Abstract
Access to human pluripotent cells theoretically provides a renewable source of cells that can give rise to any required cell type for use in cellular therapy or bioengineering. However, successfully directing this differentiation remains challenging for most desired endpoints cell type, including renal cells. This challenge is compounded by the difficultly in identifying the required cell type in vitro and the multitude of renal cell types required to build a kidney. Here we review our understanding of how the embryo goes about specifying the cells of the kidney and the progress to date in adapting this knowledge for the recreation of nephron progenitors and their mature derivatives from pluripotent cells.
Keywords: Kidney development, embryonic stem cell, directed differentiation, kidney progenitor
Introduction
The kidney is a complex organ with a highly constrained architecture and more than 20 distinct mature cellular phenotypes, all of which are required for this organ to regulate fluid balance, nitrogenous waste removal, acid-base homeostasis, haematocrit and bone density. This cellular and structural complexity represents a significant challenge with respect to repair or prolongation of function. All of the 200,000 to 1.8 million nephrons present in a human kidney (1) are formed in utero, however the progenitor population from which these nephrons are formed does not persist into postnatal life (2). Therefore, whether renal disease presents at birth or later in life, prolongation of renal function followed by replacement in the form of dialysis or transplantation is the only option available to the patient.
While preferable, only a portion of end stage renal patients will receive a transplant due to limited availability and the requirement for immunological matching. However, there have been several recent breakthroughs in the field of bioengineering that may ultimately represent feasible approaches to the recreation of functional kidneys or kidney-equivalents. These include bioprinting and recellularization of tissue scaffolds. Bioprinting is the process in which cells are suspended in a biogel and robotically ‘printed’ into three dimensional structures for the replacement of tissues (3,4). This has proven successful for tissues such as the bladder, pinna of the ear and blood vessels (5,6). While currently limited by printer resolution and methods for the culture of large complex organs, it is hoped that bioprinting may eventually result in the recreation of adult organs including kidney (4). In contrast, recellularisation involves the use of the organ itself as a scaffold into which new cells can be placed (7). Researchers recently reported the successful decellularisation of a human kidney via a process of detergent perfusion, with the resulting organ maintaining an intact extracellular scaffold (8). As a proof of concept, a decellularised rat kidney scaffold was successfully repopulated with both an intact endothelium and a tubular epithelium via cellular infusion under negative pressure. The resulting organ was successfully transplanted into a host animal without major haemorrhage and with some evidence of appropriate cellular function. The cells used for repopulation of the tubular epithelium were harvested from sacrificed neonatal rat kidney, an inappropriate cell source for human recipients.
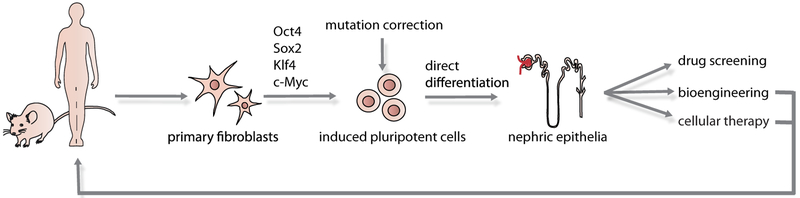
Both of these advances cannot be realised without a source of human cells. While possible as a source of scaffolds, reliance on access to cadaveric human material for renal cells is not feasible. One obvious solution would be the use of pluripotent stem cells, including either human embryonic stem cells (hESCs) or human induced pluripotent cell (hiPSC). (Figure 1). By definition, a pluripotent cell has the ability to form any mature cell type (9). Significant progress has been made in the direct differentiation of hESC/iPSC to specific somatic cell phenotypes, including neurons, cardiomyocytes, pancreatic and hematopoietic cells (10-13). If a direct differentiation protocol were developed for the generation of renal cell types, these might not only be of value for bioengineering but also for cellular therapies or as tools for nephrotoxicity screening. Indeed, the ability to generate a pluripotent state from an adult somatic cell (hiPSC) (14,15), and to edit the genome of the resulting cell line (16), heralds the prospect of cellular therapies to overcome inherited renal diseases including Alport syndrome, Finnish nephropathy and possibly even polycystic kidney disease.
Figure 1. Possible renal options for directed differentiation of pluripotent cells.
This schematic demonstrates the derivation of iPSC from adult somatic fibroblasts followed by the directed differentiation to mature kidney cells. The resulting cells might be useful for nephrotoxicity screening, disease modelling in vitro, or the generation of renal cells for use in bioengineering or cellular therapy. Gene editing to correct inherited mutations may allow the reintroduction of autologous normal cells to the original cell donor.
The directed differentiation of pluripotent cells to a given endpoint is of interest to many fields. Two main approaches have been taken. Unbiased high throughput chemical screens make no assumptions about the signalling mechanisms required. This approach has been successfully applied to screen for compounds able to maintain pluripotency or promote differentiation (17, 18). The other approach is to use our understanding of the differentiation steps used by the embryo to move from the pluripotent state to a specific tissue cell type. This has been highly successful in the directed differentiation of pluripotent cells to hematopoietic, neural and cardiomyocytic endpoints. It is also the only approach that has been reported for the generation of renal cells. Here we will review the normal embryological processes involved in kidney organogenesis, discuss how this knowledge has been used as a framework for the differentiation of pluripotent cells to renal cell types and address the remaining challenges (Figure 1).
Pluripotency: the potential to start over
An embryonic stem cell is a pluripotent stem cell which is able to give rise to all three germ layers (http://stemcells.nih.gov/info/basics/pages/basics1.aspx). The gold standard assay for testing pluripotency is the spontaneous formation of teratomas, an assay frequently performed for ES cells of any species via injection into the testis of mice (19). A more compelling evidence of pluripotency is the contribution of ES cells to the formation of all tissue types after reintroduction into a blastocyst. Germline transmission to form a clone indicates a capacity to even form gametes (20). The use of hESCs for germline transmission, referred to as reproductive cloning, is illegal in most countries. However, the use of such cells to generate specific cell types for cellular therapies opens the door for novel approaches to regenerative medicine.
The first hESCs were isolated almost 15 years ago (21). As self-renewing stem cells, such pluripotent cells may afford an ability to generate large numbers of the desired cell type for transplantation. However, the derivation of such pluripotent cell lines from the human blastocyst sparked considerable ethical debate internationally. Their use also raised the challenge of immunological rejection. In a landmark discovery, Takahashi et al demonstrated that a fully differentiated somatic cell (initially a mouse fibroblast) could be ‘reprogrammed’ to a pluripotent state via the enforced expression of four key transcription factors, Oct4, Sox2, Klf4 and c-Myc (15). This proved to be transferable to human (16) and the generation of bona fide pluripotent cells has been demonstrated to be feasible from a wide array of somatic cell types types (skin, blood, adipocytes) via a similarly wide array of gene delivery systems, including episomal systems that facilitate the generation of transgene-free and virus-free iPSCs (9).
