Abstract
EGFR-mutant lung cancer is an important molecular subtype in Asia considering that almost 40%-50% of patients with lung adenocarcinoma in Asian carry the active EGFR mutaiton. People have greatly anticipated the efficacy of PD-1/PD-L1 monoclonal antibody in lung cancer treatment but anti-PD-1/PD-L1 treatment failed to positively affect these patients. The NCCN guidelines do not recommend immunotherapy to patients with NSCLC carrying EGFR mutation at present. However, the reason why EGFR-mutant lung cancer patients show poor response to anti-PD-1/PD-L1 treatment is still unknown. Immune suppression and tolerance are the main characteristics of tumor. The PD-1/PD-L1 co-inhibitory molecule is probably not the main escape route of this tumor type. The main characteristic of EGFR-mutant lung cancer is the activation of the EGFR signaling pathway. EGFR activation is likely responsible for the uninflamed tumor microenvironment of this type tumor and particiaptes in immunosuppression and immune escape. Accumulating evidence proved that activation of EGFR signaling pathway is essential to the generation of Treg and tolerogenic DCs. In this review, we summarize the efficacy of PD-1/PD-L1 monoclonal antibiodies in patients with EGFR-mutant lung cancer patients; provide evidence to analyze the potential reason why these patients cannot benefit from anti-PD-1/PD-L1 treatment, and explore the strategy that shoud be adopted in the future.
Keywords: EGFR mutation, lung cancer, immunotherapy
Introduction
Immune checkpoint inhibitors, especially antibodies targeting programmed death-1 (PD-1) and programmed death ligand-1 (PD-L1), have shown survival benefits over chemotheapy for patients with advanced non-small-cell lung cancer (NSCLC) in several phase III trials comparing with chemotherapy [1-4]. Several anti-PD-1/PD-L1 antibodies, such as nivolumab, pembrolizumab and atezolimumab, have been approved as second- or first-line therapy in NSCLC and modified the management of patients with locally advanced or metastatic NSCLC [5,6]. Despite this progress, a considerable proportion of patients with NSCLC do not respond to anti-PD-1/PD-L1 treatment. Checkpoint inhibition is less effective in patients with EGFR mutation than in those without the mutation. In this review, we summarize the efficacy of PD-1/PD-L1 monoclonal antibiodies in patients with EGFR-mutant lung cancer patients; analyze why patients with this mutation cannot benefit from anti-PD-1/PD-L1 treatment, and explore the strategy that should be adopted in the future.
Patients with EGFR-mutant lung cancer poorly responded to anti-PD-1/PD-L1 treatment
The clinical trial checkmate 057 confirmed that patients who suffer from advanced nonsquamous NSCLC and progress during or after platinum-based chemotherapy survived longer with nivolumab than docetaxel [2]. However, subgroup analyses indicated that 82 patients with an activating EGFR mutation achieve no progression-free survival (PFS) or overall survival (OS) benefit. The KEYNOTE 010 clinical trial also revealed that pembrolizumab prolongs the OS of patients with previously treated PD-L1-positive advanced NSCLC [7]. Nevertheless, the subgroup analyses of patients with EGFR-mutant NSCLC (86 cases) still showed no apparent OS benefit from pembrolizumab (HR 0.88 [95% CI 0.45-1.70]). Atezolizumab is a humanized anti PD-L1 monoclonal antibody, and OAK is the first randomized phase 3 study that verifies its effectiveness in patients with previously treated NSCLC [4]. Similarly, EGFR-mutated patients fail to receive prolonged OS from atezolimab compared with docetaxel (HR 1.24 [95% CI 0.71-2.18]). A retrospective analysis included 58 patients who have NSCLC and are treated with PD-1/PD-L1 inhibitors. The objective responses are 1/28 (3.6%) in EGFR-mutant or ALK-positive patients and 7/30 (23.3%) in EGFR wild-type and ALK-negative/unknown patients (P=0.053) [8].
Although these data have been derived from subgroup analyses and their sample size is relatively small, a pooled analysis which included three clinical studies (checkmate 057, keynote 010, and POPLAR) has confirmed that immune checkpoint inhibitors do not enhance the OS of patients with EGFR-mutant advanced NSCLC compared with that of docetaxel (n=186, HR=1.05, 95% CI: 0.70-1.55, P < 0.81; treatment-mutation interaction P=0.03) [9]. Another pooled analysis which covered five clinical trials (Checkmate 017, Checkmate 057, Keynote 010, OAK, and POPLAR) has verified that prolonged OS can be observed in the EGFR wild-type subgroup but not in the EGFR mutant subgroup [10]. A phase II trial (NCT0287994) was conducted to test the efficacy of pembrolizumab in TKI-naive patients with EGFR mutation, advanced NSCLC, and PD-L1 positive tumors. Enrolment was ceased because of the lack of efficacy after 11 of the 25 planned patients were treated. None of the patients with EGFR-mutant lung cancer responded to pembrolizumab. Based on these data (Table 1), the NCCN clinical practice guidelines of NSCLC (version 3, 2018) clearly pointed out that immunotherapy is less effective in patients with EGFR-mutant lung cancer regardless of PD-L1 expression. Therefore, the NCCN guidelines do not recommend immunotherapy to patients with NSCLC carrying EGFR mutation.
Table 1.
