Abstract
Background: Recent studies have indicated that the default mode network (DMN) comprises at least three subsystems: The medial temporal lobe (MTL) and dorsal medial prefrontal cortex (DMPFC) subsystems and a core comprising the anterior MPFC (aMPFC) and posterior cingulate cortex (PCC). Additionally, the disruption of the DMN is related to Alzheimer’s disease (AD). However, little is known regarding the changes in these subsystems in AD, a progressive disease characterized by memory impairment. Here, we performed a resting-state functional connectivity (FC) analysis to test our hypothesis that the memory-related MTL subsystem was predominantly disrupted in AD.
Method: To reveal specific subsystem changes, we calculated the strength and number of FCS in the DMN intra- and inter-subsystems across individuals and compared the FC of the two groups. To further examine which pairs of brain regional functional connections contributed to the subsystem alterations, correlation coefficients between any two brain regions in the DMN were compared across groups. Additionally, to identify which regions made the strongest contributions to the subsystem changes, we calculated the regional FC strength (FCS), which was compared across groups.
Results: For the intra-subsystem, decreased FC number and strength occurred in the MTL subsystem of AD patients but not in the DMPFC subsystem or core. For the inter-subsystems, the AD group showed decreased FCS and number between the MTL subsystem and PCC and a decreased number between the PCC and DMPFC subsystem. Decreased inter-regional FCS were found within the MTL subsystem in AD patients relative to controls: The posterior inferior parietal lobule (pIPL) showed decreased FC with the hippocampal formation (HF), parahippocampal cortex (PHC) and ventral MPFC (vMPFC). Decreased inter-regional FCS of the inter-subsystems were also found in AD patients: The HF and/or PHC showed decreased FC with dMPFC and TPJ, located in the DMPFC subsystem, and with PCC. AD patients also showed decreased FC between the PCC and TLC of the dMPFC subsystem. Furthermore, the HF and PHC in the MTL subsystem showed decreased regional FCS.
Conclusion: Decreased intrinsic FC was mainly associated with the MTL subsystem of the AD group, suggesting that the MTL subsystem is predominantly disrupted.
Keywords: resting-state fMRI, default network, subsystems, functional connectivity, Alzheimer’s disease
Introduction
Since its name was proposed more than a decade ago (Raichle et al., 2001), the default mode network (DMN) has been characterized by its high level of metabolic activity during passive states and low activity in externally directed conditions (Shulman et al., 1997; Gusnard and Raichle, 2001; Raichle et al., 2001). Additionally, the robust activity correlations within the DMN at rest and during task performance have been confirmed in many papers (Greicius et al., 2003; Fox et al., 2005; Buckner et al., 2008; Grigg and Grady, 2010; Spreng and Grady, 2010; Allen et al., 2011). Although the precise function of the DMN is still debated, most literature has revealed that the DMN shows increased activity not only during rest but also during tasks, as long as experimental conditions involve aspects of self-generated thought (Andrews-Hanna et al., 2014), such as autobiographical memory (Svoboda et al., 2006; Spreng and Grady, 2010), future projection (Addis et al., 2007), mind wandering (Christoff et al., 2009; Smallwood et al., 2013), and social cognition (Mar, 2010). These features of self-generated thought suggest that they comprise multiple-component processes (Andrews-Hanna et al., 2014), which serve to prepare for upcoming events (Baird et al., 2011), form a sense of self-identity and continuity across time (Smallwood et al., 2011; Prebble et al., 2013), and navigate the social world (Immordino-Yang et al., 2012; Mar et al., 2012; Ruby et al., 2013). Recently, converging evidence has revealed that the DMN has a parallel level of complexity in functional-anatomical organization to support multiple-component processes of self-generated thought (Buckner et al., 2008; Sestieri et al., 2011; Andrews-Hanna, 2012; Kim, 2012; Roy et al., 2014). Andrews-Hanna et al. (2010b) explored the intrinsic functional organization of the DMN by using functional connectivity magnetic resonance imaging (fcMRI), graph analysis and hierarchical clustering techniques. The authors suggested that the DMN comprised two distinct subsystems: a dorsal medial prefrontal cortex (DMPFC) subsystem consisting of the dMPFC, the temporal parietal junction (TPJ), the lateral temporal cortex (LTC), and the temporal pole (TempP), and an MTL subsystem comprising the ventral medial prefrontal cortex (vMPFC), the posterior inferior parietal lobule (pIPL), the retrosplenial cortex (Rsp), the hippocampal formation (HF), and the parahippocampal cortex (PHC). Both subsystems converge on a core that includes the anterior MPFC (aMPFC) and the posterior cingulate cortex (PCC). The authors then proved that the two subsystems had relatively distinct functions by designing an experiment. The DMPFC subsystem was preferentially activated during decisions about one’s present situation or mental state, and the MTL subsystem was selectively activated during self-relevant predictions about one’s future.
Previous work has consistently shown that compared with healthy subjects, patients with Alzheimer’s disease (AD) have reduced FC within the DMN when at rest (Greicius et al., 2004; Rombouts et al., 2005; Celone et al., 2006; Wang K. et al., 2006) and less pronounced deactivation during cognitive tasks (Lustig et al., 2003; Celone et al., 2006). In addition, several studies of cortical hubs that performed functional and structural connectivity analysis combined with graph theoretical analysis revealed that most of the hubs are located within the DMN, such as the PCC, the medial prefrontal cortex (MPFC), and the HF (Hagmann et al., 2008; Buckner et al., 2009; Yu et al., 2017). It has been suggested that these cortical hubs in the DMN are preferentially affected in AD (Yu et al., 2017). The abovementioned studies have suggested that DMN impairment is related to AD. Moreover, although several studies have found that reductions in FC only exist in some regions, such as the PCC and the medial temporal lobe (MTL), in patients with AD (Zhou et al., 2008; Bai et al., 2009), other studies have reported widespread reductions in FC as the disease progresses (Damoiseaux et al., 2012). AD is a progressive dementia, and the predominant symptom is memory impairment, although disturbances in other cognitive functions also occur (Krajcovicova et al., 2014). Based on the above findings, distinct subsystems in the DMN engage in differential cognitive processes involving memory construction and self-oriented cognition, and these cognitive processes are influenced by AD (Greicius et al., 2004; Buckner et al., 2008, 2009; Zhou et al., 2010; Menon, 2011). Therefore, we hypothesized that the memory-related MTL subsystem was predominantly disrupted in AD and that other subsystems in the DMN may be disrupted to a certain degree. To test these hypotheses, rs-fMRI was performed on 24 patients with mild to moderate AD and on 27 healthy elderly subjects. Eleven regions of interest (ROIs) in the DMN subsystem for FC analysis were selected based on published data by Andrews-Hanna et al. (2010b). The DMN FC matrices were constructed by estimating the Pearson correlation between the time series of pairs of ROIs in the subsystems. To reveal the specific disrupted subsystem, we analyzed the strength and number of FCS in the intra- and inter-subsystems of the DMN. Then, to examine which pairs of regional FCS contributed to changes in the subsystems, each pair of correlation coefficients in the DMN was compared across groups.
