Abstract
Background
Neoadjuvant therapy (NT) for resectable pancreatic adenocarcinoma (PAC) continues to be debated. We sought to establish the relationship between pancreatic tumor size, neoadjuvant chemotherapy (NCT), neoadjuvant chemoradiation (NCRT), and definitive surgery (DS) on survival.
Methods
Utilizing the National Cancer Database we identified patients with PAC who underwent NT and DS. Patient characteristics and survival were compared with Mann-Whitney U, Pearson’s Chi-square, and the Kaplan-Meier method. Multivariable analysis (MVA) was developed to identify predictors of survival. All tests were two-sided and α <0.05 was significant.
Results
We identified 11,707 patients: 9,722 patients with tumors >2 cm and 1,985 with tumors ≤2 cm. There were 523 patients treated with NCT, 559 treated with NCRT, and 10,625 DS. Patients with tumors >2 cm were more likely to have higher T-stage, P<0.001, positive lymph nodes, P<0.001, poor histologic grade, P<0.001, and R1 resections, P<0.001. The median survival for patients with tumors ≤2 cm was 30.6 months compared to 20.5 months for those whose tumors were >2 cm, P<0.001. In the >2 cm groups the median survival for NCT, NCRT, and DS was 22.9, 25.8 and 21.3 months, P=0.01. MVA revealed that age, Charlson/Deyo score, N-stage, grade, tumor size >2 cm, R0 resection, and NT were predictors of survival. Ninety-day mortality was worse in both the NCT and NCRT compared to DS, P<0.001.
Conclusions
The size of pancreatic cancer correlates to pathologic stage and overall survival. Tumors >2 and <2 cm benefited from a NT. However, the 90-operative mortality was significantly worse in those patients receiving NT.
Keywords: Pancreatic cancer, tumor size, neoadjuvant therapy (NT)
Introduction
Pancreatic cancer is the 4th leading cause of cancer mortality in the United States, with overall survival of <5% upon diagnosis (1-3). Surgery has been proven to increase overall survival and with the addition of adjuvant chemotherapy and chemoradiation overall survival can be increased to 28–37% (3-5). Neoadjuvant therapy (NT) has shown to further increase 5-year survival in some series (6) however this approach has not been uniformly adopted.
At diagnosis, the TNM staging system by the American Joint Cancer Committee (AJCC) is used to determine prognosis of pancreatic cancer as well as guide multimodal therapy. The 7th edition of the AJCC TNM staging system includes extra-pancreatic extension as a more important prognostic factor than tumor size (7). However, there is extensive evidence demonstrating size is an independent risk factor for prognosis of pancreatic cancer patients regardless of extra-pancreatic extension (8-10). Therefore, the 8th edition of the TNM staging [2018] has been updated to exclude whether the tumor has extrapancreatic extension and focus directly on tumor size (11).
Now that tumor size has been identified as fundamental to pancreatic cancer staging and survival, it will have a role in guiding multimodality therapy. One area of evidential weakness in multimodality therapy is the effectiveness of NT. Approximately 38% of patients who have resected pancreatic cancer will have recurrence most commonly as distant metastases suggesting possible unidentified micrometastasis preoperatively (12,13). Current recommendation by the National Comprehensive Cancer Network (NCCN) for resectable and borderline resectable pancreatic cancer is to do definitive surgery followed by adjuvant therapy (14). Adjuvant therapy will be recommended for most patients following pancreatic resection. However, not all resected patients will end up receiving adjuvant therapy due to postoperative complications (15,16).
NT allows for tumor biology to declare itself and may improve the rate of resectable patients undergoing multimodal therapy despite surgical outcomes. Counter arguments that some patients will develop disease progression who would have been otherwise been potentially curable with an upfront surgery approach given the poor response rates to a neoadjuvant approach. It has been reported that neoadjuvant treatment improves median and overall survival in resectable and borderline resectable pancreatic cancer (17-20). However, there has never been a study on the survival of patients receiving NT stratified by size of the tumor. Our goal is to identify the significance of NT on survival as it pertains to tumor size and whether tumor size can influence the decision to utilize NT.
Methods
Patients
This retrospective study was approved and deemed exempt by the institutional review board at Sarasota Memorial Hospital as it did not involve patient identifiers. The National Cancer Database (NCDB) is a dataset maintained by the American College of Surgeons and the American Cancer Society and collects patient data from >1,500 centers across the United States. Our patient population was obtained from the Pancreatic Participant Use Data File (PUF). Data represents more than 70 percent of newly diagnosed cancer cases nationwide. PUF’s are entirely de-identified data files available to selected investigators at CoC-approved institutions for the advancement of patient care. We queried the NCDB for patients with a diagnosis of pancreatic adenocarcinoma (PAC) who underwent surgery between 2006 and 2013. Patients were compared by therapy, definitive surgery (DS), neoadjuvant chemotherapy only (NCT), or neoadjuvant chemoradiation (NCRT), and stratified by size of tumor, ≤2 or >2 cm.
Statistics
Baseline univariate comparisons of patient characteristics between the >2 and ≤2 cm patients were made for continuous variables using the Mann-Whitney U and Kruskal Wallis tests as appropriate. Pearson’s Chi-square test and Fisher’s exact test was used to compare categorical variables when appropriate. Overall survival was defined from the time of diagnosis to death or last contact. Survival time was censored for patients alive at the end of the study period. Kaplan-Meier survival analysis was used to generate overall survival curves and estimate median survival with 95% confidence intervals for each group. Survival distributions were compared across groups using the log-rank test.
