Highlights
-
•
Currently a vast heterogeneity in synergistic drug action scoring and analysis exists.
-
•
Novel cellular models offer robust ways to identify relevant combination treatments.
-
•
New pharmacological and genetic tools improve odds for effective combination therapies.
Abstract
Treatment of complex diseases such as cancer, cardiovascular disease, diabetes or neurological disorders frequently warrants the utilization of drug combinations for therapeutic intervention. In fact, the most successful example is the current standard of care for HIV patients. However, identification of successful drug cocktails is not a simple task and is hampered by lack of standardization in terminology, experimental protocols and models as well as data analysis. Here we discuss the most recent developments in combinatorial drug screening by covering technological advancements in screening strategies, cellular model systems as well as novel drug classes. We believe the research progress being made provides promising basis to build on and identify, develop and optimize efficacious clinically relevant combinatorial drug treatments.
Current Opinion in Pharmacology 2018, 42:102–110
This review comes from a themed issue on New technologies
Edited by Antoine Bril, Oliver Nosjean, and Patrick Genissel
For a complete overview see the Issue and the Editorial
Available online 5th September 2018
https://doi.org/10.1016/j.coph.2018.07.008
1471-4892/© 2018 The Authors. Published by Elsevier Ltd. This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
The rationale of co-administration of pharmacological agents in the treatment of cancer is that the therapy would lead to enhanced tumor-cell killing, reduced toxicity and avoidance of rapid onset of resistance. There have been ample examples of this approach benefiting patients such as combination chemotherapy for various kinds of leukemias [1] or more recently combinatorial treatment of BRAF inhibitors (e.g. dabrafenib) and MEK inhibitors (e.g. trametinib) of melanoma patients harboring BRAF V600E mutations, a regimen approved by the FDA in 2015 [2,3]. In the past 10 years, we have witnessed tremendous developments in omics technologies, which have characterized driver mutations and pathway addictions in many cancer types [4]. This knowledge has led to identification of novel drug targets and shifted the oncology drug development process from the `one-size-fits-all’ paradigm to personalized medicine approaches optimized based on molecular features of the disease [5]. Despite these advances, numerous challenges remain such as inter-patient and intra-patient genetic heterogeneity, rapid clonal evolution and difficulty to predict drug resistance mechanisms. Addressing these hurdles can be achieved by identification of the most promising drug combinations. However, this is a complex endeavor. Specifically, there is lack of nomenclature standardization defining synergy, reference methodology for screening of drug combinations as well as analysis [6••]. Thus, substantial scientific and technical difficulties have to be overcome that increased clinical translatability of experimentally identified drug combinations is achieved.
Terminology and quantification of drug combinations
Drug combinations are defined as synergistic, additive or antagonistic, depending on the divergence of the measured drug combination response from the null hypothesis (effect of non-interaction). Synergy is identified when two compounds increase each other’s effectiveness by more than the sum of their single agent responses. The advantage of designing treatment regimens of synergistic drug pairs in particular disease settings provides the opportunity to lower the dosage of the individual agents, thereby reducing toxicity while maintaining the wanted effect on the cancer cell. Synergistic effects can occur as a result of complementary drug action (different targets sites on the same protein or pathway are hit), anti-counteractive actions (one drug affects the biological response of the other one), or facilitation actions (one drug potentiates the effect of the other) [7,8]. In contrast, a drug combination is considered to be additive when the effect of each drug neither masks nor increases the efficacy of the other drug. This is also known as non-interaction and basically denotes the effect that is expected or measured when the combination of multiple drugs leads to no synergy. Lastly, a drug combination is antagonistic when the combined activity of the drugs is lower than the response of the individual agents [9]. Substantial deviation of additivity is habitually characterized as either synergy or antagonism, depending on the direction of deviation [10•].
Several models exist to simulate dose-response curves and quantify the level of drug synergy between two or more compounds such as: the highest single agent model (HAS) [11], the Loewe additivity model [12], the Bliss independence model [13], and the Chou-Talalay method [14, 15, 16] (Table 1). Each of these models has its associated limitations and many are not entirely suited for high-throughput experiments or combinations comprising more than two drugs. Moreover, it is challenging to determine the most appropriate reference model, as it is not as simple as 1 + 1 = 2. Numerous aspects need to be considered such as drug metabolism, specific target engagement as well as adverse effects, which could influence the interaction of the individual agents being used [10•]. Hence, no existing method can assess synergy under all possible experimental conditions. In fact, different methods applied to the same data may even give divergent results [17]. Historically clinically successful drug combinations have been identified and used to treat infectious diseases such as human immunodeficiency virus (HIV), but currently more and more drug combinations are being evaluated preclinically and clinically for different cancer types (Figure 1).
Table 1.
