Abstract
Ferroptosis is an iron depend cell death which caused by lipid peroxidation. Abnormal iron metabolism and high intracellular iron content are the characteristics of most cancer cells. Iron is a promoter of cell growth and proliferation. However, iron also could take part in Fenton reaction to produce reactive oxygen species (ROS). The intercellular ROS could induce lipid peroxidation, which is necessary for ferroptosis. Iron metabolism mainly includes three parts: iron uptake, storage and efflux. Therefore, iron metabolism-related genes could regulate intercellular iron content and status, which can be involved ferroptosis. In recent years, the application of nanoparticles in cancer therapy research has become more and more extensive. The iron-based nanoparticles (iron-based NPs) can release ferrous (Fe2+) or ferric (Fe3+) in acidic lysosomes and inducing ferroptosis. Magnetic field is widely used in the targeted concentration of iron-based NPs related disease therapy. Furthermore, multiple studies showed that magnetic fields can inhibit cancer cell proliferation by promoting intracellular ROS production. Herein, we focus on the relationship of between ferroptosis and iron metabolism in cancer cells, the application of nanoparticles and magnetic field in inducing ferroptosis of cancer cells, and trying to provide new ideas for cancer treatment research.
Keywords: Iron metabolism, iron-based nanoparticles, magnetic field, ferroptosis, cancer therapy
Introduction
Cell death is closely related to the development, metabolic and disease of organism. Regulated cell death (RCD) has a significant role in organismal homeostasis in both physiological and pathological settings, excessive or insufficient RCD can cause disease. including autoimmunity, neurodegeneration, even cancer [1]. The RCD contains a variety of forms, including apoptosis, necroptosis, pyroptosis, parthanatos, autosis, ferroptosis [2], and many new forms of RCD were found in succession.
Ferroptosis is a new form of RCD was found and named by the team of Dr. Brent R Stockwell in 2012 [3]. Before Ferroptosis was named, the first ferroptosis inducer was discovered in 2003, when the lab of Stockwell study the killing effect of various chemical compounds on tumor cells, the erastin can trigger a RAS-mutated dependent cell death, and quite different from apoptosis [4]. Ferroptosis was defined as a form of RCD initiated by oxidative perturbations of the intracellular microenvironment that is under constitutive control by GPX4 and can be inhibited by iron chelators and lipophilic antioxidants by The Nomenclature Committee on Cell Death (NCCD) [5]. Multiple physiological and pathological processes are associated with ferroptosis, such as neurodegenerative diseases, neurotoxicity, hepatic and heart ischemia/reperfusion injury, drug-induced hepatotoxicity, acute renal failure, et al. Understanding the molecular mechanisms of ferroptosis may provide some ideas of the diagnosis and treatment of human disease about cell death.
Ferroptosis has the special morphological characteristics compared with other forms of regulated cell death (Table 1) [6,7]. From the morphological changes of cells, the symbols of ferroptosis are smaller mitochondria, higher mitochondrial membrane density and often accompanied by reduction/vanishing of mitochondria crista [3,4,8].
Table 1.
Comparison of features between apoptosis, autophagy, necroptosis and ferroptosis
| Apoptosis | Autophagy | Necroptosis | Ferroptosis | ||
|---|---|---|---|---|---|
| Morphological features | Cell membrane | Plasma membrane blebbing; rounding-up of the cell | Lack of change | Rupture of plasma membrane | Lack of rupture and blebbing of the plasma membrane; rounding-up of the cell |
| Cytoplasm | Retraction of pseudopods; reduction of cellular volume | Accumulation of double-membraned autophagic vacuoles | Cytoplasmic swelling (oncosis); swelling of cytoplasmic organelles | Small mitochondria with condensed mitochondrial membrane densities, reduction or vanishing of mitochondria crista, as well as outer mitochondrial membrane rupture | |
| Nucleus | Reduction of nuclear volume; nuclear fragmentation; chromatin condensation | Lack of chromatin condensation | Moderate chromatin condensation | Normal nuclear size and lack of chromatin condensation | |
| Biochemical features | Activation of caspases | LC3-I to LC3-II conversion Substrate (e.g., p62) degradation | Drop in ATP levels | Iron and ROS accumulation Activation of MAPKs Inhibition of system Xc - with decreased cystine uptake GSH depletion and increased NAPDH oxidation Release of arachidonic acid mediators (e.g., 11-HETE and 15-HETE) Δψm dissipation | |
| Oligonucleosomal DNA fragmentation | Activation of RIP1, RIP3, and MLKL | ||||
| Δψm dissipation | Release of DAMPs (e.g., HMGB1) | ||||
| PS exposure | PARP1 hyperactivation | ||||
| Inhibitors | Caspase inhibitors | Autophagy inhibitors (e.g. 3-MA, wortmannin) | Necrostatins (e.g. Nec-1) | Lipophilic antioxidants (e.g. Fer-1, vitamin E) | |
| Necrosulfonamide | Iron chelators (e.g. DFO, CPX) | ||||
One important characteristic of ferroptosis is the accumulation of lipid peroxidation products, which could be produced by the Fenton reaction. The Fenton reaction is an abbreviation for the chemical reaction which participation by the iron (II or III) and hydrogen peroxide (H2O2), which was first described by H. J. H. Fenton in 1989. The Fenton reaction has been widely accepted and is described as follows [9]
Fe2+ + H2O2 = Fe3+ + •OH + HO-
Fe3+ + H2O2 = Fe2+ + •OOH + H+
Therefore, the iron content and metabolism in the tissue is closely related to the death of cell iron. Iron metabolism is one of the characteristics of cancer cells. Most cancer cells have higher levels of iron and ROS. Therefore, the relationship between iron metabolism and ferroptosis in tumor cells has also been studied in recent years. At the same time, with the development of nanotechnology, the various Iron-based nanomaterials have been used for cancer therapy research which based on Fenton reaction and ferroptosis. Caused by the excellent magnetic targeting properties and biocompatibility, iron-based NPs attracts a lot of attention to the field of magnetic resonance imaging (MRI) [10-12], computed tomography (CT) [13], biosensing [14], photothermal therapy [15], and therapeutic agent delivery [16]. The iron-based NPs can release ferrous (Fe2+) or ferric (Fe3+) ions in acidic lysosomes, and further involved in the intracellular Fenton reaction to produce ROS and induce lipid peroxidation [17]. The magnetic field can provide a target for the localization of iron-based NPs in tumor site [18]. Meanwhile, many studies have shown that magnetic fields can inhibit the proliferation of many cancer cells and tumor growth [19,20]. More interestingly, both alternating magnetic fields and static magnetic fields can promote intercellular ROS production [21,22]. This means that regulating iron metabolism, inducing the concentration and internalization of iron-based NPs in cancer cells could induce ferroptosis, and local magnetic field exposure of the tumor can promote this process. This paper focuses on the application of iron metabolism, iron-based NPs and magnetic field in ferroptosis-based cancer therapy, and attempts to explore the application potential for iron and magnetic based ferroptosis studies.
The iron metabolism and ferroptosis
The free intracellular iron can cause lipid peroxidation through the Fenton reaction, which is required for the ferroptosis. The ferroptosis induced by erastin could be inhibited by the iron chelator, such as DFO [3]. The increasing intracellular iron by the expression change of iron metabolism-related gene and iron treatment promotes ferroptosis [23]. Those results suggest that gene about iron metabolism could mediate process of ferroptosis (Table 2).