Multivariable Cox proportional hazard models were developed comparing treatment methods (definitive surgery, NCT, NCRT). Predictors of long-term survival included in the models were age, sex, pathologic T-stage, pathologic N-stage, tumor grade, tumor size, lymph nodes harvested, number of lymph of positive lymph nodes, surgical margins, institution volume, adjuvant therapy and use of induction therapy. Facility volume was calculated as the total number of cases within a facility for a given year.
To correct for baseline differences among treatment groups, propensity score matching (PSM) was used to match for age, Charlson/Deyo comorbidity score, facility volume, and NT. Matching occurred on a 1:1 basis and only exact matches were allowed. PSM creates treatment groups in a way that approximates the effect of randomization, and therefore partially removes the bias that typically accompanies treatment assignment in nonrandomized studies. All statistical tests were two-sided and α (type I) error <0.05 was considered statistically significant. Statistical analysis was performed using SPSS® version 24.0 (IBM®, Chicago, IL, USA). This study was approved as exempt by the Institutional Review Board.
Results
We identified 11,707 patients in the NCDB with PAC; 9,722 with tumors >2 cm and 1,985 with tumors ≤2 cm. The median age of both groups of patients was 67 [22–90], P=0.70 and the majority of the patients were male; 4,968 (51.1%) in >2 cm, and 989 (49.8%) in ≤2 cm (Table 1).
Table 1. Patient characteristics.
| Variable | Non-PSM | PSM | |||||
|---|---|---|---|---|---|---|---|
| ≤2 cm, N=1,985, N (%) | >2 cm, N=9,722, N (%) | P | ≤2 cm, N=1,941, N (%) | >2 cm, N=1,941, N (%) | P | ||
| Median age [range] | 67 [34–90] | 67 [22–90] | 0.7 | 67 [34–90] | 67 [34–90] | 1.0 | |
| Gender | 0.3 | 0.35 | |||||
| Male | 989 (49.8) | 4,968 (51.1) | 967 (49.8) | 996 (51.3) | |||
| Female | 996 (50.2) | 4,754 (48.9) | 974 (50.2) | 945 (48.7) | |||
| Charlson/Deyo | 0.006 | 1.0 | |||||
| 0 | 1,344 (67.7) | 6,320 (65.0) | 1,331 (68.6) | 1,331 (68.6) | |||
| 1 | 532 (26.8) | 2,691 (27.7) | 517 (26.6) | 517 (26.6) | |||
| 2 | 109 (5.5) | 711 (7.3) | 93 (4.8) | 93 (4.8) | |||
| Grade | <0.001 | <0.001 | |||||
| Low | 270 (13.6) | 840 (8.6) | 264 (13.6) | 192 (9.9) | |||
| Intermediate | 1,080 (54.4) | 5,031 (51.7) | 1,056 (54.4) | 1,027 (52.9) | |||
| High | 635 (32.0) | 3,851 (39.6) | 621 (32.0) | 722 (37.2) | |||
| Clinical T-stage | <0.001 | <0.001 | |||||
| T0 | 12 (0.6) | 35 (0.4) | 12 (0.6) | 11 (0.6) | |||
| T1 | 843 (42.5) | 953 (9.8) | 826 (42.6) | 178 (9.2) | |||
| T2 | 402 (20.3) | 3,965 (40.8) | 393 (20.2) | 805 (41.5) | |||
| T3 | 728 (36.7) | 4,760 (49.0) | 710 (36.6) | 943 (48.6) | |||
| T4 | 0 | 9 (0.1) | 0 | 4 (0.2) | |||
| Clinical N-stage | <0.001 | <0.001 | |||||
| N0 | 1,489 (75.0) | 6,610 (68.0) | 1,458 (75.1) | 1,243 (64.0) | |||
| N1 | 496 (25.0) | 3,112 (32.0) | 483 (24.9) | 698 (36.0) | |||
| Path T-stage | <0.001 | <0.001 | |||||
| T0 | 12 (0.6) | 34 (0.3) | 12 (0.6) | 12 (0.6) | |||
| T1 | 621 (31.3) | 69 (0.7) | 606 (31.2) | 5 (0.3) | |||
| T2 | 49 (2.5) | 1,523 (15.7) | 48 (2.5) | 318 (16.4) | |||
| T3 | 1,293 (65.1) | 7,962 (81.9) | 1,266 (65.2) | 1,573 (81.0) | |||
| T4 | 10 (0.5) | 134 (1.4) | 9 (0.5) | 33 (1.7) | |||
| Path N-stage | <0.001 | <0.001 | |||||
| N0 | 905 (45.6) | 2,748 (28.3) | 883 (45.5) | 544 (28.0) | |||
| N1 | 1,080 (54.4) | 6,974 (71.7) | 1,058 (54.5) | 1,397 (72.0) | |||
