Abstract
Background/Objectives
Specific maternal risk factors have recently been identified in the development of infantile hemangiomas (IH), including gestational diabetes (GDM), maternal anti-hypertensive medication use and/or gestational hypertension (GHTN), maternal progesterone use, and artificial reproductive technologies (ART). We sought to explore the change in incidence of these risk factors over time, and determine their association with the increased incidence of hemangiomas over 35 years, as previously reported.
Methods
The charts of 869 mother and infant pairs (infants previously diagnosed with IH between January 1, 1976 and December 31, 2010) were reviewed for prenatal complications. Rates of the prenatal complications over the 35-year period in birth mothers of infants diagnosed with IH were determined, and evaluated by year of diagnosis (1976–1990, 1991–2000 and 2001–2010).
Results
Over the 35-year period in which the incidence of IH was previously examined, maternal age at delivery, pre-pregnancy body mass index (BMI), use of ART, maternal progesterone use, placental abnormalities, and GDM also increased.
Conclusions
GDM, ART, and maternal progesterone use increased over the past 35 years, mirroring the previously reported trend of increasing incidence of IH. Maternal age and BMI also increased in mothers of infants with IH. Further exploration of this association may direct future research in the pathogenesis of infantile hemangiomas.
Keywords: Infantile hemangioma, temporal trend, risk factor
Introduction
Previous studies seeking to describe maternal risk factors implicated in the development of infantile hemangiomas (IH) have determined that multiple gestation, female sex, low birth weight, prematurity, use of certain prenatal medications (progesterone and corticosteroids), invasive antepartum procedures, and placental abnormalities increase infant risk for IH.1–5 Recently, gestational diabetes mellitus (GDM) and gestational hypertension were also identified as a significant risk factors.6 This population-based study was designed to explore the prenatal risk factors associated with IH, including trends over time and the association with the previously-reported increasing incidence of IH.1
Materials and Methods
Institutional Review Board approval was obtained. The resources of the Rochester Epidemiology Project (REP) were used to identify 999 children less than one year of age who were residents of Olmsted County, Minnesota, at the time of diagnosis of infantile hemangioma between January 1, 1976 and December 31, 2010. Medical records were available for the birth mother for 869 of the 999 infants. Each of the medical records was reviewed by one of the primary authors (KA, MH, or JS) to extract data, including demographics, pregnancy complications, and medications.
The REP is a medical records linkage system allowing for access to records for nearly all patient encounters in Olmsted County, Minnesota, since the 1960s. This resource provided a means to identify all diagnosed cases of IH in a geographically defined community; the REP also links maternal medical records to infant charts. The potential for the REP database to be used in a population-based study has been previously described.7–9
Categorical features were summarized with frequency counts and percentages; continuous features were summarized with medians and interquartile ranges (IQRs). Associations of features of the birth mothers over time were evaluated using Spearman rank correlation coefficients and Cochran-Armitage trend tests. Statistical analyses were performed using version 9.4 of the SAS software package (SAS Institute, Cary, NC). All tests were two-sided and p-values <0.05 were considered statistically significant.
Results
Rates of the prenatal characteristics over a 35-year period in birth mothers of infants diagnosed with IH were determined. For comparison, years of diagnosis were grouped together as follows: 1976–1990, 1991–2000 and 2001–2010 (Table 1).
Table 1.
