Abstract
Sepsis disproportionately affects the very old and the very young. IL-1 signaling is important in innate host defense but may also play a deleterious role in acute inflammatory conditions including sepsis by promulgating life-threatening inflammation. IL-1 signaling is mediated by two distinct ligands, IL-1α and IL-1β, both acting on a common receptor (IL-1R1). IL-1R1 targeting has not reduced adult human sepsis mortality despite biologic plausibility. Because the specific role of IL-1α or IL-1β in sepsis survival is unknown in any age group and the role of IL-1 signaling remains unknown in neonates, we studied the role of IL-1 signaling, including the impact of IL-1α and IL-1β, on neonatal murine sepsis survival. IL-1 signaling augments the late plasma inflammatory response to sepsis. IL-1α and not IL-1β is the critical mediator of sepsis mortality likely due to paracrine actions within the tissue. These data do not support targeting IL-1 signaling in neonates.
Introduction
Sepsis has its greatest impact at the extremes of age; preterm newborns and elderly adults. The risk of acquiring infection and the progression of infection to sepsis in neonates are attributed to distinct immune function as compared to healthy adults. We and others have shown that, in contrast to adults, neonates rely on the innate immune system for host defense(1). The majority of innate immune cells are activated by engagement of IL-1 receptor (IL-1R1)(2). Although the impact of IL-1 signaling has been well characterized in septic adult humans and animals, the role of IL-1 in neonatal sepsis remains obscure.
IL-1 signaling is mediated by two distinct ligands, IL-1α and IL-1β, both acting on a common receptor IL-1R1(3). IL-1β is critically important for innate immune cell activation but may also be detrimental if produced in excess(4, 5). In contrast to IL-1β, relatively less is known about IL-1α in neonatal inflammatory pathology. IL-1α is a nearly ubiquitous alarmin that also exists in a pro-form similar to IL-1β(6). In contrast to the biologically inactive pro-form of IL-1β, proIL-1α demonstrates biological activity similar to mature IL-1α and thus can promulgate the initial inflammatory response when released by injured tissue during sepsis(7). However, the specific contributions of these two IL-1 ligands to polymicrobial sepsis survival have not been reported in adults or neonates. Therefore, we investigated the role of IL-1 signaling mediated by both IL-1α and IL-1β in a murine model of neonatal sepsis. Unexpectedly, we discovered that IL-1β is unnecessary for lethal neonatal sepsis but rather IL-1α is the predominant IL-1R1 dependent-determinant of mortality.
Materials and Methods
Mice:
The IACUC at the University of Florida approved all studies prior to their initiation. Specific pathogen-free, male and female C57BL/6 mice [wild-type (WT) and genetically modified on a B6 background] were purchased from The Jackson Laboratory (Bar Harbor, ME), between six and eight weeks of age and allowed at least seven days to equilibrate before any further use as breeders to generate pups. Pups aged 5–7 days (P5–7) were considered neonates and used for all experiments(8). IL-1β null mice on a B6 background were previously described(8). Mixed sex litters were used.
Human subjects and sample processing.
All studies were IRB-approved prior to initiation. A description of the patient cohort including methods for gene expression profiling and plasma protein analyses were previously described(9).
Cecal slurry-induced peritonitis:
Mice were made septic using the cecal slurry model(10). Briefly, a 6-week old, non-pregnant, female WT (C57BL/6) mouse was euthanized less than two weeks after arrival from the vendor and the cecum was isolated. Cecal contents were expressed, weighed, suspended in 5% dextrose at a concentration of 80 mg/mL, and administered via intraperitoneal (IP) injection to neonatal mice at the desired lethal dose. Mice were monitored after injection as described(1).
Bacteremia and plasma cytokine analyses.
