Abstract
An increasing number of promising immunotherapies and related clinical trials have led to several major breakthroughs in multiple cancers, but a reliable and precise biomarker for evaluating efficacy and prognosis has not yet been established. As a typical representation of a liquid biopsy, circulating cell-free DNA (ctDNA) possesses the functions and advantages of tissue biopsy but its distinct advantages of convenience, real-time nature, non-invasiveness and homogeneity make it superior to tissue biopsy. Indeed, compared with routine imaging and tumor markers, ctDNA offers an earlier indication and provides more precise information. ctDNA is reportedly able to identify immunotherapy responders, evaluate efficacy and survival time, screen immune checkpoint inhibitor resistance and pseudo-progress and predict tumor recurrence and metastasis. Thus, ctDNA can act as an “Eagle Eye” by comprehensively monitoring both macro- and micro-changes in the immunotherapy process. Although ctDNA has become a research topic of interest, its limitations cannot be ignored, and improvements in its sensitivity and standardization are urgently needed. This review reveals the advantages and limitations of ctDNA as a precise biomarker and supports the feasibility of using ctDNA detection for common monitoring during immunotherapy.
Keywords: Biomarker, ctDNA, efficacy evaluation, immunotherapy, pseudo-progress
Overview of ctDNA
The emergence and gradual maturity of liquid biopsy has resulted in rapid changes to clinical diagnostic criteria and treatment measures for many tumors. The main components of a liquid biopsy include circulating tumor cells (CTCs), circulating cell-free DNA (cfDNA) and circulating tumor DNA (ctDNA). CTC is a type of tumor cell that spontaneously or passively detaches from the primary malignant tumor or site of metastasis and is released into the peripheral blood circulation [1,2]. ctDNA refers to free fragmented DNA that is released by apoptotic or necrotic tumor cells into the circulatory system [3-5], and these fragments of the tumor genome provide insight into multiple genomic features at the molecular level, such as deletion and insertion mutations. The detection of ctDNA requires only peripheral blood samples and has the additional advantages of being able to be used in real time, non-invasiveness, high reproducibility and homogeneity [6]. In the future, liquid biopsy is likely to play an important and irreplaceable role in clinical practice [7,8].
The mainstream ctDNA detection methods involve amplification refractory mutation systems (ARMSs) [9], droplet digital PCR (ddPCR) [10,11], and next-generation sequencing (NGS) [12,13]. ARMSs have a moderate cost, are simple to operate, and can sequentially detect multiple mutations in a single gene, but its sensitivity is inferior to that of ddPCR. Although ddPCR is highly sensitive, it is expensive and involves a complex procedure, and many samples and multiple tests are needed to reveal multiple mutations. NGS, which is currently the most commonly used method, has high sensitivity and is capable of simultaneously detecting multiple loci of multiple genes. There are also alternative methods for ctDNA detection, such as tagged-amplicon deep sequencing [14] and cancer personalized profiling by deep sequencing [15,16].
Application of ctDNA in clinical immunotherapy
Immunotherapy is different from traditional chemotherapy and targeted therapy because it not only targets tumor cells or immune cells but also regulates the immune microenvironment [17-19]. Immunotherapy, which can be represented by the immune checkpoint blockade (ICB), is a milestone in the progress of the ongoing struggle against cancer. Immunotherapy has the advantages of having excellent efficacy with few side effects and has thus attracted the attention of researchers. Moreover, an increasing number of new immunotherapies and related clinical trials have led to multiple major breakthroughs in metastatic melanoma, kidney cancer, bladder cancer, non-small cell lung cancer, small cell lung cancer, gastric cancer, prostate cancer, breast cancer, liver cancer and others [20-32]. With the rapid development of the immunology field and its overlap with oncology and molecular biology, the perspectives on immune escape, immune tolerance and, in particular, immunotherapy are constantly being updated.
In the age of precise treatment, a profound understanding of tumorigenesis at the molecular and genetic levels helps guide antitumor treatments effectively. ctDNA can reveal genomic changes in the primary tumor and metastatic lesions as well as during dynamic tumor progression in real time. Researchers have attempted to apply ctDNA to guide chemotherapy, targeted therapy and radiotherapy [33-35], but its clinical value in immunotherapy remains unclear. According to the published literature, studies regarding ctDNA-guided immunotherapy mostly focused on melanoma [36], and other tumors have only sporadically been addressed. Figure 1 shows a summary of the mechanisms and clinical applications of ctDNA, and the major ctDNA-related characteristics reported in previous studies are provided in Table 1. The applications of ctDNA are discussed in later sections.
Figure 1.
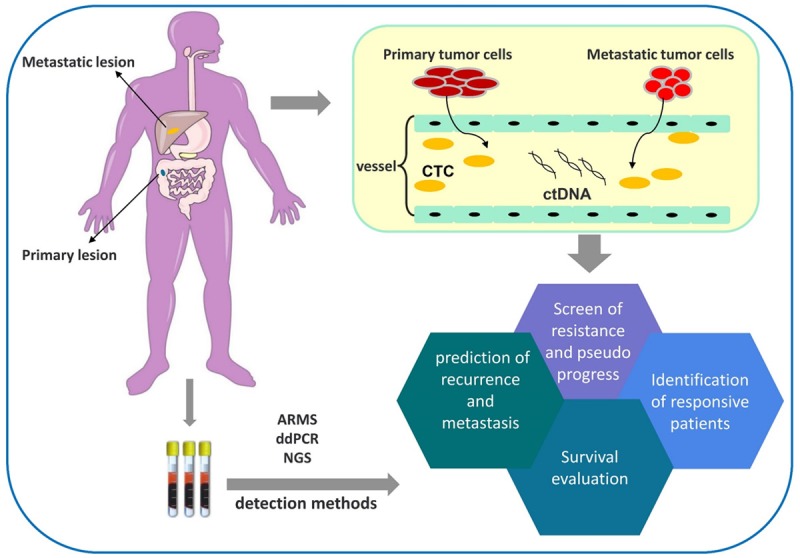
Clinical applications of ctDNA.