| Path stage group | <0.001 | <0.001 | |||||
| 0 | 11 (0.6) | 34 (0.3) | 11 (0.6) | 11 (0.6) | |||
| I | 430 (21.7) | 746 (7.7) | 416 (21.4) | 142 (7.3) | |||
| IIA | 469 (23.6) | 1,939 (19.9) | 461 (23.8) | 382 (19.7) | |||
| IIB | 1,045 (52.6) | 6,741 (69.3) | 1,024 (52.8) | 1,346 (69.3) | |||
| III | 13 (0.7) | 150 (1.5) | 12 (0.6) | 39 (2.0) | |||
| IV | 17 (0.9) | 112 (1.2) | 17 (0.9) | 21 (1.1) | |||
| Median lymph nodes removed [range] | 14 [0–63] | 16 [0–90] | <0.001 | 14 [0–63] | 15 [0–73] | 0.03 | |
| Median lymph nodes positive [range] | 1 [0–23] | 2 [0–32] | <0.001 | 1 [0–23] | 2 [0–32] | <0.001 | |
| Surgical margins | <0.001 | <0.001 | |||||
| R0 | 1,708 (86.0) | 7,313 (75.2) | 1,671 (86.1) | 1,439 (74.1) | |||
| R1/R2 | 277 (14.0) | 2,409 (24.8) | 270 (13.9) | 502 (25.9) | |||
| Neoadjuvant status | 0.14 | 1.0 | |||||
| Neoadjuvant | 166 (8.4) | 916 (9.4) | 141 (7.3) | 141 (7.3) | |||
| Def Surgery | 1,819 (91.6) | 8,806 (90.6) | 1,800 (92.7) | 1,800 (92.7) | |||
| Neo treatment | 0.23 | 0.94 | |||||
| Neo chemo | 75 (3.8) | 448 (4.6) | 62 (3.2) | 59 (3.0) | |||
| Neo chemo/rad | 91 (4.6) | 468 (4.8) | 79 (4.1) | 82 (4.2) | |||
| Def surgery | 1,819 (91.6) | 8,806 (90.6) | 1,800 (92.7) | 1,800 (92.7) | |||
| Adjuvant status | 0.01 | <0.001 | |||||
| No | 829 (41.8) | 3,771 (38.8) | 803 (41.4) | 652 (33.6) | |||
| Yes | 1,156 (58.2) | 5,951 (61.2) | 1,138 (58.6) | 1,289 (66.4) | |||
| Adjuvant treatment | 0.1 | <0.001 | |||||
| Chemo only | 654 (32.9) | 3,333 (34.3) | 641 (33.0) | 671 (34.6) | |||
| Rad only | 23 (1.2) | 129 (1.3) | 21 (1.1) | 24 (1.2) | |||
| Chemo/rad | 479 (24.1) | 2,489 (25.6) | 476 (24.5) | 594 (30.6) | |||
| None | 829 (41.8) | 3,771 (38.8) | 803 (41.4) | 652 (33.6) | |||
| Overall treatment | 0.1 | <0.001 | |||||
| Neo chemo | 75 (3.8) | 448 (4.6) | 62 (3.2) | 59 (3.0) | |||
| Neo chemo/rad | 91 (4.6) | 468 (4.8) | 79 (4.1) | 82 (4.2) | |||
| Def surgery, adjv | 1,110 (55.9) | 5,571 (57.3) | 1,100 (56.7) | 1,223 (63.0) | |||
| Def surgery, no adjv | 709 (35.7) | 3,235 (33.3) | 700 (36.1) | 577 (29.7) | |||
| Facility volume | 0.11 | 1.0 | |||||
| Low (≤10/yr) | 955 (48.1) | 4,849 (49.9) | 940 (48.4) | 940 (48.4) | |||
| Med (11–19/yr) | 589 (29.7) | 2,659 (27.4) | 572 (29.5) | 572 (29.5) | |||
| High (≥20/yr) | 441 (22.2) | 2,214 (22.8) | 429 (22.1) | 429 (22.1) | |||
NT was received in 916 (9.4%) patients in the >2 cm group and in 166 (8.4%) in the ≤2 cm group. In the >2 cm group, NT was chemotherapy in 448 (4.6%) and chemoradiation in 468 (4.8%). The ≤2 cm group NT was chemotherapy in 75 (3.8%) and chemoradiation in 91 (4.6%). There were more lymph nodes removed in the >2 cm group versus ≤2 cm group; 16 [0–90] and 14 [0–63] removed respectively, P<0.001. Histologic grade was found to be high in 3,851 (39.6%) of the >2 cm group and 635 (32.0%) in the ≤2 cm group, P<0.001. T-stage, clinical stage and pathologic stage also was found to be higher in the >2 cm group than ≤2 cm group, P<0.001 (Table 1).
R0 resection was achieved in 7,313 (75.2%) of the >2 cm group and 1,708 (86.0%) in the ≤2 cm group, P<0.001. In all there were 3,653 node negative patients and 8,054 node positive patients. Node status was negative in 2,748 (28.3%) and positive in 6,974 (71.7%) of the >2 cm group. There were more node positive patients found in the >2 cm group, P<0.001. Whereas the nodal status was negative in 905 (45.6%) and positive in 1,080 (54.4%) of the ≤2 cm group, P<0.001 (Table 1).
