Abstract
Background:
The association between obesity and rising incidence of hepatocellular carcinoma (HCC) in the United States has been documented; however, the role of bariatric surgery remains less clear.
Aim:
To evaluate the cross-sectional association of prior-bariatric surgery and HCC.
Methods:
The United States Nationwide Inpatient Sample (NIS) database was queried from 2004–2014 for discharges with a diagnosis of morbid obesity. Primary outcomes of interest were HCC and in-hospital mortality rate. Secondary outcomes were length of stay and cost. Baseline characteristics were balanced using propensity score matching (PSM). Using Poisson and logistic regressions, adjusted HCC prevalence ratio (PR) and mortality odds ratio (OR) were derived in patients with prior-bariatric surgery compared to those without bariatric surgery.
Results:
Of the 2,881,414 patients included in our study, 267,082 (9.3%) underwent bariatric surgery. From 2004–2014, there was a 3-fold increase in age-adjusted prevalence of HCC from 27 per 100,000 to 72 per 100,000 (PTrend<0.001). After PSM, 230,956 patients with prior-bariatric surgery were matched with 230,956 patients without bariatric surgery. Prior-bariatric surgery was associated with lower prevalence of HCC (PR 0.11; 95% CI, 0.03–0.48; P<0.001). In-hospital mortality was also lower for patients with surgery (OR 0.22; 95% CI, 0.20–0.26; P<0.001). The occurrence of HCC added $ 18,840 extra cost, increased mean length of stay by 2 (95% CI; 1–3) days and increased risk of death by 65% (aOR 1.65; 95% CI 1.18–2.29).
Conclusion:
In this nationwide study of morbidly obese patients, prior-bariatric surgery was associated with a lower prevalence of HCC and lower in-patient mortality.
Keywords: Obesity, Bariatric Surgery, Weight Loss, Hepatocellular Carcinoma (HCC)
INTRODUCTION
Within the last decade, hepatocellular carcinoma (HCC) has become one of the most rapidly rising causes of cancer-related death in the United States.1–3 The global rise in HCC is thought to be related to hepatitis C virus (HCV) infection; however, the concurrent intensification of the obesity epidemic, for which broadly definitive therapy is lacking, may also be a significant contributor.4–6 Although the introduction of direct-acting antiviral therapy has ushered in an era of well-tolerated and successful HCV treatments, a well-established treatment paradigm for the soaring incidence of obesity has not yet been conceived.
Obesity is recognized as a significant risk factor for various types of cancer, including HCC.7–9 Currently, United States cancer prevention guidelines include recommendations for weight loss; however, it remains unclear whether intentional weight reduction truly reduces cancer risk.10 While dietary modification and exercise are the first-line treatments available to promote weight loss in the general population, these approaches are generally unsuccessful in achieving ideal weight loss targets and sustained weight loss over time. At present, bariatric surgery provides the highest level of weight loss and improvement in weight-related comorbidities as compared to other available methods of weight reduction.11,12
While there have been population-based studies suggesting a link between obesity and development of HCC, few, if any large-scale studies have investigated the impact of weight loss surgery on clinically relevant cancer-related outcomes.5,7–9,13,14 For this reason, it is critical to investigate the potential role of bariatric surgery for cancer prevention including HCC. Therefore, we utilized data from the United States Nationwide Inpatient Sample (NIS) registry to perform a propensity score matched analysis of the relation of prior bariatric surgery and HCC. We hypothesized that bariatric surgery would be associated with a lower prevalence for HCC and improved inpatient survival for these patients.
METHODS
Data Source and Study Population
The study sample originated from the NIS database, which includes hospitalized patients in the United States during the 2004 to 2014 period. This registry is part of the Healthcare Cost and Utilization Project, sponsored by the Agency for Healthcare Research and Quality.15 The NIS is a database of hospital inpatient stays derived from billing data submitted by hospitals to statewide data organizations across the United States. Inpatient data includes clinical and resource use information typically available from discharge abstracts. Each discharge is coded with a principal diagnosis for that specific hospitalization in addition to the potential for 14 secondary diagnoses and 15 associated procedures. The NIS is the largest United States inpatient care database, encompassing hospitals from a total of 46 states, which serve 97% of the United States population.
Inclusion, Exclusion Criteria and Assessment of Bariatric Surgery History
Patients were included if they had a primary or secondary diagnosis of morbid obesity. Morbid obesity was defined by International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) codes 278.01, V85.35, V85.36, V85.37, V85.37, V85.38, V85.39, V85.40, V85.41, V85.42, V85.43, V85.44, and V85.45.16,17 Among the included patients with morbid obesity, a history of bariatric surgery was identified using the following ICD-9-CM code: 539.xx [complications of bariatric procedures], 649.2× [bariatric surgery status complicating pregnancy, childbirth, or the puerperium], or V45.86 [bariatric surgery status] as validated by previous literature.14,16,17 Cases with missing values (i.e., age, gender, and race/ethnicity) were excluded as these variables were used to perform propensity score matching analysis. According to the data user agreement, any individual table cell counts of ≤ 10 cannot be presented to preserve patient confidentiality. In such instances, data were suppressed and labeled as IS, information suppressed.
