Abstract
The optogenetic tools have been described as valuable techniques to study neural activity through light stimulation, as well as potential neuromodulator approaches in the management of several central nervous system (CNS) diseases. Since the first bacteriorhodopsin protein described as a single-component light-activated regulator of transmembrane ion flow description, in 1980’s, the focus has been on channel proteins for neurobiology; however, the advances in engineering techniques showed involvement changes in cellular biological behavior in several types of proteins involved in cell cytoskeleton regulation, motility and gene expression. Although the use of this technology has been published in many papers, a question still remains regarding real results and potential clinical applicability in CNS diseases, as well as the publications scarcity that systematically analyses the published results. Lastly, the aim of this review is to discuss the experimental results, molecular mechanisms and potential clinical applications of optogenetic tools in epilepsy and depression treatment, as well as its applicability in the treatment of CNS tumors.
Keywords: Optogenetic tools, light signaling, epilepsy, depression, central nervous system tumors
Introduction
Optogenetics is the combination of genetic and optical methods to stimulate or inhibit well-defined events in specific cells of living tissue and behaving animals [1]. The ability to systematically perturb and interrogate the intracellular protein networks that control cell behavior has lagged behind the ability to observe these behaviors [2]. Towne and Thompson (2016) [3] has defined optogenetics as “a method that uses light to control cells in living tissue, typically neurons, that have been modified to express light-sensitive ion channels and pumps”.
The standard genetic perturbation techniques as knockdown, overexpression and mutation, are extremely effective at identifying the proteins involved in a phenotype, but are less effective at explaining the mechanism. Pharmacological perturbations have been also extremely useful tools that target or block specific molecules and rapidly switch off the function of a target protein [2]. However, these approaches do not allow spatial control and considerable engineering is required to obtain highly specific inhibitors [4]. The optogenetics foundation is a combination of genetic manipulations, which renders identified populations of neurons sensitive to the action of light sensitive algae [5]. The optogenetic tools have led to an explosive proliferation of different variants of light-sensitive ion channels, G protein-coupled receptors and ion pumps [6,7]. In the last 8 years, optogenetics has become an established research tool for studying brain function [6].
Light-gated protein modules provide a potentially transformative solution to the problem of dissecting cellular network function. There are currently new light-controlled modules that can be used to control the function and localization of diverse proteins [2]. Nearly all of these modules are borrowed from organisms that have sophisticated light-sensing systems. Generally these are protein modules that contain photoisomerizable chromophores, which, when activated by the proper light wavelengths, will cause a conformational change in the protein. Broadly, these tools use two general mechanisms to link photos witching to generic protein activities. The first strategy is to allosterically link photo-activation to protein activity. For example, one can genetically insert a responsive light-oxygen-voltage-domain (LOV domain) into a protein of interest, such that this domain, in one conformation, will sterically block or perturb protein function. Photoisomerization of the LOV domain releases the allosteric block on protein function, which reverts to the inactivating state at timescales ranging from seconds to hours [8].
Another strategy for regulating cell signaling is through light-controlled protein-protein interaction. The recruitment of proteins to new locations and new complexes is frequently used to gate their function.
Similarly, photo-activated interaction factors (PIFs), which belonging to Arabidopsis basic helix-loop-helix (bHLH) subgroup of 15 key interacting proteins that play negative roles in light responses, can be used to regulate diverse cell functions. These interaction factors can be used to control protein subcellular localization [9]. Their activities are post-translationally countered by light-activated phytochromes, which promote the degradation of PIFs and directly or indirectly inhibit their binding to DNA. A way to use PIFs is to link splited portions of proteins that must associate to function [10,11].
This manuscript aims to clarify the molecular mechanisms of optogenetics related to treatment of epilepsy, depression and central nervous system (CNS) tumors described in the literature, emphasizing the potential clinical results of optogenetics regarding to pathologies’ treatments. Figure 1 represents a schematic chart with the criteria selection of the relevant publications that were included in the present review.
Figure 1.
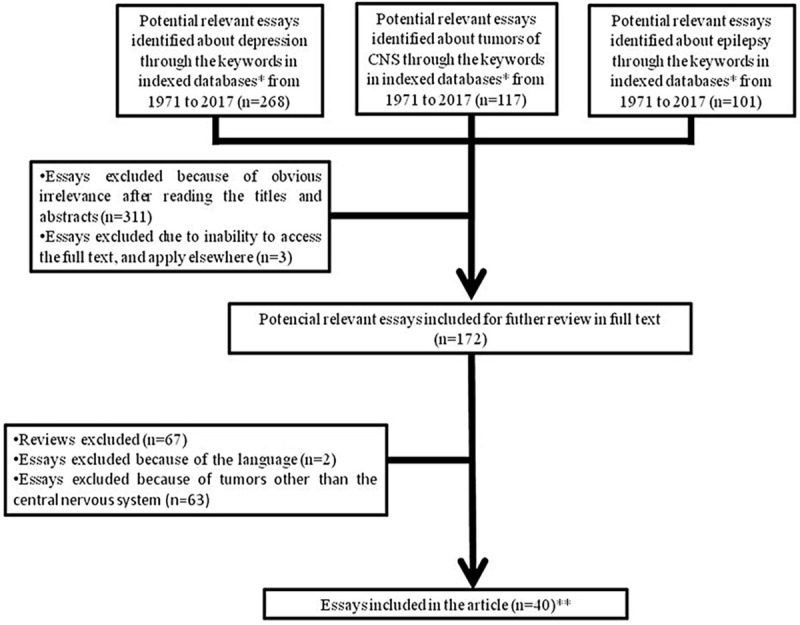
Schematic chart showing the criteria selection of the relevant publications that were included in the present review. *: MEDLINE, LILACS, SciELO, PubMed, BIREME, Cochrane Library, Scopus databases; **: Tables 1, 2 and 3.
Optogenetic tools in brain cells
Optogenetic technologies study the neural circuit underpinnings of behavior and most commonly involve three core features [8,12-15]: (i) Microbial opsins, members of an ancient, but uniquely well-suited, gene family adapted from evolutionarily distant organisms such as algae and archaebacteria; each gene encoding a distinct protein that directly elicits electrical current across cellular membranes in response to light; (ii) General methods for targeting sufficiently strong and specific opsin gene expression to well-defined cellular elements in the brain; (iii) General methods for guiding sufficiently strong and precisely timed light to specific brain regions, cells, or parts of cells, while the experimental subject carries out behaviors of interest.
Several optogenetic methods have been allowed versatile analyses and modulation over biological and molecular cellular activity in the brain cells. Thus, many neuronal excitatory (depolarization) and inhibitory (hyperpolarization) techniques have been developed. The optogenetic tools have been associated to photo-activation reversible or irreversible presenting higher rates of specificity and partial precision (micrometers), as well as the lower temporal response (milliseconds or minutes) when compared with other modulation methodologies [12]. As other methodologies it can be cited: (a) genetic-chronic, high hours, entire cell [12,13]; (b) temperature sensitive mutation-high seconds, entire cell [12,14]; (c) pharmacological-milliseconds, minutes, or micrometers, entire cell [12,15]; (d) chemical dimerization, photouncaged-high milliseconds, minutes, or micrometers, entire cell [8,12].
Combined optogenetic methods in brain cells
Although the optogenetic tools have been described associated to a significant potential with regards the manipulation and observation of cellular behavior and circuit consequences [16,17], a detailed bio-molecular analysis aiming to elucidate the biological functions of CNS require a simultaneous monitoring approaches, as well as manipulation of several types of neurons at the same cellular or circuit level [17]. The chosen method is dependent on the combined approaches of neurophysiology, on the physiopathology of the disease, and on the optogenetic tools [16,18-28] as described below: (i) Combining optogenetic methods with electrical recordings; (ii) Combining optogenetic tools monitoring and control (depolarization or hyperpolarization) neuronal activity; (iii) Combining optogenetic tools with multicolor stimulation of neural circuits; (iv) Light delivery techniques for optogenetic control of brain activity (supply light at a sufficient intensity to a defined volume of brain tissue); (v) Optoelectrodes (devices for combined light delivery and electrical recording); (vi) Combining optogenetics with pharmacological agonists and antagonists interventions; (vii) Combining optogenetics with surgical interventions.
Although some researchers have been defending the use of combined approaches (optogenetics associated to conventional techniques) [25-28], others have been debating that this methodological challenge might be more perfectly tackled by combining optogenetic tools within the same experiment [16]. However, to investigate correlations by the canonical methodology, none of those methodologies alone provide both high temporal and spatial cellular specificity [10,16,29]. Combining monitoring and control tools may establish correlation and causation in the same experiment, increasing the yield of individual experiments and raise the standards in many fields of neurobiological research [16].
Advantages and limitations of optogenetic tools in brain cells
Although thousands of optogenetic essays have already been published in brain cells from 1971 to 2017 (Figure 2A), the advantages and limitations of the procedure have not been yet adequately defined due to brain heterogeneous cell populations and highly uncontrolled protocols. At the present, the advantages of optogenetic tools can be summarized as following [12,28,30-34]: (i) Higher rates of specify stimulation that implies in highly reproducible and stable responses; (ii) Higher rates of spatial precision in the cellular stimulation; (iii) Lower temporal cellular results; (iv) Development of better transfection methods (vectors, promoters, microbial rhodopsins); (v) Improvement in the bio-molecular analyses development, neural networks of the CNS cell populations, and neuronal circuits in the pathophysiological of diseases; (vi) Development of most appropriate light sources; (vii) Versatile methodology that allow the creation of several designs in experimental studies; (viii) Development of gene therapy and potential clinical applications in CNS pathologies; (ix) Allow the creation of specific animal models aiming diseases researcher.
Figure 2.