In the present study, a regional FCS analysis was also used to identify regions that made the strongest contributions to the changes in the subsystems. Regional FCS is defined as the mean of the correlation strength of one ROI with all other target regions in a brain network and is referred to as the “degree centrality” of a weighted network in graph theory (Buckner et al., 2009; Zuo et al., 2012; Wang L. et al., 2013). Brain ROIs with higher FCS values usually indicate their central roles in the functional integrity of the brain network (Wang L. et al., 2013).
Materials and Methods
Participants
A total of 55 subjects were enrolled from Shanghai Huashan Hospital. All participants were categorized into a control group (n = 27) and an AD patient group (n = 28). AD patients were diagnosed by a qualified neurologist using criteria for amnestic AD (Fennema-Notestine et al., 2009), with mini-mental state examination (MMSE) scores between 12 and 27 (inclusive) and clinical dementia rating (CDR) scores of 1 or 2. The control groups had MMSE scores between 26 and 30 (inclusive) and CDR scores of 0. The data of 4 subjects (4 patients with AD) were excluded due to excessive motion, severe brain atrophy, hydrocephalus or large areas of cerebral infarction. Details regarding the clinical and demographic data of the remaining 51 subjects are shown in Table 1. There were no significant differences in terms of sex or age between the two groups.
Table 1.
Demographics and clinical information.
| Characteristics | HC (n = 27) | AD (n = 24) | P |
|---|---|---|---|
| Age | 63.74 ± 7.80 | 67.54 ± 10.48 | 0.146a |
| Female/Male | 11/16 | 13/11 | 0.406b |
| MMSE | 28.84 ± 1.19 | 21.46 ± 1.67 | <0.001a |
Data are presented as the mean ± standard deviation (SD). aThe P-value was obtained using a two-sample t-test. bThe P-value was obtained using the Pearson chi-square test.
Image Acquisition and Preprocessing
All subjects underwent whole-brain MRI scanning on a 3.0T SIEMENS Verio. Resting-state BOLD functional MRI data were collected using an echo-planar imaging (EPI) sequence. The scanning parameters were TR = 2000 ms, TE = 35 ms, FOV = 25.6 cm × 25.6 cm, flip angle = 90°, matrix = 256 × 256, slices = 33, thickness = 4 mm, and gap = 4 mm.
Unless specifically stated otherwise, all of the preprocessing was performed using statistical parametric mapping (SPM81). The first 5 images were discarded in consideration of magnetization equilibrium. The remaining 155 images were corrected for the acquisition time delay among different slices, and then, the images were realigned to the first volume for head-motion correction. The fMRI images were further spatially normalized to the Montreal Neurological Institute (MNI) EPI template and resampled to a 2-mm cubic voxel. Several sources of spurious variance, including the estimated motion parameters, the linear drift, and the average time series in the cerebrospinal fluid and white matter regions, were removed from the data through linear regression. Finally, temporal bandpass filtering (0.03–0.06 HZ) was performed to reduce the effects of low-frequency drift and high-frequency noise (Liu et al., 2008; Zhang et al., 2012). The time course of head motion was obtained by estimating the translations in each direction and the rotations in angular motion about each axis for each of the 155 consecutive volumes. All of the subjects included in this study exhibited a maximum displacement of less than 3 mm (smaller than the size of a voxel in plane) at each axis and an angular motion of less than 3 for each axis. Data from two subjects were excluded due to excessive motion.
Functional Connectivity Analysis
fcMRI was performed using REST2 (Song et al., 2011) to extract the time series for each ROI (spherical radius of 4 mm) (Table 2) by averaging the time courses of all voxels within an ROI. For each subject, we first calculated a Pearson’s correlation and the significance level (i.e., p-value) between all given ROIs. Then, we obtained an 11 × 11 symmetric correlation matrix and the corresponding p-value matrix for each subject. To eliminate unreliable correlations, we retained only those correlations whose corresponding p-values met a statistical threshold p < 0.001 (0.05/55, Bonferroni correction); otherwise, we set the correlations to zero (Cruse et al., 2011; Wang et al., 2016). Then, correlation coefficients in a weighted 11 × 11 FC matrix were converted into z-values with the application of Fisher’s r-to-z transformation. Fisher’s Z-transformed correlation matrix was used for the subsequent analysis of each subject. The strength of the intra-subsystem FCS was defined as the total and mean of all positive-only inter-regional FCS within the selected subsystem, while the strength of the inter-subsystem FCS was defined as the total and mean of the positive-only FCS between any two ROIs of the two selected subsystems. In this paper, we calculated the strength of the FCS of the intra- and inter-subsystems of each subject and compared them across groups using two-sample t-tests. We also analyzed the differences in the numbers of positive-only FCS of the intra- and inter-subsystems between two groups using two-sample t-tests. Notably, to explore the specific changes in FC between the core regions and subsystems, we calculated the number and strength of FCS between the two core regions and subsystems separately. To examine which pairs of brain regional FCS contributed to the alterations in the subsystems of the DMN in AD, the correlation coefficients between any two ROIs in the DMN were also compared across groups using two-sample t-tests with false discovery rate (FDR) correction. We also listed the results with p-values less than 0.05, 0.01, and 0.001 to find possible differences in physiological significance. Furthermore, to identify which regions made the highest contributions to the changes in the subsystems, we compared the regional FCS across groups by using two-sample t-tests. The FCS of a given region was calculated using the following equation (Buckner et al., 2009; Zuo et al., 2012; Wang L. et al., 2013):
Table 2.