Table 2.
Iron metabolism genes involved in ferroptosis
| Gene | Name | Function |
|---|---|---|
| TFRC | Transferrin receptor | Imports iron into cells; promotes ferroptosis |
| HSPB1 | Heat shock protein beta 1 | Regulates iron uptake and GPX4 abundance; promotes ferroptosis |
| IRP2/IREB2 | Iron-regulatory protein 2 | Post-transcriptionally repress ferritin expression and increase TFR1 expression; promotes ferroptosis |
| DMT1 | Divalent metal transporter 1 | Transporting ferrous iron; promotes ferroptosis |
| FTH1 | Ferritin heavy chain 1 | Store excess intercellular iron; inhibits ferroptosis |
| NCOA4 | Nuclear receptor coactivator 4 | Involved in ferritinophagy and control of free iron abundance; promotes ferroptosis |
| FPN | Ferroportin | mediate iron efflux; inhibits ferroptosis |
The iron uptake and ferroptosis
The cell iron uptake from extracellular environment mainly mediated by transferrin and transferrin receptor. The expression of transferrin and transferrin receptor is essential for ferroptosis [8,24]. The cytotoxicity caused by erastin can be reduced through the knockdown of transferrin receptor. Both immunodepleted transferrin and TFRC RNAi in glutaminolysis free medium can significantly inhibited ferroptosis [23]. The inactivation of HSPB1 has could accelerate the erastin-induced ferrptosis [25]. Meanwhile, the HSPB1 can inhibit the TFRC recycling and suppression the TFRC mediated iron uptake [26,27]. Iron-regulatory protein 2 (IRP2, also known as IREB2) is a gene acts to regulate iron levels in the cells by regulating the translation and stability of mRNAs that affect iron homeostasis under conditions when iron is depleted. It could inhibit the ubiquitination of transferrin receptor 1 (TfR1) and the divalent metal transporter 1 (DMT1) to upregulate cell iron uptake. It also suppresses the mRNA translation of ferritin and ferroportin (FPN) to increases the labile iron pool [28]. The study of Dixon et al. showed that IRP2 can promote erastin-induced ferroptosis and the inhibition of the IRP2 ubiquitination by FBXL5 knockdown could restrain erastin-induced ferroptosis [3].
The iron storage and ferroptosis
The excess intracellular iron mainly stores in ferritin for most cell. The downregulation of ferritin increases the labile iron pool and increasing intracellular oxidative stress [29,30]. Downregulation of ferritin increases the sensitivity of breast cancer cells to the chemotherapeutic agents doxorubicin [31], the heavy chain ferritin siRNA can increase killing effect of carmustine to breast cancer cell [32]. Ferritin can autophagic degradation by ferritinophagy to maintaining homeostasis when iron depletion. The Ferritinophagy mainly mediated by nuclear receptor coactivator 4 (NCOA4).
The ferritinophagy was acticated in the initiation of ferroptosis [33], and promote intercellular ROS accumulation by releasing the iron to LIP. Different from autophagy, ferritinophagy mediated by NCOA4 don’t need autophagic vacuoles, it can be done directly in lysosomes, but this process could inhibit by the inhibitor of autophagy. The suppression of ferritin degradation by the inhibition of NCOA4 could reduce ferroptosis, while NCOA4 overexpression could promote ferroptosis.
Induction to ferroptosis by erastin was shown to cause a time-dependent LIP increase, a process that is blocked by bafilomycin A1 (BafA1), a potent inhibitor of autophagy [34]. Meanwhile, the endogenous ferritin heavy chain 1 (FTH1) was increased during ferroptosis. Wan SY et al. studies show that overexpression of FTH1 enhanced ferritinophagy during the ferroptosis caused by erastin [34]. The regulation of ferritinopathy and the switch to ferroptosis during pathological conditions leads to cell death and this could also be beneficial therapeutically in the pathophysiology of cancer progression.
In HCT116, A549 and MIA PaCa-2 cell lines synthetic lethal screen, specificity towards oncogenic RASV12 transformed tumor cells was ensured as these cells exhibit elevated iron levels through increased expression of transferrin receptor 1 and downregulation of the iron storage protein ferritin [8]. Knockdown of the ferritinophagy-specific nuclear receptor coactivator 4 significantly decreases the ferroptotic response to erastin in human pancreas carcinoma cells (PANC1) and HT-1080 [35].
Iron efflux and ferroptosis
Ferroportin (FPN) is the only known iron efflux pump in vertebrates. Decreased expression of FPN and iron efflux are characteristic of most cancer cells. Dramatically, the S Ma et al. studies show that expression of FPN is decreased after treatment with siramesine or in combination with lapatinib in the human breast cancer cell lines MCF-7 and ZR-75-1. Meanwhile, knockdown of FPN resulted in increased ROS and ferroptosis after siramesine and lapatinib treatment, contrary to the effect of FPN overexpression [36]. The Erastin induced ferroptosis in neuroblastoma and SH-SY5Y cells, could promoted by knockdown of FPN. After the treatment of erastin, the expression of Fpn gene and protein in SH-SY5Y cells has been significate reduction [37]. Those results suggested that the decrease of FPN expression can significantly increase iron-dependent lipid ROS accumulation, which can accelerate the rate of ferroptosis inducer. Therefore, FPN could be a potential therapeutic target site for ferroptosis based cancer therapy.
The genes that indirectly regulate iron metabolism and ferroptosis
In addition to the conventional iron metabolism related genes could participate in the process of ferroptosis by regulating the intracellular iron content, there are also many genes that affect the status and distribution of intercellular iron in an indirect way. Sun X study’s shows that heat shock protein β-1 (HSPB1) could reduce intercellular iron to prevent cell ferroptosis. HSPB1 knockdown could promote the anti-tumor effect of erastin in vivo [25]. Heme oxygenase-1 has always been considered relevant to iron availability, also could regulate ferroptosis by affecting intercellular iron status or as an antioxidant [38]. The activation of nuclear factor (erythroid-derived 2)-like 2 (NRF2) which proved to have the ability to up-regulate heme oxygenase 1 and ferritin, could inhibit ferroptosis [39] Frataxin is a mitochondrial protein that is seemed to be involved in assembly of iron-sulfur clusters. Frataxin dysfunction leads to the accumulation of mitochondrial iron, and the production of ROS and oxidative stress [40]. Liver-specific knockout of frataxin impairs mitochondrial function and promotes the development of liver tumors in mice [41]. H2S acts as the second messenger in the cell. It is closely related to the iron metabolism and intercellular iron status. Cystathionine b-synthase (CBS) catalyzes the transsulfuration pathway and participate in the regulation of intracellular H2S synthesis. The team of Qian ZM found that CBS knockout (CBS-/-) mice significant increase in iron contented with severe tissue damages in the liver [42]. The condition is very similar to hemochromatosis, which the diseases accompanied by normal cell ferroptosis. The deregulation of miRNAs is entangled with the tumor, it also participates in the regulation of cancer cell’s iron phenotype [43]. The miRNA-210 has proved to inhibit the expression of TfR1, and but instead of decreased uptake of transferrin-bound iron [44]. Furthermore, some aminoferrocence-based therapies also could increase intercellular iron content and enhancing ferroptosis [45,46].