Whole blood was obtained by intracardiac puncture and evaluated for bacterial colonization as described(8). Murine blood plasma cytokine concentrations were measured using a magnetic-based multiplex assay for 32 analytes [G-CSF, GM-CSF, M-CSF, VEGF, TNF-α, IFN-γ, IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-7, IL-9, IL-10, IL-12(p40), IL-12(p70), IL-13, IL-15, IL-17A, KC(CXCL1), MIP2(CXCL2), LIX(CXCL5), MIG(CXCL9), IP10(CXCL10), LIF, MCP1(CCL2), MIP1α(CCL3), MIP1β(CCL4), RANTES(CCL5), and Eotaxin(CCL11)](Millipore) on a magnetic bead-based platform (Luminex). For all values that were less than or in excess of the endpoints of linearity for the assay, the limits of detection (minimum and maximum values) were substituted to avoid a Type I error based on extrapolation thus enabling the performance of statistical comparisons.
Western blot.
Lung and ileum were homogenized in radioimmunoprecipitation assay buffer with a protease and phosphatase inhibitor cocktail (Sigma-Aldrich, St Louis, MO), and total protein was quantified using Bio-rad DC protein assay reagents(Biorad, Hercules, CA) as per manufacturer’s instructions. Equal amounts of extracted protein from each sample were separated on a NuPAGE polyacrylamide gel(Invitrogen, Waltham, MA) and transferred to a nitrocellulose membrane(Invitrogen, Waltham, MA). After blocking with odyssey blocking buffer(LI-COR, Lincoln, NE), membranes were incubated with primary and secondary antibodies. Blots were detected using the Odyssey CLx imaging system(LI-COR, Lincoln, NE).
Immunohistochemistry:
Neonatal lung and ileum were harvested and were frozen in embedding medium(Tissue Tek, Sakura, CA) using isopentane on dry ice. Frozen tissues were sectioned(10 μm), fixed with 10% formalin(09122, Neogen Vet, Lexington, KY), permeabilized with 0.1% Triton-X-100(9410, Millipore, Burlington, MA), blocked with Background Buster(NB306, Innovex, Richmond, CA) and incubated overnight with rabbit anti-IL-1α(ab7632, Abcam, Cambridge, MA) followed by AF555 donkey anti-rabbit IgG(A31572, Life Technologies, Carlsbad, CA). Sections were mounted with Prolong Gold with 4ʹ,6-diamidino-2-phenylindole(DAPI)(P36935, Invitrogen, Carlsbad, CA) and visualized by confocal microscop using a Carl Zeiss LSM710 confocal microscope(Germany).
Reagents:
Anti-murine IL-1α(clone ALF-161, BioXCell, West Lebanon, NH), anti-murine IL-1β(BioXCell, clone B122), recombinant murine IL-1β (R&D Systems, Minneapolis, MN), and IL-1ra(anakinra, Sobi, Waltham, MA) were prepared according to manufacturer specifications. Antibodies used for western blotting include anti-murine-IL-1α(Catalog # ab7632, Abcam, Cambridge, MA), anti-murine-IL-1β(Catalog # AF-401-NA, R&D Systems, Minneapolis, MN), anti-β-actin(Catalog # A5316, Sigma-Aldrich, St Louis, MO) and IRDye secondary antibodies (LI-COR, Lincoln, NE).
Statistics:
Survival was compared using Fisher’s exact test. Values were considered significant if the two-tailed confidence level was p<0.05. Depending on whether the descriptive analyses passed normality and equal variance, a student’s t-test, Mann-Whitney, or Wilcoxon signed-rank test was used to compare results from two groups. Values were considered significant if p<0.05. Analyses were performed using Prism 7(GraphPad, La Jolla, CA).
Results
Sepsis survival is enhanced in IL-1R1-deficient neonatal mice.
To determine the role of IL-1 signaling in neonatal sepsis, we examined polymicrobial sepsis survival in IL-1R1 null(−/−) mice compared to WT mice(Figure 1A). Mice lacking IL-1R1 demonstrated a substantial sepsis survival advantage compared to WT mice (63% v 14%, p<0.005). IL-1R1 deletion did not significantly modify bacteremia at 6 and 12 hours, suggesting that the survival advantage seen in IL-1R1 null compared to WT mice was not a result of enhanced bacterial clearance or antimicrobial activity but rather could result from a decreased systemic inflammatory response(Figure 1B).