Table 1.
Common characteristics of the studies
| References Number | Types | Detection methods | Immune checkpoint inhibitors | ICB points | Mutation gene(s) |
|---|---|---|---|---|---|
| [41] | Melanoma | ddPCR | Nivolumab | PD-1 | BRAF/NRAS |
| [39] | Melanoma | ddPCR | Nivolumab | PD-1 | BRAF V600E/K/R |
| Pembrolizumab | PD-L1 | ||||
| [44] | MM | ddPCR | BMS-936559 | PD-L1 | BRAF/NRAS/TERT/ALK |
| NGS | Ipilimumab | CTLA-4 | |||
| [37] | MM | ddPCR | Nivolumab | PD-1 | BRAF V600E/K |
| Pembrolizumab | CTLA-4 | ||||
| Ipilimumab | |||||
| [38] | NSCLC/ | ddPCR | Nivolumab | PD-1 | KRAS/BRAF/GNA11/EGFR/PTPN11/NF1/KEAP11/TP53/HRAS/GNAQ/ERBB3 |
| UM/ | Bi-RAP | Pembrolizumab | |||
| MSI+CRC | NGS | ||||
| [40] | MM | castPCR | Not reported | PD-1 | BRAF V600E |
| PD-L1 | |||||
| [43] | SCAC | ddPCR | Nivolumab | PD-1 | Not reported |
| [6] | Metastatic adenocarcinoma | ddPCR | Nivolumab | PD-1 | KRAS |
Note: ICB, Immune checkpoint blockade. NGS, Next-generation sequencing. ddPCR, Droplet digital PCR. MM, Metastatic melanoma. NSCLC, Non-small cell lung cancer. UM, Uveal melanoma. MSI, Microsatellite instability. CRC, Colorectal cancer. Bi-PAP, Bidirectionalpyrophosphorolysis-activated polymerization PCR. castPCR, Competitive allele-specific 114 TaqMan PCR. SCAC, Squamous cell carcinoma of the anal canal.
Identification of patients responsive to immunotherapy
ctDNA conveys the genetic mutation information of tumors and can indicate changes in the tumor burden in real time, which would aid the identification of sensitive patients who will respond to immunotherapy and guide the treatment decisions of physicians. Previous studies have found that patients with low or undetected levels of ctDNA at the beginning of and during therapy might show a better response to immunotherapy [37,38]. The study conducted by Gray ES [39] demonstrated that the response potential of patients with low baseline ctDNA levels (< 10 copies/ml) after treatment with ipilimumab, nivolumab or pembrolizumab was five-fold higher than that of patients with high baseline ctDNA levels (95% CI 1.8-13.8, P = 0.009). After 8 weeks of these above-mentioned treatments, a decrease in the ctDNA concentration was positively correlated with the efficacy of the treatment. A study by Xi L [40] showed a strong correlation between the peak of the BRAF ctDNA level and the likelihood of an objective response, and a rapid decline in ctDNA indicated that these patients might achieve a complete response (CR) after treatment. Among 13 patients who showed this tendency, nine achieved a CR, and four exhibited a partial response (PR). Ashida A [41] studied the relationship between ctDNA and anti-PD-1 immunotherapy in five melanoma patients and found that the level of ctDNA decreased within 2-4 weeks in the three responsive patients but remained at a high level in the two unresponsive patients. Moreover, Goldberg SB examined 182 serial plasma samples from 49 metastatic non-small-cell lung cancer (NSCLC) patients receiving anti-PD-1 and/or anti-PD-L1 and found that the patients whose ctDNA levels decreased by more than 50% exhibited longer-term benefits compared with those whose ctDNA decreased by less than 50% (205.5 v 69 days, P < 0.001) [42].
Evaluation of efficacy and survival
Overall survival (OS) and progression-free survival (PFS) are indicators used to evaluate the efficacy of a treatment in patients with tumors, and studies have shown that low baseline ctDNA levels might be associated with prolonged OS and PFS. Cabel L detected the changes in ctDNA levels in 15 patients with non-small cell lung cancer, uveal melanoma, and microsatellite-instable colorectal cancer who received anti PD-1 therapy. Those with undetectable levels of ctDNA in week 0 exhibited prolonged OS compared with those with detectable levels of ctDNA, and the former patients had a 6.8-fikd higher risk of death than the former group (95% CI 1.1-41, P = 0.03) [38]. Goldberg SB’s study showed that patients with undetectable ctDNA at any point post-treatment exhibited superior OS compared with patients with detectable ctDNA (HR 0.11, 95% CI 0.02-0.88, P = 0.037) [42]. Gray ES also detected the levels of ctDNA in metastatic melanoma patients receiving anti-PD-1 and anti-CTLA-4 and found that the baseline ctDNA levels of patients with PFS > 6 months were lower than those of patients with PFS < 6 months (baseline ctDNA, 5 versus 87.2 copies/ml, P = 0.049). Thus, a lower baseline ctDNA level (< 10 copies/ml) is associated with longer PFS (HR = 3.7, 95% CI 1.2-12.5, P = 0.034) [39]. Nonetheless, only clinical examinations with a small number of samples have been published on this topic to date, and studies with larger samples are needed. The predictive value of ctDNA regarding the survival time of patients receiving extended immunotherapy has also been reported. Cabel L found that the PFS of patients with detectable ctDNA in week 8 was shorter than that of patients with undetectable ctDNA, and the recurrence risk of the former group was 10.2-fold higher than that of the latter group of patients (95% CI 2.5-4.0, median PFS, 2 v 11 months, P = 0.001) [38]. Lee JH investigated 86 melanoma patients who had received pembrolizumab or nivolumab monotherapy or ipilimumab combination therapy and found that the baseline ctDNA-/follow-up ctDNA- group and baseline ctDNA+/follow-up ctDNA- group had significantly longer PFS than the baseline ctDNA+/follow-up ctDNA+ group. Compared with the third group, the recurrence risk was decreased by 92% in the baseline ctDNA-/follow-up ctDNA- group and by 85% in the baseline ctDNA+/follow-up ctDNA- group (95% CI 0.03-0.20, P < 0.001; 95% CI 0.03-0.18, P < 0.001, respectively). The first two groups also exhibited significantly prolonged OS compared with the baseline ctDNA+/follow-up ctDNA+ group, with 89% and 88% reductions in the risk of death, respectively (95% CI 0.03-0.17, P < 0.001; 95% CI 0.05-0.43, P < 0.001) [37].
