Abstract
Minimally invasive techniques have improved post-operative outcomes, however, the majority of pancreatic surgery, known for its complexity, is still performed via open approaches. The development of robotics has improved dexterity which may allow for application in more complex surgeries. We queried a prospectively maintained robotic database to identify patients who underwent robotic pancreatic resection by a single surgeon between 2012 and 2016. Patient demographics and operative outcomes were compared using Mann-Whitney U, Kruskal Wallis and Pearson’s Chi-square test as appropriate. We identified 119 patients; 65 Whipples [Robotic Whipple (RW)], 43 distal pancreatectomies, 4 total pancreatectomies, 6 pancreatic enucleations, and 1 robotic cyst gastrostomy with a median age of 71 [24–91], median body mass index (BMI) of 27.6 (16.8–40.2), and American society of anesthesiologists (ASA) of 3. The median estimated blood loss (EBL) was 125 [25–800] and loss of heterozygosity (LOH) 6 [1–34]. Mean operative time for RW decreased after 15 cases (578 vs. 457 minutes, P<0.004). Conversions to open occurred in 5 (4.2%) patients. In total of 117 (98.3%) patients underwent R0 resections and the median lymph node (LN) harvest was 16 [0–37]. The 30 and 90 days mortality was 1 (0.8%). Major complications (Clavien-Dindo grade 3–5) were seen in 16 (13.4%) cases (20.3%) but decreased steadily as volume increased (case 30). Pancreatic leaks occurred in 14 (11.8%): A, 8 (6.7%); B, 4 (3.4%); and C, 2 (1.7%). Robotic assisted approaches to pancreatic resections is feasible. However, it takes approximately 15 cases before a decrease in operative time and 30 cases before major complications are decreased. These trends in complications are associated with surgeon experience and volume are critical to consider in robotic pancreatic surgery.
Keywords: Robotic, pancreatic resection, Whipple, distal pancreatectomy
Introduction
Pancreatic cancer remains one of the leading causes of cancer death worldwide (1). Surgical resection is a mainstay of many gastrointestinal (GI) cancers, but pancreatic surgery has significant morbidity and mortality (2). Much improvement has been achieved in minimizing mortality but pancreatic surgery remains one of the most morbid procedures (2).
The evolution of minimally invasive surgery has vastly shaped many surgical specialties. Laparoscopic pancreatic surgery was initially reported in 1994 by Pomp and Gagner and since then has proven to be clinically feasible with equal morbidity and mortality to open pancreatic surgery (3-5). The potential advantages of reduced intraoperative bleeding, postoperative recovery time and length of hospital stay have many surgeons seeking additional training with these techniques of pancreatic resection (5). However significant limitations of laparoscopic surgery exist making it difficult for many surgeons to develop the required skills necessary to maintain a minimally invasive pancreatic program.
The application of robotics to pancreatic surgery allows for improved 3D visualization and significant improvement of manual dexterity for more precise dissections. Robotic pancreatic surgery is presently being utilized at large centers and has been proven to be safe, feasible, and at least equivalent to open pancreatic surgery with regards to post-operative morbidity and oncologic outcomes (6-9). We present our series of a single surgeon’s application of robotics to pancreatic resection and identify the learning curve associated with this approach.
Methods
A retrospective review of a prospectively maintained database on all pancreatic procedures performed by a single surgeon was performed. The study was approved by the Institutional Review Board (approval # 15-onc-23). Patient and tumor characteristics as well as perioperative events were reported using mean ± standard deviation (SD) and median with interquartile range for continuous variables and frequencies for categorical variables.
We reviewed all operative events and perioperative events occurring within 90 days. All pancreatic fistulae regardless of their clinical significance were identified and classified by the international Study Group on Pancreatic Fistula (ISGPF) criteria. All post-operative complications were graded according to the Clavien-Dindo classification.
Statistical analysis was performed using SPSS version 24 (Chicago, IL, USA). Baseline univariate comparisons of patient characteristics were made for continuous variables using both the Mann-Whitney U and Kruskal Wallis tests as appropriate. Person’s Chi-square test was used to compare categorical variables.