Comparison of birth mother features by year of infantile hemangioma diagnosis
| Feature | 1976–1990 | 1991–2000 | 2001–2010 | P-value |
|---|---|---|---|---|
| N=266 | N=257 | N=346 | ||
| Median (IQR) | ||||
| Age at delivery | 27 (24–30) | 29 (26–33) | 29.5 (26–33) | <0.001 |
| Pre-pregnancy body mass index (N=815) | 21.5 (19.9–23.9) | 22.8 (20.6–26.2) | 23.9 (21.4–27.0) | <0.001 |
| Gravida (N=868)* | 2 (1–2) | 2 (1–3) | 2 (1–3) | 0.40 |
| Para (N=868)† | 2 (1–2) | 2 (1–2) | 1 (1–2) | 0.88 |
| Race (N=815) | N (%) | |||
| White | 237 (98) | 222 (93) | 285 (85) | <0.001 |
| Non-white | 4 (2) | 17 (7) | 50 (15) | |
| Prenatal care (N=836) | ||||
| Early | 232 (90) | 228 (92) | 296 (89) | 0.68 |
| Late or none | 25 (10) | 20 (8) | 35 (11) | |
| Delivery type (N=866) | ||||
| Vaginal | 160 (60) | 157 (61) | 230 (67) | <0.001 |
| Cesarean | 40 (15) | 55 (21) | 92 (27) | |
| Vaginal vacuum-assisted | 7 (3) | 12 (5) | 10 (3) | |
| Vaginal forceps-assisted | 58 (22) | 32 (13) | 13 (4) | |
| Fertility treatment | ||||
| Medication | 6 (2) | 18 (7) | 8 (2) | 0.83 |
| Assisted reproductive technology | 1 (<1) | 12 (5) | 21 (6) | <0.001 |
| Other | 1 (<1) | 0 | 0 | 0.19 |
| Any of the above | 7 (3) | 24 (9) | 28 (8) | 0.012 |
| Maternal medication | ||||
| Progesterone | 1 (<1) | 29 (11) | 64 (19) | <0.001 |
| Tocolytic | 12 (5) | 25 (10) | 20 (6) | 0.65 |
| Nifedipine (N=866) | 0 | 0 | 1 (<1) | 0.28 |
| Indomethacin (N=866) | 3 (1) | 4 (2) | 3 (1) | 0.73 |
| Terbutaline (N=866) | 10 (4) | 19 (7) | 12 (3) | 0.74 |
| Magnesium sulfate (N=866) | 0 | 0 | 0 | NA |
| Other tocolytic (N=866) | 7 (3) | 10 (4) | 7 (2) | 0.59 |
| Beta-blocker | 2 (1) | 2 (1) | 2 (1) | 0.79 |
| Non beta-blocker anti-hypertensive | 2 (1) | 1 (<1) | 2 (1) | 0.80 |
| Corticosteroid | 6 (2) | 17 (7) | 43 (12) | <0.001 |
| Any of the above | 16 (6) | 60 (23) | 99 (29) | <0.001 |
| Invasive antepartum procedures | ||||
| Amniocentesis | 20 (8) | 33 (13) | 17 (5) | 0.16 |
| Chorionic villus sampling | 0 | 1 (<1) | 8 (2) | 0.004 |
| Other | 1 (<1) | 2 (1) | 0 | 0.38 |
| Any of the above | 21 (8) | 36 (14) | 25 (7) | 0.62 |
| Placental abnormalities | ||||
| Single umbilical artery | 0 | 0 | 3 (1) | 0.059 |
| Placenta previa | 1 (<1) | 1 (<1) | 4 (1) | 0.23 |
| Placenta accrete/percreta/increta | 0 | 0 | 1 (<1) | 0.28 |
| Placental abruption | 4 (2) | 9 (4) | 11 (3) | 0.23 |
| Other | 4 (2) | 5 (2) | 9 (3) | 0.34 |
| Any of the above | 9 (3) | 15 (6) | 28 (8) | 0.015 |
| Maternal complications | ||||
| Twin gestation | 11 (4) | 18 (7) | 25 (7) | 0.13 |
| Triplet gestation | 0 | 4 (2) | 0 | 0.82 |
| Gestational diabetes | 3 (1) | 15 (6) | 26 (8) | <0.001 |
| Pre-existing diabetes | 1 (<1) | 3 (1) | 5 (1) | 0.20 |
| Gestational hypertension | 14 (5) | 13 (5) | 18 (5) | 0.98 |
| Pre-existing hypertension | 2 (1) | 4 (2) | 4 (1) | 0.68 |
| Anemia | 19 (7) | 24 (9) | 27 (8) | 0.82 |
| Preeclampsia/eclampsia | 5 (2) | 5 (2) | 9 (3) | 0.53 |
| Gestational hypertension or pre/eclampsia | 18 (7) | 16 (6) | 26 (8) | 0.69 |
| HELLP syndrome | 0 | 1 (<1) | 3 (1) | 0.11 |
| Hypothyroidism | 7 (3) | 12 (5) | 7 (2) | 0.57 |
| PPROM | 5 (2) | 16 (6) | 20 (6) | 0.031 |
| IUGR | 6 (2) | 3 (1) | 8 (2) | 0.90 |
| Breech presentation | 13 (5) | 23 (9) | 24 (7) | 0.40 |
| Vaginal bleeding | 24 (9) | 22 (9) | 30 (9) | 0.89 |
| Oligohydramnios | 2 (1) | 4 (2) | 10 (3) | 0.048 |
| Polyhydramnios | 3 (1) | 4 (2) | 1 (<1) | 0.24 |
| UTI | 14 (5) | 16 (6) | 23 (7) | 0.48 |
| Tobacco use | 38 (14) | 33 (13) | 33 (10) | 0.068 |
| Alcohol use | 0 | 0 | 3 (1) | 0.059 |
| Other substance use | 0 | 1 (<1) | 8 (2) | 0.004 |
| Other | 62 (23) | 80 (31) | 111 (32) | 0.021 |
| Any of the above | 142 (53) | 177 (69) | 221 (64) | 0.014 |
| Any of the above excluding other | 116 (44) | 143 (56) | 173 (50) | 0.16 |
Mean gravida was 2.0, 2.5, and 2.3, respectively.