Much research has focussed on the propagation and expansion of pluripotent cells without differentiation. There are a number of morphological differences between mouse and human ESCs. hESCs form flat colonies with features similar to epiblast stem cells and can be maintained in a pluripotent state in the presence of FGF2 and ActivinA. mESCs form reflective, raised colonies and require the addition of LIF and BMP4 for pluripotency (22,23). Despite these distinctions, both appear to differentiate in accordance with what we understand of normal embryology. Protocols for ESCs differentiation most commonly include monolayer culture, either on matrix (collagen, matrigel) or cellular feeder layer (usually mitotically inactivated murine embryonic fibroblasts (MEFs)), or via the formation of embryoid bodies (EBs) (19). An EB is formed via aggregation of a cluster of ESCs cultivated in bulk suspension within dishes coated with a non-adhesive material. The resulting EB undergoes spontaneous differentiation into all germ layers. Exposing EBs to distinct extrinsic factors results in differentiation that involves both the cell-autonomous response of cells within the EB and cell-cell interactions in three dimensions, as occurs during early embryogenesis. On the other hand, monolayer culture places the ESCs along a 2D surface. While this may limit the influence of neighbouring cells on differentiation, the use of highly specific culture conditions (growth factors, concentration and timing) may produce more robust and uniform differentiation to certain type of lineage.
The path from inner cell mass to mammalian kidney
The path from inner cell mass cell to kidney passes through primitive streak to definitive mesoderm and intermediate mesoderm (IM) with both the ureteric bud (UB) and metanephric mesenchyme (MM) being derived from IM. The decades of embryological research investigating the pathways involved in these processes cannot be comprehensively covered in this review. Below is a summary of the critical fate decisions required to reach kidney with a brief description of the role of growth factor families that have subsequently been employed by the field to recapitulate this process in vitro.
Primitive streak:
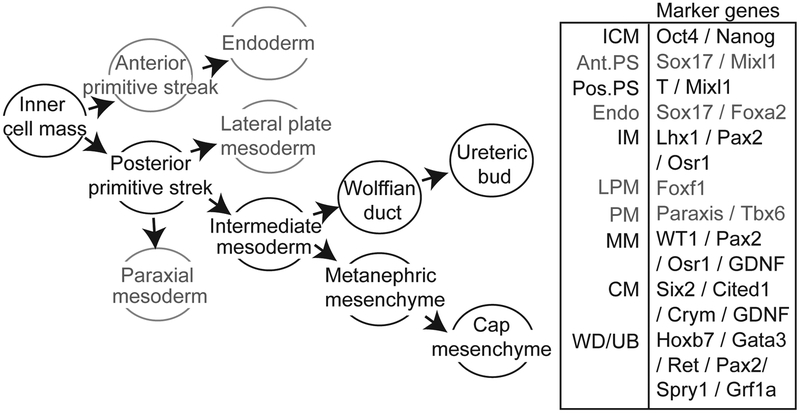
In order to direct the differentiation of pluripotent stem cells, understanding of embryogenesis and reproducing the in vivo condition is crucial. In the embryo itself, it is the inner cell mass (ICM) that represents the pluripotent cell population able to give rise to all cell lineages. The initial patterning event differentiating the ICM into the three primary germ layers (ectoderm, mesoderm and endoderm) is called gastrulation. Gastrulation begins with the generation of the primitive streak (PS) which is divided into anterior and posterior ends based on differential gene expression (Figure 2). While the anterior PS forms endoderm, giving rise to tissues including the gut, liver and lung, the posterior PS develops into definitive mesoderm, which ultimately patterns to form tissues including the heart, muscles, blood, bone, kidneys and gonad.
Figure 2. Embryonic differentiation from inner cell mass to kidney.
Illustrated are the developmental decisions required during embryonic differentiation to both nephron progenitor and ureteric bud progenitor states (left) together with marker genes that would assist in the identification of intermediate endpoints (right). ICM, inner cell mass; Mesen, mesendoderm; Mes, mesoderm; Endo, endoderm; IM, intermediate mesoderm; LPM, laternal plate mesoderm; PM, paraxial mesoderm; MM, metanephric mesenchyme; NP, nephron progenitor / cap mesenchyme; WD, Wolffian duct; UB, ureteric bud.
The BMP/Activin/Nodal gradient along the dorsoventral axis of the embryo induces and patterns these two germ layers (24,25). Activation of Activin receptors leads to phosphorylation and nuclear translocation of heterodimeric Smad proteins that activate the Mixl1 promoter (26), a marker of PS. The expression of other markers, including Brachyury (T) (27) and Eomes mark the forming mesoderm while sustained Mixl1 and Sox17 expression marks the endoderm (13, 28). Specification of anterior PS is driven by synergistic Activin/Nodal and Wnt/β-catenin signaling synergistically (29) whereas BMP signalling is considered critical in inducing mesodermal cell fates (30). Mice deficient in BMP4 (31), or the BMP receptors type I and II, failed to develop mesoderm (32,33), whereas blockade of BMP signaling in hESCs completely abolished mesoderm generation. Conversely, addition of BMP4 induced formation of a MIXL1+ mesendoderm (primitive streak) from hESC which were subsequently able to give rise to mesodermally-derived hematopoietic precursors (13).
Intermediate mesoderm:
Having reached posterior primitive streak / definitive mesoderm, this tissue is further patterned via dorsal-ventral gradients into at least four major populations: notochord (a transient ‘embryonic backbone’), paraxial mesoderm (PM; future somites ie. progenitors of certain muscles and other connective tissues), intermediate mesoderm (IM; the precursor to the kidneys), and lateral plate mesoderm (LPM; includes progenitors of the heart, blood, and vascular cells). This differentiation is again patterned via growth factor gradients, as determined from studies using a variety of embryological models, including Xenopus, chick and mouse. In the chick, BMP4 is expressed in the LPM where it functions in an autocrine manner while the BMP antagonist, Noggin, produced by the spinal cord and notochord maintain PM (34,35). Fate commitment of the IM is also controlled by the dose-dependent activation of the BMP signalling cascade along this embryonic dorso-ventral axis (34,36,37). In addition to BMP, retinoic acid (RA) is also known to regulate the body plan of the embryo along the anterior-posterior (A-P) axis. Reduced RA signalling in the posterior embryo is critical for the appropriate pattern of Hox gene expression (38,39). These Hox genes confer regional identity with Hox11 paralogs making the presumptive kidney (40). However, some level of RA in the trunk mesoderm is necessary for nephric duct formation (41). RA emanating from the paraxial mesoderm has also been suggested to be critical for the initial specification of renal progenitor cells (42,43).