Main clinical trial results concerning EGFR mutant lung cancer
| Clinical trial | Clinical trial stage | Patients number of EGFR mutation | Treatment strategy | Key results |
|---|---|---|---|---|
| Checkmate 057 [2] | Phase 3 | 82 | Nivolumab versus docetaxe | No PFS or OS benefit from nivolumab in EGFR mutation patients |
| Keynote 010 [7] | Phase 2/3 | 86 | Pembrolizumab versus docetaxel | No PFS or OS benefit from pembrolizumab in EGFR mutation patients |
| OAK [4] | Phase 3 | 42 | Atezolizumab versus docetaxel | EGFR mutant patients failed to prolong OS from atezolizumab comparing with docetaxel |
| NCT0287994 | Phase 2 | 11 | Pembrolizumab in TKI-naive patients with EGFR mutaiton, adcanced NSCLC, and PD-L1 positive tumors | None of the patients with EGFR-mutant lung cancer responded to pembrolizumab |
| Retrospective analysis [8] | - | 28 | Anti-PD-1/PD-L1 therapy | Only one patient response to anti-PD-1/PD-L1 therapy (The objective responses is 3.6%) |
| Pooled analysis 1 [9] | - | 186 | Anti-PD-1/PD-L1 therapy verus docetaxel | Anti-PD-1/PD-L1 therapy can’t improve the OS of patients with EGFR mutation |
| Pooled analysis 2 [10] | - | 271 | Anti-PD-1/PD-L1 therapy verus docetaxel | Anti-PD-1/PD-L1 therapy can’t improve the OS of patients with EGFR mutation |
Why did the EGFR-mutant lung cancer show poor response to anti-PD-1/PD-L1 treatment?
EGFR-mutant lung cancer is an important molecular subtype in Asia considering that almost 40%-50% of patients with lung adenocarcinoma in Asian carry the active EGFR mutation. People have greatly anticipated the efficacy of PD-1/PD-L1 monoclonal antibody in lung cancer treatment but anti-PD-1/PD-L1 treatment failed to positively affect patients who suffer from lung cancer and harbor the active EGFR mutation. In this regard, the benefit of anti-PD-1/PD-L1 monotherapy to EGFR-mutant lung cancer treatment is naturally questioned. Almost all lung cancer specialists globally share the same concern, and some of them have published commentary articles discussing the issue and suggesting potential reasons [11-13].
PD-L1 expression in tumor tissues and tumor mutation burden (TMB) are the most important predictive factors of the response to PD-1/PD-L1 inhibition [5,14,15]. As such, we should determine whether EGFR mutation tumor expresses low PD-L1 and carries a low TMB.
Question 1: Is EGFR-mutant lung cancer associated with low PD-L1 expression in tumor tissue?
Some studies have reported that the activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung cancer, and most studies have confirmed that high PD-L1 expression is found more frequently in EGFR-mutant lung tumor tissues than that in wild-type lung tumor tissues [16-19]. Other literature have confirmed the lack of significant difference in PD-L1 and PD-L2 expression among various EGFR mutation statuses [20]. However, there was also literature reported that lung cancer patients with mutated EGFR status showed a decreased PD-L1 expression in tumor [21]. A pooled analysis of 15 public studies have suggested that patients with EGFR mutation have a decreased PD-L1 expression [22]. The Analysis of the Cancer Genome Atlas and the GCLI cohort have also verified the inverse correlation between EGFR mutation and PD-L1 expression in tumor. Since answer to this question is conflicting, PD-L1 expression cannot explain why EGFR-mutant lung cancer exhibits a low response rate to PD-1/PD-L1 inhibition.
Question 2: Does EGFR-mutant lung cancer carry a low TMB?
Unlike the answers to question 1, the answer to question 2 is relatively uniform among studies. Studies have confirmed that EGFR mutations are associated with low TMB [22,23]. Low TMB can partly explain our dilemma. However, low TMB is merely a rough marker and hardly be the core reason.
Question 3: What other reasons can explain why EGFR-mutant lung cancer cannot respond to anti-PD-1/PD-L1 therapy?
When analyzing this question, we are more likely to fix our thought on predictive factor associated with the PD-1/PD-L1 inhibitor. This is a traditional forward thinking. Otherwise, in a reverse approach, we should try to answer how this EGFR-mutant cancer can successfully escape the immune system attacks and survive. This EGFR-mutant tumor probably utilizes its own evasion method from such attacks. The PD-1/PD-L1 co-inhibitory molecule is probably not the main escape route of this tumor type. Therefore, anti-PD-1/PD-L1 therapy cannot play a vital role in managing this kind of tumor.
In 2015, Teng et al classified tumors into four different tumor microenvironment types based on the presence or absence of tumor-infiltrating lymphocytes (TIL) and PD-L1 expression [24]. Among these tumor types (type I: TIL+, PD-L1+; type II: TIL-, PD-L1-; type III: TIL-, PD-L1+; type IV: TIL+, PD-L1-), only type I can respond to the PD-1/PD-L1 inhibitor. This observation indicates that the PD-L1 expression and the presence of TIL are important factors affecting the tumor microenvironment. Only when a tumor tissue contains a sufficient number of TIL, anti-PD-1/PD-L1 drugs can elicit anti-tumor effects. In 2017, Chen et al reported that immunity is influenced by a complex set of tumor, host, and environmental factors and divided tumor into the following types: the immune-desert phenotype, the immune-excluded phenotype, and the inflamed phenotype [25]. In this classification system, the immune-desert phenotype and the immune-excluded phenotype are naturally resistant to the PD-1/PD-L1 inhibitor. Another study has provided evidence supporting the correlation between EGFR mutation and an uninflamed tumor microenvironment [22]. Other studies also presented findings consistent with this conclusion [8] and this could perfectly explain why this type tumor cannot response to anti-PD-1/PD-L1 therapy. However, how EGFR-mutant lung cancer can transform into an immune-desert phenotype is unknown.