Screening for resistance and pseudo-progress
Tumor cells will inevitably escape ICB after a period of treatment, and immunotherapy resistance and disease progression will occur [41]. However, in the early stage of immunotherapy, the inflammatory reaction induced by ICB usually results in the appearance of larger tumor lesions or new lesions before the tumor actually shrinks, and this phenomenon is called pseudo-progression [43,44]. However, imaging technology is insufficient for distinguishing pseudo-progression from drug resistance. The Response Evaluation Criteria in Solid Tumors (RECIST) evaluates changes as progressive disease (PD) but the assessment methods for new lesions and the evidence for judging disease progression have been updated in new immune-related RECIST (irRECIST). Regardless, the judgment process is not exact. Pseudo-progression causes clinicians to mistakenly presume that immunotherapy resistance has occurred [6]. While imaging is inadequate for judging pseudo-progression, ctDNA can provide a reflection of the tumor burden in real time. Specifically, the tumor mutation burden reflects somatic mutations and the production of new antigens at the RNA and protein levels. Higher ctDNA levels indicate better ICB efficacy, and continuously high levels predict true progress [45]. Guibert N observed pseudo-progression in two cases. One patient showed high levels of KRAS-mutated ctDNA at baseline at the beginning of anti-PD-1 treatment; on the 30th day, the ctDNA level showed a significant decreased to an undetectable level, and the ctDNA levels remained low on the 60th and 120th days. However, a CT scan on the 60th day showed that the mass had significantly increased in size (28 mm), and RECIST and irRECIST identified it as PD. However, on the 120th day, the mass was markedly smaller than its initial size, and this observation confirmed that the enlargement observed on the 60th day was associated with pseudo-progression. However, in the other patients, parallel changes in imaging and ctDNA indicated the true outcome of the immunotherapy applied [6]. Ashida A’s study [41] also provided evidence supporting the notion that ctDNA monitoring can be used to distinguish real progression from pseudo-progression: for patients with pseudo-progression, the ctDNA levels decrease rapidly and persistently, whereas patients with real progression exhibit significant increases in their ctDNA levels. Therefore, ctDNA monitoring at different time points aids the identification of true resistance from pseudo-progression (Figure 2).
Figure 2.
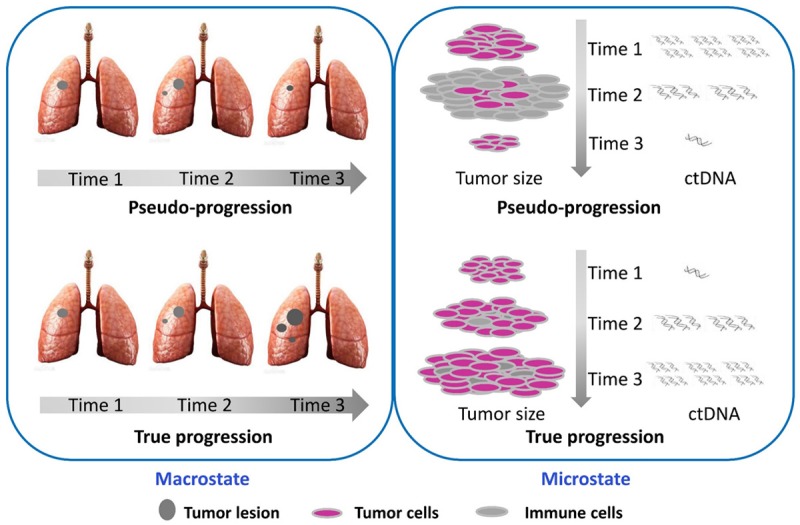
The comparison of pseudo-progression and true progression in Macrostate and microstate.
Prediction of tumor recurrence and metastasis
Resistance to immunotherapy inevitably leads to tumor progression. CTCs, which might be present in blood, form a tumor embolus after a series of migration, adhesion and aggregation steps [46,47] and eventually cause tumor recurrence and metastasis [48]. Because ctDNA is derived from necrotic or apoptotic CTCs, an increase in concentrations might predict the risk of recurrence and metastasis to a certain extent [43]. In Ashida A’s study, a decline in the ctDNA level in patients with the BRAF V600E mutation who received nivolumab indicated stable disease, whereas an increase in the ctDNA level indicated PD after 210 days, and imaging data obtained on the 240th day confirmed this result [41]. Thus, ctDNA might serve as a warning indicator before the actual occurrence of metastasis and, in the future, might help clinicians eliminate budding metastases.