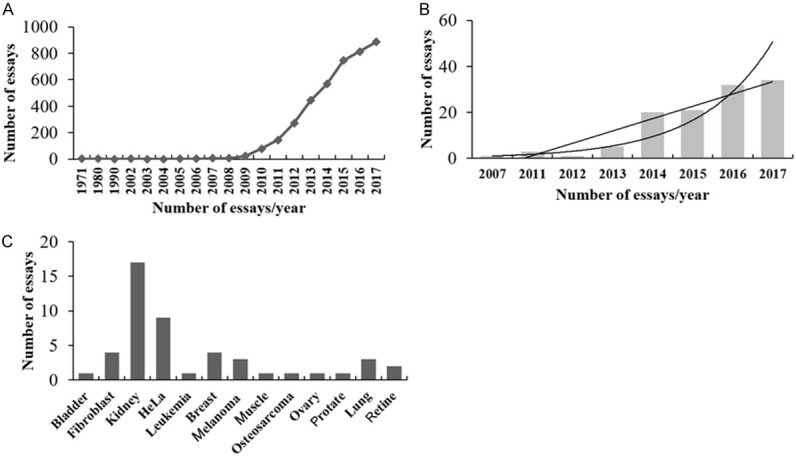
A. Number of relevant optogenetic essays in brain cells published in PubMed database per year from 1971 to 2017. B. Number of relevant optogenetic essays in cancer cells published in PubMed database from 2007 to 2017. C. Number of optogenetic essays performed in different tumor types published in PubMed database from 2007 to 2017.
The optogenetic methodological limitations [6,12,34-38] are presented below: (i) The performance of optogenetic studies requires high-end precise technology to address the issues related to data and result reproducibility; (ii) Initial experimental largely addresses in the murine system that it has been recognized as insufficient to mimic human disease; (iii) Limited range of wavelengths compatible with light microscopy; (iv) Lack of stimulation control in some cellular levels for modulation in the same cells population, as well as unknown biological interactions; (v) Some difference in the synchronization level in cells that expresses the optogenetic tools.
Microbial optogenetic tools in brain cells
The key properties of microbial optogenetic tools use seven-transmembrane proteins encoded by the type I class of opsin gene [39]. Type I opsins are protein products of microbial opsin genes and are termed rhodopsins when bound to retinal. These proteins are distinguished from their mammalian (type II) counterparts, which are single-component light-sensing systems. The same protein carries out the two operations, light sensing and ion conductance.
The type I protein is the haloarchaeal proton pump bacteriorhodopsin (BR) [40-42]. Under low-oxygen conditions, BR is highly expressed in haloarchaeal membranes and serves as part of an alternative energy-production system, pumping protons from the cytoplasm to the extracellular medium to generate a proton-motive force to drive ATP synthesis [42,43].
A second class of microbial opsin gene encodes halorhodopsins (HR) that is a light-activated chloride pump first discovered in archaebacteria [44]. The operating principles of HR are similar to those of BR [45], with the two main differences being that HR pumps chloride ions and its direction of transport is from the extracellular to the intracellular space. Specific amino acid residues have been shown to underlie the differences between BR and HR in directionality and preferred cargo ion [46]. As example, neurons can be targeted to express the light-activated chloride pumping HR from Natronomonas pharaonis (NpHR), which can be hyperpolarized and inhibited from firing action potentials when exposed to yellow light [47].
Next, a third class of conductance-regulating microbial opsin protein (channelrhodopsin or ChR) was identified (ChR1 and ChR2). Nagel and Hegemann demonstrated light-activated ion-flux properties [48] for a protein encoded by one of the genomic sequences from the green algae Chlamydomonas reinhardtii. While ChR is highly homologous to BR, especially within the transmembrane helices that constitute the retinal-binding pocket, in channelrhodopsins the ion-conducting activity is largely uncoupled from the photocycle [49]; an effective cation channel pore is opened, which implies that ion flux becomes independent of retinal isomerization and rather depends on the kinetics of channel closure. In neurons, net photocurrent due to ChR activation is dominated by cation flow down the electrochemical gradient (resulting in depolarization), rather than by the pumping of protons [48,50]. VChR1 is a cation-conducting channelrhodopsin from Volvox carteri that can drive spiking at 589 nm, with excitation maximum red-shifted approximately 70 nm compared with ChR2 [50,51].
Among other important properties, all them operate on the millisecond timescale and can function in mammalian neurons without addition of exogenous chemical cofactors, since the chromophore for these proteins, all-trans retinal, appears to be already present at sufficient levels in mammalian brains [52]. Moreover, light and gene delivery challenges have been overcome to allow specific cell types, deep within the brain, to be controlled in freely behaving mammals [51].
Targeting optogenetics vectors in brain cells
The optogenetic methodology is able to select specific cells populations; however, it is not site-specific, as a brain electric stimulation effect, such as deep brain stimulation (DBS), transcranial direct current stimulation (tDCS), magnetic seizure therapy (MST), and electroconvulsive therapy (ECT) [53,54]. Thus, in order to target specific brain cells populations, choosing the best vectors for the genetic code delivery is an important step. There are currently two methods that are the most commonly used, which are viral gene delivery and transgenic technology [53].
The viral vectors have been widely used due to its versatility, ability to infect non-dividing cells such as neurons and are relatively safe to use. The most commonly used are the adeno-associated virus (AAV) and the lentivirus (LV), even though they lack the capacity to hold larger genetic sequences that can be constructed up to 5-10 kilobases (kb) [53-55]. The sequences constructed within the viral genetic code must contain a promoter, which is responsible for the expression of the subsequent opsin gene.
There are a few gene options that are also commonly used such as Ca2+/calmodulin-dependent protein kinase IIα (CaMKIIa) [41] and Parvalbumin (PV) [56], which are to be used on nonhuman primates models, and double-floxed inverse open-reading frame (2-DIO) [55,57] that are less used in human synapsis [57,58].
The transgenic technology uses two approaches, single transgenic line and binary systems. The single transgenic line is based on the randomly placement of the transgene into a specific promoter within a specific population of cells such as neurons. A drawback from this technique is that for many genes, there is no comprehension of the cell type-specific regulation. In addition, wrongly integrated transgenes can cause multiple patterns of ectopic expressions. These transgenic constructs can be inserted into transgenic mice through bacterial artificial chromosomes (BAC) [53,59,60].
The binary systems consist on the use of two autonomous components essential for the expression of the transgene, a driver line to control the expression of the transgene and a reporter line whose activity is regulated by the driver line. The driver genes can be either site-specific recombinases (SSRs) or transcriptional activators. The Cre/Lox recombinase system allows the DNA modification to be targeted to a specific cell type, which is very useful for the manipulation of the mouse genome. After Cre expression it is possible, under the control of its specific promoter, delete the STOP cassette inducing the transgene expression [61].
Current optogenetics perspectives in epilepsy
While there is no generally-accepted definition of refractory epilepsy, this term generally designates a spectrum of pathologies characterized by recurrent seizures, which respond poorly or not at all to conventional medicines [62]. Clinical evidence indicates that some of these patients will actually benefit to some extent from add-on treatments while maintaining the antiepileptic drugs unchanged. At present, the main treatment options for refractory epilepsy are brain surgery (i.e., temporal lobe localized neocortical resection) and vagus nerve stimulation, which is a variety of neuromodulation [62-64]. Recently, researchers started to explore possible optogenetic approaches using animal models (Table 1).
Table 1.
Optogenetic tolls in the epilepsy treatment performed in in vivo models published from 2013 to 2018
| Publications | Neuron Cell Population | Light Color | Stimulation Parameters | Photoactivatable Protein | Findings |
|---|---|---|---|---|---|
| Zhang et al., 2018 [100] | 5-HT from Dorsal Raphe | Blue (473 nm) | (1) 9 mW, 20 ms, 20 Hz, 1, 5, 10, 15 min | ChR2 | Reduction of S-IRA in transgenic DBA/1 mice |
| (2) 15 mW, 20 ms, 20 Hz, 3, 5 min | |||||
| Chang et al., 2018 [101] | GABAergic interneurons from the superficial layer of the somatosensory cortex | Blue (470 nm) | 9 mW, 30 ms, 1 Hz, 50 s | ChR2 | A brief photoactivation was sufficient to initiate the transition to ictal events in cortical 4-AP models |
| Tung et al., 2018 [102] | Dorsal hippocampus | ND | ND | iLMO2 | Suppression of focal epileptic activity |
| Chang & Chang & Shyu, 2017 [103] | Cortical GABA neurons from nRT | Blue (473 nm) | (1) 9 mW, 20 ms, 10 Hz, ND | ChR2 | Attenuation of cortical seizures |
| (2) 15 mW, 50 ms, 20 Hz, ND | |||||
| (3) 9 mW, 100 ms, 50 Hz, ND | |||||
| (4) 15 mW, 1 s, 100 Hz, ND | |||||
| Wang et al., 2017 [104] | Subiculum GABAergic neurons | Blue (473 nm) | 5 mW, 10 ms, 20 Hz, 600 pulses | (1) ChR2 | (1) Retarded sGS acquisition followed by an aggravation of sGS expression by ChR2 stimulation |
| (2) Arch | (2) Photostimulation of subicular pyramidal neurons genetically targeted with Arch, not NpHR3.0, alleviates sGS Expression | ||||
| (3) NpHR3.0 | |||||
| Petersen et al., 2017 [105] | Subicular neurons from subiculum | Blue (480 nm) | ND | ChR2 | The activation of 5-HT2C receptors decreased the occurrence of epileptiform discharges in the subiculum |
| Sorokin et al., 2017 [68] | TC neurons | 488 nm/594 nm dual-wave-length laser | 8 mW (mice), 15 mW (rats), single 50 ms, 25 ms at 3, 8, 12, 20 Hz, 50 pulses (except for 3 Hz with 20 pulses) | eNpHR3.0 | (1) Unilaterally toggling TC phasic spiking via eNpHR induced bilateral SWDs |
| (2) Unilaterally toggling TC tonic spiking via SSFO bilaterally aborted SWDs | |||||
| (3) Unilaterally suppressing TC output via eNpHR bilaterally shortened SWDs | |||||