Metanephros:
The IM gives rise to the urogenital tract, comprising the kidneys and gonads. In fact, three distinct ‘kidney’ fields are formed in a specific temporospatial pattern, starting from the cranially located pronephros, then the mesonephros and finally the caudally located metanephros. It is the metanephros that forms that final postnatal kidney. For all three of these structures, organogenesis involves the interaction between the nephric duct (also called the Wolffian duct or mesonephric duct) and the adjacent nephric cord, the block of mesoderm that runs the length of the nephric duct. As well as its earlier role in dorso-ventral patterning of the embryo, RA also appears to have an effect on induction of kidney from IM. Treatment of Xenopus embryos with RA and Activin A induced pronephric differentiation (44). In mouse, ectopic expression of RA increases the size of the developing kidney, while blocking the pathway prevents kidney specification (43,45).
Formation of the nephric duct via a mesenchymal to epithelial transition (MET) within the IM is the earliest morphologic evidence of renal development. Formation of the metanephros is initiated via the secretion of glial-derived neurotrophic factor (GDNF) by MM adjacent to the caudal end of the nephric duct. This GDNF signal is transduced at the Ret receptor expressed in the nephric duct, mediating outgrowth of a Ret+ UB. Once the UB has invaded the MM, it undergoes continuous dichotomous branching. The MM condenses around each ureteric tip to form the condensed or cap mesenchyme (CM) which in turn drives continued branching via GDNF secretion. Reciprocally, distinct signals from the ureteric tip drive maintenance/self-renewal and differentiation of the CM. It is the signal to differentiate, via a mesenchyme to epithelial transition (MET), which results in the formation of the nephrons which represent the basic functional and structural units of the kidney (reviewed in 46). Reciprocal signalling between these two compartments continues until the completion of nephrogenesis. The signal from the ureteric tip regarded as promoting MET / nephron formation is Wnt9b, mediated via canonical signalling, which in turn upregulates Wnt4 to drive Ca-mediated non-canonical signalling (46). The maintenance of the CM can be supported via FGF2 and BMP7 in explant culture (47), however FGF2 is unlikely to be the FGF ligand critical for this role in vivo (48). Indeed, the combination of FGF2 and EGF or TGFa has also been proposed to sustain CM in culture (49) and more recently FGF9 expression in the ureteric tip has been attributed a major role in survival of the CM (50). BMP7 appears to induce CM differentiation via canonical Smad1/5 signalling (51), but also supports CM proliferation via JNK-MAPK signalling (52).
Mileposts of success
The successful generation of any given embryonic population requires an intimate understanding of the gene expression changes anticipated and the genes that mark specific endpoint states. While the PS expresses Mixl1, the expression of Brachyury (T) (27) and Eomes identifies the forming mesoderm while sustained Mixl1 and Sox17 expression marks the endoderm (13). One of the first specific markers upregulated in the IM of the mouse embryo is Osr1 (or Odd-skipped related-1; Odd1) (53). The homeobox gene, Lhx1 (previously known as Lim1), is also induced in the developing IM, however it is initially expressed both in LPM and IM before becoming more restricted to IM (54). Hence it may be the coexpression of Osr1, Lhx1 and Pax2 that is most specific to IM. In contrast, LPM is characterised by the expression of Foxf1 while PM expresses genes including Paraxis and Tbx6 (55-57).
A number of genes are known to be required for the induction of MM from IM. Osr1 acts upstream of genes implicated in metanephros induction, including Eya1 and WT1, thereby promoting MM formation and survival (58). The MM also expresses Six1, Sall1 and Hox11 orthologs including Hoxa11, Hoxc11 and Hoxd11 (40). Fate mapping experiments showed that Osr1+ MM gives rise to not only the CM but also the stromal cells, the vasculature and the mesangium. Another Osr1 target is the transcription factor, Pax2 (59), which functions redundantly with Pax8 and is involved in nephric duct formation and extension. Pax2 is also expressed in the MM. The nephric duct is also marked by Lhx1, Ret, Slit2, Hoxb7 and Gata3 expression, along with other markers (reviewed in 46).
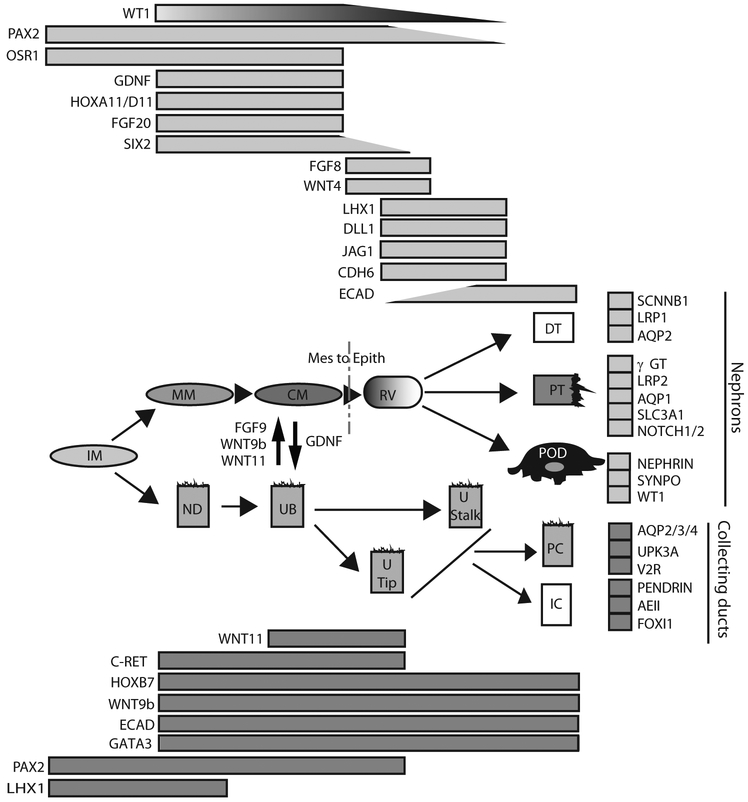
While the CM continues to express many of these genes, the co-expression of Six2, WT1 and Cited1 is characteristic of the self-renewing CM state (60), whereas the onset of differentiation of CM to the early nephron marks the onset of expression of Fgf8, Wnt4, Ffgrl1, Cdh4, Cdh6 and Lhx1 (61). This is followed by a series of morphological patterning and segmentation events that lead to the generation of distinct mature renal cell types including the proximal and distal tubular epithelium, loops of Henle and parietal (lining the Bowman’s capsule) and visceral (podocytes) epithelium. While again few of these cells express genes located at no other time or place in embryogenesis, there are a large number of segment-restricted genes described for each cell type. Recent studies have defined gene expression signatures of different nephrons segments across developmental time and space (62-64) (see Figure 3). Studies of freshly isolated or immortalised renal cell types have also identified markers of the mature differentiated cell state, particularly for cell types such as podocytes (including WT1, synaptopodin, nephrin) proximal tubule (including Aqp1, Lrp2, Slc3a1) and collecting duct (Aqp2 in principal cells; pendrin and AE1 in intercalated cells) (see Figure 3). Such mature cells also show characteristic functional attributes, such as water transport, amino acid transport or acid-base regulation, that can also assist in defining their identity. Hence the tools are in place for assessing a successful outcome from directed differentiation to kidney.