Comparison between ctDNA and known assessment methods
The evaluation methods that have been developed for immunotherapy include tissue biopsy, imaging, PD-1, PD-L1, CTC and LDH, and each of these methods has its own advantages and disadvantages.
Comparison with tissue biopsy
The detection of gene changes by tissue biopsy is the most precise method for guiding treatment. However, the routine application of this method in the clinic is limited by small tumor tissues, and repeat puncturing is not suitable for patients [49]. Moreover, a biopsy is risky when the lesion is adjacent to the heart or large vessels [50], and the characteristics of the entire tumor might not be fully revealed due to heterogeneity. Conversely, ctDNA contains information of the entire genome and overcomes the aforementioned limitations. At present, tissue biopsy is the main method, and ctDNA detection is supplementary. However, with the maturity and perfection of ctDNA detection technology, this approach might become one of the dominant diagnostic methods.
Comparison with imaging
Although CT or MRI scans are traditional and widely accepted imaging methods for evaluating the efficacy of immunotherapy, imaging cannot distinguish a tumor from inflammation in the lesion [48,51]. Additionally, imaging has difficulties in distinguishing true progression from pseudo-progression in real time, which causes interruption of effective treatment. However, ctDNA overcomes these limitations. Goldberg SB found that a change in the ctDNA level occurred 42.5 days earlier than imaging changes (P = 0.004), which suggests that ctDNA might be a better assessment method. In addition to its simple operation and minimally invasive nature, ctDNA might more accurately reflect real changes in a tumor during the entire course of treatment [6,38,39,41].
Comparison with PD-1, PD-L1
In the tumor immune microenvironment, the interaction between PD-1 and PD-L1 induces apoptosis and exhaustion of tumor-specific T cells and inhibits the proliferation and differentiation of T cells [52,53]. Therefore, blocking the PD-1/PD-L1 signaling pathway reactivates the immune system to kill tumor cells. A meta-analysis by Passiglia F, which included seven studies and 914 patients with NSCLC who received PD-1/PD-L1 inhibitors, showed that PD-L1-positive patients (cut-off > 1%) had higher response rates to immunotherapy than PD-L1-negative patients (OR = 2.44, 95% CI 1.61-3.68, P < 0.0001). PD-L1 has been used as a biomarker to predict the effectiveness of ICBs [54,55]. However, there is no uniform international standard for PD-L1 detection, and in immunohistochemistry, differences among antibodies, detection methods and cut-off values might lead to inconsistent results [56,57]. In a phase II clinical trial with atezolizumab, Herbst observed that repeated PD-L1 detection results were not consistent due to tumor heterogeneity, even though the specimens were obtained from areas adjacent to the tumor. Therefore, PD-L1 as a single-effect predictor is insufficient [58]. Moreover, the detection of PD-L1 requires tissue puncture, which results in obvious trauma to the patients. Conversely, ctDNA is detected in a simple, convenient and noninvasive manner. It can be acquired repeatedly and continuously and is capable of dynamically monitoring the tumor burden, which cannot be achieved with PD-L1 detection. Moreover, ctDNA naturally overcomes the heterogeneity of primary and metastatic lesions, which can hardly be realized with PD-L1, even when multiple biopsy specimens are analyzed.
Comparison with CTC
CTC only provides tumor information at the cellular level, which is insufficient in the age of precise treatment. Freidin MB detected KRAS mutations by ctDNA and CTC and found that the sensitivity of ctDNA and CTC was 0.96 and 0.52 and their specificity was 0.95 and 0.88, respectively, which demonstrated that CTC was significantly inferior to ctDNA with regard to diagnostic accuracy [59]. Dawson’s study also verified that ctDNA exhibits a higher correlation with the tumor burden and shows higher sensitivity for reflecting tumor burden changes compared with CTC [60].
Comparison with LDH
In melanoma patients, LDH is positively correlated with a high tumor burden and a low survival rate [61]. Because the detection of LDH is economical and convenient, it is widely used as an alternative indicator for predicting the progression of melanoma [62]. However, because LDH increases during infection, liver dysfunction, or cardiomyopathy, its specificity is poor [63]. Conversely, ctDNA is not affected by these factors. Gray ES [39] found a remarkable correlation between ctDNA and LDH in advanced metastatic melanoma patients who received ICBs (r = 0.76, P < 0.0001). Furthermore, Ashida A’s study showed that ctDNA is more sensitive than LDH in providing feedback on tumor progression in patients with advanced melanoma treated with nivolumab [41]. Lee JH’s research found that only 37% of advanced melanoma patients had a higher LDH, and the highest LDH level was only 2.5-fold higher than the normal level, which limits the usefulness of this molecule as a biomarker for predicting efficacy [37]. Therefore, ctDNA is a more accurate biomarker than LDH.
Summary
The determination of effective tumor biomarkers will help achieve a breakthrough in individualized immunotherapy. The detection of ctDNA, as a typical component of liquid biopsy, can be performed in real time and is non-invasive, repeatable and capable of overcoming the challenge of tumor heterogeneity. This approach will gradually become a part of routine clinical diagnosis. Although ctDNA is a novel method for monitoring immunotherapy efficacy, its limitations cannot be ignored. An urgent problem to overcome is improving its sensitivity, and quantification and standardization are other issues that need to be resolved. Thus far, immune clinical trials on ctDNA have only involved small samples, and the results are not highly persuasive. Prospective immunologic clinical trials involving patients with various cancers at different clinical stages and with multiple gene mutations are encouraged. We hope that in the near future, ctDNA testing will become a main method for the assessment of immunotherapy efficacy.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (Grant NO. 81301912), the Beijing Municipal Health System High-level Health Person Foundation Project (Grant NO. 2014-3-005), the Beijing Municipal Science and Technology Commission Foundation (Capital Features, Z161100000516083, to Qin Li) and the Natural Science Foundation of Capital Medical University (to Qin Li).