The main characteristic of EGFR-mutant lung cancer is the activation of the EGFR signaling pathway. EGFR, a well-accepted driver gene in lung cancer, is likely responsible for the uninflamed tumor microenvironment and may have an extensive association with immunosuppression and immune escape.
EGFR signaling pathway activation participates in immunosuppression and immune escape
Studies have mainly focused on the role of EGFR in tumor cells, but limited information has been presented regarding the effects of EGFR on immunologic effector cells. However, some studies have reported the extensive connection between EGFR signaling and immunosuppression.
EGFR signaling and Treg
Immune suppression and tolerance are the main characteristics of tumor. Regulatory T cells (Tregs) are necessary to maintaining peripheral tolerance. The most characterized Tregs are defined by the expression of the transcription factor Foxp3. The CD4+/CD25+/Foxp3+ Tregs are important immune suppressor cells that play a suppressive function in the immune system. The systemic inhibition of EGFR signaling by gefitinib can alter the immune environment of targeted cancer in vivo and in vivro. This effect is probably achieved by reducing the number of Tregs in tumors [26].
Amphiregulin (AREG) is an EGF-like growth factor and frequently up-regulated in tumor tissues. In 2013, AREG was proven to be critical for efficient Treg function in vivo, suggesting that the EGFR signaling pathway can play a substantial role in the immune system because AREG is a ligand of EGFR [27]. In 2016, Wang et al [28] first confirmed that the EGFR signaling pathway participates in the regulation of Treg, and AREG can maintain the suppressive function of Tregs via the EGFR/GSK-3β/Foxp3 axis in vitro and in vivo. In 2017, another literature also verified that a long noncoding RNA lnc-EGFR can stimulate Treg differentiation and promote hepatocellular carcinoma immune evasion via an EGFR-dependent signalling pathway [29]. Therefore, EGFR signaling activation has a vital role in the generation and activation of Tregs (Figure 1).
Figure 1.
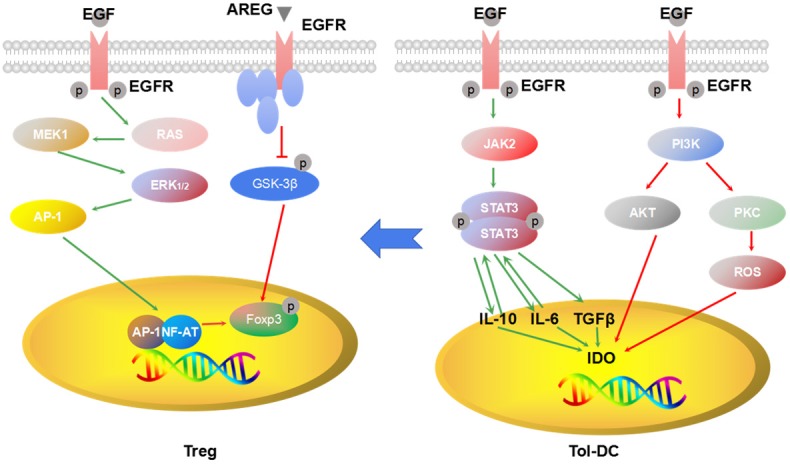
EGFR signaling and generation of Treg and tol-DC. EGFR signaling pathway activation can promote generation of Treg cells and tol-DC and maintain the suppression function of Treg cells and tol-DC.
EGFR signaling and tolerogenic dendritic cells (DCs)
DCs participate in antigen presentation to drive T cell priming and differentiation and play a vital role in the regulation of immune responses. Tolerogenic DCs can promote immune tolerance by participating in the negative selection of antoreactive T cells. Tolerogenic DCs are characterized by the low expression of co-stimulatory molecules, the highly suppressive cytokine production, and the enhanced regulation of immune responses, including impairment of T cell proliferation and promotion of Treg expansion via the upregulation of indoleamine 2,3-dioxygenase IDO [30]. IDO is involved in immune tolerance in ovarian cancer and a poor prognostic marker in serous ovarian cancer cell [31,32].
Signal transducer and activator of transcription 3 (STAT3), a downstream signaling molecule of EGFR, is a family of cytoplasmic proteins modulating various physiological functions, including cell survival and cell cycle regulation. IL-6 participates in maintaining immature DCs, and STAT3 is essential for the IL-6 suppression of bone marrow-derived DC activation/maturation [33]. Activating STAT3 can inhibit the maturation of DCs [34]. STAT3 not only prevents the maturation of DC but also induces the production of IDO. Cheng et al [30] reported that STAT3 activation in DC is essential to IDO production. IDO inhibitor or STAT3 blocking antibodies can reverse the production of IDO. The activation of other EGFR downstream molecules, such as PI3K, PKC, and NF-κB, are required for hemoglobin-induced IDO expression in bone marrow-derived myeloid DCs [35]. Collectively, the above observations indicate that the activation of EGFR signaling pathway is essential to the generation of tolerogenic DCs (Figure 1).
EGFR signaling pathway and myeloid-derived suppressor cells (MDSCs)
MDSCs with the typical phenotype of CD11b+CD33+HLA-DR- significantly increase in number of multiple cancer types and contribute to cancer development. MDSCs can inhibit IL-2 and anti-CD3/CD28 mAb-induced T cell amplification and Th1 polarization but stimulate apoptosis in T cells in an IDO-dependent manner. The phosphorylation of STAT3, an important downstream signal molecule of the EGFR signaling pathway, is required for the expression of IDO [36]. In other words, the activation of STAT3 is essential for the immune suppression of MDSCs.