| Xu et al., 2016 [106] | EC principal neurons | Blue (473 nm) | 10 mW, 5 ms, 1 or 20 Hz, 2 min | ChR2 | (1) Activation of enorhinal CaMKIIα-positive neurons inhibited hippocampal neurons activity and reduced severity of hippocampal seizures |
| (2) Inhibition of enorhinal CaMKIIα-positive neurons increased hippocampal neurons activity and promoted hippocampal seizures | |||||
| Assaf & Yitzhak, 2016 [107] | PV interneurons | Blue (473 nm) | ND, 20 ms, ND, ND | ChR2 | (1) Antiepileptic effects following PV interneurons activation during electrographic seizure |
| (2) Initiation of electrographic seizures following PV interneurons activations during interictal phase | |||||
| Tassin et al., 2016 [108] | Balb-c Mice | Blue (488 nm) | 40-200 uW, 0.1 ms, ND | ChR2 | Inactivation of the receptor activity with the mGlu7 negative allosteric modulator enhanced thalamic synaptic transmission |
| Hoffman et al., 2016 [109] | Dentate gyrus hilar somatostatin interneurons | 447 nm | 100 mW, 2 ms, 33 Hz, 25 ms | ChR2 | Inhibition of local field potential responses to perforate path stimulation |
| Lu et al., 2016 [110] | Dentate gyrus/hilus GABAergic interneurons | Blue (473 nm) | 10 mW, 5 ms, 130 Hz, 1 min | ChR2 | (1) Activation of DGH GABAergic INs significantly inhibited ictal discharges in the DGH, MEC and M1 |
| (2) Optical stimulation in the MEC suppressed seizures only in local networks, not in the DGH or M1 | |||||
| Hoffman et al., 2016 [109] | Pilocarpine animal model (mice) | 477 nm | 100 mW, 2 ms, 33 Hz, 15 s | Hilar somatostatin interneuron | Light activation of hilar somatostatin interneurons inhibited evoked responses |
| Shiri et al., 2016 [111] | Parvalbumin or somatostatin positive interneurons on EC | Blue (473 nm) | 35 mW, 1 ms, 1 Hz, 180 s | ChETA-eYFP | Shortened or delayed seizures in vitro |
| Shiri et al., 2016 [112] | PNs on EC | Blue (473 nm) | (1) 35 mW, 1 s, 0.2 Hz, 30 s | ChR2 | LVF onset ictal discharges can be triggered by optical activation of PV- and SOM-positive interneurons |
| (2) 35 mW, 20 ms, 2 Hz, 30 s | |||||
| Ladas et al., 2015 [113] | GABA interneurons in the hippocampus | Blue (473 nm) | 100 mW, 5 ms, 1 Hz, 120 s | ChR2 | (1) Low frequency optical stimulation significantly suppresses epileptiform activity |
| (2) Selective optical activation of interneurons at low frequency suppresses epileptiform activity in the hippocampus | |||||
| (3) Activation of GABA interneurons causes entrainment of hippocampal CA3 pyramidal cells by a GABAA mediated mechanism | |||||
| Kros et al., 2015 [114] | Cerebellar nuclei neurons | Blue (470 nm) | ND, 30-300 ms, ND, ND | ChR2 | A single short-lasting stimulation of cerebellar nuclei neuron activity abruptly stopped GSWDs, even when applied unilaterally |
| Yellow (590 nm) | |||||
| Krook-Magnuson et al., 2014 [115] | Parvalbumin-expressing neurons in the lateral or midline cerebellum, Purkinje cells or hippocampus | Blue (470 nm) | ND, 50 ms/1000 ms, 100 Hz, 3 s | ChR2; HR | (1) Optogenetic excitation or inhibition of parvalbumin-expressing neurons in the lateral or midline cerebellum results in a decrease in seizure duration |
| Amber (589 nm) | (2) Spontaneous seizure frequency occurs uniquely with optogenetic excitation of the midline cerebellum | ||||
| Cunningham et al., 2014 [116] | Maturing GABAergic interneurons in the hippocampus | Blue (470 nm) | ND, 1 ms, 1 Hz, ND | ChR2 | (1) The activation of human mGINs induces inhibitory synaptic responses in host neurons |
| (2) mGIN grafts suppress seizure and abnormal behavior | |||||
| Ellender et al., 2014 [117] | Hippocampal pyramidal neurons | Blue (470 nm/473 nm) | ND | ChR2; Archaerhodopsin | Inhibiting parvalbumin-expressing interneurons during epileptiform activity reduces afterdischarges incidence |
| Green (530 nm) | |||||
| Chiang et al., 2014 [118] | Pyramidal neuron or interneuron (GABA) in the hippocampus | Blue (473 nm) | 2 mW/6.1 mW, 5 s/20 s, 20 Hz/50 Hz | ChR2 | (1) Optical stimulation suppresses focal and distal epileptiform activity |
| (2) Seizure suppression decreases over time but can be reinstated by intermittent stimulation | |||||
| (3) GABA transmission activated by optical stimulation is implicated in seizure suppression | |||||
| Ledri et al., 2014 [119] | CA3 region of the hippocampus | Blue (460 nm) | ND, 1-2 ms, 20 Hz/50 Hz, 5 s | ChR2 | Simultaneous activation of mixed populations of interneurons by optogenetics brief initial action potential discharge in CA3 pyramidal neurons, followed by prolonged suppression of ongoing epileptiform activity during light exposure |
| Berglind et al., 2014 [120] | Principal neuronal population in the; Hippocampus | Orange-Yellow (593 nm) | 25 mW, ND | NpHR | (1) Bicuculline-induced seizures in vivo are reduced by principal cell optogenetic silencing |
| (2) Optogenetic silencing of principal cells arrests seizures caused by reduced inhibition | |||||
| Sukhotinsky et al., 2013 [121] | Hippocampal pyramidal neurons | Green (561 nm) | 35 mW, 5 s, ND, 35 s | NpHR | The inhibition of excitatory drive in hippocampus can delay seizure onset |
| Krook-Magnuson et al., 2013 [122] | Principal cells in the temporal lobe | Blue (473 nm) | 35 mW, 10 ms/2 s, ND, 2 h | ChR2 | Optogenetic inhibition of excitatory principal cells or activation of a subpopulation of GABAergic cells stops seizures rapidly upon light application |
| Amber (589 nm) | HR | ||||
| Red (635 nm) | |||||
| Wykes et al., 2013 [124] | Neocortical pyramidal cells | Green (561 nm) | 25 mW, ND | NpHR | Optogenetic inhibition of pyramidal neurons was sufficient to acutely attenuate seizure activity |
Stimulation parameters: Energy level, pulse duration, frequency, duration of the stimulation; ND, no Data; S-IRA, seizure induced respiratory arrest; nRT, reticular thalamic nucleus; Sgs, secondary generalized seizure; nRT, reticular thalamic nucleus; EC, entorhinal córtex; TC, thalamocortical; CT, corticothalamic; CTC, cortico-thalamo-cortical; SWD, spike-wave discharge; SSFO, stable step function opsin; PN, principal neurons; DGH, dentate gyrus/hilus; M1, primary motor cortex; VPM, glutamatergic ventro-postero-medial nucleus; HP, hippocampal; IN, interneurons; MEC, medial entorhinal cortex; M1, primary motor cortex.
It is interestingly to note that two functionally distinct classes of microbial opsin genes have been extensively used for epilepsy researchers, NpHR and ChR2 (Table 1). NpHh was used in the first demonstration of concept of seizure control by optogenetic in the temporal lobe epileptic circuit, in hippocampal slice cultures, in which eNpHR expression was driven in excitatory neurons with a viral construct containing a promoter of CaMKIIa [55,65,66]. To note, eNpHR is a molecularly engineer NpHR which high express endoplasmic reticulum (ER) accumulation when exposed to yellow light. Thus, NpHR colocalized with ER proteins containing the KDEL ER retention sequence (refers to proteins that are retained in the ER after folding being that the classical ER retention signal is the C-terminal KDEL sequence for lumen bound proteins). Also, the new enhanced NpHR (eNpHR) allows safe, high-level expression in mammalian neurons, without toxicity and with augmented inhibitory function, in vitro and in vivo [67].
Targeting multiple specific neuronal cell types can effectively reduce seizures in a model of severe epilepsy induced by intrahippocampal injection of the excitotoxin kainic acid [65]. Nevertheless, real-time detection, using software was coupled with optogenetic control to reduce seizure duration and severity. Seizure control was obtained by targeting and activating PV-containing hippocampal interneurons with ChR2 or inhibiting eNpHR-expressing hippocampal excitatory neurons [67,68].
Recent studies also showed the efficacy of optogenetic and related approaches in animal models of focal cortical epilepsy induced by focal injections into cortex [65,69]. The approach was coupled with simultaneous injection of viruses containing one of two activity-modifying therapeutic agents, eNpHR2.0 or a voltage-gated potassium channel Kv1.1 [56,65]. Each of the viral approaches was designed to infect local excitatory neurons in the region of the epileptogenic insult with an exogenous protein that would suppress excitability. Resulting spontaneous seizures and epileptiform responses were suppressed by each of these approaches, either through constitutive expression (with Kv1.1), or in a controlled fashion, with yellow light (with eNpHR) [56,65].
Advances in optogenetics versus depression
Major depressive disorder (MDD) is a common, debilitating psychiatric disorder with an estimated lifetime prevalence of approximately 16.2% [70]. MDD is the mainly cause of disability worldwide, with a global prevalence of roughly 120 million patients [71]. Depression is a leading cause of suicide and holds a correlation with several highly prevalent medical conditions, such as cardiovascular disease, obesity, diabetes, and dementia. Moreover, it is estimated that one third of the patients are not responsive to conventional pharmacological treatment [72].
The limited rate of success of the conventional therapeutic approaches makes investing in novel treatment strategies imperative. Although initially promising, DBS, a technique that uses targeted electrical stimulation to correct dysfunctional neural circuits, has been shown to have significant limitations. Electrical stimulation indiscriminately affects both neurons and fibers of passage, making the predicted spatial location difficult of the affected cells. Also, it is not possible targeting specific cell types in the heterogeneous brain regions [73]. Besides, the functional consequences of electrical stimulation are often not clear and may cause excitation, inhibition, or both, thereby limiting insight into the neurobiological mechanisms that cause or treat the disease [74].
