Abstract
Background
Exposures to DNA-damaging drugs and ionizing radiations increase risks of acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS).
Methods
9,028 recipients of hematopoietic cell autotransplants (1995–2010) for Hodgkin lymphoma (HL; n=916), non-Hodgkin lymphoma (NHL; n=3546) and plasma cell myeloma (PCM; n=4566), reported to the CIBMTR, were analyzed for risk of subsequent AML or MDS.
Results
335 MDS/AML cases were diagnosed posttransplant (3.7%). Variables associated with an increased risk for AML or MDS in multivariate analyses were: (1) conditioning with total body radiation versus chemotherapy alone for HL (HR=4.0; 95% confidence interval [1.4, 11.6]) and NHL (HR=2.5 [1.1, 2.5]); (2) ≥3 versus 1 line of chemotherapy for NHL (HR=1.9 [1.3, 2.8]); and (3) subjects with NHL transplanted in 2005–2010 versus 1995–1999 (HR=2.1[1.5,3.1]).UsingSurveillance, Epidemiology and End Results (SEER) data, we found risks for AML/MDS in HL, NHL and PCM to be 5–10 times the background rate. In contrast, relative risks were 10–50 for AML and approximately 100 for MDS in the autotransplant cohort.
Conclusions
There are substantial risks of AML and MDS after autotransplants for HL, NHL and PCM.
Keywords: Autotransplant, AML, MDS, CIBMTR, SEER, therapy-related, new cancers
1. Introduction
Acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) developing in persons exposed to DNA-damaging agents for a prior cancer are often referred to as treatment- or therapy-related myeloid neoplasms (t-MN)[1, 2]. t-MN constitute approximately 10–20% of all cases of AML and MDS[3], a proportion that may increase in the future with an increasing prevalence of cancer survivors[4, 5]. Hematopoietic cell transplants (HCT) use high doses of drugs and/or ionizing radiations and can also lead to t-MN[6, 7]. More than 60,000 allo- and autotransplants are performed annually with increasing numbers of long-term survivors.
Our aim was to determine risks of developing AML and MDS in recipients of autotransplants for Hodgkin (HL), non-Hodgkin lymphomas (NHL) and plasma cell myeloma (PCM) reported to the Center for International Blood and Marrow Transplant Research (CIBMTR). We also interrogated variables for an association with an increased likelihood of t-MN. To assess the impact of risks attributable to the autotransplant versus the disease for which the transplant was done we compared risks of developing t-MN in autotransplant recipients to risks in persons with similar diagnoses in the Surveillance, Epidemiology and End Results (SEER) database in the United States (US).
2. Methods
2.1. Data Sources:
The CIBMTR is a research collaboration between the National Marrow Donor Program/Be The Match and the Medical College of Wisconsin which collects detailed allogeneic and autologous transplant data from around the world. Patients are followed longitudinally. Studies conducted by the CIBMTR are performed in compliance with all federal regulations pertaining to the protection of human research participants. All patients and donors signed informed consent forms.
The CIBMTR collects a standard set of key variables for each patient including demographic, disease-related and transplant-related data pre-transplant. Outcome data are collected at 100 days, 6 months, annually thereafter for 6 years, and biennially until death.
The SEER dataset includes ages, sexes, races, years of diagnoses, cancer type, and if ionizing radiation and/or chemotherapy were used in the initial therapy of the disease.
2.2. Subject Selection:
All recipients of first autotransplants for HL, NHL, and PCM between 1995 and 2010 reported to the CIBMTR were included. Excluded were subjects transplanted outside the US, those missing key data fields or written informed consent, those <18 years of age at the time of transplant, those with prior ML or MDS, and those with <100 days of follow up after autotransplant.
2.3. Study Definitions:
All subjects with a new diagnosis of AML or MDS reported to the CIBMTR at any time after a first autotransplant for HL, NHL or PCM. MDS cases progressing to AML were scored as MDS. Survival was defined as the time to death from any cause with surviving subjects censored at time of last follow-up. For SEER data, cancer types were defined by International Classification of Disease Oncology 3 (ICD-O3) codes in Supplementary Table 1[8]. To minimize the impact of SEER coding therapy-related MDS (t-MDS; ICD-O3 9987) as therapy-related AML (t-AML; ICD-O3 9920) since 2010, straight lines fitted to male and female t-AML cases vs age over 2001–2009 were used to predict t-AML cases in 2010–2014. Observed minus predicted t-AML cases, for each sex and year, were then randomly reassigned as MDS cases; see[9] for details.
