Abstract
Aims
To investigate whether improved survival from non-ST-elevation myocardial infarction (NSTEMI), according to GRACE risk score, was associated with guideline-indicated treatments and diagnostics, and persisted after hospital discharge.
Methods and results
National cohort study (n = 389 507 patients, n = 232 hospitals, MINAP registry), 2003–2013. The primary outcome was adjusted all-cause survival estimated using flexible parametric survival modelling with time-varying covariates. Optimal care was defined as the receipt of all eligible treatments and was inversely related to risk status (defined by the GRACE risk score): 25.6% in low, 18.6% in intermediate, and 11.5% in high-risk NSTEMI. At 30 days, the use of optimal care was associated with improved survival among high [adjusted hazard ratio (aHR) −0.66 95% confidence interval (CI) 0.53–0.86, difference in absolute mortality rate (AMR) per 100 patients (AMR/100–0.19 95% CI −0.29 to −0.08)], and intermediate (aHR = 0.74, 95% CI 0.62–0.92; AMR/100 = −0.15, 95% CI −0.23 to −0.08) risk NSTEMI. At the end of follow-up (8.4 years, median 2.3 years), the significant association between the use of all eligible guideline-indicated treatments and improved survival remained only for high-risk NSTEMI (aHR = 0.66, 95% CI 0.50–0.96; AMR/100 = −0.03, 95% CI −0.06 to −0.01). For low-risk NSTEMI, there was no association between the use of optimal care and improved survival at 30 days (aHR = 0.92, 95% CI 0.69–1.38) and at 8.4 years (aHR = 0.71, 95% CI 0.39–3.74).
Conclusion
Optimal use of guideline-indicated care for NSTEMI was associated with greater survival gains with increasing GRACE risk, but its use decreased with increasing GRACE risk.
Keywords: Non-ST-elevation myocardial infarction , Quality of care , Mortality , GRACE risk score
Introduction
For patients with non-ST-elevation myocardial infarction (NSTEMI), evidence from international studies suggests that guideline-indicated care treatment and diagnostics are associated with improved clinical outcomes.1–4 Evidence from randomized controlled trials, suggest that the absolute effect is greater for NSTEMI at high ischaemic risk where there is reduced mortality, and lower rates of unscheduled revascularization, stroke, and hospitalization for heart failure.5–8 It is unknown, however, if beyond the setting of trials the effects of such interventions for NSTEMI (including pharmacotherapies as well as an invasive coronary strategy) are evident and, if so, whether such effects persist after discharge from hospital.
The Myocardial Ischaemia National Audit Project (MINAP) represents all acute hospitals in the single healthcare system of England and Wales and prospectively collects information about treatments provided, case mix and mortality of patients hospitalized with acute coronary syndrome over 15 years.9,10 Thus, MINAP is an optimal research conduit for understanding the impact of evidence-based NSTEMI care on clinical outcomes. We accessed anonymized patient data from MINAP to investigate whether improved survival associated with the use of NSTEMI guideline-indicated treatments was evident across the spectrum of NSTEMI risk, and whether mortality benefits were maintained over the long-term following discharge from hospital.
Methods
Data and subjects
The study was conducted using the MINAP. Data were entered electronically at each hospital where they were encrypted prior to secure transfer to a central database, anonymized and then distributed upon application for research. Each year MINAP data are validated at participating hospitals.9
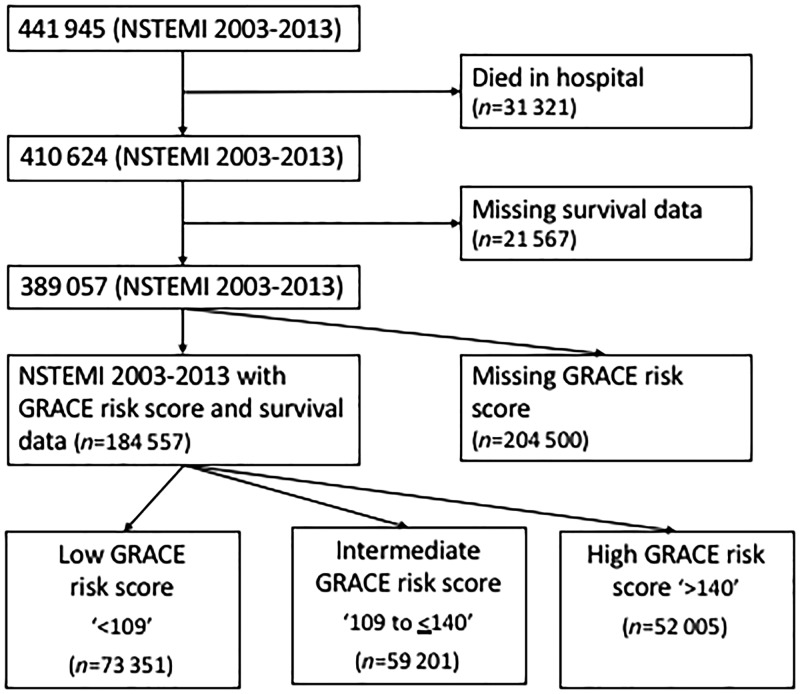
The study population included all patients (1 January 2003 to 30 June 2013) with a discharge diagnosis of NSTEMI. This diagnosis was determined by the treating team and based upon clinical presentation and investigations, including biomarkers, in keeping with the universal definition of myocardial infarction.11 Patients who died in hospital (n = 31 321) and for whom, there were no survival data (n = 21 567) were excluded from the cohort in keeping with previous work (Figure 1).3
Figure 1.
Consort diagram of exclusions of the Myocardial Ischaemia National Audit Project (MINAP) dataset.
Baseline clinical risk was determined according to the adjusted mini-GRACE risk score, which has been validated using MINAP data and endorsed by the National Institute for Health and Care Excellence (NICE).12,13 The variables included age, cardiac arrest, electrocardiographic ST-segment deviation, elevated cardiac enzymes, systolic blood pressure and heart rate at the time of hospitalization, use of a loop diuretic (substituted for Killip Class), and creatinine. In line with the American Heart Association/American College of Cardiology and European Society of Cardiology NSTEACS guidelines,14,15 we categorized patients according to their risk of in-hospital mortality using the calculated GRACE risk score as low (<109; predicted mortality <1.0%), intermediate (≥109 to ≤140; predicted mortality ≥1.0% to ≤3.0%), and high (>140; predicted mortality >3.0%).
Receipt of guideline indicated care was measured according to a composite optimal care variable. This comprised 13 care interventions, previously mapped to MINAP data by the authors,2 which were identified following review of international guidelines.16–20 The 13 interventions included receipt, if eligible, of an electrocardiogram pre- or in-hospital, pre-hospital receipt of aspirin, echocardiography, an aldosterone antagonist during admission, coronary angiography, aspirin on discharge, P2Y12 inhibition on discharge, ACE inhibitors (ACEi)/angiotensin receptor blockers (ARBs) on discharge, β-blocker on discharge, HMG Co-A reductase inhibitor (statin) on discharge, referral for cardiac rehabilitation, smoking cessation advice, and dietary advice (see Supplementary material online, Section S1).2,3 Patients were classified as ineligible if a treatment was listed as contraindicated, not indicated, not applicable, if the patient declined treatment as recorded in MINAP or if the patient was hospitalized prior to the publication year of treatment recommendation in the guidelines. If patients were deemed eligible, but there was no data regarding receipt, they were assumed to have not received that intervention. Optimal care was defined at the individual patient level, if they received all of the care opportunities for which an individual patient was eligible; thus, patients missing one or more eligible care opportunities were assigned to the suboptimal care group. Patients who were listed as having a contraindication for one care intervention were not eligible for that care intervention and only the care interventions for which a patient was eligible for were considered in the calculation of the optimal care variable.