Regions of interest (ROIs) within the DMN subsystems for functional connectivity analysis from Andrews-Hanna et al. (2010b).
| Region | Abbreviation | x | y | z |
|---|---|---|---|---|
| Core region | ||||
| Anterior medial prefrontal cortex | aMPFC | −6 | 52 | −2 |
| Posterior cingulate cortex | PCC | −8 | −56 | 26 |
| DMPFC subsystem | ||||
| Dorsal medial prefrontal cortex | dMPFC | 0 | 52 | 26 |
| Temporal parietal junction | TPJ | −54 | −54 | 28 |
| Lateral temporal cortex | LTC | −60 | −24 | −18 |
| Temporal pole | TempP | −50 | 14 | −40 |
| MTL subsystem | ||||
| Ventral medial prefrontal cortex | vMPFC | 0 | 28 | −18 |
| Posterior inferior parietal lobule | pIPL | −44 | −74 | 32 |
| Retrosplenial cortex | Rsp | −14 | −52 | 8 |
| Parahippocampal cortex | PHC | −28 | −40 | −12 |
| Hippocampal formation | HF | −22 | −20 | −26 |
Coordinates are based on the Montreal Neurological Institute coordinate system.
where N is the number of ROIs in the DMN (here N = 11), rij zij is the Fisher Z-transformed correlation coefficient, rijis the correlation coefficient between ROI i and ROI j, and r0 is a correlation threshold that was used to eliminate weak correlations possibly derived from noise (here, r0 = 0). The difference in regional FCS between the two groups revealed regions that were disrupted in patients with AD.
The Effects of Different Analysis Strategies
In our study, an FC matrix included positive and negative connections. In the positive-only strategy, we analyzed FC matrices by using the positive-only inter-regional FCS. For example, we assumed that no inter-regional FC existed if the inter-regional FC was non-positive. Given the controversies in the treatment of negative correlations in rs-fMRI network studies (Fox et al., 2009; Wang et al., 2011; Dai et al., 2015), we also calculated the strengths and numbers of positive and negative FCS of the intra- and inter-subsystems for the two groups and performed an FCS analysis including both positive and negative (absolute values) connections to assess the stability of our findings.
Results
Group Differences in the Strengths and Numbers of FCS of the Subsystems and Core
To explore specific changes in the subsystems in AD patients, the total and mean FC strengths of the intra- and inter-subsystems of the DMN were calculated. Different analysis strategies consistently produced the same results. In this study, we show the results of the positive-only strategy.
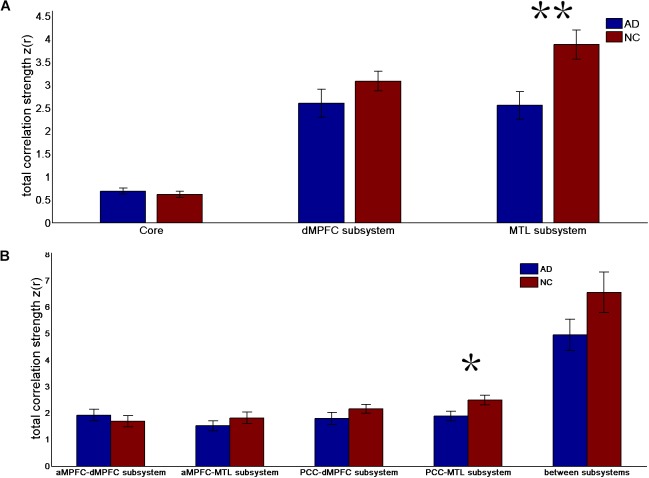
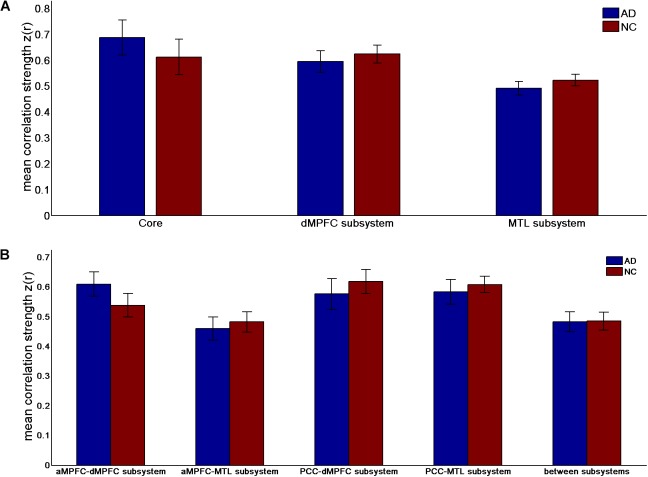
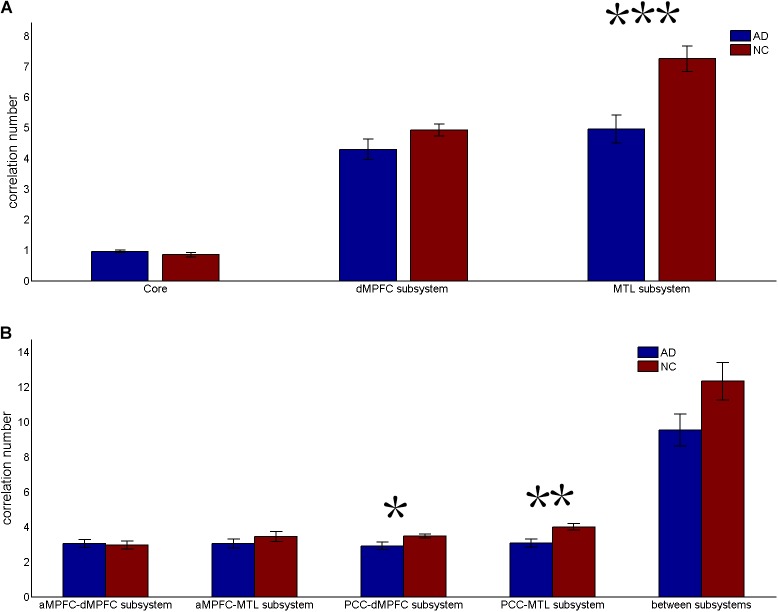
The results of the former method are as follows: Patients with AD showed decreased total FC strength (FCS) in the two subsystems (see Figure 1A) between core regions and subsystems and between subsystems relative to the controls (see Figure 1B). Two-sample t-tests were performed to statically analyze the differences between the AD group and the control group. Significant differences between the two groups were found in the MTL subsystem (t(49) = −3.00, p = 0.004) but not in the core (t(49) = 0.77, p = 0.45) or the DMPFC subsystem (t(49) = −1.31, p = 0.20). Significantly decreased total FCS was also found between the PCC and the MTL subsystem (t(49) = −2.41, p = 0.02) but not found between the subsystems (t(49) = −1.64, p = 0.11), between the PCC and the dMPFC subsystem (t(49) = −1.33, p = 0.19), between the amPFC and the dMPFC subsystem (t(49) = 0.77, p = 0.44), or between the amPFC and the MTL subsystem (t(49) = −1.01, p = 0.32). For total FCS in the core, stronger correlations were observed in the AD group, although not significant (see Figure 1A). However, there was no significant difference in the mean strength of the FC of the inter- and intra-subsystems (see Figures 2A,B) between the two groups. For the FC matrix of each subject, we also calculated the total number of intra- and inter-subsystem FCS (see Figure 3). The statistical analysis (two-sample t-tests) revealed that the AD group showed a significantly smaller total number of FCS in the MTL subsystem (t(49) = 3.74, p = 0.0005) (see Figure 3A). Moreover, a significantly reduced number of FCS was also found between the PCC and the MTL subsystem (t(49) = 3.12, p = 0.003) and between the PCC and the DMPFC subsystem (t(49) = 2.47, p = 0.02) (see Figure 3B) in AD patients relative to controls.