Optogenetics circumvents these limitations by providing temporally precise, noninvasive bidirectional control of activity in well-defined neuronal populations [6], using genetically-encodable light-sensitive proteins. With these features, optogenetics can be used to causally probe circuitries underlying complex behavior, dissect signaling pathways and construct models of psychiatric disease through gene loss- and gain-of-function experiments [38]. New branches of optogenetics, which include cellular probing of signaling mechanisms and optical readout of neuronal activity are rapidly emerging and may set the stage for precise closed-circuit control and therapeutic intervention in human disease [75].
Decades of evidence implicate the frontal cortex as a primary locus of dysfunction in affective disorders. Also, it is known dissociable roles for different frontal regions in the regulation of mood and depressive state [76]. The optogenetic results of the medial prefrontal cortex (mPFC) showed varying data. While the first study, conducted by Covington et al. (2010) [77], showed a rapid and robust antidepressant-like behavioral response measured by both the social interaction test and the sucrose preference test [77], another study published in 2012 showed no effect on depression-related behavior neither on the forced swim test (FST) nor on the open field [78].
It is important to note that in the first study the researchers used the ubiquitous IE4/5 promoter that expressed the ChR2 opsin in both inhibitory and excitatory neurons as well in the glial cells, whilst the promoter used in the second study- the CaMKIIα promoter-limited the expression of the protein only to excitatory neurons. Both studies used the well-validated social chronic social defeat animal model of depression. The study published by Warden et al. (2012) [78] also showed that stimulation of specific efferents from the mPFC to different subcortical regions elicited very different and specific behavioral responses. When the projection from the mPFC to the dorsal raphe nucleus, the major source of serotonin to the forebrain, was stimulated, immobility in the forced-swimming test (FST) decreased without affecting locomotor activity in the open field. Conversely, stimulation of the projection from the mPFC to the lateral habenula (LHb), an area known to exhibit elevated firing in response to the omission of reward increased immobility [79]. The contradictory behavioral results obtained in these studies highlight the importance of discerning the contributions of different prefrontal circuits in affective state.
Kumar et al. (2013) [80] demonstrated the intricacy of the effect of prefrontal stimulation on animal behavior. Thy1-ChR2 mice, which express ChR2 in layer 5 pyramidal neurons in the mPFC, as well as afferents from other brain regions, were implanted with optical fibers over the anterior prelimbic region. These circuits were then stimulated, producing mixed effects on depression-related behavior. Stimulated mice exhibited decreased immobility in the FST but this effect was coupled with increased distance traveled in the open field. Also, stimulation had no detectable effect on social interaction time following chronic social defeat. Additionally, mPFC stimulation in this study induced a robust anxiolytic effect in the elevated plus maze. It was also noted that therapeutic response correlates with increased synchrony among depression-relevant limbic regions.
A reduction in the ability to experience reward and pleasure (anhedonia) as well as a loss of motivation, are hallmarks of depression in humans [81]. It has been shown that these depression symptoms arise from a dysregulation in the mesolimbic dopamine (DA) system, the brain’s circuit responsible for mediating reward [82]. The mesolimbic DA system is composed of two highly interconnected regions fundamental to reward mediation: the ventral tegmental area (VTA) and the nucleus accumbens (NAc). The DA neurons in the VTA are the main effectors of reward, they fire constantly at a tonic rate, and when they fire phasically they induce reward. Optogenetic strategies are helping unveil the precise mechanisms involved in the process of reward mediation.
Nevertheless, the studies that used the optogenetics approach to modulate activity in the region seeking the improvement of depression-related symptoms have showed conflicting results. Chaudhury et al. (2013) [82] found that enhanced phasic (20 Hz), but not tonic (4 Hz), firing of VTA DA neurons induced an increased susceptibility to depression-like symptoms (social avoidance and reduced sucrose preference) even in previously resilient mice [82]. Meanwhile, Tye et al. (2012) [83] found that phasic ChR2-mediated activation of VTA DA neurons resulted in an increase in escape-related behavior.
One important factor that might account for the diverging results is the implementation of different depression animal models. Chaudhury et al. (2013) [82] exposed the mice to the chronic social defeat stress model, which stress is induced by introducing a male experimental mouse or an “intruder” into the home cage of a larger, retired breeder male mouse called “resident” for several minutes during which the experimental mouse is attacked and defeated by the resident mouse. Tye et al. (2012) [83], nonetheless, subjected the mice to the chronic mild stress model, in which foot-shock was delivered twice daily during an 8-12 week period.
Indeed, it has been reported that acute and chronic stress elicit different effects on VTA DA neurons firing: strong acute stressors can increase VTA DA neuron firing [84], but chronic stressors or incubation periods can reduce VTA DA neuron firing rates [85]. Walsh et al. (2014) [86] used Cre-inducible adenovirus-associated vectors to transduce bilaterally the VTA and express the ChR2-EYFP opsin in TH-Cre mice susceptible to the chronic stress defeat paradigm she was able to show the excessive activation of VTA DA neurons reduced the firing rate by increasing K+ currents, a phenomenon known as self-tuning compensation.
The role-played by stress exposure and constant VTA DA neurons activation was performed by phasic optogenetics, which increased brain-derived neurotrophic factor (BDNF) amounts in the NAc of socially stressed mice compared with no stressed naïve mice. This stress gating of BDNF signaling is mediated by CRF acting in the NAc [86].
Enhanced glutamatergic transmission in the NAc has been implicated in the pathophysiology of depression; however, the afferent source of this increased glutamate was not previously known. By using optogenetics, researchers have been able to identify that an increases in the glutamatergic transmission from the afferents from the ventral hippocampus (vHIP) to the NAc are associated with susceptibility to the chronic social defeat stress animal model. Furthermore, attenuation of vHIP-NAc transmission by optogenetic induction of long-term depression is pro-resilient, whereas acute enhancement of this input is pro-susceptible [87]. The results highlight an important, novel circuit-specific mechanism in depression.
The Table 2 is summarizing the studies using optogenetic tolls in the depression treatment performed in in vivo models published from 2013 to 2018.
Table 2.
Optogenetic tolls in the depression treatment performed in in vivo models published from 2013 to 2018
| Publication | Neuron Cell Population | Light Color | Stimulation Parameters | Photoactivatable Protein | Findings |
|---|---|---|---|---|---|
| Covington et al. 2010 [77] | Infralimbic and prelimbic mPFC regions | Blue (473 nm) | 40 ms 100 Hz (9.9 ms spike width), pulses every 3 s | ChR2 | Antidepressant-like effect detected by social interaction and sucrose preference tests |
| Warden et al. 2012 [78] | mPFC to dorsal raphe nucleus and lateral habenula | Blue (473 nm) | NR | ChR2 | (1) Increase in motivated scape behavior |
| (2) Increase in immobility in the FST | |||||
| Kumar et al. 2013 [80] | Layer V pyramidal neurons of the prelimbic region the mPFC | Blue (473 nm) | 40 Hz (5 ms pulse width) and 4.02 Hz, for 1 min | ChR2 | (1) Decreased immobility in the FST |
| Blue (473 nm) | Tonic (0.5 Hz) and phasic (20 Hz) stimulation. 5 spikes over each 10 s period | ChR2-eYFP | (2) Absence of significant changes on the open field | ||
| (3) No improvement found in the social interaction test | |||||
| (4) Therapeutic response correlates with increased synchrony among depression-relevant limbic regions | |||||
| Chadhury et al. 2013 [123] | VTA DA neurons projecting to the NAc and to the mPFC | Yellow (563 nm) | 8 s light on and 2 s light off | eYFP (control) | Enhanced phasic firing VTA neurons projecting to the NAc induced persistent depression-like symptoms (social avoidance and reduced sucrose preference) |
| Blue (473 nm) | 17.0 to 23.8 mW/mm2, constant | NpHR3.0-eYFP | |||
| ChR2-eYFP | |||||
| Tye et al. 2013 [125] | VTA DA neurons | Yellow (593 nm) | 8 light pulses at 30 Hz every 5 s, for 3 min. Except for anhedonia (30 min) | eYFP (control) | Bidirectional control VTA DA neurons immediately and bidirectionally modulates depression (induces or relieves) |
| NpHR3.0-eYFP | |||||
| Friedman et al. 2014 [126] | VTA DA neurons | Blue (470 nm) | Five pulses, 20 Hz every 10 s period. Pulses delivered for 20 min a day for five consecutive days | ChR2-eYFP | Excessive optogenetic activation of VTA DA neurons in susceptible mice reduced firing rate and increased K+ currents (self-tuning compensation) |
| eYFP (control) | |||||
| Walsh et al. 2013 [86] | VTA DA neurons | Blue (473 nm) | Tonic (0.5 Hz, 15 ms) or phasic (20 Hz, 40 ms) light stimulations. 5 spikes over each 10 s period | ChR2-eYFP | (1) Phasic optogenetic activation increases BDNF amounts in the NAc of socially stressed mice but not of stress naive mice |
| Yellow (561 nm) | 8 s light on and 2 s light off | eYFP (control) | |||
| NpHR3.0-eYFP | (2) The stress gating of BDNF signaling is mediated by CRF acting in the NAc | ||||
| Bargot et al. 2014 [127] | MSN connecting the vHIP, the mPFC and the AMY to the NAc | Blue (473 nm) | 1 Hz, 4 ms pulse width, 10 min | ChR2-eYFP | Attenuation of vHIP-NAc transmission by optogenetics-induced LTD is pro-resilient, whereas acute enhancement of this input is pro-susceptible. Stimulation of either mPFC or AMY afferents to the NAc yielded no result |
| eYFP (control) |
IE4/5: Immediate-early 4/5; ChR2: channelrhodopsin-2; FST, forced swim test; AAV, Adeno-associated virus; CaMKIIα, calcium/calmodulin-dependent protein kinase type II alpha chain; Thy1, thymus cell antigen 1; eYFP, enhanced yellow fluorescent protein; mPFC, medial pre-frontal cortex; VTA, ventral tegmental area; NAc, nucleus accumbens; DA, dopamine; MSN, medium spiny neurons; vHIP, ventral hippocampus; FST, forced swim test; VTA, ventral tegmental area; NAc, nucleus accumbens; DA, dopamine; LTD, long-term depression; vHIP, ventral hippocampus; HCN2, cyclic nucleotide gated channel 2; BDNF, brain-derived neurotrophic fator; CRF, corticotropin releasing fator; AMY, basolateral amygdala.
