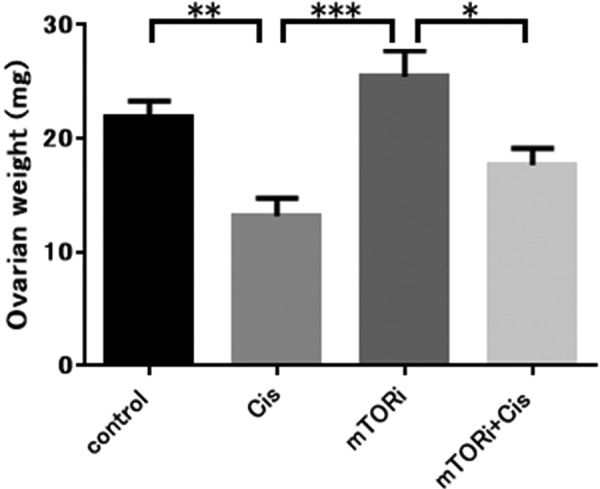
Ovarian weight. The Cis group had a significantly lower ovarian weight than the control group (**P<0.01). The mTORi group had a significantly higher ovarian weight than the Cis group (***P<0.001). The mTORi+Cis group had a significantly lower ovarian weight than the mTORi group (*P<0.05). The control group and mTORi+Cis group had equivalent ovarian weights (n.s., not significant; P>0.05). All data represent the mean ± SEM value.
Histological analysis revealed that the ovarian tissue of the control group displayed normal ovarian architecture with an orderly arrangement of follicles. Ovarian tissue of the Cis group had reduced surface area and extensive destruction of the ovarian structure, reduced numbers of follicles at all stages, and numerous degenerating follicles compared with the control group. Ovarian tissue of the mTORi group displayed normal size surface area and a larger number of various follicle types compared with the control group. Ovarian tissue of the mTORi+Cis group had normal surface area, a few degenerating follicles, and numerous normal follicle types; their histological characteristics were similar with those of the control group (Fig. 4).
Fig. 4.
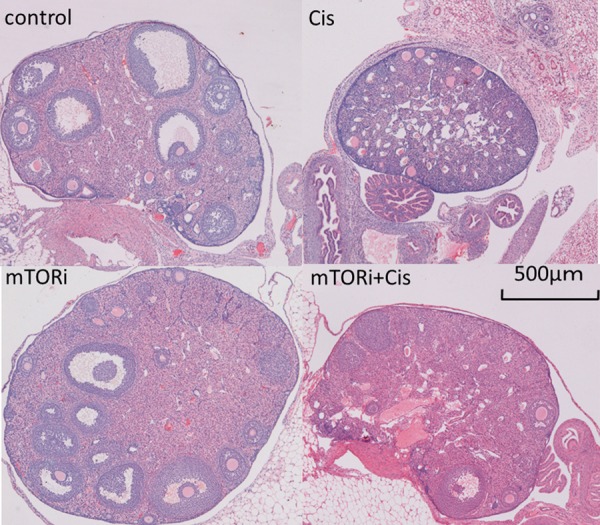
Histological examination of ovaries. The control group had follicles with normal morphology in normal-sized ovaries. The Cis group showed an obvious reduction in the ovarian surface area and number of normal follicles, increase in degenerating follicles, and destruction of the ovarian structure. The mTORi group showed many follicles with normal morphology in normal-sized ovaries. The mTORi+Cis group had many follicles with normal morphology in normal-sized ovaries and few degenerating follicles.
We investigated the effect of an mTOR inhibitor and cisplatin on the number of follicles in each group. A follicle count revealed that ovarian tissue of Cis group had significantly fewer primordial follicles (P<0.01), primary follicles (P<0.001), and antral follicles (P<0.05) than those in the control group. Ovarian tissue of the mTORi group had a significantly higher number of primordial follicles (P<0.001) and primary follicles (P<0.05) than the control group. Ovarian tissue of the mTORi+Cis group had a significantly higher number of primordial follicles (P<0.05), secondary follicles (P<0.05), and antral follicles (P<0.001) than that in the Cis group. Ovarian tissue of the mTORi+Cis and the control groups had statistical equivalent number of primordial, secondary, and antral follicles (Fig. 5).
Fig. 5.