FIGURE 1.
Total correlation strength (positive-only) in the default network subsystems in the control and AD groups. (A) Total correlation strength within the core and subsystems of the two groups. (B) Total correlation strength between core regions (including the aMPFC and PCC) and subsystems separately and between the two subsystems in the two groups (∗p < 0.05, ∗∗p < 0.01). Error bars represent the standard error of the mean.
FIGURE 2.
Mean correlation strength (positive-only) in the default network subsystems in the control and AD groups. (A) Mean correlation strength within the core and subsystems in the two groups. (B) Mean correlation strength between core regions (including the aMPFC and PCC) and subsystems separately and between the two subsystems in the two groups. Error bars represent the standard error of the mean.
FIGURE 3.
Correlation number (positive-only) in the default network subsystems in the control and AD groups. (A) Correlation number within the core and subsystems in the two groups. (B) Correlation number between core regions (including the aMPFC and the PCC) and subsystems separately and between the two subsystems in the two groups (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001). Error bars represent the standard error of the mean.
Group Differences in the Inter-Regional FCS of the DMN
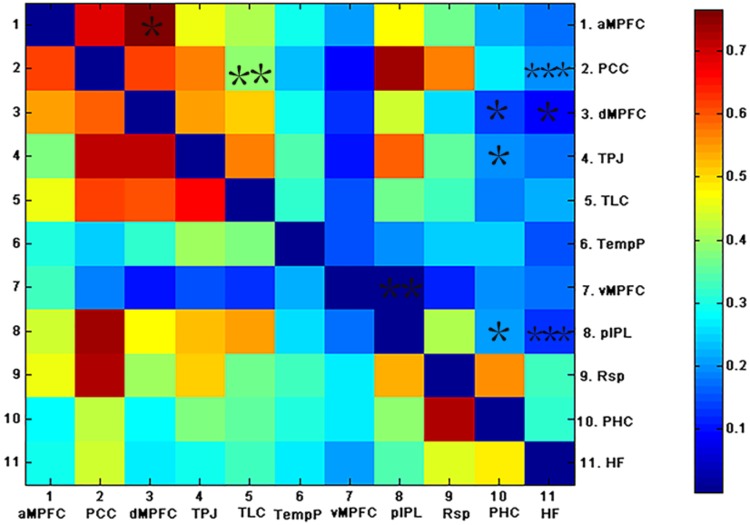
To examine which pairs of brain regional correlation coefficients contributed to the alteration in the subsystems of the DMN, correlation coefficients between pairs of ROIs in the DMN for each subject were compared across groups (see Figure 4). As shown in Figure 4, weaker correlations between ROIs in the DMN were observed in the AD group relative to the control group. Fewer inter-regional FCS were found within the MTL subsystem of AD patients than within that of controls: pIPL showed low FC with the hippocampus, the PHC and the vMPFC. In addition, fewer pairs of brain regional FCS of the inter-subsystems were found in AD patients. The hippocampus and the PHC showed decreased FC with the TPJ and the dMPFC, both of which were located in the DMPFC subsystem and the PCC. The PCC also showed decreased FC with the TLC, which exists in the dMPFC subsystem in AD. The functional connection between the hippocampus and the PCC is the only connection that was retained after FDR correction.
FIGURE 4.
The above matrices show the correlation coefficients between the ROIs in the DMN, as well as the group differences (∗p < 0.05, ∗∗p < 0.01, and ∗∗∗p < 0.001). The bottom and top triangles represent the group average correlation matrix of the control and AD groups, respectively. The color bar indicates the correlation coefficients between regions.
The Most Significantly Disrupted Regions Measured by FCS
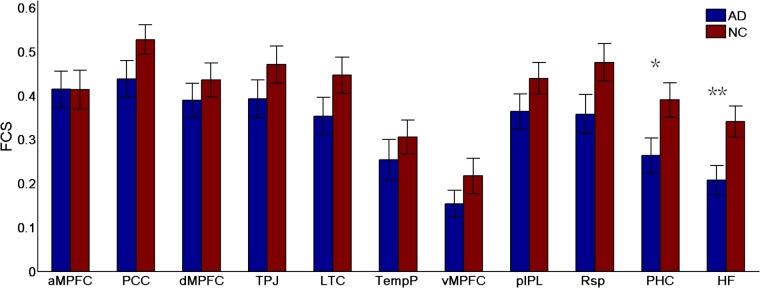
To identify which regions made the strongest contributions to the changes in subsystems of the DMN, the differences in the regional FCS between the two groups were measured (see Figure 5). Different analysis strategies consistently produced the same results. Here, we present the results of the positive-only strategy. Between-group comparison analysis (two-sample t-tests) revealed that regions showing the most significant group differences in FCS were the HF (t(49) = 2.76, p = 0.008) and the PHC (t(49) = 2.28, p = 0.03).