We also studied the impact of optimal care compared with suboptimal care for components of the care pathway separately, including pharmacological therapies [pre-hospital receipt of aspirin, aldosterone antagonist during admission, aspirin on discharge, P2Y12 inhibition on discharge, ACEi/ARBs on discharge, β-blocker on discharge and HMG Co-A reductase inhibitor (statin) on discharge), investigative and invasive coronary strategies (receipt of a pre- or in-hospital electrocardiogram, echocardiography and coronary angiography), and lifestyle care opportunities (referral for cardiac rehabilitation, receipt of smoking cessation advice and receipt of dietary advice)].
The primary outcome measure was all-cause mortality after discharge from hospital up to the maximum follow-up time of 8.4 years [median 2.3, interquartile range (IQR) 1.1–3.9 years], which represented over 1 079 044 person years. Mortality data were obtained via linkage to the United Kingdom national death records held by the Office for National Statistics.
Statistical analysis
Baseline characteristics were described using numbers and percentages for categorical data and means and standard deviations or medians and IQRs for normal and non-normally distributed continuous variables. Differences in patient characteristics according to patient demographic and baseline clinical data were compared across GRACE risk score categories using χ2, t-tests, and Wilcoxon rank-sum tests as appropriate for the data type and distribution.
Flexible parametric survival modelling21 was used to assess the association of optimal care with long-term survival according to GRACE risk score category. To model the change in hazard ratio (HR) over continuous follow-up time, optimal care, and GRACE risk score categories were included in the model as time-varying covariates. We selected flexible parametric survival modelling to overcome violation of the proportional hazards assumption and to estimate the baseline hazard function using restricted cubic splines (see Supplementary material online, Section S2, Table S2 for model selection choice). The model was adjusted for patient demographics (sex, Index of Multiple Deprivation), medical history [diabetes, smoking status, family history of coronary heart disease, hypertension, previous myocardial infarction, previous angina, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease or asthma, chronic renal failure (defined as creatinine chronically >200 µmol/L (>2.26 mg/dL)), congestive cardiac failure, previous percutaneous coronary intervention, coronary artery bypass graft surgery, and total cholesterol]. Survival differences were quantified as HRs and differences in absolute mortality rates (AMR) per 100 patients, with an AMR of zero indicating no differences in mortality rates between the optimally treated patients (received all care interventions they were eligible for) vs. those sub-optimally treated. However, an AMR less than zero indicates lower mortality rates in the optimally managed patients compared with the sub-optimally managed.
Multiple imputation by chained equations was used to produce 10 imputed datasets to minimize potential bias caused by missing data. Of the 34 MINAP variables considered in the study (Table 1), the majority had less than 10% missing data (n = 26). Overall, we had complete data across all variables included in the flexible parametric modelling for 184 390 of patients. Multiple imputation by chained equations allowed the inclusion all 389 057 patients in the main study analyses and, as such, prevented loss of information whilst mitigating potential bias from missing data. Pooled model estimates and accompanying 95% confidence intervals (CIs) were generated according to Rubin’s rules (see Supplementary material online, Section S3, Table S3).22 All imputed data were compared with complete case analyses according to imputation good practice guidelines (see Supplementary material online, Table S4).23 All tests were two-sided, and statistical significance was considered as P < 0.05. Statistical analyses were performed in Stata MP64 version 14 (http://www.stata.com/) and R version 3.1.2 (https://cran.r-project.org/).
Table 1.
Baseline characteristics and care interventions received for all NSTEMI and by GRACE risk score category
| Analytical cohort (n = 389 057) | GRACE risk score category (n = 184 557)a |
P-value for difference between GRACE risk score category | Missing data (n, % of analytical cohort) | |||
|---|---|---|---|---|---|---|
| Low (<109) (n = 73 351) (39.7%) | Intermediate (109 to <140) (n = 59 201) (32.1%) | High (>140) (n = 52 005) (28.2%) | ||||
| Patient demographics | ||||||
| Age (years), median (IQR) | 72.7 (61.7–81.2) | 59.5 (52.0–66.0) | 76.0 (70.4–81.0) | 84.0 (79.0–88.0) | NA | 638 (0.2) |
| Sex (males), n(%) | 244 837 (63.1) | 53 818 (73.4) | 35 442 (59.9) | 27 104 (52.1) | <0.001 | 258 (0.1) |
| Patient medical history and clinical measures | ||||||
| History of ischaemic heart diseaseb, n (%) | 162 064 (45.2) | 22 885 (31.4) | 29 334 (50.0) | 28 676 (55.7) | <0.001 | 23 879 (6.1) |
| Hypertension, n (%) | 188 503 (48.5) | 33 872 (46.5) | 94 894 (59.4) | 30 605 (59.5) | <0.001 | 25 991 (6.7) |
| Diabetes mellitus, n (%) | 81 469 (20.9) | 13 229 (18.2) | 15 771 (26.9) | 13 598 (26.5) | <0.001 | 27 712 (7.1) |
| Dyslipidaemia, n (%) | 121 243 (33.7) | 27 292 (38.0) | 21 893 (37.9) | 15 952 (31.6) | <0.001 | 28 771 (7.4) |
| Family history of IHD, n (%) | 77 288 (26.2) | 29 184 (44.0) | 12 302 (25.0) | 5915 (14.8) | <0.001 | 94 215 (24.2) |
| Smoking status (current or previous smoker vs. never smoked), n (%) | 217 116 (60.3) | 49 323 (68.6) | 33 327 (59.0) | 25 589 (53.1) | <0.001 | 29 219 (7.5) |
| Peripheral vascular disease, n (%) | 18 324 (5.2) | 2181 (3.1) | 3431 (6.0) | 3301 (6.5) | <0.001 | 34 467 (8.9) |
| Chronic heart failure, n (%) | 24 529 (6.9) | 1205 (1.7) | 3759 (6.4) | 7683 (15.0) | <0.001 | 33 304 (8.6) |
| COPD or asthma, n (%) | 56 708 (14.6) | 9176 (12.8) | 10 796 (18.6) | 10 176 (20.1) | <0.001 | 33 633 (8.6) |
| Chronic kidney disease, n (%) | 21 938 (6.2) | 1637 (2.3) | 4349 (7.4) | 7216 (14.1) | <0.001 | 33 448 (8.6) |
| Cerebrovascular disease, n (%) | 34 146 (9.6) | 3505 (4.8) | 7146 (12.2) | 7867 (15.3) | <0.001 | 34 302 (8.8) |
| Heart rate (b.p.m.), median (IQR) | 80 (67 -95) | 74.0 (64.0–86.0) | 79.0 (66.0–92.0) | 89.0 (74.0–107.0) | NA | 65 863 (16.9) |