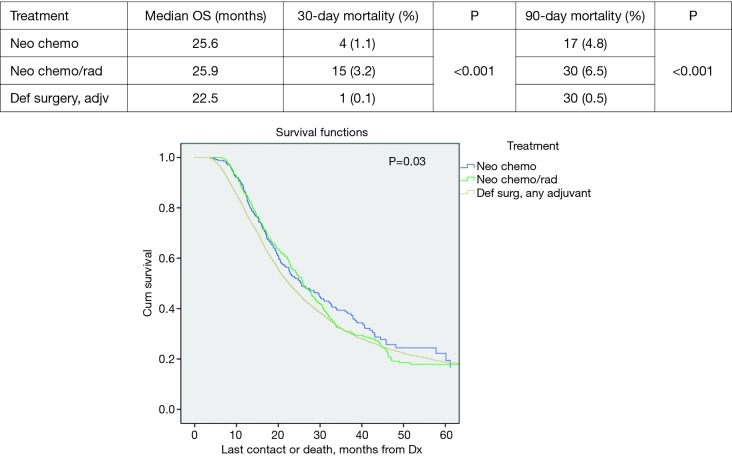
Median survival demonstrated improvement in all patients who received NT versus definitive surgery with adjuvant therapy (DS); 25.9 vs. 22.5 months respectively, P=0.03. Neoadjuvant chemoradiation (NCRT) demonstrated slightly better median survival versus NCT; 25.9 vs. 25.5 months, P=0.03 respectively (Figure 1).
Figure 1.
Overall survival by treatment.
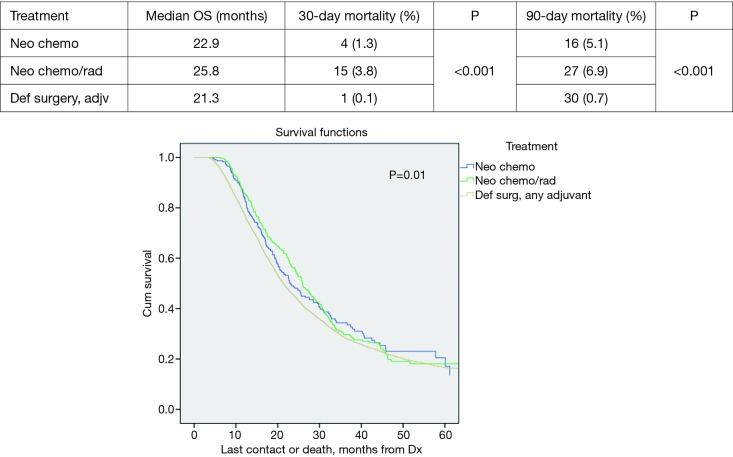
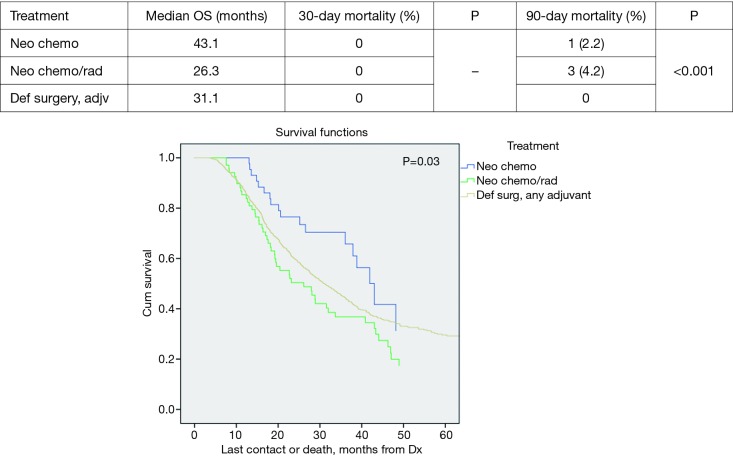
Patients with tumors >2 cm did demonstrate statistically significant improved median survival with NT vs. DS + adjuvant therapy; 25.4 vs. 21.3 months, P=0.002. The median survival in the >2 cm group for NCT, NCRT and DS with adjuvant were 22.9, 25.8, and 21.3 months respectively, P=0.01 (Figure 2). However, the NCT and NCRT had significantly worse 90-day mortality compared to the DS with adjuvant therapy 5.1%, 6.9%, and 0.7%, respectively, P<0.001. In patients with ≤2 cm who received NT, median survival was improved; NCT 43.1 months, NCRT 26.3 months and DS + adjuvant therapy 31.1 months, P=0.03 (Figure 3). NCT, NCRT similarly demonstrated significantly worse 90-day mortality for ≤2 cm group compared to the DS with adjuvant therapy; 2.2%, 4.2%, and 0%, P<0.001.
Figure 2.
Tumor >2 cm overall survival by treatment.
Figure 3.
Tumor ≤2 cm overall survival by treatment.
Multivariate analysis revealed that age, Charlson/Deyo score, pathologic T-stage, pathologic N-stage, grade, tumor size (>2 cm), surgical margins, facility volume, and NT were predictors of survival, P<0.001 (Table 2). For R1/R2 resections, hazard ratio (HR) was 1.39 (1.3–1.5), P<0.001. The HR for >2 cm was 1.35 (1.22–1.49), P<0.001.