Study Outcomes
The primary outcomes of interest in our study were age-adjusted prevalence of HCC and in-hospital mortality rate. Secondary outcomes were length of hospital stay and hospitalization cost. The diagnosis of HCC was based upon the ICD-9-CM code 155.0 [malignant neoplasm of liver primary] and excluded ICD-9-CM code 155.1 [malignant neoplasm of intrahepatic bile ducts].18
Covariates
The covariates included demographic variables (i.e., age, gender, and race/ethnicity), as well as relevant comorbidities and etiology of underlying liver disease including non-alcoholic fatty liver disease (NAFLD). Comorbid conditions were captured using the Charlson comorbidity score.19 Hospitalization data such as day of admission (weekday or weekend), median household income, location and teaching status of admission hospital as well as size, hospitalization charges, primary payer source, and information regarding in-hospital mortality was also provided. Since the NIS registry is a publicly available database and contains de-identified patient information, this study was exempt from institutional review board full review.
Statistical Analysis
The included morbidly obese participants were categorized into patients with prior bariatric surgery or patients without bariatric surgery. We described and compared demographic and clinical characteristics between these two groups. We used counts and proportions to describe categorical variables, and differences tested using Pearson’s Chi-square test. Continuous variables were presented as median (Inter-Quartile Range) and differences tested using the Mann-Whitney U test. We calculated the age-adjusted inpatient prevalence of HCC per 100,000 hospital admissions among patients with and without a history of bariatric surgery, and derived prevalence ratios (PRs) and 95% confidence intervals (CIs). Overall hospitalization cost was estimated by multiplying total hospital charges (adjusted for inflation to reflect 2014 US dollars) by hospital-specific cost-to-charge ratios and reweighted to account for the hospitals where the cost-to-charge ratio was not available.
Propensity score matching (PSM) analyses was performed on unweighted cases to match the morbidly obese patients with a history of bariatric surgery to those without bariatric surgery - Supplemental Figure 1. We performed 1:1 fixed ratio nearest neighbor matching within caliper width of 0.01, with replacement between patients with prior-bariatric surgery and non-bariatric surgery. The 1:1 ratio was chosen to minimize bias without sacrificing too much power in accordance with recommendations from previous literature.20 Because we analyzed ≥ 1 year of data, we included years 2004 to 2014 into PSM to control for year-to-year variations.
Trends in age-adjusted HCC prevalence rates were assessed using Poisson regression and fractional polynomial regression models. Generalized linear models were used to estimate cost, with inclusion of clustering at the level of the hospital. As the cost data were inherently positively skewed, the modified Park test was performed to select the appropriate family of distribution that reflected the mean variance structure that best fits the variance of the mean cost exhibited by the data. As a result, a gamma distribution was selected. Length of hospital stay was centered using the mean. A doubly robust regression analysis was performed to isolate the effect of HCC on cost and length of hospital stay.21 This method consisted of a two-stage approach. Initially, a multivariable regression was performed where the exposure (HCC) was the function of covariates and propensity scores were generated.
In the next step, a second regression analysis was performed using the inverse probability weighting based on propensity scores on matched patients with outcome as the function of covariates (i.e., age, sex, race, income, insurance status, type of admission, and relevant comorbidities). This technique combines the use of propensity scores with multivariable regression and offers greater accuracy in controlling for confounding that can occur as a result of misspecifications of models. Prior to our analysis, we tested the regression models for over-dispersion using a Pearson goodness-of-fit test and these models were not over dispersed. All tests were 2-sided, with a 5% significance level. All the statistical analyses were performed using Stata version 15.1 (StataCorp).
RESULTS
Baseline Characteristics
A total of 2,881,414 patients with a discharge diagnosis of morbid obesity were included in our study, of which 267,082 (9.3%) had a history of bariatric surgery. The characteristics of morbidly obese patients with and without a history of bariatric surgery are presented in Table 1. Prior to propensity score matching, patients who underwent bariatric surgery were younger (median age 44 ±17 versus 56 ± 22 years old; P <0.001), more likely to be men (33.8% versus 20.8%; P < 0.001), less likely to have significant comorbid disease (Charlson comorbidity Index score of 0: 54.2% versus 25.8%; P < 0.001), and more likely to have NAFLD (9.3% versus 1.6%; P < 0.001). Racial/ethnic differences were significant as well among the unmatched cohort (P < 0.001).
