Abstract
Ischemic heart disease is currently the leading cause of death globally, with coronary artery bypass grafting among the most common operations performed worldwide. More extensive use of arterial grafts has been advocated because of their high long-term patency, long-term survival benefit, and freedom from reinterventions. Despite this, the saphenous vein is the most frequently used conduit in patients undergoing coronary artery bypass surgery since its introduction over 50 years ago. Consequently, the saphenous vein remains an indispensable conduit in coronary artery bypass grafting and maintaining its long-term patency is one of the most crucial challenges in cardiovascular surgery. This situation led to the development of the no-touch saphenous vein harvesting technique, where the vein is harvested completely with its pedicle of surrounding tissue. Several studies report a superior long-term patency rate, slower progression of atherosclerosis, and better clinical outcomes whilst employing no-touch harvesting technique. The success of the technique is multifactorial, including the decreased risk for graft spasm—and the need for manual distension—preservation of the vaso vasorum and an intact endothelium, reducing neointimal hyperplasia and subsequent atherosclerosis. Furthermore, the intact perivascular tissue, including the surrounding cushion of fat, may act as a “natural external stent”, providing mechanical support preventing the graft from kinking. We are convinced that the use of arterial grafts, in combination with the no-touch saphenous vein graft, will significantly improve the results of coronary artery bypass grafting. This is important for achieving a comprehensive and evidence-based balance between the major treatment strategies of ischemic heart disease, explicitly coronary artery bypass grafting and percutaneous coronary intervention. The no-touch technique is becoming increasingly popular among surgeons, with further studies to be initiated worldwide.
Keywords: Arterial grafts, coronary artery bypass grafting (CABG), no-touch harvesting technique (NT harvesting technique), patency, saphenous vein (SV)
Introduction
Ischemic heart disease is currently the leading cause of death globally, and is expected to account for 14.2% of all deaths by 2030 (1). Coronary artery bypass grafting (CABG) is among the most common operations performed in the world (2) and is the best treatment for advanced ischemic heart disease (3-5).
In a recent perspective in the New England Journal of Medicine, Jones (6) describes the important contributions of the Argentinian cardiac surgeon, Rene Favaloro, who introduced the saphenous vein (SV) as a conduit in patients undergoing CABG. In the subsequent 50 years, this vessel has become the most commonly used conduit for revascularization. Along with the SV, the two main vessels used for CABG are the internal thoracic artery (ITA) and the radial artery (RA).
Pros and cons
Graft patency in CABG is a major determinant of clinical prognosis, measured in terms of reoperation rates and long-term survival (7,8). More extensive use of arterial grafts has been advocated because of their high long-term patency, long-term survival benefit, and freedom from reinterventions (9-11). This is compared to the high incidence of early graft occlusion, progressive intimal hyperplasia, and late graft atherosclerosis associated with the use of conventional SV grafts (SVGs) (12,13).
Even if extensive arterial revascularization is performed, SVGs still account for the majority of conduits used in CABG (14). In many centers, the SV is used for up to 80% of all grafts (15). This is due to numerous advantages of using the SV, including ease of access and manipulation, sufficient length for grafting, and short harvesting time. Clinical factors may also suggest that prolonged conduit longevity is not always the primary concern. Old age, female gender, left ventricular dysfunction, smoking, obesity, and diabetes are some of the factors that negatively impact on survival (16,17) and hence, the benefits of extensive arterial revascularization in CABG can be short-lived in these patients (18). This consideration is even more important, given that the age and comorbidities of the CABG population are increasing (19). Consequently, the SV remains an indispensable conduit in CABG and its long-term patency is one of the most crucial challenges in cardiovascular surgery.
No-touch (NT) harvesting technique
Laboratory studies have shown that damage to vessels during surgical preparation influences graft patency (20-22). Since the early 1990s, a technique for SV preparation where the vein is harvested complete with its pedicle of surrounding tissue left intact has been employed: the “NT technique” (23,24). This technique has been shown to reduce the risk of spasm and the need for distension (23) and consequently, preserves vessel wall integrity (20). This technique provides a superior patency rate (25-28), preserved left ventricular function (29), and a better clinical outcome (30) compared to conventional harvesting, in both the short and the long term.
A prospective, randomized clinical trial which compared the NT technique with two other conventional techniques demonstrated that the patency rate of SVGs harvested with its surrounding tissue is very high at 1.5, 8.5 and 16 years postoperatively (25-27,31). This patency was comparable to that of the left ITA. Such a high patency rate has not been demonstrated when using other harvesting techniques.