| Systolic blood pressure (mmHg), mean (SD) | 142.5 (28.4) | 149.1 (26.5) | 144.3 (27.3) | 131.0 (27.0) | NA | 66 688 (17.1) |
| Cardiac arrest (pre-hospital), n (%) | 1305 (0.7%) | 99 (0.1) | 354 (0.6) | 852 (1.6) | NA | 22 901 (5.9) |
| Initial creatinine (µmol/L), median (IQR) | 92.0 (76.0–114.0) | 84.0 (72.0–98.0) | 94.0 (78.0–117.0) | 110.0 (86.0–144.0) | NA | 165 622 (42.6) |
| ST-deviation on admission, n (%) | 108 189 (30.62) | 12 117 (16.5) | 16 262 (27.5) | 22 720 (43.7) | NA | 35 699 (9.2) |
| Care interventions | ||||||
| ECG during admission, n (%) | 371 149 (95.4) | 73 351 (100) | 59 201 (100) | 52 005 (100) | No difference | 9295 (4.7) |
| Receipt of pre-hospital aspirinc, n (%) | 91 679 (70.8) | 21 681 (71.1) | 13 915 (60.0) | 8721 (47.1) | <0.001 | 4917 (3.7) |
| Echocardiogramc, n (%) | 207 128 (53.3) | 44 772 (61.0) | 36 834 (62.2) | 32 404 (62.3) | <0.001 | 11 053 (2.8) |
| Receipt of angiographyc, n (%) | 198 303 (55.7) | 60 063 (85.4) | 34 691 (65.7) | 15 903 (38.0) | <0.001 | 15 656 (4.0) |
| Aspirin on dischargec, n (%) | 301 639 (88.46) | 56 130 (92.2) | 45 626 (92.6) | 39 458 (92.1) | 0.95 | 32 983 (8.5) |
| P2Y12 inhibition on dischargec, n (%) | 127 315 (93.1) | 39 858 (95.7) | 31 105 (93.1) | 24 867 (89.3) | <0.001 | 4236 (1.1) |
| ACEi/ARB on dischargec, n (%) | 169 942 (78.9) | 15 400 (91.5) | 21 360 (89.9) | 26 715 (86.2) | <0.001 | 206 719 (53.1) |
| β-Blocker on dischargec, n (%) | 138 656 (78.8) | 7625 (92.3) | 14 730 (90.5) | 24 953 (88.4) | <0.001 | 5300 (1.4) |
| Receipt of aldosterone antagonist during admissionc, n (%) | 2004 (17.5) | 191 (13.4) | 614 (19.4) | 990 (20.3) | <0.001 | 4798 (1.2) |
| Statin on dischargec, n (%) | 297 045 (85.4) | 55 965 (91.4) | 45 909 (90.8) | 38 916 (87.4) | <0.001 | 4519 (1.2) |
| Referral for cardiac rehabilitationc, n (%) | 279 027 (76.0) | 60 450 (86.1) | 44 508 (81.5) | 33 671 (74.6) | <0.001 | 4519 (1.2) |
| Smoking cessation advice receivedc, n (%) | 32 109 (19.4) | 17 405 (48.7) | 5434 (29.47) | 2350 (18.4) | <0.001 | 11 658 (3.0) |
| Dietary advice receivedc, n (%) | 119 321 (31.9) | 41 164 (58.4) | 30 048 (53.9) | 22 845 (48.3) | <0.001 | 225 444 (57.9) |
| Care by cardiologist, n (%) | 220 208 (92.9) | 67 951 (96.1) | 52 579 (92.6) | 42 969 (86.6) | <0.001 | 228 093 (58.6) |
| Optimal care received, n (%) | 44 530 11.5 | 18 785 (25.6) | 10 992 (18.6) | 5958 (11.5) | <0.001 | 140 895 (36.2) |
| Percentage of eligible care interventions received, median (IQR) | 70.0 (55.6–83.3) | 83.3 (66.7–100) | 77.8 (63.6–90.0) | 72.7 (60.0–87.5) | <0.001 | 0 (0.0) |
| Outcomes, n (%) | ||||||
| 30-Day mortality | 9097 (2.3) | 359 (0.5) | 1083 (1.8) | 2622 (5.0) | <0.001 | 0 (0.0) |
| 1-Year mortality | 55 188 (14.2) | 1854 (2.5) | 7285 (12.3) | 15 616 (30.0) | <0.001 | 0 (0.0) |
| 8-Year mortality | 113 520 (29.2) | 3417 (4.7) | 12 547 (21.2) | 23 578 (45.3) | <0.001 | 0 (0.0) |
ACEi/ARB, angiotensin converting enzyme inhibitor/angiotensin II receptor blocker; COPD, Chronic obstructive pulmonary disease; GRACE, Global Registry Acute Coronary Events; IQR, interquartile range; SD, standard deviation.
Summary data presented here are based on cases with complete GRACE risk score information only, prior to multiple imputation.
History of ischaemic heart disease refers to a history of coronary artery bypass grafting, percutaneous coronary intervention, myocardial infarction, or angina.
Includes numbers and percentage of those eligible.
Ethical considerations
MINAP is managed by the National Institute for Cardiovascular Outcomes Research (NICOR) (ref. NIGB: ECC 1-06 (d)/2011). NICOR has support under section 251 of the NHS Act 2006 for the conduction of medical research utilizing patient information without formal consent. Formal ethical approval was not required for this study under NHS research governance arrangements for use of non-identifiable patient data. The research complies with the Declaration of Helsinki.
Results
There were 389 057 patients included in the study with median age of 73 years (IQR 62–81 years) and 143 388 (36.9%) were female (Figure 1). There were more low risk [73 351 (39.7%)] than intermediate risk [59 201 (32.1%)] and high risk [52 005 (28.2%)] patients with NSTEMI (Table 1). High-risk NSTEMI were older than intermediate and low-risk NSTEMI [84 (79–88) vs. 76 (70–81) vs. 59 (52–66) years, respectively] and more likely to be female (47.9% vs. 40.2% vs. 26.6%). In general, levels of co-morbidity increased with increasing GRACE risk score category (Table 1). However, smoking decreased with increasing GRACE risk score category, and a family history of ischaemic heart disease was more frequent in the lower GRACE risk score group. Summary data for patients in whom the GRACE risk score was missing, prior to multiple imputation, is provided in Supplementary material online, Section S4, Table S5.
In total, 44 530 (11.5%) patients received optimal care, with the median proportion of eligible care received being 70.0% (IQR 55.6–85.7%). Care interventions most frequently not provided were receipt of aldosterone antagonists during admission [9426 (82.5%)], provision of smoking cessation advice [133 726 (80.6)], provision of dietary advice [254 869 (68.1)], and receipt of echocardiogram [181 831 (46.7)]. Both receipt of optimal care and the proportion of care received decreased with increasing GRACE risk score category [optimal care 18 785 (25.6%) and proportion of care 83.3% (IQR 66.7–100) for low-risk NSTEMI vs. 5958 (11.5%) and 72.7% (IQR 60.0–87.5) for high-risk NSTEMI; P < 0.001] (Table 1, Supplementary material online, Figure S1). Receipt of care did not vary significantly by GRACE risk score for electrocardiogram and aspirin on discharge, but increased for in-hospital aldosterone receptor blocker (13.4%, 19.4%, and 20.3% for low-, intermediate-, and high-risk patients, respectively). Whilst receipt of care decreased with increasing GRACE risk score for all other care opportunities, the greatest decreases were observed for coronary angiography (85.4%, 65.7%, and 38%), pre-hospital aspirin (71.1%, 60.0%, and 47.1%), and smoking cessation advice (48.7%, 29.5%, and 18.4%) for low-, intermediate-, and high-risk patients, respectively. Patients with missing GRACE risk score data had similar characteristics to those with complete GRACE risk score data, except for optimal care (8795, 4.3%) and the proportion of care received (63%, IQR 50–75%), which were lower (see Supplementary material online, Section S4, Table S5).
