Abstract
[Purpose] The current study aimed to investigate the reliability of infrared thermography as a method of determining foot skin temperature, and to determine the relationship between foot skin temperature and blood flow in type 2 diabetes mellitus (DM) patients. [Participants and Methods] Eighty-five patients were recruited and their foot skin temperature and the ankle brachial index (ABI) were measured using infrared thermography and an automated oscillometry, respectively. A correlation between foot skin temperature and blood flow was performed. The patients were screened and classified according to two groups; diabetic peripheral neuropathy (DPN) and non-DPN. Discriminant validity was determined by comparing the foot skin temperature between the two groups. [Results] The test-retest reliability of foot skin temperature was high. A positive correlation was found between foot skin temperature and ABI in both feet. The foot skin temperatures in the DPN group were found to be significant lower when compared with those in the non-DPN group. [Conclusion] Foot skin temperature is an indirect method of evaluating blood flow in the feet of diabetic patients and can be used as a clinical outcome measurement of treatments used to improve blood flow in type 2 DM patients.
Key words: Infrared thermography, Ankle brachial index, Diabetic peripheral neuropathy
INTRODUCTION
Diabetes mellitus (DM) is a chronic disease and is a worldwide health problem currently. Lower limb ischaemia due to endothelial dysfunction (vascular involvement) is a common complication in DM1). Impaired blood circulation leads to the loss of plantar cutaneous sensation, movement perception and body balance in DM patients2). Thus, a simplified assessment of peripheral vascular condition in DM patients is required. The ankle brachial index (ABI) is a commonly used noninvasive method of accessing vascular disease in DM patients3). However, it is expensive and impractical. By contrast, measuring skin temperature in the feet of DM patients using infrared thermography is predictive of ulceration risk4, 5). It is also a suitable tool with which to indirectly assess blow flow6). However, to the best of our knowledge, research has not been conducted to evaluate the correlation between blow flow and skin temperature in the feet of DM patients. The infrared thermography is a simple, fast, and inexpensive of non-invasive and indirect method for measuring the foot-skin blood flow. Thus, the current study objectives were to determine the reliability of foot skin temperature in DM patients using infrared thermography, and to establish whether or not there is a relationship between foot skin temperature and blood flow in type 2 DM patients.
PARTICIPANTS AND METHODS
The cross-sectional study of all eligible DM patients were identified and recruited from the diabetic outpatient clinic at Sisaket Hospital, Sisaket Province, Thailand. The inclusion criteria were a diagnosis by an internal medicine doctor of type 2 DM and an age of 40–75 years. Exclusion criteria were foot complications, including ulcers, infection, amputation, fever, inflammation (i.e., a plantar fasciitis), deep vein thrombosis, uncontrolled hypertension, neurological weakness, numbness and lumbar spinal stenosis. This study was approved by the Ethics Committee of Khon Kaen University, Khon Kaen, Thailand, with the protocol number of HE 592015/2016. Each participant signed a consent form to participate prior to data collection.
A sample size was calculated with the lowest of the range of confidence interval (CI) for the Pearson’s correlation coefficient (r) of 0.30. Therefore, a sample size of 85 participants was estimated which based on a pilot study for 30 participants who had type 2 DM. One hundred and eight participants were approached and randomly selected by using simple random sampling. Eighty five of these met the inclusion criteria. The demographic characteristics of the participants were recorded after screening them for diabetic peripheral neuropathy (DPN) from medical doctor and using the Thai version of the Michigan Neuropathy Screening Instrument (MNSI)7). Accordingly, the participants were categorized into DPN (n=39, age=60.9 ± 7.4 yr; height=155.4 ± 8.1 cm; body mass=62.9 ± 11.1 kg; BMI=26.1 ± 4.3 kg/m2, mean ± SD) and non-DPN groups (n=46, age=58.3 ± 9.1 yr; height=158.4 ± 9.2 cm; body mass=63.5 ± 14.0 kg; BMI=25.2 ± 4.7 kg/m2; mean ± SD). The diagnostic criteria of DPN was used in the physical assessment section of the MNSI that the cut-off point was set for a score of ≥2.5 8). The attributes and complications of diabetic patients are shown in Table 1. The duration of diabetic patients was for longer than 5 years. About 3% of all diabetic patients reported ever having the foot ulcer for more than 6 months but no ulcer at the experiment period. Most demographic data were similar for the two groups, including habits of smoking and drinking alcohol. Patients with DPN were found to have higher blood sugar and haemoglobulin A1c levels.