Figure 1.
A. Polymicrobial sepsis survival in IL-1 receptor 1 (R1) null (−/−) mice compared to WT mice (63% v 14%, *-p<0.005 by Fisher’s exact test). B. Bacteremia at 6 and 12 hours among IL-1R1−/− and WT mice (N≥5 for all groups at all time points). Each symbol represents an individual mouse. CFU-colony forming units. Survival experiments were independently performed a minimum of twice with results combined and presented.
IL-1R1 deletion is associated with a significant reduction in the late plasma inflammatory response to sepsis.
IL-1 signaling is important for generation and amplification of the inflammatory response to sepsis(11). Therefore, we next examined the effect of IL-1R1 deletion on plasma inflammatory mediator production at 4, 6, and 12 hours after neonatal sepsis. The majority of plasma mediators examined were not significantly different in WT versus IL-1R1−/− plasma at early time points(4 and 6 hours, Figure 2) suggesting that IL-1R1 signaling was not required to generate the early plasma inflammatory response. Notable exceptions included increased CXCL2 at 4 hours, increased CXCL10 at 6 hours, and reduced IL-17A in IL-1R1−/− versus WT mice. In contrast, multiple plasma inflammatory mediators were reduced in IL-1R1−/− mice compared to WT mice at 12 hours after sepsis including TNF-α, IL-1β, IL-6, IL-12p70, IL-13, IL-17A, CCL3, CCL4, CCL11, CXCL1, CXCL2, and CXCL10(all p<0.05). Of note, plasma IL-1α concentrations did not differ between IL-1R1−/− and WT mice at any of the time points examined. Together with our studies of sepsis survival and bacteremia in IL-1R1−/− and WT neonatal mice, these data suggest that IL-1 signaling augments neonatal sepsis by sustaining the plasma inflammatory response.
Figure 2.
Plasma mediator concentrations (median with quartiles) from septic WT and IL-1R1−/− neonates at 4, 6, and 12 hours after cecal slurry. *-p<0.05. Interleukin (IL). N≥5 for all groups at all time points. Each symbol represents an individual mouse.
Plasma IL-1α and IL-1β are increased early and sustained in neonatal sepsis.
To understand the kinetics of IL-1 production after polymicrobial sepsis induction in neonatal mice, we measured plasma IL-1α and IL-1β at rest, and at 2, 4, 6, 8, and 16 hours after sepsis in WT mice(Figure 3). All plasma samples were drawn prior to initial mortality to avoid selection bias. Plasma IL-1α and IL-1β increased 25–40 fold at 2 hours after cecal slurry and remained comparably elevated at all subsequent time points. These data confirm that both IL-1α and IL-1β are not only strongly induced and present in circulation early after infection, but also remain elevated and thereby can amplify other aspects of the host inflammatory response.
Figure 3.
Plasma IL-1α and IL-1β from WT neonates at rest and timepoints shown after sepsis. N≥5 for all groups at all time points. Data represent medians with 95% confidence intervals.
