Abstract
Gastric cancer (GC) ranks as the fourth most common cancer and the third leading cause of cancer-related death worldwide. Circular RNAs (circRNAs) are a new class of long noncoding RNAs characterized by a single-stranded covalently closed loop structure. Emerging evidence reveals the essential function of circRNAs in the occurrence and development of human diseases. Among these, circRNAs are aberrantly expressed in GC and are involved in the progression of GC. In this review, we briefly summarize the current knowledge of the classification, biogenesis and biological functions of circRNAs, with an emphasis on their relationship with GC. As our understanding of the relation between circRNAs and GC advances, more diagnostic and therapeutic protocols will be developed for the prevention and treatment of GC.
Keywords: circular RNAs, gastric cancer, miRNA sponge, RBP sponge, biomarker
Introduction
Gastric cancer (GC) is one of the most common gastrointestinal malignancies, being the fourth most common cancer and the third leading cause of cancer-related death worldwide [1]. The East Asia countries are known for high incidence areas of GC, especially Japan and China [1,2]. Owing to the limitations of characteristic symptoms and appropriate molecular biomarkers, most GC patients cannot be diagnosed at an early stage. Although advanced surgical and medical management have improved the survival rates and the prognosis of early GC patients, the mortality rates of advanced GC patients remain high, with a 5-year overall survival (OS) rate of less than 30% [3]. Since carcinogenesis and progression of GC is a complex process, the mechanisms underlying the development of GC are not fully understood. The exploration of the molecular mechanisms and signalling pathways associated with GC may help identify potential diagnostic biomarkers and therapeutic targets. Accumulating studies have convincingly confirmed that many noncoding RNAs (ncRNAs), such as long noncoding RNAs (lncRNAs) and microRNAs (miRNAs), play critical roles in a wide variety of biological processes including the progression of GC, but the function of circular RNAs (circRNAs) remain to be elucidated [4-6].
CircRNAs are a new class of lncRNAs characterized with a single-stranded covalently closed loop structure without 5’ end caps or 3’ poly (A) tails [7,8]. CircRNAs were first discovered in RNA viruses by electron microscopy in 1976 [9]. Subsequently, sporadic studies reported the discovery of circRNAs, and these RNAs were regarded to be of low abundance and by-products of abnormal splicing [10-12]. With the advance of high-throughput RNA sequencing, exonuclease-based enrichment tools and bioinformatics analysis in the 21st century, circRNAs were found to be ubiquitously expressed in a variety of eukaryotic organisms, relatively stable and conserved in the control of gene expression [7,8,13]. Emerging evidence reveals the essential function of circRNAs in the occurrence and development of many human diseases. Furthermore, increasing studies have demonstrated that circRNAs are strongly related with the proliferation, apoptosis, invasion, and metastasis of human tumours, which indicates the potential of circRNAs to act as novel biomarkers and therapeutic targets [14,15].
As circRNAs continue to be studied, they have been found to be indispensable participants in the occurrence and progression of GC. In this review, we briefly summarize the current knowledge of the characteristics of circRNAs, with an emphasis on their relationship with GC.
The features of circRNAs
The classification and biogenesis of circRNAs
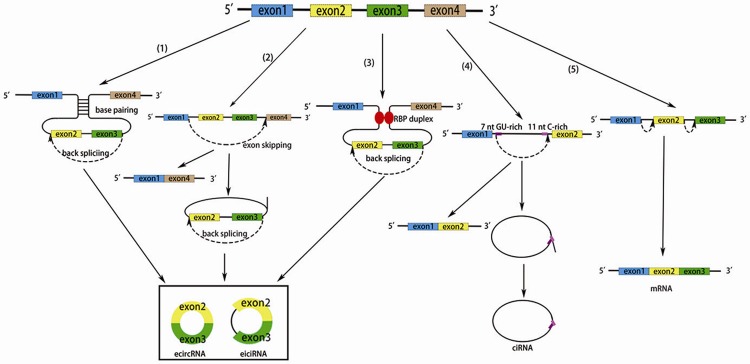
CircRNAs are generated cotranscriptionally from various protein-coding genes [16]. The rate of biogenesis can be regulated by the flanking repeat intronic complementary sequences and RNA-binding proteins (RBPs) [16-19]. Based on different generating mechanisms (Figure 1), circRNAs can be divided into three types: exonic circRNAs (ecircRNAs), circular intronic RNAs (ciRNAs) and extron-intron circRNAs (eiciRNAs). EcircRNAs consist of only exons and primarily localize to the cytoplasm [13]. The biogenesis of ecircRNAs has three potential mechanisms, including direct backsplicing, exon skipping and RBP quaking. Backsplicing during spliceosome-mediated pre-mRNA splicing occurs in each of these mechanisms as the canonical spliceosome, which connects a down splice donor site to the upstream acceptor splice site [20]. Mechanism 1 is termed direct backsplicing or intron-pairing driven circularization. Introns bordering the circularized exons carry out base pairing, which induces the two exons to undergo alternative backsplicing and forms an ecircRNA [11,13]. Mechanism 2 is termed exon skipping, or lariat-driven circularization. A downstream exon skips over one or several exons to link an upstream exon, then forms a lariat containing both exons and introns [13,21]. The skipped exons produce ecircRNA after removal of introns by internal splicing [22]. Mechanism 3 is termed RBP quaking. RBPs bind to recognition elements within introns and form a bridge between the two flanking intronic sequences, which brings exons in close proximity to undergo backsplicing and promotes ecircRNA biogenesis [16,23]. After the biogenesis, UAP56/URH49, the human homologs of Drosophila Hel25E, are key modulators that have been shown to modulate the human ecircRNA nuclear export [24]. However, the other factors involved in the localization of ecircRNA and the actual molecular mechanism remain unclear.
Figure 1.
Possible models of circRNA and mRNA biogenesis. (1) Direct backsplicing. Introns bordering the circularized exons carry out base pairing, which induces the two exons to undergo alternative backsplicing. Introns of the circRNA are removed or retained to form an ecircRNA or eiciRNA. (2) Exon skipping. A downstream exon skips over one or more exons to link an upstream exon, then forms a lariat containing both exons and introns. Introns of the circRNA are removed or retained to form an ecircRNA or eiciRNA. (3) RBP quaking. RBPs bind to recognition elements within introns, then form a bridge between the two flanking intronic sequences, which brings exons in close proximity to undergo backsplicing. Introns of the circRNA are removed or retained to form an ecircRNA or eiciRNA. (4) CiRNA biogenesis. A motif containing an 11 nt C-rich element near the branchpoint and a 7 nt GU-rich element near the 5’ splice escapes the debranching and degradation after the canonical pre-RNA splicing, and forms a ciRNA. (5) Canonical pre-mRNA splicing and mRNA biogenesis.
CiRNAs consists of only introns and associates with the nuclearinsoluble fractionation and lack of miRNA target sites [25,26]. The biogenesis of ciRNAs needs to escape from debranching, in the context of a motif containing an 11 nt C-rich element near the branchpoint and a 7 nt GU-rich element near the 5’ splice [25]. The biogenesis of eiciRNAs is complex and still uncertain because of the special constituent part of introns that have been not spliced out.