Results
We identified 119 patients who underwent robotic pancreatic procedures; 65 Whipples (RW), 43 distal pancreatectomies, 4 total pancreatectomies, 6 pancreatic enucleations and 1 robotic cyst gastrostomy. The median age was 71 [24–91] with a median body mass index (BMI) of 27.6 (16.8–40.2) and an American society of anesthesiologists (ASA) of 3. The majority of patients had adenocarcinoma (45.4%), intraductal papillary mucinous neoplasm (IPMN) (22.7%) and neuroendocrine tumors (17.6%) with about 14% other diagnoses. The R0 resection for RW was 64 (98.5%) and distal pancreatectomies 42 (97.7%). Median lymph nodes removed for RW was 17 [0–31] and distal pancreatectomy 13.5 [0–32] (Table 1).
Table 1. Patient demographics (n=119).
| Variable | n (%) |
|---|---|
| Age, year, median [range] | 71 [24–91] |
| Gender | |
| Male | 71 (59.7) |
| Female | 48 (40.3) |
| BMI, median (range) | 27.6 (16.8–40.2) |
| <20 | 3 (2.5) |
| 20–30 | 95 (79.8) |
| 31–40 | 20 (16.8) |
| >40 | 1 (0.8) |
| ASA Score | 3 [1–4] |
| 1 | 2 (1.7) |
| 2 | 35 (29.4) |
| 3 | 72 (60.5) |
| 4 | 10 (8.4) |
| Histology | |
| Adenocarcinoma | 54 (45.4) |
| IPMN | 27 (22.7) |
| Neuroendocrine | 21 (17.6) |
| Other* | 17 (14.3) |
| R0 resection | 117 (98.3) |
| Distal | 42 (97.7) |
| Whipple | 64 (98.5) |
| LN removed, median (range) | 16 [0–37] |
| Distal | 13.5 [0–32] |
| Whipple | 17 [0–31] |
| Surgery type | |
| Distal | 43 (36.1) |
| Whipple | 65 (54.6) |
| Total | 4 (3.4) |
| Enucleation | 6 (5.0) |
| RCG | 1 (0.8) |
*, other includes adenoma, fibroma, schwannoma, pseudopapillary. BMI, body mass index; ASA, American society of anesthesiologists; IPMN, intraductal papillary mucinous neoplasm; LN, lymph node; RCG, robotic cystogastrostomy.
The mean operative time for all 119 patients was 389 minutes; 498 minutes for RW, 244 minutes for distal pancreatectomy, 567 minutes for total pancreatectomy, 145 minutes for enucleation and 271 minutes for robotic cystogastrostomy (RCG). The median estimated blood loss for all cases was 125 mL (25–800 mL). There were 5 conversions to open (4.2%) and 2 (1.7%) re-operations. The median hospital length of stay was 6 days [1–34] with 24 (20.2%) readmissions (Table 2).
Table 2. Operative outcomes.
| Variables | Entire cohort (n=119) | Distal (n=43) | Whipple (n=65) | Total (n=4) | Enucleation (n=6) | RCG (n=1) |
|---|---|---|---|---|---|---|
| Operative time (min) mean [SD] | 389 [181] | 242 [78] | 498 [144] | 567 [156] | 145 [80] | 271 |
| Conversion, n (%) | 5 (4.2) | 1 (2.3) | 3 (4.6) | 1 (25.0) | 0 | 0 |
| Reoperation, n (%) | 2 (1.7) | 2 (4.7) | 0 | 0 | 0 | 0 |
| Median length of hospitalization, day [range] | 6 [1–34] | 5 [2–19] | 7 [4–34] | 8 [7–10] | 2 [1–5] | 7 |
| Readmission, n (%) | 24 (20.2) | 8 (18.6) | 16 (24.6) | 0 | 0 | 0 |
| Median estimated blood loss, mL [range] | 125 [25–800] | 50 [25–800] | 150 [25–600] | 275 [175–800] | 25 [25–75] | 25 |
RCG, robotic cystogastrostomy; SD, standard deviation.