The iron-based NPs related tumor therapy and ferroptosis
In recent years, more and more nanoparticle research in the direction of cancer treatment. Moreover, the most research was based on the iron-based NPs, cause by the special physical and chemical properties of nanostructures, such as active targeting et al. Due to the magnetic field targeting, the concentration of nanoparticles could be significantly improving in the tumor site, and the reduce the side effects on other tissue.
The iron in iron-based NPs can be released as ferrous (Fe2+) or ferric (Fe3+) ions in acidic lysosomes. Moreover, the pH in most tumor cells is more acidic than normal cell. Owing to the poor perfusion under hypoxic condition and increased anaerobic glycolysis, the tumor tissue is overall acidic [47]. Previous studies have shown that the extracellular pH values approaching 6.0 in the human and animal tumours [48]. This means that the iron-based NPs releases ferrous (Fe2+) or ferric (Fe3+) ions in the tumor site is more pronounced than at the normal tissue. Released iron can participate in the Fenton reaction and induce ferroptosis of tumor cell. Therefore, there are many types of nanoparticles used as inducers of ferroptosis in the study of cancer therapy (Figure 1).
Figure 1.
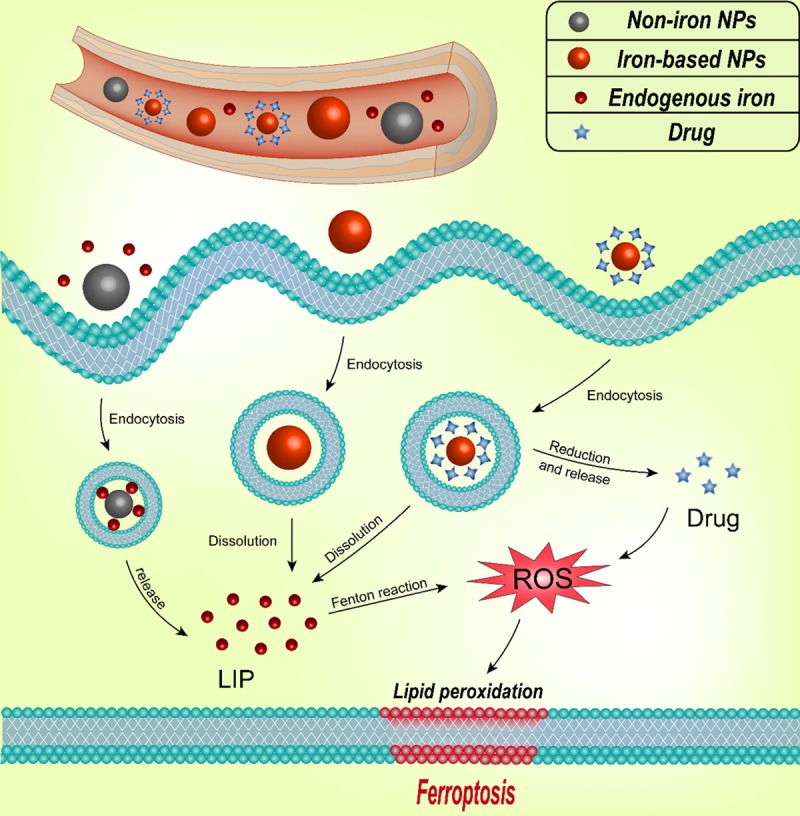
Mechanism of the iron-based NPs and Non-iron NPs for ferroptosis-based cancer therapy. The Non-iron NPs can loaded endogenous iron and the iron-based NPs can release iron in lysosome after endocytosis, which can be involved in the Fenton reaction to produce ROS and induce ferroptosis. The drugs carried by nanoparticles can facilitate the production of ROS, which caused by excess iron. Adapted from Shen ZY, et al. [17].
Iron oxide NPs
The iron oxide nanoparticles (IO NPs) alone has anti-cancer effect by inducing cell ferroptosis [17]. By studying the PLGA-coated Fe3O4 nanoparticles and the pure PLGA nanoparticles on MCF-7, Zhang XD and Mei L determined that the iron core rather than the nanoparticle structure caused endoplasmic reticulum stress and mitochondrial damage [49].
Ferumoxytol is an intravenous preparation as iron supplementation in patients with renal insufficiency and approved by the U.S. Food and Drug Administration (FDA) [50,51]. Zanganeh et al. study focus on the intrinsic therapeutic effect of ferumoxytol on the growth of early mammary cancers, and lung cancer metastases in liver and lungs [52]. The research showed that adenocarcinoma cells caspase-3 activity has significantly increased from co-incubated with ferumoxytol and macrophages, and macrophages exposed to ferumoxytol displayed increased mRNA associated with pro-inflammatory Th1-type responses in vitro. In their previous study, the M1 macrophage subtype has been shown can induce a Fenton reaction in cancer cell. Furthermore, the growth of subcutaneous adenocarcinomas in mice has been significantly inhibited by ferumoxytol and accompanied by an increased presence of pro-inflammatory M1 macrophages in the tumor tissues which detect by Fluorescence-activated cell sorting (FACS) and histopathology studies. Therefore, the ferumoxytol could trigger the ferroptosis in cancer cell by inducing tumor-associated macrophage (TAM) transformation to M1 subtype.
Chemotherapeutic agents -loaded iron-based NPs
Combining iron-based NPs with conventional chemotherapeutic agents are currently one of the main research methods about cancer therapy. The most current method is to modify the drug into nanoparticles to make it a whole, and using the iron-induced ferroptosis enhances the therapeutic effect of conventional drugs.
Zhu XL et al. found that the DOX-Cit/CuS@Fe3O4 nanoparticle could show higher cytotoxicity than DOX at the same concentration in MCF-7 cells. DOX-Cit/CuS@Fe3O4 nanoparticle could rapidly increase intracellular ROS levels under of 980 nm laser irradiation [53]. Sorafenib has been used as anti-cancer drugs in clinical for Liver cancer, kidney cancer and osteosarcoma et al. It has also been identified as a ferroptosis inducer. Zhang L et al. studies found that Sorafenib-modified iron-based NPs is more effective at inhibiting proliferation and inducing death of HepG2 cells in vitro than sorafenib alone [54]. The intracellular ROS generation plays a critical role in therapeutic effects of cisplatin [55,56]. The cisplatin-loaded iron-based NPs has been designed by Ma et al. to study its anticancer efficacy. Human ovarian carcinoma A2780 cells (cisplatin-sensitive) and cisplatin-resistant A2780DDP cells (denoted ACP) has been used to testing the anticancer efficacy of designed IO NPs in Ma P et al. study [57]. The results showed that the A2780 and ACP cells death could be significant increasing by the treatment of Cisplatin-Loaded IO NPs than Cisplatin alone which can be blocked by iron chelator and ROS scavenger. Caused the Cisplatin mediates activation of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX), which triggers oxygen (O2) to superoxide radical (O2•-) and its downstream H2O2. Through the Fenton reaction, H2O2 could be catalyzed by Fe2+/Fe3+ which released from IO NPs to the toxic hydroxyl radicals (•OH), which cause oxidative damages to lipids, proteins, and DNA. The result is also demonstrated tumor site-specific conversion of ROS generation induced by released cisplatin and Fe2+/Fe3+ from iron-oxide nanocarriers with cisplatin(IV) prodrugs for enhanced anticancer activity but minimized systemic toxicity. Interestingly, the IC50 (half maximal inhibitory concentration) values of carboplatin, oxaliplatin, doxorubicin, and artesunate in A2780 and ACP cells showed a significant decrease with iron treatment.
