Abstract
Aims/hypothesis
Ceramides are sphingolipids that contribute to insulin resistance in preclinical studies. We hypothesised that plasma ceramides would be associated with body fat distribution, insulin resistance and incident type 2 diabetes in a multi-ethnic cohort.
Methods
A total of 1557 participants in the Dallas Heart Study without type 2 diabetes underwent measurements of metabolic biomarkers, fat depots by MRI, and plasma ceramides by liquid chromatography-mass spectrometry. Diabetes outcomes were assessed after 7 years. Associations of body fat and insulin resistance with ceramides at baseline and of ceramides with incident diabetes outcomes were analysed.
Results
The cohort had mean age 43 years, with 58% women, 45% African-Americans and mean BMI 28 kg/m2. Total cholesterol levels were associated with all ceramides, but higher triacylglycerols and lower HDL-cholesterol and adiponectin were associated only with saturated fatty acid chain ceramides (p<0.0003). After adjusting for clinical characteristics and total body fat, visceral adipose tissue was positively associated with saturated fatty acid ceramides (per SD, β= 0.16 to 0.18) and inversely associated with polyunsaturated fatty acid ceramides (β= −0.14 to −0.16, p<0.001 for all). Lower-body subcutaneous fat showed an opposite pattern to that for visceral fat. HOMA-IR was positively associated with saturated (β=0.08 to 0.09, p<0.001) and inversely with polyunsaturated ceramides (β=−0.06 to −0.07, p<0.05). Ceramides were not associated with incident type 2 diabetes after adjustment for clinical factors.
Conclusions/interpretation
Plasma ceramides demonstrate a biologically complex relationship with metabolic and imaging indicators of dysfunctional adiposity. The role of ceramides in a shared pathway of metabolic dysfunction linking visceral adiposity and insulin resistance requires further investigation.
Keywords: Ceramides, Liver fat, Obesity, Prediabetes, Type 2 diabetes mellitus, Visceral adiposity
Introduction
Obesity predisposes to the development of insulin resistance when storage capacity in adipocytes is exceeded and fat accumulates in tissues not suited for lipid storage, such as the visceral adipose tissue (VAT) depot and other ectopic sites [1, 2]. VAT is linked to adipose tissue dysfunction, inflammation, stimulation of lipolysis and delivery of excess gluconeogenic substrates such as NEFA and glycerol to the liver [3–5], resulting in inhibition of insulin signal transduction, hepatic and peripheral insulin resistance, and type 2 diabetes mellitus. Chronic exposure to excess saturated NEFA in the setting of visceral obesity can also lead to de novo biosynthesis of ceramides [6], members of the sphingolipid family that are ubiquitous lipid constituents of cell membranes and play a key role in the regulation of signal transduction pathways [7]. Abnormal accumulation of ceramides may contribute to lipid-induced skeletal muscle and hepatic insulin resistance [8, 9], especially among those with obesity [10], and is a putative intermediate link between excess adiposity, inflammation and metabolic diseases, such as type 2 diabetes mellitus [11, 12].
However, existing evidence regarding the role of ceramides in insulin resistance syndromes and type 2 diabetes comes mostly from in vitro experiments, animal models or translational studies with limited sample sizes [13, 14]. There are no large-scale epidemiological cohort studies evaluating the relationship of ceramides to visceral adiposity or development of type 2 diabetes. Therefore, in order to better understand the aetiology of type 2 diabetes and the role of ceramides in this process, we aimed to investigate: (1) the association of various body fat depots, including visceral, abdominal subcutaneous, lower-body subcutaneous and liver fat with circulating ceramide moieties previously implicated in the insulin resistance syndrome; and (2) the association between ceramides, insulin resistance and incident type 2 diabetes and prediabetes (i.e. impaired fasting glucose) in the large multi-ethnic Dallas Heart Study (DHS) cohort. We hypothesised that visceral adiposity would be associated with ceramide species and that ceramides would be associated with insulin resistance and diabetes outcomes independent of traditional risk factors (including BMI), implicating a visceral adiposity–ceramide link in the development of insulin resistance and type 2 diabetes.
Methods
Study population
The DHS is a single-site multi-ethnic population-based probability sample of Dallas County residents (aged 18–65 years), with deliberate oversampling of black participants. Details regarding the design of the DHS have been previously described [15]. The current study population included 3072 participants who completed DHS phase 1 (DHS-1) visits from 2000 to 2002, which included a computerassisted survey, anthropometric and blood pressure measurements, laboratory testing and imaging assessments. Participants with prevalent type 2 diabetes mellitus (defined by clinical history and/or laboratory assessment) or cardiovascular disease (defined by clinical history of coronary heart disease, heart failure or ischaemic stroke) and those without ceramide measurements or without imaging assessment of VAT were excluded. Participants were subsequently followed over time for clinical outcomes and a repeat visit, in DHS phase 2 (DHS-2), was completed during the period from 2007 to 2009, at which metabolic measures were repeated and assessment of diabetes status was performed. Participants who did not attend a follow-up appointment during DHS-2 were excluded. There were no major differences in medical history, demographics or biomarker data between eligible participants who did and did not complete DHS-2 [3].
All participants provided written informed consent and the University of Texas Southwestern Medical Center Institutional Review Board approved the protocol.
Demographics, lifestyle and other risk factors were determined from a baseline questionnaire. BMI was calculated as kg/m2. Hypertension was defined as BP ≥ 140/90 mmHg or taking antihypertensive medication(s). Smoking was defined as cigarette use within the previous 30 days and/or a lifetime history of having smoked ≥ 100 cigarettes. Comorbid conditions were determined from selfreport, medication history and clinical assessment.
Body fat distribution assessments
Participants were scanned by a 1.5 T MRI scanner (Intera, Philips Healthcare, Best, the Netherlands). Retroperitoneal, intraperitoneal and subcutaneous adipose tissue abdominal fat masses were quantified by a single MRI slice taken at the L2–L3 level using manual contours, as previously validated against cadaveric samples [16]. Areas were converted to mass (kg) using previously determined regression equations [17]. VAT was defined as the combination of both retroperitoneal and intraperitoneal fat masses [3]. Participants also underwent 1H-magnetic resonance spectroscopy for hepatic triacylglycerol quantification, as previously described [18]. Non-alcoholic fatty liver disease (NAFLD) was defined as hepatic triacylglycerol content ≥ 5.5% [19]. Dual x-ray absorptiometry (DXA) was performed with a Delphi W scanner (Hologic, Bedford, MA, USA) and analysed using Apex 13.4.2 version software to determine total and segmental fat and lean mass. Lowerbody fat was quantified as the total fat mass from the lower extremities, hips and buttocks, as previously described [20].