Actually, substantial literature has proven the intimate relationship between the activation of STAT3 and generation of MDSCs. In 2010, Poschke et al confirmed that increased STAT3 levels is an important regulator in MDSC development and function, and inhibition of STAT3 can abolish MDSCs’ suppressive activity almost completely [37]. Other researchers demonstrated that the persistent activation of STAT3 can promote MDSC-mediated immune suppression in lung cancer [38].
How does EGFR activation in tumor cells transfer to immune cells?
Exosomes are spherical to cup-shaped nanoparticles (30-100 nm) and can be present in nearly all human body fluids. The main function of exosomes is to participate in cell-to-cell communication by transferring bioactive molecules to recipient cells close to or distant from original cells [39]. For example, exosomes secreted from hepatitis C virus-infected cells contain full-length viral RNA and protein, and these exosomes can successfully transmit infection to other hepatoma cells and establish a protective infection [40,41]. Tumor cells can share some malignant characteristics through the exchange of exosomes. For example, exosomes from mutant KRAS-expressing colon cancer cells can transfer their invasiveness to recipient cells expressing wild-type KRAS gene [42].
Recently, accumulating evidence has proven that the presence of active EGFR protein in exosomes derived from cancer cells and, more importantly, exosomal EGFR protein, can be transferred between cells and contribute tumor development [43-45]. For example, EGFR in exosomes secreted from gastric cancer cells can be delivered to liver cells, and the EGFR molecules can be integrated into the plasma membrane of liver stromal cells and then promote gastric cancer liver metastasis [45]. Interestingly, in 2010, Chalmin et al found that hsp72 derived from tumor exosomes can mediate the STAT3-dependent immunosuppressive function of human MDSCs [46]. Recently, some researchers observed that EGFR can be transferred by exosomes between tumor cells and immune cells and induce the generation of tumor-specific Treg cells [47]. These studies may partly explain how EGFR mutant cancer cells can affect the activation of Treg cells.
What strategy should we adopt?
Because anti-PD-1/PD-L1 monoclonal antibody exhibits limited activity toward EGFR-mutant lung cancer patients. NCCN guidelines do not recommend immunotherapy to patients with active mutation. Is this the final end of the war? We believe that this conclusion is only preliminary because many researchers still focus on this field. Furthermore, immunotherapy is expected to go beyond anti-PD-1/PD-L1 monoclonal antibody.
PD-1/PD-L1 monoclonal antibody as third-line or later treatment for selective EGFR-mutant lung cancer
The activation of the EGFR signaling pathway is the hallmark of EGFR-mutant lung cancer and is the potential reason why tumor type cannot respond to anti-PD-1/PD-L1 therapy. As EGFR-TKI therapy continuously develops from first generation to third generation, cancer cells with an activated of EGFR signaling pathway are subjected to EGFR-TKI treatment. Most of the surviving tumor cells are independent of the EGFR signaling pathway. In this situation, when a “clean” tumor becomes relatively “dirty”, anti-PD-1/PD-L1 therapy may play a role in this tumor.
Actually, osimertinib can decrease the expression of pEGFR in tumor and increase CD8+ T cell infiltration, which faciliates the anti-tumor effect of PD-1/PD-L1 therapy [48]. In the ATLANTIC clinical trial, patients were divided into three cohorts on the basis of EGFR/ALK status and PD-L1 expression and received durvalumab (anti-PD-L1) as third-line or later treatment. A total of 111 patients were included in cohort 1 and harbored EGFR+ or ALK+ NSCLC with at least 25% or less than 25%, of tumor cells with PD-L1 expression. The patients with EGFR-/ALK- NSCLC achieved a response higher than that in cohort 1. Even so, the ORR among the patients with EGFR+ NSCLC with ≥ 25% of tumor cells expressing PD-L1 remained encouraging (12.2%) relative to that (4%) of patients with EGFR+ NSCLC with < 25% tumor cells expressing PD-L1 [49]. Considering this result, we can utilize anti-PD-1/PD-L1 therapy in EGFR-mutant and PD-L1 overexpression lung cancer patients heavily treated with anti-EGFR treatment.
PD-1/PD-L1 monoclonal antibody combined with other therapy
EGFR-TKI is the standard therapy for EGFR-mutant lung cancer. However, the combination of EGFR-TKI with PD-1/PD-L1 monoclonal antibody appears more attractive. Several phase I trials are intended to study the possibility of combining of EGFR-TKI with PD-1/PD-L1 monoclonal antibody, and some trials have attained a preliminary result.
TATTON is a phase Ib trial aiming to investigate the tolerability and safety of combining therapies with osimertinib and durvalumab. The response rate in patients with EGFR mutation was encouraging (12/21). However, 38% of the patients enrolled developed serious interstitial pneumonitis, and the poor safety profile ended the development of the osimertinib-durvalumab combination for further study. Another two gefitinib + durvalumab combination regimens were also designed to test in patients with EGFR-mutant and EGFR-TKI-naïve lung cancer patients. Tolerance to the combination was acceptable and the ORR was 77.9% and 80%, respectively. Because the treatment effect of EGFR-TKI alone was relatively high, improving the patients’ ORR by adding duralumab appeared difficult.
Adding anti-PD-1/PD-L1 treatment to standard chemotherapy results in a significantly longer OS and PFS than those of chemotherapy alone patients with lung cancer without targetable mutation [3]. Adding anti-PD-1/PD-L1 treatment to chemotherapy in patients with EGFR mutation may achieve desirable results. Although no clinical trial has focused on patients with EGFR-mutant lung cancer patients, information can still be acquired from the subgroup analysis of other clinical trials. The PACIFIC study was a phase III study that compared durvalumab (PD-L1 antibody) as consolidation therapy with placebo in patients with stage III NSCLC who did not present disease progression after two or more cycles of platinum-based chemotherapy [50]. This clinical trial attained a positive result and demonstrated a longer PFS in the durvalumab cohort than that in the placebo cohort. In the subgroup analysis, patients with EGFR-mutant patients also slightly benefited from durvalumab after chemoradiotherapy.