Table 1. The clinical characteristics of non-diabetic peripheral neuropathy (Non DPN, n=46) and diabetic peripheral neuropathy (DPN, n=69) patients.
| Non-DPN | DPN | Total | ||
|---|---|---|---|---|
| Duration of DM (years) | 7.46 ± 5.49 | 13.15 ± 4.54* | 10.07 ± 5.08 | |
| Comorbidities , n (%) | None | 23 (50.00) | 8 (20.51) | 31 (36.47) |
| Hypertension | 19 (41.30) | 28 (71.79) | 47 (55.29) | |
| Heart disease | 1 (2.17) | 1 (2.56) | 2 (2.35) | |
| Renal disorder | 3 (6.52) | 2 (5.13) | 5 (5.88) | |
| Blood sugar (mg/dl) | 142.93 ± 30.55 | 191.62 ± 65.57* | 165.27 ± 55.14 | |
| Haemoglobulin A1c (mg/dl) | 6.98 ± 1.19 | 8.81 ± 1.65* | 7.82 ± 1.68 | |
| Cholesterol (mg/dl) | 170.30 ± 49.68 | 207.10 ± 74.18* | 187.19 ± 64.44 | |
| Ever having foot ulcer (%) | 2 (4.34) | 1 (2.56) | 3 (3.53) | |
| MNSI | Subjective aspect (13 scores) | 1.52 ± 1.39 | 3.05 ± 1.96* | 2.22 ± 1.83 |
| Objective aspect (10 scores) | 1.27 ± 0.75 | 4.06 ± 1.14* | 2.55 ± 1.69 | |
Data are shown as mean ± SD. DM: diabetes mellitus; MNSI: Michigan Neuropathy Screening Instrument.
Outcome measurements were including of plantar foot surface temperature and the ankle brachial index (ABI). Plantar foot surface temperatures were taken using infrared thermography (Ti10 Fluke Thermal Imaging Camera; Fluke Corporation, WA, USA). Picture-in-picture technology isolates the problem areas within a user-defined temperature range, and features 320 × 240 pixels with an infrared spectral band of 7.5–14.0 μm. The participants were placed in a supine position on the examining table with their feet placed on a pillow to keep their knees in a neutral position in a quiet room in a thermoneutral environment of 25 °C with an average humidity of 38%rh. Prior to taking the measurements, the feet of the participants were cleaned with a dry towel. Either foot was randomly selected for the first measurement. An infrared thermographic image was captured at a distance of 80 cm from the foot. The images were taken thrice of six specific areas of each foot within five minutes. The mean values of each area were used for further analysis.
Peripheral vascular condition was measured by the ankle brachial index (ABI) using an automated oscillometry (Omron VP-1000 Vascular Profiler; Omron Healthcare Co., Ltd., Kyoto, Japan). ABI is a noninvasive technique that is commonly used to detect the peripheral vascular diseases. The details of the methodology and reproducibility of the measurements have been described elsewhere9). The participants were placed in a supine position on the examining table. Four blood pressure cuffs with size 20–32 cm bladders were applied to their arms and ankles. A single ABI measurement was taken immediately after taking the skin temperature measurement in the feet in all participants, according to hospital policy. The ratio between systolic blood pressure in the brachial artery in each arm and that in the dorsalis pedis and posterior tibial artery in the ankle was calculated for further analysis.
The demographic data of participants are presented as mean ± standard deviation (SD) and number (percentage). The Shapiro-Wilks test was performed to determine the normality of the data. Reproducibility of measurement of the foot skin temperatures was evaluated using intra-class correlation coefficient (ICC). ICC (1,3) values of 3 successive measurements using model of a two way random effects analysis of variance with a 95% confidence interval (CI) was used to examine the test-retest reliability of the foot skin temperatures, determined by infrared thermography. To perform the construct validity, Pearson’s correlation coefficient (r) and 95% CI were used to explore a correlation between foot skin temperature and ABI. For the correlation analysis, the foot skin temperature obtained was regarded as the average for the entire foot area. The independent t-test and the effect size (Cohen’s d) were used to compare foot skin temperature in DPN and non-DPN. The level of significance was set at p<0.05.