Table 1:
Characteristics of Hospitalized Patients with/without Bariatric Surgery before and after Propensity Score Matching
| Before Propensity Score Matching | After Propensity Score Matching | |||||
|---|---|---|---|---|---|---|
| Variable | Bariatric Surgery (N=267,082; 9.3%) | No Bariatric Surgery (N=2,614,332; 90.7%) | P Value | Bariatric Surgery (N=230,956; 50%) | No Bariatric Surgery (N=230,956; 50%) | P Value |
| Patient Characteristics | ||||||
| Age, median (IQR), Years | 44 (17) | 56 (22) | <0.001 | 44 (17) | 44 (17) | 0.99 |
| Gender | <0.001 | 0.98 | ||||
| Male | 66.2% | 33.8% | 21.0 | 21.0 | ||
| Female | 79.2% | 20.8% | 79.0 | 79.0 | ||
| Race /Ethnicity | <0.001 | 0.50 | ||||
| White | 70.6% | 67.2% | 70.6 | 70.7 | ||
| Black | 14.2% | 20.0% | 14.2 | 14.4 | ||
| Hispanic | 10.6% | 9.1% | 10.6 | 10.6 | ||
| Other | 4.6% | 3.7% | 4.6 | 4.3 | ||
| Charlson Comorbidity Index score | <0.001 | 0.56 | ||||
| 0 | 54.2% | 25.8% | 53.9 | 53.9 | ||
| 1–2 | 34.6% | 26.0% | 34.8 | 34.9 | ||
| >=3 | 11.2% | 48.2% | 11.3 | 11.2 | ||
| Comorbidities | ||||||
| Acute Myocardial Infarction | 1.2% | 7.2% | <0.001 | 1.1 | 1.1 | 0.94 |
| Congestive Heart Failure | 1.5% | 23.4% | <0.001 | 1.7 | 1.6 | 0.54 |
| Peripheral Vascular Disease | 0.4% | 3.7% | <0.001 | 0.4 | 0.4 | 0.99 |
| Cerebrovascular Disease | 0.3% | 4.3% | <0.001 | 0.2 | 0.2 | 0.95 |
| Dementia | 0.01% | 0.2% | <0.001 | 0.01 | # | 0.1 |
| COPD | 18.6% | 33.6% | <0.001 | 18.7 | 18.8 | 0.46 |
| Rheumatoid Disease | 1.3% | 2.6% | <0.001 | 1.3 | 1.2 | 0.22 |
| Peptic Ulcer Disease | 0.4% | 1.2% | <0.001 | 0.4 | 0.3 | 0.58 |
| Diabetes | 29.6% | 37.9% | <0.001 | 29.9 | 30.0 | 0.81 |
| Diabetes with complications | 1.7% | 8.6% | <0.001 | 1.7 | 1.7 | 0.99 |
| Hemiplegia/Paraplegia | 0.03% | 0.9% | <0.001 | 0.03 | 0.03 | 0.98 |
| Renal Disease | 1.4% | 15.3% | <0.001 | 1.4 | 1.4 | 0.89 |
| AIDS | 0.02% | 0.13% | <0.001 | 0.02 | 0.02 | 0.98 |
| Mild Liver Disease | 0.6% | 0.9% | <0.001 | 0.6 | 0.5 | 0.14 |
| Moderate/Severe Liver Disease | 0.1% | 0.9% | <0.001 | 0.1 | 0.1 | 0.99 |
| Etiology of Liver Disease | ||||||
| Hepatitis C | 0.4% | 1.1% | <0.001 | 0.4 | 0.3 | 0.11 |
| Hepatitis B | 0.07% | 0.1% | <0.001 | 0.08 | 0.07 | 0.09 |
| Alcohol Liver Disease | 0.02% | 0.5% | <0.001 | 0.02 | 0.02 | 0.99 |
| Primary Biliary Cholangitis | 0.01% | 0.02% | <0.001 | 0.02 | 0.01 | 0.20 |
| Primary Sclerosing Cholangitis | IS | 0.1% | <0.001 | IS | IS | 0.32 |
| Autoimmune Hepatitis | 0.02% | 0.04% | <0.001 | 0.02 | 0.02 | 0.98 |
| NAFLD | 9.3% | 1.6% | <0.001 | 9.7 | 9.1 | 0.12 |
| Type of Admission | ||||||
| Elective admission | 92.7% | 24.3% | <0.001 | 92.3 | 90.0 | 0.08 |
| Weekend Admission | 0.4% | 18.9% | <0.001 | 0.41 | 0.52 | 0.06 |
| Primary Payer Source | <0.001 | 0.31 | ||||
| Private Insurance | 12.2% | 42.6% | 12.3 | 12.7 | ||
| Medicaid | 8.3% | 17.8% | 8.5 | 9.0 | ||
| Medicare | 70.6% | 31.2% | 70.4 | 69.1 | ||
| Other Payment Source | 5.3% | 4.9% | 5.4 | 5.5 | ||
| Self-Pay | 0.1% | 0.5% | 0.1 | 0.1 | ||
| No Charge | 3.5% | 3.0% | 3.3 | 3.6 | ||
| Median household income, $ | <0.001 | 0.40 | ||||
| <38 999 | 21.4% | 32.8% | 21.2 | 21.0 | ||
| 39 000–47 999 | 25.8% | 28.1% | 25.4 | 26.1 | ||
| 48 000–62 999 | 26.5% | 22.7% | 26.0 | 25.9 | ||
| >63 000 | 26.3% | 16.4% | 27.5 | 27.0 | ||
| Location/Teaching status | <0.001 | 0.55 | ||||
| Rural | 3.6% | 11.8% | 3.3 | 3.3 | ||
| Urban Non-Teaching | 40.7% | 41.1% | 42.2 | 43.0 | ||
| Urban Teaching | 47.1% | 47.1% | 54.6 | 53.7 | ||
| Hospital Size | <0.001 | 0.29 | ||||
| Small | 16.2% | 13.9% | 16.2 | 16.1 | ||
| Medium | 28.1% | 25.7% | 29.3 | 29.3 | ||
| Large | 55.7% | 60.4% | 54.5 | 54.6 | ||
Abbreviation: IQR, interquartile range. Weighted counts using
Nationwide Inpatient Sample complex survey weights; numbers may not sum to group totals or percentages may not add to 100 owing to the need for rounding. Numbers are rounded to nearest integral number and percentages are based on rounded numbers.