The most commonly utilized drug synergy detection and quantification methods
| Concept | Assumptions | Equations | Limitations | Reference | |
|---|---|---|---|---|---|
| Bliss independence | Idea of no interaction (each drug is acting independently of one another) | (i) The drug effect achieved by the probability that two drugs do not interfere with each other (ii) The combined drugs have a different site/ mechanism of action in achieving the effect (iii) Drugs have exponential dose–effect curves |
EA + EB(1 − EA) = EA + EB − EAEB CI = (EA + EB − EA x EB)/EAB |
Model is applicable solely to effects expressed as probabilities between 0 and 1 In many drug interactions drug dependence cannot be excluded The model must be applicable along the entire dose response curve The model does not work in a `sham mixture’ situation |
[13] |
| Loewe additivity | `Sham mixture’ — no expectation of any type of interaction | (i) Drug cannot interact with itself (ii) Constant potency ratio at all doses (iii) Equal individual drug maximum effects |
EAB = EA(a + ab) = EB(ba + b) a + ab = A ↔ a + b x R = A ↔ a + b x A/B = A a/A + b/B = 1 CI = a/A + b/B |
Relies on precisely estimated dose–effect curves — thus not applicable when a dose-effect curve is not available R is not always constant and is rather the exception |
[12] |
| Highest single agent (HAS) | The resulting effect of a drug combination is superior than the effects achieved by the individual drugs | Synergistic drug combination should produce additional result on top of what its components can produce alone |
EAB = max(EA,EB) CI = max(EA,EB)/EAB |
Often a drug combined with itself can produce an excess over HAS Frequently fails to establish an improved drug combination effect in comparison to the expected additive effect of its components Any extra effect over the higher single drug will be considered as synergy |
[11] |
| Chou-Talalay | Based on the median-effect equation and mass-action law | Drugs should have a constant potency ratio |
fa/fu = (D/Dm)m* CI = D1/E1 + D2/E2 |
Dose response curves are primarily non-linear, thus difficult to correctly calculate the median effect dose and the sigmoidicity of the dose–effect curve | [14, 15, 16] |
a + ab: dose a giving the effect EAB; ba + b: dose b giving the effect EAB; CI: combination index (CI > 1 antagonism; CI = 1 additivity; CI < 1 synergy); D: dose of the drug given; Dm: median-effect dose; D1 and D2: actual drug doses used; EA: effect of drug A; EB: effect of drug B; EAB: effect of the combination of drug A and drug B; E1 and E2: theoretically individual drug levels expected to be required to produce the experimentally measured effect; fa: fraction of cells killed; fu: fraction of living cells; m: sigmoidicity of the dose–effect curve; R: potency ratio; `Sham mixture’: drug mixed with itself; *: median effect equation.
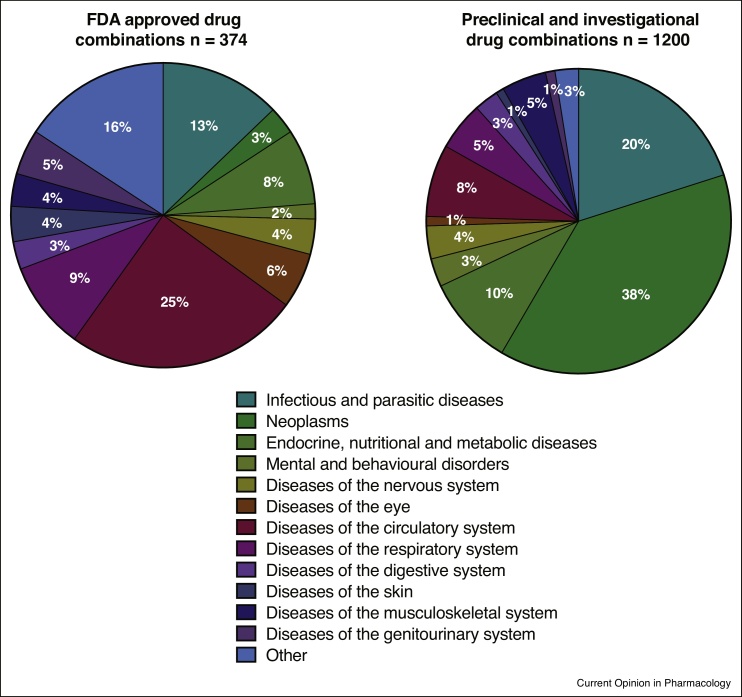
Figure 1.
Distribution of approved, preclinical and investigational drug combinations per disease area. The pie charts illustrate that currently most of the approved drug combinations are for treatment of infectious diseases (e.g. HIV, tuberculosis) whereas much of the research and development is targeting different cancer types. Data is retrieved from the Drug Combination Database (http://www.cls.zju.edu.cn/dcdb/) [52].
The concept of independent drug action as an alternative to drug synergy
One of the difficulties in translating experimentally identified effective drug therapies is cell-to-cell heterogeneity within a patient as well as the patient-to-patient variability in response to drugs. These factors can cause development of drug resistance, disease progression, and make the effectiveness of treatment challenging to envisage even for patients. The solution to these issues has been seen in combinatorial drug treatment with increased chances of at least one drug eliciting the wanted effect. This phenomenon is known as ‘independent action’ and defined as the response of a patient to the combination therapy equivalent to the response to the more efficacious drug alone with no added advantage from the less effective drug [18•]. Hence, it is imperative to make a difference between therapeutic benefit of a drug combination through drug interaction (e.g. additivity or synergy) versus drug independence. In the first case, benefit is most likely observed as a result of drug interaction within cancer cells, whereas in the second case benefit results from variability of drug response in patient populations [18•].