A number of underlying mechanisms have been suggested as contributories to the success of NT vein grafts. For example, the decreased risk of graft spasm and the associated requirement for graft distension reduces endothelial cell loss and the resulting long-term damage (20,32,33). Other aspects include the preservation of the vasa vasorum [allowing retrograde blood flow from the graft lumen to perfuse through the vein wall (Figure 1)], thereby maintaining transmural flow and reducing ischemic damage (34). In addition, preservation of an intact luminal endothelium and local nitric oxide levels (20,34) are suggested to reduce vasospasm at harvesting and to reduce neointimal hyperplasia and subsequent atherosclerosis, decreasing the incidence of long-term graft failure (35). Furthermore, the intact perivascular tissue, including the surrounding cushion of fat, may act as a “natural external stent” that provides mechanical support, thus reducing neointimal and medial thickening of the vein graft (36,37). Importantly, preservation of the pronounced cushion of perivascular fat prevents the graft from kinking—a feature which is especially important when using sequential grafts, vital in patients with small target vessels, such as in the case of diabetics (Figure 2). In addition, perivascular adipose tissue is a source of adipocyte-derived, anticontractile factors, such as nitric oxide and leptin, which may play a role in reducing spasm and maintaining graft patency (20).
Figure 1.
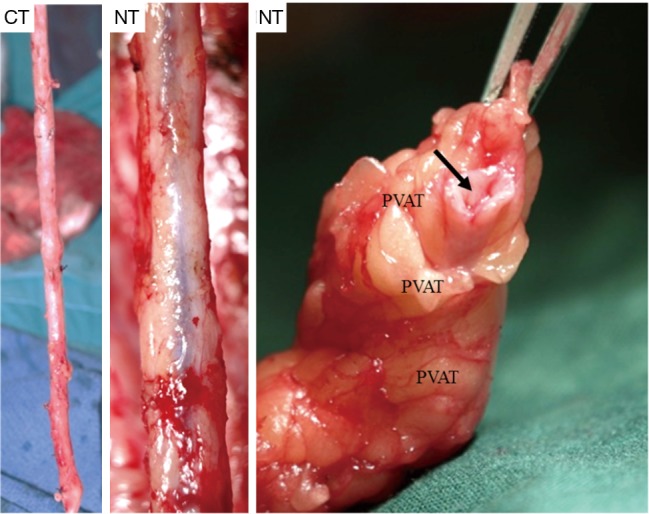
Representative examples of “no-touch” (NT) and conventional (CT) saphenous veins as seen at harvesting. The NT vein is removed complete with its cushion of surrounding fat whereas this fat is removed in CT vein grafts that are often also distended at high pressure to overcome spasm. The right-hand panel shows an example of a segment of NT vein with its surrounding cushion of perivascular adipose tissue (PVAT) and adventitial layer intact and a patent lumen. Scale bars =2.5 mm for left panels and 10 mm for right panel.
Figure 2.
A sequential no-touch vein graft anastomosed to three small target vessels.
In several post-mortem biopsies, we observed that there are significant differences in the macroscopic aspects of atherosclerotic disease between SVGs that are harvested with the NT, versus the conventional technique (27), with the latter showing more extensive atherosclerosis. This leads us to postulate that the success of percutaneous coronary intervention (PCI) in those SVGs that develop stenosis might be significantly higher in the NT SVGs. Therefore, we propose a study to evaluate the results of PCI in SVGs of approximately 300 consecutive patients during the period 2004–2017. We aim to compare the results between those veins that were treated with the NT versus the conventional technique. Our hypothesis is that the NT technique protects the SV from developing atherosclerosis and when it occurs, the process is often limited to a specific area of the vein graft, increasing the chance of a successful PCI. This slower and more limited atherosclerosis in NT SVGs was shown in angiographic and intravascular ultrasound studies of the grafts (38).
Concerns have been raised regarding the increase in leg wound complications observed in patients receiving SVGs harvested by the NT technique. Wound complications may occur at higher rates if the technique is not employed correctly [i.e., as previously described (24)]. We would certainly prefer to provide a superior conduit with increased patency, even if a higher risk of wound infection occurs. An ideal situation would be to produce a superior conduit, combined with minimal risk of harvesting complications (39). In a recent study, Mannion et al. report that NT vein grafts, which were harvested by an open technique, had a higher patency rate compared to the conventionally harvested endoscopic veins; nonetheless, the NT group had significantly higher harvest site complications (40). However, another report demonstrated that functional wound healing was similar between NT and conventional harvesting techniques 12 months after surgery (22). With these points in consideration, our future aim must be to develop an endoscopic, minimally invasive NT SVG harvesting technique.