Figure 3. Temporal gene expression patterns during renal development from posterior primitive streak to mature renal cell types.
This framework illustrates the steps involved in forming renal cell types from the IM stage illustrating gene expression by compartment / cell type across development, as well as the interplay between the UB and NP populations that allows nephron formation via a mesenchyme to epithelial (Mes to Epith) transition. It also shows the spectrum of different mature epithelial cell types generated from the renal vesicle or from the UB after this event and illustrates markers used to identify stages of differentiation as well as mature cell types. IM, intermediate mesoderm; ND, nephric duct; UB, ureteric bud; UTip, ureteric tip; U Stalk, ureteric stalk / branch; PC, Principal cell; IC, intercalated cell; MM, metanephric mesenchyme; CM, cap mesenchyme (nephron progenitor); RV, renal vesicle (Stage 1 nephron); DT, distal tubule; PT, proximal tubule; POD, podocyte. Note that many of the genes involved in MET in the mesonephros are shared with that of the metanephros, however no CM or UB form (84). The mesonephric mesenchyme is also lacking expression of Hoxa11/d11.
Directed differentiation of mouse ES cells to kidney
The preceding literature describing factors known to drive kidney development has been used to guide most attempts at direct ESC differentiation to kidney. Table 1 summarises the approaches taken using mouse ESCs. While most studies investigating the differentiation of mESC to kidney derivatives have relied on an understanding of embryology, early studies drew on spontaneous differentiation. Yamamoto et al (65) examined teratomas for evidence of kidney differentiation, while Steenhard et al (66) injected undifferentiated mESC into developing kidney showing integration into proximal tubules. The first specific attempt to generate kidney from mESC drew on the evidence that Activin A and RA are key growth factors for intermediate mesoderm differentiation. The addition of these growth factors together with BMP7 to EB cultures resulted in the induction of Pax2+ cells that could integrate into proximal tubules in an embryonic kidney (67). In most subsequent attempts, some combination of these three factors has been added to mESC-derived EBs (68-72). The assessment of success has varied between studies, and has included evidence for Pax2+ or Aqp+ cells, as determined by gene expression of presence of protein, and/or integration into developing murine kidneys (see Table 1). However, neither of these genes identifies a specific cell type within the kidney and both are also expressed in many other tissues. Indeed, none of these studies provided clear evidence of the formation of nephron progenitor cells with the potential to differentiate into all components of the nephron. This reflected the lack of known CM markers at the time and the complexity of the differentiation process within EBs, the latter making them relatively intractable for monitoring outcomes or the regulation of cell fate induction towards the desired phenotype in a stepwise manner. Other studies performed stepwise differentiation under monolayer culture conditions (73,74), showing evidence for gene expression changes consistent with differentiation through definitive mesoderm and IM to MM. Nishikawa et al (73) used a successive combination of ActivinA, BMP4, LiCl and RA to induce Brachyury (T)-expressing mesoderm followed by Pax2+ intermediate mesoderm. The subsequent addition of conditioned media from a ureteric bud cell line (CMUB-1) induced the metanephric mesenchymal genes GDNF, WT1 and Cdh11. This suggested that the secretion of unknown factors from the UB cells contributed to MM development, highlighting the critical nature of reciprocal interactions between the UB and the MM in kidney differentiation. However, none of these studies included analyses of Six2. It is very likely that additional factors are required for the optimal generation and subsequent support of a cap mesenchymal state. As noted, recent studies have implicated FGF9 (50), low levels of canonical Wnt signaling (75) and inhibition of pSMAD1/5/8 signaling (51) in CM maintenance, suggesting further options for the optimization of this stage.
Table 1.
Overview of directed differentiation mouse and human embryonic stem cells to renal cell types.
| Ref. | Year | Starting cell | Differentiation method | Growth factors | Time | Endpoint | Assays used | |
|---|---|---|---|---|---|---|---|---|
| Mouse ESCs | 65 | 2005 | mESCs | Teratoma formation in the mouse retroperitoneum | 2–3w | Pax2+/DBA+ tubules | IF | |
| 66 | 2005 | ROSA26 mESCs | Injection into E12.5 kidney. | 3–5d | Integration into NA+/K+ ATPase+ tubules | IF | ||
| 67 | 2005 | ROSA26 mESCs | EB formation | 10%FCS-LIF 10%FCS +ActivA/BMP7/RA |
2–5 d 5–7 d |
Intermediate mesoderm |
Pax2, WT1, Lhx1 in EBs by RT-PCR. Pax2+ by IF. Injection into E12.5 kidney showed the integration into LTA+ PT |
|
| 68 | 2005 | Wnt4 expressing mESCs | EB formation | 15%FCS-LIF 15%FCS +ActivA/HGF |
2d 20d |
AQP2+ cells in EBs | 3D culture showed tubule formations using Wnt4-ESCs | |
| 69 | 2007 |
Pax2-GFP mESCs |
EB formation | Serum free +LIF/BMP4 | 16d | Pax2+ tubules in EBs | RT-PCR and IHC of EBs | |
| 70 | 2007 | T-GFP mESCs | EB formation | Serum free-LIF +ActivA | 4d | Mesoderm | T-GFP+ cells are injected into P0 kidney then integrated into AQP1+ tubules. | |
| 71 | 2009 | mESCs miPSCs | EB formation | 10%FCS 10%FCS +GDNF or BMP7 or ActivA |
3d 15d |
Unknown |
WT1/PAX2 upregulated by GDNF or BMP7. KSP upregulated by ActivinA |
|
| 74 | 2010 | mESCs | Monolayer | Serum free +JAK inhibitor/PI3K inhibitor/RhoA inhibitor |
8d | Intermediate mesoderm | IF showed Osr1+/Pax2+. | |
| 73 | 2010 | mESCs | Monolayer | 10%FCS +ActivA +BMP4 +LiCl +RA MM-CM or UB-CM |
2d 2d 2d 2d 4–8d |
UB markers ON by MM-CM. MM markers ON by UB-CM. | RT-PCR showed, T for PS, Pax2/Lhx1 for IM, GDNF/WT1/Cdh11 for MM and Hoxb7/Wnt11/C-ret for UB. | |
| 72 | 2010 | mESCs | EB formation | 15%FCS-LIF 15%FCS +ActivinA/RA UB-CM |
2d 6d 10d |
Renal lineage marker+ cells | IF of EBs showed WT1+, Pax2+, Pod1+ or DBA+ cells | |
| Human ESCs | 78 | 2010 | hESCs | Monolayer spontaneous differentiation followed by FACS by CD24+, Podocalyxin+ and GCTM2− | On MEF 20%FCS 5%FCS |
2d 12d |
WT1+/PAX2+ intermediate mesoderm cells |
IF showed WT1+/PAX2+. Microarray showed renal genes upregulated. |
| 80 | 2013 | hiPSCs | Monolayer or EB formation | Serum free +ActivA/CHIR BMP7/CHIR |
2d 8d |
Intermediate mesoderm | IF showed renal markers. Integration assay into mouse kidney. | |
| 82 | 2013 | hESCs | Monolayer |
0.5%FCS+BMP2+BMP7 |
20d | AQP+ cells | In vitro functional assay and integration assay for PT. | |
| 83 | 2012 | hiPSCs | EB formation | 2.5%FCS+ActivA/BMP7/RA, plated down | 3d/7d | Podocyte | IF and Functional assay. |
While there has been significant attention paid to the CM as the nephron progenitor population, the kidney contains at least two other progenitor populations; the ureteric progenitors which give rise to the collecting ducts / ureter and the stromally-located vascular progenitors (46). Little attention has been paid to the generation of the ureteric progenitor population from mESCs. GDNF-Ret signaling is regarded as essential for the support of this compartment during development. Hence, success might be identified as the formation of a Ret+ epithelial population, presumably in response to the addition of GDNF. While in vitro culture conditions for mouse UB have not been well established, rat primary UB cells can be maintained with TGFα, EGF and low concentrations FBS (1-2%) (76). Ret expression in the UB has been reported to rely upon expression of the RA-synthesizing enzyme retinaldehyde dehydrogenase (Raldh2) in the adjacent stroma and freshly isolated mouse UB can remain Ret+ in vitro only in the presence of RA (77). To date, these conditions have not been investigated for the purposes of mESC differentiation into ureteric progenitors.