NOTE. According to the data user agreement, any individual table cell counts of 10 or fewer cannot be presented to preserve patient confidentiality. In such instances, data are suppressed. IS, information suppressed.
The characteristics of the propensity matched sample are displayed in Table 1. The matched cohort included 230,956 bariatric surgery patients and 230,956 patients without bariatric surgery with a similar distribution of propensity score as shown in Supplemental Figure 1. In the matched cohort, the patient characteristics were similar between groups including age, gender, race, Charlson comorbidity index score, relevant comorbidities etiology of liver disease, and hospitalization data.
Hepatocellular Carcinoma and In-Hospital Mortality Trends
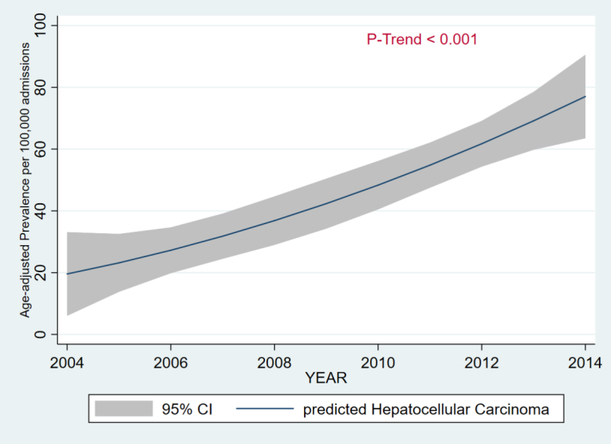
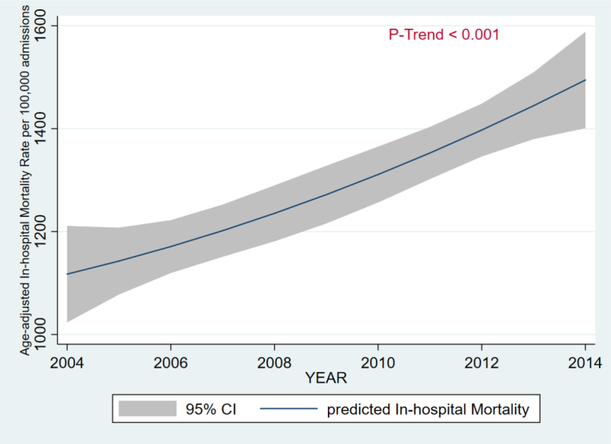
From 2004 to 2014, there was a 3-fold increase in the age-adjusted inpatient prevalence of HCC from 27 per 100,000 to 72 per 100,000 (PTrend < 0.001) - Figure 1. From 2004 to 2014, age-adjusted inpatient mortality rate increased from 1,110 per 100,000 to 1,471 per 100,000 (PTrend < 0.001) - Figure 2.
Figure 1.
Age-Adjusted Prevalence of Hepatocellular in Patients with Morbid Obesity, 2004 – 2014.
Figure 2.
Age-Adjusted In-Hospital Mortality in Patients with Morbid Obesity, 2004 – 2014.
