Abstract
Purpose of Review
This review provides an update on the current status of platelet-rich plasma (PRP). Topics covered include the current regulatory environment, economic outlook, and current clinical evidence.
Recent Findings
The global PRP market is expected to grow to between 380 million and 4.5 billion (USD) over the next 5–10 years. The cost of a single treatment, which is not covered by most insurance, is roughly $500–$2500, with patients often returning for additional treatments.
Summary
While PRP is not ‘FDA-approved’, it can be legally offered in the clinic ‘off-label’ in the USA for a myriad of musculoskeletal indications. Recently published meta-analyses have demonstrated statistically significant improvements that, in some cases, suggest that PRP may have clinically meaningful effects. However, given the fact that clearance is not synonymous with approval, PRP is a costly treatment not covered by insurance, and clinical trials have not demonstrated definitive efficacy, we recommend informing patients when providing PRP ‘off-label’.
Keywords: HCT/Ps, Platelet-rich plasma, PRP, Orthopedics, Sports medicine, Regenerative medicine
Introduction
What is PRP?
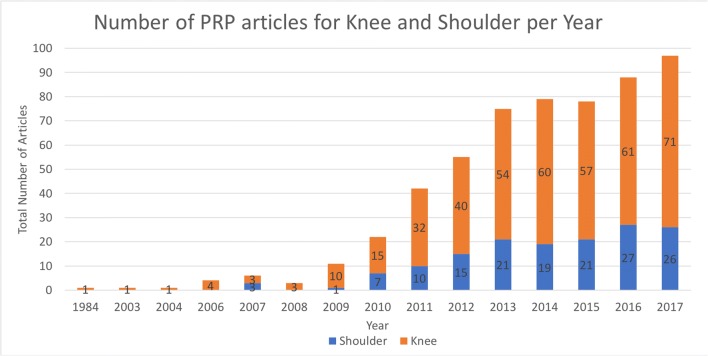
Platelet-rich plasma (PRP) has seen increased interest and utilization over the past decade, particularly in the field of orthopedics (Fig. 1). At its simplicity, PRP is autologous blood with concentrations of platelets above baseline values [1]. Regulatory bodies have established more precise definitions, including the US Food and Drug Administration (FDA), which requires that the blood be collected by a single, uninterrupted venipuncture, centrifuged, and contain at least 250,000 platelets per microliter [2]. PRP categories that are based on the leukocyte and fibrin content of the preparation have also been proposed [3].
Fig. 1.
PubMed search for articles published on PRP for the “knee” and “shoulder”
When considering the broader economic context of PRP, it is important to understand that the term ‘PRP’ is used to refer to broad range of different treatments and that more than 40 commercial PRP systems exist [4]. The final concentration of platelets, leukocytes, and growth factors varies between and within different techniques, as well as between and within patients [5, 6], and different protocols yield products with different compositions and characteristics [7•, 8•]. This leads to a lack of comparability that has major implications for the PRP market at large. Attempts to affirm or discredit the efficacy of PRP have largely failed, which has spurred numerous low-powered studies that are likely to represent wasted research investment [9].
How is PRP Produced?
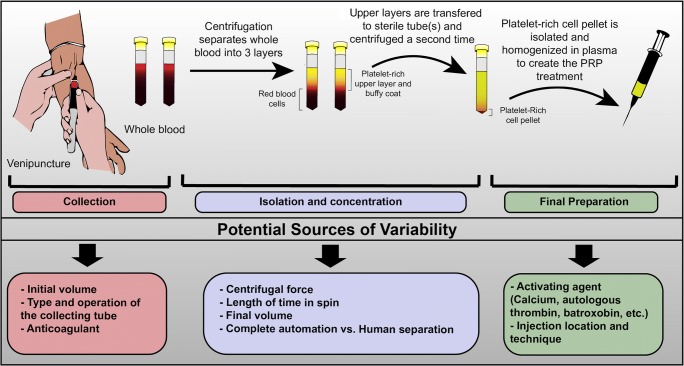
PRP is prepared by a process known as differential centrifugation, which separates the cellular constituents of autologous whole blood based on their specific gravity (Fig. 2). The process is usually carried out in two phases [4]. In the first phase, a preliminary “soft spin” separates anticoagulated blood into three layers. The upper layers, comprised of mostly platelets and leukocytes, are transferred to an empty sterile tube and the lower layer (mostly erythrocytes) is discarded. The remaining mixture is then subjected to a second spin that further concentrates platelets into a cell pellet that is extracted and homogenized in leftover plasma. A detailed overview of the principles and methods used to prepare PRP can be found elsewhere [8•].
Fig. 2.
PRP collection, isolation, and sources of variability
Treatment heterogeneity can be introduced at every stage of even the most basic PRP protocols. The type of collecting tube used, centrifugation speed, number of steps, and use (or lack thereof) of activating agents, produce PRP preparations of varying volumes, platelet numbers, growth factor content, and residual white and red blood cells [6, 10, 11]. It is not clear how these differences influence PRP’s efficacy [8•]. For example, higher concentrations of platelets do not necessarily lead to an enhanced tissue healing effect [12]. The lack of comparability between different treatments is further exacerbated by the failure to characterize PRP and methodological under-reporting in the literature [13].