The overall complication rate was 32 (26.9%), 10 (23.3%) in the robotic distal pancreatectomy (RDP) and 20 (30.8%) in the RW. The incidence of pancreatic leak was 14 (11.8%); 8 (6.7%) grade A leaks, 4 (3.4%) grade B leak, and 2 (1.7%) grade C leaks (Table 3). Clavien-Dindo complications were 21 (17.6) grade II, 10 (8.4) grade III, and 1 (0.8%) grade IV (Table 4). Clavien-Dindo grade 3–5 complications were 4 (3.4%) abcess, 10 (8.5%) pancreatic leak, 1 (0.8%) pneumothorax, 1 (0.8%) pneumonia, 1 (0.8%) pulmonary embolism, 1 (0.8%) sepsis, 1 (0.8%) fluid collection, 1 (0.8%) post-operative bleeding, and 1 (0.8%) cerebellar stroke (Table 5). Mortality was 1 (0.8%) for all 119 patients.
Table 3. Incidence of pancreatic leak.
| Variables | Entire cohort (n=119) | Distal (n=43) | Whipple (n=65) | Total (n=4) | Enucleation (n=6) | RCG (n=1) |
|---|---|---|---|---|---|---|
| Pancreatic leak, n (%) | 14 (11.8) | 5 (11.6) | 9 (13.8) | 0 | 0 | 0 |
| Grade A | 8 (6.7) | 1 (2.3) | 7 (10.8) | |||
| Grade B | 4 (3.4) | 2 (4.6) | 2 (3.1) | |||
| Grade C | 2 (1.7) | 2 (4.6) | 0 |
RCG, robotic cystogastrostomy.
Table 4. Morbidity and mortality.
| Variables | Entire cohort (n=119) | Distal (n=43) | Whipple (n=65) | Total (n=4) | Enucleation (n=6) | RCG (n=1) |
|---|---|---|---|---|---|---|
| Mortality, 30-day, n (%) | 1 (0.8) | 1 (2.3) | 0 | 0 | 0 | 0 |
| Mortality, 90-day, n (%) | 0 | 1 (2.3) | 0 | 0 | 0 | 0 |
| Morbidity, n (%) | 32 (26.9) | 10 (23.3) | 20 (30.8) | 2 (50.0) | 0 | 0 |
| Grade I | 0 | 0 | 0 | 0 | 0 | 0 |
| Grade II | 21 (17.6) | 6 (13.9) | 13 (20.0) | 2 (50.0) | 0 | 0 |
| Grade III | 10 (8.4) | 4 (9.3) | 6 (9.2) | 0 | 0 | 0 |
| Grade IV | 1 (0.8) | 0 | 1 (1.5) | 0 | 0 | 0 |
RCG, robotic cystogastrostomy.
Table 5. Clavien-Dindo grade 3–5 complications.
| Major complications (grade 3–5) | n (%) |
|---|---|
| Abscess | 4 (3.4) |
| Pancreatic leak | 10 (8.5) |
| Pneumothorax | 1 (0.8) |
| Pneumonia | 1 (0.8) |
| Pulmonary embolism | 1 (0.8) |
| Sepsis | 1 (0.8) |
| Fluid collection | 1 (0.8) |
| Post-op bleeding | 1 (0.8) |
| Cerebellar stroke | 1 (0.8) |
Overall, operative time for RW began to decrease steadily after 10 cases and operative time for Distal pancreatectomies also steadily decreased after 5 cases (Figure 1). All complication rate decreased steadily after 15 cases (Figure 2) and major complications (Clavien-Dindo grade 3–5) were seen in 16 (13.6%) of cases but decreased steadily after case 30 (Figure 3).
Figure 1.