2.4. Statistical Analyses:
Autotransplant data analyses used the following methods. Survival probability was estimated by the Kaplan-Meier method. The cumulative incidences of subsequent AML or MDS were calculated accounting for the competing risk of death. Cox regression models were used to identify factors significant in predicting development of subsequent t-MN posttransplant. Covariates of interest were age at transplant, sex, race, transplant year, Karnofsky performance score, interval from diagnosis to transplant, receipt of chemotherapy, conditioning regimen and receipt of total body irradiation (TBI). Multivariable models were built separately for HL, NHL and CM. A proportional hazard assumption was tested for each factor using a time-dependent covariate approach. Backward elimination procedures were used to identify significant variables to include in the final model. SAS 9.4 was used to implement the aforementioned analyses.
Risks relative to background/expected rates in SEER were computed using the R package SEERaBomb, which calculates background/expected cancer incidences assuming a null hypothesis that neoplasm incidences are not impacted by prior neoplasm diagnoses. Background/expected incidences were thus computed using all cases, regardless of being 1st, 2nd, or later neoplasms. Incidences were sex-, age- and year specific. To compute risks relative to such background/expected rates, person years (PY) after lymphoma and PCM diagnoses (SEER) or autotransplants (CIBMTR) at risk of AML or MDS were aggregated across subjects into single-year-resolution (age)x(year) matrices. Such matrices were multiplied into background/expected incidence surfaces and results were summed to form expected AML and MDS cases (E). Relative risks (RR) were then observed cases (O) divided by E. For AML and MDS incidences (i.e. absolute risks) after HL, NHL or PCM, PY matrices described above were summed over calendar years but not age, and incidences were formed as O/PY. Throughout, 95% confidence intervals (CI) were formed assuming that the observed number of cases O was a sample of a Poisson distribution with mean O.
3. Results
3.1. Study Cohort
9,028 subjects met the study criteria including 916 HL, 3,546 NHL, and 4,566 PCM. Subject, disease and therapy-related variables are displayed in Table 1. Median follow-ups for HL, NHL and PCM were 90 months (mo) (range, 3–246), 110 mo (range, 3–243), and 97 mo (range, 3–236).
Table 1:
Characteristics of the autotransplant CIBMTR cohorts for Hodgkin (HL), non-Hodgkin (NHL) lymphoma and plasma cell myeloma (PCM)
| Diagnosis | |||
|---|---|---|---|
| Characteristics | HL | NHL | PCM |
| Subjects (n) | 916 | 3546 | 4566 |
| Centers (n) | 103 | 118 | 118 |
| Median age at transplant (y; range) | 35(18-77) | 56(18-82) | 58(22-80) |
| 18 - 29 | 328(36) | 147(4) | 15(<1) |
| 30 - 39 | 255(28) | 307(9) | 152(3) |
| 40 - 49 | 166(18) | 708(20) | 756(17) |
| 50 - 59 | 93(10) | 1094(31) | 1719(38) |
| 60 - 69 | 65(7) | 1098(31) | 1653(36) |
| ≥70 | 9(<1) | 192(5) | 271(6) |
| Male, n (%) | |||
| 528(58) | 2194(62) | 2706(59) | |
| Race/Ethnicity, n (%) | |||
| Caucasian/White | 727(79) | 3140(89) | 3494(77) |
| Others | 184(20) | 384(11) | 1043(23) |
| Unknown/Declined | 5(<1) | 22(<1) | 29(<1) |
| N prior lines of chemotherapy regimens, n (%) | |||
| 1 | 156(17) | 847(24) | 2472(54) |
| 2 | 488(53) | 1425(40) | 1254(27) |
| 3 | 172(19) | 712(20) | 451(10) |
| ≥4 | 72(8) | 416(12) | 205(4) |
| Missing | 28(3) | 146(4) | 184(4) |