How Does PRP Work?
The biological complexity of PRP makes firm efficacy conclusion hard to pin down. The therapeutic effects of PRP are largely attributed the “bioactive factors” it contains [14, 15]. While unsatisfyingly opaque, this sort of explanation is not entirely without merit. PRP contains signaling proteins, including growth factors, chemokines, and cytokines, as well as adhesive proteins, proteases, small molecules, and ions that interact with multiple cell types. Endogenous cells within the joint respond to these agents, further contributing to the biological milieu by secreting their own biologically active molecules [7•].
The beneficial biologic effects of PRP are most commonly associated with the high concentration of growth factors it contains. However, many of these growth factors are pleiotropic and the same growth factor may provide benefits to one tissue, while having deleterious effects on the surrounding tissues [15]. Moreover, growth factor signaling alone cannot account for the biological response to PRP, suggesting that additional mediators play an important role [7•].
Is the Use of PRP for Musculoskeletal Indications Safe?
One of the few aspects of PRP that is reasonably well understood is its safety profile. Adverse events associated with PRP, including pain and stiffness, tend to be similar to comparative treatments (or only slightly increased [16, 17]), and recent meta-analyses have shown that severe adverse events attributable to the treatment are almost never reported [18•, 19]. While some in the transfusion medicine community have expressed discomfort with the widespread, unregulated use of PRP in musculoskeletal medicine [20], no compelling evidence of systemic effects resulting from locally administered PRP have been demonstrated [7•, 21].
PRP in Musculoskeletal Medicine
Regulatory Overview
Title 21 United States Code of Federal Regulations Part 1271 (21 CFR 1271) governs transplantation of human cells, tissues, and cell and tissue-based products (HCT/Ps). HCT/Ps are “articles containing or consisting of human cells or tissues that are intended for implantation, transplantation, infusion, or transfer into a human recipient” [2] and, unless they qualify for a 361-product exemption, require premarket approval (PMA) before they can be sold commercially in the USA. In contrast, products regulated solely under Section 361 of the Public Health Service Act follow a different regulatory pathway, which requires animal studies and clinical trials [22•].
Is PRP Approved?
Much of the confusion surrounding the regulation of biologic drugs stems from the FDA’s tiered, risk-based approach. However, PRP is a particularly special case because, despite being a biologic drug, PRP is not considered a HCT/P [23]. PRP is regulated by regulating the device used to manufacture it and the wide-range PRP systems that are currently available have been brought to market using the 510(k) pathway [22•]. The 510(k) pathway “clears” products that are “substantially equivalent” to an already cleared predicate device [24]. In the case of PRP, the original predicate device is a platelet and plasma separator that produces PRP that is intended to be mixed with bone graft materials to enhance handling properties [25], or in the case of PRP gel, to ‘maintain moisture in a wound [26]. In other words, PRP has been cleared for use as a sticky substance and PRP gel has been cleared for use as a wet substance.
FDA clearance allows PRP to be used for a wide range of different orthopedic indications [15, 27, 28]. However, clearance is not synonymous with approval for a specific indication [22•]. As such, most of the PRP treatments offered for musculoskeletal indications are considered ‘off-label’ use, which transfers liability from the manufacturers of the device to the individual providing it [20].
While off-label treatments (including PRP) are not FDA-approved, it is important to understand that their use of off-label drug is not necessarily improper or illicit. Indeed, off-label prescriptions account for roughly half of all prescriptions written today [29], and the off-label use of certain drugs is well-accepted within standards [30]. However, within the broader economic context of PRP, it is also important to understand that off-label use is not necessarily supported by sound scientific evidence and has the potential to drive up healthcare costs [29, 31].
Production Cost and the Global Market
Because PRP is not covered by insurance, the production and treatment costs are hard to pin down. Growth estimates by various market research and consulting agencies have estimated that the global market will grow to between 380 million and 4.5 billion (USD) over the next 5–10 years [32–34]. A 2009 review by Hall et al., estimated that the one-time preparation cost per syringe was roughly $150 [1]. However, Hall was quick to point out that the cost is dependent on both the distributing company and on institutional relationships and, in a more recent review, the price per kit was found to vary more widely (range: $50–$500 USD) [35]
It is important to understand that the aforementioned costs do not include the indirect costs of the centrifuge, clinic staff, or facilities. This is particularly important given the constraints of the Current Procedural Terminology (CPT) code that is used for PRP injections. In 2010, PRP was given the category III CPT code, ‘0232T’—a temporary code used for emerging technologies, services, and procedures [21]. This code does not apply to PRP injections that are given during the course of surgical procedures, but should be used in stand-alone cases, such as in the office, ambulatory surgical center, or outpatient facility [36]. Because the code encompasses a number of the procedures that support the actual injection, including the bloodwork, harvesting, preparation, and imaging guidance [21], these procedures cannot be billed separately. Moreover, the existence of a CPT code does not mean that payers/carriers reimburse the service. In fact, most payers/carriers have internal policies of non-coverage for PRP-type services [36]. Within these constraints, costs are passed directly onto patients.