Mortality, guideline-indicated treatments, and ischaemic risk
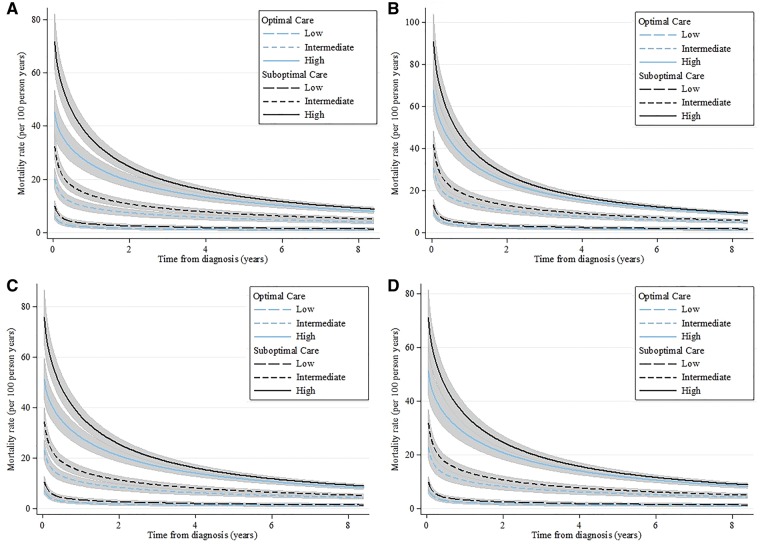
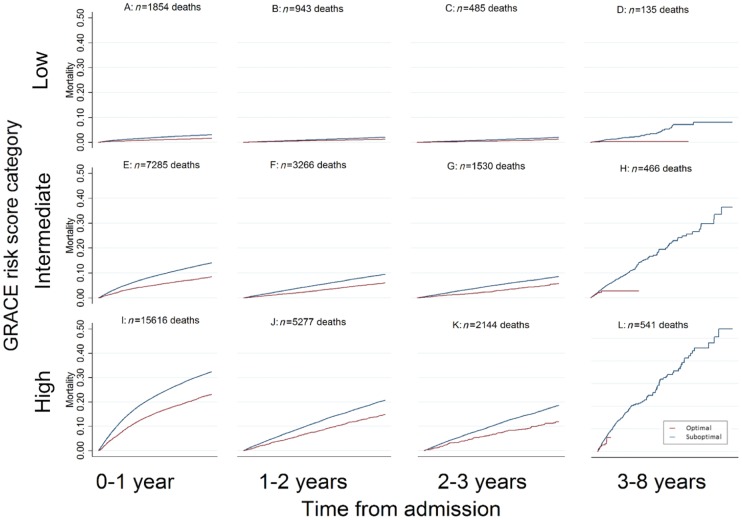
There were 113 856 (29.2%) deaths corresponding to 10.5 deaths per 100 person years. A pattern of greater early hazard for death was evident across the spectrum of NSTEMI risk, and accentuated among high-risk NSTEMI (Figure 2). Across all GRACE risk score groups for landmark time periods 0–1 years, 1–2 years, and 2–3 years, but not 3–8 years, unadjusted mortality rates were significantly higher for patients who did not receive optimal care (Figure 3). After adjustment, there remained a benefit in receiving optimal care regardless of estimated ischaemic risk. [Adjusted hazard ratio (aHR) = 0.62 (95% CI 0.56–0.68) difference in absolute mortality rate per 100 patients (AMR/100) −0.01 (95% CI −0.01 to 0.00) Table 2].
Figure 2.
Adjusted* time-varying mortality rates by receipt of optimal care and clinical risk obtained from a flexible parametric model (odds scale, five degrees of freedom) with time varying covariates by GRACE risk score category for optimal care vs. suboptimal care across the full care pathway (A) and by the following subgroups of the care pathway: pharmacological therapies (B)†, investigative and invasive coronary strategies (C)‡, and lifestyle (D)§,^. P = 0.004 for interaction. GRACE, Global registry of Acute Coronary Events, categorized into low (<109), intermediate (109 to ≤140), and high (>140) risk. *Model adjusted for demographic characteristics including sex, year, deprivation, previous acute myocardial infarction, previous angina, previous PCI, previous CABG, hypertension, peripheral vascular disease, chronic renal failure, chronic heart failure, cerebrovascular disease, diabetes mellitus, smoking status, and elevated cholesterol. †Including pre-hospital receipt of aspirin, aldosterone antagonist during admission, aspirin on discharge, P2Y12 inhibition on discharge, ACE inhibitors (ACEi)/angiotensin receptor blockers (ARBs) on discharge, β-blocker on discharge, and HMG Co-A reductase inhibitor (statin) on discharge. ‡Including receipt of a pre- or in-hospital electrocardiogram, echocardiography and coronary angiography. §Including referral for cardiac rehabilitation, smoking cessation advice and dietary advice.
Figure 3.
Unadjusted landmark Kaplan–Meier survival curves and crude mortality rates by GRACE risk score category and receipt of optimal care vs. suboptimal care. This figure demonstrates the benchmarked crude mortality rates for the following time periods; 0–1 year, 1–2 years, 2–3 years, 3–8 years, across GRACE risk category by the receipt of optimal care. The percentages represent the crude mortality rates for each time period with the 95% confidence interval for each of the respective GRACE risk score categories. Population at risk at baseline; low GRACE risk category: 73 351, intermediate GRACE risk category: 59 201, high GRACE risk category: 52 005. Population at risk 3–8 years; low GRACE risk category: 70 069 intermediate GRACE risk category: 47 120, high GRACE risk category: 28 698.
Table 2.
Time-varying adjusted hazard ratios and absolute difference in mortality rate per 100 for patients receiving optimal care compared with suboptimal care after multiple imputation for missing data
| Optimal care vs. suboptimal care |
||
|---|---|---|
| aHRa | Difference in AMR/100 | |
| HR over total follow-up time | 0.62 (0.56–0.68) | −0.01 (−0.01 to 0.00) |
| 30 days | 0.72 (0.63–0.84) | −0.02 (−0.03 to −0.01) |
| 1 | 0.57 (0.47–0.73) | −0.01 (−0.02 to −0.01) |
| 2 | 0.56 (0.44–0.77) | −0.01 (−0.02 to −0.01) |
| 3 | 0.56 (0.43–0.80) | −0.01 (−0.01 to 0.00) |
| 4 | 0.56 (0.42–0.82) | −0.01 (−0.01 to 0.00) |
| 5 | 0.56 (0.42–0.84) | −0.01 (−0.01 to 0.00) |
| 6 | 0.56 (0.42–0.86) | −0.01 (−0.01 to 0.00) |
| 7 | 0.56 (0.42–0.87) | −0.01 (−0.01 to 0.00) |
| 8 | 0.57 (0.42–0.89) | −0.01 (−0.01 to 0.00) |
aHR, adjusted hazard ratio; AMR, absolute mortality rate.
aaHR—adjusted hazard ratio obtained from flexible parametric survival modelling on the odds scale with five degrees of freedom and time-varying covariates for optimal care and GRACE risk, adjusted for: patient demographics (sex, year, and Index of Multiple Deprivation) and medical history (history of diabetes, smoking status, family history of coronary heart disease, hypertension, previous myocardial infarction, previous angina, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease or asthma, chronic renal failure, congestive cardiac failure, previous percutaneous coronary intervention, previous coronary artery bypass graft surgery, and total cholesterol).