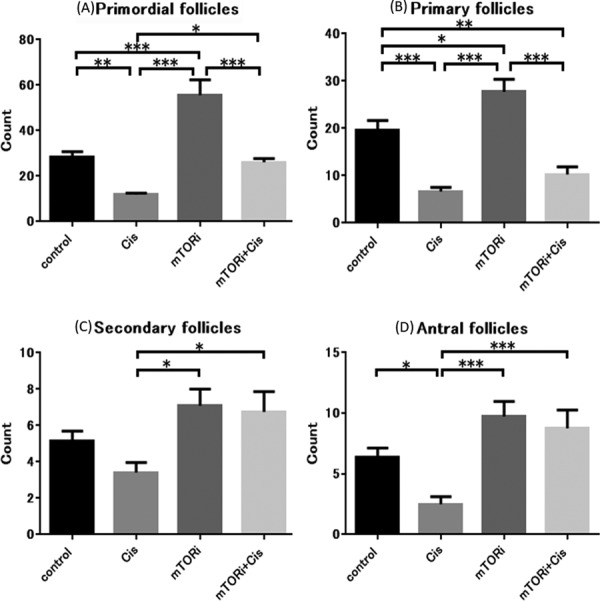
Follicle counts. (A) Primordial follicle count: the Cis group had significantly fewer primordial follicles than the control group (**P<0.01). The mTORi+Cis group had significantly more primordial follicles than the Cis group (*P<0.05). The mTORi+Cis group and control group had equivalent primordial follicle counts (n.s., not significant; P>0.05). (B) Primary follicle count: the Cis group had significantly fewer primary follicles than the control group (***P<0.001). (C) Secondary follicle count: The mTORi+Cis group had significantly more secondary follicles than the Cis group (*P<0.05). The mTORi+Cis and control groups had equivalent secondary follicle counts (n.s., not significant; P>0.05). (D) Antral follicle count: the Cis group had significantly fewer antral follicles than the control group (*P<0.05). The mTORi+Cis group had significantly more follicles than the Cis group (***P<0.001). The mTORi+Cis group and control group had equivalent antral follicle counts (n.s., not significant; P>0.05). All data represent the mean ± SEM value.
Discussion
The present results indicate that daily administration of an mTOR inhibitor can preserve ovarian function and protect against cisplatin chemotherapy-induced dysfunction with minimal toxicity. Our results indicate that cisplatin caused intense ovarian toxicity by increasing the number of degenerating follicles and decreasing the number of primordial and other types of follicles. The number of primordial, secondary, and antral follicles increased with the addition of the mTOR inhibitor during cisplatin administration, and the numbers of primordial and other types of follicles between the mTOR+Cis and control groups were equivalent. Since the primordial follicle pool size is the primary indicator of ovarian function in mice [13], mTOR inhibitors are effective ferto-protective adjuvant agents in combating the effects of cisplatin. The mTORi group presented a larger primordial follicle pool size than the control group, consistent with previous reports. This mechanism was explained by the decreased expression of mTOR and augmentation of the resting follicle reserves [31]. The result of ovarian weight measurement supported the histological analysis. Results of ovarian weight change due to cisplatin-induced ovarian toxicity were similar to those reported in a previous study [5]. The final body weight indicated systemic minimal toxicity associated with mTOR inhibitor treatment in mice.
The mechanism underlying the improvement of primordial follicles as ferto-protective effect of mTOR inhibitors against cisplatin-induced ovarian toxicity may include preventing follicular activation and preventing apoptosis in growing follicles, through interference of the mTOR pathway. Recently, new mechanisms underlying cisplatin and cyclophosphamide chemotherapy-induced ovarian toxicity have been identified. Follicle activation occurs through activation of the PI3K/PTEN/AKT pathway, resulting in the pooling of many growing follicles, and many of these growing follicles undergo apoptosis [14]. Thereafter, Goldman et al. and Zhou et al. focused on mTOR, a downstream protein in the PI3K/PTEN/AKT pathway, and they reported that co-treatment with an mTOR inhibitor prevents cyclophosphamide-induced ovarian dysfunction by reducing both follicle activation and apoptosis, specifically targeting the follicles [9, 32]. In the present study, loss of primordial follicles and an increased number of degenerating and growing follicles was observed in the cisplatin treatment group. A normal number of primordial follicles, a few degenerating follicles, and a normal number of growing follicles were observed in the mTOR inhibitor and cisplatin co-treatment group compared with the control group. Considering previous literature and the present results, the mechanism underlying the improvement of primordial follicles as ferto-protective of mTOR inhibitors against cisplatin-induced ovarian toxicity probably involves both prevention of follicular activation and prevention of apoptosis in growing follicles, through interference of the mTOR pathway. Further studies are needed to elucidate the mechanism underlying the protection of cisplatin-induced ovarian toxicity by mTOR inhibitors.