Conclusions
We are disappointed at the reluctance of surgeons to adopt the NT harvesting technique, despite considerable evidence supporting its contribution in improving SVG patency in CABG. We believe the main issue for preventing the NT technique from being internationally embraced is that, like all other surgical techniques, considerable time is required for surgeons to become familiar with and eventually implement them. Even though the NT harvesting technique was first introduced in 1996 (23), the vast majority of SVGs are still being severely damaged during harvesting.
We are convinced that the use of arterial grafts in combination with the NT SVG will significantly improve the results of CABG. This is important for achieving a comprehensive and evidence-based balance between the major treatment strategies of ischemic heart disease, explicitly, CABG and PCI.
Following the excellent patency results reported for the NT SVG, interest in the NT technique has increased dramatically worldwide. A number of multicenter randomized studies are either ongoing or are to be initiated in Canada, Sweden and China. The final results of these studies will be crucial for the future acceptance of the technique.
We close with the final message; when using an SVG in CABG, we would encourage harvesting with the NT technique.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.WHO. The top 10 causes of death. World Health Statistics 2012. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/
- 2.Head SJ, Kieser TM, Falk V, et al. Coronary artery bypass grafting: Part 1—the evolution over the first 50 years. Eur Heart J 2013;34:2862-72. 10.1093/eurheartj/eht330 [DOI] [PubMed] [Google Scholar]
- 3.Kolh P, Windecker S, Alfonso F, et al. 2014 ESC/EACTS Guidelines on myocardial revascularization. Eur J Cardiothorac Surg 2014;46:517-92. 10.1093/ejcts/ezu366 [DOI] [PubMed] [Google Scholar]
- 4.Benedetto U, Amrani M, Bahrami T, et al. Survival probability loss from percutaneous coronary intervention compared with coronary artery bypass grafting across age groups. J Thorac Cardiovasc Surg 2015;149:479-84.e3. 10.1016/j.jtcvs.2014.10.032 [DOI] [PubMed] [Google Scholar]
- 5.Samano N, Bodin L, Karlsson J, et al. Graft patency is associated with higher health-related quality of life after coronary artery bypass surgery†. Interact Cardiovasc Thorac Surg 2017;24:388-94. [DOI] [PubMed] [Google Scholar]
- 6.Jones DS. CABG at 50 (or 107?) - The Complex Course of Therapeutic Innovation. N Engl J Med 2017;376:1809-11. 10.1056/NEJMp1702718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fitzgibbon GM, Kafka HP, Leach AJ, et al. Coronary bypass graft fate and patient outcome: angiographic follow-up of 5,065 grafts related to survival and reoperation in 1,388 patients during 25 years. J Am Coll Cardiol 1996;28:616-26. 10.1016/0735-1097(96)00206-9 [DOI] [PubMed] [Google Scholar]
- 8.Taggart DP, Altman DG, Gray AM, et al. Randomized trial to compare bilateral vs. single internal mammary coronary artery bypass grafting: 1-year results of the Arterial Revascularisation Trial (ART). Eur Heart J 2010;31:2470-81. 10.1093/eurheartj/ehq318 [DOI] [PubMed] [Google Scholar]
- 9.Sabik JF, 3rd, Blackstone EH, Gillinov AM, et al. Influence of patient characteristics and arterial grafts on freedom from coronary reoperation. J Thorac Cardiovasc Surg 2006;131:90-8. 10.1016/j.jtcvs.2005.05.024 [DOI] [PubMed] [Google Scholar]
- 10.Zacharias A, Schwann TA, Riordan CJ, et al. Late results of conventional versus all-arterial revascularization based on internal thoracic and radial artery grafting. Ann Thorac Surg 2009;87:19-26.e2. 10.1016/j.athoracsur.2008.09.050 [DOI] [PubMed] [Google Scholar]