IMpower150 was a randomized phase III study of atezolizumab + chemotheray ± bevacizumab vesus chemotherapy + bevacizumab in first-line nonsquamous NSCLC. The study showed a significant OS benefit with atezolizumab + chemotherapy + bevacizumab vesus chemotherapy + bevacizumab in first-line NSCLC. More importantly, patients with EGFR/ALK+ patients also benefited from the addition of atezolizumab. This finding implied that bevacizumab and chemotherapy can enhance atezolizumab efficacy in EGFR-mutant lung cancer patients.
Pegilodecakin (AM0010) can stimulate the survival and expansion of intratumoral, antigen-activated CD8+ T cells, which provided a rationale for combining anti-PD-1 agents with pegilodecakin. A total of 34 pretreated patients with NSCLC received pegilodecakin (10-20 μg/kg daily, SC) with pembrolizumab (2 mg/kg, every 3 weeks, IV) or nivolumab (3 mg/kg, every 2 weeks, IV). In 26 subjects who can be evaluated for the response, the ORR was 41%, and another 12 patients (46%) achieved SD as the best response. The responses were also observed when anti-PD-1 therapy has demonstrated limited benefit, such as in absent PD-L1 expression, low TMB, and/or the presence of liver metastasis. Although no similar data have been found concerning on EGFR-mutant patients, the data from this research appear optimistic for patients with EGFR mutation.
Other immunotherapy treatments
PD-1/PD-L1 is a highly typical immune checkpoint inhibitor. Recently, HHLA2, a newly discovered member of the B7/CD28 family, was found to be widely expressed in human lung cancer. More importantly, the expression of HHLA2 was noted to be higher in patients with EGFR mutation than in other patients; this finding indicated that HHLA2 is a potentially novel target for lung cancer immunotherapy, especially in patients with EGFR mutation [51].
There were also other immune therapy drugs some of which have entered to clinical trials. IDO is a key regulator of immune tolerance in multiple cancers. IDO expression in DCs can suppress T-cell responses and promote tolerance by either a direct effect on T-cells mediated by tryptophan depletion or cytotoxic effects on T-cells from tryptophan metabolites. INCB24360 (epacadostat) is a highly potent and selective IDO1 inhibitor for immuno-oncology [52]. Epacadostat was proven to be generally well tolerated in clinical trials [53]. ECHO-306/Keynote-715, a phase III study of first-line epacadostat plus pembrolizumab with or without platinum-based chemotherapy vesus pembrolizumab plus platinum-based chemotherapy plus placebo for metastatic NSCLC patients has already been designed. Another phase III randomized double-blind study of first-line epacadostat plus pembrolizumab vesus pembrolizumab plus placebo for metastatic NSCLC was also designed.
LYC-55716, a first-in-class oral, small-molecule agonist of the retinoic acid receptor-related orphan receptor γ (RORγ) was developed to treat solid tumor. A phase Ib trial of this drug in combination with pembrolizumab has been designed. In preclinical study, adding RORγ agonists increased the activity of PD-1/PD-L1 inhibitors augmented the number and activation of tumor-infiltrating lymphocytes and diminished immune suppression.
Conclusion
Patients with EGFR-mutant lung cancer patients cannot benefit from monotherapy with PD-1/PD-L1 monoclonal antibody. The activation of EGFR signalling pathway in immunologic effector cells may participate in the formation of an immunosuppressive microenvironment in lung cancer and finally result in the nonresponsiveness of this type of lung cancer to anti-PD-1/PD-L1 treatment. The present data suggest that combination therapy showed potential for treatment applications. As such, new immune drugs for EGFR-mutant lung cancer should be developed.
Acknowledgements
This work was supported by the grand from the National Natural Science Foundation of China (81871873) and the Project of Invigorating Health Care through Science, Technology and Education, Jiangsu Provincial Medical Youth Talent (QNRC2016646); China Postdoctoral Science Foundation (2017M621680) and the talents program of Jiangsu Cancer Hospital.
Disclosure of conflict of interest
None.