Out of Pocket Cost to Patients
There is insufficient data to perform a cost-benefit analysis of PRP in the USA for musculoskeletal pathology, although it has been estimated that the cost of a single PRP treatment in Europe is approximately twice as much as a corticosteroid treatment [37]. Based on the clinical experience of the authors, the average cost of a single PRP injection is roughly $750 (range: $300 to $2500), not including the initial consult, which usually costs over $200. The number of injections recommended to patients varies widely, with most clinics recommending 2–3 injections. While far from a definitive estimate (and also likely influenced by regional factors), these numbers are consistent with other informal estimates, which have pegged the out-the-door cost for a PRP procedure at roughly $500–$2500 [21, 36].
What Does the Clinical Data Tell Us?
Overview
Even among high-level studies, PRP clinical trials tend to be underpowered, averaging fewer than 30 patients (control and treatment groups combined) [18•]. However, in recent years several meta-analyses have been published (Table 1). While these meta-analyses cannot tell us if a particular PRP treatment works, they are beginning to shed light on the field more broadly. The following sections highlight notable meta-analyses that have been published in recent years. To provide additional context, we discuss these findings in the context of a clinically meaningful change.
Table 1.
Notable meta-analysis published in the last 5 years. No studies reported severe adverse events. Outcomes marked with a single “*” indicate outcomes that were not significant. Outcomes marked with two “**” indicate outcomes where PRP was outperformed by its comparator
| Study | Pathology | Studies included | Total patients | Treatment COMPARISON | Outcome (PRP vs comparator) | 95% confidence intervals | |
|---|---|---|---|---|---|---|---|
| Tendon/ligament | 2018 Hurley [38] | Rotator cuff tear | 18 | 1147 | PRP vs. RCR | VAS: − 1.41 (at 30 days) | − 1.69 to − 1.13 |
| VAS: − 0.12 (at final) | − 0.20 to − 0.05 | ||||||
| CS: 2.51 (at final) | 1.04 to − 3.97 | ||||||
| *ASES: 1.21 (at final) | − 0.65 to 3.09 | ||||||
| *PS: 1.12 (at final) | 0.98 to 1.29 | ||||||
| 2017 Chen [18] | Tendon/ligament pathology/injury | 21 | 1031 | PRP vs. various control | VAS: − 0.72 (short-term) | − 1.10 to − 0.34 | |
| VAS: − 0.84 (long-term) | − 1.23 to − 0.44 | ||||||
| 2017 Mi [17] | Lateral epicondylitis | 8 | 511 | PRP vs. corticosteroid | VAS: 1.02 (2–4 weeks)** | 0.34 to 0.88 | |
| *VAS: 0.73 (6–8 weeks)** | 0.27 to 0.78 | ||||||
| *VAS: − 0.28 (12 weeks) | − 0.77 to − 0.28 | ||||||
| VAS: − 1.60 (6 months) | − 0.84 to − 0.28 | ||||||
| VAS: − 1.45 (1 year) | − 1.00 to − 0.40 | ||||||
| Articular cartilage | 2013 Khoshbin [16] | Knee OA | 6 | 577 | PRP vs. control (5 HA and 1 saline) | *VAS: 0.46 (6 months) | −0.52 to 1.43 |
| WOMAC: − 18.0 (6 months) | − 27.75 to − 8.30 | ||||||
| IKDC: − 8.26 (6 months) | 2.58 to 13.98 | ||||||
| *PS: 8.97 (6 months) | 0.54 to 149.25 | ||||||
| 2017 Leite [39] | Hip OA | 8 | 807 | PRP vs. HA | VAS: 1.00 (6 months) | − 1.50 to 3.50 | |
| VAS: 0.81 (12 months) | − 1.11 to 2.73 | ||||||
| 2016 Kanchanatawan [40] | Knee OA | 9 | 551 | PRP vs. HA or placebo | WOMAC: − 15.4 (12 months) | − 28.57 to − 2.30 | |
| Other indications | 2018 Grassi [41] | Acute muscle injuries | 6 | 374 | PRP vs. PT or placebo | RS: − 7.17 days | − 12.26 to − 2.08 |
| 2012 Sheth [42] | Mixed | 9 | 446 | PRP vs. various control | *VAS: − 0.62 (6 weeks) | − 1.25 to 0.02 | |
| *VAS: − 0.24 (6 months) | − 1.03 to 0.55 | ||||||
| *VAS: − 0.30 (1 year) | − 0.85 to 0.25 | ||||||
| 2014 Moraes VY [43] | Mixed | 8 | 175 | PRP vs. various control | VAS: − 0.95 (3 month) | − 1.41 to − 0.48 |
OA osteoarthritis, HA hyaluronic acid, RCR rotator cuff repair, PT physical therapy, PS patient satisfaction, RS return to sport, VAS Visual Analog Scale, WOMAC Western Ontario and McMaster University Osteoarthritis Index, IKDC International Knee Documentation Committee, ASES American Shoulder and Elbow Society Score
Tendon and Ligament Injury/Pathology
A recent meta-analysis published by Chen et al. [18•] assessed the ability of PRP to reduce pain in patients with tendon and ligament injuries. They found that patients treated with PRP had significantly less pain, as determined using the 10-point visual analogue scale (VAS), at final follow-up (weighted mean difference [WMD]: − 0.84; 95% CI, − 1.23 to − 0.44; P < .01). While these results seem promising at the outset, the magnitude of the difference suggests that, even among patients who improve, the potential benefits of PRP may not be clinically meaningful [38, 39]. A recent review published by Hurley et al. included many of the studies from Chen’s work and focused exclusively on the rotator cuff [40]. They found that patients treated with PRP had lower VAS scores at final follow-up (− 0.22 [95% CI, − 0.37 to − 0.06]; P < .05). These findings were even less favorable than those reported in the rotator cuff subset analysis of Chen’s work, which found that the weighted mean difference at final follow-up was − 0.53 (95% CI, − 0.98 to − 0.09; P = .02).