| Anthracyclines pre-conditioning, n (%) | |||
| No | 21(2) | 54(2) | 2409(53) |
| Yes | 849(93) | 3180(90) | 1954(43) |
| Missing | 46(5) | 312(9) | 203(4) |
| Alkylating drug pre-conditioning, n (%) | |||
| No | 2(<1) | 22(<1) | 1656(36) |
| Yes | 874(95) | 3338(94) | 2569(56) |
| Missing | 40(4) | 186(5) | 341(7) |
| Etoposide pre-conditioning, n (%) | |||
| No | 214(23) | 1153(33) | 3670(80) |
| Yes | 602(66) | 1964(55) | 592(13) |
| Missing | 100(11) | 429(12) | 304(7) |
| Cyclophosphamide/melphalan pre-conditioning | |||
| No | N/A | 1798(39) | |
| Yes | 2553(56) | ||
| Missing | 215(5) | ||
| Radiation therapy pre-conditioning, n (%) | |||
| No | 451(49) | 2038(57) | 3157(69) |
| Yes | 345(38) | 782(22) | 1259(28) |
| Missing | 120(13) | 726(20) | 150(3) |
| Total body radiation (TBI) conditioning, n (%) | |||
| No | 871(95) | 3034(86) | 4338(95) |
| Yes | 45(5) | 512(14) | 228(5) |
| TBI conditioning dose, n (%) | |||
| None | 871(95) | 3034(86) | 4338(95) |
| ≤10 Gy | 5(<1) | 100(3) | 60(1) |
| >10 Gy | 40(4) | 408(12) | 167(4) |
| Missing | 0 | 4(<1) | 1(<1) |
| Lymphoma conditioning type, n (%) | |||
| TBI-based | 45(5) | 512(14) | N/A |
| BEAM and similar | 577(63) | 2319(65) | |
| CBV and similar | 124(14) | 381(11) | |
| BuMel/BuCy | 87(9) | 197(6) | |
| Others | 83(9) | 137(4) | |
| PCM conditioning type, n (%) | |||
| Melphalan only | N/A | N/A | 3844(84) |
| Melphalan+TBI+/−other | 182(4) | ||
| Melphalan+other (no TBI) | 285(6) | ||
| Bu+ y+/−other | 185(4) | ||
| Others | 70(2) | ||
| Melphalan conditioning dose (mg/m2), n (%) | |||
| None | N/A | N/A | 256(6) |
| 140 | 1430(31) | ||
| 200 | 2660(58) | ||
| Missing | 220(5) | ||
| Median interval diagnosis to transplant (mo; range) | 21(<1−374) | 16(<1−362) | 8(<1−295) |
| <12 | 156(17) | 1323(37) | 3304(72) |
| ≥12 | 758(83) | 2217(63) | 1260(28) |
| Missing | 2(<1) | 6(<1) | 2(<1) |
| Tandem autotransplant, n (%) | |||
| No | 877(96) | 3521(99) | 3806(84) |
| Yes | 39(4) | 25(<1) | 760(17) |
| Year of transplant, n (%) | |||
| 1995-1999 | 338(37) | 1352(38) | 615(13) |
| 2000-2004 | 165(18) | 737(21) | 1244(27) |
| 2005-2010 | 413(45) | 1457(41) | 2707(59) |
| Median follow-up of survivors (mo; range) | 90(3−246) | 110(3−243) | 97(3−236) |
| Kaplan-Meier 5-year survival rate estimates (95% CI) | 67%(64, | 57%(55, | 57(55, |
| 70% | 58%) | 58%) | |
TBI = total body irradiation; BCNU = Bis-chloroethylNitrosourea (i.e. carmustine); BEAM = BCNU, etoposide, cytarabine, and melphalan (Mel); CBV = yclophosphamide, BCNU and etoposide; Bu = Busulfan.
3.2. AML and MDS
Numbers of t-MN posttransplant by diagnosisaredisplayedinTable 2.Cumulative incidences (CI) of developing t-MN are shown in Table 3a and vary between an upper CI limit of 7% for NHL and a lower limit of 2% for PCM. umbers of cases segregated by whether or not an additional major posttransplant event (defined as relapse, subsequent transplant, new cancer), which likely results in increased exposures, occurred (Supplement Table 2).
Table 2:
Number of t-MN after autotransplant according to diagnosis groups and competitive eventsa (n=3335)
| t-MN | Subtotal Nb | Total(N) | ||
|---|---|---|---|---|
| HL | NHL | PCM | ||
| AML | 7 | 45 | 23 | 75 |
| MDS transformed to AMLc | 5 | 26 | 8 | 39 |
| MDS | 19 | 127 | 75 | 221 |
| Total | 31 | 198 | 106 | 335 |
Events = relapse, unplanned 2nd transplant, non-myeloid new malignancy
Two MN cases were excluded because they were diagnosed before day 100
In our analyses these are scored as MDS, so there are 39 + 221 = 260 MDS cases.