Among ferto-protective adjuvant agents, mTOR inhibitors have many advantages over other drugs. Different types of ferto-protective adjuvant agents have been reported in in vivo models. Briefly, hormonal therapies and non- hormonal therapies have been reported [23, 24, 27]. In in vivo models, gonadotropin releasing hormone agonist (GnRHa) have has been the most well-studied candidate drug in this field. However, there have been conflicting results regarding GnRHa in human clinical trials [6, 23]. There are four advantages of using mTOR inhibitors as ferto-protective adjuvant agents. First, mTOR inhibitors have an antitumor effect, and they are used to treat breast cancer [1] and renal cancer [19]. A considerable number of studies have evaluated the oncological outcomes of mTOR inhibitor treatment in cervical and other gynaecological cancers [7]. Second, pregnancy is possible during oral administration of mTOR inhibitors, whereas it is difficult in many hormonal therapies. Third, pregnancy prognosis might be improved. Miscarriage and preterm birth rates would increase after cancer treatment [16, 29]. mTOR inhibitors have been reported to cause remission of respiratory distress syndrome induced by preterm delivery [12] and to decrease the preterm birth rate [4]. Fourth, mTOR inhibitors are clinically used for various diseases and proven to be safe for long-term use. In the present study, everolimus was used as an mTOR inhibitor, which is a derivative of sirolimus, a known “anti-aging agent” [11, 31]. Everolimus is used clinically as an immunosuppressant to prevent the immunological rejection of organ transplants [2], restenosis of coronary stents [26], and as an anticancer-drug in the treatment of malignant tumours [1, 19].
Our study has several limitations. Although our study was limited by the use of a murine model, our drug dosage and experiment protocol were determined in accordance with the amount that is clinically applied in human cancer treatment and we used the same dose that has an anti-tumour effect on tumour-bearing mice in an in vivo model. Everolimus and cisplatin dosages used for our murine model were almost equivalent to and greater than those used to treat cancer in humans [5, 9]. The administration protocol and dosage of cisplatin in our study were exactly the same as those reported previously [5]. Considering the time lag of reaching to steady-state everolimus concentrations [15], everolimus administration was initiated 1 week before cisplatin administration, as in a previous study [32]. Furthermore, we conducted experiments with healthy mice rather than tumour-bearing mice, and future studies using tumour-bearing mice are necessary. The primary focus of the present study was ovarian function; however, evaluations of perinatal prognosis, such as litter size and/or percentage of live births and/or pup weight, are also necessary in our experimental system. Although some human clinical case reports describe normal fertility outcomes in patients treated with mTOR inhibitors [9], more studies are needed to assess the long-term effects of mTOR inhibitors on offspring.
In conclusion, the present study report that an mTOR inhibitor, when used in combination with the chemotherapeutic drug cisplatin, attenuated cisplatin-mediated primordial follicular loss in mouse ovaries.
Acknowledgments
This study was supported by JSPS KAKENHI Grant Number JP17K11272. We are grateful for technical assistance from Takefumi Yamamoto and Yasuhiro Mori from the Central Research Laboratory, and Ichiro Terakado from the Research Center for Animal Life Science, Shiga University of Medical Science.
References
- 1.Breast Cancer Guideline (Version 3. 2015). NCCN Clinical Practice Guidelines in Oncology http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 2.Budde K., Rath T., Sommerer C., Haller H., Reinke P., Witzke O., Suwelack B., Baeumer D., May C., Porstner M., Arns W.2015. Renal, efficacy and safety outcomes following late conversion of kidney transplant patients from calcineurin inhibitor therapy to everolimus: the randomized APOLLO study. Clin. Nephrol. 83: 11–21. doi: 10.5414/CN108444 [DOI] [PubMed] [Google Scholar]