Progress on forming kidney from human pluripotent cells
In comparison to mESC, there have been fewer reports of attempts to direct the differentiation of human pluripotent cells to kidney (Table 1). Lin et al (78) approached the problem by reducing the concentration of serum to encourage spontaneous mesoderm differentiation and then fractionating EB cultures based on their expression of three markers; presence of CD24 and podocalyxin, both proposed to mark MM (79), and absence of GCTM2, a cell surface marker of pluripotency. The CD24+Podocalyxin+GCTM2− fraction contained WT1+PAX2+ cells and displayed an expression profile more similar to embryonic kidney than other fractions. This suggests that hESC are able to form a nephrogenic IM. This is supported by Mae et al (80) who report the directed differentiation of hESCs and iPSCs into IM using a combination of Activin A and the GSK-3β inhibitor, CHIR. Subsequent addition of BMP7 and CHIR resulted in evidence of an OSR1+ IM and further differentiation suggested isolated instances of mature kidney cell types, however this was not extensively analysed. As noted previously, in mouse Osr1 is known to be expressed in the MM of the kidney but also prior to that in IM and, to a lesser extent, LPM. Indeed, Osr1 marks trunk mesoderm and is relatively broadly expressed in cells in culture, including mesenchymal stem cells (81). Hence, while the hESC differentiated to an OSR1+ state may include MM, further analyses are required to assess whether a robust CM was induced. Narayanan et al (82) showed hESCs could spontaneously differentiate into AQP1+ cells at 12% efficiency after 20 days monolayer culture on Matrigel in a renal epithelial supportive media. This efficiency was improved to more than 30% by the addition of BMP2 and BMP7. These authors compared the expression pattern and functional properties of the resulting differentiated hESCs with primary human proximal tubule cells. They report a similar expression profile and functional evidence for response to parathyroid hormone, γ-glutamyl transferase (γGT) activity, ammonia production and water transport. Finally, Song et al (83) took a single step approach to the directed differentiation of human iPSC to mature kidney cell types. iPSC-derived EBs were cultivated with Activin A, BMP7 and RA for 20 days, forming cells with a podocytic morphology and positive for SYNPO expression. Moreover, these cells actively endocytosed albumin uptake in a similar fashion to a primary podocyte cell line.
The challenge of defining success
While these studies are beginning to show evidence of differentiation towards potential renal endpoints, a major imperative for the field is to define and identify success. In vitro, the identification of a CM/nephron progenitor population is challenged by the lack of any one definitive gene or protein unique to this cell type. Gene expression analysis must involve multiple markers and accompanying co-immunoreactivity for more than one protein within the same cell is required to support any claim of a successful phenotype. More importantly, functional evidence that a CM/nephron progenitor state has been reached must be provided and this will involve demonstration of nephron formation. Present evidence for this does not extend beyond in vitro epithelialisation or poorly characterised evidence for contribution to an embryonic explant. Some studies only provide data on the relative levels of gene expression of one of more renal cell types within what is a mixed culture.
The generation of a mature cell type with characteristic morphology and functional properties would appear to be an easier target than differentiation to a progenitor. However, in contrast to endpoints such as neurons or cardiomyocytes, properties such as the capacity to endocytose albumin or express a water channel are not unique to podocytes or proximal tubule cells. In vivo, functional ‘proof of concept’ has relied upon evidence for integration into embryonic or neonatal explant cultures. Few studies have carefully identified the phenotypes of the introduced cells and even fewer studies present comparisons with the introduction of undifferentiated pluripotent cells into the same tissue. The field has also not settled on what cell types need to be generated for what purposes. The advent of iPSC opens the door for gene editing to correct inherited mutations within patient-derived lines (16) (Figure 1). Podocyte differentiation of such lines might allow cellular therapies for conditions such as Alport syndrome or Finnish nephropathy. The proof of differentiation to this endpoint would be evidence for function in the recipient adult organ, however delivery of the resulting cells remains a major obstacle. A much broader repertoire of renal cell types would be required for the seeding of a decellularized renal scaffold or the bioprinting of an adult organ. To achieve this, the specificity and efficiency of differentiation approaches must improve, together with the tools used to assess success and to purify / isolate the desired cellular endpoints.
Despite these major hurdles, progress towards the generation of renal cells from pluripotent cell sources is gaining momentum. The advances required to turn the possible into the plausible will include further insights into the specification, maintenance and differentiation of renal cellular compartments. Hence, we continue to need to learn from the embryo itself.
Acknowledgements:
ML is a Senior Principal Research Fellow of the National Health and Medical Research Council of Australia. BM is a Rosamond Siemon Postgraduate Scholar. This work is supported by Stem Cells Australia (Australian Research Council SRI110001002) and the National Health and Medical Research Council (APP1041277).