Table 3a:
Cumulative incidence over time (and 95% CI) of AML or MDS after autotransplant according to diagnosis group (with death as the competing risk)
| Diagnosis | |||
|---|---|---|---|
| Time post transplant |
HL (n = 915) |
NHL (n = 3,540) |
PCM (n = 4,563) |
| 1-year | 0(0–1)% | 1(0–1)% | 0(0–0)% |
| 3-year | 1(1–2)% | 2(2–3)% | 1(0–1)% |
| 5-year | 3(2–4)% | 4(3–5)% | 1(1–2)% |
| 10-year | 4(3–6)% | 6(5–7)% | 3(2–3)% |
3.3. Risk factors for developing AML or MDS
Hazard ratios (HR) for developing t-MN that were statistically significant in multivariable analyses are displayed in Tables 3b, 3c and 3d for HL, NHL and PCM. In subjects with HL, variables associated with an increased risk of t-MN included: (1) age ≥45 versus <45 years (HR = 5.6 [2.7, 11.7]); and (2) conditioning with TBI versus no TBI (HR = 4.0 [1.4, 11.6]). In subjects with NHL, increased risk of t-MN included: (1) age >55 years (HR = 4.5 [2.6–7.7] versus 45–54 years (HR = 2.7 [1.5–4.9]) versus <45 years; (2) ≥3 versus 1 prior chemotherapy regimens (HR = 1.9 [1.3, 2.8]); (3) transplants in 2005–2010 versus 1995–1999 (HR = 2.1 [1.5, 3.1]); and (4) conditioning by TBI (HR = 1.7 [1.1, 2.5]) or by busulfan and melphalan or cyclophosphamide (HR = 1.8 [1.0, 3.0]) versus BEAM (bischloroethylnitrosourea [BCNU], etoposide, cytarabine, and melphalan). Finally, in subjects with PCM, risk factors for t-MN included: (1) age ≥55 years versus ≤55 years (HR = 2.5 [1.5, 3.9]); (2) ≥3 versus 1 prior chemotherapy regimens (HR = 1.8 [1.0, 3.0]); and (3) male sex (HR = 2.3 [1.4, 3.5]). HR forest plots are given in Supplement Figure 1.
Table 3b.
Risks of developing t-MN after autotransplant for Hodgkin lymphoma (n=915)
| Parameter | n | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|
| Age at transplant, years | |||
| 18–44 | 674 | 1.00 | |
| 45+ | 241 | 5.59(2.68 – 11.70) | <.01 |
| Total body irradiation | |||
| No | 870 | 1.00 | |
| Yes | 45 | 4.02(1.40 – 11.55) | <.01 |
Table 3c:
Risks of developing t-MN after autotransplant for NHL (n=3540)
| Parameter | n | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|
| Age at transplant, years | <.01 | ||
| 18-44 | 748 | 1.00 | |
| 45-54 | 938 | 2.77(1.55 - 4.93) | <.01 |
| 55+ | 1854 | 4.52(2.63 - 7.77) | <.01 |
| No. of prior lines of chemotherapy | <.01 | ||
| 1 | 846 | 1.00 | |
| 2 | 1422 | 0.98(0.67 - 1.44) | 0.91 |
| 3+ | 1128 | 1.93(1.34 - 2.78) | <.01 |
| Missing | 144 | 0.55(0.20 - 1.53) | 0.25 |
| Conditioning | 0.04 | ||
| BEAM or similar | 2316 | 1.00 | |
| TBI based | 510 | 1.69(1.14 - 2.49) | 0.008 |
| CBV or similar | 381 | 0.96(0.59 - 1.54) | 0.86 |
| Bu+Mel/Bu+Cy | 197 | 1.75(1.03 - 2.96) | 0.04 |
| Others | 136 | 1.30(0.60 - 2.82) | 0.51 |
| Year of transplant | <.01 | ||
| 1995-1999 | 1350 | 1.00 | |
| 2000-2004 | 735 | 1.36 (0.91 - 2.05) | 0.14 |
| 2005-2010 | 1455 | 2.12 (1.47 - 3.08) | <.01 |
Table 3d:
Risks of developing t-MN after autotransplants for plasma cell myeloma (n=4653)
| Parameter | n | Hazard Ratio (95% CI) | P-value |
|---|---|---|---|
| Age at transplant, years | |||
| 18-54 | 1661 | 1.00 | |
| 55+ | 2902 | 2.47(1.55 - 3.93) | <.01 |
| Prior Lines of chemotherapy | 0.08 | ||
| 1 | 2472 | 1.00 | |
| 2 | 1252 | 1.21(0.78 - 1.88) | 0.39 |
| 3+ | 655 | 1.77(1.06 - 2.96) | 0.03 |
| Missing | 184 | 0.28(0.04 - 2.06) | 0.21 |
| Sex | |||
| Female | 1859 | 1.00 | |
| Male | 2704 | 2.27(1.45-3.53) | <.01 |
Figure 1.

Relative risks (RR) of AML and MDS after autotransplants (CIBMTR data) or diagnoses (SEER data). A) RR associate with autotransplants, more for MDS than AML. B) MDS/AML ratios of RR are 3–5 for CIBMTR data and 1–2 for SEER data. A-B) 95% CI assume observed cases are Poisson distributed. Dx = diagnosis, Tx = transplant.