Clinical Outcomes
Unmatched Sample:
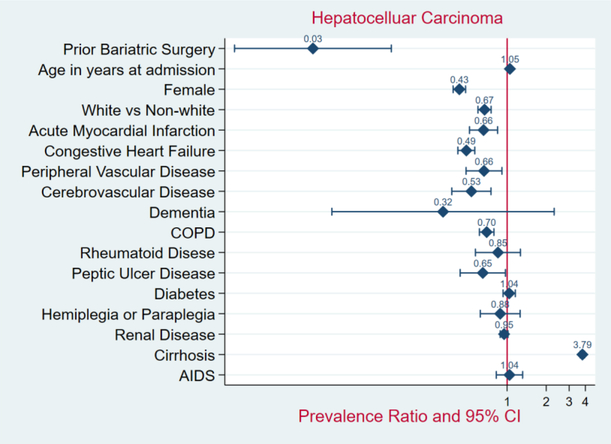
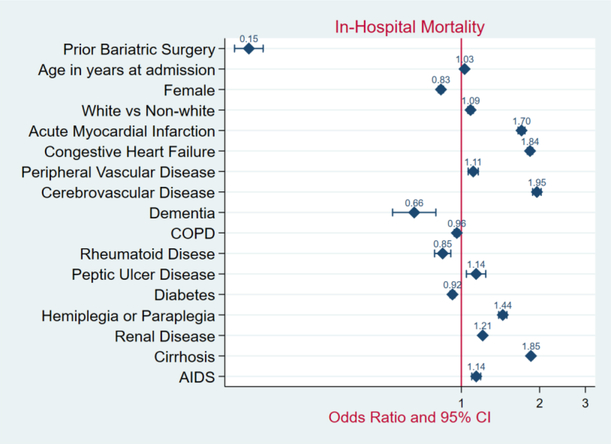
In the unmatched sample, the patients with prior bariatric surgery demonstrated a significantly lower prevalence of HCC (PR 0.03; 95% CI, 0.01–0.13; P < 0.001) - Figure 3. The in-hospital mortality rate was lower among patients with prior bariatric surgery compared to those without bariatric surgery (0.1% versus 1.5%; OR 0.15; 95% CI, 0.13–0.17; P < 0.001) - Figure 4. In comparison to patients without weight loss surgery, morbidly obese patients with prior bariatric surgery had higher median hospitalization costs ($35,420 versus $24,886; P < 0.001), but shorter length of hospital stay (2.0 versus 4.0 days; P< 0.001).
Figure 3.
Forrest Plot of Adjusted Prevalence Ratios and 95%CIs in Patients with Morbid Obesity, 2004 – 2014.
Figure 4.
Forrest Plot of Adjusted Mortality Odds Ratios and 95%CIs in Patients with Morbid Obesity, 2004 – 2014.
Propensity Score Sample:
Using the propensity matched sample, patients with prior-bariatric surgery demonstrated a significantly lower prevalence of HCC (PR 0.11; 95% CI, 0.03–0.48; P < 0.001). In-hospital mortality remained lower for patients with a history of bariatric surgery (0.1% versus 0.5%; OR 0.22; 95% CI, 0.20–0.26; P < 0.001). While the median overall hospitalization cost was significantly higher for the obese patient with bariatric surgery as compared to non-bariatric surgery patients ($36,406 versus $22,415; P < 0.001), the length of hospital stay was lower (2.0 versus 3.0 days; P < 0.001) - Table 2.
Table 2:
Outcomes between Patients with/without History of Bariatric Surgery before and after Propensity Score Matching
| Before Propensity Score Matching | After Propensity Score Matching | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Bariatric Surgery (N=267,082; 9.3%) | No Bariatric Surgery (N=2,614, 332; 90.7%) | PR or OR or RR (95% CI) | P Value | Bariatric Surgery (N=230,9 56; 50%) | No Bariatric Surgery (N=230,9 56; 50%) | PR or OR or RR (95% CI) | P Value |
| Hepatocellular Carcinoma, No. (%) | IS | 1,478 (0.06) | 0.03 (0.01 0.13)* | <0.001 | IS | 18 (0.01) | 0.11 (0.03 0.48)* | <0.001 |
| In Hospital Mortality, No. (%) | 303 (0.1) | 38,101 (1.5) | 0.15 (0.13 0.17)*** | <0.001 | 244 (0.1) | 1,088 (0.5) | 0.22 (0.20 0.26)*** | <0.001 |
| LOS, median (IQR), d | 2 (2) | 4 (4) | 0.44 (0.43 0.45)** | <0.001 | 2 (1) | 3 (3) | 0.51 (0.50 0.52) ** | <0.001 |
| Overall cost, Median (IQR), USD, $ | 35,420(26,202) | 24,886(33,532) | 1.03(1.02 1.04) ** | <0.001 | 36,406(28,005) | 22,415(28,802) | 1.04(1.05 1.07) ** | <0.001 |
PR = Prevalence ratios or
RR=Risk Ratio, using Poisson regression or linear regression
OR= Odds ratio using logistic regression CI = Confidence interval NOTE. According to the data user agreement, any individual table cell counts of 10 or fewer cannot be presented to preserve patient confidentiality. In such instances, data are suppressed. IS, information suppressed.
Incremental Cost, Length of Hospital Stay, and Mortality Associated with Hepatocellular Carcinoma
After double robust regression analysis, a diagnosis of HCC was associated with an incremental overall hospitalization cost of $ 18,840 (95% CI $11,1118-$26,562), incremental mean length of stay of 2 days (95% CI; 1day-3days) and increased risk of death by 65% (aOR 1.65; 95% CI 1.18–2.29).