Biological functions of circRNAs
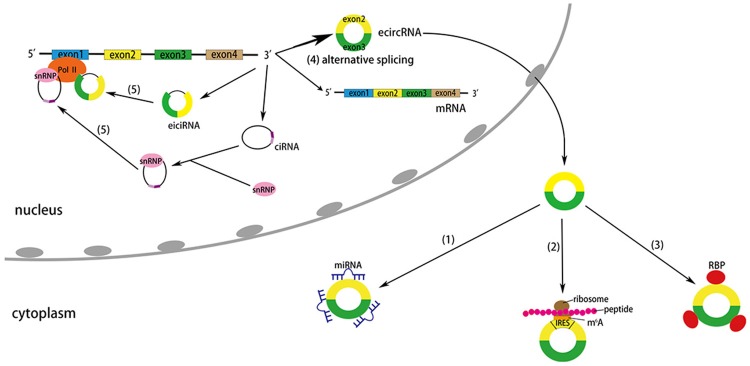
Emerging studies have demonstrated that circRNAs may be involved in various biological functions, including miRNA sponges, transcription regulation, protein translation, interaction with RBPs, alternative splicing, and probably other unknown functions (Figure 2).
Figure 2.
Putative functions of circRNAs. (1) CircRNAs as miRNA sponges. Some circRNAs can act as miRNA sponges by competing for miRNA binding sites. (2) CircRNAs as protein translators. Some circRNAs containing IRES have the ability to bind with ribosome and translate into proteins and peptides. M6A can act as IRES to translate circRNAs in human cells. (3) Interaction with RBPs. Some circRNAs can bind RBPs to form RNA-protein complexes and act as RBP sponges. (4) Alternative splicing. Some circRNAs can compete with the biogenesis and processing of mRNA in the nucleus. (5) Transcriptional regulation. Some eiciRNAs and ciRNAs can interact with transcription complexes and promote their parental gene transcription in the nucleus.
MiRNA sponges
MiRNAs are a class of small RNAs that are important post-transcription regulators by targeting mRNAs [27]. Recent studies suggests that circRNAs are enriched in conserved nucleotides and contain miRNA response elements (MREs), indicating the potential of circRNAs to act as competing endogenous RNAs (ceRNAs) to compete for miRNA binding sites, thereby reducing the binding of miRNA and its target sites so that they counteract the effect of miRNAs on their target mRNAs [7,28]. This function of miRNA inhibitors has also been termed miRNA sponges. The circular RNA sponge for miR-7 (ciRS-7), also known as cerebellar degeneration-related protein 1 transcript (CDR1as), was the first ecircRNA identified to have miRNA sponge effects. CiRS-7 contains 74 miR-7 binding sites, which can strongly decrease miR-7 activity, causing the expression of miR-7 targets to increase [28]. An in vivo experiment indicated that ciRS-7 can act as a miRNA antagonist with high miRNA-binding capacity and participate in post-transcriptional regulation [7]. Moreover, the testis-specific circRNA, sex-determining region Y (Sry) can serve as a miR-138 sponge with 16 miR-138 binding sites [28]. However, a study estimated a large set of circRNAs and found that most identified circRNAs in mammalian cells, except ciRS-7 and Sry, lack the characteristic feature of more than ten miRNA binding sites [29]. Recent accumulated evidence suggests that an increasing number of functional circRNAs with less than ten miRNA binding sites have the potential to serve as miRNA sponges and are relevant to human diseases [30,31]. Therefore, miRNA sponges may not require a large number of miRNA sites, and more potential interactions between circRNAs, miRNAs and their target mRNAs are still being evaluated [20].
Transcription regulation
EcircRNAs participate in regulatory functions in the cytoplasm, whereas the ciRNAs and eiciRNAs are retained during the transcription of parental genes in the nucleus [7,25,26,28]. A study has suggested that two eiciRNAs, circEIF3J, and circPAIP2, can combine with U1 small nuclear tibonucleic proteins (snRNPs) via RNA-RNA interaction, and with RNA polymerase II (Pol II) complex and promote the expression of parental genes in cis [26]. Ci-ankrd52, an abundant ciRNA, can also combine with elongation Pol II and enhance the transcription of RNA Pol II [25]. The expression of their parental genes was reduced after removing ciRNAs, further indicating the positive effects of ciRNAs on the transcription regulation.
Protein translation
EcircRNAs located in the cytoplasm might have the potential to be translated into proteins or peptides [32,33]. The presence of Internal Ribosomal Entry Sites (IRES) and open reading frame (ORF) in some ecircRNAs allows for the formation of proteins [32,34,35]. A study demonstrated that N6-methyladenosine (m6A), the most abundant internal modification of RNA, is enriched in circRNAs and can act as IRES to translate circRNAs in human cells [36]. The widespread m6A translation in circRNAs reveals the large number of endogenous circRNAs can translate proteins [36]. Whether circRNAs-driven proteins lack molecular activity or can act as biologically functional molecules in cells has not been determined. CircRNAs with protein translation functions not only can be regarded as conventional ncRNAs, but might also act as a novel type of protein-coding RNAs.
Interaction with RBPs
CircRNAs can interact with multiple RBPs to form an RNA-protein complex, and engage in multiple functions. For instance, the circ-Dnmt1 can promote the nuclear translation of P53 and AUF1 by interacting with them, resulting in cellular autophagy or reduction of target mRNA instability [37]. A study identified that hundreds of circRNAs were regulated in the process of human epithelial-mesenchymal transition (EMT) [23]. Quaking (QKI) protein is the pivotal regulator of enhanced production of circRNAs in EMT [23]. A bioinformatics analysis showed that the IRES regions of circRNAs are predicted binding sites for many RBPs, suggesting the role of RBPs in the initiation of protein translation from circRNAs [35]. The brain-related ecircRNA CDR1as can associate with Argonaute (AGO) and act in a way similar to miRNA sponges [7]. The ecircRNA circ-Foxo3, which is highly expressed in noncancer cells, can interact with the cell cycle proteins cyclin-dependent kinase 2 (CDK2) and cyclin-dependent kinase inhibitor 1 (p21), arrest the cell cycle progression and inhibit cell proliferation [38]. Additionally, circ-Foxo3 can bind murine double minute 2 (MDM2) and p53 to promote the ubiquitination function of MDM2, and induce cell apoptosis [39]. These RBP sponge functions have also been discovered in circRNAs with other RBPs, such as MBL [16], Pol II [25,26], eukaryotic initiation factor 4A-III (EIF4A3) [35] and ELAV-like protein 1 (HUR) [40]. CircRNAs with a high density of binding sites for a given RBP can be regarded as “super-sponges” with enhanced sponging functions [35]. The interactions between circRNAs and RBPs may link circRNAs to diverse biological processes.
Alternative splicing
Pre-mRNA splicing can result in the biogenesis of a linear mRNA or a circRNA. The short repeat elements in the flanking introns can facilitate backsplicing to promote the production of circRNAs and reduce the linear splicing of flanking exons [16,17]. This function of alternative splicing has been shown in the circular muscleblind (circMBL) biosynthesis [16]. The circMBL can strongly compete with linear splicing in the context of flanking introns with abundant MBL-specific binding sites [16]. Another study demonstrated that the production of an ecircRNA from the mouse formin (Fmn) gene can act as “mRNA trap”, similar to “alternative splicing” [12]. The Fmn circRNA with translation-initiation site can trap the Fmn gene transcripts in a nonfunctional form and reduce the expression of the normal linear RNA transcripts [12].