- 3.Cervical Cancer Guideline (Version 1. 2017). NCCN Clinical Practice Guidelines in Oncology http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 4.Cha J., Bartos A., Egashira M., Haraguchi H., Saito-Fujita T., Leishman E., Bradshaw H., Dey S.K., Hirota Y.2013. Combinatory approaches prevent preterm birth profoundly exacerbated by gene-environment interactions. J. Clin. Invest. 123: 4063–4075. doi: 10.1172/JCI70098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chang E.M., Lim E., Yoon S., Jeong K., Bae S., Lee D.R., Yoon T.K., Choi Y., Lee W.S.2015. Cisplatin induces overactivation of the dormant primordial follicle through PTEN/AKT/FOXO3a pathway which leads to loss of ovarian reserve in mice. PLoS One 10: e0144245. doi: 10.1371/journal.pone.0144245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Demeestere I., Brice P., Peccatori F.A., Kentos A., Dupuis J., Zachee P., Casasnovas O., Van Den Neste E., Dechene J., De Maertelaer V., Bron D., Englert Y.2016. No evidence for the benefit of gonadotropin-releasing hormone agonist in preserving ovarian function and fertility in lymphoma survivors treated with chemotherapy: final long-term report of a prospective randomized trial. J. Clin. Oncol. 34: 2568–2574. doi: 10.1200/JCO.2015.65.8864 [DOI] [PubMed] [Google Scholar]
- 7.de Melo A.C., Paulino E., Garces Á.H.I.2017. A review of mTOR pathway inhibitors in gynecologic cancer. Oxid. Med. Cell. Longev. 2017: 4809751. doi: 10.1155/2017/4809751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du X.L., Sheng X.G., Jiang T., Li Q.S., Yu H., Pan C.X., Lu C.H., Wang C., Song Q.Q.2011. Sentinel lymph node biopsy as guidance for radical trachelectomy in young patients with early stage cervical cancer. BMC Cancer 11: 157. doi: 10.1186/1471-2407-11-157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldman K.N., Chenette D., Arju R., Duncan F.E., Keefe D.L., Grifo J.A., Schneider R.J.2017. mTORC1/2 inhibition preserves ovarian function and fertility during genotoxic chemotherapy. Proc. Natl. Acad. Sci. USA 114: 3186–3191. doi: 10.1073/pnas.1617233114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goorin A.M., Schwartzentruber D.J., Devidas M., Gebhardt M.C., Ayala A.G., Harris M.B., Helman L.J., Grier H.E., Link M.P., Pediatric Oncology Group2003. Presurgical chemotherapy compared with immediate surgery and adjuvant chemotherapy for nonmetastatic osteosarcoma: Pediatric Oncology Group Study POG-8651. J. Clin. Oncol. 21: 1574–1580. doi: 10.1200/JCO.2003.08.165 [DOI] [PubMed] [Google Scholar]
- 11.Harrison D.E., Strong R., Sharp Z.D., Nelson J.F., Astle C.M., Flurkey K., Nadon N.L., Wilkinson J.E., Frenkel K., Carter C.S., Pahor M., Javors M.A., Fernandez E., Miller R.A.2009. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460: 392–395. doi: 10.1038/nature08221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ikeda H., Shiojima I., Oka T., Yoshida M., Maemura K., Walsh K., Igarashi T., Komuro I.2011. Increased Akt-mTOR signaling in lung epithelium is associated with respiratory distress syndrome in mice. Mol. Cell. Biol. 31: 1054–1065. doi: 10.1128/MCB.00732-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones E.C., Krohn P.L.1961. The relationships between age, numbers of ocytes and fertility in virgin and multiparous mice. J. Endocrinol. 21: 469–495. doi: 10.1677/joe.0.0210469 [DOI] [PubMed] [Google Scholar]
- 14.Kalich-Philosoph L., Roness H., Carmely A., Fishel-Bartal M., Ligumsky H., Paglin S., Wolf I., Kanety H., Sredni B., Meirow D.2013. Cyclophosphamide triggers follicle activation and “burnout”; AS101 prevents follicle loss and preserves fertility. Sci. Transl. Med. 5: 185ra62. doi: 10.1126/scitranslmed.3005402 [DOI] [PubMed] [Google Scholar]
- 15.Kirchner G.I., Meier-Wiedenbach I., Manns M.P.2004. Clinical pharmacokinetics of everolimus. Clin. Pharmacokinet. 43: 83–95. doi: 10.2165/00003088-200443020-00002 [DOI] [PubMed] [Google Scholar]
- 16.Landa A., Kuller J., Rhee E.2015. Perinatal considerations in women with previous diagnosis of cancer. Obstet. Gynecol. Surv. 70: 765–772. doi: 10.1097/OGX.0000000000000255 [DOI] [PubMed] [Google Scholar]
- 17.Lewis D.R., Seibel N.L., Smith A.W., Stedman M.R.2014. Adolescent and young adult cancer survival. J. Natl. Cancer Inst. Monogr. 2014: 228–235. doi: 10.1093/jncimonographs/lgu019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loren A.W., Mangu P.B., Beck L.N., Brennan L., Magdalinski A.J., Partridge A.H., Quinn G., Wallace W.H., Oktay K., American Society of Clinical Oncology2013. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 31: 2500–2510. doi: 10.1200/JCO.2013.49.2678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Motzer R.J., Escudier B., Oudard S., Hutson T.E., Porta C., Bracarda S., Grünwald V., Thompson J.A., Figlin R.A., Hollaender N., Kay A., Ravaud A., RECORD‐1 Study Group2010. Phase 3 trial of everolimus for metastatic renal cell carcinoma : final results and analysis of prognostic factors. Cancer 116: 4256–4265. doi: 10.1002/cncr.25219 [DOI] [PubMed] [Google Scholar]
- 20.Myers M., Britt K.L., Wreford N.G., Ebling F.J., Kerr J.B.2004. Methods for quantifying follicular numbers within the mouse ovary. Reproduction 127: 569–580. doi: 10.1530/rep.1.00095 [DOI] [PubMed] [Google Scholar]
- 21.Ovarian Cancer Guideline (Version 1. 2016). NCCN Clinical Practice Guidelines in Oncology http://www.nccn.org/professionals/physician_gls/f_guidelines.asp.