Reduction inhibitors/oxide -tethered iron-based NPs
Since the release of iron from iron-based NPs can increase intracellular ROS levels, the oxides or reducing inhibitors can synergize with iron in cells. Many studies focus on the modifying oxides on iron-based NPs to promote ferroptosis caused by nanoparticle ingestion.
Ascorbic acid, known an antioxidant, is able to produce endogenous H2O2 to result in the oxidative stress by the generation of ROS [58]. The combination of Ascorbic acid with iron oxide particles was used as a new source of ROS manipulating anticancer drugs. This nanoparticle was ionized in acidic tumors and released iron, which in turn induced localized Fenton reaction. The rate of OH• generation using free Fe2+ ions was found to be faster than Fe2+ on the surface of Fe3O4 nanoparticles [59,60].
β-lapachone (β-lap), a novel anticancer drug, has shown considerable cancer specificity by selectively increasing reactive oxygen species (ROS) stress in cancer cells. A 10-fold increase in ROS stress was detected in β-lap-exposed cells pretreated with iron oxide nanoparticle over those treated with β-lap alone in A549 non-small cell lung carcinoma (NSCLC) cells, which also correlates with significantly increased cell death [61].
Zhou et al. have designed an activatable singlet oxygen (1O2)-generating system for specific cancer therapy under tumor acidic pH environment through engineering the reaction between linoleic acid hydroperoxide (LAHP) and catalytic iron (II) ions. LAHP is one of the primary products of lipid peroxidation, which is associated with several diseases by decomposition into ROS and 1O2 in the presence of Fe2+ through the Russell mechanism. The iron could release from nanoparticle under tumor acidic pH environment, and LAHP can react with Fe2+ to produce 1O2, which is much more efficient than Fenton reaction without LAHP participation. The result showed that IO-LAHP nanoparticles are able to induce efficient cancer cell death in U87MG cells through tumor-specific 1O2 generation and subsequent ROS mediated mechanism, and the tumor growth has been significant inhibited in vivo [62].
No iron-based NPs
There are also special cases in which non-iron nanoparticles can induce cancer cells ferroptosis. Sung EK et al. studies show that ultrasmall αMSH-PEG-C’ dots could suppress tumor growth, the function could be reserved liproxstatin-1, which has been determined as an inhibitor of ferroptosis. Their results also demonstrated that αMSH-PEG-C’ dots could induce nutrient-deprived cancer cells ferroptosis, while the generally cancer cells are resistant to it. In this study, the anti-cancer ability of αMSH-PEG-C’ dots come from its promotion of iron uptake of cancer cell [63]. Ou WJ et al. designed a Low-Density Lipoprotein Docosahexaenoic Acid Nanoparticles (LDL-DHA NPs) and found that it could induce hepatoma cells death selectively and inhibit orthotopic liver tumors growth in vivo. This anti-cancer effect is accompanied by lipid peroxidation with the iron-dependent characteristics of ferroptosis [64].
By the way, even besides iron, other particles such as silver, gold, and FeOx-MSNs22 were reported to produce OH• from H2O2 in the acidic lysosomes. But the drawback of these studies is that these nanoparticles only produced OH• at the surface via a heterogeneous reaction and unable to treat the cancer cells using the endogenous H2O2.
The iron-based NPs has been used as contrast agents for magnetic resonance imaging (MRI) in clinical [65]. Therefore, its security has a certain guarantee of cancer therapy. Furthermore, iron-based NPs can be easily for targeted concentration in tumor sites due to their special physicochemical properties. It cannot be ignored that function and magnetic-targeting efficiency of nanoparticle is closely related to iron-particle size. Guo XM et al. found that smaller Fe3O4 nanoparticles are easier internalized by cells, while larger Fe3O4 nanoparticles are easier to accumulate in the tumor [66].
In addition to releasing iron to increase the intracellular ROS level, the iron-based NPs can also participate in intercellular redox metabolism as a “nanozyme” [67,68]. Ultrasmall Fe3O4 nanoparticles could be described as an inorganic nanozyme and can participate in intercellular redox metabolism as a Fenton reaction catalyst in the mildly acidic microenvironment of tumor [69]. Huo MF et al. has designed a nanoparticle constructed from glucose oxidase (GOD), synthetic ultrasmall Fe3O4 nanoparticle, and dendritic MSN combinations to investigate the role and mechanism of GOD and ultrasmall Fe3O4 nanoparticles on tumors [70]. Their result shows that GOD-Fe3O4@DMSNs nanocatalysts (GFD NCs) could induce death of 4T1 and U87 cells in vitro and inhibit tumor growth in vivo accompanied by elevated levels of intracellular ROS.
Application potential of magnetic field in ferroptosis-based cancer therapy
The magnetic field often plays a guiding role in the process of iron-based NPs induce ferroptosis, allowing nanoparticles to be enriched in the tumor site. In some special structure of nanoparticle treatment, the magnetic field can also change the arrangement of nanoparticles in the body to promote the local release of the drug. They build two types nanoparticle as enzyme and substrate, the nanoparticle could merge and forced to interact with the generated nanocompartment during the external magnetic field loading [71].
In some studies, the magnetic field can be used as a stirrer of nanoparticles to promote the production of ROS in tumor cells. The Flanagan SW et al. showed that the magnetic field can heat the IO NPs, lead the hyperthermia in tumor area, and the ROS production to suppress the proliferation of pancreatic cancer cells (PANC-1 and BxPC-3 cells) and reduced tumor volumes. In this process, ROS is not only produced by the release of Fe from the nanoparticles, but also be induced via hyperthermia [72,73]. Furthermore, numerous studies have shown that the magnetic field itself can also inhibit tumor cell proliferation by promoting intracellular ROS production. Magnetic field treatment can increase the concentration, viability and longevity of paramagnetic intercellular free radicals, and changes the conformation and activity of oxidative balance related enzymes. These changes may lead to a series of subsequent changes in oxidative stress levels even cell death [74].
Sabo J et al. found that after 1.0 T strong magnetic field treatment for 72 h, the rat leukemia HL-60 cell’s metabolic activity has been significate inhibited. This phenomenon probably due to the strong magnetic field induced intracellular ROS increase, then ROS destroyed the cell’s metabolic process, thus making it metabolic activity is inhibited [22]. Spyridopoulou K et al. found that both the static magnetic field and the rotating magnetic field can inhibit the activity of human colon cancer HT29 cells, and the inhibition is positively correlated with the magnetic field strength. Interestingly, during this experiment they also found that the magnetic field promoted the absorption of nanoparticles by the cells [75]. The research of Hajipour VB et al. shows that the 5, 10, 15 and 20 mT magnetic field can promote the accumulation of iron in MCF-7 and HFF cells, increase of production of ROS, inhibit its cellular activity and has a synergistic effect with doxorubicin [76]. At the same time, previous research in our laboratory showed that high statics magnetic field can promote iron uptake in osteosarcoma cell line MG63 (unpublish). This means that the magnetic field has great potential and application value in the study of cancer therapy based on ferroptosis.