References
- 1.Bertram JF, Douglas-Denton RN, Diouf B, Hughson MD, Hoy WE. (2011) Human nephron number: implications for health and disease. Pediatr Nephrol 26(9):1529–33. [DOI] [PubMed] [Google Scholar]
- 2.Rumballe BA, Georgas KM, Combes A, Ju A, Gilbert T, Little MH. (2011) Nephron formation adopts a novel spatial topology at cessation of nephrogenesis. DevBiol 360(1):110–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang CC, Boland ED, Williams SK, Hoying JB (2011) Direct-write bioprinting three-dimensional biohybrid systems for future regenerative therapies. J Biomed Mater Res B Appl Biomater 98(1):160–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mironov V, Drake C, Wen X. (2006) Research project: Charleston Bioengineered Kidney Project. Biotechnol J 1(9):903–905. [DOI] [PubMed] [Google Scholar]
- 5.Skardal A, Zhang JX, Prestwich GD (2010) Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 31: 6173–6181. [DOI] [PubMed] [Google Scholar]
- 6.Reiffel AJ, Kafka C, Hernandez KA, Popa S, Perez JL, Zhou S, Pramanik S, Brown BN, Ryu WS, Bonassar LJ, Spector JA (2013) High-fidelity tissue engineering of patient-specific auricles for reconstruction of pediatric microtia and other auricular deformities. PLoS One 8(2):e56506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Badylak SF (2007) The extracellular matrix as a biologic scaffold material. Biomaterials 28(25):3587–3593. [DOI] [PubMed] [Google Scholar]
- 8.Song JJ, Guyette JP, Gilpin SE, Gonzalez G, Vacanti JP, Ott HC (2013) Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat Med 19(5):646–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Okita K, Yamanaka S (2011) Induced pluripotent stem cells: opportunities and challenges. Philos Trans R Soc Lond B Biol Sci 366(1575):2198–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chambers SM, Fasano CA, Papapetrou EP, Tomishima M, Sadelain M, Studer L (2009) Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol 27(3):275–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM (2008) Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature 453(7194):524–528. [DOI] [PubMed] [Google Scholar]
- 12.Chen S, Borowiak M, Fox JL, Maehr R, Osafune K, Davidow L, Lam K, Peng LF, Schreiber SL, Rubin LL, Melton D (2009) A small molecule that directs differentiation of human ESCs into the pancreatic lineage. Nat Chem Biol (4):258–265. [DOI] [PubMed] [Google Scholar]
- 13.Davis RP, Ng ES, Costa M, Mossman AK, Sourris K, Elefanty AG, Stanley EG (2007) Targeting a GFP reporter gene to the MIXL1 locus of human embryonic stem cells identifies human primitive streak-like cells and enables isolation of primitive hematopoietic precursors. Blood 111(4):1876–1884. [DOI] [PubMed] [Google Scholar]
- 14.Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126(4):663–676. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131(5):861–872. [DOI] [PubMed] [Google Scholar]
- 16.Collin J, Lako M (2011) Concise review: putting a finger on stem cell biology: zinc finger nuclease-driven targeted genetic editing in human pluripotent stem cells. Stem Cells 29(7):1021–1033 [DOI] [PubMed] [Google Scholar]
- 17.Sachinidis A, Sotiriadou I, Seelig B, Berkessel A, Hescheler J (2008) A chemical genetics approach for specific differentiation of stem cells to somatic cells: a new promising therapeutical approach. Comb Chem High Throughput Screen. 11(1):70–82. [DOI] [PubMed] [Google Scholar]
- 18.Zhu S, Wurdak H, Schultz PG (2010) Directed embryonic stem cell differentiation with small molecules. Future Med Chem 2(6):965–973 [DOI] [PubMed] [Google Scholar]
- 19.Daley GQ (2003) From embryos to embryoid bodies: generating blood from embryonic stem cells. Ann N Y Acad Sci 996:122–131. [DOI] [PubMed] [Google Scholar]
- 20.Bradley A, Evans M, Kaufman MH, Robertson E (1984) Formation of germ-line chimaeras from embryo-derived teratocarcinoma cell lines. Nature 309(5965):255–256. [DOI] [PubMed] [Google Scholar]
- 21.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science. 282(5391):1145–1147. [DOI] [PubMed] [Google Scholar]
- 22.Brons IG, Smithers LE, Trotter MW, Rugg-Gunn P, Sun B, Chuva de Sousa Lopes SM, Howlett SK, Clarkson A, Ahrlund-Richter L, Pedersen RA, Vallier L (2007) Derivation of pluripotent epiblast stem cells from mammalian embryos. Nature 448(7150):191–195. [DOI] [PubMed] [Google Scholar]
- 23.Tesar PJ, Chenoweth JG, Brook FA, Davies TJ, Evans EP, Mack DL, Gardner RL, McKay RD (2007) New cell lines from mouse epiblast share defining features with human embryonic stem cells. Nature 448(7150):196–199. [DOI] [PubMed] [Google Scholar]
- 24.Schier AF (2003) Nodal signaling in vertebrate development. Ann Rev Cell Devel Biol 19:589–621 [DOI] [PubMed] [Google Scholar]
- 25.Shen MM (2007) Nodal signaling: developmental roles and regulation. Development 134: 1023–1034 [DOI] [PubMed] [Google Scholar]
- 26.Pereira LA, Wong MS, Mei Lim S, Stanley EG, Elefanty AG (2012) The Mix family of homeobox genes--key regulators of mesendoderm formation during vertebrate development. Dev Biol 367:163–177 [DOI] [PubMed] [Google Scholar]
- 27.Wilson V, Manson L, Skarnes WC, Beddington RS (1995) The T gene is necessary for normal mesodermal morphogenetic cell movements during gastrulation. Development 121: 877–886. [DOI] [PubMed] [Google Scholar]
- 28.Hart AH, Hartley L, Sourris K, Stadler ES, Li R, Stanley EG, Tam PP, Elefanty AG, Robb L (2002) Mixl1 is required for axial mesendoderm morphogenesis and patterning in the murine embryo. Development 129(15):3597–3608. [DOI] [PubMed] [Google Scholar]
- 29.Sumi T, Tsuneyoshi N, Nakatsuji N, Suemori H (2008) Defining early lineage specification of human embryonic stem cells by the orchestrated balance of canonical Wnt/beta-catenin, Activin/Nodal and BMP signaling. Development 135(17):2969–2979. [DOI] [PubMed] [Google Scholar]
- 30.Zhang P, Li J, Tan Z, Wang C, Liu T, Chen L, Yong J, Jiang W, Sun X, Du L, Ding M, Deng H (2008) Short-term BMP-4 treatment initiates mesoderm induction in human embryonic stem cells. Blood 111:1933–1941 [DOI] [PubMed] [Google Scholar]
- 31.Winnier G, Blessing M, Labosky PA, Hogan BL (1995) Bone morphogenetic protein-4 is required for mesoderm formation and patterning in the mouse. Genes Dev 9: 2105–2116. [DOI] [PubMed] [Google Scholar]