Disclosure of conflict of interest
None.
Abbreviations
- ICB
immune checkpoint blockade
- CTC
circulating tumor cells
- cfDNA
circulating cell-free DNA
- ctDNA
circulating tumor DNA
- NSCLC
non-small-cell lung cancer
- ARMS
amplification refractory mutation system
- ddPCR
droplet digital PCR
- NGS
next-generation sequencing
- CR
complete response
- PR
partial response
- OS
Overall survival
- PFS
progression-free survival
- RECIST
Response Evaluation Criteria in Solid Tumors
References
- 1.Azevedo AS, Follain G, Patthabhiraman S, Harlepp S, Goetz JG. Metastasis of circulating tumor cells: favorable soil or suitable biomechanics, or both. Cell Adh Migr. 2015;9:345–356. doi: 10.1080/19336918.2015.1059563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hong B, Zu Y. Detecting circulating tumor cells: current challenges and new trends. Theranostics. 2013;3:377–394. doi: 10.7150/thno.5195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melo SA, Luecke LB, Kahlert C, Fernandez AF, Gammon ST, Kaye J, LeBleu VS, Mittendorf EA, Weitz J, Rahbari N, Reissfelder C, Pilarsky C, Fraga MF, Piwnica-Worms D, Kalluri R. Glypican-1 identifies cancer exosomes and detects early pancreatic cancer. Nature. 2015;523:177–182. doi: 10.1038/nature14581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schwarzenbach H, Hoon DS, Pantel K. Cell-free nucleic acids as biomarkers in cancer patients. Nat Rev Cancer. 2011;11:426–437. doi: 10.1038/nrc3066. [DOI] [PubMed] [Google Scholar]
- 5.Cheng F, Su L, Qian C. Circulating tumor DNA: a promising biomarker in the liquid biopsy of cancer. Oncotarget. 2016;7:48832–48841. doi: 10.18632/oncotarget.9453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guibert N, Mazieres J, Delaunay M, Casanova A, Farella M, Keller L, Favre G, Pradines A. Monitoring of KRAS-mutated ctDNA to discriminate pseudo-progression from true progression during anti-PD-1 treatment of lung adenocarcinoma. Oncotarget. 2017;8:38056–38060. doi: 10.18632/oncotarget.16935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ilié M, Hofman P. Pros: can tissue biopsy be replaced by liquid biopsy. Transl Lung Cancer Res. 2016;5:420–423. doi: 10.21037/tlcr.2016.08.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu M, Chia D, Wei F, Wong D. Liquid biopsy for detection of actionable oncogenic mutations in human cancers and electric field induced release and measurement liquid biopsy (eLB) Analyst. 2016;141:393–402. doi: 10.1039/c5an01863c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Song Z, Zhang Y. A comparison of ddPCR and ARMS for detecting EGFR T790M status in ctDNA from advanced NSCLC patients with acquired EGFR-TKI resistance. Cancer Med. 2017;6:154–162. doi: 10.1002/cam4.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sacher AG, Paweletz C, Dahlberg SE, Alden RS, O’Connell A, Feeney N, Mach SL, Jänne PA, Oxnard GR. Prospective validation of rapid plasma genotyping for the detection of EGFR and KRAS mutations in advanced lung cancer. JAMA Oncol. 2016;2:1014–1022. doi: 10.1001/jamaoncol.2016.0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koepfli C, Nguitragool W, Hofmann NE, Robinson LJ, Ome-Kaius M, Sattabongkot J, Felger I, Mueller I. Sensitive and accurate quantification of human malaria parasites using droplet digital PCR (ddPCR) Sci Rep. 2016;6:39183. doi: 10.1038/srep39183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aly SM, Sabri DM. Next generation sequencing (NGS): a golden tool in forensic toolkit. Arch Med Sadowej Kryminol. 2015;65:260–271. doi: 10.5114/amsik.2015.61029. [DOI] [PubMed] [Google Scholar]
- 13.Li J, Batcha AM, Grüning B, Mansmann UR. An NGS workflow blueprint for DNA sequencing data and its application in individualized molecular oncology. Cancer Inform. 2015;14:87–107. doi: 10.4137/CIN.S30793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Forshew T, Murtaza M, Parkinson C, Gale D, Tsui DW, Kaper F, Dawson SJ, Piskorz AM, Jimenez-Linan M, Bentley D, Hadfield J, May AP, Caldas C, Brenton JD, Rosenfeld N. Noninvasive identification and monitoring of cancer mutations by targeted deep sequencing of plasma DNA. Sci Transl Med. 2012;4:136ra68. doi: 10.1126/scitranslmed.3003726. [DOI] [PubMed] [Google Scholar]