At 30 days, the use of all eligible guideline-indicated treatments was associated with improved survival among high risk NSTEMI [aHR = 0.66 (95% CI 0.53–0.86) AMR/100 −0.19 (95% CI −0.29 to −0.08)], and intermediate risk NSTEMI [aHR = 0.74 (95% CI 0.62–0.92); AMR/100 −0.15 (95% CI −0.23 to −0.08) Table 3]. At the end of follow-up (8.4 years), the significant association between the use of all eligible guideline-indicated treatments and improved survival remained for high-risk NSTEMI (aHR = 0.66, 95% CI 0.50–0.96; AMR/100 = −0.03, 95% CI −0.06 to −0.01), but not for intermediate-risk NSTEMI (aHR = 1.04, 95% CI 0.74–1.71; AMR/100 = 0.002, 95% CI −0.02 to 0.03). For the low-risk NSTEMI, there was no association between use of all compared with the use of some eligible guideline-indicated treatments and improved survival at 30-days (aHR = 0.92, 95% CI 0.69–1.38) and at 8.4 years (aHR = 0.71, 95% CI 0.39–3.74). A sensitivity analysis, which included in-hospital deaths made minimal difference to the effect directions and magnitudes (see Supplementary material online, Section S5, Table S6).
Table 3.
Time-varying adjusted hazard ratios and absolute difference in mortality rate per 100 for patients receiving optimal care compared with suboptimal care according to low, intermediate and high GRACE risk score category after multiple imputation for missing data*
| Optimal care vs. suboptimal care |
Optimal care vs. suboptimal care |
Optimal care vs. suboptimal care |
||||
|---|---|---|---|---|---|---|
| Low GRACE risk |
Intermediate GRACE risk |
High GRACE risk |
||||
| aHRa | Difference in AMR/100 | aHRa | Difference in AMR/100 | aHRa | Difference in AMR/100 | |
| HR over total follow-up time | 0.76 (0.60–0.96) | −0.01 (−0.02 to −0.002) | 0.66 (0.56–0.77) | −0.03 (−0.04 to −0.02) | 0.55 (0.48–0.63) | −0.07 (−0.09 to −0.05) |
| Time varying HRs | ||||||
| 30 days | 0.92 (0.69–1.38) | −0.01 (−0.06 to 0.03) | 0.74 (0.62–0.92) | −0.15 (−0.23 to −0.08) | 0.66 (0.53–0.86) | −0.19 (−0.29 to −0.08) |
| 1 | 0.71 (0.47–1.49) | −0.02 (−0.04 to 0.01) | 0.85 (0.64–1.25) | −0.03 (−0.08 to 0.02) | 0.53 (0.42–0.74) | −0.18 (−0.25 to −0.12) |
| 2 | 0.71 (0.43–1.93) | −0.01 (−0.04 to 0.01) | 0.92 (0.66–1.47) | −0.01 (−0.06 to 0.04) | 0.56 (0.42–0.82) | −0.12 (−0.17 to −0.07) |
| 3 | 0.70 (0.42–2.31) | −0.01 (−0.03 to 0.01) | 0.96 (0.68–1.58) | −0.0050 (−0.05 to 0.04) | 0.58 (0.44–0.87) | −0.09 (−0.13 to −0.05) |
| 4 | 0.70 (0.41–2.66) | −0.01 (−0.03 to 0.01) | 0.98 (0.70–1.65) | −0.0017 (−0.04 to 0.04) | 0.60 (0.45–0.90) | −0.07 (−0.1 to −0.03) |
| 5 | 0.71 (0.40–2.97) | −0.01 (−0.03 to 0.01) | 1.00 (0.71–1.68) | 0.0001 (−0.03 to 0.03) | 0.62 (0.46–0.92) | −0.06 (−0.09 to −0.02) |
| 6 | 0.71 (0.40–3.25) | −0.01 (−0.03 to 0.01) | 1.02 (0.72–1.70) | 0.0012 (−0.03 to 0.03) | 0.63 (0.48–0.94) | −0.05 (−0.07 to −0.2) |
| 7 | 0.71 (0.39–3.51) | −0.01 (−0.02 to 0.01) | 1.03 (0.74–1.71) | 0.0019 (−0.03 to 0.03) | 0.64 (0.49–0.95) | −0.04 (−0.06 to −0.02) |
| 8 | 0.71 (0.39–3.74) | −0.01 (−0.02 to 0.01) | 1.04 (0.74–1.71) | 0.0023 (−0.02 to 0.03) | 0.66 (0.50–0.96) | −0.03 (−0.06 to −0.01) |
aHR, adjusted hazard ratio; AMR, absolute mortality rate; GRACE, Global registry of Acute Coronary Events, categorized into low (<109), intermediate (109 to ≤140), and high (>140) risk.
aHR—adjusted hazard ratio obtained from flexible parametric survival modelling on the odds scale with five degrees of freedom and time-varying covariates for optimal care and GRACE risk, adjusted for: patient demographics (sex, year, and Index of Multiple Deprivation) and medical history (history of diabetes, smoking status, family history of coronary heart disease, hypertension, previous myocardial infarction, previous angina, peripheral vascular disease, cerebrovascular disease, chronic obstructive pulmonary disease or asthma, chronic renal failure, congestive cardiac failure, previous percutaneous coronary intervention, previous coronary artery bypass graft surgery, and total cholesterol).
P < 0.001 for interaction.
Of the three subgroups of guideline-indicated care treatments and diagnostics, investigative and invasive coronary strategy was associated with the most comprehensive impact on survival—including beneficial effects among low-, intermediate-, and high-risk NSTEMI as well as effects that persisted the longest for the high-risk group (Figure 2, Supplementary material online, Tables S7a–S7c). For intermediate-risk NSTEMI, investigative and invasive coronary strategies were associated with a 28% relative survival improvement up to 3 years (aHR = 0.72, 95% CI 0.57–0.98; AMR/100 = −0.03, 95% CI −0.06 to −0.01), and for high-risk NSTEMI a 19% survival improvement at 8 years (aHR = 0.81, 95% CI 0.69–0.97; AMR/100 = −0.02, 95% CI −0.03 to −0.01). Pharmacological therapies were associated with a 46% survival improvement at 6 years (aHR = 0.54, 95% CI 0.37–0.97; AMR/100 = −0.02, 95% CI −0.04 to −0.01) for low-risk NSTEMI, and a 25% survival improvement at 5 years (aHR = 0.75, 95% CI 0.61–0.99; AMR/100 = −0.02, 95% CI −0.04 to 0.00) for intermediate-risk NSTEMI, with no persisting effect for high-risk NSTEMI. Lifestyle care opportunities were associated with a 25% survival improvement at 8 years (aHR = 0.75, 95% CI 0.63–0.92; AMR/100 = −0.02, 95% CI −0.04 to −0.01) for high-risk NSTEMI, but no persisting effect for low- or intermediate-risk NSTEMI.