- 32.Kimelman D (2006) Mesoderm induction: from caps to chips. Nat Rev Genet 7:360–372. [DOI] [PubMed] [Google Scholar]
- 33.Beppu H, Kawabata M, Hamamoto T, Chytil A, Minowa O, Noda T, Miyazono K (2000) BMP type II receptor is required for gastrulation and early development of mouse embryos. Dev Biol 221:249–258 [DOI] [PubMed] [Google Scholar]
- 34.James RG, Schultheiss TM (2003) Patterning of the avian intermediate mesoderm by lateral plate and axial tissues. Dev Biol 253:109–124 [DOI] [PubMed] [Google Scholar]
- 35.Wijgerde M, Karp S, McMahon J, McMahon AP (2005) Noggin antagonism of BMP4 signaling controls development of the axial skeleton in the mouse. Dev Biol 286(1):149–157. [DOI] [PubMed] [Google Scholar]
- 36.James R, Schultheiss T (2005) Bmp signaling promotes intermediate mesoderm gene expression in a dose-dependent, cell-autonomous and translation-dependent manner. Dev Biol 288:113–125. [DOI] [PubMed] [Google Scholar]
- 37.Obara-Ishihara T, Kuhlman J, Niswander L, Herzlinger D (1999) The surface ectoderm is essential for nephric duct formation in intermediate mesoderm. Development 126(6):1103–1108. [DOI] [PubMed] [Google Scholar]
- 38.Sakai T, Larsen M, Yamada KM (2003) Fibronectin requirement in branching morphogenesis. Nature 423(6942):876–881. [DOI] [PubMed] [Google Scholar]
- 39.Abu-Abed S, Dollé P, Metzger D, Beckett B, Chambon P, Petkovich M (2001) The retinoic acid-metabolizing enzyme, CYP26A1, is essential for normal hindbrain patterning, vertebral identity, and development of posterior structures. Genes Dev 15(2):226–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wellik DM, Hawkes PJ, Capecchi MR (2002) Hox11 paralogous genes are essential for metanephric kidney induction. Genes Dev 16(11):1423–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niederreither K, Subbarayan V, Dollé P, Chambon P (1999) Embryonic retinoic acid synthesis is essential for early mouse post-implantation development. Nat Genet 21(4):444–448. [DOI] [PubMed] [Google Scholar]
- 42.Serluca FC, Fishman MC (2001). Pre-pattern in the pronephric kidney field of zebrafish. Development 128:2233–2241 [DOI] [PubMed] [Google Scholar]
- 43.Cartry J, Nichane M, Ribes V, Colas A, Riou JF, Pieler T, Dollé P, Bellefroid EJ, Umbhauer M (2006) Retinoic acid signalling is required for specification of pronephric cell fate. Dev Biol 299:35–51 [DOI] [PubMed] [Google Scholar]
- 44.Osafune K, Nishinakamura R, Komazaki S, Asashima M (2002) In vitro induction of the pronephric duct in Xenopus explants. Dev Growth Differ 44:161–167 [DOI] [PubMed] [Google Scholar]
- 45.Wingert RA, Davidson AJ (2008) The zebrafish pronephros: a model to study nephron segmentation. Kidney Int 73:1120–1127 [DOI] [PubMed] [Google Scholar]
- 46.Little MH, McMahon AP (2012) Mammalian kidney development: principles, progress and projections. Cold Spring Harb Perspect Biol doi: 10.1101/cshperspect.a008300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dudley AT, Godin RE, Robertson EJ (1999) Interaction between FGF and BMP signaling pathways regulates development of metanephric mesenchyme. Genes Dev 13(12):1601–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Barasch J, Qiao J, McWilliams G, Chen D, Oliver JA, Herzlinger D (1997) Ureteric bud cells secrete multiple factors, including bFGF, which rescue renal progenitors from apoptosis. Am J Physiol 273:F757–F767. [DOI] [PubMed] [Google Scholar]
- 49.Brown AC, Adams D, de Caestecker M, Yang X, Friesel R, Oxburgh L.(2011) FGF/EGF signaling regulates the renewal of early nephron progenitors during embryonic development. Development. 138(23):5099–5112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barak H, Huh SH, Chen S, Jeanpierre C, Martinovic J, Parisot M, Bole-Feysot C, Nitschké P, Salomon R, Antignac C, Ornitz DM, Kopan R (2013) FGF9 and FGF20 maintain the stemness of nephron progenitors in mice and man. Dev Cell 22(6):1191–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brown AC, Muthukrishnan SD, Guay JA, Adams DC, Schafer DA, Fetting JL, Oxburgh L (2013) Role for compartmentalization in nephron progenitor differentiation. Proc Natl Acad Sci U S A. 110(12):4640–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Blank U, Brown A, Adams DC, Karolak MJ, Oxburgh L (2009) BMP7 promotes proliferation of nephron progenitor cells via a JNK-dependent mechanism. Development 136(21):3557–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.So PL, Danielian PS (1999) Cloning and expression analysis of a mouse gene related to Drosophila odd-skipped. Mech Dev 84:157–160 [DOI] [PubMed] [Google Scholar]
- 54.Cirio MC, Hui Z, Haldin CE, Cosentino CC, Stuckenholz C, Chen X, Hong SK, Dawid IB, Hukriede NA (2011) Lhx1 is required for specification of the renal progenitor cell field. PloS One 6:e18858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Burgess R, Cserjesi P, Ligon KL, Olson EN (1995) Paraxis: a basic helix-loop-helix protein expressed in paraxial mesoderm and developing somites. Dev Biol 168(2):296–306. [DOI] [PubMed] [Google Scholar]
- 56.Chapman DL, Agulnik I, Hancock S, Silver LM, Papaioannou VE (1996) Tbx6, a mouse T-Box gene implicated in paraxial mesoderm formation at gastrulation. Dev Biol 180(2):534–542. [DOI] [PubMed] [Google Scholar]
- 57.Mahlapuu M, Ormestad M, Enerbäck S, Carlsson P (2001) The forkhead transcription factor Foxf1 is required for differentiation of extra-embryonic and lateral plate mesoderm. Development 128(2):155–166. [DOI] [PubMed] [Google Scholar]
- 58.James RG, Kamei CN, Wang Q, Jiang R, Schultheiss TM (2006) Odd-skipped related 1 is required for development of the metanephric kidney and regulates formation and differentiation of kidney precursor cells. Development 133:2995–3004 [DOI] [PubMed] [Google Scholar]
- 59.Dressler GR, Deutsch U, Chowdhury K, Nornes HO, Gruss P (1990) Pax2, a new murine paired-box-containing gene and its expression in the developing excretory system. Development 109:787–795 [DOI] [PubMed] [Google Scholar]