- 15.Newman AM, Bratman SV, To J, Wynne JF, Eclov NC, Modlin LA, Liu CL, Neal JW, Wakelee HA, Merritt RE, Shrager JB, Loo BW, Alizadeh AA, Diehn M. An ultrasensitive method for quantitating circulating tumor DNA with broad patient coverage. Nat Med. 2014;20:548–554. doi: 10.1038/nm.3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Newman AM, Lovejoy AF, Klass DM, Kurtz DM, Chabon JJ, Scherer F, Stehr H, Liu CL, Bratman SV, Say C, Zhou L, Carter JN, West RB, Sledge GW, Shrager JB, Loo BW, Neal JW, Wakelee HA, Diehn M, Alizadeh AA. Integrated digital error suppression for improved detection of circulating tumor DNA. Nat Biotechnol. 2016;34:547–555. doi: 10.1038/nbt.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pardoll D. Cancer and the immune system: basic concepts and targets for intervention. Semin Oncol. 2015;42:523–538. doi: 10.1053/j.seminoncol.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Topalian SL, Drake CG, Pardoll DM. Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell. 2015;27:450–461. doi: 10.1016/j.ccell.2015.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–287. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hodi FS, O’Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC, Akerley W, van den Eertwegh AJ, Lutzky J, Lorigan P, Vaubel JM, Linette GP, Hogg D, Ottensmeier CH, Lebbé C, Peschel C, Quirt I, Clark JI, Wolchok JD, Weber JS, Tian J, Yellin MJ, Nichol GM, Hoos A, Urba WJ. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS, Shaheen M, Ernstoff MS, Minor D, Salama AK, Taylor M, Ott PA, Rollin LM, Horak C, Gagnier P, Wolchok JD, Hodi FS. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med. 2015;372:2006–2017. doi: 10.1056/NEJMoa1414428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, Desrichard A, Walsh LA, Postow MA, Wong P, Ho TS, Hollmann TJ, Bruggeman C, Kannan K, Li Y, Elipenahli C, Liu C, Harbison CT, Wang L, Ribas A, Wolchok JD, Chan TA. Genetic basis for clinical response to CTLA-4 blockade in melanoma. N Engl J Med. 2014;371:2189–2199. doi: 10.1056/NEJMoa1406498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Allen EM, Miao D, Schilling B, Shukla SA, Blank C, Zimmer L, Sucker A, Hillen U, MHG F, Goldinger SM, Utikal J, Hassel JC, Weide B, Kaehler KC, Loquai C, Mohr P, Gutzmer R, Dummer R, Gabriel S, Wu CJ, Schadendorf D, Garraway LA. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science. 2015;350:207–211. doi: 10.1126/science.aad0095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nathanson T, Ahuja A, Rubinsteyn A, Aksoy BA, Hellmann MD, Miao D, Van Allen E, Merghoub T, Wolchok JD, Snyder A, Hammerbacher J. Somatic mutations and neoepitope homology in melanomas treated with CTLA-4 blockade. Cancer Immunol Res. 2017;5:84–91. doi: 10.1158/2326-6066.CIR-16-0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rizvi NA, Hellmann MD, Snyder A, Kvistborg P, Makarov V, Havel JJ, Lee W, Yuan J, Wong P, Ho TS, Miller ML, Rekhtman N, Moreira AL, Ibrahim F, Bruggeman C, Gasmi B, Zappasodi R, Maeda Y, Sander C, Garon EB, Merghoub T, Wolchok JD, Schumacher TN, Chan TA. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science. 2015;348:124–128. doi: 10.1126/science.aaa1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vandekerkhove G, Todenhöfer T, Annala M, Struss WJ, Wong A, Beja K, Ritch E, Brahmbhatt S, Volik SV, Hennenlotter J, Nykter M, Chi KN, North S, Stenzl A, Collins CC, Eigl BJ, Black PC, Wyatt AW. Circulating tumor DNA reveals clinically actionable somatic genome of metastatic bladder cancer. Clin Cancer Res. 2017;23:6487–6497. doi: 10.1158/1078-0432.CCR-17-1140. [DOI] [PubMed] [Google Scholar]
- 27.Giaccone G, Debruyne C, Felip E, Chapman PB, Grant SC, Millward M, Thiberville L, D’addario G, Coens C, Rome LS, Zatloukal P, Masso O, Legrand C. Phase III study of adjuvant vaccination with Bec2/bacille Calmette-Guerin in responding patients with limited-disease small-cell lung cancer (European Organisation for Research and Treatment of Cancer 08971-08971B; Silva Study) J. Clin. Oncol. 2005;23:6854–6864. doi: 10.1200/JCO.2005.17.186. [DOI] [PubMed] [Google Scholar]
- 28.Song Z, Wu Y, Yang J, Yang D, Fang X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017;39:1010428317714626. doi: 10.1177/1010428317714626. [DOI] [PubMed] [Google Scholar]
- 29.Hammerstrom AE, Cauley DH, Atkinson BJ, Sharma P. Cancer immunotherapy: sipuleucel-T and beyond. Pharmacotherapy. 2011;31:813–828. doi: 10.1592/phco.31.8.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lin XL, Wang XL, Ma B, Jia J, Yan Y, Di LJ, Yuan YH, Wan FL, Lu YL, Liang X, Shen T, Ren J. HER2-specific T lymphocytes kill both trastuzumab-resistant and trastuzumab-sensitive breast cell lines in vitro. Chin J Cancer Res. 2012;24:143–150. doi: 10.1007/s11670-012-0143-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, Biedrzycki B, Donehower RC, Zaheer A, Fisher GA, Crocenzi TS, Lee JJ, Duffy SM, Goldberg RM, de la Chapelle A, Koshiji M, Bhaijee F, Huebner T, Hruban RH, Wood LD, Cuka N, Pardoll DM, Papadopoulos N, Kinzler KW, Zhou S, Cornish TC, Taube JM, Anders RA, Eshleman JR, Vogelstein B, Diaz LA. PD-1 Blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nishida N, Kudo M. Alteration of epigenetic profile in human hepatocellular carcinoma and its clinical implications. Liver Cancer. 