Table 2. Multivariate analysis.
| Variable | Non-PSM | PSM | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | 1.01 | 1.00–1.01 | <0.001 | 1.01 | 1.01–1.02 | <0.001 | |
| Gender | |||||||
| Male | REF | REF | REF | REF | REF | REF | |
| Female | 0.99 | 0.93–1.05 | 0.73 | 0.94 | 0.84–1.04 | 0.22 | |
| Charlson/Deyo | |||||||
| 0 | REF | REF | REF | REF | REF | REF | |
| 1 | 1.07 | 1.00–1.14 | 0.06 | 1.14 | 1.01–1.28 | 0.04 | |
| 2 | 1.11 | 0.97–1.25 | 0.12 | 1.35 | 1.03–1.77 | 0.03 | |
| Path T-stage | |||||||
| T0 | REF | REF | REF | REF | REF | REF | |
| T1 | 0.49 | 0.20–1.20 | 0.12 | 0.46 | 0.15–1.45 | 0.19 | |
| T2 | 0.47 | 0.19–1.13 | 0.09 | 0.4 | 0.13–1.27 | 0.12 | |
| T3 | 0.52 | 0.22–1.25 | 0.15 | 0.48 | 0.15–1.50 | 0.21 | |
| T4 | 0.71 | 0.28–1.76 | 0.45 | 0.55 | 0.16–1.85 | 0.33 | |
| Path N-stage | |||||||
| N0 | REF | REF | REF | REF | REF | REF | |
| N1 | 1.59 | 1.50–1.69 | <0.001 | 1.48 | 1.31–1.68 | <0.001 | |
| Grade | |||||||
| Low | REF | REF | REF | REF | REF | REF | |
| Intermediate | 1.22 | 1.09–1.37 | 0.001 | 1.25 | 1.03–1.51 | 0.02 | |
| High | 1.58 | 1.41–1.77 | <0.001 | 1.76 | 1.44–2.13 | <0.001 | |
| Tumor size | |||||||
| ≤2 cm | REF | REF | REF | REF | REF | REF | |
| >2 cm | 1.35 | 1.22–1.49 | <0.001 | 1.45 | 1.28–1.64 | <0.001 | |
| Surgical margins | |||||||
| R0 | REF | REF | REF | REF | REF | REF | |
| R1/R2 | 1.39 | 1.30–1.50 | <0.001 | 1.54 | 1.35–1.75 | <0.001 | |
| Facility volume | |||||||
| Low (<10/yr) | REF | REF | REF | REF | REF | REF | |
| Med (11–19/yr) | 0.96 | 0.89–1.03 | 0.22 | 1.02 | 0.91–1.16 | 0.7 | |
| High (>20/yr) | 0.92 | 0.85–0.99 | 0.03 | 0.91 | 0.79–1.06 | 0.22 | |
| Treatment | |||||||
| Def surgery, adjv | REF | REF | REF | REF | REF | REF | |
| Neo chemo | 0.92 | 0.80–1.06 | 0.27 | 0.69 | 0.49–0.99 | 0.04 | |
| Neo chemo/rad | 1.1 | 0.98–1.25 | 0.12 | 1.35 | 1.09–1.67 | 0.006 | |
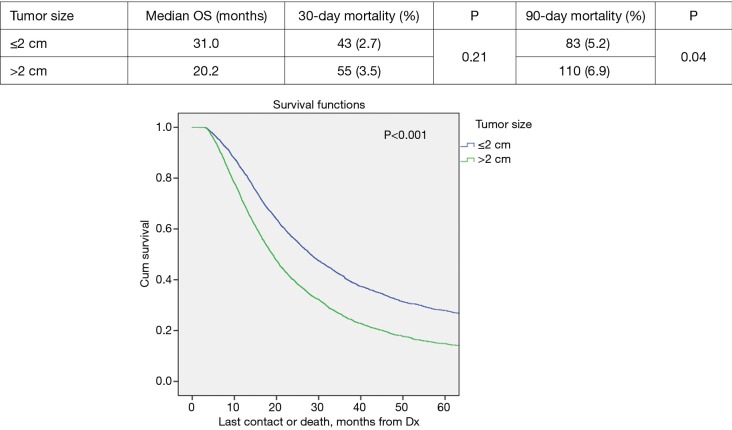
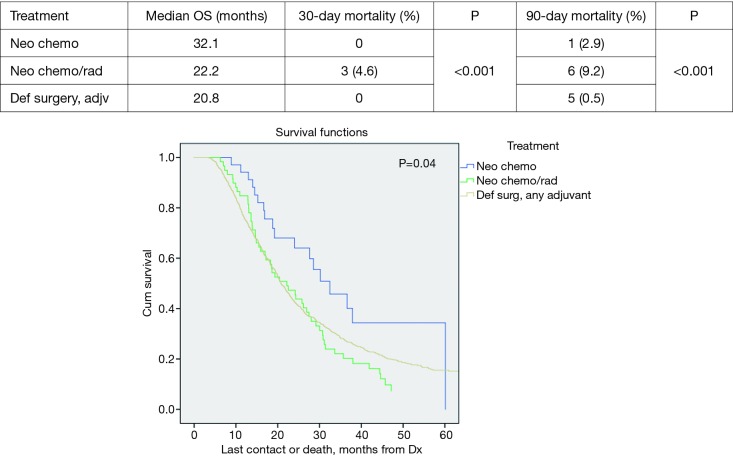
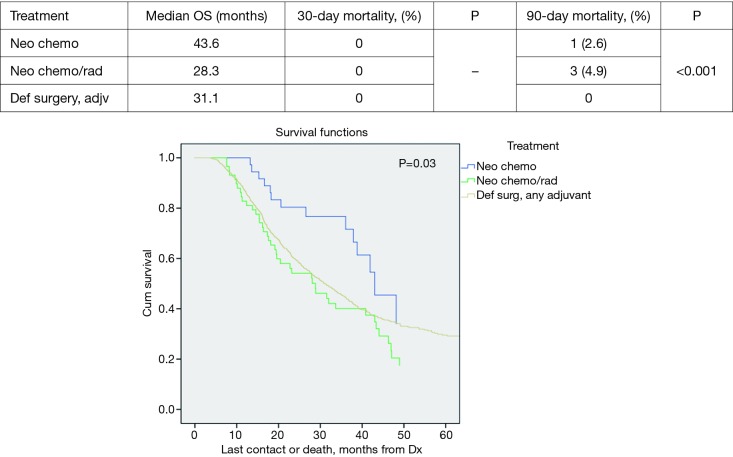
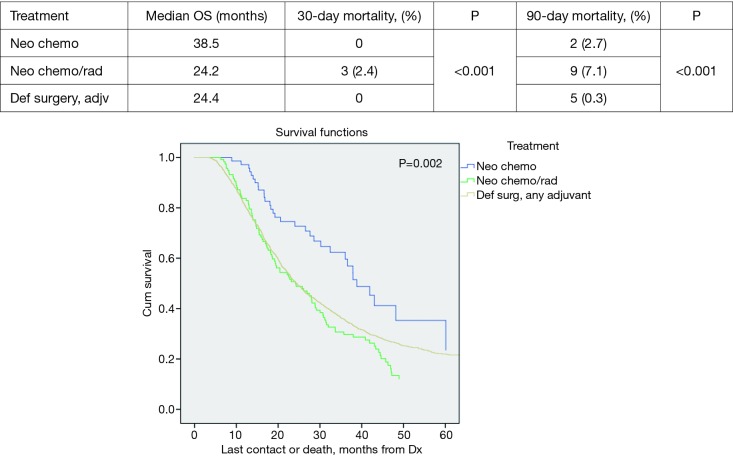
Propensity score matched analysis for age, Charlson/Deyo comorbidity score, facility volume, and NT demonstrated that tumor size significantly influenced nodal positivity (P<0.001), T-stage (P<0.001), N-stage (P<0.001), grade (P<0.001), and surgical margins (Table 1). Median survival stratified by size for >2 cm was 20.2 months and for ≤2 cm was 31 months, P<0.001 (Figure 4). In the >2 cm group, median survival for NCT, NCRT, and definitive surgery with adjuvant therapy were 32.1, 22.2, and 20.8 months respectively, P=0.04 (Figure 5). The ≤2 cm group demonstrated significant difference between median survival of NCT, NCRT, and definitive surgery with addition of adjuvant therapy; 43.6, 28.3, and 31.1 months respectively, P=0.03 (Figure 6). Overall median survival by therapy was best with NCT versus NCRT or definitive surgery with adjuvant therapy; 38.5, 24.2, and 24.4 months, P=0.002 (Figure 7). Similar to the non-propensity score cohort, NCT and NCRT demonstrated significantly worse 90-day mortality compared to DS in patients with tumors >2 and <2 cm (<2 cm 2.6%, 4.9%, and 0%, P<0.001), and (>2 cm, 2.9%, 9.2%, and 0.5%, P<0.001).