References
- 1.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, Clingan P, Hochmair MJ, Powell SF, Cheng SY, Bischoff HG, Peled N, Grossi F, Jennens RR, Reck M, Hui R, Garon EB, Boyer M, Rubio-Viqueira B, Novello S, Kurata T, Gray JE, Vida J, Wei Z, Yang J, Raftopoulos H, Pietanza MC, Garassino MC. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378:2078–2092. doi: 10.1056/NEJMoa1801005. [DOI] [PubMed] [Google Scholar]
- 4.Rittmeyer A, Barlesi F, Waterkamp D, Park K, Ciardiello F, von Pawel J, Gadgeel SM, Hida T, Kowalski DM, Dols MC, Cortinovis DL, Leach J, Polikoff J, Barrios C, Kabbinavar F, Frontera OA, De Marinis F, Turna H, Lee JS, Ballinger M, Kowanetz M, He P, Chen DS, Sandler A, Gandara DR. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi: 10.1016/S0140-6736(16)32517-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR. Pembrolizumab versus Chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 6.Langer CJ, Gadgeel SM, Borghaei H, Papadimitrakopoulou VA, Patnaik A, Powell SF, Gentzler RD, Martins RG, Stevenson JP, Jalal SI, Panwalkar A, Yang JC, Gubens M, Sequist LV, Awad MM, Fiore J, Ge Y, Raftopoulos H, Gandhi L. Carboplatin and pemetrexed with or without pembrolizumab for advanced, non-squamous non-small-cell lung cancer: a randomised, phase 2 cohort of the open-label KEYNOTE-021 study. Lancet Oncol. 2016;17:1497–1508. doi: 10.1016/S1470-2045(16)30498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 8.Gainor JF, Shaw AT, Sequist LV, Fu X, Azzoli CG, Piotrowska Z, Huynh TG, Zhao L, Fulton L, Schultz KR, Howe E, Farago AF, Sullivan RJ, Stone JR, Digumarthy S, Moran T, Hata AN, Yagi Y, Yeap BY, Engelman JA, Mino-Kenudson M. EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non-small cell lung cancer: a retrospective analysis. Clin Cancer Res. 2016;22:4585–4593. doi: 10.1158/1078-0432.CCR-15-3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee CK, Man J, Lord S, Links M, Gebski V, Mok T, Yang JC. Checkpoint inhibitors in metastatic EGFR-mutated non-small cell lung cancer-a meta-analysis. J Thorac Oncol. 2017;12:403–407. doi: 10.1016/j.jtho.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Lee CK, Man J, Lord S, Cooper W, Links M, Gebski V, Herbst RS, Gralla RJ, Mok T, Yang JC. Clinical and molecular characteristics associated with survival among patients treated with checkpoint inhibitors for advanced non-small cell lung carcinoma: a systematic review and meta-analysis. JAMA Oncol. 2018;4:210–216. doi: 10.1001/jamaoncol.2017.4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heigener DF, Reck M. PD-1 axis inhibition in EGFR positives: a blunt sword? J Thorac Oncol. 2017;12:171–172. doi: 10.1016/j.jtho.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 12.Zarogoulidis P, Papadopoulos V, Maragouli E, Papatsibas G, Huang H. Checkpoint inhibitors in metastatic epidermal growth factor receptor-mutated non-small cell lung cancer patients: where we treating the wrong cancer? J Thorac Dis. 2017;9:2771–2773. doi: 10.21037/jtd.2017.08.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gainor JF. Square peg, round hole? Programmed death-1 inhibitors in epidermal growth factor receptor-mutant non-small cell lung cancer. J Thorac Dis. 2018;10:25–29. doi: 10.21037/jtd.2017.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herbst RS, Morgensztern D, Boshoff C. The biology and management of non-small cell lung cancer. Nature. 2018;553:446–454. doi: 10.1038/nature25183. [DOI] [PubMed] [Google Scholar]
- 15.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, Christensen CL, Mikse OR, Cherniack AD, Beauchamp EM, Pugh TJ, Wilkerson MD, Fecci PE, Butaney M, Reibel JB, Soucheray M, Cohoon TJ, Janne PA, Meyerson M, Hayes DN, Shapiro GI, Shimamura T, Sholl LM, Rodig SJ, Freeman GJ, Hammerman PS, Dranoff G, Wong KK. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi: 10.1158/2159-8290.CD-13-0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Incecco A, Andreozzi M, Ludovini V, Rossi E, Capodanno A, Landi L, Tibaldi C, Minuti G, Salvini J, Coppi E, Chella A, Fontanini G, Filice ME, Tornillo L, Incensati RM, Sani S, Crino L, Terracciano L, Cappuzzo F. PD-1 and PD-L1 expression in molecularly selected non-small-cell lung cancer patients. Br J Cancer. 2015;112:95–102. doi: 10.1038/bjc.2014.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, Hoshino T, Nakanishi Y, Okamoto I. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 19.Tang Y, Fang W, Zhang Y, Hong S, Kang S, Yan Y, Chen N, Zhan J, He X, Qin T, Li G, Tang W, Peng P, Zhang L. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6:14209–14219. doi: 10.18632/oncotarget.3694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koh J, Go H, Keam B, Kim MY, Nam SJ, Kim TM, Lee SH, Min HS, Kim YT, Kim DW, Jeon YK, Chung DH. Clinicopathologic analysis of programmed cell death-1 and programmed cell death-ligand 1 and 2 expressions in pulmonary adenocarcinoma: comparison with histology and driver oncogenic alteration status. Mod Pathol. 2015;28:1154–1166. doi: 10.1038/modpathol.2015.63. [DOI] [PubMed] [Google Scholar]