RESULTS
The test-retest reliability of foot skin temperature using infrared thermography was high for all the six plantar foot surfaces measured in both feet (ICC ≥0.90, p<0.001). The ICC average foot skin temperature value obtained for the entire area was 1.00 (95% CI: 0.99–1.00, p<0.001) for the right foot and 0.97 (95% CI: 0.96–0.98, p<0.001) for the left foot.
The average foot skin temperature for the entire plantar surface for the right and left feet was 31.30 ± 2.00°C and 31.21 ± 2.19°C, respectively, with corresponding ABI values of 1.10 ± 0.14 and 1.09 ± 0.16. A statistically significant difference was not found between the right and left foot (p>0.05) with respect to foot skin temperature and ABI values. Individual ABI values were between 0.99 and 1.17 in both legs and the right foot, and were considered to be normal (ABI=1.00–1.40)10).
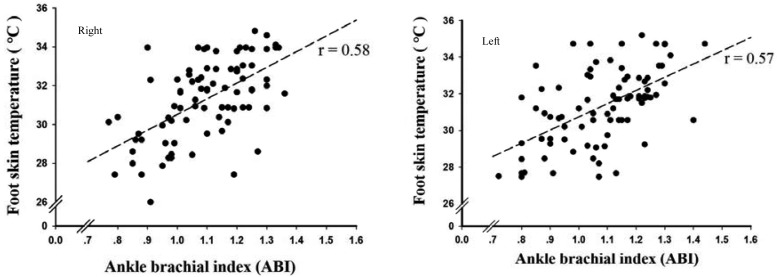
As shown in Fig. 1, a positive correlation was established between the average foot skin temperature for the entire foot and the ABI. A value of r=0.58 was obtained for the right foot (95% CI: 0.42–0.71, p<0.001) and that of r=0.57 for the left foot (95% CI: 0.41–0.71, p<0.001).
Fig. 1.
Correlation between plantar foot temperature and the Ankle Brachial Index in the left and right feet of patients (n=85) using Pearson’s correlation coefficient (r).
The foot skin temperatures and ABI values obtained for DM in the non-DPN and DPN groups are shown in Table 2. The foot skin temperature values obtained for the DPN were lower than those reported for the non-DPN (p<0.05). In addition, the ABI values showed for both feet in the DPN participants were lower than those obtained for both feet in the non-DPN group (p<0.05). Moreover, when presented according to Cohen’s d effect size, the foot skin temperatures and ABI values for the DPN were lower than those taken for the non-DPN (a large effect size of ≥0.80).
Table 2. A comparison of skin temperatures in the feet and the Ankle Brachial Index obtained in non-diabetic peripheral neuropathy (Non DPN, n=46) and diabetic peripheral neuropathy (DPN, n=39) patients .
| Measures | Non DPN | DPN | Differences | 95% CI | Effect size | ||
|---|---|---|---|---|---|---|---|
| ST (° C) | Hallux | R | 32.33 ± 1.79 | 30.12 ± 2.26 | 2.21* | 1.33–3.08 | 1.11 |
| L | 32.56 ± 1.77 | 29.38 ± 2.55 | 3.18* | 2.25–4.12 | 1.49 | ||
| Lesser toes | R | 31.93 ± 1.97 | 29.43 ± 2.57 | 2.50* | 1.52–3.48 | 1.12 | |
| L | 32.11 ± 1.78 | 28.92 ± 2.43 | 3.19* | 2.28–4.10 | 1.54 | ||
| MMH | R | 32.25 ± 1.51 | 30.53 ± 1.71 | 1.72* | 1.03–2.42 | 1.08 | |
| L | 32.54 ± 1.54 | 30.08 ± 1.93 | 2.46* | 1.71–3.21 | 1.44 | ||
| LMH | R | 31.95 ± 1.57 | 30.33 ± 1.77 | 1.62* | 0.90–2.34 | 0.98 | |
| L | 32.14 ± 1.62 | 29.73 ± 1.94 | 2.41* | 1.64–3.18 | 1.38 | ||
| Midfoot | R | 32.50 ± 1.27 | 31.06 ± 1.41 | 1.44* | 0.86–2.02 | 1.09 | |
| L | 32.75 ± 1.35 | 30.55 ± 1.63 | 2.20* | 1.56–2.84 | 1.50 | ||
| Rearfoot | R | 32.29 ± 1.38 | 30.65 ± 1.78 | 1.64* | 0.96–2.32 | 1.05 | |
| L | 32.49 ± 1.52 | 30.02 ± 1.84 | 2.47* | 1.74–3.19 | 1.49 | ||
| Ave | R | 32.21 ± 1.54 | 30.34 ± 1.85 | 1.87* | 1.15–2.60 | 1.12 | |
| L | 32.43 ± 1.55 | 29.78 ± 1.96 | 2.65* | 1.89–3.41 | 1.53 | ||
| ABI | R | 1.16 ± 0.11 | 1.02 ± 0.14 | 0.14** | 0.09–0.20 | 1.14 | |
| L | 1.17 ± 0.12 | 0.99 ± 0.15 | 0.18** | 0.12–0.24 | 1.35 | ||
CI: confidence interval; ST: skin temperature; ABI: ankle brachial index; MMH: medical metatarsal head; LMH: lateral metatarsal head; Ave: average of 6 foot areas; R: right foot; L: left foot, *p<0.05, **p<0.0001.