DISCUSSION
Using the United States NIS database, we found that bariatric surgery was associated with a lower risk of HCC and lower overall in-hospital mortality among matched cohorts of morbidly obese patients. Despite these improved outcomes, our analysis of more than 2,881,414 patients found bariatric surgery to have not kept pace with the skyrocketing epidemic of obesity. In fact, only 9.3% of patients in this national database sample had a history of bariatric surgery, despite a prevalence of obesity greater than 35% of the United States adult population.22 Even after propensity score matching the patients who underwent bariatric surgery continued to demonstrate a lower age-adjusted prevalence of HCC and reduction of in-hospital mortality.
Potential Mechanisms of the relation of Obesity and Hepatocellular Carcinoma
Obesity is a well-established risk factor for several types of cancer, predominantly in the gastrointestinal tract, where one of the strongest associations is with liver cancer.7,23 In a landmark study, Calle et al,7 linked 14% of all cancer deaths in women and 20% in men with overweight or obesity. A subsequent meta-analysis by Larsson et al found that compared with persons of normal weight, the relative risk of liver cancer was 17% higher for overweight patients and 89% higher for those who were obese.24 Similarly, Hassan and colleagues, previously demonstrated that obesity in early adulthood increased risk of HCC but did not affect overall survival of patients with HCC.25
While theories to explain the underlying mechanisms of liver cancer in relation to obesity are abundant, these largely remain unclear.26 Although the presence of NAFLD certainly may influence the occurrence of HCC, it is important to highlight that our study included multiple alternative etiologies for HCC in the sample population, suggesting obesity itself is an independent risk factor for the development of HCC. The pathogenesis of HCC with or without NAFLD is multi-factorial and involves obesity-mediated mechanisms including lipid accumulation within hepatocytes, leading to chronic low-grade liver inflammation, as a result of various cytokines and adipokines.26,27 However, the transition from low-grade chronic hepatocellular inflammation to a neoplastic cellular process involves mechanisms that have yet to be definitively identified.
Obesity itself may directly predispose patients to HCC, but also may promote progression of other factors, including impaired glucose tolerance and chronic liver disease which confer additional HCC risk.28 Obesity may lead to a systemic increase in non-esterified fatty acids, insulin, glucose, leptin, and inflammatory cytokines.29 These imbalances may promote cancer cell survival, proliferation, and malignant progression. Additional mechanisms include the fact that adipocytes in obese individuals are swollen and de-differentiated, thereby releasing less adiponectin.28,29 This, in turn, results in macrophage infiltration, contributing to a systemic inflammatory state. This ongoing inflammation, excess fatty acid, and generation of reactive oxygen species create an environment within the liver that promotes DNA damage, an impaired immune response, and the subsequent development of HCC.
Weight Loss and Impact on Hepatocellular Carcinoma
While weight reduction surgery has been demonstrated to improve liver fibrosis, there remains a paucity of data regarding whether weight loss lowers the risk for HCC.26 Targeting obesity-related inflammation and improvement of insulin resistance with the aim of chemoprevention of hepatocarcinogenesis has been previously studied.26,30,31 The results of these prior studies are consistent with our findings. Bougoulia et al found that a 19% weight loss in obese women led to reduced plasma TNF, interleukin-6, leptin, and increased plasma adiponectin.32 While this study examined the effect of orlistat, a more effective weight loss modality such as bariatric surgery would be expected to be even more efficacious. Indeed, bariatric surgery is considered to be the best treatment for morbid obesity and related comorbidities in the general population.33,34 Bariatric surgery provides the highest level of excess weight loss as compared to other available methods, achieving approximately 62% with gastric bypass, 41% with laparoscopic adjustable gastric banding, and 66% with laparoscopic sleeve gastrectomy.35–38 It is therefore not surprising that bariatric surgery would be associated with reduced HCC risk.
Clinical Implications and Future Directions
HCC incidence in the United States tripled from the 1970’s to the 2000’s with approximately 30% of patients found to be hepatitis virus negative.29,39 Given that obesity is now an established independent risk factor for HCC suggests a need to investigate the impact of weight loss surgery on cancer-related outcomes. Future well-designed prospective studies are needed to confirm our findings and possibly define exact neuro-hormonal mechanisms for a potential reduction in HCC risk. Additionally, surgeons and physicians alike must work together to better identify patients at risk who might benefit from life-saving weight loss surgery. As the prevalence of obesity among United States adults continues to increase to approximately 40%, greater consideration for obesity-related cancers is needed in addition to effective treatment options to potentially mitigate this risk.22
Strengths and Limitations
Our study is inherently limited by its retrospective and observational nature. Additionally, using an inpatient sample database did not allow us to assess long-term and post-discharge outcomes. Furthermore, given the reliance upon ICD-9-CM diagnoses codes, administrative databases such as the NIS carry the risk of coding errors. Nevertheless, these codes have been previously validated by prior studies.14,16−18 An important challenge to the validity of our results is the lack of clinically relevant information on dietary habits and dietary modifications, pharmacological therapy, and other weight loss reduction methods possibly applied by these patients. Furthermore, we did not have data regarding HCC characteristics as well as the true effectiveness of bariatric surgery (i.e., body mass index or percent weight loss).