3.4. Relative risks of developing AML or MDS
The SEER database from 2001–2014 contained 32,059, 77,990 and 217,602 cases of HL, NHL and PCM, and 50,310 and 53,722 cases of AML and MDS. SEER RRs of AML and MDS were 5–10 compared to CIBMTR RRs after autotransplants of 10–50 for AML and approximately 100 for MDS (Figure 1A), i.e. CIBMTR MDS RRs are 3–5-fold higher than CIBMTR AML RRs but SEER MDS and AML RR are roughly similar (Figure 1B).
Comparing AML and MDS log incidences after HL, NHL or PCM diagnoses to background rates across ages in SEER data, gaps for AML and MDS behave similarly (Figure 2). Younger age of transplant recipients in CIBTMR versus SEER is thus unlikely to be the cause of increased MDS:AML ratios. Further supporting this notion, when we stratified SEER data by age-at-diagnosis, the MDS RR of even the youngest age group was well below that of CIBMTR autotransplant recipients (Figure 3).
Figure 2.
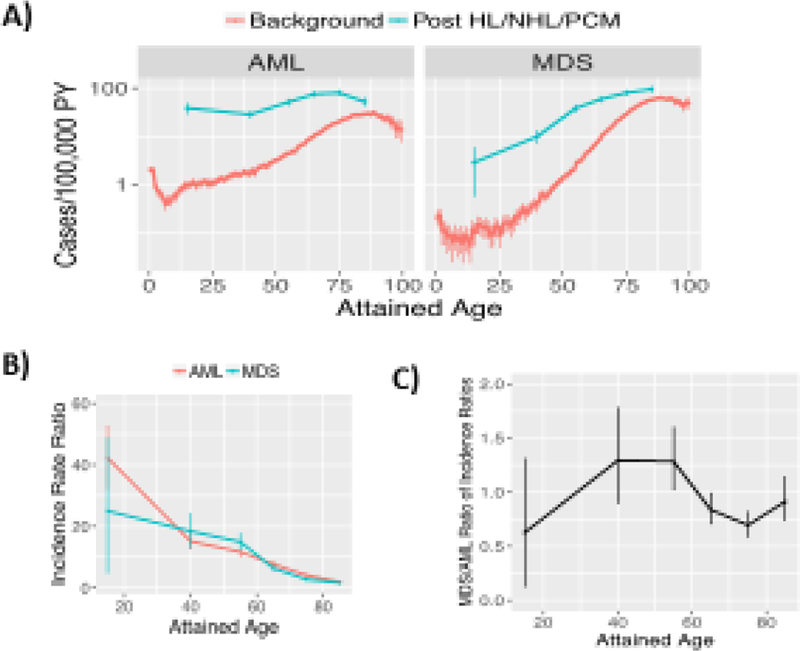
AML and MDS risks vs. age in SEER. A) Absolute AML and MDS risks (incidences) of those exposed to a prior HL, NHL, or PCM versus background rates expected under a null that prior cancers do not matter. [O/PY=observed cases / person years at risk] B) Exposed/background ratios of incidences in . For MDS and AML, at younger ages risks after prior diagnoses of HL, NHL, or PCM, i.e. Op/PYp, are greater multiples (Op/PYp)/(O/PY) of background rates O/PY. C) MDS/AML ratios of ratios in B. These are (Omp/Om)/(Oap/Oa) where m stands for MDS, a for AML, and PY cancel out. A-C) 95% CI (bars) were formed via simulations that assumed O is Poisson distributed.
Figure 3.
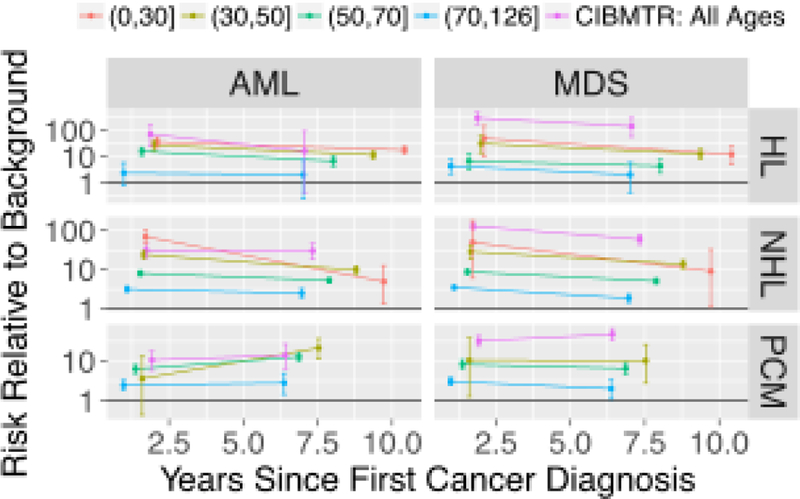
Relative risk (RR) of AML and MDS with SEER data stratified by age-at-diagnosis groups.