Mean para was 1.8, 1.9, and 1.8, respectively.
NA: Not applicable
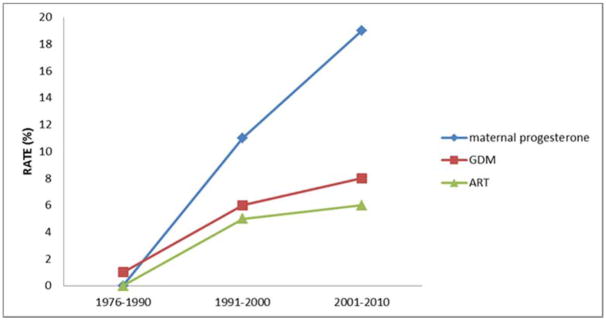
Rates of ART in this population increased from <1% to 6% (p<0.001) between 1976 and 2010. The use of prenatal maternal medications also increased over the 35-year period. Of the maternal medications studied, prenatal progesterone use significantly increased from <1% to 19% (p<0.001) over the study period; corticosteroid use also significantly increased from 2% to 12% (p<0.001). Placental abnormalities increased from 3% to 8% (p=0.015). The prevalence of birth mothers with a diagnosis of GDM increased from 1% to 8% (p< 0.001) over the 35-year period (Figure 1), correlating with an overall increase in median maternal BMI, which increased from 21.5 to 23.9 (p<0.001). Median age of mothers to infants with IH also increased throughout the study period from 27-years-old at delivery to 29.5-years-old at delivery (p<0.001) (Table 1).
Figure 1. Temporal trends of select prenatal risk factors between 1976–2010.
Rate of assisted reproductive technologies increased from <1% to 6% (p<0.001) over the 35 years. The use of maternal progesterone increased from <1% to 19% (p<0.001). The percentage of birth mothers with a diagnosis of GDM increased from 1% to 8% (p< 0.001) over the 35-year period.
Discussion
The incidence of IH is steadily increasing and positively correlates with decreasing gestational age at birth and birth weight.1,6 Over the same time period, maternal age, maternal BMI, the use of ART, maternal progesterone and corticosteroid use, placental abnormalities, and GDM also increased in our cohort of mothers to infants with IH.
ART use increased over time, likely due to technology advances and increased availability of reproductive medicine in the same time period. Progesterone and corticosteroid use also increased throughout the study period. These factors are likely closely related, as progesterone may be given to maintain pregnancy in the setting of previous pregnancy loss, and corticosteroids may be given to accelerate fetal lung development in women at risk for premature birth.10
GDM prevalence increased over the 35-year period, correlating with increasing incidence of IH over the same period. The increasing prevalence of GDM mirrors the GDM trend observed in the general U.S population and may be partially attributable to the increase in median maternal BMI also observed over the 35-year period.11,12 Several of the known fetal complications in GDM are likely secondary to placental abnormalities13; interestingly the prevalence of placental abnormalities also increased over the study period. Therefore, increasing maternal BMI likely explains the increase in GDM, leading to the increase in placental abnormalities, and thus IH.
Increasing maternal age may be the underlying factor that links these prenatal risk factors. Median maternal age increased throughout the study period from 27-years-old at delivery to 29.5-years-old at delivery (p<0.001). Increased maternal age was significantly associated with ART use, maternal progesterone use, maternal corticosteroid use, placental abnormalities, and GDM (Table 2). Maternal age also negatively correlated with gestational age (r = −0.11, p = 0.001) and positively correlated with BMI (r = 0.09, p = 0.009). The underlying causality of this association warrants further investigation.
Table 2.
Association of select prenatal risk factors and maternal age
| Feature | Median Age (IQR) | Median Age (IQR) | P-value |
|---|---|---|---|
| Without risk factor | With risk factor | ||
| Assisted reproductive therapy | 28 (25–32) | 33 (29–35) | <0.001 |
| Progesterone | 28 (25–32) | 31 (28–34) | <0.001 |
| Corticosteroids | 28 (25–32) | 30 (27–35) | 0.039 |
| Placental abnormalities | 28 (25–32) | 31 (27–34) | 0.012 |
| Gestational diabetes mellitus | 28 (25–32) | 32 (28–36) | <0.001 |
Limitations of this study include that the population of Olmsted County is predominantly a non-Hispanic white population, which may lead to population bias when reporting the occurrence of prenatal characteristics. The retrospective nature of this study may limit the ability to accurately characterize some maternal risk factors. Strengths of this study include the ability to utilize the REP to identify birth mother medical records of infants with IH, and to examine the trends of prenatal risk factors over a 35-year period.