DISCUSSION
This study was the first to explore a correlation between foot skin temperature and blood flow in type 2 DM patients. Excellent reliability was demonstrated following the determination of foot skin temperatures measured by infrared thermography. The foot skin temperature measurements were also shown to positively correlate with blood flow evaluated using the ABI measured by an automated oscillometry. Foot skin temperatures were significantly lower in DM patients with DPN than in those with non-DPN, suggestive that measuring foot skin temperature is an indirect method that could be used to evaluate the risk of diabetic foot in DM patients.
The excellent reliability of foot skin temperatures obtained using infrared thermography in DM patients in the current study is in line with the findings of another study in this regard11). The range of foot skin temperatures in the present study was from 27–35°C, similar to that reported elsewhere4, 12). Foot skin temperatures were found to have a positive correlation with blood flow evaluated by ABI in the current study, in support of a similar association reported in other research between hand skin temperature and blood flow in healthy participamts6). Accordingly, skin temperatures taken in the feet of DM patients could reflect blood flow status in the lower limbs, probably due to a reduction in blood flow which leads to vasoconstriction of the peripheral blood vessels, causing a decrease in skin temperature.
In the current study, the DPN patients showed lower temperature about 1.5–3.0°C than the non-DPN with the large effect size (≥0.8) and considered to be the clinical significant with the value of the difference greater than 2.2°C13). Similar results were shown in another study in which a comparison was made of DPN and non-DPN patients13). The skin temperature of diabetic patients was also demonstrated to be lower than that of healthy participants in the previous study14). Enduring lower skin temperatures in DM patients are associated with vascular insufficiency15). Vasodilation in DPN patients is also usually impaired, leading to deficiencies in microcirculation. By contrast, the skin temperature of DPN patients was found to be higher than that of non-DPN patients in another study4). A chronic increase in skin temperature in the feet of diabetic patients with peripheral neuropathy may be due to an increase in arteriovenous shunt flow16), while an acute increase in foot skin temperature is a predisposing sign of pre-ulcer inflammation13, 17).
There were limitations to the current study. Only DM patients were selected and a comparison was not made of DM patients and healthy participants. However, the DM patients were classified into DPN and non-DPN groups, and significant differences were found between them, with a large effect size. In addition, confounding factors, such as the levels of diabetes foot problems and daily physical activity were not taken into consideration. Thus, further studies are warranted to confirm an association between foot skin temperature in the severely affected feet of diabetic patients or blood circulation impairment. Despite its limitations, the current study findings have advanced an understanding of the relationship between foot skin temperature and blood flow in type 2 DM patients.
In conclusion, foot skin temperature, measured by infrared thermography, was found to correlate positively with blood flow determined by the ABI measured by an automated oscillometry in type 2 DM patients. This suggests that foot skin temperatures measured by infrared thermography could be a useful method to use when evaluating the risk of diabetic foot in the clinical setting.
Funding
Research Center in BNOJPH, Faculty of AMS, Khon Kaen University.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgments
The staff and personnel at Sisaket Hospital, Sisaket Province, Thailand are gratefully thanked for their cooperation. Sincere gratitude and appreciation is extended to the patients for their generosity of spirit and willingness to participate in this study.