PRP has demonstrated perhaps the most promising results for lateral epicondylitis (LE). A 2017 meta-analysis published by Mi et al. comparing platelet-rich plasma (PRP) to corticosteroids found that PRP offered significant improvements over corticosteroids after 6 months (10-point VAS-pain: − 1.60, [− 1.97 to − 1.22]; P < .001) [17]. These findings were similar to those reported in the LE subset analysis of Chen’s work, which found improvements of − 1.14 (− 1.85 to − 0.43, P < 0.01) in VAS up to 6.5 months. Additionally, the subset analysis of LE patients in Chen’s work found longer-term improvements (> 1 year) of − 1.39 ([− 2.49 to − 0.29]; P = 0.01) [18•]. Overall, these findings suggest that PRP may provide an “appreciable” reduction in LE-associated pain for a number of patients [41]. However, given the intrinsic lack of comparability between different PRP treatments, it is not possible to make definitive judgements at this time.
Articular Cartilage
The limited regenerative capacity of articular cartilage [42, 43] and need for non-surgical treatment alternatives [44] have spurred a number of clinical investigations into the efficacy of PRP and other autologous cell therapies for the treatment of osteoarthritis (OA) [45]. A 2013 review by Khoshbin et al. found that OA patients that had been treated with PRP had better Western Ontario and McMaster Universities Arthritis Index (WOMAC) scores at 6 months than those treated with hyaluronic acid or saline (mean difference, 18.0 [95% confidence interval, 28.8 to 8.3]) [16]. A more recent review published by Kanchanatawan et al. supported Khoshbin’s findings [46]. Kanchanatawan’s group found that short-term (≤ 1 year) WOMAC scores of patients that had been treated with PRP were lower than those treated with HA or placebo (− 15.4 [95% CI − 28.6, − 2.3], p = 0.021) [46]. With respect to some of the most commonly cited values for minimal important change (MID or MCID) for WOMAC (total change: 9.1 [47], 15 [48]; percent change: 17–22% [49]), these differences are particularly notable.
PRP has produced less favorable results in patients with hip OA than it has in patients with knee OA. In a paper evaluating the effectiveness of HA compared to other intra-articular injections, Leite et al. found that the mean differences in the VAS scores at 6 and 12 months were only 1.0 [95% CI, − 1.5 to 3.50] and .81 [95% CI, − 1.11 to 2.73], respectively, which leads the authors to conclude that there is very low evidence that PRP is superior to HA for pain [50]. Leite’s conclusions stand in sharp contrast with those made by Khoshbin’s and Kanchanatawan’s [16]. The difference could be due to biological differences between the pathologies themselves or the fact that Leite’s analysis only included 3 studies.
Other Musculoskeletal Indications
There is less evidence to support the use of PRP for other musculoskeletal indications. Grassi et al. performed a meta-analysis of randomized, controlled trials investigating the use of PRP for acute muscle injuries [51]. Patients treated with PRP showed a significant improvement in return to sport (the outcome with the most robust data reporting). However, when they excluded non-blinded studies, the difference was no longer significant, which leads the authors to conclude that there is little-to-no evidence that treatment with PRP results in significant differences in pain, function, healing, or strength.