- 22.Pareja R., Rendón G.J., Vasquez M., Echeverri L., Sanz-Lomana C.M., Ramirez P.T.2015. Immediate radical trachelectomy versus neoadjuvant chemotherapy followed by conservative surgery for patients with stage IB1 cervical cancer with tumors 2cm or larger: A literature review and analysis of oncological and obstetrical outcomes. Gynecol. Oncol. 137: 574–580. doi: 10.1016/j.ygyno.2015.03.051 [DOI] [PubMed] [Google Scholar]
- 23.Roness H., Kalich-Philosoph L., Meirow D.2014. Prevention of chemotherapy-induced ovarian damage: possible roles for hormonal and non-hormonal attenuating agents. Hum. Reprod. Update 20: 759–774. doi: 10.1093/humupd/dmu019 [DOI] [PubMed] [Google Scholar]
- 24.Roness H., Kashi O., Meirow D.2016. Prevention of chemotherapy-induced ovarian damage. Fertil. Steril. 105: 20–29. doi: 10.1016/j.fertnstert.2015.11.043 [DOI] [PubMed] [Google Scholar]
- 25.Suzuki N.2016. Oncofertility in Japan: advances in research and the roles of oncofertility consortia. Future Oncol. 12: 2307–2311. doi: 10.2217/fon-2016-0187 [DOI] [PubMed] [Google Scholar]
- 26.Townsend J.C., Rideout P., Steinberg D.H.2012. Everolimus-eluting stents in interventional cardiology. Vasc. Health Risk Manag. 8: 393–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsuyoshi H., Orisaka M., Fukuda S., Hattori K., Tsang B.K., Yoshida Y.2015. Protective effect of dienogest on chemotherapy-induced reduced fertility in female rats. Steroids 93: 1–7. doi: 10.1016/j.steroids.2014.10.010 [DOI] [PubMed] [Google Scholar]
- 28.Visser J.A., Durlinger A.L., Peters I.J., van den Heuvel E.R., Rose U.M., Kramer P., de Jong F.H., Themmen A.P.2007. Increased oocyte degeneration and follicular atresia during the estrous cycle in anti-Müllerian hormone null mice. Endocrinology 148: 2301–2308. doi: 10.1210/en.2006-1265 [DOI] [PubMed] [Google Scholar]
- 29.Wethington S.L., Cibula D., Duska L.R., Garrett L., Kim C.H., Chi D.S., Sonoda Y., Abu-Rustum N.R.2012. An international series on abdominal radical trachelectomy: 101 patients and 28 pregnancies. Int. J. Gynecol. Cancer 22: 1251–1257. doi: 10.1097/IGC.0b013e318263eee2 [DOI] [PubMed] [Google Scholar]
- 30.Woodruff T.K.2009. Preserving fertility during cancer treatment. Nat. Med. 15: 1124–1125. doi: 10.1038/nm1009-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X.M., Li L., Xu J.J., Wang N., Liu W.J., Lin X.H., Fu Y.C., Luo L.L.2013. Rapamycin preserves the follicle pool reserve and prolongs the ovarian lifespan of female rats via modulating mTOR activation and sirtuin expression. Gene 523: 82–87. doi: 10.1016/j.gene.2013.03.039 [DOI] [PubMed] [Google Scholar]
- 32.Zhou L., Xie Y., Li S., Liang Y., Qiu Q., Lin H., Zhang Q.2017. Rapamycin Prevents cyclophosphamide-induced Over-activation of Primordial Follicle pool through PI3K/Akt/mTOR Signaling Pathway in vivo. J. Ovarian Res. 10: 56. doi: 10.1186/s13048-017-0350-3 [DOI] [PMC free article] [PubMed] [Google Scholar]