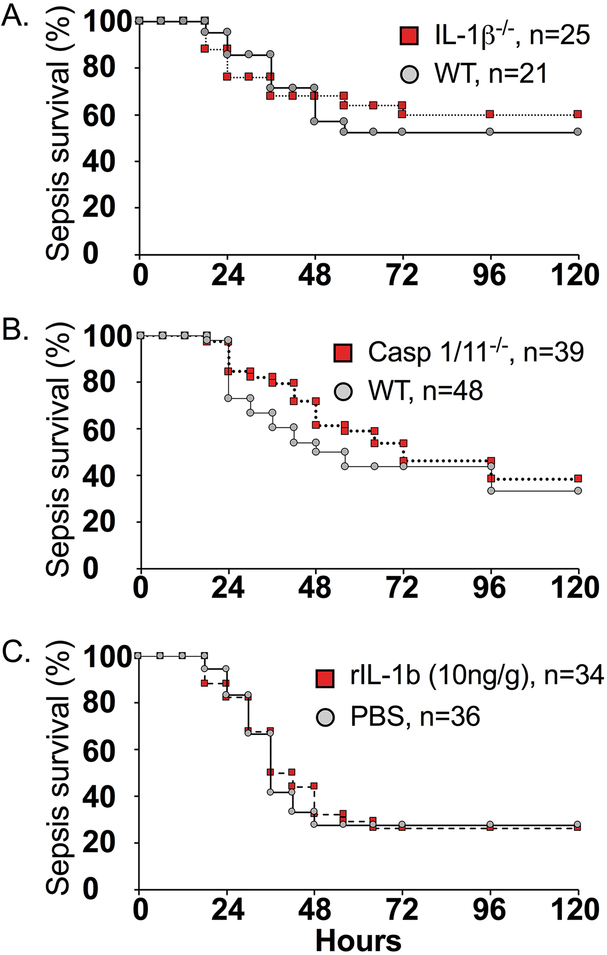
IL-1β does not play a significant role in sepsis survival.
To better understand the specific contribution of IL-1β to sepsis mortality, we utilized genetic(IL-1β−/− and caspase-1/11−/−) and pharmacologic(prophylactic treatment IP with 100μg/mouse of anti-IL-1β immediately prior to slurry injection) targeting of IL-1β. None of these strategies modifed sepsis mortality(Figure 4A–B, Figure 6B) as compared to WT mice treated with or without an isotype control-antibody. Although excessive IL-1β production can be detrimental to the host, IL-1β can also enhance innate immune function(12, 13). To determine whether the IL-1β response was inadequate in neonates and could be enhanced, concurrent treatment with rIL-1β(10ng/g IP)(13–15) was performed but also did not modify subsequent sepsis survival(26%, n = 34) compared to septic WT mice treated with PBS(28%, n = 36)(Figure 4C). Taken together, these data do not support a significant role for IL-1β in sepsis survival using this model.
Figure 4.
Impact of genetic and pharmacologic interventions that target or enhance IL-1β. A. IL-1β−/− v WT. B. Sepsis survival among WT neonates vs caspase-1/11−/− (WT 33% vs caspase-1/11−/− 38%). C. Sepsis survival among WT neonates that received 10ng/g body weight rIL-1β concurrently with cecal slurry (26%) or PBS (28%). Survival experiments were independently performed a minimum of twice with results combined and presented.
Figure 6.
A. Sepsis survival in IL-1α−/− mice versus WT mice. B. Sepsis survival among IL-1α−/− and WT mice pretreated with anti-IL-1β or isotype antibody. C. Sepsis survival among IL-1β−/− and WT mice pretreated with anti-IL-1β or isotype antibody. Survival experiments were independently performed a minimum of twice with results combined and presented.
ProIL-1α is present in healthy ileum and lung.
The failure to attenuate sepsis mortality by genetic and pharmacologic targeting of IL-1β suggests that deleterious IL-1α signaling mediates the IL-1R1-dependent sepsis pathophysiology in this model. Tissue injury and release of alarmins including IL-1α are well-described with sepsis(16). As expected(6), neither pro-IL-1β nor mature IL-1β protein was present in healthy neonatal lung and ileum(Figure 5A). In contrast, pro-IL-1α protein was detectable in both lung and ileum from healthy neonatal mice(Figure 5BC).
Figure 5.
A. Representative immunoblots of murine recombinant IL-1β (rIL-1β) as well as lung and ileum from healthy neonatal mice. B. Representative immunofluorescent image (200×) of total IL-1α in lung and ileum from healthy neonatal mice. C. Representative immunoblots for IL-1α in lung and ileum from healthy neonatal mice. Each experiment was independently performed a minimum of twice on a minimum of 3 mice.
IL-1α plays a significant role in sepsis survival.