REFERENCES
- 1.Guirro EC, Guirro RR, Dibai-Filho AV, et al. : Immediate effects of electrical stimulation, diathermy, and physical exercise on lower limb arterial blood flow in diabetic women with peripheral arterial disease: a randomized crossover trial. J Manipulative Physiol Ther, 2015, 38: 195–202. [DOI] [PubMed] [Google Scholar]
- 2.Chatchawan U, Eungpinichpong W, Plandee P, et al. : Effects of Thai foot massage on balance performance in diabetic patients with peripheral neuropathy: a randomized parallel-controlled trial. Med Sci Monit Basic Res, 2015, 21: 68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Herráiz-Adillo Á, Martínez-Vizcaíno V, Cavero-Redondo I, et al. : Diagnostic accuracy study of an oscillometric ankle-brachial index in peripheral arterial disease: the influence of oscillometric errors and calcified legs. PLoS One, 2016, 11: e0167408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bagavathiappan S, Philip J, Jayakumar T, et al. : Correlation between plantar foot temperature and diabetic neuropathy: a case study by using an infrared thermal imaging technique. J Diabetes Sci Technol, 2010, 4: 1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Houghton VJ, Bower VM, Chant DC: Is an increase in skin temperature predictive of neuropathic foot ulceration in people with diabetes? A systematic review and meta-analysis. J Foot Ankle Res, 2013, 6: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nilsson AL: Blood flow, temperature, and heat loss of skin exposed to local radiative and convective cooling. J Invest Dermatol, 1987, 88: 586–593. [DOI] [PubMed] [Google Scholar]
- 7.Damri TC: Validity and reliability of the Michigan Neuropathy Screening Instrument (MNSI) on the Diabetic type II patients (Thai version). J Med Tech Phy Ther, 2015, 27: 307–319. [Google Scholar]
- 8.Herman WH, Pop-Busui R, Braffett BH, et al. DCCT/EDIC Research Group: Use of the Michigan Neuropathy Screening Instrument as a measure of distal symmetrical peripheral neuropathy in Type 1 diabetes: results from the Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications. Diabet Med, 2012, 29: 937–944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan CR, Staessen JA, Li Y, et al. : Comparison of three measures of the ankle-brachial blood pressure index in a general population. Hypertens Res, 2007, 30: 555–561. [DOI] [PubMed] [Google Scholar]
- 10.Wohlfahrt P, Palouš D, Ingrischová M, et al. : A high ankle-brachial index is associated with increased aortic pulse wave velocity: the Czech post-MONICA study. Eur J Cardiovasc Prev Rehabil, 2011, 18: 790–796. [DOI] [PubMed] [Google Scholar]
- 11.Rodrigues-Bigaton D, Dibai Filho AV, Costa AC, et al. : Accuracy and reliability of infrared thermography in the diagnosis of arthralgia in women with temporomandibular disorder. J Manipulative Physiol Ther, 2013, 36: 253–258. [DOI] [PubMed] [Google Scholar]
- 12.Boyko EJ, Ahroni JH, Stensel VL: Skin temperature in the neuropathic diabetic foot. J Diabetes Complications, 2001, 15: 260–264. [DOI] [PubMed] [Google Scholar]
- 13.Lavery LA, Higgins KR, Lanctot DR, et al. : Preventing diabetic foot ulcer recurrence in high-risk patients: use of temperature monitoring as a self-assessment tool. Diabetes Care, 2007, 30: 14–20. [DOI] [PubMed] [Google Scholar]
- 14.Sivanandam S, Anburajan M, Venkatraman B, et al. : Medical thermography: a diagnostic approach for type 2 diabetes based on non-contact infrared thermal imaging. Endocrine, 2012, 42: 343–351. [DOI] [PubMed] [Google Scholar]
- 15.Ring F: Thermal imaging today and its relevance to diabetes. J Diabetes Sci Technol, 2010, 4: 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagase T, Sanada H, Takehara K, et al. : Variations of plantar thermographic patterns in normal controls and non-ulcer diabetic patients: novel classification using angiosome concept. J Plast Reconstr Aesthet Surg, 2011, 64: 860–866. [DOI] [PubMed] [Google Scholar]
- 17.Armstrong DG, Holtz-Neiderer K, Wendel C, et al. : Skin temperature monitoring reduces the risk for diabetic foot ulceration in high-risk patients. Am J Med, 2007, 120: 1042–1046. [DOI] [PubMed] [Google Scholar]