By analyzing available clinical trial data comparing mono-therapy and combinatorial therapy, Palmer and Sorger concluded that for numerous effective and approved combinations the benefit observed in patients is likely due to independent drug action rather than drug additivity/synergy [18•]. For example, the results of a recent phase III clinical trial for melanoma patients comparing the effects of single agent ipilimumab (anti-CTLA-4) and nivolumab (anti-PD-1) as well as the combination illustrated that the positive outcome observed in patients treated with combination was due to the concept of independent action [18•,19]. Nonetheless, it is important to note that therapeutic benefit as a consequence of independent action in patients treated with a combination of drugs can still be clinically and statistically significant relative to the outcome of treatment with single agents. Thus, in applicable cases it would be imperative to optimize drug combinations based on boosting the likelihood of the effect of at least one drug.
Current and novel methodology for combinatorial drug therapy identification
The predominant approach for combinatorial drug screening is testing the effect of two different drugs in dose-response matrices (Figure 2a). This experimental setup allows for generation of single drug dose response data as well as systematic assessment of the effect of all possible tested combination concentrations for each drug pair. This method is amenable to high-throughput screening in vitro enabling the assessment of up to 6 different drug combinations in one 384 multi-well plate using and 8 × 8 dose matrix format. Recent studies utilized this methodology to identify combination partners to ibrutinib in hematological malignancies [20], explore novel drug combinations for Ewing Sarcoma [21] and for novel targeting approaches of androgen receptor signaling [22]. Importantly, small-scale or hypothesis-driven drug combinations can be tested in animal models of disease (e.g. recent studies; [23,24]), but might require in vitro pre-testing in cell lines to determine effective drug concentrations as a dose matrix setup is practically unfeasible in vivo. Currently, it is still cost-prohibitive as well as unfeasible to test for all possible drug combinations for a particular disease. In fact, identification of effective drug combinations is rather the exception than the rule and a process directed by serendipity as well as trial and error. These limitations necessitate the incorporation of systematic drug synergy prediction models as well as the utilization of novel genetic screening methodology and cellular model systems to quicken hypothesis generation and direct research undertakings. Several of these recent developments will be discussed in further detail in the following paragraphs.
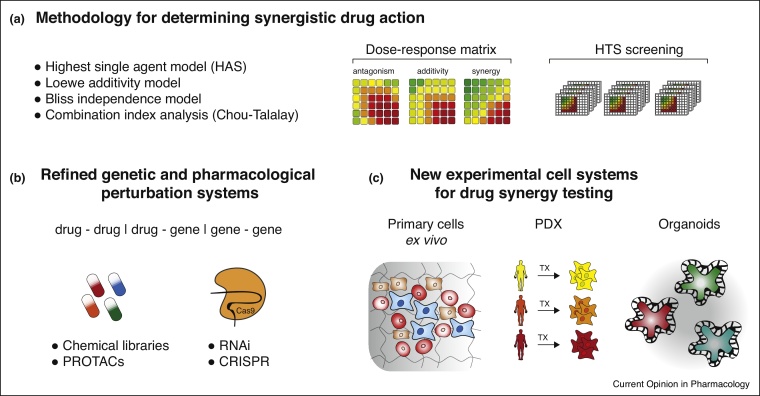
Figure 2.
Drug synergism analysis and technological advancements for drug combination screening. (a) Different methodologies for the statistical analysis and scoring of synergist drug effects, (b) functional genetic tools and novel drug candidates for the identification of combinatorial drug effects. RNAi: RNA interference, CRISPR: clustered regularly interspaced short palindromic repeats, PROTAC: proteolysis targeting chimera compounds, (c) novel cellular model systems for screening and identification of synergistic drug combinations, TX: transplant.
In recent years, the fields of functional genomic and genetic screening have been revolutionized both by improvements in RNA interference (RNAi) technology as well as the wide-spread adaption of clustered regularly interspaced short palindromic repeats (CRISPR) systems [25] (Figure 2b). Cell survival screens, performed in a genome-wide manner (‘essentialomes’), are uncovering novel cancer targets and provide a means to instruct inhibitor design as well as select combinatorial treatments for in vivo testing [26, 27, 28]. Especially CRISPR-based screening has recently been extended to combinatorial gene knock-out screening using multiple gene-targeting single guide RNA (sgRNAs) within the same cell [29]. A study by Han et al. has utilized this approach to screen for effective drug combinations of 207 possible drug targets in the BCR-ABL1 expressing chronic myeloid leukemia (CML) cell line K-562 [30••]. This enabled the generation of a genetic interaction map identifying contextual synthetic lethal gene pairs in BCR-ABL1-driven disease that could be efficaciously phenocopied by using existing drug candidates (e.g. BCL2 and MCL1 inhibitor) [30••]. Thus, this strategy has the potential of identifying clinically actionable drug-combination with genetic precision in defined disease models highlighting potentially promising drug combinations for compound development and in vivo testing.