Ceramides and other laboratory measurements
Fasting plasma samples were obtained from participants by venepuncture and collected into EDTA-containing tubes and stored at −80°C. Ceramides and NEFA were measured by ultra-performance liquid chromatography coupled with a hybrid quadrupole orthogonal time of flight mass spectrometer (UPLC-QTOF Synapt G2, Waters, Milford, MA, USA). The data were collected in positive and negative ion electrospray using accurate mass mode so that elemental compositions were obtained < 5ppm. Quantification of lipids was achieved by reference to appropriate heavy labelled deuterated internal standards for each lipid class detected. Ceramides were identified on the basis of total acyl carbon content and degrees of saturation. Ceramides detected were derived from Cer(d18:1) in sn-1 position. The sn-2 fatty acid chain length in ceramides varies from 16 to 34 carbon atoms [21]. A total of 13 distinct species of ceramides and nine NEFA were identified and analysed in the present study. Ceramides are annotated as number of carbon (sn-2 position) atoms:number of double bonds in the fatty acid chain; ceramides with very long-chain fatty acids likely represent in vivo modifications of dietary fatty acids that do not exist in natural food sources. Ceramides with saturated fatty acid chains are those with no double bonds; monounsaturated and polyunsaturated fatty acid chains are those with one double bond and ≥ 2 double bonds in their fatty acid chains, respectively. Ceramide values that were lower than the limit of detection of the assay were set to ‘missing’ in the dataset. Samples were analysed for plasma glucose and cholesterol using standard assays; levels of high-sensitivity C-reactive protein (hs-CRP), adiponectin, HbA1c) and insulin were measured as previously described [3, 22].
Ascertainment of insulin resistance, prediabetes and type 2 diabetes outcomes
Insulin resistance was determined using the HOMA-IR index. The HOMA-IR value was calculated as level of fasting glucose (measured in mmol/l) × level of fasting insulin (measured in μU/ml)/22.5 [23]. An index threshold of 3.0 was chosen to define abnormal insulin resistance because it identifies the highest quartile among populations without diabetes [24], was recently used to select a population at high risk for myocardial infarction or stroke in a randomised trial [25] and represents the approximate median value in the baseline DHS population.
At baseline, diabetes was defined by prevalent medical treatment for diabetes, a fasting blood glucose (FBG) of 7.0 mmol/l or greater or a non-FBG level 11.1 mmol/l or greater. At follow-up, incident diabetes was defined by initiation of medical treatment for diabetes during the study interval, an FBG of 7.0 mmol/l or greater, a non-FBG of 11.1 mmol/l or greater or HbA1c 48 mmol/mol (6.5%) or greater, according to updated guidelines [26] (HbA1c was not measured in DHS-1). No information was available regarding the time of diagnosis or onset of incident diabetes. At baseline, prediabetes was defined by the 2003 American Diabetes Association criteria for impaired fasting glucose (IFG) as an FBG of 5.6–6.9 mmol/l [27]. At follow-up, incident prediabetes was defined as either new IFG with an FBG of 5.6 to 6.9 mmol/l or HbA1c of 39 mmol/mol to 46 mmol/mol (5.7–6.4%) [26]. Oral glucose tolerance testing was not performed.
Statistical analysis
To minimise the amount of non-biological variation and increase precision, ceramide levels were normalised by plate, subtracting the plate-specific median from each measurement and dividing by the plate-specific median absolute deviation. Ceramide concentrations were transformed using an inverse-normal transformation to render the distributions approximately Gaussian and to stabilise the variance. To assess correlations among ceramide species and between ceramides and NEFA species, ceramide levels were adjusted for age, sex and self-reported race/ethnicity and correlations among the adjusted measurements were calculated using Spearman rank correlation coefficient. The relationships between blood lipid levels, adiponectin and body fat distribution (including visceral, abdominal subcutaneous, lower-body subcutaneous and liver fat [both as a continuous variable and defined by NAFLD]) with ceramides at baseline were assessed using multivariable adjusted linear regression models, with lipids, adiponectin and body fat depots as predictors and ceramide levels as the outcomes. When the associations of ceramides with fasting glucose, insulin and HOMA-IR were assessed, ceramides were treated as the predictor and the metabolic variables were treated as the outcomes. All models were adjusted for age, sex, self-reported race/ethnicity and other metabolic factors as specified. Subgroup analyses were performed by sex, race (black/non-black), age (< or ≥ 50 years) and obesity status (< or ≥ 30 kg/m2).
Sensitivity analyses were performed in the body fat models by additionally adjusting for total cholesterol, NEFA and hs-CRP levels in the model of HOMA-IR by additionally adjusting for visceral adiposity and in the diabetes outcomes models by removing smoking as a covariate. The relationships between baseline ceramides (exposure) and follow-up HOMA-IR (adjusted for baseline values), incident insulin resistance (HOMA-IR > 3 at follow-up among those with values < 3 at baseline), incident prediabetes and incident type 2 diabetes (outcomes) were further assessed using multivariable linear and logistic regression adjusted for age, sex, race/ethnicity and other covariates as indicated. Significance levels were corrected for multiple testing using the Bonferroni method. We calculated the study-wise significance level with Bonferroni correction for the total number of ceramides and metabolic variables tested, which resulted in a significance level of 0.0003 (13 ceramides × 13 metabolic traits). The statistical analyses were performed using R version 3.2.1 statistical analysis software (www.r-project.org) and SAS version 9.4 software (SAS Institute, Cary, NC, USA).