In contrast to our results targeting IL-1β, sepsis survival was dramatically increased in IL-1α−/− mice as compared to WT mice(52% vs 17%, p<0.02, Figure 6A). Prophylactic treatment with anti-IL-1β(100μg/mouse of anti-IL-1β IP immediately prior to slurry injection) to neutralize circulating IL-1β further enhanced sepsis survival in IL-1α−/− mice but had no effect on WT mice over isotype control treatment(Figure 6B). While prophylactic treatment of WT and IL-1β null mice with anti-IL-1α(100μg/mouse IP immediately prior to slurry injection) to neutralize circulating IL-1α) resulted in a small reduction in sepsis mortality, this effect was not statistically significant and likely reflects the local production and paracrine action of IL-1α which is difficult to neutralize with systemic treatments(Figure 6C).
Discussion
There has been a longstanding interest in the deleterious role of IL-1R1 signaling in sepsis(3, 17–21). This report is the first study in which the roles of both IL-1α and IL-1β are examined in a comparative manner using an established and clinically-relevant model of sepsis rather than endotoxemia. We show IL-1 signaling through IL-1R1 is detrimental primarily because it sustains the late plasma inflammatory response. Intriguingly, a deleterious role on neonatal sepsis mortality for IL-1β by itself was not supported by genetic or pharmacologic attenuation approaches in this study, and exogenous IL-1β did not increase sepsis mortality. These studies strongly argue that it is IL-1α, not IL-1β, that mediates the detrimental effects of IL-1R1-signaling on neonatal sepsis survival.
Prior studies have suggested a deleterious role for IL-1β in adults using either knockout mice or antibody mediated neutralization(22). Importantly, these studies were not done in accepted polymicrobial sepsis models such as cecal ligation and puncture, but rather focused on lipopolysaccharide to mimic septic shock(22). Although nearly ubiquitous, less emphasis has been placed on the role of IL-1α in sepsis. Relevant to neonates specifically, IL-1α is the top upregulated gene in LPS-stimulated enriched neutrophils isolated from human neonatal cord blood(23). Further, IL-1α replicates many of the effects of endotoxemia in primates including hypotension(24).
Attenuation of IL-1R1 signaling via the IL-1R1 antagonist(IL-1ra) has been well studied but produced only minor reductions in mortality in adult mice after cecal ligation and puncture and in adult humans with sepsis(25, 26). Similarly, we have found that a single prophylactic administration of IL-1ra(100μg/mouse IP immediately prior to slurry injection) did not modify sepsis survival in neonatal WT mice (Supplemental Figure 1). Because pro-IL-1α is present in tissues at rest, does not require synthesis and signals rapidly and potently, we also attempted to pharmacologically block IL-1α - IL1R1 signaling early by administering an anti-IL-1α antibody prior to the slurry injection. However, pharmacologic targeting of IL-1α (prophylaxis immediately prior to slurry injection) was also not as effective as genetic deletion of IL-1α on sepsis survival. We have previously used the intraperitoneal route to successfully administer antibodies to murine neonates(27). The anti-IL-1α antibody used in these experiments has been previously used in vivo(28) and the dose used for pharmacological neutralization (100μg/mouse) was sufficient(13); thus, technical limitations do not readily explain the discrepancy between knockout mice and pharmacologically-treated WT mice. Although neutralizing antibodies and receptor antagonist treatments can act on circulating ligands and easily accessible receptors in well vascularized tissues, they may not effectively penetrate all tissues to inhibit local IL-1α-mediated IL-1R1 signaling. Thus, it is likely that paracrine (tissue-level) signaling mediated by IL-1α(pro or mature) released early as a result of tissue necrosis secondary to shifts in perfusion may be the critical determinant of sepsis mortality(29). Local IL-1α release and rapid IL-1R1 signaling following tissue injury with sepsis may also explain why IL-1ra treatment has failed to reduce mortality in humans.