Another way whereby CRISPR technology can be instrumental for the identification of drug combinations is carrying out screens in defined genetic cellular backgrounds, thereby identifying specific pharmacologically exploitable vulnerabilities in molecularly defined disease entities. The study by Wang et al. has utilized CRISPR-based essentiality screening in RAS mutant and wild-type acute myeloid leukemia (AML) cell lines to identify synthetic lethal gene dependencies. RAS mutant cell lines were found to be highly sensitive to inhibition of the group I p21 activated serine/threonine kinases (PAK1-3) potentially guiding new combinatorial treatments [31]. Similar studies have been performed using RNAi technology and identified a specific vulnerability of KRAS-mutant lung cancer to combined inhibition of MEK1/2 and fibroblast growth factor receptor (FGFR) signaling employing trametinib and ponatinib [32].
In general, functional genetic screening represents a powerful means for optimizing combinatorial cancer treatment as it (i) detects drug sensitizers with high accuracy, (ii) is highly scalable in robust cellular culture systems and (iii) allows to map mutation-cooperation sensitivity landscapes in different cancer types and/or diseases thereby guiding the development of combination treatment strategies capable of overcoming drug resistance.
Novel cellular systems for the identification of drug combinations
As previously mentioned, high-throughput drug sensitivity profiling in large cell line collections has traditionally been utilized to identify single agent and combination treatments for different cancer entities [33,34]. However, these efforts often suffered from difficulties translating obtained findings into clinical therapeutic routine [35]. In order to move (combinatorial) drug sensitivity assessment closer to the patient and optimize personalized treatment strategies influential studies have been performed focusing on primary hematopoietic malignancy samples. Given that the cancer is easily accessible, ex vivo drug sensitivity testing using cell viability readouts of single agent and combination treatment is readily feasible [36,37•] (Figure 2c). For example, the study by Pemovska et al. has demonstrated that the combination treatment using dasatinib, sunitinib and temsirolimus can induce clinical responses and complete remissions in a subgroup of AML patients albeit with short duration [36]. In a more recent study, Tyner and colleagues have specifically looked for drug combination effects in a broad panel of myeloid and lymphoid leukemia derived ex vivo samples. They found several translatable compound combinations with BCL2 inhibitor-based regimens preferentially enriched in myeloid malignancies and PI3K and bromodomain inhibitor-based treatments in lymphoid cancers [37•].
However, liquid biopsies rarely represent homogenous defined cell populations and rather are composed of a complex mixture of various different cell types, including healthy and diseased subclones. In an attempt to address this heterogeneity, a novel approach has been developed based on high-content fluorescent microscopy combined with pharmacological perturbation, called pharmacoscopy [38]. The hallmark feature of this new screening approach is the integration of total cell counts with the abundance measurement of different marker-stained positive subpopulations after drug perturbation or control treatment. Scoring the survival of the CD34+ CD117+ cellular fractions in de novo AML upon etoposide, daunorubicin and cytarabine combination treatment allowed for clear distinction between responders and non-responders in comparison to overall cell survival measurement [39•]. Furthermore, targeted single and combination treatment in lymphoid malignancies guided by image-based screening results led to prolonged progression-free survival compared to the most recent therapeutic regimen [39•].
Although in the case of hematopoietic malignancies access to tumor cells for ex vivo drug combination testing is readily achievable, obtaining and propagating solid tumors with their cellular complexity and three-dimensional architecture has been difficult to achieve under laboratory culture conditions. To address some of these limitations, patient derived xenograft (PDX) models have been developed that enable to preserve and provide niche factors and structural tissue architecture upon successful engraftment [40,41] (Figure 2c). Such models have been established for various different cancer types and tissues allowing for the identification of efficient combination treatments, as exemplified by the administration of the BRAF inhibitor encorafenib and inhibitor of cell cycle regulatory kinases CDK4/6 LEE011 for colorectal cancer (CRC) and non-small cell lung carcinoma (NSCLC) PDX models [42]. One important restrictive factor of PDX models in comparison to ex vivo drug sensitivity testing as described before is the duration from tumor sampling to obtaining drug sensitivity results. Whereas in ex vivo screening settings, results can ideally be provided to the treating physician within one week, PDX approaches face a more prolonged time span in assessing drug response patterns. Another limiting factor in light of discovering novel synergistic drug pairings is the number of drugs that can be tested at the same time.
Facing these challenges, the development of organoid culture systems has provided tremendous progress in establishing new platforms for the testing of drug action in patient derived solid cancer tissue biopsies like esophagus, stomach, liver, pancreas, colon or breast [43] (Figure 2c). Clevers and colleagues have built one of the first CRC-derived cancer organoid biobanks that can be utilized for compound screening [44••]. A recent study performed by Jabs et al. has employed high-content fluorescent microscopy to study the effects of single agent and combination treatment in ovarian cancer derived cell monolayers and organoids emphasizing the importance of differential culture conditions on drug response [45]. Whereas most of the aforementioned novel culture systems have relied on small molecule libraries of variable size, novel compound classes and therapeutic agents will be of great relevance. With the advent and first clinical approval of many new immunotherapeutic antibodies, complex ex vivo or organoid-derived culture systems might be able to capture the cellular complexity necessary to answer the question how to most beneficially combine these new different therapeutic modalities [38,46].