Despite these limitations, our study possesses several strengths. The most noticeable of which is its sample size that allows for generalizability to the broader United States population. Additionally, prior studies have shown an association with obesity and HCC, but have not controlled consistently for well-known risk factors.40 Our study has the advantage of utilizing a propensity score matched cohort to minimize confounding. While use of propensity score matching attempts to minimize confounding of measured variables available in the NIS database, it does not preclude residual confounding by unmeasured variables, such as individual body mass index or other obesity-related or procedure-specific variables.
Conclusion
Our cross-sectional study showed an association between a history of bariatric surgery and an 89% lower prevalence of HCC and 78% lower in-hospital mortality compared with no history of bariatric surgery. HCC was associated with $18,840 extra cost, longer hospital stay by 2 days, and a 65% increased risk of death. These findings suggest that bariatric surgery may play an important role in HCC and related mortality prevention among morbidly obese patients in the United States. Future prospective studies are needed to evaluate the impact of weight loss on the occurrence of HCC and related outcomes. This may provide robust evidence for expanding the pool of ideal candidates for bariatric surgery.
Supplementary Material
Supplemental Figure 1: Graph of Propensity Score Densities before and after 1:1 Fixed Ratio nearest Neighbor Matching
Acknowledgements:
None
Financial Support: Supported by NIH 5 T32 DK 7356–37 (BN)
Footnotes
STROBE Statement: All checklist items for cross-sectional studies have been completed.
Ethical Approval Statement: For this type of study formal consent is not required.
Informed Consent Statement: Does not apply.
Potential Conflicts of Interest: The authors have no conflicts to disclose.
Conflict of Interest Statement: The authors have no conflicts to disclose.
REFERENCES
- 1.El-Serag HB. Hepatocellular carcinoma. The New England journal of medicine 2011;365:1118–27. [DOI] [PubMed] [Google Scholar]
- 2.El-Serag HB, Kanwal F. Epidemiology of hepatocellular carcinoma in the United States: where are we? Where do we go? Hepatology 2014;60:1767–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Njei B, Rotman Y, Ditah I, Lim JK. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology 2015;61:191–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.El-Serag HB, Davila JA, Petersen NJ, McGlynn KA. The continuing increase in the incidence of hepatocellular carcinoma in the United States: an update. Ann Intern Med 2003;139:817–23. [DOI] [PubMed] [Google Scholar]
- 5.Caldwell SH, Crespo DM, Kang HS, Al-Osaimi AM. Obesity and hepatocellular carcinoma. Gastroenterology 2004;127:S97–103. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell NS, Catenacci VA, Wyatt HR, Hill JO. Obesity: overview of an epidemic. Psychiatr Clin North Am 2011;34:717–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med 2003;348:1625–38. [DOI] [PubMed] [Google Scholar]
- 8.Moller H, Mellemgaard A, Lindvig K, Olsen JH. Obesity and cancer risk: a Danish record-linkage study. Eur J Cancer 1994;30A:344–50. [DOI] [PubMed] [Google Scholar]
- 9.Wolk A, Gridley G, Svensson M, et al. A prospective study of obesity and cancer risk (Sweden). Cancer Causes Control 2001;12:13–21. [DOI] [PubMed] [Google Scholar]
- 10.Food, Nutrition, Physical Activity and the Prevention of Cancer: A Global Perspective. Washington DC: AICR: World Cancer Research Fund/American Institute for Cancer Research; 2007. [Google Scholar]
- 11.Dudekula A, Rachakonda V, Shaik B, Behari J. Weight loss in nonalcoholic Fatty liver disease patients in an ambulatory care setting is largely unsuccessful but correlates with frequency of clinic visits. PLoS One 2014;9:e111808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colquitt JL, Pickett K, Loveman E, Frampton GK. Surgery for weight loss in adults. Cochrane Database Syst Rev 2014:CD003641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rapp K, Schroeder J, Klenk J, et al. Obesity and incidence of cancer: a large cohort study of over 145,000 adults in Austria. Br J Cancer 2005;93:1062–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang B, Yang HP, Ward KK, Sahasrabuddhe VV, McGlynn KA. Bariatric Surgery and Liver Cancer in a Consortium of Academic Medical Centers. Obes Surg 2016;26:696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lam PH, Obirieze AC, Ortega G, et al. Characterization of Hepatitis B and C Among Liver Transplant Recipients With Hepatocellular Carcinoma: An Analysis of the Nationwide Inpatient Sample Database. Transplant Proc 2016;48:123–7. [DOI] [PubMed] [Google Scholar]