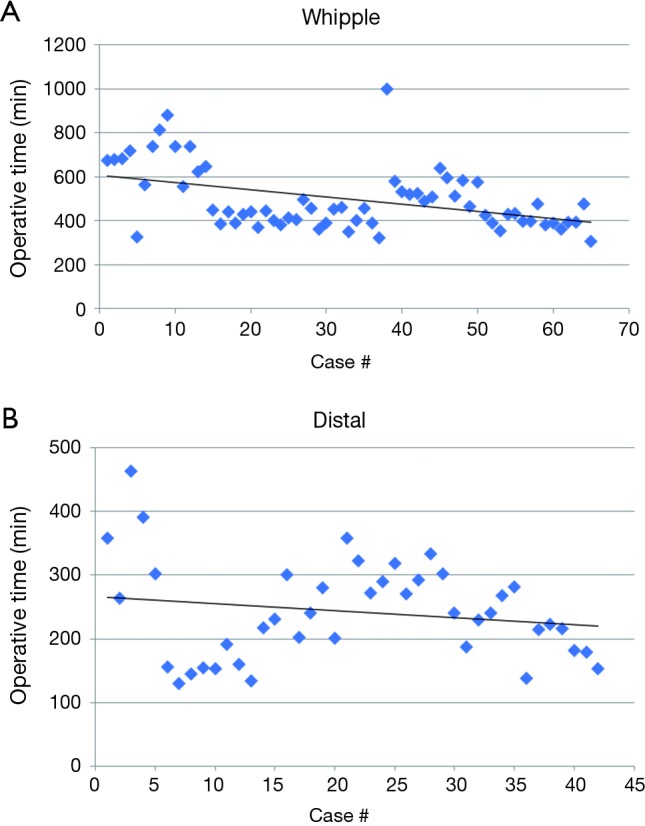
Operative time over course of experience (A) Whipple and (B) distal.
Figure 2.
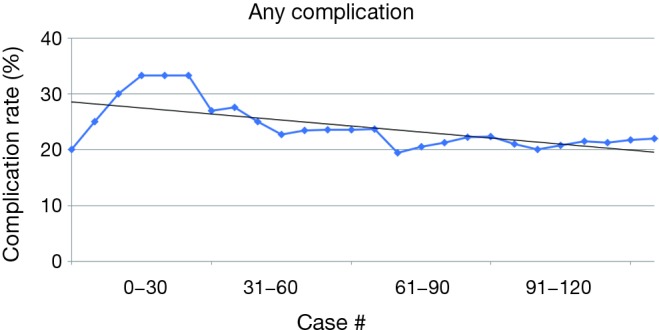
Any complication rate.
Figure 3.
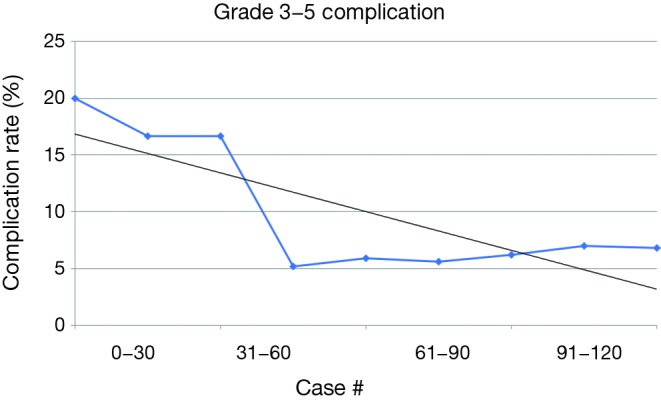
Clavien-Dindo grade 3–5 complication rate.
Discussion
This study demonstrates the safety, efficacy and feasibility of utilizing robotics for pancreatic surgery. We demonstrated a 0.8% mortality for 119 patients and 13.6% for major complications. We have also demonstrated that outcomes begin to show major improvement at increased volume of about 30 cases.
Pancreatic surgery continues to have high morbidity despite the improvements in mortality over the past decade or so (10). Surgical technique has evolved to become more minimally invasive and laparoscopic pancreatic surgery has been proven to have similar operative morbidity to open pancreatic surgery (4,11). For example, a study done comparing laparoscopic distal pancreatectomy to open distal pancreatectomy found improved estimated blood loss, decrease hospital length of stay but no statistically significant difference in complications (12).
The Da Vinci robot has continued to make headway in the field of minimally invasive surgery proving to be safe and feasible (7). It overcomes the disadvantages of poor dexterity and 2D visualization encountered with laparoscopic surgery; two tools that are useful in pancreatic surgery. Although utilized more frequently in other fields of surgery, Giullianotti et al. demonstrated both safety and feasibility of robotic pancreatic surgery in a study of 134 patients (8). They reported a mean operative time of 331 [75–660] min, 14 (10.4%) conversions to open, mean length of stay of 9.3 [3–85] days, postoperative morbidity of 26% and mortality of 2.2%. In our study the length of stay, conversion to open, and mortality were lower with similar rates of post-operative morbidity.