The application of optogenetic tools have made possible a more intricate understanding of the circuits and mechanisms associated with different hallmark symptoms of depression, such as apathy, anhedonia and psychomotor retardation. Further investigation will elucidate the specific cell subpopulations and circuits responsible for the strong antidepressant-like responses elicited by optogenetic neuromodulation of different brain regions, setting the stage for precise closed-circuit control and therapeutic intervention in human disease that may revolutionize depression treatment.
Optogenetic applications in Neuro-oncology
The use of optogenetics technology in the treatment of cancer is still in the initial stages (Figure 2B and 2C). However, there are some promising bio-molecular approaches by which optogenetics showed an effect in preventing CNS tumors advancement and even the elimination of oncogenic cells (Table 3). Optogenetics tools in neuro-oncology are directly used for light-activated ion-transporting proteins to modify voltage transmembrane [88,89] though ChR2 protein has been adapted for neuro-oncology (Table 3).
Table 3.
Optogenetic tolls in the nervous central system tumors treatment performed in in vitro and in vivo models published in 2013 and 2014
| Publications | Neuron Cell Population | Light Color | Light Stimulation | Photoactivatable protein | Tumor Effect |
|---|---|---|---|---|---|
| Yang et al., 2013 [128] | Cell lines U87, U251, Ai72 and H4 | Blue | 30 min, 1 cycle, 10 m/mm2 | opsin ChETA | (1) Decreased cell progression and proliferation: down-regulated expression levels of Cyclin D1 and Cyclin E |
| (2) Increased mitochondria-dependent apoptosis: increased expression of Caspase-3 and cytosolic cytochrome-C | |||||
| (3) Increased ChETA expression | |||||
| Figueiredo et al., 2014 [129] | Cerebral cortices, cerebellum and brainstem of Wistar rat pups | Blue | 60 sec, 1 cycle, 561 nm | ChR2 | (1) Increased ChR2 [Ca2+] elevations |
| (2) Increased CatCh [Ca2+] elevations | |||||
| (3) Increased optoα1AR (Gq-coupled) and optoβ2AR (Gs-coupled) [Ca2+] elevations and activation of phospholipase C and adenylate cyclase signals, respectively | |||||
| (4) Autocrine action of ATP (blocking of the bulk of [Ca2+] responses evoked using either optoAR | |||||
| Venkatesh et al., 2015 [90] | SU-pcGBM2 | Blue | 30 sec, 1 cycle, 473 nm, 20 Hz | NLGN3 | (1) Decreased NLGN3: inhibition of PI3K-mTOR pathway and mitotic activity |
| (2) Increased animal model survival: Neuroligin-3 expression was inversely correlated with survival in human high grade glioblastoma | |||||
| Johung et al., 2014 [130] | Pediatric high-grade gliomas | Blue | 30 min, 470 nm, 20 Hz | ChR2 | (1) ChR2 expression: increased the mitotic index by around 50% (P<0.05) |
| (2) Increased animal model survival: Neuroligin-3 expression was inversely correlated with survival in human high grade glioblastoma |
Opsin ChETA: gene Channelrhodopsin-2 variant; CatCh: Ca2+ translocating channelrhodopsin; optoα1AR: opto-adrenoceptors; optoβ2AR: opto-betaceptors; NLGN3: Neuroligin-3.
Venkatesh et al., 2015 [90] have used optogenetic control of cortical neuronal activity in a patient-derived pediatric glioblastoma xenograft model. The authors demonstrated active neurons promote high grade-tumors (HGG) proliferation and growth in vivo, indicating secretion of activity-regulated mitogens. The synaptic protein neuroligin-3 (NLGN3) was identified as the leading candidate mitogen. NLGN3 induced PI3K-mTOR pathway activity. These findings indicate the important role of active neurons in the brain tumor microenvironment and identify secreted NLGN3 as an unexpected mechanism promoting neuronal activity-regulated cancer growth.
More recently, optically activated variants of son of sevenless 1, Raf 1, Rho A, Rac 1, phosphoinositide 3 kinase p85α, and low-density lipoprotein receptor-related protein 6 (activating the Wnt pathway) have been developed and used to study cellular signaling events with an unprecedented degree of spatial and temporal precision [88]. Also, it was described opto-receptor tyrosine kinases (RTKs) that can be controlled with light. RTKs consist of an extracellular ligand-binding domain, a single-pass transmembrane domain, and an intracellular tyrosine kinase domain. These reports demonstrated blue light-induced, spatio-temporally precise activation of several members of this crucial cell surface receptor family [91-95].
In all published opto-RTKs, photoreceptors LOV domains of plants, bacteria, and fungi were attached to the far C-terminus of the RTK, whereas the original extracellular domains were either retained or replaced by heterologous domains. Recently, three aureochrome LOV domains were identified that are capable of activating the RTKs murine fibroblast growth factor receptor 1 (mFGFR1), human epidermal growth factor receptor, and human ret proto-oncogene [91]. Another recently study used the photolyase homology region of cryptochrome 2 (CRY2) from Arabidopsis thaliana to drive the activation of the RTKs neurotrophin tyrosine kinase receptor type 1/2/3 (NTRK1/2/3) and human FGFR1 [95,96].
Both systems LOV domains and CRY2 have been shown to form oligomeric complexes and demonstrated light-induced simultaneous activation of the mitogen activated protein kinase, phosphoinositide 3 kinase, and phospholipase Cγ pathways, as expected for canonical RTK [97,98]. Furthermore, these observations are contrasting with methods for activation of a single pathway and no activation of signaling in the absence of light is observed in either of the two systems.
Ingles-Prieto et al. (2016) [99] focused on the role of FGFR1 in malignant growth and demonstrated that light-induced activation of opto-mFGFR1 was sufficient to quantitatively control cell behaviors that are directly relevant to cancer by enhancing proliferation, epithelial-mesenchymal transition of cancer cells, and sprouting of blood endothelial cells. Opto-RTKs may also enable detailed studies of pathway-specific temporal and spatial dose-effect relationships between RTK activation and cell fates ranging from proliferation and migration to differentiation, senescence, and apoptosis. Since aberrant RTK signals contribute to most functional hallmarks of cancer, these experiments, combined with rapid and sensitive read-outs of cell signaling and behavior, will lead to a new understanding of key events underlying cancer in real-time and with high resolution [99].
All optogenetic stimulation resulted in bioelectrical changes that culminate in regulation of cellular processes including the rates of differentiation, morphology, proliferation and migration of CNS tumors [89,90].
Conclusions
The optogenetics tools are valuable techniques applied in the study of physiology and bio-molecular mechanisms in the neurons, as well as the physiopathology and cellular behavior in CNS diseases. Based on literature, ChR2 and NpHR proteins are common bio-molecular pathway presented in the CNS diseases evaluated in optogenetic essays. However, new studies are needed for the standardization of methodologies and reproducibility experimental in vitro and in vivo results before being implied in the clinical practice in humans.
Disclosure of conflict of interest
None.
References
- 1.Deisseroth K. Optogenetics. Nat Methods. 2011;8:26–29. doi: 10.1038/nmeth.f.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Toettcher JE, Voigt CA, Weiner OD, Lim WA. The promise of optogenetics in cell biology: interrogating molecular circuits in space and time. Nat Methods. 2011;8:35–38. doi: 10.1038/nmeth.f.326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Towne C, Thompson KR. Overview on research and clinical applications of optogenetics. Curr Prot Pharmacol. 2016;75:11.19.1–11.19.21. doi: 10.1002/cpph.13. [DOI] [PubMed] [Google Scholar]
- 4.Bishop AC, Ubersax JA, Petsch DT, Matheos DP, Gray NS, Blethrow J, Shimizu E, Tsien JZ, Schultz PG, Rose MD, Wood JL, Morgan DO, Shokat KM. A chemical switch for inhibitor-sensitive alleles of any protein kinase. Nature. 2000;407:395–401. doi: 10.1038/35030148. [DOI] [PubMed] [Google Scholar]
- 5.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 6.Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- 7.Lima SQ, Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;8:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 8.Wu YI, Frey D, Lungu OI, Jaehrig A, Schlichting I, Kuhlman B, Hahn KM. A genetically encoded photoactivatable Rac controls the motility of living cells. Nature. 2009;461:104–108. doi: 10.1038/nature08241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haruki H, Nishikawa J, Laemmli UK. The anchor-away technique: rapid, conditional establishment of yeast mutant phenotypes. Mol Cell. 2008;31:925–932. doi: 10.1016/j.molcel.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 11.Shimizu-Sato S, Huq E, Tepperman JM, Quail PH. A light-switchable gene promoter system. Nat Biotechnol. 2002;20:1041–1044. doi: 10.1038/nbt734. [DOI] [PubMed] [Google Scholar]
- 12.Kamps D, Dehmelt L. De-blurring signal network dynamics. Chem Biol. 2017;4:1–12. doi: 10.1021/acschembio.7b00451. [DOI] [PubMed] [Google Scholar]
- 13.Casquillas GV, Fu C, Le Berre M, Cramer J, Meance S, Plecis A, Baigl D, Greffet JJ, Chen Y, Piel M, Tran PT. Fast microfluidic temperature control for high resolution live cell imaging. Lab Chip. 2011;11:484–489. doi: 10.1039/c0lc00222d. [DOI] [PubMed] [Google Scholar]