4. Discussion
Our data show a markedly increased risk of t-MN in recipients of autotransplants for HL, NHL and PCM compared to persons with similar diagnoses reported in SEER, less then 20% of whom received an autotransplants [10–13]. Several variables were identified in the autotransplant cohort to be associated with increased risks of developing t-MN, including exposures to more chemotherapy regimens and TBI and receiving a transplant recently. It was previously reported that the risks of developing t-MN as new cancers are higher in breast and lung cancer patients given radiation therapy versus not; in these cases AML RRs were approximately 2-fold higher than MDS RRs [9]. Also in that study, after HL, NHL and PCM as primary cancer diagnoses, AML and MDS relative risks were similar in magnitude. These data suggest a probable association between lymphoid and myeloid cancers which is stronger for MDS than for AML in the current study.
The current study further confirms the role of TBI as a strong risk factor for developing t-MNs. Metayer et al. conducted a case-control study in subjects receiving autotransplants for HL or NHL; they reported a statistically significant increased risk for t-MN with TBI in subjects >45 years (RR=4.9; 95% CI, 1.32, 24.78)[14]. Given current results and prior studies it may be prudent to avoid TBI-based pretransplant conditioning regimens.
It is unclear why autotransplants performed more recently associated with higher t-MN risks in NHL patients. This was not found in subjects with PCM. It is also unclear why in PCM, the use of TBI was not associatedwithasignificantincreaseint-MN. Perhaps drugs used preferentially in lymphoma conditioning interact preferentially with TBI[13].
An important finding of our study is that autotransplant recipients have high RR (up to 100) for developing subsequent t-MN compared to similar subjects most of whom did not receive an autotransplant (RRs = 5–10). These increases may be related to exposure to high doses of DNA-damaging drugs given for the autotransplant but this hypothesis can only be tested in a prospective study. Other limitations of our study include possible reporting bias inherent to all voluntary reporting registries. trengths of our study include its large sample size and its comparisons to risks in SEER data.
In an early study of 206 subjects receiving autotransplants, 8 developed MDS and 1 AML [7]. Given MDS and AML risks are approximately equal in SEER data [11], these data suggest autotransplants may cause more MDS than AML. Compared with a recent study in which cytogenetically-defined MDS was detected in approximately 3% of approximately 3000 persons with PCM receiving autotransplants[6], our study differs in that it: 1) includes subjects with HL and NHL; 2) estimates effects of prior therapies and conditioning regimens; 3) estimates risks relative to background rates in SEER; and 4) compares MDS and AML risks.
The reason(s) for the high relative risks of AML and MDS after autotransplants (~10–50 for AML and ~100 for MDS) in our analysis is unknown. Approximately 2-fold higher ML than MDS risks after breast and lung cancer diagnoses[9], similar risks post thyroid cancer diagnoses[15, 16], and an MDS RR of ~13[17] versus an AML RR of ~11[18] for persons exposed to 3 Gy of atomic bomb radiation, suggest that radiation, and by extension, radiomimetic drugs (e.g. cyclophosphamide and melphalan), are unlikely causes of the higher MDS risks, relative to AML risks, observed here after transplants. One possible explanation is that many cases of MDS evolve to AML, and that earlier diagnosis from increased post-transplant surveillance resulted in a deficiency of AML. A second is based on steeper MDS versus AML incidences versus age (Figure 2A) and the possibility that transplantation recipient marrow ages (i.e. marrow biological ages) are perhaps decades older than calendar ages. In this scenario, the process of repopulating the bone marrow causes hematopoietic cell aging along a system state dimension associated with AML and MDS risks. In healthy elderly, clonally expanded mutations in TET2 have a stronger age-dependence than mutations in DNMT3A and are found in MDS more than in AML[19–21]. This suggests that the transplantation process favors expansions of clones with TET2 mutations over clones with DNMT3A mutations, i.e. clones that have a greater propensity to evolve to MDS than AML.
Supplementary Material
Highlights.
TBI conditioning for autotransplantation is associated with higher risks of t-MN
Risks of t-MN after autotransplantation for NHL are increasing
Autotransplantation increases risks of MDS more than AML
Acknowledgements.
We thank the R community for the provision of free software.