Discussion
In this prospective observational cohort study of 389 057 patients with NSTEMI using data for all acute hospitals in a single health care system, optimal use of guideline-indicated care for NSTEMI was associated with greater survival gains with increasing GRACE risk, but its use decreased with increasing GRACE risk. Of note, is that the mortality benefit associated with optimal care found in high-risk NSTEMI persisted for over eight years from the time of discharge from hospital. Whilst there was a preponderance of low-risk NSTEMI patients who had high rates of survival, these patients proportionally received more evidence-based care compared with intermediate and high-risk NSTEMI. Moreover, a pattern of early death was evident across the NSTEMI risk spectrum, which was accentuated for those with the highest GRACE risk scores. Taken together, these findings suggest that providing all eligible care opportunities to NSTEMI patients has the potential to improve survival, and that those at highest risk will derive greater and more sustained benefit.
To our knowledge, this is the first study to investigate how long the impact of the pathway of guideline-indicated care according to baseline ischaemic risk among eligible patients with NSTEMI persists. Previous work has demonstrated that guideline-directed therapy results in improved outcomes at 30 days and 3 years, yet is limited because it focuses on the performance of finite quality indicators or interventions rather than cumulative care.3,24–26 This is important because the treatment of NSTEMI follows a journey of care and defined by evidence from randomized controlled trials and observational studies.14,15 Whilst earlier studies have demonstrated excess mortality associated with the non-receipt of guideline indicated interventions along the pathway of care, the potential persistence of effect sizes was not studied.2
Low-risk NSTEMI who received all eligible care interventions did not have a significant survival advantage compared with their counterparts who received some or none of the eligible care interventions. Whilst this seems counterintuitive, especially when randomized data have demonstrated clinical benefit from evidence—based treatments for NSTEMI,27 there are a number of possible explanations. First, the low GRACE risk score comparator group had high rates of receipt of many care interventions; therefore, although care was not optimal for 54 566 patients in this group, it was still high overall (median receipt of care 83.3%), and indeed, those with a low GRACE risk score comprised the greatest proportion of patients with the highest receipt of care. Second, the low rates of death in the low GRACE risk score group created a ‘floor effect’ whereby the discrimination of differences between optimal and suboptimal care was not possible.
We found that an invasive coronary strategy was associated with the most comprehensive and persistent impact on survival. Such an approach to the treatment of NSTEMI improved survival for intermediate and high-risk patients—with effects lasting for many years after hospital discharge following treatment. We noted that the beneficial survival effect associated with pharmacotherapies was restricted to low- and intermediate-risk NSTEMI for approximately 6 years after hospital discharge, whereas lifestyle modifications were associated with improved survival for up to 8 years among high-risk NSTEMI. We speculate that this differential association with survival may be because the effect of other care interventions such as an invasive strategy is greater than that of pharmacotherapies.3 Indeed, the advantages of an invasive strategy on early and mid-term clinical outcomes have been demonstrated in randomized studies,7,24 yet until now the evidence for its impact on longer-term outcomes has been limited.28 Our ‘real world’ national study supports these mid-term outcomes data, but also suggests that the impact of coronary angiography and revascularization for NSTEMI extends to at least 8 years.
The utilization of risk scores is recommended by international guidelines.13–15 In part, this is because physicians underestimate future ischaemic risk for NSTEMI which in turn contributes to suboptimal use of treatments.30,31 Our research supports the use of accurate risk estimation for NSTEMI, and is in keeping with evidence indicating that the GRACE risk score may be used to predict long-term outcomes.31–34 Using a validated risk stratification tool such as the GRACE risk score, may enable earlier mobilization of care interventions, and therefore, reduce fatal and non-fatal cardiovascular events.35,36
Moreover, the international burden of NSTEMI burden is high and is set to increase, with associated high mortality rates in the medium to longer-term.29,30 It is evident that the opportunities to improve care, and therefore realize reductions in cardiovascular endpoints following acute myocardial infarction, are unmet.1,2,25,36–38 Given that all of the interventions selected in this study were based on Class 1 recommendations that have been demonstrated to improve outcomes for NSTEMI, a decline in mortality rates should follow an increase in adherence to guideline-indicated care, with greater and persisting benefits among the higher risk.
To our knowledge, MINAP is the largest whole-country, single health system, prospective observational cohort of the quality of care and clinical outcomes across the spectrum of acute coronary syndromes. It is designed to be representative of the management of acute coronary syndrome in a clinical setting and has standardized criteria for defining case mix and treatments. Nevertheless, there were limitations to our study. First, the study was reliant on accurate recording of data, receipt of dietary and smoking advice was low this may be because the receipt of verbal advice is not well recorded. Second, MINAP does not collect all diagnosed cases of NSTEMI within England and Wales, whereby estimates of cases of acute myocardial infarction may be reduced by half compared with a multi-electronic health records approach.39 Third, missing data could have biased the estimates. However, an imputation strategy to minimize bias was implemented.37,40 Fourth, it is probable that other factors beyond the hospital stay (such as drug adherence and primary care visits) may also have influenced survival. Fifth, all-cause mortality was studied because cause specific mortality data were not available. This is a limitation because non-cardiovascular deaths may not be attributable to NSTEMI care.41 Sixth, in-hospital deaths were excluded, which could have resulted in survivorship bias—even so, a sensitivity analysis revealed that exclusion of these cases did not affect the conclusions drawn. Seventh is that the non-receipt of guideline care treatment and diagnostics may be a marker of higher NSTEMI risk. Indeed, we found that higher attainment of care occurred for lower risk NSTEMI. Finally, this observational study cannot demonstrate causation.
Conclusion
For nearly 400 000 NSTEMI hospital survivors in England and Wales, guideline-indicated treatment was less frequent among the high-risk NSTEMI, but when provided was significantly associated with improved survival which persisted over the longer-term. There was benefit seen in those at lower risk, though this was not significant over the whole study period. The provision of ‘up to standard’ guideline-indicated care for high-risk NSTEMI has the potential to improve their longer-term survival.
Supplementary Material
Acknowledgements
MINAP is commissioned by the Health Quality Improvement Partnership as part of the National Clinical Audit and Patient Outcomes Programme. Marlous Hall and Tatendashe Dondo were funded by the British Heart Foundation (project grant PG/13/81/30474). Chris P. Gale is a senior British Heart Foundation researcher.
Funding
M.H. and T.B.D. were supported by grant (PG/13/81/30474) from the British Heart Foundation.