- 11.Weiss AJ, Zhao S, Tian DH, et al. A meta-analysis comparing bilateral internal mammary artery with left internal mammary artery for coronary artery bypass grafting. Ann Cardiothorac Surg 2013;2:390-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shah PJ, Gordon I, Fuller J, et al. Factors affecting saphenous vein graft patency: clinical and angiographic study in 1402 symptomatic patients operated on between 1977 and 1999. J Thorac Cardiovasc Surg 2003;126:1972-7. 10.1016/S0022-5223(03)01276-5 [DOI] [PubMed] [Google Scholar]
- 13.Harskamp RE, Lopes RD, Baisden CE, et al. Saphenous vein graft failure after coronary artery bypass surgery: pathophysiology, management, and future directions. Ann Surg 2013;257:824-33. 10.1097/SLA.0b013e318288c38d [DOI] [PubMed] [Google Scholar]
- 14.Bello SO, Peng EW, Sarkar PK. Conduits for coronary artery bypass surgery: the quest for second best. J Cardiovasc Med (Hagerstown) 2011;12:411-21. 10.2459/JCM.0b013e328345a20d [DOI] [PubMed] [Google Scholar]
- 15.Schwann TA, Tatoulis J, Puskas J, et al. Worldwide Trends in Multi-arterial Coronary Artery Bypass Grafting Surgery 2004-2014: A Tale of 2 Continents. Semin Thorac Cardiovasc Surg 2017;29:273-80. 10.1053/j.semtcvs.2017.05.018 [DOI] [PubMed] [Google Scholar]
- 16.Weintraub WS, Clements SD, Jr, Crisco LV, et al. Twenty-year survival after coronary artery surgery: an institutional perspective from Emory University. Circulation 2003;107:1271-7. 10.1161/01.CIR.0000053642.34528.D9 [DOI] [PubMed] [Google Scholar]
- 17.Myers WO, Blackstone EH, Davis K, et al. CASS registry: Long term surgical survival. J Am Coll Cardiol 1999;33:488-98. 10.1016/S0735-1097(98)00563-4 [DOI] [PubMed] [Google Scholar]
- 18.Mohammadi S, Dagenais F, Doyle D, et al. Age cut-off for the loss of benefit from bilateral internal thoracic artery grafting. Eur J Cardiothorac Surg 2008;33:977-82. 10.1016/j.ejcts.2008.03.026 [DOI] [PubMed] [Google Scholar]
- 19.Cornwell LD, Omer S, Rosengart T, et al. Changes Over Time in Risk Profiles of Patients Who Undergo Coronary Artery Bypass Graft Surgery: The Veterans Affairs Surgical Quality Improvement Program (VASQIP). JAMA Surg 2015;150:308-15. 10.1001/jamasurg.2014.1700 [DOI] [PubMed] [Google Scholar]
- 20.Dashwood MR, Tsui JC. 'No-touch' saphenous vein harvesting improves graft performance in patients undergoing coronary artery bypass surgery: a journey from bedside to bench. Vascul Pharmacol 2013;58:240-50. 10.1016/j.vph.2012.07.008 [DOI] [PubMed] [Google Scholar]
- 21.Soyombo AA, Angelini GD, Bryan AJ, et al. Surgical preparation induces injury and promotes smooth muscle cell proliferation in a culture of human saphenous vein. Cardiovasc Res 1993;27:1961-7. 10.1093/cvr/27.11.1961 [DOI] [PubMed] [Google Scholar]
- 22.Verma S, Lovren F, Pan Y, et al. Pedicled no-touch saphenous vein graft harvest limits vascular smooth muscle cell activation: the PATENT saphenous vein graft study. Eur J Cardiothorac Surg 2014;45:717-25. 10.1093/ejcts/ezt560 [DOI] [PubMed] [Google Scholar]
- 23.Souza D. A new no-touch preparation technique. Technical notes. Scand J Thorac Cardiovasc Surg 1996;30:41-4. 10.3109/14017439609107239 [DOI] [PubMed] [Google Scholar]
- 24.Souza DS, Arbeus M, Botelho Pinheiro B, et al. The no-touch technique of harvesting the saphenous vein for coronary artery bypass grafting surgery. Multimed Man Cardiothorac Surg 2009;2009:mmcts.2008.003624. [DOI] [PubMed]
- 25.Souza DS, Dashwood MR, Tsui JC, et al. Improved patency in vein grafts harvested with surrounding tissue: results of a randomized study using three harvesting techniques. Ann Thorac Surg 2002;73:1189-95. 10.1016/S0003-4975(02)03425-2 [DOI] [PubMed] [Google Scholar]
- 26.Souza DS, Johansson B, Bojö L, et al. Harvesting the saphenous vein with surrounding tissue for CABG provides long-term graft patency comparable to the left internal thoracic artery: results of a randomized longitudinal trial. J Thorac Cardiovasc Surg 2006;132:373-8. 10.1016/j.jtcvs.2006.04.002 [DOI] [PubMed] [Google Scholar]