More heterogenous meta-analysis evaluating PRP for musculoskeletal indications have also been published. Sheth et al. performed meta-analysis of randomized controlled trials and prospective cohort studies for ‘orthopedic injuries’ broadly [52]. They found no differences in VAS between the treatment and control groups. Another recently published, highly heterogenous meta-analysis looked at the benefits and harms of ‘platelet-rich therapies’ for treating ‘musculoskeletal soft tissue injuries’ [53]. Data pooled from four trials showed a small reduction in short-term pain (VAS) favoring PRP (MD − 0.95, [95% CI − 1.41 to − 0.48]); however, the evidence was characterized as being of ‘very low quality’ by the authors and they did not find significant differences between the functional scores of patients treated with PRP at the short-term (up to 3 months), medium-term (6 months), or long-term (1 year) follow-up.
Conclusions and Recommendations
Three key factors are often associated with the high demand for PRP and similar blood derived products: (1) its scientific underpinnings, (2) the ease and relative non-invasive nature in which it can be obtained, and (3) its well-established safety record [7•]. As this article has discussed, these 3 factors are indeed important aspects driving the current PRP market. However, the hopes of patients, critical need for new therapies, and persuasive advertising are also likely to be factors driving the global market for PRP [54].
In a review of the current use and regulatory underpinnings of PRP, Beitzel et al. share their approach [22]. They recommend (1) discussing the treatment and the regulations extensively with the patient, (2) letting the patient know that there is no guarantee that the PRP injection will provide relief, (3) encouraging patients to discuss PRP with their family and primary care physician, and (4) collecting quality care information, including adverse events. Overall, these are excellent recommendations. However, we do not agree with the author’s decision to highlight the fact that PRP devices are “cleared” for safe and effective processing of PRP when discussing the treatment with patients. Clearance has very specific and technical meaning that is likely to be misinterpreted by patients. Indeed, even among clinicians, FDA clearance is often misconstrued as FDA approval. Given that PRP is not covered by insurance and clinical trials have not demonstrated clear and definitive efficacy, we believe that it is more appropriate to highlight the fact that the treatment is being provided to the patient ‘off-label’ based on what the practitioner believes is in the patient’s best interest (Table 2).
Table 2.
Overview
| Key concepts: | – PRP is not a standardized treatment – The method used to prepare PRP is a major source of treatment variability – The complex and diverse milieu of chemical mediators that comprise PRP makes it difficult to validate purported therapeutic mechanisms – In clinical studies to date, no major complications have been associated with PRP – PRP is biologic but is not subject to the same regulatory scrutiny as most other biologic products – The vast majority of PRP treatments are offered ‘off-label’ – The global PRP market is expected to grow to between $380 million and $4.5 billion (USD) over the next 5–10 years – PRP is not covered by most insurance and the out-of-pocket cost to patients is roughly $500–$2500 per treatment, with patients often receiving multiple treatments |
| Recommendations: | – Adequately powered studies aimed at showing clinically meaningful improvement are needed. Further investment in low-powered studies is likely to represent research waste – When PRP is administered for unapproved indications, practitioners should inform patients that the treatment is being administered ‘off-label’ based on their provider’s professional medical opinion |
| Future outlook: | – Encouraging preliminary results suggest that PRP may be an effective treatment alternative for specific indications under certain conditions – Improved reporting and study methodology will allow PRP formulations to be refined, which may improve efficacy. |
Conflict of Interest
Ian A. Jones and Ryan C. Togashi declare that they have no conflict of interests. C. Thomas Vangsness is a shareholder of CarthroniX.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Footnotes
This article is part of the Topical Collection on Protein-Rich Plasma: From Bench to Treatment of Arthritis
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
- 1.Hall MP, Band PA, Meislin RJ, Jazrawi LM, Cardone DA. Platelet-rich plasma: current concepts and application in sports medicine. J Am Acad Orthop Surg. 2009;17:602–608. doi: 10.5435/00124635-200910000-00002. [DOI] [PubMed] [Google Scholar]
- 2.FDA. Code of Federal Regulations - Title 21, Volume 7 [Internet]. Code of Federal Regulations Code of Federal Regulations; Apr 1, 2017. Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=640.34.