Biological roles of circRNAs in GC
Profiles of circRNA expression in GC
The RNA sequence analysis and microarray analysis of the expression of circRNA in human cells have shown that many circRNAs were aberrantly expressed in GC and may have several potential functions. Sui et al. [41] discovered that a total of 1285 circRNAs were differentially expressed in GC tissues compared with adjacent tissues based on microarray chip technology. Of these, 691 circRNAs were upregulated and 594 were downregulated. Sixty-nine of these circRNAs were found to have the potential to serve as miRNA sponge to regulate the expression of target mRNAs. Another result of the circRNA microarray showed that 16 circRNAs were upregulated whereas 84 circRNAs were downregulated in GC [42]. Among these circRNAs, only circ_0000026 expression was significantly downregulated 2.8-fold change in GC by quantitative reverse transcription polymerase chain reaction (qRT-PCR) [42]. Dang et al. [43] revealed that a total of 713 circRNAs showed differential expression in GC tissues as screened by the expression profiles of 5 pairs of GC and matched non-GC tissues. Of these circRNAs, 191 and 522 were upregulated and downregulated, respectively. Vidal et al. [44] found 736 differentially expressed circRNAs on RNA sequence analysis. They revealed the over-expression of circRNAs in both tumour-adjacent and GC samples compared with healthy samples, indicating the presence of field cancerization in GC. Shen et al. [45] performed a circRNA microarray analysis and confirmed that a total of 347 upregulated and 603 downregulated circRNAs in GC compared with normal gastric tissue. Ten out of 20 randomly selected circRNAs were verified to have differential expression. However, the expression of these circRNAs was not correlated with the expression of host genes, indicating the independent regulation in circRNA formation against transcription [45].
Gu et al. [46] performed a microarray analysis and further researched the circRNA-miRNA-mRNA regulation network. They found that circRNA_101504 may be a central part in the regulation network, by sponging the miR-454-3p and miR-301a-3p to affect several mRNAs. Lai et al. [47] studied the microarray data and found 240 circRNAs and 169 mRNAs were differentially expressed in GC tissues. Among these circRNAs, 71 were upregulated while 133 were downregulated. The co-expression network predicted the correlation of one circRNA or mRNA with one to many circRNAs or mRNAs. Li et al. [48] first performed a microarray analysis of both GC tissues and plasma and found 343 differentially expressed circRNAs. However, only 3 and 14 circRNAs were elevated and reduced in both GC tissues and plasma. These expression profiles of circRNAs in GC further confirmed that circRNAs are closely associated to GC. However, only a small number of circRNAs have been ascertained to regulate carcinogenesis in GC. While researchers can use bioinformatics to analyse these expression profiles to predict more accurately the roles of circRNAs, these results reveal that the study of GC-related circRNAs is still full of challenges.
CircRNAs as potential biomarkers in GC
Due to their special characteristics such as conservation, abundance, and long half-lives, circRNAs may act as special and stable molecular markers to predict GC [13]. Li et al. [14] first demonstrated that circ_002059 was highly stable in mammalian cells and downregulated in GC tissues and plasma by qRT-PCR. These finding demonstrated that circ_002059 may be a potential stable biomarker for the diagnosis of GC. They also reported that circ_0000096 was significantly downregulated in GC with an area under receiver operating characteristic (ROC) curve of 0.82 [49]. The combination of circ_002059 and circ_0000096 results can increase the AUC to 0.91. Fang et al. [50] demonstrated that the upregulated circ_0058246 in tumour specimens of patients with poor clinical outcomes by qRT-PCR. Huang et al. [51] revealed that the expression of circ_0000745 was significantly downregulated in GC and correlated with tumour differentiation and tumour nodal metastasis. Zhao et al. [52] showed that circ_00000181 was downregulated in GC and associated with multiple clinicopathologic factors, such as TNM classification and differentiation. As mentioned above, Lai et al. [47] not only studied the microarray data but also validated the expression of circ_0047905, circ_0138960, and circRNA7690-15 by qRT-PCR. The area under the ROC curve (AUC) for these three circRNAs was 0.85, 0.647 and 0.681, respectively. Similarly, Shao et al. [53] reported that a total of 308 circRNAs were aberrantly expressed in GC tissues by microarray, 107 of which were upregulated and 201 of which were downregulated. Circ_0014717 was one of the most downregulated circRNAs and its downregulation in GC tissues and stable existence in human gastric juice has been confirmed. QRT-PCR results also showed that circ_0001895 were downregulated in both GC tissue and gastric precancerous lesions compared with healthy control tissues, with a significant correlation with cell differentiation [54]. In further studies, Sun et al. [55] demonstrated that the expression level of circ_0000520 was downregulated in GC tissues, plasma, and GC cell lines and may serve as a novel biomarker with the area under the ROC curve (AUC) was 0.8967 in plasma. Lu et al. [56] showed that circ_0006633 was downregulated in GC samples and correlated with cancer distal metastasis and tissue carcinoembryonic antigen level. Tian et al. [57] reported a study that circ_0003159 had a down-regulated expression in GC tissues compared with adjacent noncancerous tissues and negatively associated with gender, distal metastasis, and the TNM stage. The expression of circ_0000190 was downregulated in GC tissues and plasma samples and related with tumour diameter, lymphatic metastasis, distal metastasis and TNM stage [58]. Li et al. [59] found that the expression of circ_0001649 in GC tissues and preoperative serum was notably decreased and AUC was up to 0.834. CircPVRL3, also termed circ_0066779, was downregulated in GC tissues and associated with TNM stage [60]. The AUC of circPVRL3 was 0.7626 in all samples, and up to 0.805 in GC patients with advanced (III-IV) TNM stages [60]. Rong et al. [61] showed that circ_0066444 was upregulated in GC and associated with lymph node metastasis. Li et al. [48] performed RT-droplet digital PCR (ddPCR) to validate microarray results and confirmed the downregulation of circ_0001017 and circ_0061276. The AUC for combined circ_0001017 and circ_0061276 results was up to 0.966 with 95.5% sensitivity and 95.7% specificity, indicating the potential role of these circRNAs as biomarkers to screen GC and evaluate prognosis. A larger cohort of patients is still needed to confirm its clinical value.
In summary, various aberrantly expressed circRNAs have been reported to have the potential to serve as prognostic biomarkers for the diagnosis of GC (Table 1). However, it is pivotal to develop more functional investigation of these circRNAs to confirm their relation with GC and discover novel therapeutic targets.
Table 1.