Conclusions and prospective
Ferroptosis is essentially destroying the intracellular redox balance by the ROS accumulation. Most of the previous studies about ferroptosis focused on how to maintain or destroy the intercellular redox balance by regulating “reduction part” of the redox balance, such as System Xc -, Glutathione peroxidase 4 (GPX4), glutathione metabolism and dysregulation of lipid metabolism et al. [77-81]. The role of iron in ferroptosis mainly focused on “oxidation part”. The intercellular iron status affects the production of ROS. Abnormal iron metabolism is characteristic of most tumor cells [82]. The overexpression of genes related to iron uptake and the low expression of genes related to iron efflux lead to a much higher intercellular iron content of most cancer cells than normal cells [83,84]. At the same time, the iron content of the LIP in cancer cells is much higher than that in normal cells. The Fenton reaction involved in iron is one of the main pathways to intracellular ROS production. Therefore, cancer cells have higher levels of ROS, and the redox balance is also more fragile [82]. As a new form of cell death and closely related to intracellular redox balance, ferroptosis introduces iron from the field of nutrition to the cancer therapy. In addition to regulating iron metabolism in cells to induce cancer cells ferroptosis, put the cancer cell in a special high-iron environment is another way to make it ferroptosis. With the development of nanotechnology and the special physical and chemical properties of nanomaterials, the various nanoparticle has also been widely used in the research of cancer treatment. At the same time, iron-based NPs could ferrous (Fe2+) or ferric (Fe3+) ions in acidic lysosomes and increase ROS levels in tumor cells rapidly. This suggests that iron-based NPs has great potential for the ferroptosis based cancer therapy. Due to the tendency towards iron-based NPs to concentrate in the tumor site under the action of a magnetic field, the magnetic field is widely used in tumor therapy research as drugs “guide”. The magnetic field not only can play a role in the targeted concentration of nanoparticles, but also promotes the production of ROS in cells (Figure 2). Therefore, iron metabolism, iron-based NPs and magnetic field can mutual assistance in ferroptosis-based cancer therapy.
Figure 2.
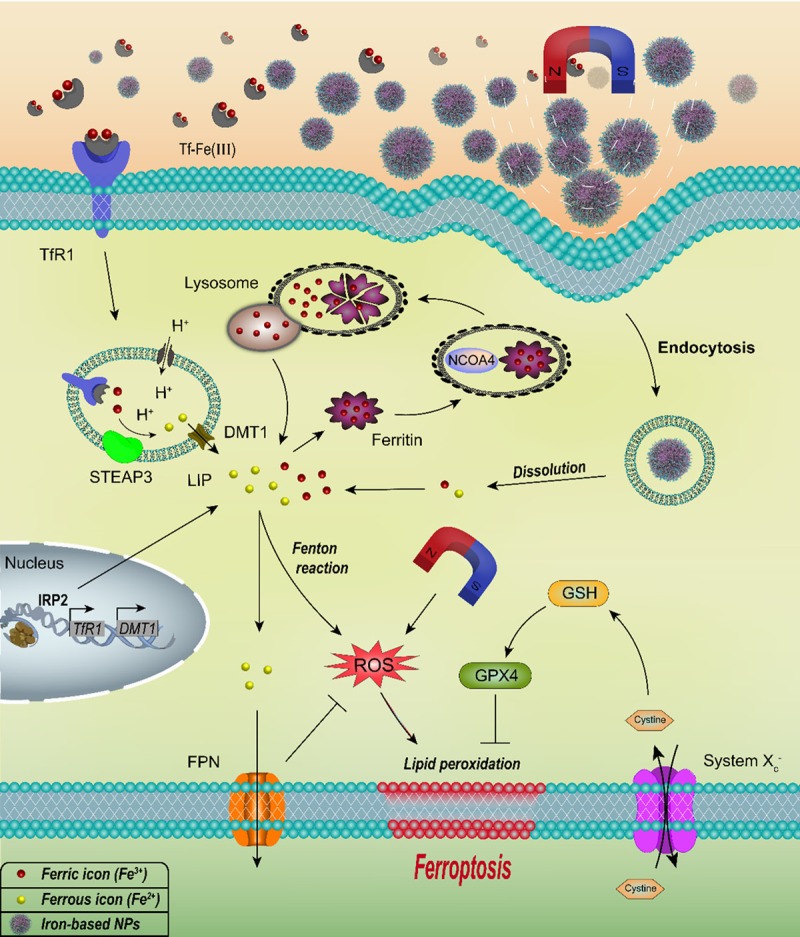
The mechanism of iron metabolism, iron-based NPs and magnetic field in the ferroptosis-based cancer therapy. Iron metabolism affects ferroptosis by regulating cellular iron uptake (TfR1, DMT1, IRP2), storage (Ferritin, NCOA4), and efflux (FPN). Iron-based NPs could release ferrous (Fe2+) or ferric (Fe3+) in acidic lysosomes. Excess intercellular iron can cause lipid peroxidation by participating in Fenton reaction, which is necessary for ferroptosis. The magnetic field can be used to concentrate iron-based NPs in the tumor site, meanwhile magnetic field can directly promote the intracellular ROS production.
In the future, the research of ferroptosis through the iron metabolism and iron preparations will provide more extensive research ideas of cancer therapy. The used of iron-based NPs combination with other chemotherapeutics have great potential for future research. Furthermore, the magnetic field not only serves as a guide in the ferroptosis-based cancer therapy to have a wide application prospect, but also its own influence on the process of ferroptosis deserves further exploration.
Acknowledgements
This work sponsored by grants from the Science and Technology Planning Project of Shenzhen of China (JCYJ20170412140904406) and Shenzhen Virtual University Park Special Funds (YFJGJS1.0).
Disclosure of conflict of interest
None.