Results
Baseline characteristics
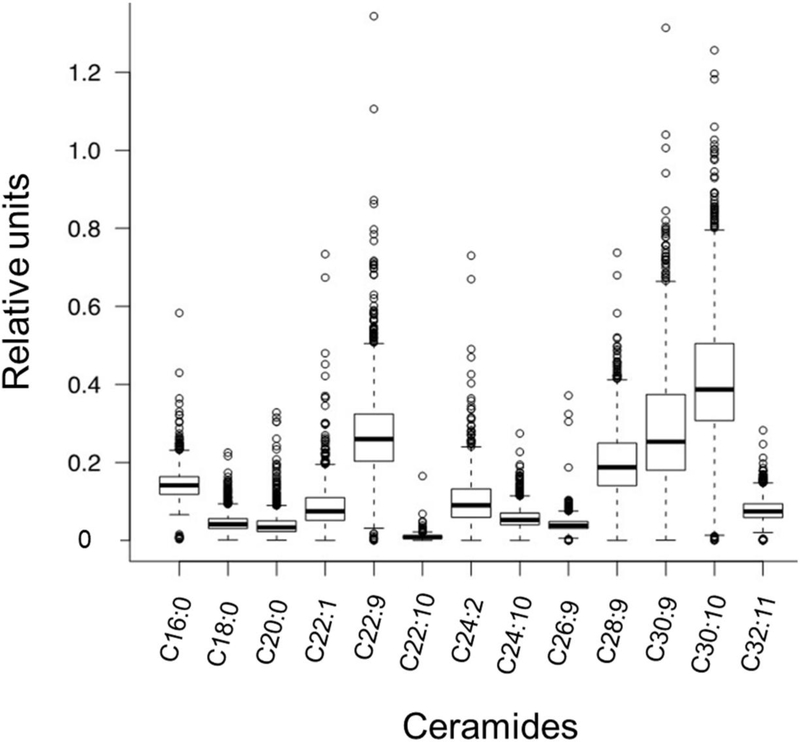
The final study population included 1557 participants; baseline characteristics are described in Table 1. Ceramide species were variably distributed in the population: ceramide C30:10 had the highest relative concentration, whereas ceramide C22:10 had the lowest concentration. (Fig. 1). As some ceramide levels differed systematically between men and women and across races/ethnicities, all analyses were adjusted for age, sex and race/ethnicity. Ceramides with saturated fatty acid chains were highly correlated with each other and correlated less well with ceramides with mono- or polyunsaturated fatty acid chains (ESM Fig. 1). Correlations between ceramides and NEFA species were generally modest (all ρ<0.25, ESM Fig. 2). Participants with adverse lipid profiles (e.g. higher total cholesterol, LDLcholesterol and triacylglycerols and lower HDL-cholesterol) generally had higher levels of shorter-chain saturated and monounsaturated ceramides (p<0.0003 for all), whereas HDL-cholesterol and triacylglycerols had more variable relationships with longer-chain polyunsaturated ceramides. In multivariable models adjusted for age, sex and race/ethnicity, higher total and LDL-cholesterol levels were significantly associated with higher relative concentrations of all ceramides, whereas higher triacylglycerol levels and lower levels of HDL-cholesterol and adiponectin were consistently associated with shorter-chain saturated fatty acid ceramides (C16:0 and C18:0) (p<0.0003 for all). In contrast, lower levels of triacylglycerol were significantly associated with longer-chain polyunsaturated fatty acid ceramides (C30:10 and C32:11) (Table 2).
Table 1.
Baseline characteristics
| Characteristic | Result |
|---|---|
| Age, years | 43.4±9.5 |
| Female | 897 (57.6) |
| Race/ethnicity | |
| African-American | 695 (44.6) |
| Non-Hispanic white | 610 (39.2) |
| Hispanic | 223 (14.3) |
| Other | 29 (1.9) |
| Systolic BP (mmHg) | 119 (111–130) |
| Anti-hypertensive medication use | 196 (12.6) |
| FBG (mmol/l) | 5.0 (4.7–5.4) |
| Insulin (pmol/l) | 79.2 (47.2–127.8) |
| HOMA-IR | 2.5 (1.5–4.2) |
| Total cholesterol (mmol/l) | 9.9 (8.6–11.3) |
| HDL-cholesterol (mmol/l) | 2.7 (2.2–3.3) |
| LDL-cholesterol (mmol/l) | 5.9 (4.7–7.0) |
| Triacylglycerols (mmol/l) | 5.2 (3.7–7.6) |
| Adiponectin (ng/mL) | 7.0 (4.7–10.1) |
| Hs-CRP(nmol/l) | 22.9 (9.5–52.4) |
| Adiposity measure | |
| BMI (kg/m2) | 28.4 (24.8–33.1) |
| Total body fat (kg) | 24.7 (18.2–32.4) |
| Total abdominal fat (kg) | 6.3 (4.6–8.4) |
| Abdominal subcutaneous fat (kg) | 4.1 (2.7–6) |
| Subcutaneous/total body fat (%) | 17 (14.0–19.0) |
| Subcutaneous/total abdominal fat (%) | 69.0 (59.0–76.0) |
| Visceral fat (kg) | 1.9 (1.4–2.7) |
| Visceral/total body fat (%) | 8.0 (5.0–12.0) |
| Visceral/total abdominal fat (%) | 31.0 (24.0–41.0) |
| Lower-body subcutaneous fat (kg) | 8.8 (6.3–12.1) |
| Lower-body/total body fat (%) | 36.0 (31.0–41.0) |
| Liver fat (%) | 3.5 (2.0–6.0) |
| NAFLD | 354 (22.7) |
| Ceramides (RU × 100) | |
| C16:0 | 14.1 (11.9–16.4) |
| C18:0 | 4.1 (3.1–5.6) |
| C20:0 | 3.4 (2.3–5.0) |
| C22:1 | 7.5 (5.2–11.0) |
| C22:9 | 26.0 (20.3–32.4) |
| C22:10 | 0.9 (0.6–0.12) |
| C24:2 | 9.0 (5.9–13.2) |
| C24:10 | 5.2 (4.1–7.0) |
| C26:9 | 3.9 (3.1–4.9) |
| C28:9 | 18.7 (14.1–24.9) |
| C30:9 | 25.3 (18.0–37.4) |
| C30:10 | 38.7 (30.7–50.5) |
| C32:11 | 7.4 (5.9–9.4) |
Data are mean ± SD, median (interquartile range) or proportion (%) as appropriate
n for ceramides missing or < lower limit of detection: C16:0, 91; C18:0, 51; C20:0, 87; C22:1, 81; C24:2, 131
RU, relative units
Fig. 1.