FIGURE 5.
Regional functional connectivity strength (FCS) in the control and AD groups (∗p < 0.05, ∗∗p < 0.01). Error bars represent the standard error of the mean.
Discussion
Our results suggest that the memory-related MTL subsystem is predominantly disrupted in AD. First, we explored the changes in FC of the intra- and inter-subsystems in patients with AD. The strength and number of FCS were significantly decreased, specifically in the MTL subsystems but not in the core or the DMPFC subsystem. Moreover, the strength and number of FCS were significantly decreased between the MTL subsystems and the PCC. We also examined which pairs of brain regional functional connections contributed to the changes in the subsystems and found that the HF and the PHC showed extensively decreased FCS with other regions in the DMN. Furthermore, we identified the HF and the PHC as making the strongest contributions to the alterations in the subsystems. Additionally, our data show that in addition to the changes in the MTL subsystem, the core and DMPFC subsystems in the default network also have some degree of disruption. A significantly decreased number of FCS between the core and the DMPFC subsystem was found in AD patients. Inter-regional FC analysis revealed that there are a few pairs of decreased brain regional FCS between the subsystems and between the core and the dMPFC in patients with AD.
Predominantly Disrupted Memory-Related Subsystems in Patients With AD
As consistently reported in the literature, the MTL subsystem is reliably activated when engaged in autobiographical memory and episodic future thought (Andrews-Hanna et al., 2014). These spontaneous thoughts may allow individuals to construct and simulate scenarios, mentally organize their plans, and prepare for what may lie ahead (Immordino-Yang et al., 2012). Additionally, spontaneous thoughts may facilitate consolidation of the most self-relevant information into long-term memory. In the present study, we found that the MTL subsystem is predominantly disrupted. Within the MTL subsystem, the PIPL showed decreased FC with the HF, the PHC and the vMPFC. This finding was supported by the research of Wang L. et al. (2006) and Wang Z. et al. (2013). The HF and the PHC located in the MTL subsystem were mostly disrupted in AD patients. Consistent with our findings, previous researchers have proposed that pathological amyloid depositions and atrophy in AD patients are located in cortical regions such as the HF and the PHC (Buckner et al., 2005, 2009; Stricker et al., 2012). fMRI studies have revealed that AD patients have reduced activity in regions of the DMN. Greicius et al. (2004) found decreased resting-state activity in the hippocampus of AD patients. The PHC and the HF possibly play significant roles in the MTL subsystem because the cognitive processes in which the MTL subsystem is involved might be supported by the MTL (including the PHC and the HF), which dynamically interacts with cortical regions within and outside the default network. Recent studies have supported the possibility of resting MTL activity or MTL-cortical FC related to individual differences in spontaneous episodic thoughts (Andrews-Hanna et al., 2010a), memory ability (Wig et al., 2008), and the consolidation of recent experiences (Tambini et al., 2010). Thus, the changes in the FC of the HF and the PHC in the DMN probably reflect cognitive impairment in recent episodic memory. Our findings support the hypothesis that the memory-related MTL subsystem of the DMN is primarily disrupted in AD.
In addition to the changes within the MTL subsystem itself, in the present study, we found that the MTL subsystem also showed decreased number and strength of FCS with the PCC in patients with AD. A number of studies have also suggested that the PCC plays an essential role in spatial orientation, self-appraisal and internal monitoring as well as memory processing (Gusnard and Raichle, 2001; Greicius et al., 2003; Whishaw and Wallace, 2003; Ries et al., 2006). As the core of the DMN, the PCC, which exhibits strong functional coherence with both subsystems (Andrews-Hanna et al., 2014) in coordination with the MTL subsystems, may facilitate memory processing. As consistently reported in previous literature, in the early stage of AD, FC between the PCC and the hippocampus, the anterior cingulate cortex, the MPFC and the precuneus are disrupted (Greicius et al., 2004; Wang L. et al., 2006; Sorg et al., 2007). It is possible that impairment between the PCC and the MTL subsystem may damage memory processing. These findings indicate that AD predominantly disrupts the memory-related MTL subsystem.
More Extensive Disruption of Subsystems in Patients With AD
In addition to the predominantly decreased FC associated with the memory-related MTL subsystem, we also found more extensively reduced FC in the inter-subsystems. Compared with the control group, the number of FCS between the PCC and the DMPFC subsystem was significantly reduced in AD, and inter-regional FC revealed that a few pairs of brain regional FCS between the subsystems and between the core region and the dMPFC subsystem exhibited significant decreases in the AD group. In line with our findings, previous studies have found that dissociated FC occurs between the PCC and the inferior temporal cortex in AD patients (Zhang et al., 2010). AD patients also have decreased hippocampus connectivity with the TPJ (Greicius et al., 2004) and the MPFC (Grady, 2001). These results provide powerful evidence for the possibility of the deterioration of all subsystems of the DMN as AD progresses, and previous studies support this possibility. A resting-state FC study suggested that early in the disease, regions of the posterior DMN, including the PCC/Rsp, the IPL, and the LTC (Buckner et al., 2005), begin to disengage their connectivity; however, as the disease progresses, connectivity within all systems eventually deteriorates (Damoiseaux et al., 2012). Zhang et al. (2010) suggested that impairment in the PCC FC changes with AD progression. In contrast to the constructive functions of the MTL subsystem, the DMPFC subsystem may play an important role in examining the mental states of social agents (Andrews-Hanna, 2012), including the ability to reflect on, evaluate, or appraise social information. The PCC shares functional properties with both subsystems, possibly interacting with the DMPFC to facilitate examination of the mental state. The DMN subsystems supporting component processes of self-generated thought work together but independently to complete internal information processing and ultimately guide and motivate behavior. For example, the DMPFC subsystem may allow individuals to reflect on the mental states elicited by the stimulus, and the MTL subsystem may allow individuals to integrate this introspective information into a goal-directed plan (Andrews-Hanna, 2012). Therefore, more extensive disruption of the subsystems of the DMN in patients with AD in the DMN may account for additional cognitive impairments other than memory deficits to some degree. Our findings support previous studies indicating that the DMN is an important target network to be impaired (Buckner et al., 2009) and specify that different subsystems of the default network are not equivalently damaged in AD but that memory-related MTL subsystems are selectively damaged. As the disease progresses, other subsystems of the DMN may also have a certain degree of damage.