Our findings in mice point to a prominent deleterious role for IL-1α-mediated IL-1R1 signaling. Because IL-1R2 reduces IL-1α-induced inflammation that occurs with necrosis(30), and IL-1α was the most upregulated gene in LPS-stimulated human neonatal neutrophils(23), we re-examined whole blood gene expression profiles from septic human neonates with specific attention to IL-1R2 and IL-1-related gene expression as well as IL-1-related plasma concentrations(9). Compared to uninfected neonates, IL-1R2 was among the top 5 upregulated genes in the blood from septic neonates(8.2 fold over uninfected, Supplemental Figure 2). Similarly, sIL-1RII was increased by 50% in the plasma of septic neonates over uninfected neonates. In contrast, plasma IL-1α and IL-1β were not different when compared to uninfected neonates. Strong negative regulation of IL-1 signaling in the blood in the absence of robust increases in plasma IL-1 proteins in these human neonates strongly suggests IL-1 signaling may occur in tissue compartments as we saw in our murine neonatal studies.
The identification of early paracrine IL-1α-mediated IL-1R1-dependent signaling that leads to sepsis mortality but is not amenable to IL-1-targeting therapy underscores the need to pursue targeted sepsis therapeutics downstream of IL-1, especially in the setting of significant and evolving tissue injury. We recently described a novel IL-18/IL-1/IL-17a axis that foments sepsis, and demonstrated a significant reduction in sepsis mortality with transient IL-17R blockade(8). Similar benefits in sepsis survival were provided by IL-17R blockade following cecal ligation and puncture in adult mice(31) and a deleterious role for IL-17A also occurs in necrotizing enterocolitis(32). These data provide a strong rationale for the selective use of IL-17A or IL-17 receptor(IL-17R) monoclonal antibodies in septic patients with the highest risk of mortality that renews hope for effective interventions in this vulnerable population.
In conclusion, we show IL-1α, not IL-1β, is the IL-1R1 ligand portending mortality in sepsis. Because the biologically active proform of IL-1α is released locally within tissue compartments and acts immediately with the tissue injury inherent to sepsis, IL-1R1 blocking or IL-1α-specific attenuation strategies we employed were not as effective as results seen with IL-1R1 or IL-1α null mice. Further investigation into targets downstream of IL-1R1 signaling such as IL-17A may hold more promise to attenuate pathologic inflammation in the setting of sepsis.
Supplementary Material
Acknowledgments:
We thank Professor Linc Moldawer for his comments on the manuscript.
Support:
Support was received from the National Institutes of Health (NIH) National Heart, Lung, and Blood Institute (K08HL133484-Benjamin), National Institute of Allergy and Infectious Diseases (R21AI121549-Moore), National Institute of General Medical Sciences (R01GM128452-Wynn) and Eunice Kennedy Shriver National Institute of Child Health and Human Development (R01HD089939-Wynn) as well as the JDRF Career Development Award (Moore).