Box-and-whiskers plot of the distribution of ceramide species measured in the DHS. Ceramides are annotated as number of carbon (sn-2 position) atoms:number of double bonds in the fatty acid chain and plotted against relative units of concentration. Boxes represent the 25th, 50th, and 75th percentiles, and whiskers represent the 10th and 90th percentiles. Circles represent outlier values
Table 2.
Associations between lipids, adiponectin and ceramides
| Ceramides | Total cholesterol | LDL-cholesterol | HDL-cholesterol | Triacylglycerol | Adiponectin |
|---|---|---|---|---|---|
| C16:0 | 0.44 (0.39, 0.49) † | 0.42 (0.37, 0.46) † | −0.22 (−0.27, −0.17) † | 0.33 (0.28, 0.38) † | −0.13 (−0.18, −0.07) † |
| C18:0 | 0.35 (0.30, 0.40) † | 0.32 (0.27, 0.37) † | −0.20 (−0.26, −0.15) † | 0.33 (0.28, 0.38) † | −0.21 (−0.27, −0.16) † |
| C20:0 | 0.31 (0.26, 0.36) † | 0.30 (0.25, 0.34) † | −0.11 (−0.16, −0.06) † | 0.21 (0.16, 0.26) † | −0.07 (−0.12, −0.02)* |
| C22:1 | 0.28 (0.23, 0.33) † | 0.32 (0.27, 0.37) † | −0.20 (−0.25, −0.15) † | 0.13 (0.08, 0.18) † | −0.09 (−0.15, −0.04)*** |
| C22:9 | 0.45 (0.41, 0.50) † | 0.51 (0.46, 0.55) † | −0.03 (−0.08, 0.02) | −0.04 (−0.09, 0.02) | −0.02 (−0.08, 0.03) |
| C22:10 | 0.42 (0.37, 0.46) † | 0.40 (0.36, 0.45) † | −0.01 (−0.06, 0.04) | 0.09 (0.04, 0.14)*** | −0.05 (−0.1, 0) |
| C24:2 | 0.24 (0.19, 0.29) † | 0.28 (0.23, 0.33) † | −0.13 (−0.19, −0.08) † | 0.07 (0.02, 0.12)* | −0.04 (−0.1, 0.02) |
| C24:10 | 0.45 (0.40, 0.49) † | 0.50 (0.46, 0.55) † | −0.04 (−0.09, 0.01) | −0.03 (−0.08, 0.02) | −0.03 (−0.09, 0.02) |
| C26:9 | 0.29 (0.25, 0.34) † | 0.35 (0.31, 0.4) † | 0.06 (0.01, 0.11)* | −0.17 (−0.22, −0.12) † | 0.04 (−0.01, 0.09) |
| C28:9 | 0.48 (0.44, 0.53) † | 0.51 (0.46, 0.55)† | −0.04 (−0.09, 0.01) | 0.04 (−0.01, 0.1) | −0.07 (−0.12, −0.01)* |
| C30:9 | 0.47 (0.42, 0.52) † | 0.51 (0.47, 0.55) † | −0.02 (−0.07, 0.04) | −0.01 (−0.06, 0.04) | 0 (−0.06, 0.05) |
| C30:10 | 0.43 (0.39, 0.48) † | 0.50 (0.46, 0.55) † | −0.01 (−0.06, 0.04) | −0.11 (−0.16, −0.06) † | 0 (−0.05, 0.06) |
| C32:11 | 0.39 (0.34, 0.44) † | 0.47 (0.43, 0.52) † | −0.02 (−0.07, 0.04) | −0.14 (−0.19, −0.09) † | 0.01 (−0.05, 0.06) |
Data represent the β coefficient and 95% CI of the association adjusted for age, sex and race/ethnicity
p<0.05
p<0.001 and
p<0.0003
Body fat distribution and plasma ceramides
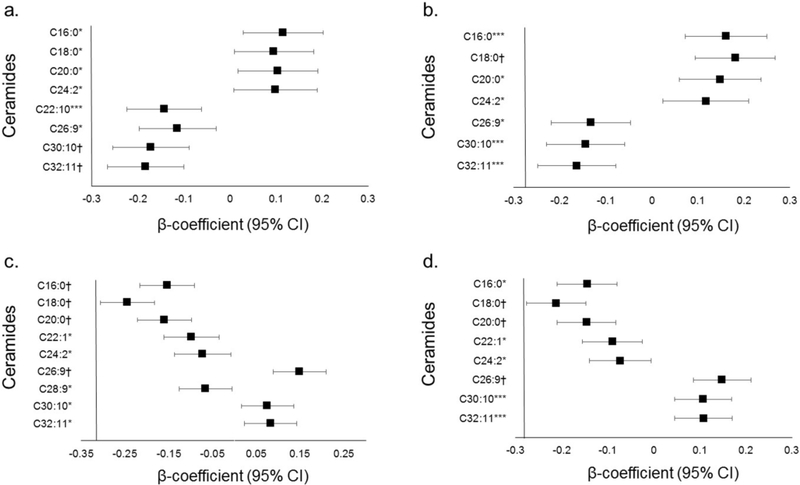
Higher BMI and total abdominal fat were positively associated with many ceramides in models adjusted for age, sex and race/ethnicity (ESM Table 1). In order to evaluate the independent associations of different body fat depots with ceramides, each body fat depot (except liver fat) was indexed to total body fat. After multivariable adjustment for age, sex, and race/ethnicity, higher VAT/total fat ratio was inversely associated with C30:10 and C32:11 (p<0.0003 for both; Fig. 2). After additional adjustment for BMI, higher VAT/total fat ratio was positively associated with saturated fatty acid ceramide C18:0 (p<0.0003), but the inverse associations with C30:10 and C32:11 were no longer significant after Bonferroni correction (p<0.001 for both; Fig. 2). In contrast, after adjustment for age, sex, and race/ethnicity, higher lower-body fat/total fat was positively associated with the longer-chain polyunsaturated fatty acid ceramide C26:9 and inversely associated with the shorter-chain saturated fatty acid ceramides (C16:0, C18:0 and C20:0) (p<0.0003 for all; Fig. 2). After additional adjustment for BMI, higher lower-body fat/total fat ratio remained significantly positively associated with C26:9 and inversely associated with C18:0 and C20:0 (p<0.0003 for all; Fig. 2). Subgroup analyses by sex, race (black/non-black) and age (< or ≥ 50 years) were generally consistent and demonstrated only rare heterogeneity of effects between groups of borderline statistical significance (ESM Table 2). Conversely, subgroup analyses by obesity status (< or ≥ 30 kg/m2) demonstrated several significant interaction p values (ESM Table 2), with generally stronger relationships between fat depots and ceramides among those with obesity. A higher abdominal subcutaneous/total fat ratio was positively associated with ceramides C16:0, C18:0, C20:0, C22:1 and C22:10 in models adjusted for age, sex and race/ethnicity, but associations were attenuated and no longer statistically significant after adjustment for BMI (ESM Table 1). Increasing liver fat was inversely associated with ceramides C24:2, C26:9, C30:10 and C32:11, even after adjusting for VAT (ESM Table 1). Increased concentrations of ceramides C30:10 and C32:11 were associated with lower odds of NAFLD, even after adjustment for traditional risk factors (ESM Table 3), with consistent effects across subgroups. Ceramides C16:0 and C18:0 were not statistically significantly associated with higher liver fat (p=0.40 and p=0.001, respectively) or odds of NAFLD (p=0.53 and p=0.09, respectively; ESM Tables 1 and 3). In sensitivity analyses, most associations between body fat and ceramides remained significant, independent of total cholesterol, and results were not materially different after additional adjustment for total NEFA levels or hs-CRP.