Zureikat et al. reported their series of 250 consecutive robotic pancreatic surgeries and demonstrated conversion rates of only 6%, improved operative times and complications as volume increased, and a pancreatic leak rate of 30% (9). We similarly demonstrated improved operative times after 15 cases, low rate of conversion to open (4.2%) and improved complication rates with higher volume of cases performed. However, the pancreatic leak rate was lower at 11.8% with similar distribution of grade A, B, and C pancreatic fistulae. The aforementioned study had a significant number of central pancreatectomies with a leak rate of 92% which would explain the higher overall leak rate in their series.
Conclusions
Over the course of 119 consecutive robotic pancreatic cases, we have demonstrated the effectiveness, feasibility and safety of utilizing robotics for pancreatic surgery. The dexterity and 3D visualization of the robotics system is advantageous and there is improvement in operative time and post-operative complications with increased case volume. The learning curve associated with robotic pancreatic surgery is extensive and dependent on surgeon volume and experience with robotic surgery.
Acknowledgements
None.
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. 10.3322/caac.21387 [DOI] [PubMed] [Google Scholar]
- 2.Wittel UA, Makowiec F, Sick O, et al. Retrospective analyses of trends in pancreatic surgery: indications, operative techniques, and postoperative outcome of 1,120 pancreatic resections. World J Surg Oncol 2015;13:102. 10.1186/s12957-015-0525-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gagner M, Pomp A. Laparoscopic pylorus-preserving pancreatoduodenectomy. Surg Endosc 1994;8:408-10. 10.1007/BF00642443 [DOI] [PubMed] [Google Scholar]
- 4.Kendrick ML, Cusati D. Total laparoscopic pancreaticoduodenectomy: feasibility and outcome in an early experience. Arch Surg 2010;145:19-23. 10.1001/archsurg.2009.243 [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, Uchida E, Nomura T, et al. Laparoscopic pancreatic resection: some benefits of evolving surgical techniques. J Hepatobiliary Pancreat Surg 2009;16:741-8. 10.1007/s00534-009-0140-4 [DOI] [PubMed] [Google Scholar]
- 6.de Vasconcellos Macedo AL, Schraibman V, Okazaki S, et al. Treatment of intraductal papillary mucinous neoplasms, neuroendocrine and periampullary pancreatic tumors using robotic surgery: a safe and feasible technique. J Robot Surg 2011;5:35-41. 10.1007/s11701-010-0238-3 [DOI] [PubMed] [Google Scholar]
- 7.Giulianotti PC, Coratti A, Angelini M, et al. Robotics in general surgery: personal experience in a large community hospital. Arch Surg 2003;138:777-84. 10.1001/archsurg.138.7.777 [DOI] [PubMed] [Google Scholar]
- 8.Giulianotti PC, Sbrana F, Bianco FM, et al. Robot-assisted laparoscopic pancreatic surgery: single-surgeon experience. Surg Endosc 2010;24:1646-57. 10.1007/s00464-009-0825-4 [DOI] [PubMed] [Google Scholar]
- 9.Zureikat AH, Moser AJ, Boone BA, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg 2013;258:554-9; discussion 559-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cameron JL, Riall TS, Coleman J, et al. One thousand consecutive pancreaticoduodenectomies. Ann Surg 2006;244:10-5. 10.1097/01.sla.0000217673.04165.ea [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mabrut JY, Fernandez-Cruz L, Azagra JS, et al. Laparoscopic pancreatic resection: results of a multicenter European study of 127 patients. Surgery 2005;137:597-605. 10.1016/j.surg.2005.02.002 [DOI] [PubMed] [Google Scholar]
- 12.Stauffer JA, Rosales-Velderrain A, Goldberg RF, et al. Comparison of open with laparoscopic distal pancreatectomy: a single institution’s transition over a 7-year period. HPB (Oxford) 2013;15:149-55. 10.1111/j.1477-2574.2012.00603.x [DOI] [PMC free article] [PubMed] [Google Scholar]