Conflict of interest: The following authors report disclosures outside the submitted work. A.T.Y reports grants from Astra-Zeneca, outside the submitted work. S.G.G reports grants and personal fees from Sanofi, personal fees from Regeneron, grants and personal fees from Amgen, grants and personal fees from Lilly, grants and personal fees from Merck, grants and personal fees from Pfizer, grants and personal fees from AstraZeneca, grants and personal fees from Bayer, grants and personal fees from Boehringer Ingelheim, grants and personal fees from Bristol-Myers Squibb, personal fees from Fenix Group International, grants from Novartis, personal fees from Servier, outside the submitted work. D.P.C reports grants from Roche Diagnostics, outside the submitted work. K.A.A.F reports grants and personal fees from AstraZeneca, grants and personal fees from Bayer/Janssen, personal fees from Sanofi/Regeneron, personal fees from Verseon, outside the submitted work. H.B reports grants and personal fees from ASTRA ZENECA, personal fees from DAICHII-SANKYO, personal fees from ELI-LILLY, personal fees from BAYER, personal fees from SANOFI, during the conduct of the study; personal fees from NOVARTIS, personal fees from BMS-PFIZER, from SERVIER, outside the submitted work. C.P.G reports personal fees and non-financial support from AstraZeneca, personal fees from Novartis, personal fees from Vicor Pharma, outside the submitted work. A.T reports personal fees from Novo Nordisk, outside the submitted work. All other authors declared no conflict of interest.
Footnotes
See page 3807 for the editorial comment on this article (doi: 10.1093/eurheartj/ehy577)
References
- 1. Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, Smith SC, Pollack CV, Newby LK, Harrington RA, Gibler WB, Ohman EM.. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA 2006;295:1912–1920. [DOI] [PubMed] [Google Scholar]
- 2. Dondo TB, Hall M, Timmis AD, Gilthorpe MS, Alabas OA, Batin PD, Deanfield JE, Hemingway H, Gale CP.. Excess mortality and guideline-indicated care following non-ST-elevation myocardial infarction. Eur Heart J Acute Cardiovasc Care 2017;6:412.. [DOI] [PubMed] [Google Scholar]
- 3. Hall M, Dondo TB, Yan AT, Goodman SG, Bueno H, Chew DP, Brieger D, Timmis A, Batin PD, Deanfield JE, Hemingway H, Fox KAA, Gale CP.. Association of clinical factors and therapeutic strategies with improvements in survival following non–ST-elevation myocardial infarction, 2003-2013. JAMA 2016;316:1073–1082. [DOI] [PubMed] [Google Scholar]
- 4. Zaman MJ, Stirling S, Shepstone L, Ryding A, Flather M, Bachmann M, Myint PK.. The association between older age and receipt of care and outcomes in patients with acute coronary syndromes: a cohort study of the Myocardial Ischaemia National Audit Project (MINAP). Eur Heart J 2014;35:1551–1558. [DOI] [PubMed] [Google Scholar]
- 5. Mukherjee D, Fang J, Chetcuti S, Moscucci M, Kline-Rogers E, Eagle KA.. Impact of combination evidence-based medical therapy on mortality in patients with acute coronary syndromes. Circulation 2004;109:745–749. [DOI] [PubMed] [Google Scholar]
- 6. Nallamothu B, Fox KA, Kennelly BM, Van de Werf F, Gore JM, Steg PG, Granger CB, Dabbous OH, Kline-Rogers E, Eagle KA.. Relationship of treatment delays and mortality in patients undergoing fibrinolysis and primary percutaneous coronary intervention. The Global Registry of Acute Coronary Events. Heart 2007;93:1552–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fox K, Poole-Wilson P, Clayton T, Henderson R, Shaw T, Wheatley D, Knight R, Pocock S.. 5-Year outcome of an interventional strategy in non-ST-elevation acute coronary syndrome: the British Heart Foundation RITA 3 randomised trial. Lancet 2005;366:914–920. [DOI] [PubMed] [Google Scholar]
- 8. Fox KA, Clayton TC, Damman P, Pocock SJ, de Winter RJ, Tijssen JG, Lagerqvist B, Wallentin L.. Long-term outcome of a routine versus selective invasive strategy in patients with non–ST-segment elevation acute coronary syndrome: a meta-analysis of individual patient data. J Am Coll Cardiol 2010;55:2435–2445. [DOI] [PubMed] [Google Scholar]
- 9. Herrett E, Smeeth L, Walker L, Weston C.. The myocardial ischaemia national audit project (MINAP). Heart 2010;96:1264–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Simms A, Weston C, West R, Hall A, Batin P, Timmis A, Hemingway H, Fox K, Gale C.. Mortality and missed opportunities along the pathway of care for ST-elevation myocardial infarction: a national cohort study. Eur Heart J Acute Cardiovasc Care 2015;4:241–253. [DOI] [PubMed] [Google Scholar]
- 11. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Thygesen K, Alpert JS, White HD, Jaffe AS, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BR, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand J-P, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Simoons ML, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S.. Third universal definition of myocardial infarction. J Am Coll Cardiol 2012;60:1581–1598. [DOI] [PubMed] [Google Scholar]
- 12. Simms AD, Reynolds S, Pieper K, Baxter PD, Cattle BA, Batin PD, Wilson JI, Deanfield JE, West RM, Fox KAA, Hall AS, Gale CP.. Evaluation of the NICE mini-GRACE risk scores for acute myocardial infarction using the Myocardial Ischaemia National Audit Project (MINAP) 2003–2009: National Institute for Cardiovascular Outcomes Research (NICOR). Heart 2013;99:35–40. [DOI] [PubMed] [Google Scholar]
- 13. Royal College of Physcians. Unstable angina and NSTEMI: early management In NICE Guidelines CG94. London: National Institute for Health and Clinical Excellence; 2010. [PubMed] [Google Scholar]
- 14. Roffi M, Patrono C, Collet J-P, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S.. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2016;37:267–315. [DOI] [PubMed] [Google Scholar]
- 15. Amsterdam EA, Wenger NK, Brindis RG, Casey DE, Ganiats TG, Holmes DR, Jaffe AS, Jneid H, Kelly RF, Kontos MC, Levine GN, Liebson PR, Mukherjee D, Peterson ED, Sabatine MS, Smalling RW, Zieman SJ.. 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2014;64:e139–e228. [DOI] [PubMed] [Google Scholar]
- 16. Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K, Ohman M, Petrie MC, Sonntag F, Uva MS, Storey RF, Wijns W, Zahger D.. ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2011;32:2999–3054. [DOI] [PubMed] [Google Scholar]
- 17. Bertrand M, Simoons M, Fox K, Wallentin L, Hamm C, de Feyter P, Specchia G, Ruzyllo W, McFadden E.. Management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J 2002;23:1809–1840. [DOI] [PubMed] [Google Scholar]
- 18. Bassand JP, Hamm CW, Ardissino D, Boersma E, Budaj A, Fernandez-Aviles F, Fox KA, Hasdai D, Ohman EM, Wallentin L, Wijns W.. Guidelines for the diagnosis and treatment of non-ST-segment elevation acute coronary syndromes. Eur Heart J 2007;28:1598–1660. [DOI] [PubMed] [Google Scholar]