The potential biomarkers and diagnostic value of circRNAs in GC
| CircRNA (alians) | Chromosome | Regulation | Gene symbol | Functions | Diagnostic ability | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Patients (No.) | AUC | SEN | SPE | Cut-off | ||||||
| hsa_circ_002059 (hsa_circ_0000140) | chr1 | ↓ | KIAA0907 | --- | 101 (tissues) | 0.730 | 0.810 | 0.620 | 12.90 | [14] |
| 36 (plasma) | --- | --- | --- | --- | ||||||
| hsa_circ_0000096 | chr1 | ↓ | HIAT1 | Proliferation (+), Migration (+) | 101 (tissues) | 0.820 | 0.880 | 0.560 | 12.90 | [49] |
| hsa_circ_0058246 | chr2 | ↑ | VIL1 | --- | 43 (tissues) | --- | --- | --- | --- | [50] |
| hsa_circ_0000745 | chr17 | ↓ | SPECC1 | --- | 60 (tissues) | --- | --- | --- | --- | [51] |
| 60 ( plasma) | 0.683 | 0.855 | 0.450 | --- | ||||||
| hsa_circ_0000181 | chr1 | ↓ | TATDN3 | --- | 115 (tissues) | 0.756 | 0.590 | 0.852 | 9.40 | [52] |
| 102 (plasma) | 0.756 | 0.990 | 0.206 | 7.27 | ||||||
| hsa_circ_0047905 | chr9 | ↑ | SERPINB5 | Proliferation (+), Invasion (+) | 31 (tissues) | 0.850 | --- | --- | --- | [47] |
| hsa_circ_0138960 | chr9 | ↑ | GDA | Proliferation (+), Invasion (+) | 31 (tissues) | 0.647 | --- | --- | --- | [47] |
| circRNA7690-15 | Chr18 | ↑ | GDA | Proliferation (+), Invasion (+) | 31 (tissues) | 0.681 | --- | --- | --- | [47] |
| hsa_circ_0014717 | chr1 | ↓ | CCT3 | --- | 96 (tissues) | 0.696 | 0.594 | 0.813 | --- | [53] |
| hsa_circ_0001895 | chr9 | ↓ | PRRC2B | --- | 96 (tissues) | 0.792 | 0.678 | 0.857 | 9.53 | [54] |
| hsa_circ_0000520 | chr14 | ↓ | RPPH1 | --- | 56 (tissues) | 0.613 | 0.536 | 0.857 | --- | [55] |
| 45 (plasma) | 0.897 | 0.824 | 0.844 | --- | ||||||
| hsa_circ_0006633 | chr1 | ↓ | FGGY | --- | 96 (tissues) | 0.741 | 0.600 | 0.810 | 8.17 | [56] |
| 20 (plasma) | --- | --- | --- | --- | ||||||
| hsa_circ_0003159 | chr7 | ↓ | CACNA2D1 | --- | 108 (tissues) | 0.750 | 0.852 | 0.565 | 12.31 | [57] |
| hsa_circ_0000190 | chr1 | ↓ | CNIH4 | --- | 104 (tissues) | 0.750 | 0.721 | 0.683 | 6.83 | [58] |
| 104 (plasma) | 0.600 | 0.414 | 0.875 | 3.07 | ||||||
| hsa_circ_0001649 | chr6 | ↓ | SHPRH | --- | 76 (tissues) | 0.834 | 0.711 | 0.816 | 0.23 | [59] |
| hsa_circ_0066779 (circPVRL3) | chr3 | ↓ | PVRL3 | Proliferation (-), Migration (-) | 62 (tissues) | 0.763 | 0.903 | 0.564 | --- | [60] |
| hsa_circ_0066444 | chr3 | ↑ | ADAMTS9 | Proliferation (+), Invasion (+), Migration (+) | 88 (tissues) | 0.733 | 0.708 | 0.689 | --- | [61] |
| hsa_circ_0001017 | chr2 | ↓ | XPO1 | --- | 121 (tissues) | 0.732 | 0.702 | 0.620 | --- | [48] |
| 121 (plasma) | 0.849 | 0.758 | 0.959 | --- | ||||||
| hsa_circ_0061276 | chr21 | ↓ | NRIP1 | --- | 121 (tissues) | 0.780 | 0.636 | 0.769 | --- | [48] |
| 121 (plasma) | 0.851 | 0.676 | 0.897 | --- | ||||||
“↓”: down-regulation; “↑”: up-regulation; “(+)”: stimulatory roles; “(-)”: inhibitory roles.
CircRNAs act as miRNA sponge in GC
Recently, emerging evidence has shown that some circRNAs act as miRNA sponge and interact with RBPs involved in the progression and metastasis of GC (Table 2).
Table 2.
The validated circRNA with potential therapeutic functions in GC
| CircRNA (alians) | Chromosome | Regulation | Gene symbol | Functions | Possible mechanisms | Ref. |
|---|---|---|---|---|---|---|
| hsa_circ_101057 (circLARP4) | chr12 | ↓ | LARP4 | Proliferation (-), Invasion (-) | miR-424-5p sponge/AGO2 | [62] |
| circPVT1 | chr8 | ↑ | PVT1 | Proliferation (+) | miR-125 sponge/E2F2; let-7b sponge/MYC | [63] |
| hsa_circ_0000096 | chr1 | ↓ | HIAT1 | Proliferation (+), Migration (+) | miR-224 sponge/cyclin D1, CKD6, MMP-2, MMP-9 | [49] |
| hsa_circ_100269 | chr1 | ↓ | LPHN2 | Proliferation (-) | miR-630 sponge | [65,66] |
| hsa_circ_0066779 (circPVRL3) | chr3 | ↓ | PVRL3 | Proliferation (-), Migration (-) | miRNA sponge/AGO2, FUS, LIN28A, EIF4A3; protein translation | [60] |
| hsa_circ_0066444 | chr3 | ↑ | ADAMTS9 | Proliferation (+), Invasion (+), Migration (+) | miRNA sponge | [61] |
| circ_ZFR | chr10 | ↓ | PTEN | Proliferation (-), Apoptosis (+), Tumorigenesis (+) | miR-130a sponge; miR-107 sponge/p53 | [67] |
| hsa_circ_0001946 (ciRS-7, cdr1as) | chrX | ↑ | Cdr1 | Proliferation (+), Invasion (+), Migration (+), Apoptosis (-), Tumorigenesis (+) | miR-7 sponge/PTEN, PI3K, AKT | [69] |
| circHIPK3 | chr11 | ↑ | HIPK3 | Proliferation (+) | miR-124 sponge; miR-29b sponge | [71] |
| hsa_circ_104916 | chr9 | ↓ | NEK6 | Proliferation (-), Invasion (-), Migration (-) | EMT/E-cadherin, N-cadherin, vimentin, slug | [75] |
| hsa_circ_0023642 | chr11 | ↑ | UVRAG | Proliferation (+), Invasion (+), Migration (+), Apoptosis (-) | EMT/vimentin, snail, E-cadherin, N-cadherim | [77] |
“↓”: down-regulation; “↑”: up-regulation; “(+)”: stimulatory roles; “(-)”: inhibitory roles.
A study on the expression of circLARP4 in GC tissues has revealed that circLARP4 was downregulated and can act as an independent prognostic factor for overall survival of GC patients and patients with adjuvant chemotherapy [62]. Further studies revealed that circLARP4 can serve as a sponge of miR-424-5p and regulate the expression of LATS1 and YAP gene, subsequently inhibiting DNA synthesis, proliferation, and invasion of GC cells [62].
CircPVT1, a circRNA screened by circRNA microarray and validated by qRT-PCR, was upregulated in patients with GC [63]. The target mRNA PVT1 was involved in many human cancers and associated with poor prognosis via the regulation of protein stability of oncogenes, especially the myelocytomatosis (MYC) [64]. CircPVT1 also can facilitate the expression of MYC protein by sponging let-7b and promote the proliferation of GC cells [63]. Additionally, circPVT1 can facilitate the expression of miR-125 target E2F2 by acting as a sponge of the tumour suppressor miR-125 [63]. Although circRVT1 acted as an oncogene in vitro, circPVT1 was negatively associated with T4 stage and perineural invasion, and high expression was associated to the overall survival of GC patients [63]. The positive correlation between circPVT1 and tumour suppressor miR-125 may account for this discordant result.