Novel pharmacological agents for combination therapy
Pharmacological exploration of the ubiquitin-proteasome system has been a long-standing scientific effort pioneered by the introduction of bortezomib and newer generations of proteasome inhibitors into the clinical routine [47]. An exciting new field of drug development that will undoubtedly offer new ways for combination therapy has been sparked by the discovery and development of proteolysis targeting chimera (PROTAC) compounds (Figure 2b). These act by drug-induced recruitment of E3 ubiquitin ligase complexes to induce target protein degradation [48]. This novel drug class has specifically been inspired by the discovery of the molecular mode of action of IMiDs (immunomodulatory imide drugs; e.g. thalidomide). Utilization of phthalimide conjugation to transform targeting-binding drug molecules into selective target protein degrading agents sparked the development of an entirely novel class of (anti-cancer) agents [49,50]. In the future it will be of great interest to uncover how these agents can be used in conjunction with already approved agents as well as with immunotherapeutic modalities to improve efficiency and durability of combination therapy [51].
Conclusion
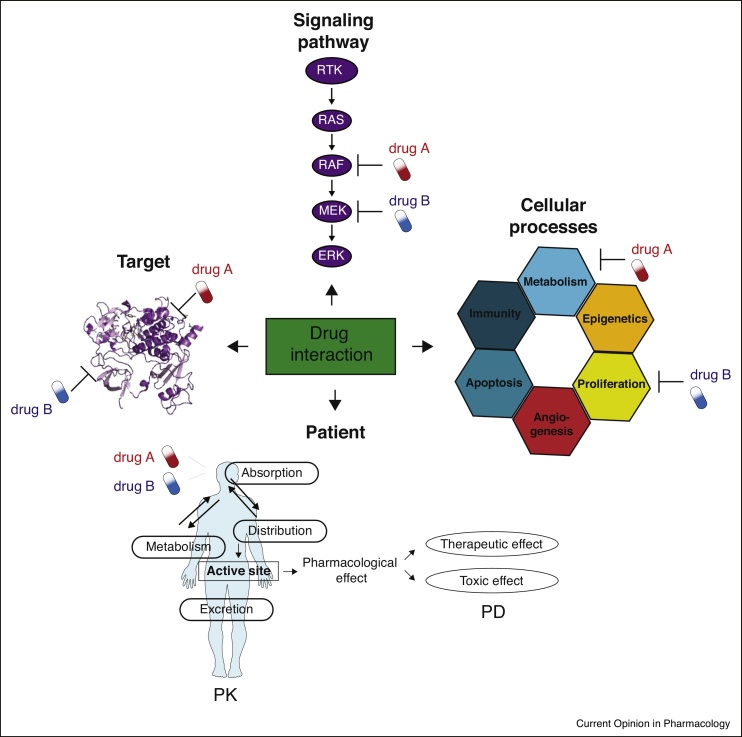
With this concise review, we call on further standardization of the nomenclature and definition of drug synergy scoring as well as harmonization of drug combination screening and analysis protocols to enhance comparability between different analysis platforms. Technological advancements in screening strategies (e.g. CRISPR genome editing), development of novel drug classes (e.g. immunotherapeutics, PROTACs, etc.) and further optimization of cellular, animal and primary models (e.g. organoids, PDXs, liquid biopsies) hold great promise to uncover highly efficacious and translatable combinatorial therapies that will pave the way to next generation cancer treatments. Last but not least, it is important to note that drug-drug interactions, whether synergistic or antagonistic, can occur at the level of target, pathway, and process and organism (e.g. patient; Figure 3). Thus, in later stages of preclinical or early clinical testing pharmacodynamics and pharmacokinetics of the prioritized drug combinations need to be considered for optimization of successful clinically utilizable therapies.
Figure 3.
Different levels of drug-drug interactions. The figure shows that the effect of combining two drugs can be elicited at the level of target (drugs targeting different sites within the same target via similar or different mechanisms), pathway (drugs targeting different signaling proteins within the same cascade), processes (drugs targeting different processes contributing to the disease phenotype) and patient (where the effect of the drug combination will depend on how the drugs will influence each other’s ADME properties and pharmacological effects).
Conflict of interest statement
GS-F is a shareholder of Allcyte, has a patent WO2016046346 licensed to Allcyte, and is a scientific cofounder of Allcyte. All other authors declare no competing interests.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as
• of special interest
•• of outstanding interest
Acknowledgements
We acknowledge funding from the Austrian Academy of Sciences, an ERC GAME of GATES Advanced Investigator Grant (GS-F), Austrian Science Fund grant F4711-B20 (GS-F) and an EMBO long-term Fellowship (TP ALTF 733-2016).