Figure 4.
Overall survival by tumor size.
Figure 5.
Tumor >2 cm overall survival by treatment.
Figure 6.
Tumor ≤2 cm overall survival by treatment.
Figure 7.
Overall survival by treatment.
Discussion
Between the 7th edition and 8th edition of the AJCC TNM staging guidelines, many studies have reported the significance of pancreatic tumor size on survival, influencing the 8th edition to base T-staging solely off tumor size (11). However, there has been little advancement in the use of tumor size for guiding multimodality therapy, specifically NT. Our study is among the first to compare NT amongst pancreatic tumor size. Multivariate analysis demonstrates that NT is an independent predictor of prognosis, as is tumor size. NCT showed the most improved survival as compared to neoadjuvant chemoradiation and definitive surgery. Furthermore, this study identifies the significance of utilizing NT in patients with pancreatic cancer tumors >2 and <2 cm to significantly improve survival however the 90-day mortality in these patients are also increased.
NT in pancreatic cancer continues to be controversial. Its use in pancreatic cancer has previously demonstrated higher likelihood of achieving R0 margins in borderline resectable disease (18,21-25). Achieving an R0 resection over R1 has demonstrated a significant improvement in median survival by 6 months (26). NT in resectable pancreatic cancer has also proven to have a higher R0 resection rate. A few prospective studies have demonstrated up to 100% R0 resection rates with the use of NT in resectable pancreatic cancer (6,20). These studies are limited by their small sample sizes. However, our study corroborates these findings in a much larger series.
Another advantage to utilizing NT is to ensure that all patients receive adequate multimodal therapy. The NCCN recommends as of April 2017, to utilize definitive surgery in all resectable and borderline resectable pancreatic cancer followed by adjuvant therapy (14). Many previous studies that supported definitive surgery then adjuvant therapy were found to have selection bias by excluding patients who were not able to receive all adjuvant therapy (up to 60% in some cases) due to post-operative complications (3,4,27,28). A study by Tzeng et al. of 167 patients, 115 who underwent NT and 52 who underwent definitive surgery and adjuvant therapy, discovered that 83% of the NT group completed all multimodality therapy, whereas only 58% of the definitive surgery group was able to complete adjuvant therapy (29). The utilization of NT will allow more patients to receive all necessary multimodality therapy upfront thereby obviating post-operative complications as an impact on adjuvant therapies administered.