Fig. 2.
Associations between body fat depots and ceramides. Plots demonstrating the association between plasma ceramides and (a, b) VAT and (c, d) lower-body fat, normalised to total body fat. β coefficients and 95% CIs represent the difference in ceramide concentration per 1 SD (kg) of body fat in the depot. Data in (a, c) are adjusted for age, sex and race/ethnicity; (b, d) are additionally adjusted for BMI. *p<0.05, ***p<0.001 and †p<0.0003
When the relationships between body fat depots and specific ceramide ratios were examined, including those ceramide ratios previously demonstrated by Tarasov et al [28] and Laaksonen et al [29] to have prognostic value for cardiovascular disease, we found a similar pattern for individual ceramide ratios such that VAT/total fat was positively associated with the ratio of C18:0/24:2 (β=0.19, p=7.4×10−5) and lower-body fat/total fat was inversely associated with the same ceramide ratio (β= −0.16, p=1.1×10−5) (ESM Table 4). Similar findings were seen when additional ratios of saturated/polyunsaturated moieties (e.g. C18:0/C26:9, C18:0/C30:10 and C:18:0/C32:11) were examined.
Plasma ceramide concentrations and insulin resistance
The relationship between ceramides and markers of dysglycaemia and insulin resistance at baseline are shown in Table 3. Shorter-chain saturated fatty acid ceramides (C16:0, C18:0) were positively associated with fasting insulin and HOMA-IR and, to a lesser degree, fasting glucose, and longer-chain polyunsaturated ceramides (C24:2, C30:10 and C32:11) were inversely associated with these markers, a pattern similar to that seen with the relationship between visceral adiposity and ceramides. The associations with insulin resistance persisted even after adjustment for BMI and VAT.
Table 3.
Associations between ceramide species and insulin resistance
| Ceramides | Fasting glucose |
Fasting insulin |
HOMA-IR |
|||
|---|---|---|---|---|---|---|
| Age, sex, ethnicity | Age, sex, ethnicity, BMI, VAT | Age, sex, ethnicity | Age, sex, ethnicity, BMI, VAT | Age, sex, ethnicity | Age, sex, ethnicity, BMI, VAT | |
| C16:0 | 0.06 (0.01, 0.10)* | 0.06 (0.01, 0.10)* | 0.09 (0.05, 0.14) † | 0.09 (0.05, 0.14) † | 0.08 (0.04, 0.13) † | 0.08 (0.04, 0.12) † |
| C18:0 | 0.06 (0.02, 0.11)* | 0.06 (0.01, 0.11)* | 0.11 (0.07, 0.15) † | 0.10 (0.06, 0.14) † | 0.10 (0.06, 0.14) † | 0.09 (0.05, 0.13) † |
| C20:0 | 0.04 (−0.01, 0.08) | 0.03 (−0.01, 0.08) | 0.02 (−0.02, 0.06) | 0.02 (−0.02, 0.06) | 0.02 (−0.02, 0.06) | 0.02 (−0.02, 0.06) |
| C22:1 | 0.01 (−0.04, 0.06) | 0.01 (−0.04, 0.06) | −0.02 (−0.06, 0.02) | −0.02 (−0.06, 0.02) | −0.02 (−0.06, 0.03) | −0.02 (−0.06, 0.03) |
| C22:9 | −0.01 (−0.05, 0.04) | −0.01 (−0.06, 0.03) | 0.002 (−0.04, 0.04) | −0.01 (−0.05, 0.03) | −0.01 (−0.06, 0.03) | −0.03 (−0.07, 0.02) |
| C22:10 | 0.03 (−0.02, 0.07) | 0.01 (−0.03, 0.06) | 0.02 (−0.02, 0.06) | −0.01 (−0.05, 0.03) | 0.01 (−0.04, 0.05) | −0.02 (−0.07, 0.02) |
| C24:2 | −0.02 (−0.06, 0.03) | −0.01 (−0.06, 0.03) | −0.07 (−0.12, −0.03)*** | −0.07 (−0.11, −0.03)* | −0.07 (−0.11, −0.03)* | −0.07 (−0.11, −0.02)* |
| C24:10 | 0.004 (−0.05, 0.04) | −0.01 (−0.05, 0.04) | 0.01 (−0.03, 0.05) | 0.004 (−0.04, 0.04) | −0.01 (−0.05, 0.04) | −0.02 (−0.06, 0.02) |
| C26:9 | 0.002 (−0.05, 0.04) | 0 (−0.05, 0.04) | −0.03 (−0.07, 0.01) | −0.03 (−0.07, 0.01) | −0.02 (−0.07, 0.02) | −0.03 (−0.07, 0.01) |
| C28:9 | 0.03 (−0.02, 0.07) | 0.02 (−0.02, 0.07) | 0.03 (−0.01, 0.07) | 0.02 (−0.02, 0.06) | 0.03 (−0.01, 0.08) | 0.03 (−0.01, 0.07) |
| C30:9 | 0.01 (−0.03, 0.06) | 0.01 (−0.03, 0.06) | 0.004 (−0.04, 0.04) | −0.001 (−0.04, 0.04) | 0.004 (−0.04, 0.05) | 0.0001 (−0.04,0.04) |
| C30:10 | −0.02 (−0.07, 0.03) | −0.03 (−0.08, 0.02) | −0.05 (−0.09, −0.01)* | −0.07 (−0.11, −0.03)*** | −0.05 (−0.09, 0)* | −0.06 (−0.11, −0.02)* |
| C32:11 | −0.02 (−0.07, 0.02) | −0.03 (−0.08, 0.02) | −0.05 (−0.09, −0.01)* | −0.07 (−0.11, −0.03)*** | −0.05 (−0.09, 0)* | −0.06 (−0.1, −0.02)* |
Data represent the β coefficient and 95% CI of the association adjusted for covariates as noted
p<0.05
p<0.001 and
p<0.0003
Incident diabetes-related outcomes