- 14.Ellis-Davies GC. Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat Methods. 2007;4:619–628. doi: 10.1038/nmeth1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Umeda N, Ueno T, Pohlmeyer C, Nagano T, Inoue T. A photocleavable rapamycin conjugate for spatiotemporal control of small GTPase activity. J Am Chem Soc. 2011;133:12–14. doi: 10.1021/ja108258d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Knopfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2012;30:14998–15004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carter ME, de Lecea L. Optogenetic investigation of neural circuits in vivo. Trends Mol Med. 2011;17:197–206. doi: 10.1016/j.molmed.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Atasoy D, Aponte Y, Su HH, Sternson SM. A FLEX switch targets Channelrhodopsin-2 to multiple cell types for imaging and long-range circuit mapping. J Neurosci. 2008;28:7025–7030. doi: 10.1523/JNEUROSCI.1954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cruikshank SJ, Urabe H, Nurmikko AV, Connors BW. Pathway-specific feedforward circuits between thalamus and neocortex revealed by selective optical stimulation of axons. Neuron. 2010;65:230–245. doi: 10.1016/j.neuron.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaneda K, Kasahara H, Matsui R, Katoh T, Mizukami H, Ozawa K, Watanabe D, Isa D. Selective optical control of synaptic transmission in the subcortical visual pathway by activation of viral vector-expressed halorhodopsin. PLoS One. 2011;6:e18452. doi: 10.1371/journal.pone.0018452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Petreanu L, Mao T, Sternson S, Svoboda K. The subcellular organization of neocortical excitatory connections. Nature. 2009;457:1142–1145. doi: 10.1038/nature07709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Varga V, Losonczy A, Zemelman BV, Borhegyi Z, Nyiri G, Domonkos A, Hangya B, Holderith N, Magee JC, Freund TF. Fast synaptic subcortical control of hippocampal circuits. Science. 2009;326:449–453. doi: 10.1126/science.1178307. [DOI] [PubMed] [Google Scholar]
- 23.Jerome J, Foehring RC, Armstrong WE, Spain WJ, Heck DH. Parallel optical control of spatiotemporal neuronal spike activity using high speed digital light processing. Front Syst Neurosci. 2011;5:70–73. doi: 10.3389/fnsys.2011.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grossman N, Poher V, Grubb MS, Kennedy GT, Nikolic K, McGovern B, Berlinguer Palmini R, Gong Z, Drakakis EM, Neil MA, Dawson MD, Burrone J, Degenaar P. Multi-site optical excitation using ChR2 and micro-LED array. J Neural Eng. 2010;7:16004. doi: 10.1088/1741-2560/7/1/016004. [DOI] [PubMed] [Google Scholar]
- 25.Anikeeva P, Andalman AS, Witten I, Warden M, Goshen I, Grosenick L, Gunaydin LA, Frank LM, Deisseroth K. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat Neurosci. 2011;15:163–170. doi: 10.1038/nn.2992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Diester I, Kaufman MT, Mogri M, Pashaie R, Goo W, Yizhar O, Ramakrishnan C, Deisseroth K, Shenoy KV. An optogenetic toolbox designed for primates. Nat Neurosci. 2011;14:387–397. doi: 10.1038/nn.2749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang J, Laiwalla F, Kim JA, Urabe H, Van Wagenen R, Song YK, Connors BW, Zhang F, Deisseroth K, Nurmikko AV. Integrated device for optical stimulation and spatiotemporal electrical recording of neural activity in light-sensitized brain tissue. J Neural Eng. 2009;6:055007. doi: 10.1088/1741-2560/6/5/055007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang F, Gradinaru V, Adamantidis AR, Durand R, Airan RD, de Lecea L, Deisseroth K. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc. 2010;5:439–456. doi: 10.1038/nprot.2009.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Levin M. Bioelectric mechanisms in regeneration: unique aspects and future perspectives. Semin Cell Dev Biol. 2009;20:543–556. doi: 10.1016/j.semcdb.2009.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Malyshev A, Goz R, LoTurco JJ, Volgushev M. Advantages and limitations of the use of optogenetic approach in studying fast-scale spike encoding. PLoS One. 2015;10:e0122286. doi: 10.1371/journal.pone.0122286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han X, Qian X, Bernstein JG, Zhou HH, Franzesi GT, Stern P, Bronson RT, Graybiel AM, Desimone R, Boyden ES. Millisecond-timescale optical control of neural dynamics in the non human primate brain. Neuron. 2009;62:191–198. doi: 10.1016/j.neuron.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maguire AM, High KA, Auricchio A, Wright JF, Pierce EA, Testa F, Mingozzi F, Bennicelli JL, Ying GS, Rossi S, Fulton A, Marshall KA, Banfi S, Chung DC, Morgan JI, Hauck B, Zelenaia O, Zhu X, Raffini L, Coppieters F, De Baere E, Shindler KS, Volpe NJ, Surace EM, Acerra C, Lyubarsky A, Redmond TM, Stone E, Sun J, McDonnell JW, Leroy BP, Simonelli F, Bennett J. Age-dependent effects of RPE65 gene therapy for Leber’s congenital amaurosis: a phase 1 dose-escalation trial. Lancet. 2009;374:1597–1605. doi: 10.1016/S0140-6736(09)61836-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lagali PS, Balya D, Awatramani GB, Münch TA, Kim DS, Busskamp V, Cepko CL, Roska B. Light-activated channels targeted to ON bipolar cells restore visual function in retinal degeneration. Nat Neurosci. 2008;11:667–675. doi: 10.1038/nn.2117. [DOI] [PubMed] [Google Scholar]
- 34.Elsea SH, Lucas RE. The mousetrap: what we can learn when the mouse model does not mimic the human disease. ILAR J. 2002;43:66–79. doi: 10.1093/ilar.43.2.66. [DOI] [PubMed] [Google Scholar]
- 35.Nagel G, Szellas T, Huhn W, Kateriya S, Adeishvili N, Berthold P, Ollig D, Hegemann P, Bamberg E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci U S A. 2003;100:13940–13945. doi: 10.1073/pnas.1936192100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Repina NA, Rosenbloom A, Mukherjee A, Schaffer DV, Kane RS. At light speed: advances in optogenetic systems for regulating cell signaling and behavior. Annu Rev Chem Biomol Eng. 2017;8:13–39. doi: 10.1146/annurev-chembioeng-060816-101254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guntas G, Hallett RA, Zimmerman SP, Williams T, Yumerefendi H, Bear JE, Kuhlman B. Engineering an improved light-induced dimer (iLID) for controlling the localization and activity of signaling proteins. PNAS. 2015;112:112–117. doi: 10.1073/pnas.1417910112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mei Y, Zhang F. Molecular tools and approaches for optogenetics. Biol Psychiatry. 2012;71:1033–1038. doi: 10.1016/j.biopsych.2012.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yizhar O, Fenno L, Zhang F, Hegemann P, Diesseroth K. Microbial opsins: a family of single-component tools for optical control of neural activity. Cold Spring Harb Protoc. 2011;3:top102. doi: 10.1101/pdb.top102. [DOI] [PubMed] [Google Scholar]
- 40.Oesterhelt D, Stoeckenius W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat New Biol. 1971;233:149–152. doi: 10.1038/newbio233149a0. [DOI] [PubMed] [Google Scholar]
- 41.Oesterhelt D, Stoeckenius W. Functions of a new photoreceptor membrane. Proc Natl Acad Sci USA. 1973;70:2853–2857. doi: 10.1073/pnas.70.10.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Racker E, Stoeckenius W. Reconstitution of purple membrane vesicles catalyzing light-driven proton uptake and adenosine triphosphate formation. J Biol Chem. 1974;249:662–663. [PubMed] [Google Scholar]
- 43.Michel H, Oesterhelt D. Light-induced changes of the pH gradient and the membrane potential in H. halobium. FEBS Lett. 1976;65:175–178. doi: 10.1016/0014-5793(76)80473-5. [DOI] [PubMed] [Google Scholar]
- 44.Matsuno-Yagi A, Mukohata Y. Two possible roles of bacteriorhodopsin: a comparative study of strains of Halobacterium halobium differing in pigmentation. Biochem Biophys Res Commun. 1977;78:237–243. doi: 10.1016/0006-291x(77)91245-1. [DOI] [PubMed] [Google Scholar]
- 45.Essen LO. Halorhodopsin: light-driven ion pumping made simple? Curr Opin Struct Biol. 2002;12:516–522. doi: 10.1016/s0959-440x(02)00356-1. [DOI] [PubMed] [Google Scholar]
- 46.Sasaki J, Brown LS, Chon YS, Kandori H, Maeda A, Needleman R, Lanyi JK. Conversion of bacteriorhodopsin into a chloride ion pump. Science. 1995;269:73–75. doi: 10.1126/science.7604281. [DOI] [PubMed] [Google Scholar]
- 47.Zhang F, Wang LP, Brauner M, Liewald JF, Kay K, Watzke N, Wood PG, Bamberg E, Nagel G, Gottschalk A, Deisseroth K. Multimodal fast optical interrogation of neural circuitry. Nature. 2007;446:633–639. doi: 10.1038/nature05744. [DOI] [PubMed] [Google Scholar]
- 48.Nagel G, Ollig D, Fuhrmann M, Kateriya S, Musti AM, Bamberg E, Hegemann P. Channelrhodopsin-1: a light-gated proton channel in green algae. Science. 2002;296:2395–2398. doi: 10.1126/science.1072068. [DOI] [PubMed] [Google Scholar]
- 49.Feldbauer K, Zimmermann D, Pintschovius V, Spitz J, Bamann C, Bamberg E. Channelrhodopsin-2 is a leaky proton pump. PNAS. 2009;106:12317–12322. doi: 10.1073/pnas.0905852106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang F, Prigge M, Beyrière F, Tsunoda SP, Mattis J, Yizhar O, Hegemann P, Deisseroth K. Red-shifted optogenetic excitation: a tool for fast neural control derived from volvox carteri. Nat Neurosci. 2008;11:631–633. doi: 10.1038/nn.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gradinaru V, Thompson KR, Zhang F, Mogri M, Kay K, Schneider MB, Deisseroth K. Targeting and readout strategies for fast optical neural control in vitro and in vivo. J Neurosci. 2007;27:14231–14238. doi: 10.1523/JNEUROSCI.3578-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang F, Wang LP, Boyden ES, Deisseroth K. Channelrhodopsin-2 and optical control of excitable cells. Nat Methods. 2006;3:785–792. doi: 10.1038/nmeth936. [DOI] [PubMed] [Google Scholar]