- 3.Dohan Ehrenfest DM, Rasmusson L, Albrektsson T. Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF) Trends Biotechnol. 2009;27:158–167. doi: 10.1016/j.tibtech.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 4.Hsu WK, Mishra A, Rodeo SR, Fu F, Terry MA, Randelli P, et al. Platelet-rich plasma in orthopaedic applications: evidence-based recommendations for treatment. J Am Acad Orthop Surg. 2013;21:739–471. doi: 10.5435/JAAOS-21-12-739. [DOI] [PubMed] [Google Scholar]
- 5.Mazzucco L, Balbo V, Cattana E, Guaschino R, Borzini P. Not every PRP-gel is born equal. Evaluation of growth factor availability for tissues through four PRP-gel preparations: Fibrinet, RegenPRP-Kit, Plateltex and one manual procedure. Vox Sang. Blackwell Publishing Ltd; 2009;97:110–8. [DOI] [PubMed]
- 6.Castillo TN, Pouliot MA, Kim HJ, Dragoo JL. Comparison of growth factor and platelet concentration from commercial platelet-rich plasma separation systems. Am J Sports Med. 2011;39:266–271. doi: 10.1177/0363546510387517. [DOI] [PubMed] [Google Scholar]
- 7.Andia Isabel, Maffulli Nicola. Platelet-rich plasma for managing pain and inflammation in osteoarthritis. Nature Reviews Rheumatology. 2013;9(12):721–730. doi: 10.1038/nrrheum.2013.141. [DOI] [PubMed] [Google Scholar]
- 8.Dhurat Rachita, Sukesh MS. Principles and methods of preparation of platelet-rich plasma: A review and author′s perspective. Journal of Cutaneous and Aesthetic Surgery. 2014;7(4):189. doi: 10.4103/0974-2077.150734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buchbinder R, Maher C, Harris IA. Setting the research agenda for improving health care in musculoskeletal disorders. Nat Rev Rheumatol. 2015;11:597–605. doi: 10.1038/nrrheum.2015.81. [DOI] [PubMed] [Google Scholar]
- 10.Mazzocca AD, McCarthy MBR, Chowaniec DM, Cote MP, Romeo AA, Bradley JP, et al. Platelet-rich plasma differs according to preparation method and human variability. J Bone Joint Surg Am. 2012;94:308–316. doi: 10.2106/JBJS.K.00430. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Vidriero E, Goulding KA, Simon DA, Sánchez M, Johnson DH. The use of platelet-rich plasma in arthroscopy and sports medicine: optimizing the healing environment. Arthroscopy. 2010;26:269–278. doi: 10.1016/j.arthro.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 12.Giusti I, Rughetti A, D'Ascenzo S, Millimaggi D, Pavan A, Dell'Orso L, et al. Identification of an optimal concentration of platelet gel for promoting angiogenesis in human endothelial cells. Transfusion. 2009;49:771–778. doi: 10.1111/j.1537-2995.2008.02033.x. [DOI] [PubMed] [Google Scholar]
- 13.Murray IR, LaPrade RF. Platelet-rich plasma: renewed scientific understanding must guide appropriate use. Bone Joint Res. 2016;5:92–94. doi: 10.1302/2046-3758.53.BJR-2016-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Foster TE, Puskas BL, Mandelbaum BR, Gerhardt MB, Rodeo SA. Platelet-rich plasma: from basic science to clinical applications. Am J Sports Med. 2009;37:2259–2272. doi: 10.1177/0363546509349921. [DOI] [PubMed] [Google Scholar]
- 15.LaPrade RF, Geeslin AG, Murray IR, Musahl V, Zlotnicki JP, Petrigliano F, et al. Biologic treatments for sports injuries II think tank-current concepts, future research, and barriers to advancement, part 1: biologics overview, ligament injury, tendinopathy. Am J Sports Med. 2016;44:3270–3283. doi: 10.1177/0363546516634674. [DOI] [PubMed] [Google Scholar]
- 16.Khoshbin A, Leroux T, Wasserstein D, Marks P, Theodoropoulos J, Ogilvie-Harris D, Gandhi R, Takhar K, Lum G, Chahal J. The efficacy of platelet-rich plasma in the treatment of symptomatic knee osteoarthritis: a systematic review with quantitative synthesis. Arthroscopy. 2013;29:2037–2048. doi: 10.1016/j.arthro.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Mi B, Liu G, Zhou W, Lv H, Liu Y, Wu Q, Liu J. Platelet rich plasma versus steroid on lateral epicondylitis: meta-analysis of randomized clinical trials. Phys Sportsmed. 2017;45:97–104. doi: 10.1080/00913847.2017.1297670. [DOI] [PubMed] [Google Scholar]
- 18.Chen X, Jones IA, Park C, Vangsness CT. The efficacy of platelet-rich plasma on tendon and ligament healing: a systematic review and meta-analysis with bias assessment. Am J Sports Med. 2017;2016:363546517743746. doi: 10.1177/0363546517743746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang-Saegusa A, Cugat R, Ares O, Seijas R, Cuscó X, Garcia-Balletbó M. Infiltration of plasma rich in growth factors for osteoarthritis of the knee short-term effects on function and quality of life. Arch Orthop Trauma Surg. 2011;131:311–317. doi: 10.1007/s00402-010-1167-3. [DOI] [PubMed] [Google Scholar]
- 20.Harm SK, Fung MK. Platelet-rich plasma injections: out of control and on the loose? Transfusion. 2015;55:1596–1598. doi: 10.1111/trf.13160. [DOI] [PubMed] [Google Scholar]
- 21.Dhillon RS, Schwarz EM, Maloney MD. Platelet-rich plasma therapy - future or trend? Arthritis Res Ther. 2012;14:219. doi: 10.1186/ar3914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Beitzel K, Allen D, Apostolakos J, Russell RP, McCarthy MB, Gallo GJ, et al. US definitions, current use, and FDA stance on use of platelet-rich plasma in sports medicine. J Knee Surg. 2015;28:29–34. doi: 10.1055/s-0034-1390030. [DOI] [PubMed] [Google Scholar]
- 23.Marks P, Gottlieb S. Balancing safety and innovation for cell-based regenerative medicine. N Engl J Med. 2018;378:954–959. doi: 10.1056/NEJMsr1715626. [DOI] [PubMed] [Google Scholar]
- 24.Sweet BV, Schwemm AK, Parsons DM. Review of the processes for FDA oversight of drugs, medical devices, and combination products. J Manag Care Pharm. 2011;17:40–50. doi: 10.18553/jmcp.2011.17.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gutman SI. 510(k) SUMMARY [internet]. The United States Department of Health and Human Services; 2000 Feb. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf/K994148.pdf.