Several authors have reported that NT improves median and overall survival of pancreatic cancer patients with resectable or borderline resectable disease (30-32). A retrospective study by Artinyan et al. of 458 patients demonstrated improved median and overall 5-year survival in patients receiving NT versus definitive surgery (31). Our study similarly identifies NT as an independent predictor of survival. We also found improved survival with NCT over NCRT. With more studies geared towards NT, this finding has potential to influence the decision between chemotherapy and chemoradiation for neoadjuvant treatment.
Tumor size has been an integral part of staging pancreatic cancer according to the AJCC guidelines. However, only recently did tumor size come under scrutiny as a prognostic factor for survival. A retrospective study by Park et al. of 6,145 patients from the Surveillance Epidemiology and End Result database sought to identify whether the 7th edition of AJCC TNM staging accurately represented tumor size versus extrapancreatic extension. What they demonstrated was that tumor size was more determinant of prognosis than extrapancreatic extension (8). Other studies, including our own, also show that tumor size is an independent risk factor of survival in patients with pancreatic cancer (9,10). Therefore, the 8th edition of the AJCC TNM staging guidelines to be used starting January 1st 2018 will solely utilizes tumor size in its T-stage system (11).
The new T-stage will invariably alter the way multimodality therapy will be guided. Since the current NCCN guidelines recommends surgical therapy followed by adjuvant therapy, most studies are geared towards adjuvant therapy as an independent factor for survival (3,14,27). Aoyama et al. retrospectively studied 76 patients, stratified by pancreatic tumor size, who underwent surgical resection of pancreatic cancer and received adjuvant therapy. They discovered that tumor size was the most important prognostic factor for survival specifically in patients receiving adjuvant therapy (33). We similarly found size to be an independent factor of survival and further studied those patients who received NT. Our data demonstrated that NT was a statistically significant predictor of survival in patients with tumor size >2 cm.
Our study is limited by inherent selection bias by being a retrospective analysis, but we attempted to limit this bias by including propensity score matching. We also are unable to standardize data input across institutions and are unable to monitor the guidelines used for collecting data and making diagnosis. Additionally we are unable to account for the accuracy of imaging staging prior to surgery between institutions.
Conclusions
NT demonstrated a survival benefit in all pancreatic cancer patients. NCT demonstrated the most significant improvements in both tumor size >2 and <2 cm. However, in both the NCT and NCRT groups the 30- and 90-day mortality was significantly worse. While pancreatic cancer tumor size is a prognostic factor for survival it should not be used to determine which patients should receive NT. This data supports the use of NT in all pancreatic cancer patients.
Acknowledgements
None.
Ethical Statement: This retrospective study was approved and deemed exempt by the institutional review board at Sarasota Memorial Hospital as it did not involve patient identifiers.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Trede M, Schwall G, Saeger HD. Survival after pancreatoduodenectomy. 118 consecutive resections without an operative mortality. Ann Surg 1990;211:447-58. 10.1097/00000658-199004000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Corsini MM, Miller RC, Haddock MG, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975-2005). J Clin Oncol 2008;26:3511-6. 10.1200/JCO.2007.15.8782 [DOI] [PubMed] [Google Scholar]
- 4.Winter JM, Brennan MF, Tang LH, et al. Survival after resection of pancreatic adenocarcinoma: results from a single institution over three decades. Ann Surg Oncol 2012;19:169-75. 10.1245/s10434-011-1900-3 [DOI] [PubMed] [Google Scholar]
- 5.Neoptolemos JP, Dunn JA, Stocken DD, et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 2001;358:1576-85. 10.1016/S0140-6736(01)06651-X [DOI] [PubMed] [Google Scholar]
- 6.Takahashi H, Ohigashi H, Gotoh K, et al. Preoperative gemcitabine-based chemoradiation therapy for resectable and borderline resectable pancreatic cancer. Ann Surg 2013;258:1040-50. 10.1097/SLA.0b013e31829b3ce4 [DOI] [PubMed] [Google Scholar]
- 7.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol 2010;17:1471-4. [DOI] [PubMed] [Google Scholar]
- 8.Park H, An S, Eo SH, et al. Survival effect of tumor size and extrapancreatic extension in surgically resected pancreatic cancer: proposal for improved T classification. Hum Pathol 2014;45:2341-6. 10.1016/j.humpath.2014.06.030 [DOI] [PubMed] [Google Scholar]
- 9.Lim JE, Chien MW, Earle CC. Prognostic factors following curative resection for pancreatic adenocarcinoma: a population-based, linked database analysis of 396 patients. Ann Surg 2003;237:74-85. 10.1097/00000658-200301000-00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marchegiani G, Andrianello S, Malleo G, et al. Does Size Matter in Pancreatic Cancer?: Reappraisal of Tumour Dimension as a Predictor of Outcome Beyond the TNM. Ann Surg 2017;266:142-8. 10.1097/SLA.0000000000001837 [DOI] [PubMed] [Google Scholar]