- 53.Naso MF, Tomkowicz B, Perry WL, Strohl WR. Adeno-associated virus (AAV) as a vector for gene therapy. Bio Drugs. 2017;31:317–334. doi: 10.1007/s40259-017-0234-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bentley JN, Chestek C, Stacey WC, Patil PG. Optogenetics in epilepsy. Neurosurg Focus. 2013;34:E4. doi: 10.3171/2013.3.FOCUS1364. [DOI] [PubMed] [Google Scholar]
- 55.Galvan A, Stauffer WR, Acker L, El-Shamayleh Y, Inoue KI, Ohayon S, Schmid MC. Nonhuman primate optogenetics: recent advances and future directions. J Neurosci. 2017;37:10894–10903. doi: 10.1523/JNEUROSCI.1839-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choy MK, Duffy BA, Lee JH. Optogenetic study of networks in epilepsy. J Neurosci Res. 2017;95:2325–2335. doi: 10.1002/jnr.23767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Depuy SD, Kanbar R, Coates M, Stornetta RL, Guyenet PG. Control of breathing by raphe obscures serotonergic neurons in mice. J Neurosci. 2011;31:1981–1990. doi: 10.1523/JNEUROSCI.4639-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ye H, Kaszuba S. Inhibitory or excitatory? Optogenetic interrogation of the functional roles of GABAergic interneurons in epileptogenesis. J Biomed Sci. 2017;24:93–95. doi: 10.1186/s12929-017-0399-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong S, Doughty M, Harbaugh CR, Cummins A, Hatten ME, Heintz N, Gerfen CR. Targeting Cre recombinase to specific neuron populations with bacterial artificial chromosome constructs. J Neurosci. 2007;27:9817–9823. doi: 10.1523/JNEUROSCI.2707-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ting JT, Feng G. Recombineering strategies for developing next generation BAC transgenic tools for optogenetics and beyond. Front Behav Neurosci. 2014;8:1–13. doi: 10.3389/fnbeh.2014.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Madisen L, Mao T, Koch H, Zhuo JM, Berenyi A, Fujisawa S, Hsu YW, Garcia AJ 3rd, Gu X, Zanella S, Kidney J, Gu H, Mao Y, Hooks BM, Boyden ES, Buzsáki G, Ramirez JM, Jones AR, Svoboda K, Han X, Turner EE, Zeng H. A toolbox of Cre-dependent optogenetic transgenic mice for light-induced activation and silencing. Nat Neurosci. 2012;15:793–802. doi: 10.1038/nn.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.French JA. Refractory epilepsy: one size does not fit all. Epilepsy Curr. 2006;6:177–180. doi: 10.1111/j.1535-7511.2006.00137.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Krauss GL, Sperling MR. Treating patients with medically resistant epilepsy. Neurol Clin Pract. 2011;1:14–23. doi: 10.1212/CPJ.0b013e31823d07d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Treiman DM. Management of refractory complex partial seizures: current state of the art. Neuropsychiatr Dis Treat. 2010;6:297–308. doi: 10.2147/ndt.s4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Paz JT, Huguenard JR. Optogenetics and epilepsy: past, present and future. Epilepsy Curr. 2015;15:34–38. doi: 10.5698/1535-7597-15.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Weitz AJ, Fang Z, Lee HJ, Fisher RS, Smith WC, Choy M, Liu J, Lin P, Rosenberg M, Lee JH. Optogenetic fMRI reveals distinct, frequency dependent networks recruited by dorsal and intermediate hippocampus stimulations. Neuroimage. 2015;107:229–241. doi: 10.1016/j.neuroimage.2014.10.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gradinaru V, Thompson KR, Deisseroth K. eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol. 2018;36:129–139. doi: 10.1007/s11068-008-9027-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sorokin JM, Davidson TJ, Frechette E, Abramian AM, Deisseroth K, Huguenard JR, Paz JT. Bidirectional control of generalized epilepsy networks via rapid real-time switching of firing mode. Neuron. 2017;93:194–210. doi: 10.1016/j.neuron.2016.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Osawa SI, Iwasaki M, Hosaka R, Matsuzaka Y, Tomita H, Ishizuka T, Sugano E, Okumura E, Yawo H, Nakasato N, Tominaga T, Mushiake H. Optogenetically induced seizure and the longitudinal hippocampal network dynamics. PLoS One. 2013;8:e60928. doi: 10.1371/journal.pone.0060928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS National Comorbidity Survey Replication. The epidemiology of major depressive disorder-results from the national comorbidity survey replication (NCS-R) JAMA. 2003;289:3095–3105. doi: 10.1001/jama.289.23.3095. [DOI] [PubMed] [Google Scholar]
- 71.Murray CJL, Lopez AD. The global burden of disease: a comprehensive assessment of mortality and disability from deceases, injuries and risk factors in 1990 and projected to 2010. Harvard University Press. 1996;1:1–35. [Google Scholar]
- 72.Lozano AM, Gildenberg PL, Tasker RR. Impedance recording in functional neurosurgery. Textbook of stereotactic and functional neurosurgery. 2009;79:1325–1330. [Google Scholar]
- 73.Histed MH, Bonin V, Reid RC. Direct activation of sparse, distributed populations of cortical neurons by electrical microstimulation. Neuron. 2009;63:508–522. doi: 10.1016/j.neuron.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Steinberg EE, Christoffel DJ, Deisseroth K, Malenka RC. Illuminating circuitry relevant to psychiatric disorders with optogenetics. Curr Opin Neurobiol. 2015;30:9–16. doi: 10.1016/j.conb.2014.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Touriño C, Eban-Rothschild A, de Lecea L. Optogenetics in psychiatric diseases. Curr Opin Neurobiol. 2013;23:430–435. doi: 10.1016/j.conb.2013.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Covington HE, Lobo MK, Maze I, Vialou V, Hyman JM, Zaman S, LaPlant Q, Mouzon E, Ghose S, Tamminga CA, Neve RL, Deisseroth K, Nestler EJ. Antidepressant effect of optogenetic stimulation of the medial prefrontal cortex. J Neurosci. 2010;30:16082–16090. doi: 10.1523/JNEUROSCI.1731-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, Adhikari A, Tye KM, Frank LM, Deisseroth K. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 80.Kumar S, Black SJ, Hultman R, Szabo ST, DeMaio KD, Du J, Katz BM, Feng G, Covington HE, Dzirasa K. Cortical control of affective networks. J Neurosci. 2013;33:1116–1129. doi: 10.1523/JNEUROSCI.0092-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Berton O, Hahn CG, Thase ME. Are we getting closer to valid translational models for major depression? Science. 2012;338:75–79. doi: 10.1126/science.1222940. [DOI] [PubMed] [Google Scholar]
- 82.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2012;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valenti O, Lodge DJ, Grace AA. Aversive stimuli alter ventral tegmental area dopamine neuron activity via a common action in the ventral hippocampus. J Neurosci. 2011;31:4280–4289. doi: 10.1523/JNEUROSCI.5310-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Moore H, Rose HJ, Grace AA. Chronic cold stress reduces the spontaneous activity of ventral tegmental dopamine neurons. Neuropsychopharmacology. 2001;24:410–419. doi: 10.1016/S0893-133X(00)00188-3. [DOI] [PubMed] [Google Scholar]
- 86.Walsh JJ, Friedman AK, Sun H, Heller EA, Ku SM, Juarez B, Burnham VL, Mazei-Robison MS, Ferguson D, Golden SA, Koo JW, Chaudhury D, Christoffel DJ, Pomeranz L, Friedman JM, Russo SJ, Nestler EJ, Han MH. Stress and CRF gate neural activation of BDNF in the mesolimbic reward pathway. Nat Neurosci. 2014;17:27–29. doi: 10.1038/nn.3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bagot RC, Parise EM, Peña CJ, Zhang HX, Maze I, Chaudhury D, Persaud B, Cachope R, Bolaños-Guzmán CA, Cheer JF, Deisseroth K, Han MH, Nestler EJ. Ventral hippocampal afferents to the nucleus accumbens regulate susceptibility to depression. Nat Commun. 2015;6:7062. doi: 10.1038/ncomms8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Knopfel T, Boyden E. A comprehensive concept of optogenetics. Optogenetics: tools for controlling and monitoring neuronal activity. Elsevier. 2012;196:1. [Google Scholar]
- 89.Adams DS, Tseng AS, Levin M. Light-activation of the archaerhodopsin H+-pump reverses age-dependent loss of vertebrate regeneration: sparking system-level controls in vivo. Biol Open. 2012;2:306–313. doi: 10.1242/bio.20133665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Venkatesh HS, Johung TB, Caretti V, Noll A, Tang Y, Nagaraja S, Gibson EM, Mount CW. Activity promotes glioma growth through neuroligin-3 secretion. Cell. 2015;161:803–816. doi: 10.1016/j.cell.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. Bioessays. 2012;34:205–217. doi: 10.1002/bies.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tischer D, Weiner OD. Illuminating cell signalling with optogenetic tools. Nat Rev Mol Cell Biol. 2014;15:551–558. doi: 10.1038/nrm3837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Grusch M, Schelch K, Riedler R, Reichhart E, Differ C, Berger W, Inglés-Prieto Á, Janovjak H. Spatio-temporally precise activation of engineered receptor tyrosine kinases by light. EMBO J. 2014;33:1713–1726. doi: 10.15252/embj.201387695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chang KY, Woo D, Jung H, Lee S, Kim S, Won J, Kyung T, Park H, Kim N, Yang HW, Park JY, Hwang EM, Kim D, Heo WD. Light-inducible receptor tyrosine kinases that regulate neurotrophin signaling. Nat Commun. 2014;5:4057. doi: 10.1038/ncomms5057. [DOI] [PubMed] [Google Scholar]
- 95.Kim N, Kim JM, Lee M, Kim CY, Chang KY, Heo WD. Spatiotemporal control of fibroblast growth factor receptor signals by blue light. Chem Biol. 2014;21:903–912. doi: 10.1016/j.chembiol.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 96.Chang KY, Woo D, Jung H, Lee S, Kim S, Won J, Kyung T, Park H, Kim N, Yang HW, Park JY, Hwang EM, Kim D, Heo WD. Light-inducible receptor tyrosine kinases that regulate neurotrophin signalling. Nat Commun. 2014;5:4057. doi: 10.1038/ncomms5057. [DOI] [PubMed] [Google Scholar]