References
- 1.Wynn JL, Scumpia PO, Winfield RD, Delano MJ, Kelly-Scumpia K, Barker T, Ungaro R, Levy O, and Moldawer LL. 2008. Defective innate immunity predisposes murine neonates to poor sepsis outcome but is reversed by TLR agonists. Blood 112: 1750–1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garlanda C, Dinarello CA, and Mantovani A. 2013. The interleukin-1 family: back to the future. Immunity 39: 1003–1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dinarello CA 2018. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol Rev 281: 8–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dinarello CA 2009. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27: 519–550. [DOI] [PubMed] [Google Scholar]
- 5.Broz P, and Dixit VM. 2016. Inflammasomes: mechanism of assembly, regulation and signalling. Nat Rev Immunol 16: 407–420. [DOI] [PubMed] [Google Scholar]
- 6.Di Paolo NC, and Shayakhmetov DM. 2016. Interleukin 1alpha and the inflammatory process. Nat Immunol 17: 906–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim B, Lee Y, Kim E, Kwak A, Ryoo S, Bae SH, Azam T, Kim S, and Dinarello CA. 2013. The Interleukin-1alpha Precursor is Biologically Active and is Likely a Key Alarmin in the IL-1 Family of Cytokines. Front Immunol 4: 391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wynn JL, Wilson CS, Hawiger J, Scumpia PO, Marshall AF, Liu JH, Zharkikh I, Wong HR, Lahni P, Benjamin JT, Plosa EJ, Weitkamp JH, Sherwood ER, Moldawer LL, Ungaro R, Baker HV, Lopez MC, McElroy SJ, Colliou N, Mohamadzadeh M, and Moore DJ. 2016. Targeting IL-17A attenuates neonatal sepsis mortality induced by IL-18. Proc Natl Acad Sci U S A 113: E2627–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wynn JL, Guthrie SO, Wong HR, Lahni P, Ungaro R, Lopez MC, Baker HV, and Moldawer LL. 2015. Postnatal Age Is a Critical Determinant of the Neonatal Host Response to Sepsis. Mol Med 21: 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wynn JL, Scumpia PO, Delano MJ, O’Malley KA, Ungaro R, Abouhamze A, and Moldawer LL. 2007. Increased mortality and altered immunity in neonatal sepsis produced by generalized peritonitis. Shock 28: 675–683. [DOI] [PubMed] [Google Scholar]
- 11.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, and Apte RN. 2011. IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation. J Immunol 187: 4835–4843. [DOI] [PubMed] [Google Scholar]
- 12.Hagiwara K, Yamanaka H, Higuchi H, Nagahata H, Kirisawa R, and Iwai H. 2001. Oral administration of IL-1 beta enhanced the proliferation of lymphocytes and the O(2)(−) production of neutrophil in newborn calf. Vet Immunol Immunopathol 81: 59–69. [DOI] [PubMed] [Google Scholar]
- 13.Mancilla J, Garcia P, and Dinarello CA. 1993. The interleukin-1 receptor antagonist can either reduce or enhance the lethality of Klebsiella pneumoniae sepsis in newborn rats. Infect Immun 61: 926–932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shi J, Goodband RD, Chengappa MM, Nelssen JL, Tokach MD, McVey DS, and Blecha F. 1994. Influence of interleukin-1 on neutrophil function and resistance to Streptococcus suis in neonatal pigs. J Leukoc Biol 56: 88–94. [DOI] [PubMed] [Google Scholar]
- 15.Hagiwara K, Yamanaka H, Higuchi H, Nagahata H, Kirisawa R, and Iwai H. 2001. Oral administration of IL-1 beta enhanced the proliferation of lymphocytes and the O(2)(−) production of neutrophil in newborn calf. Vet Immunol Immunopathol 81: 59–69. [DOI] [PubMed] [Google Scholar]
- 16.Rider P, Voronov E, Dinarello CA, Apte RN, and Cohen I. 2017. Alarmins: Feel the Stress. J Immunol 198: 1395–1402. [DOI] [PubMed] [Google Scholar]
- 17.Granowitz EV, Santos AA, Poutsiaka DD, Cannon JG, Wilmore DW, Wolff SM, and Dinarello CA. 1991. Production of interleukin-1-receptor antagonist during experimental endotoxaemia. Lancet 338: 1423–1424. [DOI] [PubMed] [Google Scholar]