- 19. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Chavey WE, Fesmire FM, Hochman JS, Levin TN.. ACC/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines for the Management of Patients With Unstable Angina/Non-ST-Elevation Myocardial Infarction) developed in collaboration with the American College of Emergency Physicians, the Society for Cardiovascular Angiography and Interventions, and the Society of Thoracic Surgeons endorsed by the American Association of Cardiovascular and Pulmonary Rehabilitation and the Society for Academic Emergency Medicine. J Am Coll Cardiol 2007;50:e1–e157. [DOI] [PubMed] [Google Scholar]
- 20. Braunwald E, Antman EM, Beasley JW, Califf RM, Cheitlin MD, Hochman JS, Jones RH, Kereiakes D, Kupersmith J, Levin TN.. ACC/AHA guidelines for the management of patients with unstable angina and non-ST-segment elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association task force on practice guidelines (committee on the management of patients with unstable angina). J Am Coll Cardiol 2000;36:970–1062. [DOI] [PubMed] [Google Scholar]
- 21. Royston P, Lambert PC.. Flexible Parametric Survival Analysis Using Stata: Beyond the Cox Model College Station (Texas): Stata Press; 2011. [Google Scholar]
- 22. Rubin DB. Inference and missing data. Biometrika 1976;63:581–592. [Google Scholar]
- 23. Sterne JA, White IR, Carlin JB, Spratt M, Royston P, Kenward MG, Wood AM, Carpenter JR.. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bebb O, Hall M, Fox KAA, Dondo T, Timmis A, Bueno H, Schiele F, Gale CP.. Performance of hospitals according to the ESC ACCA quality indicators and 30-day mortality for acute myocardial infarction: national cohort study using the United Kingdom Myocardial Ischaemia National Audit Project (MINAP) register. Eur Heart J 2017;38:974–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schiele F, Gale CP, Simon T, Fox KAA, Bueno H, Lettino M, Tubaro M, Puymirat E, Ferrières J, Meneveau N, Danchin N.. Assessment of quality indicators for acute myocardial infarction in the FAST-MI (French Registry of Acute ST-Elevation or Non-ST-Elevation Myocardial Infarction) Registries. Circ Cardiovasc Qual Outcomes 2017;10:e003336.. [DOI] [PubMed] [Google Scholar]
- 26. Jobs A, Mehta SR, Montalescot G, Vicaut E, van't Hof AWJ, Badings EA, Neumann FJ, Kastrati A, Sciahbasi A, Reuter PG, Lapostolle F, Milosevic A, Stankovic G, Milasinovic D, Vonthein R, Desch S, Thiele H.. Optimal timing of an invasive strategy in patients with non-ST-elevation acute coronary syndrome: a meta-analysis of randomised trials. Lancet 2017;390:737–746. [DOI] [PubMed] [Google Scholar]
- 27. Fox K, Poole-Wilson P, Henderson R, Clayton T, Chamberlain D, Shaw T, Wheatley D, Pocock S.. Interventional versus conservative treatment for patients with unstable angina or non-ST-elevation myocardial infarction: the British Heart Foundation RITA 3 randomised trial. Lancet 2002;360:743–751. [DOI] [PubMed] [Google Scholar]
- 28. Henderson RA, Jarvis C, Clayton T, Pocock SJ, Fox KA.. 10-year mortality outcome of a routine invasive strategy versus a selective invasive strategy in non-ST-segment elevation acute coronary syndrome: the British Heart Foundation RITA-3 Randomized Trial. J Am Coll Cardiol 2015;66:511–520. [DOI] [PubMed] [Google Scholar]
- 29. Yan AT, Yan RT, Huynh T, Casanova A, Raimondo FE, Fitchett DH, Langer A, Goodman SG.. Understanding physicians' risk stratification of acute coronary syndromes: insights from the Canadian ACS 2 Registry. Arch Intern Med 2009;169:372–378. [DOI] [PubMed] [Google Scholar]
- 30. Lee CH, Tan M, Yan AT, Yan RT, Fitchett D, Grima EA, Langer A, Goodman SG.. Use of cardiac catheterization for non-ST-segment elevation acute coronary syndromes according to initial risk: reasons why physicians choose not to refer their patients. Arch Intern Med 2008;168:291–296. [DOI] [PubMed] [Google Scholar]
- 31. Goldberg RJ, Currie K, White K, Brieger D, Steg PG, Goodman SG, Dabbous O, Fox KA, Gore JM.. Six-month outcomes in a multinational registry of patients hospitalized with an acute coronary syndrome (the Global Registry of Acute Coronary Events [GRACE]). Am J Cardiol 2004;93:288–293. [DOI] [PubMed] [Google Scholar]
- 32. Tang EW, Wong C-K, Herbison P.. Global Registry of Acute Coronary Events (GRACE) hospital discharge risk score accurately predicts long-term mortality post acute coronary syndrome. Am Heart J 2007;153:29–35. [DOI] [PubMed] [Google Scholar]
- 33. Fox KA, Dabbous OH, Goldberg RJ, Pieper KS, Eagle KA, Van de Werf F, Avezum A, Goodman SG, Flather MD, Anderson FA, Granger CB.. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: prospective multinational observational study (GRACE). BMJ 2006;333:1091.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Alnasser SM, Huang W, Gore JM, Steg PG, Eagle KA, Anderson FA, Fox KAA, Gurfinkel E, Brieger D, Klein W, van de Werf F, Avezum Á, Montalescot G, Gulba DC, Budaj A, Lopez-Sendon J, Granger CB, Kennelly BM, Goldberg RJ, Fleming E, Goodman SG.. Late consequences of acute coronary syndromes: global registry of acute coronary events (GRACE) follow-up. Am J Med 2015;128:766–775. [DOI] [PubMed] [Google Scholar]
- 35. Chew DP, Astley CM, Luker H, Alprandi-Costa B, Hillis G, Chow CK, Quinn S, Yan AT, Gale CP, Goodman S, Fox KAA, Brieger D.. A cluster randomized trial of objective risk assessment versus standard care for acute coronary syndromes: rationale and design of the Australian GRACE Risk score Intervention Study (AGRIS). Am Heart J 2015;170:995–1004.e1. [DOI] [PubMed] [Google Scholar]
- 36. Dondo TB, Hall M, Timmis AD, Yan AT, Batin PD, Oliver G, Alabas OA, Norman P, Deanfield JE, Bloor K, Hemingway H, Gale CP.. Geographic variation in the treatment of non ST-segment myocardial infarction in the English National Health Service: a cohort study. BMJ Open 2016;6:e011600.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hall M, Laut K, Dondo TB, Alabas OA, Brogan RA, Gutacker N, Cookson R, Norman P, Timmis A, de Belder M, Ludman PF, Gale CP.. Patient and hospital determinants of primary percutaneous coronary intervention in England, 2003–2013. Heart 2016;102:313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bradley EH, Herrin J, Elbel B, McNamara RL, Magid DJ, Nallamothu BK, Wang Y, Normand S-LT, Spertus JA, Krumholz HM.. Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short-term mortality. JAMA 2006;296:72–78. [DOI] [PubMed] [Google Scholar]
- 39. Herrett E, Shah AD, Boggon R, Denaxas S, Smeeth L, van Staa T, Timmis A, Hemingway H.. Completeness and diagnostic validity of recording acute myocardial infarction events in primary care, hospital care, disease registry, and national mortality records: cohort study. BMJ 2013;346:f2350.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cattle BA, Baxter PD, Greenwood DC, Gale CP, West RM.. Multiple imputation for completion of a national clinical audit dataset. Stat Med 2011;30:2736–2753. [DOI] [PubMed] [Google Scholar]
- 41. Hall M, Alabas OA, Dondo TB, Jernberg T, Gale CP.. Use of relative survival to evaluate non-ST-elevation myocardial infarction quality of care and clinical outcomes. Eur Heart J Qual Care Clin Outcomes 2015;1:85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.