Unlike other downregulated circRNAs acting as suppressor genes, Li et al. [49] showed that the knockdown of circ_0000096 greatly inhibited cell proliferation and migration in vitro and in vivo by reducing the expression of cyclin D1, cyclin-dependent kinase 6 (CKD6), matrix metalloproteinase-2 (MMP-2) and MMP-9. The existence of ceRNA may help explain for this discordant result. The qRT-PCR results showed that circ_0000096 can serve as miRNA sponges and inhibit the expression of miR-224 [49].
Additionally, Zhang et al. [65] identified 46 differently expressed circRNAs by microarray and further screened 4 circRNAs by qRT-PCR. A four-circRNA-based classifier was constructed to evaluate the early recurrence of stage III GC after radical surgery [65]. CircRNA_100269, one of the predictor circRNA in the classifier, was negatively correlated with miR-630 [66]. These results suggest a correlation between circRNA_100269 and miR-630 in inhibiting the proliferation of GC cells [66].
Sun et al. [60] revealed that the downregulation of circPVRL3 in GC patients and the knockdown of circPVRL3 by small interfering RNAs (siRNAs) promoted proliferation and migration in GC cells. The prediction and annotation revealed that circPVRL3 can interact with target mRNA and bind to AGO2, FUS, LIN28A, and EIF4A3 due to the potential interaction with 9 miRNAs. Rong et al. [61] showed that an upregulated circRNA in GC, circ_0066444, can promote cell proliferation, invasion, and migration. The network prediction prognosis of 5 miRNAs, including miR_1282, miR-1243, miR-1178, miR-638 and miR-451 interact with circ_0066444. However, these functions in circPVRL3 and circ_0066444 still need more biologic evidence to confirm these effects.
Circ_ZFR is transcribed from PTEN, a known tumor suppressor [67]. Circ_ZFR was found to be decreased in GC tissues and cells compared with negative control [68]. Acting as a sponge for miR-107 and miR-130a, circ_ZFR promoted p53 expression and impeded cell propagation, induced cell cycle arrest and promoted apoptosis in vitro, and curbed GC tumour growth in vivo [68]. These data suggest that the circZFR-miR-130a/miR-107-PTEN axis may be of interest as a potential therapeutic target for GC.
CiRS-7 is a special circRNA that functions as a super sponge of miR-7 and is aberrantly expressed in many cancers [28]. In GC, Pan et al. [69] reported that ciRS-7 was significantly upregulated in tissues and correlated with poor survival of GC patients. The over-expression of ciRS-7 can repress the activity of miR-7, subsequently increasing the level of miR-7 targets PI3K, AKT phosphorylation and decreasing the expression of PTEN. Further studies demonstrated that ciRS-7 blocked the miR-7-mediated cell proliferation suppression, migration suppression, apoptosis promotion and tumourigenesis inhibition in vitro and in vivo.
CircHIPK3 is an abundant circRNA with multiple miRNA binding sites [70]. Cheng et al. [71] reported that circHIPK3 can serve as sponges of miR-124 and miR-29b and is involved in the proliferation of GC cells. The circHIPK3 level was upregulated in the GC tissues and closely correlated with T stage and Ming’s classification. The level of circHIPK3 negatively correlated with the levels of miR-124 and miR-29b. The authors emphasized the significant function of circRNA-miRNA-mRNA network in the progression of GC.
circRNA act as RBP sponge in EMT in GC
In the EMT program, epithelial cells transition into mesenchymal cells that can invade the extracellular matrix [72]. Because this process is related to the invasion and metastasis of cancer cells, the EMT-targeting strategies may reveal new therapeutic intervention in cancer initiation and progression [73]. Many complex mechanisms regulate the process of EMT, including the interaction of QKI protein and circRNAs [23,74]. Circ_104916 was downregulated in GC and associated with deep invasion, tumour stage and lymphatic metastasis [65,75]. Li et al. [75] showed that circ_104916 could suppress the proliferation, invasion and migration abilities of GC cells by decreasing the expression of Slug. Slug is one of the zinc-finger transcription factors and can act as the repressor of the epithelial molecule E-cadherin [76]. They found the upregulation of E-cadherin and the downregulation of the mesenchymal molecule N-cadherin by Western blot after over-expression of circ_104916, suggesting the regulation of circ_104916 in EMT [75].
An upregulated circRNA in GC, CircRNA_0023642, was screened by circRNA microarray and validated by qRT-PCR [42,77]. The downregulation of circRNA_0023642 by siRNA can reduce cell proliferation, invasion, and migration and promote cell apoptosis in GC [77]. The downregulation of EMT-related gene N-cadherin, vimentin, and snail, and the upregulation of E-cadherin indicated the involvement of circRNA-0023462 in EMT signalling pathway [77].
Conclusion and future perspectives
As high throughput sequencing technologies and bioinformatics are advancing, growing evidence has demonstrated that numerous circRNAs are aberrantly expressed in GC tissues. Some differentially expressed circRNAs are correlated with the clinicopathological features in GC patients. Moreover, the differential expression exists and increases during the tumourigenesis, proliferation, metastasis, and apoptosis of GC. Recent studies confirmed that circRNAs can function as miRNA sponges, RBP sponges and EMT regulators that modulate target miRNAs and proteins and contribute to GC progression. Given the characteristics of circRNAs, such as abundance, stability, and presence in different body fluids, some dysregulated circRNAs are promising diagnostic biomarkers and therapeutic targets.
The complete biological and molecular functions of circRNAs in GC remain uncertain. Although recent studies demonstrated that a number of circRNAs have the potential to serve as noninvasive diagnostic and prognostic biomarkers, suitable circRNAs acting as independent biomarkers of GC have not yet been discovered. One circRNA may be combined with multiple other circRNAs or conventional cancer biomarkers to improve the sensitivity and specificity of diagnosis of GC [49,65]. More extensive studies are needed to fully understand the specific expression of circRNAs, eliminate the effects of secondary expression in other tissues and identify the best body fluids for circRNA detection. It is also important to find more suitable circRNAs with high diagnostic abilities of GC in the future.
Although microarray chip technology identified a large number of dysregulated circRNAs in GC, only a small number of functional circRNAs have been validated and elucidated. Most of the recent studies focused on the functions of circRNAs as miRNA sponge and interactor with RBPs in the proliferation, invasion, apoptosis, and migration of GC cells. As one circRNA can interact with several miRNAs, including tumour suppressor, tumour oncogene, and drug resistance-related gene, circRNA can perform diverse functions via the action of ceRNA [63]. It is possible that unlike traditional ncRNAs, not all upregulated circRNAs act as oncogenes and not all downregulated circRNAs act as suppressor genes [49,63]. Therefore, enhancing the interaction of circRNAs with oncogene miRNAs can weaken carcinogenicity whereas attenuating the interaction of circRNAs with suppressor miRNAs can enhance the antitumour effect, subsequently providing novel insight into therapeutic targets for GC. It is also urgent to explore more functional circRNAs and elucidate their biological functions and molecular mechanisms in the progression of GC. As our understanding of the relation between circRNAs and GC advances, more diagnostic and therapeutic protocols will be developed to contribute to the prevention and early treatment of GC.