Limitations
Several limitations in the present work should be considered. First, our study had a small sample size. Second, our research focused only on subjects with mild to moderate AD, which did not allow us to determine the specific changes in the DMN subsystems during different stages of the disease. Third, we did not perform related behavioral tests. Specific self-referential cognitive ability changes in AD patients may be related to the corresponding FC in the DMN subsystems. Four, our processing of the functional matrix of each subject may remove some of the weaker connections that are actually present. Considering these limitations, future work should involve a longitudinal study (i.e., from mild cognitive impairment (MCI) to mild, moderate, and serious AD) and include more participants in each group to enhance stability of the results. In addition, we should explore the relationship between the changes in the DMN subsystems in the functional and structural network and specific self-referential cognitive ability. Furthermore, we will explore more reasonable ways to implement our research.
Conclusion
In summary, this study shows that the memory-related MTL subsystem of the DMN is predominantly disrupted in patients with AD and further that the core and DMPFC subsystems in the DMN are also disrupted to some degree. These findings indicate the different levels of disruption of distinct subsystems of the DMN, possibly providing novel insight into the potential pathogenesis of AD and a possible imaging biomarker for the early diagnosis of AD.
Ethics Statement
This study was carried out in accordance with the recommendations of “GCP and SFDA, Ethics Review Committee of Huashan Hospital affiliated to Fudan University” with written informed consent from all subjects. All subjects gave written informed consent in accordance with the Declaration of Helsinki. The protocol was approved by the “Ethics Review Committee of Huashan Hospital affiliated to Fudan University.”
Author Contributions
HQ and XZ designed the study. HQ analyzed and interpreted the data and drafted the manuscript. HQ and HL revised the manuscript and interpreted the data. XZ, HMH, and HJH acquired the data. All the authors read and approved the final manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We wish to thank several people for providing helpful discussion or data analysis, namely, Ye Xie, Zixuan Wang, Ru Wang, and Jiali Liang. This manuscript was edited for English language by American Journal Experts (AJE). We also thank AJE for the English language editing.
Funding. This work was partially supported by the National Natural Science Foundation of China (Grant Nos. 30970818, 81271552) and the Science and Technology Commission of Shanghai Municipality (Grant No. 124119a5000), and the Health Industry Clinical Research of Shanghai Health and Family Planning Committee (Grant No. 201840018).
References
- Addis D. R., Wong A. T., Schacter D. L. (2007). Remembering the past and imagining the future: common and distinct neural substrates during event construction and elaboration. Neuropsychologia 45 1363–1377. 10.1016/j.neuropsychologia.2006.10.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E. A., Erhardt E. B., Damaraju E., Gruner W., Segall J. M., Silva R. F., et al. (2011). A baseline for the multivariate comparison of resting-state networks. Front. Syst. Neurosci. 5:2 10.3389/fnsys.2011.00002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R. (2012). The brain’s default network and its adaptive role in internal mentation. Neuroscientist 18 251–270. 10.1177/1073858411403316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Reidler J. S., Huang C., Buckner R. L. (2010a). Evidence for the default network’s role in spontaneous cognition. J. Neurophysiol. 104 322–335. 10.1152/jn.00830.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Reidler J. S., Sepulcre J., Poulin R., Buckner R. L. (2010b). Functional-anatomic fractionation of the brain’s default network. Neuron 65 550–562. 10.1016/j.neuron.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Hanna J. R., Smallwood J., Spreng R. N. (2014). The default network and self-generated thought: component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 1316 29–52. 10.1111/nyas.12360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai F., Watson D. R., Yu H., Shi Y., Yuan Y., Zhang Z. (2009). Abnormal resting-state functional connectivity of posterior cingulate cortex in amnestic type mild cognitive impairment. Brain Res. 1302 167–174. 10.1016/j.brainres.2009.09.028 [DOI] [PubMed] [Google Scholar]
- Baird B., Smallwood J., Schooler J. W. (2011). Back to the future: autobiographical planning and the functionality of mind-wandering. Conscious. Cogn. 20 1604–1611. 10.1016/j.concog.2011.08.007 [DOI] [PubMed] [Google Scholar]
- Buckner R. L., Andrews-Hanna J. R., Schacter D. L. (2008). The brain’s default network: anatomy, function and relevance to disease. Ann. N. Y. Acad. Sci. 1124 1–38. 10.1196/annals.1440.011 [DOI] [PubMed] [Google Scholar]
- Buckner R. L., Sepulcre J., Talukdar T., Krienen F. M., Liu H., Hedden T., et al. (2009). Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. 29 1860–1873. 10.1523/JNEUROSCI.5062-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner R. L., Snyder A. Z., Shannon B. J., LaRossa G., Sachs R., Fotenos A. F., et al. (2005). Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J. Neurosci. 25 7709–7717. 10.1523/JNEUROSCI.2177-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celone K. A., Calhoun V. D., Dickerson B. C., Atri A., Chua E. F., Miller S. L., et al. (2006). Alterations in memory networks in mild cognitive impairment and Alzheimer’s disease: an independent component analysis. J. Neurosci. 26 10222–10231. 10.1523/JNEUROSCI.2250-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K., Gordon A. M., Smallwood J., Smith R., Schooler J. W. (2009). Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proc. Natl. Acad. Sci. U.S.A. 106 8719–8724. 10.1073/pnas.0900234106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruse D., Chennu S., Chatelle C., Bekinschtein T. A., Fernández-Espejo D., Pickard J. D., et al. (2011). Bedside detection of awareness in the vegetative state: a cohort study. Lancet 378 2088–2094. 10.1016/S0140-6736(11)61224-5 [DOI] [PubMed] [Google Scholar]