After a median follow-up of 7 years (interquartile range 6.6 to 7.6), metabolic measurements were repeated. Higher ceramide levels were not statistically associated with higher HOMA-IR at follow-up after adjustment for age, sex, race/ethnicity, BMI, VAT and baseline HOMA-IR after adjustment for multiple comparisons. There was no significant association of the baseline ceramide species with incident insulin resistance (HOMA-IR > 3) at follow-up (ESM Table 5), after adjustment for age, sex and race/ethnicity. A total of 112 participants (7.2%) developed type 2 diabetes mellitus over the follow-up period. Higher VAT (OR 1.72, 95% CI 1.29, 2.29) and VAT/total fat ratio (OR 1.69, 95% CI 1.22, 2.35) were positively associated with incident type 2 diabetes and lower-body fat (OR 0.48, 95% CI 0.31, 0.75) and lower-body fat/total fat ratio (OR 0.56, 95% CI 0.42, 0.76) were inversely associated with type 2 diabetes in models adjusted for age, sex, race/ethnicity, systolic BP, antihypertensive medication use and BMI. Adiponectin was not independently associated with incident diabetes in our dataset, after adjusting for traditional risk factors, BMI and VAT (p=0.074). None of the ceramide species was independently associated with incident type 2 diabetes after adjustment for age, sex and race/ethnicity. Among those with normal fasting plasma glucose at baseline (n= 1240), 240 (19.4%) developed prediabetes over the follow-up period. VAT/total fat (OR 1.31, 95% CI 1.02, 1.68) and lower-body fat/total fat ratios (OR 0.67, 95% CI 0.54, 0.82) were also significantly and oppositely associated with incident prediabetes; however, none of the ceramides was associated with incident prediabetes in models adjusted for clinical factors. We also assessed the relationships between baseline ceramides and insulin resistance, prediabetes (IFG) and type 2 diabetes at follow-up among subgroups of sex, race, age and obesity status. We did not find any evidence of effect modification on these relationships and we did not find any significant associations between ceramides and incident diabetes outcomes in any subgroup after adjusting for multiple testing. Sensitivity analyses removing smoking as a covariate in the models did not impact the results.
Discussion
In this prospective study of a large population-based multi-ethnic cohort, we observed that an unfavourable adiposity, lipid and insulin resistance profile was associated with shorter-chain saturated fatty acid ceramide moieties, while this phenotype was inversely associated with the longer-chain unsaturated fatty acid ceramide species. These opposing associations of saturated and unsaturated ceramide species were consistent across multiple fat depots, including visceral, lower-body and liver fat. We did not find that ceramides were independently associated with incident prediabetes or type 2 diabetes after adjustment for clinical factors.
To our knowledge, this is the first study to evaluate the relationship between ceramide species, visceral adiposity and incidence of type 2 diabetes in a large epidemiological cohort. Previous studies have demonstrated increased saturated ceramide moieties and skeletal muscle ceramide accumulation in individuals with generalised obesity [30, 31]. C16:0 ceramide has been shown to be elevated in the white adipose tissue of obese humans, as well as in the white adipose tissue and liver of mice fed a high-fat diet [32], and C16:0 ceramide in human subcutaneous adipose tissue was found to correlate with insulin resistance by HOMA-IR [33]. Our study extends these findings to include systemically circulating C16:0 and other short-chain saturated ceramide species. These findings suggest that a circulating ceramide pattern reflecting a poor nutritional state of excess saturated NEFA, rather than a general increase in ceramide content, may be a more specific and meaningful marker of a dysmetabolic obesogenic state leading to insulin resistance. As the detrimental effect of higher ceramide concentrations on cardiometabolic risk may be modified by dietary intervention (such as the Mediterranean diet) [34], it is possible that dietary modification of visceral adiposity, even in the absence of weight loss (as demonstrated in the PREDIMED [Prevencion con Dieta Mediterranea] trial), could improve cardiometabolic risk through alteration in ceramides.
Longer-chain unsaturated ceramide species were positively associated with lower-body fat and negatively correlated with visceral and liver fat. Epidemiological studies have demonstrated that lower-body fat is associated with a lower cardiac risk factor burden [35] and a lower risk for incident cardiovascular disease [36] and cancer [37], when adjusted for body size. These data suggest that long-chain unsaturated ceramide species may actually reflect a healthy adiposity/metabolic phenotype. Higher circulating levels of these ceramides could result from a specific dietary pattern in parallel with expansion of the lower-body fat depot to store excess fat. Alternatively, lower-body subcutaneous fat depot expansion could alter de novo ceramide synthesis, with beneficial downstream effects on systemic metabolism.