- 21.Ji M, Liu Y, Li Q, Li X, Ning Z, Zhao W, Shi H, Jiang J, Wu C. PD-1/PD-L1 expression in non-small-cell lung cancer and its correlation with EGFR/KRAS mutations. Cancer Biol Ther. 2016;17:407–413. doi: 10.1080/15384047.2016.1156256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong ZY, Zhang JT, Liu SY, Su J, Zhang C, Xie Z, Zhou Q, Tu HY, Xu CR, Yan LX, Li YF, Zhong WZ, Wu YL. EGFR mutation correlates with uninflamed phenotype and weak immunogenicity, causing impaired response to PD-1 blockade in non-small cell lung cancer. Oncoimmunology. 2017;6:e1356145. doi: 10.1080/2162402X.2017.1356145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haratani K, Hayashi H, Tanaka T, Kaneda H, Togashi Y, Sakai K, Hayashi K, Tomida S, Chiba Y, Yonesaka K, Nonagase Y, Takahama T, Tanizaki J, Tanaka K, Yoshida T, Tanimura K, Takeda M, Yoshioka H, Ishida T, Mitsudomi T, Nishio K, Nakagawa K. Tumor immune microenvironment and nivolumab efficacy in EGFR mutation-positive non-small-cell lung cancer based on T790M status after disease progression during EGFR-TKI treatment. Ann Oncol. 2017;28:1532–1539. doi: 10.1093/annonc/mdx183. [DOI] [PubMed] [Google Scholar]
- 24.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature. 2017;541:321–330. doi: 10.1038/nature21349. [DOI] [PubMed] [Google Scholar]
- 26.Mascia F, Schloemann DT, Cataisson C, McKinnon KM, Krymskaya L, Wolcott KM, Yuspa SH. Cell autonomous or systemic EGFR blockade alters the immune-environment in squamous cell carcinomas. Int J Cancer. 2016;139:2593–2597. doi: 10.1002/ijc.30376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zaiss DM, van Loosdregt J, Gorlani A, Bekker CP, Grone A, Sibilia M, van Bergen en Henegouwen PM, Roovers RC, Coffer PJ, Sijts AJ. Amphiregulin enhances regulatory T cell-suppressive function via the epidermal growth factor receptor. Immunity. 2013;38:275–284. doi: 10.1016/j.immuni.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang S, Zhang Y, Wang Y, Ye P, Li J, Li H, Ding Q, Xia J. Amphiregulin confers regulatory T cell suppressive function and tumor invasion via the EGFR/GSK-3beta/Foxp3 axis. J Biol Chem. 2016;291:21085–21095. doi: 10.1074/jbc.M116.717892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang R, Tang J, Chen Y, Deng L, Ji J, Xie Y, Wang K, Jia W, Chu WM, Sun B. The long noncoding RNA lnc-EGFR stimulates T-regulatory cells differentiation thus promoting hepatocellular carcinoma immune evasion. Nat Commun. 2017;8:15129. doi: 10.1038/ncomms15129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheng JT, Deng YN, Yi HM, Wang GY, Fu BS, Chen WJ, Liu W, Tai Y, Peng YW, Zhang Q. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5:e198. doi: 10.1038/oncsis.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ino K. Indoleamine 2,3-dioxygenase and immune tolerance in ovarian cancer. Curr Opin Obstet Gynecol. 2011;23:13–18. doi: 10.1097/GCO.0b013e3283409c79. [DOI] [PubMed] [Google Scholar]
- 32.Okamoto A, Nikaido T, Ochiai K, Takakura S, Saito M, Aoki Y, Ishii N, Yanaihara N, Yamada K, Takikawa O, Kawaguchi R, Isonishi S, Tanaka T, Urashima M. Indoleamine 2,3-dioxygenase serves as a marker of poor prognosis in gene expression profiles of serous ovarian cancer cells. Clin Cancer Res. 2005;11:6030–6039. doi: 10.1158/1078-0432.CCR-04-2671. [DOI] [PubMed] [Google Scholar]
- 33.Park SJ, Nakagawa T, Kitamura H, Atsumi T, Kamon H, Sawa S, Kamimura D, Ueda N, Iwakura Y, Ishihara K, Murakami M, Hirano T. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173:3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 34.Rizzuti D, Ang M, Sokollik C, Wu T, Abdullah M, Greenfield L, Fattouh R, Reardon C, Tang M, Diao J, Schindler C, Cattral M, Jones NL. Helicobacter pylori inhibits dendritic cell maturation via interleukin-10-mediated activation of the signal transducer and activator of transcription 3 pathway. J Innate Immun. 2015;7:199–211. doi: 10.1159/000368232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogasawara N, Oguro T, Sakabe T, Matsushima M, Takikawa O, Isobe K, Nagase F. Hemoglobin induces the expression of indoleamine 2,3-dioxygenase in dendritic cells through the activation of PI3K, PKC, and NF-kappaB and the generation of reactive oxygen species. J Cell Biochem. 2009;108:716–725. doi: 10.1002/jcb.22308. [DOI] [PubMed] [Google Scholar]
- 36.Yu J, Du W, Yan F, Wang Y, Li H, Cao S, Yu W, Shen C, Liu J, Ren X. Myeloid-derived suppressor cells suppress antitumor immune responses through IDO expression and correlate with lymph node metastasis in patients with breast cancer. J Immunol. 2013;190:3783–3797. doi: 10.4049/jimmunol.1201449. [DOI] [PubMed] [Google Scholar]
- 37.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 38.Wu L, Du H, Li Y, Qu P, Yan C. Signal transducer and activator of transcription 3 (Stat3C) promotes myeloid-derived suppressor cell expansion and immune suppression during lung tumorigenesis. Am J Pathol. 2011;179:2131–2141. doi: 10.1016/j.ajpath.2011.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu S, Cao H, Shen B, Feng J. Tumor-derived exosomes in cancer progression and treatment failure. Oncotarget. 2015;6:37151–37168. doi: 10.18632/oncotarget.6022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, Willemsen R, Demmers JA, Stalin Raj V, Jenster G, Kwekkeboom J, Tilanus HW, Haagmans BL, Baumert TF, van der Laan LJ. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A. 2013;110:13109–13113. doi: 10.1073/pnas.1221899110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu Z, Zhang X, Yu Q, He JJ. Exosome-associated hepatitis C virus in cell cultures and patient plasma. Biochem Biophys Res Commun. 2014;455:218–222. doi: 10.1016/j.bbrc.2014.10.146. [DOI] [PubMed] [Google Scholar]