- Dai Z., Yan C., Li K., Wang Z., Wang J., Cao M., et al. (2015). Identifying and mapping connectivity patterns of brain network hubs in Alzheimer’s disease. Cereb. Cortex 25 3723–3742. 10.1093/cercor/bhu246 [DOI] [PubMed] [Google Scholar]
- Damoiseaux J. S., Prater K. E., Miller B. L., Greicius M. D. (2012). Functional connectivity tracks clinical deterioration in Alzheimer’s disease. Neurobiol. Aging 33 828.e19–828.e30. 10.1016/j.neurobiolaging.2011.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennema-Notestine C., Hagler D. J., McEvoy L. K., Fleisher A. S., Wu E. H., Karow D. S., et al. (2009). Structural MRI biomarkers for preclinical and mild Alzheimer’s disease. Hum. Brain Mapp. 30 3238–3253. 10.1002/hbm.20744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D., Snyder A. Z., Vincent J. L., Corbetta M., Van Essen D. C., Raichle M. E. (2005). The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc. Natl. Acad. Sci. U.S.A. 102 9673–9678. 10.1073/pnas.0504136102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox M. D., Zhang D., Snyder A. Z., Raichle M. E. (2009). The global signal and observed anticorrelated resting state brain networks. J. Neurophysiol. 101 3270–3283. 10.1152/jn.90777.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady C. L. (2001). Altered brain functional connectivity and impaired short-term memory in Alzheimer’s disease. Brain 124 739–756. 10.1093/brain/124.4.739 [DOI] [PubMed] [Google Scholar]
- Greicius M. D., Krasnow B., Reiss A. L., Menon V. (2003). Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. U.S.A. 100 253–258. 10.1073/pnas.0135058100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius M. D., Srivastava G., Reiss A. L., Menon V. (2004). Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc. Natl. Acad. Sci. U.S.A. 101 4637–4642. 10.1073/pnas.0308627101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg O., Grady C. L. (2010). The default network and processing of personally relevant information: converging evidence from task-related modulations and functional connectivity. Neuropsychologia 48 3815–3823. 10.1016/j.neuropsychologia.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusnard D. A., Raichle M. E. (2001). Searching for a baseline: functional imaging and the resting human brain. Nat. Rev. Neurosci. 2 685–694. 10.1038/35094500 [DOI] [PubMed] [Google Scholar]
- Hagmann P., Cammoun L., Gigandet X., Meuli R., Honey C. J., Wedeen V. J., et al. (2008). Mapping the structural core of human cerebral cortex. PLoS Biol. 6:e159. 10.1371/journal.pbio.0060159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immordino-Yang M. H., Christodoulou J. A., Singh V. (2012). Rest is not idleness: implications of the brain’s default mode for human development and education. Perspect. Psychol. Sci. 7 352–364. 10.1177/1745691612447308 [DOI] [PubMed] [Google Scholar]
- Kim H. (2012). A dual-subsystem model of the brain’s default network: self-referential processing, memory retrieval processes, and autobiographical memory retrieval. Neuroimage 61 966–977. 10.1016/j.neuroimage.2012.03.025 [DOI] [PubMed] [Google Scholar]
- Krajcovicova L., Mikl M., Marecek R., Rektorova I. (2014). Disturbed default mode network connectivity patterns in Alzheimer’s disease associated with visual processing. J. Alzheimers Dis. 41 1229–1238. 10.3233/JAD-131208 [DOI] [PubMed] [Google Scholar]
- Liu Y., Liang M., Zhou Y., He Y., Hao Y., Song M., et al. (2008). Disrupted small-world networks in schizophrenia. Brain 131 945–961. 10.1093/brain/awn018 [DOI] [PubMed] [Google Scholar]
- Lustig C., Snyder A. Z., Bhakta M., O’Brien K. C., McAvoy M., Raichle M. E., et al. (2003). Functional deactivations: change with age and dementia of the Alzheimer type. Proc. Natl. Acad. Sci. U.S.A. 100 14504–14509. 10.1073/pnas.2235925100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mar R. A. (2010). The neural bases of social cognition and story comprehension. Annu. Rev. Psychol. 62 103–134. 10.1146/annurev-psych-120709-145406 [DOI] [PubMed] [Google Scholar]
- Mar R. A., Mason M. F., Litvack A. (2012). How daydreaming relates to life satisfaction, loneliness, and social support: the importance of gender and daydream content. Conscious. Cogn. 21 401–407. 10.1016/j.concog.2011.08.001 [DOI] [PubMed] [Google Scholar]
- Menon V. (2011). Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn. Sci. 15 483–506. 10.1016/j.tics.2011.08.003 [DOI] [PubMed] [Google Scholar]
- Prebble S. C., Addis D. R., Tippett L. J. (2013). Autobiographical memory and sense of self. Psychol. Bull. 139 815–840. 10.1037/a0030146 [DOI] [PubMed] [Google Scholar]
- Raichle M. E., MacLeod A. M., Snyder A. Z., Powers W. J., Gusnard D. A., Shulman G. L. (2001). A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 98 676–682. 10.1073/pnas.98.2.676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ries M. L., Schmitz T. W., Kawahara T. N., Torgerson B. M., Trivedi M. A., Johnson S. C. (2006). Task-dependent posterior cingulate activation in mild cognitive impairment. Neuroimage 29 485–492. 10.1016/j.neuroimage.2005.07.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rombouts S. A. R. B., Barkhof F., Goekoop R., Stam C. J., Scheltens P. (2005). Altered resting state networks in mild cognitive impairment and mild Alzheimer’s disease: an fMRI study. Hum. Brain Mapp. 26 231–239. 10.1002/hbm.20160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy S., Rubi L. D., Rafael M. (2014). Deconstructing the default: cortical subdivision of the default mode/intrinsic system during self-related processing. Hum. Brain Mapp. 35 1491–1502. 10.1002/hbm.22268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby F. J., Smallwood J., Sackur J., Singer T. (2013). Is self-generated thought a means of social problem solving? Front. Psychol. 4:962. 10.3389/fpsyg.2013.00962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sestieri C., Corbetta M., Romani G. L., Shulman G. L. (2011). Episodic memory retrieval, parietal cortex, and the default mode network: functional and topographic analyses. J. Neurosci. 31 4407–4420. 10.1523/JNEUROSCI.3335-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shulman G. L., Fiez J. A., Corbetta M., Buckner R. L., Miezin F. M., Raichle M. E., et al. (1997). Common blood flow changes across visual tasks: II. Decreases in cerebral cortex. J. Cogn. Neurosci. 9 648–663. 10.1162/jocn.1997.9.5.648 [DOI] [PubMed] [Google Scholar]