From a mechanistic standpoint, ceramides are theorised to be important modulators of obesity-induced metabolic disorders [38]. Studies have revealed altered ceramide metabolism and accumulation of specific saturated ceramides (C16:0 and C18:0) in obese individuals due to reduced fatty acid oxidation, causing increased levels of saturated NEFA and resulting in de novo synthesis of these specific ceramide species. These data provide insight into a link between variation in body fat distribution and metabolic status vis à vis ceramide physiology. They suggest a more nuanced understanding of global ceramide synthesis and signalling pathways, given the clear dichotomy between various ceramide species in relation to metabolic phenotypes. Assessment of circulating ceramide species may be a more feasible approach to population-based investigation and confirmation that ceramides previously linked to dysfunctional adiposity only at the tissue level now show similar relationships when measured in plasma suggests a potential role for blood-based ceramide screening in metabolic risk stratification. Given the cross-sectional nature of our study with regard to fat depots and metabolic markers, however, we are unable to discern whether variation in body fat distribution impacts de novo ceramide synthesis or if modification of circulating ceramide levels could modulate metabolic outcomes. Further studies should examine the temporal variation in ceramide levels and their impact on adiposity and diabetes outcomes.
The robust associations between ceramides and body fat/metabolic status did not translate to significant relationships between circulating ceramide species and diabetes outcomes in our study. This may be due to multiple factors, such as a lack of power from insufficient diabetes event rates to detect a statistically significant relationship with ceramides. Alternatively, there may be a discordance between ceramides measured at the tissue level and those measured in the plasma. Indeed, there are limited clinical data available that compare plasma ceramides with tissue ceramides, most importantly in adipose tissue and liver, and there may be significant differences between tissue and plasma ceramides depending on the species. Although plasma ceramide measurements did not indicate higher risk for clinical diabetes in our study, they were nevertheless strongly associated with multiple markers of altered glucose and lipid metabolism.
Our results should be interpreted in the context of several limitations. First, as our study cohort oversampled black participants and did not include South or East Asians, the results may not be generalisable to these racial/ethnic groups or the general US population. Second, there may have been other factors/unmeasured confounders that could have influenced both adiposity and ceramide levels, such as dietary habits, that we are unable to account for in this study. Third, because all of our participants were free of clinically apparent diabetes and cardiovascular disease at baseline, the participants represent a relatively healthy population-based sample and results may not be applicable to those with established cardiometabolic disease. Fourth, as some ceramides were lower than the limit of detection of the assay, there were some missing ceramide values for individual participants; however, it is unlikely that missing values impacted the results as all ceramides were detectable in > 94% of study participants. Last, after adjusting for multiple comparisons, several of the p values were of borderline statistical significance. This limitation is of particular importance for interpretation of the multiple subgroup analyses and should be viewed as hypothesis generating. However, the consistency of these results across the plasma ceramides supports the reproducibility of these observations.
In summary, our study documented a biologically complex relationship of metabolic and imaging indicators of dysfunctional adiposity with plasma ceramide concentrations in a large multi-ethnic cohort of adults free from cardiovascular disease or diabetes. An adverse adiposity and metabolic profile was associated with shorter-chain saturated fatty acid ceramide species and a healthier metabolic profile was associated with longer-chain polyunsaturated fatty acid ceramides. Plasma ceramides were not associated with incident diabetes outcomes after accounting for clinical factors. Further studies are needed to replicate these results in alternative populations and to investigate the impact of changes in body fat depots and ceramide concentrations on diabetes outcomes over longitudinal follow-up.
Supplementary Material
Acknowledgments
Funding
IJN is supported by grant K23 DK106520 from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institute of Health and by the Dedman Family Scholarship in Clinical Care from UT Southwestern. Research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001105 and by the Donald W. Reynolds Foundation. Ceramide measurements were supported by investigator-initiated grants from Merck & Co., Inc., Kenilworth, NJ, USA.
Abbreviations
- DHS
Dallas Heart Study
- DHS-2
DHS phase 2
- FBG
Fasting blood glucose
- hs-CRP
High-sensitivity C-reactive protein
- IFG
Impaired fasting glucose
- NAFLD
Non-alcoholic fatty liver disease
- VAT
Visceral adipose tissue
Footnotes
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Duality of interest
IJN has received honoraria, consulting and speaking fees and travel support from Boehringer Ingelheim/Lilly Alliance (significant), a research grant from Novo Nordisk (significant) and is a member of the scientific advisory board of Advanced MR Analytics (modest). DKM reports clinical trial leadership for AstraZeneca (modest), Sanofi Aventis (modest), Janssen (modest), Boehringer Ingelheim (significant), Merck & Co (modest), Novo Nordisk (significant), Lexicon (modest), Eisai (modest), GlaxoSmithKline (modest), Esperion (modest); consultancy for AstraZeneca (modest), Sanofi Aventis (significant), Lilly US (modest), Boehringer Ingelheim (significant), Merck & Co, Pfizer (modest), Novo Nordisk (significant), Metavant (modest). TR, DFR and JC-P are employees of Merck & Co. or were employees when the ceramide measurements were performed.