2014;3:417–427. doi: 10.1159/000343860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tie J, Wang Y, Tomasetti C, Li L, Springer S, Kinde I, Silliman N, Tacey M, Wong HL, Christie M, Kosmider S, Skinner I, Wong R, Steel M, Tran B, Desai J, Jones I, Haydon A, Hayes T, Price TJ, Strausberg RL, Diaz LA, Papadopoulos N, Kinzler KW, Vogelstein B, Gibbs P. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med. 2016;8:346ra92. doi: 10.1126/scitranslmed.aaf6219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ratajska M, Koczkowska M, Żuk M, Gorczyński A, Kuźniacka A, Stukan M, Biernat W, Limon J, Wasąg B. Detection of BRCA1/2 mutations in circulating tumor DNA from patients with ovarian cancer. Oncotarget. 2017;8:101325–101332. doi: 10.18632/oncotarget.20722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chaudhuri AA, Binkley MS, Osmundson EC, Alizadeh AA, Diehn M. Predicting radiotherapy responses and treatment outcomes through analysis of circulating tumor DNA. Semin Radiat Oncol. 2015;25:305–312. doi: 10.1016/j.semradonc.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oellerich M, Schütz E, Beck J, Kanzow P, Plowman PN, Weiss GJ, Walson PD. Using circulating cell-free DNA to monitor personalized cancer therapy. Crit Rev Clin Lab Sci. 2017;54:205–218. doi: 10.1080/10408363.2017.1299683. [DOI] [PubMed] [Google Scholar]
- 37.Lee JH, Long GV, Boyd S, Lo S, Menzies AM, Tembe V, Guminski A, Jakrot V, Scolyer RA, Mann GJ, Kefford RF, Carlino MS, Rizos H. Circulating tumour DNA predicts response to anti-PD1 antibodies in metastatic melanoma. Ann Oncol. 2017;28:1130–1136. doi: 10.1093/annonc/mdx026. [DOI] [PubMed] [Google Scholar]
- 38.Cabel L, Riva F, Servois V, Livartowski A, Daniel C, Rampanou A, Lantz O, Romano E, Milder M, Buecher B, Piperno-Neumann S, Bernard V, Baulande S, Bieche I, Pierga JY, Proudhon C, Bidard FC. Circulating tumor DNA changes for early monitoring of anti-PD1 immunotherapy: a proof-of-concept study. Ann Oncol. 2017;28:1996–2001. doi: 10.1093/annonc/mdx212. [DOI] [PubMed] [Google Scholar]
- 39.Gray ES, Rizos H, Reid AL, Boyd SC, Pereira MR, Lo J, Tembe V, Freeman J, Lee JH, Scolyer RA, Siew K, Lomma C, Cooper A, Khattak MA, Meniawy TM, Long GV, Carlino MS, Millward M, Ziman M. Circulating tumor DNA to monitor treatment response and detect acquired resistance in patients with metastatic melanoma. Oncotarget. 2015;6:42008–42018. doi: 10.18632/oncotarget.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xi L, Pham TH, Payabyab EC, Sherry RM, Rosenberg SA, Raffeld M. Circulating tumor DNA as an early indicator of response to T-cell transfer immunotherapy in metastatic melanoma. Clin Cancer Res. 2016;22:5480–5486. doi: 10.1158/1078-0432.CCR-16-0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ashida A, Sakaizawa K, Uhara H, Okuyama R. Circulating tumour DNA for monitoring treatment response to anti-pd-1 immunotherapy in melanoma patients. Acta Derm Venereol. 2017;97:1212–1218. doi: 10.2340/00015555-2748. [DOI] [PubMed] [Google Scholar]
- 42.Goldberg SB, Narayan A, Kole AJ, Decker RH, Teysir J, Carriero NJ, Lee A, Nemati R, Nath SK, Mane SM, Deng Y, Sukumar N, Zelterman D, Boffa DJ, Politi K, Gettinger SN, Wilson LD, Herbst RS, Patel AA. Early assessment of lung cancer immunotherapy response via circulating tumor DNA. Clin Cancer Res. 2018;24:1872–1880. doi: 10.1158/1078-0432.CCR-17-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cabel L, Bidard FC, Servois V, Cacheux W, Mariani P, Romano E, Minsat M, Bieche I, Farkhondeh F, Jeannot E, Buecher B. HPV circulating tumor DNA to monitor the efficacy of anti-PD-1 therapy in metastatic squamous cell carcinoma of the anal canal: a case report. Int J Cancer. 2017;141:1667–1670. doi: 10.1002/ijc.30863. [DOI] [PubMed] [Google Scholar]
- 44.Lipson EJ, Velculescu VE, Pritchard TS, Sausen M, Pardoll DM, Topalian SL, Diaz LA. Circulating tumor DNA analysis as a real-time method for monitoring tumor burden in melanoma patients undergoing treatment with immune checkpoint blockade. J Immunother Cancer. 2014;2:42. doi: 10.1186/s40425-014-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 inhibition. N Engl J Med. 2017;377:2500–2501. doi: 10.1056/NEJMc1713444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spring BQ, Palanisami A, Hasan T. Microscale receiver operating characteristic analysis of micrometastasis recognition using activatable fluorescent probes indicates leukocyte imaging as a critical factor to enhance accuracy. J Biomed Opt. 2014;19:066006. doi: 10.1117/1.JBO.19.6.066006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Massagué J, Obenauf AC. Metastatic colonization by circulating tumour cells. Nature. 2016;529:298–306. doi: 10.1038/nature17038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tjensvoll K, Nordgård O, Smaaland R. Circulating tumor cells in pancreatic cancer patients: methods of detection and clinical implications. Int J Cancer. 2014;134:1–8. doi: 10.1002/ijc.28134. [DOI] [PubMed] [Google Scholar]