- 26.Melkerson MN. 510(k) SUMMARY [Internet]. The United States Department of Health and Human Services; 2009 Nov. Report No.: K082333. Available from: https://www.accessdata.fda.gov/cdrh_docs/pdf8/K082333.pdf.
- 27.Murray IR, LaPrade RF, Musahl V, Geeslin AG, Zlotnicki JP, Mann BJ, et al. Biologic treatments for sports injuries II think tank-current concepts, future research, and barriers to advancement, part 2: rotator cuff. Orthop J Sports Med. 2016;4:2325967116636586. doi: 10.1177/2325967116636586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zlotnicki JP, Geeslin AG, Murray IR, Petrigliano FA, LaPrade RF, Mann BJ, et al. Biologic treatments for sports injuries II think tank-current concepts, future research, and barriers to advancement, part 3: articular cartilage. Orthop J Sports Med. 2016;4:2325967116642433. doi: 10.1177/2325967116642433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilkes M, Johns M. Informed consent and shared decision-making: a requirement to disclose to patients off-label prescriptions. PLoS Med Public Libr Sci. 2008;5:e223. doi: 10.1371/journal.pmed.0050223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wittich CM, Burkle CM, Lanier WL. Ten common questions (and their answers) about off-label drug use. Mayo Clin Proc. 2012;87:982–990. doi: 10.1016/j.mayocp.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Radley DC, Finkelstein SN, Stafford RS. Off-label prescribing among office-based physicians. Arch Intern Med. 2006;166:1021–1026. doi: 10.1001/archinte.166.9.1021. [DOI] [PubMed] [Google Scholar]
- 32.Research GV. Platelet Rich Plasma (PRP) Market analysis by product (pure, leukocyte-rich, leukocyte-rich fibrin), by application (orthopedics, cosmetic surgery, ophthalmic surgery, neurosurgery), and segment forecasts, 2014–2025 [Internet]. www.grandviewresearch.com. 2017 [cited 2018 Mar 17]. p. 100. Available from: https://www.grandviewresearch.com/industry-analysis/platelet-rich-plasma-prp-market.
- 33.Engine MR. Platelet rich plasma market by type analysis (pure-PRP, leukocyte-rich-PRP, pure-PRF); by origin analysis (autologous PRP, allogeneic PRP, homologous PRP); by applications analysis (orthopedic surgery, cosmetic surgery, general surgery, neurosurgery) and by regional analysis – global forecast by 2016 - 2024 [Internet]. www.marketresearchengine.com; 2017 Jun. Report no.: PPRPM617. Available from: https://www.marketresearchengine.com/platelet-rich-plasma-market.
- 34.Platelet-rich plasma (PRP) market was valued at USD 201.2 Mn in 2016. SA-BRC; 2017.
- 35.Oudelaar BW, Peerbooms JC, Huis In't Veld R, Vochteloo AJH. Concentrations of blood components in commercial platelet-rich plasma separation systems: a review of the literature. Am J Sports Med. 2018;14:363546517746112. doi: 10.1177/0363546517746112. [DOI] [PubMed] [Google Scholar]
- 36.Vaught MS, Cole BJ. Coding and reimbursement issues for platelet-rich plasma. Operative Techniques in Sports Medicine. 2011;19:185–189. doi: 10.1053/j.otsm.2011.03.005. [DOI] [Google Scholar]
- 37.Gosens T, Peerbooms JC, van Laar W, Oudsten den BL. Ongoing positive effect of platelet-rich plasma versus corticosteroid injection in lateral epicondylitis: a double-blind randomized controlled trial with 2-year follow-up. Am J Sports Med. 2011;39:1200–1208. doi: 10.1177/0363546510397173. [DOI] [PubMed] [Google Scholar]
- 38.Tashjian RZ, Deloach J, Porucznik CA, Powell AP. Minimal clinically important differences (MCID) and patient acceptable symptomatic state (PASS) for visual analog scales (VAS) measuring pain in patients treated for rotator cuff disease. J Shoulder Elb Surg. 2009;18:927–932. doi: 10.1016/j.jse.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 39.Tashjian RZ, Hung M, Keener JD, Bowen RC, McAllister J, Chen W, et al. Determining the minimal clinically important difference for the American Shoulder and Elbow Surgeons score, Simple Shoulder Test, and visual analog scale (VAS) measuring pain after shoulder arthroplasty. J Shoulder Elb Surg. 2017;26:144–148. doi: 10.1016/j.jse.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 40.Hurley ET, Lim Fat D, Moran CJ, Mullett H. The efficacy of platelet-rich plasma and platelet-rich fibrin in arthroscopic rotator cuff repair: a meta-analysis of randomized controlled trials. Am J Sports Med. 2018;363546517751397:79. doi: 10.1177/0363546517751397. [DOI] [PubMed] [Google Scholar]