References
- [1].Bastien M, Poirier P, Lemieux I, Despres JP (2014) Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog Cardiovasc Dis 56: 369–381 [DOI] [PubMed] [Google Scholar]
- [2].Despres JP (2012) Body fat distribution and risk of cardiovascular disease: an update. Circulation 126: 1301–1313 [DOI] [PubMed] [Google Scholar]
- [3].Neeland IJ, Turer AT, Ayers CR, et al. (2012) Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA 308: 1150–1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Neeland IJ, Ayers CR, Rohatgi AK, et al. (2013) Associations of visceral and abdominal subcutaneous adipose tissue with markers of cardiac and metabolic risk in obese adults. Obesity (Silver Spring) 21: E439–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Neeland IJ, Hughes C, Ayers CR, Malloy CR, Jin ES (2017) Effects of visceral adiposity on glycerol pathways in gluconeogenesis. Metabolism 67: 80–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Summers SA (2006) Ceramides in insulin resistance and lipotoxicity. Prog Lipid Res 45: 42–72 [DOI] [PubMed] [Google Scholar]
- [7].Galadari S, Rahman A, Pallichankandy S, Galadari A, Thayyullathil F (2013) Role of ceramide in diabetes mellitus: evidence and mechanisms. Lipids Health Dis 12: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chavez JA, Summers SA (2012) A ceramide-centric view of insulin resistance. Cell Metab 15: 585–594 [DOI] [PubMed] [Google Scholar]
- [9].Petersen MC, Shulman GI (2017) Roles of Diacylglycerols and Ceramides in Hepatic Insulin Resistance. Trends Pharmacol Sci 38: 649–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Luukkonen PK, Zhou Y, Sadevirta S, et al. (2016) Hepatic ceramides dissociate steatosis and insulin resistance in patients with non-alcoholic fatty liver disease. J Hepatol 64: 1167–1175 [DOI] [PubMed] [Google Scholar]
- [11].Haus JM, Kashyap SR, Kasumov T, et al. (2009) Plasma ceramides are elevated in obese subjects with type 2 diabetes and correlate with the severity of insulin resistance. Diabetes 58: 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wigger L, Cruciani-Guglielmacci C, Nicolas A, et al. (2017) Plasma Dihydroceramides Are Diabetes Susceptibility Biomarker Candidates in Mice and Humans. Cell Rep 18: 2269–2279 [DOI] [PubMed] [Google Scholar]
- [13].Samuel VT, Shulman GI (2012) Mechanisms for insulin resistance: common threads and missing links. Cell 148: 852–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Gorski J (2012) Ceramide and insulin resistance: how should the issue be approached? Diabetes 61: 3081–3083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Victor RG, Haley RW, Willett DL, et al. (2004) The Dallas Heart Study: a population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 93: 1473–1480 [DOI] [PubMed] [Google Scholar]
- [16].Abate N, Burns D, Peshock RM, Garg A, Grundy SM (1994) Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res 35: 14901496. [PubMed] [Google Scholar]
- [17].Abate N, Garg A, Coleman R, Grundy SM, Peshock RM (1997) Prediction of total subcutaneous abdominal, intraperitoneal and retroperitoneal adipose tissue masses in men by a single axial magnetic resonance imaging slice. Am J Clin Nutr 65: 403–408 [DOI] [PubMed] [Google Scholar]
- [18].Szczepaniak LS, Babcock EE, Schick F, et al. (1999) Measurement of intracellular triglyceride stores by H spectroscopy: validation in vivo. Am J Physiol 276: E977–989 [DOI] [PubMed] [Google Scholar]
- [19].Szczepaniak LS, Nurenberg P, Leonard D, et al. (2005) Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab 288: E462–468 [DOI] [PubMed] [Google Scholar]
- [20].Vega GL, Adams-Huet B, Peshock R, Willett D, Shah B, Grundy SM (2006) Influence of body fat content and distribution on variation in metabolic risk. J Clin Endocrinol Metab 91: 4459–4466 [DOI] [PubMed] [Google Scholar]
- [21].Bouwstra JA, Gooris GS, Dubbelaar FE, Weerheim AM, Ijzerman AP, Ponec M (1998) Role of ceramide 1 in the molecular organization of the stratum corneum lipids. J Lipid Res 39: 186–196 [PubMed] [Google Scholar]
- [22].Chandra A, Neeland IJ, Berry JD, et al. (2014) The relationship of body mass and fat distribution with incident hypertension: observations from the Dallas Heart Study. J Am Coll Cardiol 64: 997–1002 [DOI] [PubMed] [Google Scholar]
- [23].Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419 [DOI] [PubMed] [Google Scholar]
- [24].Ascaso JF, Pardo S, Real JT, Lorente RI, Priego A, Carmena R (2003) Diagnosing insulin resistance by simple quantitative methods in subjects with normal glucose metabolism. Diabetes Care 26: 3320–3325 [DOI] [PubMed] [Google Scholar]
- [25].Kernan WN, Viscoli CM, Furie KL, et al. (2016) Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med 374: 1321–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].(2009)Diagnosis and classification of diabetes mellitus. Diabetes Care 32(Suppl 1): S62–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Genuth S, Alberti KG, Bennett P, et al. (2003) Follow-up report on the diagnosis of diabetes mellitus. Diabetes Care 26: 3160–3167 [DOI] [PubMed] [Google Scholar]
- [28].Tarasov K, Ekroos K, Suoniemi M, et al. (2014) Molecular lipids identify cardiovascular risk and are efficiently lowered by simvastatin and PCSK9 deficiency. J Clin Endocrinol Metab 99: E45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Laaksonen R, Ekroos K, Sysi-Aho M, et al. (2016) Plasma ceramides predict cardiovascular death in patients with stable coronary artery disease and acute coronary syndromes beyond LDL-cholesterol. Eur Heart J 37: 1967–1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hanamatsu H, Ohnishi S, Sakai S, et al. (2014) Altered levels of serum sphingomyelin and ceramide containing distinct acyl chains in young obese adults. Nutr Diabetes 4: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].de la Maza MP, Rodriguez JM, Hirsch S, Leiva L, Barrera G, Bunout D (2015) Skeletal muscle ceramide species in men with abdominal obesity. J Nutr Health Aging 19: 389–396 [DOI] [PubMed] [Google Scholar]
- [32].Turpin SM, Nicholls HT, Willmes DM, et al. (2014) Obesity-induced CerS6-dependent C16:0 ceramide production promotes weight gain and glucose intolerance. Cell Metab 20: 678–686 [DOI] [PubMed] [Google Scholar]
- [33].Blachnio-Zabielska AU, Baranowski M, Hirnle T, et al. (2012) Increased bioactive lipids content in human subcutaneous and epicardial fat tissue correlates with insulin resistance. Lipids 47: 1131–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Wang DD, Toledo E, Hruby A, et al. (2017) Plasma Ceramides, Mediterranean Diet and Incident Cardiovascular Disease in the PREDIMED Trial (Prevencion con Dieta Mediterranea). Circulation 135: 2028–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Manolopoulos KN, Karpe F, Frayn KN (2010) Gluteofemoral body fat as a determinant of metabolic health. Int J Obes (Lond) 34: 949–959 [DOI] [PubMed] [Google Scholar]
- [36].Neeland IJ, Turer AT, Ayers CR, et al. (2015) Body fat distribution and incident cardiovascular disease in obese adults. J Am Coll Cardiol 65: 2150–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Gupta A, Pandey A, Ayers C, et al. (2017) An Analysis of Individual Body Fat Depots and Risk of Developing Cancer: Insights From the Dallas Heart Study. Mayo Clin Proc 92: 536–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fucho R, Casals N, Serra D, Herrero L (2017) Ceramides and mitochondrial fatty acid oxidation in obesity. FASEB J 31: 1263–1272 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.