References
- 1.Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yuan JY, Kroemer G. Alternative cell death mechanisms in development and beyond. Genes Dev. 2010;24:2592–2602. doi: 10.1101/gad.1984410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dixon SJ, Lemberg KM, Lamprecht MR, Skouta R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS, Morrison B, Stockwell BR. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dolma S, Lessnick SL, Hahn WC, Stockwell BR. Identification of genotype-selective antitumor agents using synthetic lethal chemical screening in engineered human tumor cells. Cancer Cell. 2003;3:285–296. doi: 10.1016/s1535-6108(03)00050-3. [DOI] [PubMed] [Google Scholar]
- 5.Galluzzi L, Vitale I, Aaronson SA, Abrams JM, Adam D, Agostinis P, Alnemri ES, Altucci L, Amelio I, Andrews DW, Annicchiarico-Petruzzelli M, Antonov AV, Arama E, Baehrecke EH, Barlev NA, Bazan NG, Bernassola F, Bertrand MJ, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Boya P, Brenner C, Campanella M, Candi E, Carmona-Gutierrez D, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Cohen GM, Conrad M, Cubillos-Ruiz JR, Czabotar PE, D’Angiolella V, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, DeBerardinis RJ, Deshmukh M, Di Daniele N, Di Virgilio F, Dixit VM, Dixon SJ, Duckett CS, Dynlacht BD, El-Deiry WS, Elrod JW, Fimia GM, Fulda S, Garcia-Saez AJ, Garg AD, Garrido C, Gavathiotis E, Golstein P, Gottlieb E, Green DR, Greene LA, Gronemeyer H, Gross A, Hajnoczky G, Hardwick JM, Harris IS, Hengartner MO, Hetz C, Ichijo H, Jaattela M, Joseph B, Jost PJ, Juin PP, Kaiser WJ, Karin M, Kaufmann T, Kepp O, Kimchi A, Kitsis RN, Klionsky DJ, Knight RA, Kumar S, Lee SW, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lowe SW, Luedde T, Lugli E, MacFarlane M, Madeo F, Malewicz M, Malorni W, Manic G, Marine JC, Martin SJ, Martinou JC, Medema JP, Mehlen P, Meier P, Melino S, Miao EA, Molkentin JD, Moll UM, Munoz-Pinedo C, Nagata S, Nunez G, Oberst A, Oren M, Overholtzer M, Pagano M, Panaretakis T, Pasparakis M, Penninger JM, Pereira DM, Pervaiz S, Peter ME, Piacentini M, Pinton P, Prehn JHM, Puthalakath H, Rabinovich GA, Rehm M, Rizzuto R, Rodrigues CMP, Rubinsztein DC, Rudel T, Ryan KM, Sayan E, Scorrano L, Shao F, Shi YF, Silke J, Simon HU, Sistigu A, Stockwell BR, Strasser A, Szabadkai G, Tait SWG, Tang DL, Tavernarakis N, Thorburn A, Tsujimoto Y, Turk B, Vanden Berghe T, Vandenabeele P, Heiden MGV, Villunger A, Virgin HW, Vousden KH, Vucic D, Wagner EF, Walczak H, Wallach D, Wang Y, Wells JA, Wood W, Yuan JY, Zakeri Z, Zhivotovsky B, Zitvogel L, Melino G, Kroemer G. Molecular mechanisms of cell death: recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018;25:486–541. doi: 10.1038/s41418-017-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xie Y, Hou W, Song X, Yu Y, Huang J, Sun X, Kang R, Tang D. Ferroptosis: process and function. Cell Death Differ. 2016;23:369–379. doi: 10.1038/cdd.2015.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao JY, Dixon SJ. Mechanisms of ferroptosis. Cell Mol Life Sci. 2016;73:2195–2209. doi: 10.1007/s00018-016-2194-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang WS, Stockwell BR. Synthetic lethal screening identifies compounds activating iron-dependent, nonapoptotic cell death in oncogenic-RAS-harboring cancer cells. Chem Biol. 2008;15:234–245. doi: 10.1016/j.chembiol.2008.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dunford HB. Oxidations of iron(II)/(III) by hydrogen peroxide: from aquo to enzyme. Coordination Chemistry Reviews. 2002;233:311–318. [Google Scholar]
- 10.Shin TH, Choi Y, Kim S, Cheon J. Recent advances in magnetic nanoparticle-based multi-modal imaging. Chem Soc Rev. 2015;44:4501–4516. doi: 10.1039/c4cs00345d. [DOI] [PubMed] [Google Scholar]
- 11.Singamaneni S, Bliznyuk VN, Binek C, Tsymbal EY. Magnetic nanoparticles: recent advances in synthesis, self-assembly and applications. Chem Soc Rev. 2011;21:16819–16845. [Google Scholar]
- 12.Thorat ND, Lemine OM, Bohara RA, Omri K, El Mir L, Tofail SA. Superparamagnetic iron oxide nanocargoes for combined cancer thermotherapy and MRI applications. Phys Chem Chem Phys. 2016;18:21331–21339. doi: 10.1039/c6cp03430f. [DOI] [PubMed] [Google Scholar]
- 13.Liang SY, Zhou Q, Wang M, Zhu YH, Wu QZ, Yang XL. Water-soluble L-cysteine-coated FePt nanoparticles as dual MRI/CT imaging contrast agent for glioma. Int J Nanomedicine. 2015;10:2325–33. doi: 10.2147/IJN.S75174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen S, Wang LJ, Duce SL, Brown S, Lee S, Melzer A, Cuschieri SA, Andre P. Engineered biocompatible nanoparticles for in vivo imaging applications. Int J Nanomedicine. 2010;132:15022–15029. doi: 10.1021/ja106543j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu XL, Choo ESG, Ahmed AS, Zhao LY, Yang Y, Ramanujan RV, Xue JM, Di Fan D, Fan HM, Ding J. Magnetic nanoparticle-loaded polymer nanospheres as magnetic hyperthermia agents. Journal of Materials Chemistry B. 2014;2:120–128. doi: 10.1039/c3tb21146k. [DOI] [PubMed] [Google Scholar]
- 16.Ulbrich K, Hola K, Subr V, Bakandritsos A, Tucek J, Zboril R. Targeted drug delivery with polymers and magnetic nanoparticles: covalent and noncovalent approaches, release control, and clinical studies. Chem Rev. 2016;116:5338–5431. doi: 10.1021/acs.chemrev.5b00589. [DOI] [PubMed] [Google Scholar]
- 17.Shen ZY, Song JB, Yung BC, Zhou ZJ, Wu AG, Chen XY. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30:e1704007. doi: 10.1002/adma.201704007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi JW, Park JW, Na Y, Jung SJ, Hwang JK, Choi D, Lee KG, Yun CO. Using a magnetic field to redirect an oncolytic adenovirus complexed with iron oxide augments gene therapy efficacy. Biomaterials. 2015;65:163–174. doi: 10.1016/j.biomaterials.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 19.Raylman RR, Clavo AC, Wahl RL. Exposure to strong static magnetic field slows the growth of human cancer cells in vitro. Bioelectromagnetics. 1996;17:358–363. doi: 10.1002/(SICI)1521-186X(1996)17:5<358::AID-BEM2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 20.Ghibelli L, Cerella C, Cordisco S, Clavarino G, Marazzi S, De Nicola M, Nuccitelli S, D’Alessio M, Magrini A, Bergamaschi A, Guerrisi V, Porfiri LM. NMR exposure sensitizes tumor cells to apoptosis. Apoptosis. 2006;11:359–365. doi: 10.1007/s10495-006-4001-1. [DOI] [PubMed] [Google Scholar]