- 27.Samano N, Geijer H, Liden M, et al. The no-touch saphenous vein for coronary artery bypass grafting maintains a patency, after 16 years, comparable to the left internal thoracic artery: A randomized trial. J Thorac Cardiovasc Surg 2015;150:880-8. 10.1016/j.jtcvs.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 28.Samano N, Geijer H, Bodin L, et al. The no-touch saphenous vein graft in elderly coronary bypass patients with multiple comorbidities is a promising conduit to substitute the left internal thoracic artery. J Thorac Cardiovasc Surg 2017;154:457-66.e3. 10.1016/j.jtcvs.2017.03.048 [DOI] [PubMed] [Google Scholar]
- 29.Johansson B, Samano N, Souza D, et al. The no-touch vein graft for coronary artery bypass surgery preserves the left ventricular ejection fraction at 16 years postoperatively: long-term data from a longitudinal randomised trial. Open Heart 2015;2:e000204. 10.1136/openhrt-2014-000204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Johansson BL, Souza DS, Bodin L, et al. No touch vein harvesting technique for CABG improves the long-term clinical outcome. Scand Cardiovasc J 2009;43:63-8. 10.1080/14017430802140104 [DOI] [PubMed] [Google Scholar]
- 31.Souza DSR, Bomfim V, Skoglund H, et al. High early patency of saphenous vein graft for coronary artery bypass harvested with surrounding tissue. Ann Thorac Surg 2001;71:797-800. 10.1016/S0003-4975(00)02508-X [DOI] [PubMed] [Google Scholar]
- 32.Stigler R, Steger C, Schachner T, et al. The impact of distension pressure on acute endothelial cell loss and neointimal proliferation in saphenous vein grafts. Eur J Cardiothorac Surg 2012;42:e74-9. 10.1093/ejcts/ezs402 [DOI] [PubMed] [Google Scholar]
- 33.Ozturk N, Sucu N, Comelekoglu U, et al. Pressure applied during surgery alters the biomechanical properties of human saphenous vein graft. Heart Vessels 2013;28:237-45. 10.1007/s00380-012-0245-6 [DOI] [PubMed] [Google Scholar]
- 34.Dreifaldt M, Souza D, Bodin L, et al. The vasa vasorum and associated endothelial nitric oxide synthase is more important for saphenous vein than arterial bypass grafts. Angiology 2013;64:293-9. 10.1177/0003319712443729 [DOI] [PubMed] [Google Scholar]
- 35.Osgood MJ, Hocking KM, Voskresensky IV, et al. Surgical vein graft preparation promotes cellular dysfunction, oxidative stress, and intimal hyperplasia in human saphenous vein. J Vasc Surg 2014;60:202-11. 10.1016/j.jvs.2013.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vijayan V, Shukla N, Johnson JL, et al. Long-term reduction of medial and intimal thickening in porcine saphenous vein grafts with a polyglactin biodegradable external sheath. J Vasc Surg 2004;40:1011-9. 10.1016/j.jvs.2004.08.047 [DOI] [PubMed] [Google Scholar]
- 37.Taggart D, Nir RR, Bolotin G. New technologies in coronary artery surgery. Rambam Maimonides Med J 2013;4:e0018. 10.5041/RMMJ.10118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Johansson BL, Souza DS, Bodin L, et al. Slower progression of atherosclerosis in vein grafts harvested with 'no touch' technique compared with conventional harvesting technique in coronary artery bypass grafting: an angiographic and intravascular ultrasound study. Eur J Cardiothorac Surg 2010;38:414-9. 10.1016/j.ejcts.2010.02.007 [DOI] [PubMed] [Google Scholar]
- 39.Souza D, Samano N. Long-term patency versus leg wound healing in coronary artery bypass surgery: Surgical aspects of the no-touch harvesting technique. J Thorac Cardiovasc Surg 2016;151:276. 10.1016/j.jtcvs.2015.10.025 [DOI] [PubMed] [Google Scholar]
- 40.Mannion JD, Marelli D, Brandt T, et al. "No-touch" versus "endo" vein harvest: early patency on symptom-directed catheterization and harvest site complications. Innovations (Phila) 2014;9:306-11. 10.1097/IMI.0000000000000084 [DOI] [PubMed] [Google Scholar]