Acknowledgements
This study was supported by grants from the Natural Science Foundation of Ningbo (No. 2016A610158), the Natural Science Foundation of Ningbo (No. 2014A610226), the Zhejiang Medical and Health Project (No. 2018ZH025) and the Scientific benefit for people Project of Ningbo (No. 2014C51001).
Disclosure of conflict of interest
None.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 3.Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, Bannon F, Ahn JV, Johnson CJ, Bonaventure A, Marcos-Gragera R, Stiller C, Azevedo e Silva G, Chen WQ, Ogunbiyi OJ, Rachet B, Soeberg MJ, You H, Matsuda T, Bielska-Lasota M, Storm H, Tucker TC, Coleman MP. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2) Lancet. 2015;385:977–1010. doi: 10.1016/S0140-6736(14)62038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang YK, Yu JC. Circulating microRNAs and long non-coding RNAs in gastric cancer diagnosis: an update and review. World J Gastroenterol. 2015;21:9863–9886. doi: 10.3748/wjg.v21.i34.9863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang Z, Guo X, Li G, Shi Y, Li L. Long noncoding RNAs as potential biomarkers in gastric cancer: opportunities and challenges. Cancer Lett. 2016;371:62–70. doi: 10.1016/j.canlet.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 7.Memczak S, Jens M, Elefsinioti A, Torti F, Krueger J, Rybak A, Maier L, Mackowiak SD, Gregersen LH, Munschauer M, Loewer A, Ziebold U, Landthaler M, Kocks C, le Noble F, Rajewsky N. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature. 2013;495:333–338. doi: 10.1038/nature11928. [DOI] [PubMed] [Google Scholar]
- 8.Salzman J, Gawad C, Wang PL, Lacayo N, Brown PO. Circular RNAs are the predominant transcript isoform from hundreds of human genes in diverse cell types. PLoS One. 2012;7:e30733. doi: 10.1371/journal.pone.0030733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanger HL, Klotz G, Riesner D, Gross HJ, Kleinschmidt AK. Viroids are single-stranded covalently closed circular RNA molecules existing as highly base-paired rod-like structures. Proc Natl Acad Sci U S A. 1976;73:3852–3856. doi: 10.1073/pnas.73.11.3852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hsu MT, Coca-Prados M. Electron microscopic evidence for the circular form of RNA in the cytoplasm of eukaryotic cells. Nature. 1979;280:339–340. doi: 10.1038/280339a0. [DOI] [PubMed] [Google Scholar]
- 11.Cocquerelle C, Mascrez B, Hetuin D, Bailleul B. Mis-splicing yields circular RNA molecules. FASEB J. 1993;7:155–160. doi: 10.1096/fasebj.7.1.7678559. [DOI] [PubMed] [Google Scholar]
- 12.Chao CW, Chan DC, Kuo A, Leder P. The mouse formin (Fmn) gene: abundant circular RNA transcripts and gene-targeted deletion analysis. Mol Med. 1998;4:614–628. [PMC free article] [PubMed] [Google Scholar]
- 13.Jeck WR, Sorrentino JA, Wang K, Slevin MK, Burd CE, Liu J, Marzluff WF, Sharpless NE. Circular RNAs are abundant, conserved, and associated with ALU repeats. RNA. 2013;19:141–157. doi: 10.1261/rna.035667.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li P, Chen S, Chen H, Mo X, Li T, Shao Y, Xiao B, Guo J. Using circular RNA as a novel type of biomarker in the screening of gastric cancer. Clin Chim Acta. 2015;444:132–136. doi: 10.1016/j.cca.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 15.Chen LL. The biogenesis and emerging roles of circular RNAs. Nat Rev Mol Cell Biol. 2016;17:205–211. doi: 10.1038/nrm.2015.32. [DOI] [PubMed] [Google Scholar]
- 16.Ashwal-Fluss R, Meyer M, Pamudurti NR, Ivanov A, Bartok O, Hanan M, Evantal N, Memczak S, Rajewsky N, Kadener S. circRNA biogenesis competes with pre-mRNA splicing. Mol Cell. 2014;56:55–66. doi: 10.1016/j.molcel.2014.08.019. [DOI] [PubMed] [Google Scholar]
- 17.Liang D, Wilusz JE. Short intronic repeat sequences facilitate circular RNA production. Genes Dev. 2014;28:2233–2247. doi: 10.1101/gad.251926.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liang D, Tatomer DC, Luo Z, Wu H, Yang L, Chen LL, Cherry S, Wilusz JE. The output of protein-coding genes shifts to circular RNAs when the Pre-mRNA processing machinery is limiting. Mol Cell. 2017;68:940–954. e943. doi: 10.1016/j.molcel.2017.10.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ivanov A, Memczak S, Wyler E, Torti F, Porath HT, Orejuela MR, Piechotta M, Levanon EY, Landthaler M, Dieterich C, Rajewsky N. Analysis of intron sequences reveals hallmarks of circular RNA biogenesis in animals. Cell Rep. 2015;10:170–177. doi: 10.1016/j.celrep.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 20.Jeck WR, Sharpless NE. Detecting and characterizing circular RNAs. Nat Biotechnol. 2014;32:453–461. doi: 10.1038/nbt.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zaphiropoulos PG. Circular RNAs from transcripts of the rat cytochrome P450 2C24 gene: correlation with exon skipping. Proc Natl Acad Sci U S A. 1996;93:6536–6541. doi: 10.1073/pnas.93.13.6536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelly S, Greenman C, Cook PR, Papantonis A. Exon skipping is correlated with exon circularization. J Mol Biol. 2015;427:2414–2417. doi: 10.1016/j.jmb.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 23.Conn SJ, Pillman KA, Toubia J, Conn VM, Salmanidis M, Phillips CA, Roslan S, Schreiber AW, Gregory PA, Goodall GJ. The RNA binding protein quaking regulates formation of circRNAs. Cell. 2015;160:1125–1134. doi: 10.1016/j.cell.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 24.Huang C, Liang D, Tatomer DC, Wilusz JE. A length-dependent evolutionarily conserved pathway controls nuclear export of circular RNAs. Genes Dev. 2018;32:639–644. doi: 10.1101/gad.314856.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Y, Zhang XO, Chen T, Xiang JF, Yin QF, Xing YH, Zhu S, Yang L, Chen LL. Circular intronic long noncoding RNAs. Mol Cell. 2013;51:792–806. doi: 10.1016/j.molcel.2013.08.017. [DOI] [PubMed] [Google Scholar]
- 26.Li Z, Huang C, Bao C, Chen L, Lin M, Wang X, Zhong G, Yu B, Hu W, Dai L, Zhu P, Chang Z, Wu Q, Zhao Y, Jia Y, Xu P, Liu H, Shan G. Exon-intron circular RNAs regulate transcription in the nucleus. Nat Struct Mol Biol. 2015;22:256–264. doi: 10.1038/nsmb.2959. [DOI] [PubMed] [Google Scholar]
- 27.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 28.Hansen TB, Jensen TI, Clausen BH, Bramsen JB, Finsen B, Damgaard CK, Kjems J. Natural RNA circles function as efficient microRNA sponges. Nature. 2013;495:384–388. doi: 10.1038/nature11993. [DOI] [PubMed] [Google Scholar]