References
- 1.Frei E., Holland J.F., Schneiderman M.A., Pinkel D., Selkirk G., Freireich E.J., Silver R.T., Gold G.L., Regelson W. A comparative study of two regimens of combination chemotherapy in acute leukemia. Blood. 1958;13:1126–1148. [PubMed] [Google Scholar]
- 2.Chabner B.A., Roberts T.G. Timeline: chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65–72. doi: 10.1038/nrc1529. [DOI] [PubMed] [Google Scholar]
- 3.Spain L., Julve M., Larkin J. Combination dabrafenib and trametinib in the management of advanced melanoma with BRAFV600 mutations. Expert Opin Pharmacother. 2016;17:1031–1038. doi: 10.1517/14656566.2016.1168805. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence M.S., Stojanov P., Mermel C.H., Robinson J.T., Garraway L.A., Golub T.R., Meyerson M., Gabriel S.B., Lander E.S., Getz G. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Al-Lazikani B., Banerji U., Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30:679–692. doi: 10.1038/nbt.2284. [DOI] [PubMed] [Google Scholar]
- 6••.Foucquier J., Guedj M. Analysis of drug combinations: current methodological landscape. Pharmacol Res Perspect. 2015;3:e00149. doi: 10.1002/prp2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review on the most prevalent analysis methods for drug combinatorial screening providing detailed overview of nomenclature, methodology landscapes as well as analysis pitfalls.
- 7.Jia J., Zhu F., Ma X., Cao Z., Cao Z.W., Li Y., Li Y.X., Chen Y.Z. Mechanisms of drug combinations: interaction and network perspectives. Nat Rev Drug Discov. 2009;8:111–128. doi: 10.1038/nrd2683. [DOI] [PubMed] [Google Scholar]
- 8.Sun X., Vilar S., Tatonetti N.P. High-throughput methods for combinatorial drug discovery. Sci Transl Med. 2013;5 doi: 10.1126/scitranslmed.3006667. 205rv1–205rv1. [DOI] [PubMed] [Google Scholar]
- 9.Gibbs B.K., Sourbier C. Detecting the potential pharmacological synergy of drug combination by viability assays in vitro. Methods Mol Biol. 2018;1709:129–137. doi: 10.1007/978-1-4939-7477-1_10. [DOI] [PubMed] [Google Scholar]
- 10•.Roell K.R., Reif D.M., Motsinger-Reif A.A. An introduction to terminology and methodology of chemical synergy-perspectives from across disciplines. Front Pharmacol. 2017;8:158. doi: 10.3389/fphar.2017.00158. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors review the different terminology that exists in terms of drug combination effects in various fields and cover the most abundant analysis methods with their advantages and disadvantages.
- 11.Berenbaum M.C. What is synergy? Pharmacol Rev. 1989;41:93–141. [PubMed] [Google Scholar]
- 12.Loewe S. The problem of synergism and antagonism of combined drugs. Arzneimittelforschung. 1953;3:285–290. [PubMed] [Google Scholar]
- 13.Bliss C.I. The toxicity of poisons applied jointly. Annals Appl Biol. 1939;26:585–615. [Google Scholar]
- 14.Chou T.-C. Theoretical basis, experimental design, and computerized simulation of synergism and antagonism in drug combination studies. Pharmacol Rev. 2006;58:621–681. doi: 10.1124/pr.58.3.10. [DOI] [PubMed] [Google Scholar]
- 15.Chou T.-C. Drug combination studies and their synergy quantification using the Chou–Talalay method. Cancer Res. 2010;70:440–446. doi: 10.1158/0008-5472.CAN-09-1947. [DOI] [PubMed] [Google Scholar]
- 16.He L., Kulesskiy E., Saarela J., Turunen L., Wennerberg K., Aittokallio T., Tang J. Methods for high-throughput drug combination screening and synergy scoring. Methods Mol Biol. 2018;1711:351–398. doi: 10.1007/978-1-4939-7493-1_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doern C.D. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol. 2014;52:4124–4128. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18•.Palmer A.C., Sorger P.K. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017;171:1678–1691.e13. doi: 10.1016/j.cell.2017.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; A compelling theory article putting forward the concept that clinical effectiveness of combinatorial drug treatment may be robustly forecasted without drug synergy.