Thus, the recent increase in the incidence of infantile hemangiomas may be wholly or partially explained by a concomitant increase in placental abnormalities, as well as an increase in maternal age. Better understanding of the association and trends of these risk factors in the development of IH will direct further research in the pathogenesis of IH.
Acknowledgments
FUNDING SOURCE
This study was made possible using the resources of the Rochester Epidemiology Project, which is supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS
- ART
artificial reproductive technologies
- BMI
body mass index
- GDM
gestational diabetes
- GHTN
gestational hypertension
- IH
infantile hemangiomas
- IQR
interquartile range
- REP
Rochester Epidemiology Project
Footnotes
CONFLICTS OF INTEREST STATEMENT
The authors of this manuscript have no conflicts of interest including any financial, personal, academic or intellectual issues.
PREVIOUS PUBLICATION
Portions of this data were previously presented at the Society of Pediatric Dermatology Annual Meeting, Minneapolis, MN; July 14–17, 2016.
References
- 1.Anderson KR, Schoch JJ, Lohse CM, Hand JL, Davis DM, Tollefson MM. Increasing incidence of infantile hemangiomas (IH) over the past 35 years: Correlation with decreasing gestational age at birth and birth weight. J Am Acad Dermatol. 2016;74(1):120–126. doi: 10.1016/j.jaad.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Drolet BA, Swanson EA, Frieden IJ Hemangioma Investigator Group. Infantile hemangiomas: an emerging health issue linked to an increased rate of low birth weight infants. J Pediatr. 2008;153(5):712–715. 715.e1. doi: 10.1016/j.jpeds.2008.05.043. [DOI] [PubMed] [Google Scholar]
- 3.Munden A, Butschek R, Tom WL, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol. 2014;170(4):907–913. doi: 10.1111/bjd.12804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J, Chen X, Zhao S, et al. Demographic and clinical characteristics and risk factors for infantile hemangioma: a Chinese case-control study. Arch Dermatol. 2011;147(9):1049–1056. doi: 10.1001/archdermatol.2011.122. [DOI] [PubMed] [Google Scholar]
- 5.Burton BK, Schulz CJ, Angle B, Burd LI. An increased incidence of haemangiomas in infants born following chorionic villus sampling (CVS) Prenat Diagn. 1995;15(3):209–214. doi: 10.1002/pd.1970150302. [DOI] [PubMed] [Google Scholar]
- 6.Hunjan MK, Schoch JJ, Anderson KR, et al. Prenatal Risk Factors for the Development of Infantile Hemangiomas. J Invest Dermatol. 2016 Dec; doi: 10.1016/j.jid.2016.10.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.St Sauver JL, Grossardt BR, Yawn BP, et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol. 2012;41(6):1614–1624. doi: 10.1093/ije/dys195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Melton LJ. History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71(3):266–274. doi: 10.1016/S0025-6196(11)63966-9. [DOI] [PubMed] [Google Scholar]
- 9.Rocca WA, Yawn BP, St Sauver JL, Grossardt BR, Melton LJ. History of the Rochester Epidemiology Project: half a century of medical records linkage in a US population. Mayo Clin Proc. 2012;87(12):1202–1213. doi: 10.1016/j.mayocp.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts D, Brown J, Medley N, Dalziel SR. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2017;3:CD004454. doi: 10.1002/14651858.CD004454.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dabelea D, Snell-Bergeon JK, Hartsfield CL, et al. Increasing prevalence of gestational diabetes mellitus (GDM) over time and by birth cohort: Kaiser Permanente of Colorado GDM Screening Program. Diabetes Care. 2005;28(3):579–584. doi: 10.2337/diacare.28.3.579. [DOI] [PubMed] [Google Scholar]
- 12.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991–2000. Obstet Gynecol. 2004;103(3):526–533. doi: 10.1097/01.AOG.0000113623.18286.20. [DOI] [PubMed] [Google Scholar]
- 13.Jarmuzek P, Wielgos M, Bomba-Opon D. Placental pathologic changes in gestational diabetes mellitus. Neuro Endocrinol Lett. 2015;36(2):101–105. [PubMed] [Google Scholar]