- 29.Guo JU, Agarwal V, Guo H, Bartel DP. Expanded identification and characterization of mammalian circular RNAs. Genome Biol. 2014;15:409. doi: 10.1186/s13059-014-0409-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang M, Yu F, Wu W, Zhang Y, Chang W, Ponnusamy M, Wang K, Li P. Circular RNAs: a novel type of non-coding RNA and their potential implications in antiviral immunity. Int J Biol Sci. 2017;13:1497–1506. doi: 10.7150/ijbs.22531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas LF, Saetrom P. Circular RNAs are depleted of polymorphisms at microRNA binding sites. Bioinformatics. 2014;30:2243–2246. doi: 10.1093/bioinformatics/btu257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pamudurti NR, Bartok O, Jens M, Ashwal-Fluss R, Stottmeister C, Ruhe L, Hanan M, Wyler E, Perez-Hernandez D, Ramberger E, Shenzis S, Samson M, Dittmar G, Landthaler M, Chekulaeva M, Rajewsky N, Kadener S. Translation of CircRNAs. Mol Cell. 2017;66:9–21. e27. doi: 10.1016/j.molcel.2017.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Perriman R, Ares M Jr. Circular mRNA can direct translation of extremely long repeating-sequence proteins in vivo. RNA. 1998;4:1047–1054. doi: 10.1017/s135583829898061x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Granados-Riveron JT, Aquino-Jarquin G. The complexity of the translation ability of circRNAs. Biochim Biophys Acta. 2016;1859:1245–1251. doi: 10.1016/j.bbagrm.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 35.Dudekula DB, Panda AC, Grammatikakis I, De S, Abdelmohsen K, Gorospe M. CircInteractome: a web tool for exploring circular RNAs and their interacting proteins and microRNAs. RNA Biol. 2016;13:34–42. doi: 10.1080/15476286.2015.1128065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang Y, Fan X, Mao M, Song X, Wu P, Zhang Y, Jin Y, Yang Y, Chen LL, Wang Y, Wong CC, Xiao X, Wang Z. Extensive translation of circular RNAs driven by N(6)-methyladenosine. Cell Res. 2017;27:626–641. doi: 10.1038/cr.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Du WW, Yang W, Li X, Awan FM, Yang Z, Fang L, Lyu J, Li F, Peng C, Krylov SN, Xie Y, Zhang Y, He C, Wu N, Zhang C, Sdiri M, Dong J, Ma J, Gao C, Hibberd S, Yang BB. A circular RNA circ-DNMT1 enhances breast cancer progression by activating autophagy. Oncogene. 2018 doi: 10.1038/s41388-018-0369-y. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 38.Du WW, Yang W, Liu E, Yang Z, Dhaliwal P, Yang BB. Foxo3 circular RNA retards cell cycle progression via forming ternary complexes with p21 and CDK2. Nucleic Acids Res. 2016;44:2846–2858. doi: 10.1093/nar/gkw027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Du WW, Fang L, Yang W, Wu N, Awan FM, Yang Z, Yang BB. Induction of tumor apoptosis through a circular RNA enhancing Foxo3 activity. Cell Death Differ. 2017;24:357–370. doi: 10.1038/cdd.2016.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Abdelmohsen K, Panda AC, Munk R, Grammatikakis I, Dudekula DB, De S, Kim J, Noh JH, Kim KM, Martindale JL, Gorospe M. Identification of HuR target circular RNAs uncovers suppression of PABPN1 translation by CircPABPN1. RNA Biol. 2017;14:361–369. doi: 10.1080/15476286.2017.1279788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sui W, Shi Z, Xue W, Ou M, Zhu Y, Chen J, Lin H, Liu F, Dai Y. Circular RNA and gene expression profiles in gastric cancer based on microarray chip technology. Oncol Rep. 2017;37:1804–1814. doi: 10.3892/or.2017.5415. [DOI] [PubMed] [Google Scholar]
- 42.Huang YS, Jie N, Zou KJ, Weng Y. Expression profile of circular RNAs in human gastric cancer tissues. Mol Med Rep. 2017;16:2469–2476. doi: 10.3892/mmr.2017.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dang Y, Ouyang X, Zhang F, Wang K, Lin Y, Sun B, Wang Y, Wang L, Huang Q. Circular RNAs expression profiles in human gastric cancer. Sci Rep. 2017;7:9060. doi: 10.1038/s41598-017-09076-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vidal AF, Ribeiro-Dos-Santos AM, Vinasco-Sandoval T, Magalhaes L, Pinto P, Anaissi AKM, Demachki S, de Assumpcao PP, Dos Santos SEB, Ribeiro-Dos-Santos A. The comprehensive expression analysis of circular RNAs in gastric cancer and its association with field cancerization. Sci Rep. 2017;7:14551. doi: 10.1038/s41598-017-15061-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shen Y, Zhang J, Fu Z, Zhang B, Chen M, Ling X, Zou X. Gene microarray analysis of the circular RNAs expression profile in human gastric cancer. Oncol Lett. 2018;15:9965–9972. doi: 10.3892/ol.2018.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gu W, Sun Y, Zheng X, Ma J, Hu XY, Gao T, Hu MJ. Identification of gastric cancer-related circular RNA through microarray analysis and bioinformatics analysis. Biomed Res Int. 2018;2018:2381680. doi: 10.1155/2018/2381680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lai Z, Yang Y, Yan Y, Li T, Li Y, Wang Z, Shen Z, Ye Y, Jiang K, Wang S. Analysis of co-expression networks for circular RNAs and mRNAs reveals that circular RNAs hsa_circ_0047905, hsa_circ_0138960 and has-circRNA7690-15 are candidate oncogenes in gastric cancer. Cell Cycle. 2017;16:2301–2311. doi: 10.1080/15384101.2017.1380135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li T, Shao Y, Fu L, Xie Y, Zhu L, Sun W, Yu R, Xiao B, Guo J. Plasma circular RNA profiling of patients with gastric cancer and their droplet digital RT-PCR detection. J Mol Med (Berl) 2018;96:85–96. doi: 10.1007/s00109-017-1600-y. [DOI] [PubMed] [Google Scholar]
- 49.Li P, Chen H, Chen S, Mo X, Li T, Xiao B, Yu R, Guo J. Circular RNA 0000096 affects cell growth and migration in gastric cancer. Br J Cancer. 2017;116:626–633. doi: 10.1038/bjc.2016.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fang Y, Ma M, Wang J, Liu X, Wang Y. Circular RNAs play an important role in late-stage gastric cancer: Circular RNA expression profiles and bioinformatics analyses. Tumour Biol. 2017;39:1010428317705850. doi: 10.1177/1010428317705850. [DOI] [PubMed] [Google Scholar]
- 51.Huang M, He YR, Liang LC, Huang Q, Zhu ZQ. Circular RNA hsa_circ_0000745 may serve as a diagnostic marker for gastric cancer. World J Gastroenterol. 2017;23:6330–6338. doi: 10.3748/wjg.v23.i34.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhao Q, Chen S, Li T, Xiao B, Zhang X. Clinical values of circular RNA 0000181 in the screening of gastric cancer. J Clin Lab Anal. 2018;32:e22333. doi: 10.1002/jcla.22333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shao Y, Li J, Lu R, Li T, Yang Y, Xiao B, Guo J. Global circular RNA expression profile of human gastric cancer and its clinical significance. Cancer Med. 2017;6:1173–1180. doi: 10.1002/cam4.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao Y, Chen L, Lu R, Zhang X, Xiao B, Ye G, Guo J. Decreased expression of hsa_circ_0001895 in human gastric cancer and its clinical significances. Tumour Biol. 2017;39:1010428317699125. doi: 10.1177/1010428317699125. [DOI] [PubMed] [Google Scholar]