- 21.Watanabe Y, Nakagawa M, Miyakoshi Y. Enhancement of lipid peroxidation in the liver of mice exposed to magnetic fields. Ind Health. 1997;35:285–290. doi: 10.2486/indhealth.35.285. [DOI] [PubMed] [Google Scholar]
- 22.Sabo J, Mirossay L, Horovcak L, Sarissky M, Mirossay A, Mojzis J. Effects of static magnetic field on human leukemic cell line HL-60. Bioelectrochemistry. 2002;56:227–231. doi: 10.1016/s1567-5394(02)00027-0. [DOI] [PubMed] [Google Scholar]
- 23.Gao MH, Monian P, Quadri N, Ramasamy R, Jiang XJ. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59:298–308. doi: 10.1016/j.molcel.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galluzzi L, Bravo-San Pedro JM, Vitale I, Aaronson SA, Abrams JM, Adam D, Alnemri ES, Altucci L, Andrews D, Annicchiarico-Petruzzelli M, Baehrecke EH, Bazan NG, Bertrand MJ, Bianchi K, Blagosklonny MV, Blomgren K, Borner C, Bredesen DE, Brenner C, Campanella M, Candi E, Cecconi F, Chan FK, Chandel NS, Cheng EH, Chipuk JE, Cidlowski JA, Ciechanover A, Dawson TM, Dawson VL, De Laurenzi V, De Maria R, Debatin KM, Di Daniele N, Dixit VM, Dynlacht BD, El-Deiry WS, Fimia GM, Flavell RA, Fulda S, Garrido C, Gougeon ML, Green DR, Gronemeyer H, Hajnoczky G, Hardwick JM, Hengartner MO, Ichijo H, Joseph B, Jost PJ, Kaufmann T, Kepp O, Klionsky DJ, Knight RA, Kumar S, Lemasters JJ, Levine B, Linkermann A, Lipton SA, Lockshin RA, Lopez-Otin C, Lugli E, Madeo F, Malorni W, Marine JC, Martin SJ, Martinou JC, Medema JP, Meier P, Melino S, Mizushima N, Moll U, Munoz-Pinedo C, Nunez G, Oberst A, Panaretakis T, Penninger JM, Peter ME, Piacentini M, Pinton P, Prehn JH, Puthalakath H, Rabinovich GA, Ravichandran KS, Rizzuto R, Rodrigues CM, Rubinsztein DC, Rudel T, Shi Y, Simon HU, Stockwell BR, Szabadkai G, Tait SW, Tang HL, Tavernarakis N, Tsujimoto Y, Vanden Berghe T, Vandenabeele P, Villunger A, Wagner EF, Walczak H, White E, Wood WG, Yuan J, Zakeri Z, Zhivotovsky B, Melino G, Kroemer G. Essential versus accessory aspects of cell death: recommendations of the NCCD 2015. Cell Death Differ. 2015;22:58–73. doi: 10.1038/cdd.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun X, Ou Z, Xie M, Kang R, Fan Y, Niu X, Wang H, Cao L, Tang D. HSPB1 as a novel regulator of ferroptotic cancer cell death. Oncogene. 2015;34:5617–5625. doi: 10.1038/onc.2015.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Arrigo AP, Virot S, Chaufour S, Firdaus W, Kretz-Remy C, Diaz-Latoud C. Hsp27 consolidates intracellular redox homeostasis by upholding glutathione in its reduced form and by decreasing iron intracellular levels. Antioxid Redox Signal. 2005;7:414–424. doi: 10.1089/ars.2005.7.414. [DOI] [PubMed] [Google Scholar]
- 27.Chen HY, Zheng CL, Zhang Y, Chang YZ, Qian ZM, Shen X. Heat shock protein 27 downregulates the transferrin receptor 1-mediated iron uptake. Int J Biochem Cell Biol. 2006;38:1402–1416. doi: 10.1016/j.biocel.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 28.Wu KJ, Polack A, Dalla-Favera R. Coordinated regulation of iron-controlling genes, H-ferritin and IRP2, by c-MYC. Science. 1999;283:676–679. doi: 10.1126/science.283.5402.676. [DOI] [PubMed] [Google Scholar]
- 29.Kakhlon O, Gruenbaum Y, Cabantchik ZI. Repression of ferritin expression modulates cell responsiveness to H-ras-induced growth. Biochem Soc Trans. 2002;30:777–780. doi: 10.1042/bst0300777. [DOI] [PubMed] [Google Scholar]
- 30.Kakhlon O, Gruenbaum Y, Cabantchik ZI. Ferritin expression modulates cell cycle dynamics and cell responsiveness to H-ras-induced growth via expansion of the labile iron pool. Biochem J. 2002;363:431–436. doi: 10.1042/0264-6021:3630431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shpyleva SI, Tryndyak VP, Kovalchuk O, Starlard-Davenport A, Chekhun VF, Beland FA, Pogribny IP. Role of ferritin alterations in human breast cancer cells. Breast Cancer Res Treat. 2011;126:63–71. doi: 10.1007/s10549-010-0849-4. [DOI] [PubMed] [Google Scholar]
- 32.Liu XL, Madhankumar AB, Slagle-Webb B, Sheehan JM, Surguladze N, Connor JR. Heavy chain ferritin siRNA delivered by cationic liposomes increases sensitivity of cancer cells to chemotherapeutic agents. Cancer Res. 2011;71:2240–2249. doi: 10.1158/0008-5472.CAN-10-1375. [DOI] [PubMed] [Google Scholar]
- 33.Gao MH, Monian P, Pan QH, Zhang W, Xiang J, Jiang XJ. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26:1021–1032. doi: 10.1038/cr.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang WS, Kim KJ, Gaschler MM, Patel M, Shchepinov MS, Stockwell BR. Peroxidation of polyunsaturated fatty acids by lipoxygenases drives ferroptosis. Proc Natl Acad Sci U S A. 2016;113:E4966–E4975. doi: 10.1073/pnas.1603244113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hou W, Xie YC, Song XX, Sun XF, Lotze MT, Zeh HJ, Kang R, Tang DL. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12:1425–1428. doi: 10.1080/15548627.2016.1187366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma S, Henson EE, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Geng N, Shi BJ, Li SL, Zhong ZY, Li YC, Xua WL, Zhou H, Cai JH. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci. 2018;22:3826–3836. doi: 10.26355/eurrev_201806_15267. [DOI] [PubMed] [Google Scholar]
- 38.Kwon MY, Park E, Lee SJ, Chung SW. Heme oxygenase-1 accelerates erastin-induced ferroptotic cell death. Oncotarget. 2015;6:24393–24403. doi: 10.18632/oncotarget.5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun XF, Ou ZH, Chen RC, Niu XH, Chen D, Kang R, Tang DL. Activation of the p62-Keap1-NRF2 pathway protects against ferroptosis in hepatocellular carcinoma cells. Hepatology. 2016;63:173–184. doi: 10.1002/hep.28251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shoichet SA, Baumer AT, Stamenkovic D, Sauer H, Pfeiffer AF, Kahn CR, Muller-Wieland D, Richter C, Ristow M. Frataxin promotes antioxidant defense in a thiol-dependent manner resulting in diminished malignant transformation in vitro. Hum Mol Genet. 2002;11:815–821. doi: 10.1093/hmg/11.7.815. [DOI] [PubMed] [Google Scholar]
- 41.Thierbach R, Schulz TJ, Isken F, Voigt A, Mietzner B, Drewes G, von Kleist-Retzow JC, Wiesner RJ, Magnuson MA, Puccio H, Pfeiffer AF, Steinberg P, Ristow M. Targeted disruption of hepatic frataxin expression causes impaired mitochondrial function, decreased life span and tumor growth in mice (vol 14, pg 3857, 2005) Hum Mol Genet. 2007;16:2987–2987. doi: 10.1093/hmg/ddi410. [DOI] [PubMed] [Google Scholar]