- 19.Larkin J., Chiarion-Sileni V., Gonzalez R., Grob J.J., Cowey C.L., Lao C.D., Schadendorf D., Dummer R., Smylie M., Rutkowski P. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N Engl J Med. 2015;373:23–34. doi: 10.1056/NEJMoa1504030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schaffer M., Chaturvedi S., Davis C., Aquino R., Stepanchick E., Versele M., Liu Y., Yang J., Lu R., Balasubramanian S. Identification of potential ibrutinib combinations in hematological malignancies using a combination high-throughput screen. Leuk Lymphoma. 2018;59:931–940. doi: 10.1080/10428194.2017.1349899. [DOI] [PubMed] [Google Scholar]
- 21.Radic-Sarikas B., Tsafou K.P., Emdal K.B., Papamarkou T., Huber K.V.M., Mutz C., Toretsky J.A., Bennett K.L., Olsen J.V., Brunak S. Combinatorial drug screening identifies ewing sarcoma-specific sensitivities. Mol Cancer Ther. 2017;16:88–101. doi: 10.1158/1535-7163.MCT-16-0235. [DOI] [PubMed] [Google Scholar]
- 22.Licciardello M.P., Ringler A., Markt P., Klepsch F., Lardeau C.-H., Sdelci S., Schirghuber E., Müller A.C., Caldera M., Wagner A. A combinatorial screen of the CLOUD uncovers a synergy targeting the androgen receptor. Nat Chem Biol. 2017;13:771–778. doi: 10.1038/nchembio.2382. [DOI] [PubMed] [Google Scholar]
- 23.Simmons J.K., Michalowski A.M., Gamache B.J., DuBois W., Patel J., Zhang K., Gary J., Zhang S., Gaikwad S., Connors D. Cooperative targets of combined mTOR/HDAC Inhibition promote MYC degradation. Mol Cancer Ther. 2017;16:2008–2021. doi: 10.1158/1535-7163.MCT-17-0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beug S.T., Beauregard C.E., Healy C., Sanda T., St-Jean M., Chabot J., Walker D.E., Mohan A., Earl N., Lun X. Smac mimetics synergize with immune checkpoint inhibitors to promote tumour immunity against glioblastoma. Nat Commun. 2017;8 doi: 10.1038/ncomms14278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barrangou R., Doudna J.A. Applications of CRISPR technologies in research and beyond. Nat Biotechnol. 2016;34:933–941. doi: 10.1038/nbt.3659. [DOI] [PubMed] [Google Scholar]
- 26.Wang T., Birsoy K., Hughes N.W., Krupczak K.M., Post Y., Wei J.J., Lander E.S., Sabatini D.M. Identification and characterization of essential genes in the human genome. Science. 2015;350:1096–1101. doi: 10.1126/science.aac7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hart T., Chandrashekhar M., Aregger M., Steinhart Z., Brown K.R., MacLeod G., Mis M., Zimmermann M., Fradet-Turcotte A., Sun S. High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell. 2015;163:1515–1526. doi: 10.1016/j.cell.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 28.Meyers R.M., Bryan J.G., McFarland J.M., Weir B.A., Sizemore A.E., Xu H., Dharia N.V., Montgomery P.G., Cowley G.S., Pantel S. Computational correction of copy number effect improves specificity of CRISPR–Cas9 essentiality screens in cancer cells. Nat Genet. 2017;49:1779–1784. doi: 10.1038/ng.3984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Najm F.J., Strand C., Donovan K.F., Hegde M., Sanson K.R., Vaimberg E.W., Sullender M.E., Hartenian E., Kalani Z., Fusi N. Orthologous CRISPR–Cas9 enzymes for combinatorial genetic screens. Nat Biotechnol. 2018;36:179–189. doi: 10.1038/nbt.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30••.Han K., Jeng E.E., Hess G.T., Morgens D.W., Li A., Bassik M.C. Synergistic drug combinations for cancer identified in a CRISPR screen for pairwise genetic interactions. Nat Biotechnol. 2017;35:463–474. doi: 10.1038/nbt.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]; A key paper demonstrating the utility of CRISPR technology in screening and identification of promising clinically relevant drug combinations in defined cellular genetic backgrounds.
- 31.Wang T., Yu H., Hughes N.W., Liu B., Kendirli A., Klein K., Chen W.W., Lander E.S., Sabatini D.M. Gene essentiality profiling reveals gene networks and synthetic lethal interactions with oncogenic Ras. Cell. 2017;168:890–903.e15. doi: 10.1016/j.cell.2017.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manchado E., Weissmueller S., Morris J.P., Chen C.-C., Wullenkord R., Lujambio A., de Stanchina E., Poirier J.T., Gainor J.F., Corcoran R.B. A combinatorial strategy for treating KRAS-mutant lung cancer. Nature. 2016;534:647–651. doi: 10.1038/nature18600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barretina J., Caponigro G., Stransky N., Venkatesan K., Margolin A.A., Kim S., Wilson C.J., Lehar J., Kryukov G.V., Sonkin D. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garnett M.J., Edelman E.J., Heidorn S.J., Greenman C.D., Dastur A., Lau K.W., Greninger P., Thompson I.R., Luo X., Soares J. Systematic identification of genomic markers of drug sensitivity in cancer cells. Nature. 2012;483:570–575. doi: 10.1038/nature11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lieu C.H., Tan A.-C., Leong S., Diamond J.R., Eckhardt S.G. From bench to bedside: lessons learned in translating preclinical studies in cancer drug development. J Natl Cancer Inst. 2013;105:1441–1456. doi: 10.1093/jnci/djt209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pemovska T., Kontro M., Yadav B., Edgren H., Eldfors S., Szwajda A., Almusa H., Bespalov M.M., Ellonen P., Elonen E. Individualized systems medicine strategy to tailor treatments for patients with chemorefractory acute myeloid leukemia. Cancer Discov. 2013;3:1416–1429. doi: 10.1158/2159-8290.CD-13-0350. [DOI] [PubMed] [Google Scholar]
- 37•.Kurtz S.E., Eide C.A., Kaempf A., Khanna V., Savage S.L., Rofelty A., English I., Ho H., Pandya R., Bolosky W.J. Molecularly targeted drug combinations demonstrate selective effectiveness for myeloid- and lymphoid-derived hematologic malignancies. Proc Natl Acad Sci U S A. 2017;114:E7554–E7563. doi: 10.1073/pnas.1703094114. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show how primary liquid biopsies of patient with hematological malignancies can be used to screen for combinations of drugs that affect specific cell signaling pathways.