- 11.Chun YS, Pawlik TM, Vauthey JN. 8th Edition of the AJCC Cancer Staging Manual: Pancreas and Hepatobiliary Cancers. Ann Surg Oncol 2018;25:845-7. [DOI] [PubMed] [Google Scholar]
- 12.Aoyama T, Murakawa M, Katayama Y, et al. Impact of postoperative complications on survival and recurrence in pancreatic cancer. Anticancer Res 2015;35:2401-9. [PubMed] [Google Scholar]
- 13.Fischer R, Breidert M, Keck T, et al. Early recurrence of pancreatic cancer after resection and during adjuvant chemotherapy. Saudi J Gastroenterol 2012;18:118-21. 10.4103/1319-3767.93815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2017, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2017;15:1028-61. 10.6004/jnccn.2017.0131 [DOI] [PubMed] [Google Scholar]
- 15.Bilimoria KY, Bentrem DJ, Ko CY, et al. Multimodality therapy for pancreatic cancer in the U.S.: utilization, outcomes, and the effect of hospital volume. Cancer 2007;110:1227-34. 10.1002/cncr.22916 [DOI] [PubMed] [Google Scholar]
- 16.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative complications reduce adjuvant chemotherapy use in resectable pancreatic cancer. Ann Surg 2014;260:372-7. 10.1097/SLA.0000000000000378 [DOI] [PubMed] [Google Scholar]
- 17.Kim EJ, Ben-Josef E, Herman JM, et al. A multi-institutional phase 2 study of neoadjuvant gemcitabine and oxaliplatin with radiation therapy in patients with pancreatic cancer. Cancer 2013;119:2692-700. 10.1002/cncr.28117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. 10.1371/journal.pmed.1000267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Geus SW, Eskander MF, Bliss LA, et al. Neoadjuvant therapy versus upfront surgery for resected pancreatic adenocarcinoma: A nationwide propensity score matched analysis. Surgery 2017;161:592-601. 10.1016/j.surg.2016.08.040 [DOI] [PubMed] [Google Scholar]
- 20.Mellon EA, Strom TJ, Hoffe SE, et al. Favorable perioperative outcomes after resection of borderline resectable pancreatic cancer treated with neoadjuvant stereotactic radiation and chemotherapy compared with upfront pancreatectomy for resectable cancer. J Gastrointest Oncol 2016;7:547-55. 10.21037/jgo.2016.03.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christians KK, Tsai S, Mahmoud A, et al. Neoadjuvant FOLFIRINOX for borderline resectable pancreas cancer: a new treatment paradigm? Oncologist 2014;19:266-74. 10.1634/theoncologist.2013-0273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhan HX, Xu JW, Wu D, et al. Neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of prospective studies. Cancer Med 2017;6:1201-19. 10.1002/cam4.1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang X, Knoble JL, Zeng M, et al. Neoadjuvant Gemcitabine Chemotherapy followed by Concurrent IMRT Simultaneous Boost Achieves High R0 Resection in Borderline Resectable Pancreatic Cancer Patients. PLoS One 2016;11:e0166606. 10.1371/journal.pone.0166606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mellon EA, Hoffe SE, Springett GM, et al. Long-term outcomes of induction chemotherapy and neoadjuvant stereotactic body radiotherapy for borderline resectable and locally advanced pancreatic adenocarcinoma. Acta Oncol 2015;54:979-85. 10.3109/0284186X.2015.1004367 [DOI] [PubMed] [Google Scholar]
- 25.Takahashi H, Akita H, Tomokuni A, et al. Preoperative Gemcitabine-based Chemoradiation Therapy for Borderline Resectable Pancreatic Cancer: Impact of Venous and Arterial Involvement Status on Surgical Outcome and Pattern of Recurrence. Ann Surg 2016;264:1091-7. 10.1097/SLA.0000000000001547 [DOI] [PubMed] [Google Scholar]
- 26.Raut CP, Tseng JF, Sun CC, et al. Impact of resection status on pattern of failure and survival after pancreaticoduodenectomy for pancreatic adenocarcinoma. Ann Surg 2007;246:52-60. 10.1097/01.sla.0000259391.84304.2b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herman JM, Swartz MJ, Hsu CC, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol 2008;26:3503-10. 10.1200/JCO.2007.15.8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aloia TA, Aloia TE, Lee JE, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg 2007;204:347-55. 10.1016/j.jamcollsurg.2006.12.011 [DOI] [PubMed] [Google Scholar]
- 29.Tzeng CW, Tran Cao HS, Lee JE, et al. Treatment sequencing for resectable pancreatic cancer: influence of early metastases and surgical complications on multimodality therapy completion and survival. J Gastrointest Surg 2014;18:16-24; discussion 24-5. 10.1007/s11605-013-2412-1 [DOI] [PubMed] [Google Scholar]
- 30.Mokdad AA, Minter RM, Zhu H, et al. Neoadjuvant Therapy Followed by Resection Versus Upfront Resection for Resectable Pancreatic Cancer: A Propensity Score Matched Analysis. J Clin Oncol 2017;35:515-22. 10.1200/JCO.2016.68.5081 [DOI] [PubMed] [Google Scholar]
- 31.Artinyan A, Anaya DA, McKenzie S, et al. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer 2011;117:2044-9. 10.1002/cncr.25763 [DOI] [PubMed] [Google Scholar]
- 32.Varadhachary GR, Wolff RA, Crane CH, et al. Preoperative gemcitabine and cisplatin followed by gemcitabine-based chemoradiation for resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3487-95. 10.1200/JCO.2007.15.8642 [DOI] [PubMed] [Google Scholar]
- 33.Aoyama T, Yoshikawa T, Watanabe T, et al. Macroscopic tumor size as an independent prognostic factor for stage II/III gastric cancer patients who underwent D2 gastrectomy followed by adjuvant chemotherapy with S-1. Gastric Cancer 2011;14:274-8. 10.1007/s10120-011-0038-0 [DOI] [PubMed] [Google Scholar]