- 42.Demory Beckler M, Higginbotham JN, Franklin JL, Ham AJ, Halvey PJ, Imasuen IE, Whitwell C, Li M, Liebler DC, Coffey RJ. Proteomic analysis of exosomes from mutant KRAS colon cancer cells identifies intercellular transfer of mutant KRAS. Mol Cell Proteomics. 2013;12:343–355. doi: 10.1074/mcp.M112.022806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Antonyak MA, Cerione RA. Microvesicles as mediators of intercellular communication in cancer. Methods Mol Biol. 2014;1165:147–173. doi: 10.1007/978-1-4939-0856-1_11. [DOI] [PubMed] [Google Scholar]
- 44.Sanderson MP, Keller S, Alonso A, Riedle S, Dempsey PJ, Altevogt P. Generation of novel, secreted epidermal growth factor receptor (EGFR/ErbB1) isoforms via metalloprotease-dependent ectodomain shedding and exosome secretion. J Cell Biochem. 2008;103:1783–1797. doi: 10.1002/jcb.21569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang H, Deng T, Liu R, Bai M, Zhou L, Wang X, Li S, Yang H, Li J, Ning T, Huang D, Li H, Zhang L, Ying G, Ba Y. Exosome-delivered EGFR regulates liver microenvironment to promote gastric cancer liver metastasis. Nat Commun. 2017;8:15016. doi: 10.1038/ncomms15016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chalmin F, Ladoire S, Mignot G, Vincent J, Bruchard M, Remy-Martin JP, Boireau W, Rouleau A, Simon B, Lanneau D, De Thonel A, Multhoff G, Hamman A, Martin F, Chauffert B, Solary E, Zitvogel L, Garrido C, Ryffel B, Borg C, Apetoh L, Rebe C, Ghiringhelli F. Membrane-associated Hsp72 from tumor-derived exosomes mediates STAT3-dependent immunosuppressive function of mouse and human myeloid-derived suppressor cells. J Clin Invest. 2010;120:457–471. doi: 10.1172/JCI40483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang SH, Li Y, Zhang J, Rong J, Ye S. Epidermal growth factor receptor-containing exosomes induce tumor-specific regulatory T cells. Cancer Invest. 2013;31:330–335. doi: 10.3109/07357907.2013.789905. [DOI] [PubMed] [Google Scholar]
- 48.Thress KS, Jacobs V, Angell HK, Yang JC, Sequist LV, Blackhall F, Su WC, Schuler M, Wolf J, Gold KA, Cantarini M, Barrett JC, Janne PA. Modulation of biomarker expression by osimertinib: results of the paired tumor biopsy cohorts of the AURA phase I trial. J Thorac Oncol. 2017;12:1588–1594. doi: 10.1016/j.jtho.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 49.Garassino MC, Cho BC, Kim JH, Mazieres J, Vansteenkiste J, Lena H, Corral Jaime J, Gray JE, Powderly J, Chouaid C, Bidoli P, Wheatley-Price P, Park K, Soo RA, Huang Y, Wadsworth C, Dennis PA, Rizvi NA. Durvalumab as third-line or later treatment for advanced non-small-cell lung cancer (ATLANTIC): an open-label, single-arm, phase 2 study. Lancet Oncol. 2018;19:521–536. doi: 10.1016/S1470-2045(18)30144-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Antonia SJ, Villegas A, Daniel D, Vicente D, Murakami S, Hui R, Yokoi T, Chiappori A, Lee KH, de Wit M, Cho BC, Bourhaba M, Quantin X, Tokito T, Mekhail T, Planchard D, Kim YC, Karapetis CS, Hiret S, Ostoros G, Kubota K, Gray JE, Paz-Ares L, de Castro Carpeno J, Wadsworth C, Melillo G, Jiang H, Huang Y, Dennis PA, Ozguroglu M. Durvalumab after chemoradiotherapy in Stage III non-small-cell lung cancer. N Engl J Med. 2017;377:1919–1929. doi: 10.1056/NEJMoa1709937. [DOI] [PubMed] [Google Scholar]
- 51.Cheng H, Janakiram M, Borczuk A, Lin J, Qiu W, Liu H, Chinai JM, Halmos B, Perez-Soler R, Zang X. HHLA2, a new immune checkpoint member of the B7 family, is widely expressed in human lung cancer and associated with EGFR mutational status. Clin Cancer Res. 2017;23:825–832. doi: 10.1158/1078-0432.CCR-15-3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yue EW, Sparks R, Polam P, Modi D, Douty B, Wayland B, Glass B, Takvorian A, Glenn J, Zhu W, Bower M, Liu X, Leffet L, Wang Q, Bowman KJ, Hansbury MJ, Wei M, Li Y, Wynn R, Burn TC, Koblish HK, Fridman JS, Emm T, Scherle PA, Metcalf B, Combs AP. INCB24360 (Epacadostat), a highly potent and selective indoleamine-2,3-dioxygenase 1 (IDO1) inhibitor for immuno-oncology. ACS Med Chem Lett. 2017;8:486–491. doi: 10.1021/acsmedchemlett.6b00391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kristeleit R, Davidenko I, Shirinkin V, El-Khouly F, Bondarenko I, Goodheart MJ, Gorbunova V, Penning CA, Shi JG, Liu X, Newton RC, Zhao Y, Maleski J, Leopold L, Schilder RJ. A randomised, open-label, phase 2 study of the IDO1 inhibitor epacadostat (INCB024360) versus tamoxifen as therapy for biochemically recurrent (CA-125 relapse)-only epithelial ovarian cancer, primary peritoneal carcinoma, or fallopian tube cancer. Gynecol Oncol. 2017;146:484–490. doi: 10.1016/j.ygyno.2017.07.005. [DOI] [PubMed] [Google Scholar]