- 18.Moldawer LL 1994. Biology of proinflammatory cytokines and their antagonists. Crit Care Med 22: S3–7. [PubMed] [Google Scholar]
- 19.Stearns-Kurosawa DJ, Osuchowski MF, Valentine C, Kurosawa S, and Remick DG. 2011. The pathogenesis of sepsis. Annu Rev Pathol 6: 19–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Netea MG, van de Veerdonk FL, van der Meer JW, Dinarello CA, and Joosten LA. 2015. Inflammasome-independent regulation of IL-1-family cytokines. Annu Rev Immunol 33: 49–77. [DOI] [PubMed] [Google Scholar]
- 21.Casanova JL, Abel L, and Quintana-Murci L. 2011. Human TLRs and IL-1Rs in host defense: natural insights from evolutionary, epidemiological, and clinical genetics. Annu Rev Immunol 29: 447–491. [DOI] [PubMed] [Google Scholar]
- 22.Vanden Berghe T, Demon D, Bogaert P, Vandendriessche B, Goethals A, Depuydt B, Vuylsteke M, Roelandt R, Van Wonterghem E, Vandenbroecke J, Choi SM, Meyer E, Krautwald S, Declercq W, Takahashi N, Cauwels A, and Vandenabeele P. 2014. Simultaneous targeting of IL-1 and IL-18 is required for protection against inflammatory and septic shock. American journal of respiratory and critical care medicine 189: 282–291. [DOI] [PubMed] [Google Scholar]
- 23.Mathias B, Mira JC, Rehfuss JP, Rincon JC, Ungaro R, Nacionales DC, Lopez MC, Baker HV, Moldawer LL, and Larson SD. 2017. LPS Stimulation of Cord Blood Reveals a Newborn-Specific Neutrophil Transcriptomic Response and Cytokine Production. Shock 47: 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fischer E, Marano MA, Barber AE, Hudson A, Lee K, Rock CS, Hawes AS, Thompson RC, Hayes TJ, and Anderson TD. 1991. Comparison between effects of interleukin-1 alpha administration and sublethal endotoxemia in primates. Am J Physiol 261: R442–452. [DOI] [PubMed] [Google Scholar]
- 25.Remick DG, Call DR, Ebong SJ, Newcomb DE, Nybom P, Nemzek JA, and Bolgos GE. 2001. Combination immunotherapy with soluble tumor necrosis factor receptors plus interleukin 1 receptor antagonist decreases sepsis mortality. Crit Care Med 29: 473–481. [DOI] [PubMed] [Google Scholar]
- 26.Minnich DJ, and Moldawer LL. 2004. Anti-cytokine and anti-inflammatory therapies for the treatment of severe sepsis: progress and pitfalls. Proc Nutr Soc 63: 437–441. [DOI] [PubMed] [Google Scholar]
- 27.Wynn JL, Scumpia PO, Stocks BT, Romano-Keeler J, Alrifai MW, Liu JH, Kim AS, Alford CE, Matta P, Weitkamp JH, and Moore DJ. 2015. Neonatal CD71+ Erythroid Cells Do Not Modify Murine Sepsis Mortality. Journal of immunology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Copenhaver AM, Casson CN, Nguyen HT, Duda MM, and Shin S. 2015. IL-1R signaling enables bystander cells to overcome bacterial blockade of host protein synthesis. Proc Natl Acad Sci U S A 112: 7557–7562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen CJ, Kono H, Golenbock D, Reed G, Akira S, and Rock KL. 2007. Identification of a key pathway required for the sterile inflammatory response triggered by dying cells. Nat Med 13: 851–856. [DOI] [PubMed] [Google Scholar]
- 30.Zheng Y, Humphry M, Maguire JJ, Bennett MR, and Clarke MC. 2013. Intracellular interleukin-1 receptor 2 binding prevents cleavage and activity of interleukin-1alpha, controlling necrosis-induced sterile inflammation. Immunity 38: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Flierl MA, Rittirsch D, Gao H, Hoesel LM, Nadeau BA, Day DE, Zetoune FS, Sarma JV, Huber-Lang MS, Ferrara JL, and Ward PA. 2008. Adverse functions of IL-17A in experimental sepsis. Faseb J 22: 2198–2205. [DOI] [PubMed] [Google Scholar]
- 32.Egan CE, Sodhi CP, Good M, Lin J, Jia H, Yamaguchi Y, Lu P, Ma C, Branca MF, Weyandt S, Fulton WB, Nino DF, Prindle T Jr., Ozolek JA, and Hackam DJ. 2016. Toll-like receptor 4-mediated lymphocyte influx induces neonatal necrotizing enterocolitis. J Clin Invest 126: 495–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.