- 38.Vladimer G.I., Snijder B., Krall N., Bigenzahn J.W., Huber K.V.M., Lardeau C.-H., Sanjiv K., Ringler A., Berglund U.W., Sabler M. Global survey of the immunomodulatory potential of common drugs. Nat Chem Biol. 2017;13:681–690. doi: 10.1038/nchembio.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39•.Snijder B., Vladimer G.I., Krall N., Miura K., Schmolke A.-S., Kornauth C., Lopez de la Fuente O., Choi H.-S., van der Kouwe E., Gültekin S. Image-based ex-vivo drug screening for patients with aggressive haematological malignancies: interim results from a single-arm, open-label, pilot study. Lancet Haematol. 2017;4:e595–e606. doi: 10.1016/S2352-3026(17)30208-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors report a novel imaging-based drug screening platform and its applicability to screen for effective drug combinations in patients with hematological malignancies as well as separate responding from non-responding AML patients to induction chemotherapy regimens.
- 40.Tentler J.J., Tan A.-C., Weekes C.D., Jimeno A., Leong S., Pitts T.M., Arcaroli J.J., Messersmith W.A., Eckhardt S.G. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrne A.T., Alférez D.G., Amant F., Annibali D., Arribas J., Biankin A.V., Bruna A., Budinská E., Caldas C., Chang D.K. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254–268. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
- 42.Gao H., Korn J.M., Ferretti S., Monahan J.E., Wang Y., Singh M., Zhang C., Schnell C., Yang G., Zhang Y. High-throughput screening using patient-derived tumor xenografts to predict clinical trial drug response. Nat Med. 2015;21:1318–1325. doi: 10.1038/nm.3954. [DOI] [PubMed] [Google Scholar]
- 43.Drost J., Clevers H. Organoids in cancer research. Nat Rev Cancer. 2018;65:87. doi: 10.1038/s41568-018-0007-6. [DOI] [PubMed] [Google Scholar]
- 44••.van de Wetering M., Francies H.E., Francis J.M., Bounova G., Iorio F., Pronk A., van Houdt W., van Gorp J., Taylor-Weiner A., Kester L. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]; A seminal paper showing the feasibility of combinatorial drug screening in living organoid samples of colorectal cancer patients.
- 45.Jabs J., Zickgraf F.M., Park J., Wagner S., Jiang X., Jechow K., Kleinheinz K., Toprak U.H., Schneider M.A., Meister M. Screening drug effects in patient-derived cancer cells links organoid responses to genome alterations. Mol Syst Biol. 2017;13:955. doi: 10.15252/msb.20177697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zappasodi R., Merghoub T., Wolchok J.D. Emerging concepts for immune checkpoint blockade-based combination therapies. Cancer Cell. 2018;33:581–598. doi: 10.1016/j.ccell.2018.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nalepa G., Rolfe M., Harper J.W. Drug discovery in the ubiquitin–proteasome system. Nat Rev Drug Discov. 2006;5:596–613. doi: 10.1038/nrd2056. [DOI] [PubMed] [Google Scholar]
- 48.Sakamoto K.M., Kim K.B., Kumagai A., Mercurio F., Crews C.M., Deshaies R.J. Protacs: chimeric molecules that target proteins to the Skp1–Cullin–F box complex for ubiquitination and degradation. Proc Natl Acad Sci U S A. 2001;98:8554–8559. doi: 10.1073/pnas.141230798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Winter G.E., Buckley D.L., Paulk J., Roberts J.M., Souza A., Dhe-Paganon S., Bradner J.E. Drug development. Phthalimide conjugation as a strategy for in vivo target protein degradation. Science. 2015;348:1376–1381. doi: 10.1126/science.aab1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bondeson D.P., Crews C.M. Targeted protein degradation by small molecules. Annu Rev Pharmacol Toxicol. 2017;57:107–123. doi: 10.1146/annurev-pharmtox-010715-103507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moser S.C., Voerman J.S.A., Buckley D.L., Winter G.E., Schliehe C. Acute pharmacologic degradation of a stable antigen enhances its direct presentation on MHC class I molecules. Front Immunol. 2017;8:1920. doi: 10.3389/fimmu.2017.01920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu Y., Wei Q., Yu G., Gai W., Li Y., Chen X. DCDB 2.0: a major update of the drug combination database. Database (Oxford) 2014;2014:bau124. doi: 10.1093/database/bau124. [DOI] [PMC free article] [PubMed] [Google Scholar]