- 55.Sun H, Tang W, Rong D, Jin H, Fu K, Zhang W, Liu Z, Cao H, Cao X. Hsa_circ_0000520, a potential new circular RNA biomarker, is involved in gastric carcinoma. Cancer Biomark. 2018;21:299–306. doi: 10.3233/CBM-170379. [DOI] [PubMed] [Google Scholar]
- 56.Lu R, Shao Y, Ye G, Xiao B, Guo J. Low expression of hsa_circ_0006633 in human gastric cancer and its clinical significances. Tumour Biol. 2017;39:1010428317704175. doi: 10.1177/1010428317704175. [DOI] [PubMed] [Google Scholar]
- 57.Tian M, Chen R, Li T, Xiao B. Reduced expression of circRNA hsa_circ_0003159 in gastric cancer and its clinical significance. J Clin Lab Anal. 2018:32. doi: 10.1002/jcla.22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chen S, Li T, Zhao Q, Xiao B, Guo J. Using circular RNA hsa_circ_0000190 as a new biomarker in the diagnosis of gastric cancer. Clin Chim Acta. 2017;466:167–171. doi: 10.1016/j.cca.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 59.Li WH, Song YC, Zhang H, Zhou ZJ, Xie X, Zeng QN, Guo K, Wang T, Xia P, Chang DM. Decreased expression of Hsa_circ_00001649 in gastric cancer and its clinical significance. Dis Markers. 2017;2017:4587698. doi: 10.1155/2017/4587698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun HD, Xu ZP, Sun ZQ, Zhu B, Wang Q, Zhou J, Jin H, Zhao A, Tang WW, Cao XF. Down-regulation of circPVRL3 promotes the proliferation and migration of gastric cancer cells. Sci Rep. 2018;8:10111. doi: 10.1038/s41598-018-27837-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rong D, Dong C, Fu K, Wang H, Tang W, Cao H. Upregulation of circ_0066444 promotes the proliferation, invasion, and migration of gastric cancer cells. Onco Targets Ther. 2018;11:2753–2761. doi: 10.2147/OTT.S156516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhang J, Liu H, Hou L, Wang G, Zhang R, Huang Y, Chen X, Zhu J. Circular RNA_LARP4 inhibits cell proliferation and invasion of gastric cancer by sponging miR-424-5p and regulating LATS1 expression. Mol Cancer. 2017;16:151. doi: 10.1186/s12943-017-0719-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chen J, Li Y, Zheng Q, Bao C, He J, Chen B, Lyu D, Zheng B, Xu Y, Long Z, Zhou Y, Zhu H, Wang Y, He X, Shi Y, Huang S. Circular RNA profile identifies circPVT1 as a proliferative factor and prognostic marker in gastric cancer. Cancer Lett. 2017;388:208–219. doi: 10.1016/j.canlet.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 64.Tseng YY, Moriarity BS, Gong W, Akiyama R, Tiwari A, Kawakami H, Ronning P, Reuland B, Guenther K, Beadnell TC, Essig J, Otto GM, O’Sullivan MG, Largaespada DA, Schwertfeger KL, Marahrens Y, Kawakami Y, Bagchi A. PVT1 dependence in cancer with MYC copy-number increase. Nature. 2014;512:82–86. doi: 10.1038/nature13311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang Y, Li J, Yu J, Liu H, Shen Z, Ye G, Mou T, Qi X, Li G. Circular RNAs signature predicts the early recurrence of stage III gastric cancer after radical surgery. Oncotarget. 2017;8:22936–22943. doi: 10.18632/oncotarget.15288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang Y, Liu H, Li W, Yu J, Li J, Shen Z, Ye G, Qi X, Li G. CircRNA_100269 is downregulated in gastric cancer and suppresses tumor cell growth by targeting miR-630. Aging (Albany NY) 2017;9:1585–1594. doi: 10.18632/aging.101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Milella M, Falcone I, Conciatori F, Cesta Incani U, Del Curatolo A, Inzerilli N, Nuzzo CM, Vaccaro V, Vari S, Cognetti F, Ciuffreda L. PTEN: multiple functions in human malignant tumors. Front Oncol. 2015;5:24. doi: 10.3389/fonc.2015.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu T, Liu S, Xu Y, Shu R, Wang F, Chen C, Zeng Y, Luo H. Circular RNA-ZFR inhibited cell proliferation and promoted apoptosis in gastric cancer by sponging miR-130a/miR-107 and modulating PTEN. Cancer Res Treat. 2018 doi: 10.4143/crt.2017.537. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Pan H, Li T, Jiang Y, Pan C, Ding Y, Huang Z, Yu H, Kong D. Overexpression of circular RNA ciRS-7 abrogates the tumor suppressive effect of miR-7 on gastric cancer via PTEN/PI3K/AKT signaling pathway. J Cell Biochem. 2018;119:440–446. doi: 10.1002/jcb.26201. [DOI] [PubMed] [Google Scholar]
- 70.Zheng Q, Bao C, Guo W, Li S, Chen J, Chen B, Luo Y, Lyu D, Li Y, Shi G, Liang L, Gu J, He X, Huang S. Circular RNA profiling reveals an abundant circHIPK3 that regulates cell growth by sponging multiple miRNAs. Nat Commun. 2016;7:11215. doi: 10.1038/ncomms11215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cheng J, Zhuo H, Xu M, Wang L, Xu H, Peng J, Hou J, Lin L, Cai J. Regulatory network of circRNA-miRNA-mRNA contributes to the histological classification and disease progression in gastric cancer. J Transl Med. 2018;16:216. doi: 10.1186/s12967-018-1582-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest. 2009;119:1438–1449. doi: 10.1172/JCI38019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Qi X, Zhang L, Lu X. New insights into the epithelial-to-mesenchymal transition in cancer. Trends Pharmacol Sci. 2016;37:246–248. doi: 10.1016/j.tips.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 74.Nieto MA. Epithelial plasticity: a common theme in embryonic and cancer cells. Science. 2013;342:1234850. doi: 10.1126/science.1234850. [DOI] [PubMed] [Google Scholar]
- 75.Li J, Zhen L, Zhang Y, Zhao L, Liu H, Cai D, Chen H, Yu J, Qi X, Li G. Circ-104916 is downregulated in gastric cancer and suppresses migration and invasion of gastric cancer cells. Onco Targets Ther. 2017;10:3521–3529. doi: 10.2147/OTT.S136347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hajra KM, Chen DY, Fearon ER. The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res. 2002;62:1613–1618. [PubMed] [Google Scholar]
- 77.Zhou LH, Yang YC, Zhang RY, Wang P, Pang MH, Liang LQ. CircRNA_0023642 promotes migration and invasion of gastric cancer cells by regulating EMT. Eur Rev Med Pharmacol Sci. 2018;22:2297–2303. doi: 10.26355/eurrev_201804_14818. [DOI] [PubMed] [Google Scholar]