- 16.McCarty TR, Echouffo-Tcheugui JB, Lange A, Haque L, Njei B. Impact of bariatric surgery on outcomes of patients with nonalcoholic fatty liver disease: a nationwide inpatient sample analysis, 2004–2012. Surg Obes Relat Dis 2018;14:74–80. [DOI] [PubMed] [Google Scholar]
- 17.Sharma P, McCarty TR, Njei B. Impact of Bariatric Surgery on Outcomes of Patients with Inflammatory Bowel Disease: a Nationwide Inpatient Sample Analysis, 2004–2014. Obes Surg 2017. [DOI] [PubMed] [Google Scholar]
- 18.Abbas A, Medvedev S, Shores N, et al. Epidemiology of metastatic hepatocellular carcinoma, a nationwide perspective. Dig Dis Sci 2014;59:2813–20. [DOI] [PubMed] [Google Scholar]
- 19.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol 1992;45:613–9. [DOI] [PubMed] [Google Scholar]
- 20.Austin PC. Statistical criteria for selecting the optimal number of untreated subjects matched to each treated subject when using many-to-one matching on the propensity score. Am J Epidemiol 2010;172:1092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Funk MJ, Westreich D, Wiesen C, Sturmer T, Brookhart MA, Davidian M. Doubly robust estimation of causal effects. Am J Epidemiol 2011;173:761–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marengo A, Rosso C, Bugianesi E. Liver Cancer: Connections with Obesity, Fatty Liver, and Cirrhosis. Annu Rev Med 2016;67:103–17. [DOI] [PubMed] [Google Scholar]
- 24.Larsson SC, Wolk A. Overweight, obesity and risk of liver cancer: a meta-analysis of cohort studies. Br J Cancer 2007;97:1005–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hassan MM, Abdel-Wahab R, Kaseb A, et al. Obesity Early in Adulthood Increases Risk but Does Not Affect Outcomes of Hepatocellular Carcinoma. Gastroenterology 2015;149:119–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schutte K, Balbisi F, Malfertheiner P. Prevention of Hepatocellular Carcinoma. Gastrointest Tumors 2016;3:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun B, Karin M. Obesity, inflammation, and liver cancer. J Hepatol 2012;56:704–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reeves HL, Zaki MY, Day CP. Hepatocellular Carcinoma in Obesity, Type 2 Diabetes, and NAFLD. Dig Dis Sci 2016;61:1234–45. [DOI] [PubMed] [Google Scholar]
- 29.Font-Burgada J, Sun B, Karin M. Obesity and Cancer: The Oil that Feeds the Flame. Cell Metab 2016;23:48–62. [DOI] [PubMed] [Google Scholar]
- 30.Sakai H, Shirakami Y, Shimizu M. Chemoprevention of obesity-related liver carcinogenesis by using pharmaceutical and nutraceutical agents. World J Gastroenterol 2016;22:394–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shimizu M, Tanaka T, Moriwaki H. Obesity and hepatocellular carcinoma: targeting obesity-related inflammation for chemoprevention of liver carcinogenesis. Semin Immunopathol 2013;35:191–202. [DOI] [PubMed] [Google Scholar]
- 32.Bougoulia M, Triantos A, Koliakos G. Effect of weight loss with or without orlistat treatment on adipocytokines, inflammation, and oxidative markers in obese women. Hormones (Athens) 2006;5:259–69. [DOI] [PubMed] [Google Scholar]
- 33.Colquitt JL, Picot J, Loveman E, Clegg AJ. Surgery for obesity. Cochrane Database Syst Rev 2009:CD003641. [DOI] [PubMed] [Google Scholar]
- 34.Sjostrom L, Narbro K, Sjostrom CD, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52. [DOI] [PubMed] [Google Scholar]
- 35.Fischer L, Hildebrandt C, Bruckner T, et al. Excessive weight loss after sleeve gastrectomy: a systematic review. Obes Surg 2012;22:721–31. [DOI] [PubMed] [Google Scholar]
- 36.Khan S, Rock K, Baskara A, Qu W, Nazzal M, Ortiz J. Trends in bariatric surgery from 2008 to 2012. Am J Surg 2016;211:1041–6. [DOI] [PubMed] [Google Scholar]
- 37.O’Brien PE, McPhail T, Chaston TB, Dixon JB. Systematic review of medium-term weight loss after bariatric operations. Obes Surg 2006;16:1032–40. [DOI] [PubMed] [Google Scholar]
- 38.Phillips E, Ponce J, Cunneen SA, et al. Safety and effectiveness of Realize adjustable gastric band: 3-year prospective study in the United States. Surg Obes Relat Dis 2009;5:588–97. [DOI] [PubMed] [Google Scholar]
- 39.Davila JA, El-Serag H. The Rising Incidence of Hepatocellular Carcinoma in the United States: an Update. Gastroenterology. 2012:142(5), Supp 1:S–914. [Google Scholar]
- 40.Saunders D, Seidel D, Allison M, Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma - epidemiological evidence. Aliment Pharmacol Ther 2010;31:1051–63. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: Graph of Propensity Score Densities before and after 1:1 Fixed Ratio nearest Neighbor Matching