- 60.Mugford JW, Yu J, Kobayashi A, McMahon AP (2009) High-resolution gene expression analysis of the developing mouse kidney defines novel cellular compartments within the nephron progenitor population. Dev Biol 333(2):312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Georgas KM, Rumballe BA, Valerius MT, Chiu HS, Thiagarajan RD, Lesieur E, Aronow BJ, Brunskill EW, Combes AN, Tang D, Taylor D, Grimmond SM, Potter SS, McMahon AP, Little MH. (2009) Analysis of early nephron patterning reveals a role for distal RV proliferation in fusion to the ureteric tip via a cap mesenchyme-derived connecting segment. Dev Biol 332(2):273–286. [DOI] [PubMed] [Google Scholar]
- 62.Thiagarajan R, Georgas K, Rumballe B, Lesieur E, Chiu H, Taylor D, Tang D, Grimmond SM, Little MH (2011) Identification of anchor genes during kidney development defines ontological relationships, molecular subcompartments and regulatory pathways. PLoS ONE 6(2):e17286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Brunskill EW, Georgas K, Rumballe B, Little MH, Potter SS (2011) Defining the molecular character of the developing and adult kidney podocyte. PLoS One. 6(9):e24640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Georgas K, Rumballe B, Wilkinson L, Chiu HS, Lesieur E, Gilbert T, Little MH (2008) Use of dual section mRNA in situ hybridisation/immunohistochemistry to clarify gene expression patterns during the early stages of nephron development in the embryo and in the mature nephron of the adult mouse kidney. Histochem Cell Biol 130(5):927–942. [DOI] [PubMed] [Google Scholar]
- 65.Yamamoto M, Cui L, Johkura K, Asanuma K, Okouchi Y, Ogiwara N, Sasaki K (2005) Branching ducts similar to mesonephric ducts or ureteric buds in teratomas originating from mouse embryonic stem cells. Am J Physiol Renal Physiol 290(1):F52–60. [DOI] [PubMed] [Google Scholar]
- 66.Steenhard B, Isom K, Cazcarro P, Dunmore J, Godwin A, St John P, Abrahamson D (2005) Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol 16:1623–1631. [DOI] [PubMed] [Google Scholar]
- 67.Kim D, Dressler GR (2005) Nephrogenic factors promote differentiation of mouse embryonic stem cells into renal epithelia. J Am Soc Nephrol 16(12):3527–3534. [DOI] [PubMed] [Google Scholar]
- 68.Kobayashi T, Tanaka H, Kuwana H, Inoshita S, Teraoka H, Sasaki S, Terada Y (2005) Wnt4-transformed mouse embryonic stem cells differentiate into renal tubular cells. Biochem Biophys Res Commun 336:585–595. [DOI] [PubMed] [Google Scholar]
- 69.Bruce S, Rea R, Steptoe A, Busslinger M, Bertram J, Perkins A (2007) In vitro differentiation of murine embryonic stem cells toward a renal lineage. Differentiation 75:337–349. [DOI] [PubMed] [Google Scholar]
- 70.Vigneau C, Polgar K, Striker G, Elliott J, Hyink D, Weber O, Fehling HJ, Keller G, Burrow C, Wilson P (2007) Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo. J Am Soc Nephrol 18:1709–1720. [DOI] [PubMed] [Google Scholar]
- 71.Morizane R, Monkawa T, Itoh H (2009) Differentiation of murine embryonic stem and induced pluripotent stem cells to renal lineage in vitro. Biochem Biophys Res Commun 390:1334–1339. [DOI] [PubMed] [Google Scholar]
- 72.Ren X, Zhang J, Gong X, Niu X, Zhang X, Chen P, Zhang X (2010) Differentiation of murine embryonic stem cells toward renal lineages by conditioned medium from ureteric bud cells in vitro. Acta Biochim Biophys Sin (Shanghai). 42(7):464–471. [DOI] [PubMed] [Google Scholar]
- 73.Nishikawa M, Yanagawa N, Kojima N, Yuri S, Hauser PV, Jo OD, Yanagawa N (2012) Stepwise renal lineage differentiation of mouse embryonic stem cells tracing in vivo development. Biochem Biophys Res Commun 417(2):897–902. [DOI] [PubMed] [Google Scholar]
- 74.Mae S, Shirasawa S, Yoshie S, Sato F, Kanoh Y, Ichikawa H, Yokoyama T, Yue F, Tomotsune D, Sasaki K (2010) Combination of small molecules enhances differentiation of mouse embryonic stem cells into intermediate mesoderm through BMP7-positive cells. Biochem Biophys Res Commun 393(4):877–882. [DOI] [PubMed] [Google Scholar]
- 75.Karner CM, Das A, Ma Z, Self M, Chen C, Lum L, Oliver G, Carroll TJ (2011) Canonical Wnt9b signaling balances progenitor cell expansion and differentiation during kidney development. Development 138(7):1247–1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Perantoni AO (2003) The ureteric bud. Tissue-culture approaches to branching morphogenesis and inductive signaling. Methods Mol Med 86(0):179–192. [DOI] [PubMed] [Google Scholar]
- 77.Rosselot C, Spraggon L, Chia I, Batourina E, Riccio P, Lu B, Niederreither K, Dolle P, Duester G, Chambon P, Costantini F, Gilbert T, Molotkov A, Mendelsohn C (2011) Non-cell-autonomous retinoid signaling is crucial for renal development. Development 137(2):283–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin SA, Kolle G, Grimmond SM, Zhou Q, Doust E, Little MH, Aronow B, Ricardo SD, Pera MF, Bertram JF, Laslett AL (2010) Subfractionation of differentiating human embryonic stem cell populations allows the isolation of a mesodermal population enriched for intermediate mesoderm and putative renal progenitors. Stem Cells Dev 19(10):1637–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Challen GA, Martinez G, Davis M, Teasdale R, Grimmond S. Little MH. (2004) Identifying the molecular phenotype of renal progenitor cells. J Am SocNephrol 15(9):2344–2357. [DOI] [PubMed] [Google Scholar]
- 80.Mae S, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, Arai S, Sato-Otubo A, Toyoda T, Takahashi K, Nakayama N, Cowan CA, Aoi T, Ogawa S, McMahon AP, Yamanaka S, Osafune K (2013) Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun 4:1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lusis M, Li J, Ineson J, Christensen ME, Rice A, Little MH (2010) Isolation of clonogenic, long-term self renewing embryonic renal stem cells. Stem Cell Research 5(1):23–39. [DOI] [PubMed] [Google Scholar]
- 82.Narayanan K, Schumacher KM, Tasnim F, Kandasamy K, Schumacher A, Ni M, Gao S, Gopalan B, Zink D, Ying JY (2013) Human embryonic stem cells differentiate into functional renal proximal tubular-like cells. Kidney Int 83(4):593–603 [DOI] [PubMed] [Google Scholar]
- 83.Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD (2012) The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 7(9):e46453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Georgas KM, Chiu HS, Lesieur E, Rumballe BA, Little MH (2011) Expression of metanephric nephron-patterning genes in differentiating mesonephric tubules. Dev Dyn 240(6):1600–1612. [DOI] [PMC free article] [PubMed] [Google Scholar]