Funding. The CIBMTR is supported primarily by Public Health Service Grant/Cooperative Agreement 5U24CA076518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 4U10HL069294 from NHLBI and NCI; a contract HHSH250201200016C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014–17-1–2388 and N0014–17-1–2850 from the Office of Naval Research; and grants from *Actinium Pharmaceuticals, Inc.; *Amgen, Inc.; *Amneal Biosciences; *Angiocrine Bioscience, Inc.; Anonymous donation to the Medical College of Wisconsin; Astellas harma US; Atara Biotherapeutics, Inc.; Be the Match Foundation; *bluebird bio, Inc.; *Bristol Myers Squibb Oncology; *Celgene Corporation; Cerus Corporation; * himerix, Inc.; Fred Hutchinson Cancer Research Center; Gamida Cell Ltd.; Gilead Sciences, Inc.; HistoGenetics, Inc.; Immucor; *Incyte Corporation; Janssen Scientific Affairs, LLC; *Jazz Pharmaceuticals, Inc.; Juno Therapeutics; Karyopharm Therapeutics, Inc.; Kite Pharma, Inc.; Medac, GmbH; MedImmune; The Medical College of Wisconsin; *Mediware; *Merck & Co, Inc.; *Mesoblast; MesoScale Diagnostics, Inc.; Millennium, the Takeda Oncology Co.; *Miltenyi Biotec, Inc.; National Marrow Donor Program; *Neovii Biotech NA, Inc.; Novartis Pharmaceuticals Corporation; Otsuka Pharmaceutical Co, Ltd. – Japan; PCORI; *Pfizer, Inc; *Pharmacyclics, LLC; PIRCHE AG; *Sanofi Genzyme; *Seattle Genetics; Shire; Spectrum Pharmaceuticals, Inc.; St. Baldrick’s Foundation; *Sunesis Pharmaceuticals, Inc.; Swedish Orphan Biovitrum, Inc.; Takeda Oncology; Telomere Diagnostics, Inc.; and University of Minnesota. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, theDepartmentofDefense,HealthResources and Services Administration (HRSA) or any other agency of the U.S. Government. TR is supported by the National Aeronautics and Space Administration (NNJ13ZSA001N). RPG acknowledges support from the National Institute of Health Research (NIH ) Biomedical Research Centre funding scheme. *Corporate Members
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: No relevant COI by any author
REFERENCES
- [1].Gale RP, Bennett JM, Hoffman FO, Who Has Therapy-Related AML?, Mediterranean journal of hematology and infectious diseases 9(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lam CJ, Curtis RE, Dores GM, Engels EA, Caporaso NE, Polliack A, Warren JL, Young HA, Levine PH, Elmi AF, Risk factors for second acute myeloid leukemia/myelodysplastic syndrome among survivors of non-Hodgkin lymphoma, Leukemia 30(5) (2016) 1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Granfeldt Østgård LS, Medeiros BC, Sengeløv H, Nørgaard M, Andersen MK, Dufva IH, Friis LS, Kjeldsen E, Marcher CW, Preiss B, Epidemiology and clinical significance of secondary and therapy-related acute myeloid leukemia: a national population-based cohort study, Journal of Clinical Oncology 33(31) (2015) 3641–3649. [DOI] [PubMed] [Google Scholar]
- [4].Miller KD, Siegel RL, Lin CC, Mariotto AB, Kramer JL, Rowland JH, Stein KD, Alteri R, Jemal A, Cancer treatment and survivorship statistics, 2016, CA: a cancer journal for clinicians 66(4) (2016) 271–289. [DOI] [PubMed] [Google Scholar]
- [5].Majhail NS, Tao L, Bredeson C, Davies S, Dehn J, Gajewski JL, Hahn T, Jakubowski A, Joffe S, Lazarus HM, Prevalence of hematopoietic cell transplant survivors in the united States, Biology of Blood and Marrow Transplantation 19(10) (2013) 1498–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Barlogie B, Tricot G, Haessler J, van Rhee F,Cottler-Fox M,Anaissie E,Waldron J,Pineda-Roman M, Thertulien R, Zangari M, Cytogenetically defined myelodysplasia after melphalan-based autotransplantation for multiple myeloma linked to poor hematopoietic stem-cell mobilization: the Arkansas experience in more than 3000 patients treated since 1989, Blood 111(1) (2008) 94–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miller JS, Arthur DC, Litz CE, Neglia JP, iller WJ, Weisdorf DJ, Myelodysplastic syndrome after autologous bone marrow transplantation: an additional late complication of curative cancer therapy, Blood 83(12) (1994) 3780–3786. [PubMed] [Google Scholar]
- [8].SEER, Surveillance, Epidemiology, and End Results (SEER) Program Research Data (1973–2012), National Cancer Institute, TEDDCCPS, Surveillance Research Program, Surveillance Systems Branch (www.seer.cancer.gov), released April 2016, based on the November 2015 submission, (2016).