- 49.Mao C, Yuan JQ, Yang ZY, Fu XH, Wu XY, Tang JL. Blood as a substitute for tumor tissue in detecting egfr mutations for guiding EGFR TKIs treatment of nonsmall cell lung cancer: a systematic review and meta-analysis. Medicine (Baltimore) 2015;94:e775. doi: 10.1097/MD.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lim C, Sung M, Shepherd FA, Nouriany N, Sawczak M, Paul T, Perera-Low N, Foster A, Zawisza D, Feld R, Liu G, Leighl NB. Patients with advanced non-small cell lung cancer: are research biopsies a barrier to participation in clinical trials. J Thorac Oncol. 2016;11:79–84. doi: 10.1016/j.jtho.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 51.Tachtsidis A, McInnes LM, Jacobsen N, Thompson EW, Saunders CM. Minimal residual disease in breast cancer: an overview of circulating and disseminated tumour cells. Clin Exp Metastasis. 2016;33:521–550. doi: 10.1007/s10585-016-9796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Meng X, Huang Z, Teng F, Xing L, Yu J. Predictive biomarkers in PD-1/PD-L1 checkpoint blockade immunotherapy. Cancer Treat Rev. 2015;41:868–876. doi: 10.1016/j.ctrv.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Xia B, Herbst RS. Immune checkpoint therapy for non-small-cell lung cancer: an update. Immunotherapy. 2016;8:279–298. doi: 10.2217/imt.15.123. [DOI] [PubMed] [Google Scholar]
- 54.Passiglia F, Bronte G, Bazan V, Natoli C, Rizzo S, Galvano A, Listì A, Cicero G, Rolfo C, Santini D, Russo A. PD-L1 expression as predictive biomarker in patients with NSCLC: a pooled analysis. Oncotarget. 2016;7:19738–19747. doi: 10.18632/oncotarget.7582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garon EB, Rizvi NA, Hui R, Leighl N, Balmanoukian AS, Eder JP, Patnaik A, Aggarwal C, Gubens M, Horn L, Carcereny E, Ahn MJ, Felip E, Lee JS, Hellmann MD, Hamid O, Goldman JW, Soria JC, Dolled-Filhart M, Rutledge RZ, Zhang J, Lunceford JK, Rangwala R, Lubiniecki GM, Roach C, Emancipator K, Gandhi L. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med. 2015;372:2018–2028. doi: 10.1056/NEJMoa1501824. [DOI] [PubMed] [Google Scholar]
- 56.Remon J, Chaput N, Planchard D. Predictive biomarkers for programmed death-1/programmed death ligand immune checkpoint inhibitors in nonsmall cell lung cancer. Curr Opin Oncol. 2016;28:122–129. doi: 10.1097/CCO.0000000000000263. [DOI] [PubMed] [Google Scholar]
- 57.Fusi A, Festino L, Botti G, Masucci G, Melero I, Lorigan P, Ascierto PA. PD-L1 expression as a potential predictive biomarker. Lancet Oncol. 2015;16:1285–1287. doi: 10.1016/S1470-2045(15)00307-1. [DOI] [PubMed] [Google Scholar]
- 58.Herbst RS, Soria JC, Kowanetz M, Fine GD, Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger SN, Kohrt HE, Horn L, Lawrence DP, Rost S, Leabman M, Xiao Y, Mokatrin A, Koeppen H, Hegde PS, Mellman I, Chen DS, Hodi FS. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Freidin MB, Freydina DV, Leung M, Montero FA, Nicholson AG, Lim E. Circulating tumor DNA outperforms circulating tumor cells for KRAS mutation detection in thoracic malignancies. Clin Chem. 2015;61:1299–1304. doi: 10.1373/clinchem.2015.242453. [DOI] [PubMed] [Google Scholar]
- 60.Dawson SJ, Tsui DW, Murtaza M, Biggs H, Rueda OM, Chin SF, Dunning MJ, Gale D, Forshew T, Mahler-Araujo B, Rajan S, Humphray S, Becq J, Halsall D, Wallis M, Bentley D, Caldas C, Rosenfeld N. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N Engl J Med. 2013;368:1199–1209. doi: 10.1056/NEJMoa1213261. [DOI] [PubMed] [Google Scholar]
- 61.Balch CM, Buzaid AC, Soong SJ, Atkins MB, Cascinelli N, Coit DG, Fleming ID, Gershenwald JE, Houghton A, Kirkwood JM, McMasters KM, Mihm MF, Morton DL, Reintgen DS, Ross MI, Sober A, Thompson JA, Thompson JF. Final version of the American Joint Committee on Cancer staging system for cutaneous melanoma. J. Clin. Oncol. 2001;19:3635–3648. doi: 10.1200/JCO.2001.19.16.3635. [DOI] [PubMed] [Google Scholar]
- 62.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, Buzaid AC, Cochran AJ, Coit DG, Ding S, Eggermont AM, Flaherty KT, Gimotty PA, Kirkwood JM, McMasters KM, Mihm MC, Morton DL, Ross MI, Sober AJ, Sondak VK. Final version of 2009 AJCC melanoma staging and classification. J. Clin. Oncol. 2009;27:6199–6206. doi: 10.1200/JCO.2009.23.4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calapre L, Warburton L, Millward M, Ziman M, Gray ES. Circulating tumour DNA (ctDNA) as a liquid biopsy for melanoma. Cancer Lett. 2017;404:62–69. doi: 10.1016/j.canlet.2017.06.030. [DOI] [PubMed] [Google Scholar]