- 97.Toyooka T, Hisatomi O, Takahashi F, Kataoka H, Terazima M. Photoreactions of aureochrome-1. Biophys J. 2011;100:2801–2809. doi: 10.1016/j.bpj.2011.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bugaj LJ, Choksi AT, Mesuda CK, Kane RS, Schaffer DV. Optogenetic protein clustering and signaling activation in mammalian cells. Nat Methods. 2013;10:249–252. doi: 10.1038/nmeth.2360. [DOI] [PubMed] [Google Scholar]
- 99.Ingles-Prieto A, Reichhart E, Schelch K, Janovjak H, Grusch M. The optogenetic promise for oncology: episode I. Mol Cell Oncol. 2014;1:e964045. doi: 10.4161/23723548.2014.964045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang H, Zhao H, Zeng C, Van Dort C, Faingold CL, Taylor NE, Solt K, Feng HJ. Optogenetic activation of 5-ht neurons in the dorsal raphe suppresses seizure-induced respiratory arrest and produces anticonvulsant effect in the DBA/1 Mouse SUDEP model. Neurobiol Dis. 2018;110:47–58. doi: 10.1016/j.nbd.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chang M, Joshua AD, Suzie D, Lihua W, Homeira MC, Meera R, Liang Z, Peter LC, Thilo W, Taufik AV. Brief activation of GABAergic interneurons initiates the transition to ictal events through post-inhibitory rebound excitation. Neurobiol Dis. 2018;109:102–116. doi: 10.1016/j.nbd.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 102.Tung JK, Shiu FH, Ding K, Gross RE. Chemically activated luminopsins allow optogenetic inhibition of distributed nodes in an epileptic network for non-invasive and multi-site suppression of seizure activity. Neurobiol Dis. 2018;109:1–10. doi: 10.1016/j.nbd.2017.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chang WJ, Chang WP, Shyu BC. Suppression of cortical seizures by optic stimulation of the reticular thalamus in PV-mhChR2-YFP BAC transgenic mice. Mol Brain. 2017;10:42–45. doi: 10.1186/s13041-017-0320-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wang Y, Xu C, Xu Z, Ji C, Liang J, Wang Y, Chen B, Wu X, Gao F, Wang S, Guo Y, Li X, Luo J, Duan S, Chen Z. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron. 2017;95:92–105. doi: 10.1016/j.neuron.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 105.Petersen V, Jensen CS, Crépel V, Falkerslev M, Perrier JF. Serotonin regulates the firing of principal cells of the subiculum by inhibiting a T-Type Ca2+ current. Front Cell Neurosci. 2017;11:60. doi: 10.3389/fncel.2017.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xu Z, Wang Y, Chen B, Xu CM, Wu XM, Wang Y, Zhang S, Hu W, Wang S, Guo Y, Zhang X, Luo J, Duan S, Chena Z. Entorhinal principal neurons mediate brain-stimulation treatments for epilepsy. EBioMedicine. 2016;14:148–60. doi: 10.1016/j.ebiom.2016.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Assaf F, Yitzhak S. The antiepileptic and ictogenic effects of optogenetic neurostimulation of PV-expressing interneurons. J Neurophysiol. 2016;116:1694–1704. doi: 10.1152/jn.00744.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tassin V, Girard B, Chotte A, Fontanaud P, Rigault D, Kalinichev M, Perroy J, Acher F, Fagni L, Bertaso F. Phasic and tonic mGlu7 receptor activity modulates the thalamocortical network. Front Neural Circuits. 2016;10:31–32. doi: 10.3389/fncir.2016.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Hoffman G, Reeder SB, Hu H, Sirlin CB. Hilar somatostatin interneuron loss reduces dentate gyrus inhibition in a mouse model of temporal lobe epilepsy. Epilepsia. 2016;36:1011–1014. doi: 10.1111/epi.13376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lu Y, Zhong C, Wang L, Wei P, He W, Huang K, Zhang Y, Zhan Y, Feng G, Wang L. Optogenetic dissection of ictal propagation in the hippocampal-entorhinal cortex structures. Nat Commun. 2016;7:1–11. doi: 10.1038/ncomms10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shiri Z, Manseau F, Lévesque M, Williams S, Avoli M. Activation of specific neuronal networks leads to different seizure onset types. Ann Neurol. 2016;41:86–106. doi: 10.1002/ana.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shiri Z, Manseau F, Lévesque M, Williams S, Avoli M. Activation of specific neuronal networks leads to different seizure onset types. Ann Neurol. 2016;79:354–65. doi: 10.1002/ana.24570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ladas TP, Chiang CC, Gonzalez-Reyes LE, Nowak T, Durand DM. Seizure reduction through interneuron-mediated entrainment using low frequency optical stimulation. Exp Neurol. 2015;269:120–132. doi: 10.1016/j.expneurol.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kros L, Eelkman Rooda OH, Spanke JK, Alva P, van Dongen MN, Karapatis A, Tolner EA, Strydis C, Davey N, Winkelman BH, Negrello M, Serdijn WA, Steuber V, van den Maagdenberg AM, De Zeeuw CI, Hoebeek FE. Cerebellar output controls generalized spike-and-wave discharge occurrence. Ann Neurol. 2015;77:1027–1049. doi: 10.1002/ana.24399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Krook-Magnuson E, Szabo GG, Armstrong C, Oijala M, Soltesz I. Cerebellar directed optogenetic intervention inhibits spontaneous hippocampal seizures in a mouse model of temporal lobe epilepsy. eNeuro. 2014;1:1–10. doi: 10.1523/ENEURO.0005-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cunningham M, Cho JH, Leung A, Savvidis G, Ahn S, Moon M, Lee PK, Han JJ, Azimi N, Kim KS, Bolshakov VY, Chung S. hPSC-derived maturing gabaergic interneurons ameliorate seizures and abnormal behavior in epileptic mice. Cell Stem Cell. 2014;15:559–573. doi: 10.1016/j.stem.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ellender TJ, Raimondo JV, Irkle A, Lamsa KP, Akerman CJ. Excitatory effects of parvalbumin-expressing interneurons maintain hippocampal epileptiform activity via synchronous after discharges. J Neurosc. 2014;34:15208–15220. doi: 10.1523/JNEUROSCI.1747-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chiang CC, Ladas TP, Gonzalez-Reyes LE, Durand DM. Seizure suppression by high frequency optogenetic stimulation using in vitro and in vivo animal models of epilepsy. Brain Stimul. 2014;7:890–899. doi: 10.1016/j.brs.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ledri M, Madsen MG, Nikitidou L, Kirik D, Kokaia M. Global optogenetic activation of inhibitory interneurons during epileptiform activity. J Neurosci. 2014;34:3364–33677. doi: 10.1523/JNEUROSCI.2734-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Berglind F, Ledri M, Sørensen AT, Nikitidou L, Melis M, Bielefeld P, Kirik D, Deisseroth K, Andersson M, Kokaia M. Optogenetic inhibition of chemically induced hypersynchronized bursting in mice. Neurobiol Dis. 2014;65:133–141. doi: 10.1016/j.nbd.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 121.Sukhotinsky I, Chan AM, Ahmed OJ, Rao VR, Gradinaru V, Ramakrishnan C, Deisseroth K, Majewska AK, Cash SS. Optogenetic delay of status epilepticus onset in an in vivo rodent epilepsy model. PLoS One. 2013;8:e62013. doi: 10.1371/journal.pone.0062013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Krook-Magnuson E, Armstrong C, Oijala M, Soltesz I. On-demand optogenetic control of spontaneous seizures in temporal lobe epilepsy. Nat Commun. 2013;4:1376. doi: 10.1038/ncomms2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chaudhury D, Walsh JJ, Friedman AK, Juarez B, Ku SM, Koo JW, Ferguson D, Tsai HC, Pomeranz L, Christoffel DJ, Nectow AR, Ekstrand M, Domingos A, Mazei-Robison MS, Mouzon E, Lobo MK, Neve RL, Friedman JM, Russo SJ, Deisseroth K, Nestler EJ, Han MH. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493:532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wykes RC, Heeroma JH, Mantoan L, Zheng K, MacDonald DC, Deisseroth K, Hashemi KS, Walker MC, Schorge S, Kullmann DM. Optogenetic and potassium channel gene therapy in a rodent model of focal neocortical epilepsy. Sci Transl Med. 2013;4:161ra152. doi: 10.1126/scitranslmed.3004190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tye KM, Mirzabekov JJ, Warden MR, Ferenczi EA, Tsai HC, Finkelstein J, Kim SY, Adhikari A, Thompson KR, Andalman AS, Gunaydin LA, Witten IB, Deisseroth K. Dopamine neurons modulate neural encoding and expression of depression-related behaviour. Nature. 2013;493:537–541. doi: 10.1038/nature11740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Friedman AK, Walsh JJ, Juarez B, Ku SM, Chaudhury D, Wang J, Li X, Dietz DM, Pan N, Vialou VF, Neve RL, Yue Z, Han MH. Enhancing depression mechanisms in midbrain dopamine neurons achieves homeostatic resilience. Science. 2014;344:313–319. doi: 10.1126/science.1249240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bagot RC, Labonté B, Peña CJ, Nestler EJ. Epigenetic signaling in psychiatric disorders: stress and depression. Dialogues Clin Neurosci. 2014;16:281–295. doi: 10.31887/DCNS.2014.16.3/rbagot. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang F, Tu J, Pan JQ, Luo HL, Liu YH, Wan J, Zhang J, Wei PF, Jiang T, Chen YH, Wang LP. Light-controlled inhibition of malignant glioma by opsin gene transfer. Cell Death Dis. 2013;4:1–14. doi: 10.1038/cddis.2013.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Figueiredo M, Lane S, Stout RF Jr, Liu B, Parpura V, Teschemacher AG, Kasparov S. Comparative analysis of optogenetic actuators in cultured astrocytes. Cell Calcium. 2014;56:208–214. doi: 10.1016/j.ceca.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Johung T, Venkatesh H, Caretti V, Noll A, Monje M. Neurons promote glioma growth in an activity-dependent manner. Neuro-Oncology. 2014;16:120–124. [Google Scholar]