- [9].Radivoyevitch T, Sachs R, Gale R, Molenaar R, Brenner D, Hill B, Kalaycio ME, Carraway HE, Mukherjee S, Sekeres M, Defining AML and MDS second cancer risk dynamics after diagnoses of first cancers treated or not with radiation, Leukemia 30(2) (2016) 285. [DOI] [PubMed] [Google Scholar]
- [10].Shah GL, Winn A, .Lin P-J, Klein A, Sprague KA, Smith HP, Buchsbaum R, Cohen JT, Miller KB, Comenzo R, Cost implications of comorbidity for autologous stem cell transplantation in elderly patients with multiple myeloma using SEER-Medicare, Bone marrow research 2016 (2016). [DOI] [PMC free article] [PubMed]
- [11].Winn AN, Shah GL, Cohen JT, Lin P-J, Parsons SK, The real world effectiveness of hematopoietic transplant among elderly individuals with multiple myeloma, Journal of the National Cancer Institute 107(8) (2015) djv139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Warren JL, Harlan LC, Stevens J, Little RF, Abel GA, Multiple myeloma treatment transformed: a population-based study of changes in initial management approaches in the United States, Journal of Clinical Oncology 31(16) (2013) 1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Krishnan A, Bhatia S, Slovak ML, Arber DA, Niland JC, Nademanee A, Fung H, Bhatia R, Kashyap A, Molina A, Predictors of therapy-related leukemia and myelodysplasia following autologous transplantation for lymphoma: an assessment of risk factors, Blood 95(5) (2000) 1588–1593. [PubMed] [Google Scholar]
- [14].Metayer C, Curtis RE, Vose J, Sobocinski KA, Horowitz MM, Bhatia S, Fay JW, Freytes CO, Goldstein SC, Herzig RH, Myelodysplastic syndrome and acute myeloid leukemia after autotransplantation for lymphoma: a multicenter case-control study, Blood 101(5) (2003) 2015–2023. [DOI] [PubMed] [Google Scholar]
- [15].Molenaar R, Pleyer C, Radivoyevitch T, Sidana S, Godley A, Advani A, Gerds A, Carraway H, Kalaycio M, Nazha A, Risk of developing chronic myeloid neoplasms in well-differentiated thyroid cancer patients treated with radioactive iodine, Leukemia 32(4) (2018) 952. [DOI] [PubMed] [Google Scholar]
- [16].Molenaar RJ, Sidana S, Radivoyevitch T, Advani AS, Gerds AT, Carraway HE, Angelini D, Kalaycio M, Nazha A, Adelstein DJ, Risk of Hematologic MalignanciesAfterRadioiodineTreatmentof Well-Differentiated Thyroid Cancer, Journal of Clinical Oncology (2017) JCO 201775 0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hsu W-L, Preston DL, Soda M, Sugiyama H, Funamoto S, Kodama K, Kimura A, Kamada N, Dohy H, Tomonaga M, The incidence of leukemia, lymphoma and multiple myeloma among atomic bomb survivors: 1950–2001, Radiation research 179(3) (2013) 361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Iwanaga M, Hsu W-L, Soda M, Takasaki Y, Tawara M, Joh T, Amenomori T, Yamamura M, Yoshida Y, Koba T, Risk of myelodysplastic syndromes in people exposed to ionizing radiation: a retrospective cohort study of Nagasaki atomic bomb survivors, Journal of Clinical Oncology 29(4) (2010) 428–434. [DOI] [PubMed] [Google Scholar]
- [19].Buscarlet M, Provost S, Zada YF, Barhdadi A, Bourgoin V, Lépine G, Mollica L, Szuber N, Dubé M-P, Busque L, DNMT3A and TET2 dominate clonal hematopoiesis and demonstrate benign phenotypes and different genetic predispositions, Blood 130(6) (2017) 753–762. [DOI] [PubMed] [Google Scholar]
- [20].Hirsch CM, Przychodzen BP, Radivoyevitch T, Patel B, Thota S, Clemente MJ, Nagata Y, LaFramboise T, Carraway HE, Nazha A, Molecular features of early onset adult myelodysplastic syndrome, haematologica 102(6) (2017) 1028–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sperling AS, Gibson CJ, Ebert BL, The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia, Nature Reviews Cancer 17(1) (2017) 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Agathocleous M, Meacham CE, Burgess RJ, Piskounova E, Zhao Z, Crane GM, Cowin BL, Bruner E, Murphy MM, Chen W, Ascorbate regulates haematopoietic stem cell function and leukaemogenesis, Nature 549(7673) (2017) 476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Cimmino L,Dolgalev I,Wang Y,Yoshimi A, Martin GH, Wang J, Ng V, Xia B, Witkowski MT, Mitchell-Flack M, Restoration of TET2 function blocks aberrant self-renewal and leukemia progression, Cell 170(6) (2017) 1079–1095. e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