- 41.Johnston BC, Thorlund K, Schünemann HJ, Xie F, Murad MH, Montori VM, et al. Improving the interpretation of quality of life evidence in meta-analyses: the application of minimal important difference units. Health Qual Life Outcomes. 2010;8:116. doi: 10.1186/1477-7525-8-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas RJ, Hourd PC, Williams DJ. Application of process quality engineering techniques to improve the understanding of the in vitro processing of stem cells for therapeutic use. J Biotechnol. 2008;136:148–155. doi: 10.1016/j.jbiotec.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 43.Tran-Khanh N, Chevrier A, Lascau-Coman V, Hoemann CD, Buschmann MD. Young adult chondrocytes proliferate rapidly and produce a cartilaginous tissue at the gel-media interface in agarose cultures. Connect Tissue Res. 2010;51:216–223. doi: 10.3109/03008200903281683. [DOI] [PubMed] [Google Scholar]
- 44.Maniar KH, Jones IA, Gopalakrishna R, JR CTV Lowering side effects of NSAID usage in osteoarthritis: recent attempts at minimizing dosage. Expert Opin Pharmacother. 2017;6:14656566.2017.1414802–null. doi: 10.1080/14656566.2017.1414802. [DOI] [PubMed] [Google Scholar]
- 45.McIntyre JA, Jones IA, Han B, Vangsness CT., Jr Intra-articular Mesenchymal Stem Cell Therapy for the human joint: a systematic review. Am J Sports Med. 2017;11:036354651773584. doi: 10.1177/0363546517735844. [DOI] [PubMed] [Google Scholar]
- 46.Kanchanatawan W, Arirachakaran A, Chaijenkij K, Prasathaporn N, Boonard M, Piyapittayanun P, et al. Short-term outcomes of platelet-rich plasma injection for treatment of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2016;24:1665–1677. doi: 10.1007/s00167-015-3784-4. [DOI] [PubMed] [Google Scholar]
- 47.Tubach F, Ravaud P, Baron G, Falissard B, Logeart I, Bellamy N, et al. Evaluation of clinically relevant changes in patient reported outcomes in knee and hip osteoarthritis: the minimal clinically important improvement. Ann Rheum Dis. 2005;64:29–33. doi: 10.1136/ard.2004.022905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Escobar A, Quintana JM, Bilbao A, Aróstegui I, Lafuente I, Vidaurreta I. Responsiveness and clinically important differences for the WOMAC and SF-36 after total knee replacement. Osteoarthr Cartil. 2007;15:273–280. doi: 10.1016/j.joca.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 49.Angst F, Aeschlimann A, Michel BA, Stucki G. Minimal clinically important rehabilitation effects in patients with osteoarthritis of the lower extremities. J Rheumatol. 2002;29:131–138. [PubMed] [Google Scholar]
- 50.Leite VF, Daud Amadera JE, Buehler AM. Viscosupplementation for hip osteoarthritis: a systematic review and meta-analysis of the efficacy on pain and disability, and the occurrence of adverse events. Arch Phys Med Rehabil. 2018;99:574–583.e1. doi: 10.1016/j.apmr.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 51.Grassi A, Napoli F, Romandini I, Samuelsson K, Zaffagnini S, Candrian C, et al. Is platelet-rich plasma (PRP) effective in the treatment of acute muscle injuries? A systematic review and meta-analysis. Sports Med. 2018;39:1226–1219. doi: 10.1007/s40279-018-0860-1. [DOI] [PubMed] [Google Scholar]
- 52.Sheth U, Simunovic N, Klein G, Fu F, Einhorn TA, Schemitsch E, et al. Efficacy of autologous platelet-rich plasma use for orthopaedic indications: a meta-analysis. J Bone Joint Surg Am. 2012;94:298–307. doi: 10.2106/JBJS.K.00154. [DOI] [PubMed] [Google Scholar]
- 53.Moraes VY, Lenza M, Tamaoki MJ, Faloppa F, Belloti JC. Platelet-rich therapies for musculoskeletal soft tissue injuries. In: Moraes VY, editor. Cochrane Database Syst Rev. Chichester: John Wiley & Sons, Ltd; 2014. p. CD010071. [Google Scholar]
- 54.Turner L. US stem cell clinics, patient safety, and the FDA. Trends Mol Med. 2015;21:271–273. doi: 10.1016/j.molmed.2015.02.008. [DOI] [PubMed] [Google Scholar]