- Smallwood J., Schooler J. W., Turk D. J., Cunningham S. J., Burns P., Macrae C. N. (2011). Self-reflection and the temporal focus of the wandering mind. Conscious. Cogn. 20 1120–1126. 10.1016/j.concog.2010.12.017 [DOI] [PubMed] [Google Scholar]
- Smallwood J., Tipper C., Brown K., Baird B., Engen H., Michaels J. R., et al. (2013). Escaping the here and now: evidence for a role of the default mode network in perceptually decoupled thought. Neuroimage 69 120–125. 10.1016/j.neuroimage.2012.12.012 [DOI] [PubMed] [Google Scholar]
- Song X. W., Dong Z. Y., Long X. Y., Li S. F., Zuo X. N., Zhu C. Z., et al. (2011). REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One 6:e25031. 10.1371/journal.pone.0025031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg C., Riedl V., Muhlau M., Calhoun V. D., Eichele T., Laer L., et al. (2007). Selective changes of resting-state networks in individuals at risk for Alzheimer’s disease. Proc. Natl. Acad. Sci. U.S.A. 104 18760–18765. 10.1073/pnas.0708803104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spreng R. N., Grady C. L. (2010). Patterns of brain activity supporting autobiographical memory, prospection, and theory of mind, and their relationship to the default mode network. J. Cogn. Neurosci. 22 1112–1123. 10.1162/jocn.2009.21282 [DOI] [PubMed] [Google Scholar]
- Stricker N. H., Dodge H. H., Dowling N. M., Han S. D., Erosheva E. A., Jagust W. J. (2012). CSF biomarker associations with change in hippocampal volume and precuneus thickness: implications for the Alzheimer’s pathological cascade. Brain Imaging Behav. 6 599–609. 10.1007/s11682-012-9171-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svoboda E., McKinnon M. C., Levine B. (2006). The functional neuroanatomy of autobiographical memory: a meta-analysis. Neuropsychologia 44 2189–2208. 10.1016/j.neuropsychologia.2006.05.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tambini A., Ketz N., Davachi L. (2010). Enhanced brain correlations during rest are related to memory for recent experiences. Neuron 65 280–290. 10.1016/j.neuron.2010.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Lu M., Fan Y., Wen X., Zhang R., Wang B., et al. (2016). Exploring brain functional plasticity in world class gymnasts: a network analysis. Brain Struct. Funct. 221 3503–3519. 10.1007/s00429-015-1116-6 [DOI] [PubMed] [Google Scholar]
- Wang J.-H., Zuo X.-N., Gohel S., Milham M. P., Biswal B. B., He Y. (2011). Graph theoretical analysis of functional brain networks: test-retest evaluation on short- and long-term resting-state functional MRI data. PLoS One 6:e21976. 10.1371/journal.pone.0021976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K., Liang M., Wang L., Tian L., Zhang X., Li K., et al. (2006). Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum. Brain Mapp. 28 967–978. 10.1002/hbm.20324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Zang Y., He Y., Liang M., Zhang X., Tian L., et al. (2006). Changes in hippocampal connectivity in the early stages of Alzheimer’s disease: evidence from resting state fMRI. Neuroimage 31 496–504. 10.1016/j.neuroimage.2005.12.033 [DOI] [PubMed] [Google Scholar]
- Wang L., Dai Z., Peng H., Tan L., Ding Y., He Z., et al. (2013). Overlapping and segregated resting-state functional connectivity in patients with major depressive disorder with and without childhood neglect. Hum. Brain Mapp. 35 1154–1166. 10.1002/hbm.22241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Xia M., Dai Z., Liang X., Song H., He Y., et al. (2013). Differentially disrupted functional connectivity of the subregions of the inferior parietal lobule in Alzheimer’s disease. Brain Struct. Funct. 220 745–762. 10.1007/s00429-013-0681-9 [DOI] [PubMed] [Google Scholar]
- Whishaw I. Q., Wallace D. G. (2003). On the origins of autobiographical memory. Behav. Brain Res. 138 113–119. 10.1016/S0166-4328(02)00236-X [DOI] [PubMed] [Google Scholar]
- Wig G. S., Grafton S. T., Demos K. E., Wolford G. L., Petersen S. E., Kelley W. M. (2008). Medial temporal lobe bold activity at rest predicts individual differences in memory ability in healthy young adults. Proc. Natl. Acad. Sci. U.S.A. 105 18555–18560. 10.1073/pnas.0804546105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M., Engels M. M. A., Hillebrand A., van Straaten E. C. W., Gouw A. A., Teunissen C., et al. (2017). Selective impairment of hippocampus and posterior hub areas in Alzheimer’s disease: an MEG-based multiplex network study. Brain 140 1466–1485. 10.1093/brain/awx050 [DOI] [PubMed] [Google Scholar]
- Zhang H.-Y., Wang S.-J., Liu B., Ma Z.-L., Yang M., Zhang Z.-J., et al. (2010). Resting brain connectivity: changes during the progress of alzheimer disease. Radiology 256 598–606. 10.1148/radiol.10091701 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Liu Y., Jiang T., Zhou B., An N., Dai H., et al. (2012). Altered spontaneous activity in Alzheimer’s disease and mild cognitive impairment revealed by regional homogeneity. Neuroimage 59 1429–1440. 10.1016/j.neuroimage.2011.08.049 [DOI] [PubMed] [Google Scholar]
- Zhou J., Greicius M. D., Gennatas E. D., Growdon M. E., Jang J. Y., Rabinovici G. D., et al. (2010). Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 133 1352–1367. 10.1093/brain/awq075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y., Dougherty J. H., Hubner K. F., Bai B., Cannon R. L., Hutson R. K. (2008). Abnormal connectivity in the posterior cingulate and hippocampus in early Alzheimer’s disease and mild cognitive impairment. Alzheimers Dement. 4 265–270. 10.1016/j.jalz.2008.04.006 [DOI] [PubMed] [Google Scholar]
- Zuo X.-N., Ehmke R., Mennes M., Imperati D., Castellanos F. X., Sporns O., et al. (2012). Network centrality in the human functional connectome. Cereb. Cortex 22 1862–1875. 10.1093/cercor/bhr269 [DOI] [PubMed] [Google Scholar]