- 42.Zhou YF, Wu XM, Zhou G, Mu MD, Zhang FL, Li FM, Qian C, Du F, Yung WH, Qian ZM, Ke Y. Cystathionine beta-synthase is required for body iron homeostasis. Hepatology. 2018;67:21–35. doi: 10.1002/hep.29499. [DOI] [PubMed] [Google Scholar]
- 43.Jansson MD, Lund AH. MicroRNA and cancer. Mol Oncol. 2012;6:590–610. doi: 10.1016/j.molonc.2012.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshioka Y, Kosaka N, Ochiya T, Kato T. Micromanaging iron homeostasis hypoxia-inducible micro-rna-210 suppresses iron homeostasis-related proteins. J Biol Chem. 2012;287:34110–34119. doi: 10.1074/jbc.M112.356717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marzenell P, Hagen H, Sellner L, Zenz T, Grinyte R, Pavlov V, Daum S, Mokhir A. Aminoferrocene-based prodrugs and their effects on human normal and cancer cells as well as bacterial cells. J Med Chem. 2013;56:6935–6944. doi: 10.1021/jm400754c. [DOI] [PubMed] [Google Scholar]
- 46.Hagen H, Marzenell P, Jentzsch E, Wenz F, Veldwijk MR, Mokhir A. Aminoferrocene-based prodrugs activated by reactive oxygen species. J Med Chem. 2012;55:924–934. doi: 10.1021/jm2014937. [DOI] [PubMed] [Google Scholar]
- 47.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 48.Raghunand N, Gatenby RA, Gillies RJ. Microenvironmental and cellular consequences of altered blood flow in tumours. Br J Radiol. 2003;76:S11–S22. doi: 10.1259/bjr/12913493. [DOI] [PubMed] [Google Scholar]
- 49.Zhang XD, Zhang HQ, Liang X, Zhang JX, Tao W, Zhu XB, Chang DF, Zeng XW, Mei L. Iron oxide nanoparticles induce autophagosome accumulation through multiple mechanisms: lysosome impairment, mitochondrial damage, and ER stress. Mol Pharm. 2016;13:2578–2587. doi: 10.1021/acs.molpharmaceut.6b00405. [DOI] [PubMed] [Google Scholar]
- 50.Rubio-Navarro A, Carril M, Padro D, Guerrero-Hue M, Tarin C, Samaniego R, Cannata P, Cano A, Villalobos JMA, Sevillano AM, Yuste C, Gutierrez E, Praga M, Egido J, Moreno JA. CD163-macrophages are involved in rhabdomyolysis-induced kidney injury and may be detected by MRI with targeted gold-coated iron oxide nanoparticles. Theranostics. 2016;6:896–914. doi: 10.7150/thno.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Su YL, Fang JH, Liao CY, Lin CT, Li YT, Hu SH. Targeted mesoporous iron oxide nanoparticles-encapsulated perfluorohexane and a hydrophobic drug for deep tumor penetration and therapy. Theranostics. 2015;5:1233–1248. doi: 10.7150/thno.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zanganeh S, Hutter G, Spitler R, Lenkov O, Mahmoudi M, Shaw A, Pajarinen JS, Nejadnik H, Goodman S, Moseley M, Coussens LM, Daldrup-Link HE. Iron oxide nanoparticles inhibit tumour growth by inducing pro-inflammatory macrophage polarization in tumour tissues. Nat Nanotechnol. 2016;11:986–994. doi: 10.1038/nnano.2016.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhu XL, Huang HQ, Zhang YJ, Zhang HJ, Hou L, Zhang ZZ. Cit/CuS@Fe3O4-based and enzyme-responsive magnetic nanoparticles for tumor chemotherapy, photothermal, and photodynamic therapy. J Biomater Appl. 2017;31:1010–1025. doi: 10.1177/0885328216676159. [DOI] [PubMed] [Google Scholar]
- 54.Zhang L, Gong FM, Zhang F, Ma J, Zhang PD, Shen J. Targeted therapy for human hepatic carcinoma cells using folate-functionalized polymeric micelles loaded with superparamagnetic iron oxide and sorafenib in vitro. Int J Nanomedicine. 2013;8:1517–1524. doi: 10.2147/IJN.S43263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Marullo R, Werner E, Degtyareva N, Moore B, Altavilla G, Ramalingam SS, Doetsch PW. Cisplatin induces a mitochondrial-ROS response that contributes to cytotoxicity depending on mitochondrial redox status and bioenergetic functions. PLoS One. 2013;8:e81162. doi: 10.1371/journal.pone.0081162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim SJ, Hur JH, Park C, Kim HJ, Oh GS, Lee JN, Yoo SJ, Choe SK, So HS, Lim DJ, Moon SK, Park R. Bucillamine prevents cisplatin-induced ototoxicity through induction of glutathione and antioxidant genes. Exp Mol Med. 2015;47:e142. doi: 10.1038/emm.2014.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma PA, Xiao HH, Yu C, Liu JH, Cheng ZY, Song HQ, Zhang XY, Li CX, Wang JQ, Gu Z, Lin J. Enhanced cisplatin chemotherapy by iron oxide nanocarrier-mediated generation of highly toxic reactive oxygen species. Nano Lett. 2017;17:928–937. doi: 10.1021/acs.nanolett.6b04269. [DOI] [PubMed] [Google Scholar]
- 58.Chen Q, Espey MG, Sun AY, Pooput C, Kirk KL, Krishna MC, Khosh DS, Drisko J, Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc Natl Acad Sci U S A. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hermanek M, Zboril R, Medrik N, Pechousek J, Gregor C. Catalytic efficiency of iron(III) oxides in decomposition of hydrogen peroxide: Competition between the surface area and crystallinity of nanoparticles. J Am Chem Soc. 2007;129:10929–10936. doi: 10.1021/ja072918x. [DOI] [PubMed] [Google Scholar]
- 60.Wang B, Yin JJ, Zhou XY, Kurash I, Chai ZF, Zhao YL, Feng WY. Physicochemical origin for free radical generation of iron oxide nanoparticles in biomicroenvironment: catalytic activities mediated by surface chemical states. Journal of Physical Chemistry C. 2013;117:383–392. [Google Scholar]
- 61.Huang G, Chen HB, Dong Y, Luo XQ, Yu HJ, Moore Z, Bey EA, Boothman DA, Gao JM. Superparamagnetic iron oxide nanoparticles: amplifying ROS stress to improve anticancer drug efficacy. Theranostics. 2013;3:116–126. doi: 10.7150/thno.5411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou ZJ, Song JB, Tian R, Yang Z, Yu GC, Lin LS, Zhang GF, Fan WP, Zhang FW, Niu G, Nie LM, Chen XY. Activatable singlet oxygen generation from lipid hydroperoxide nanoparticles for cancer therapy. Angew Chem Int Ed Engl. 2017;56:6492–6496. doi: 10.1002/anie.201701181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim SE, Zhang L, Ma K, Riegman M, Chen F, Ingold I, Conrad M, Turker MZ, Gao MH, Jiang XJ, Monette S, Pauliah M, Gonen M, Zanzonico P, Quinn T, Wiesner U, Bradbury MS, Overholtzer M. Ultrasmall nanoparticles induce ferroptosis in nutrient-deprived cancer cells and suppress tumour growth. Nat Nanotechnol. 2016;11:977–985. doi: 10.1038/nnano.2016.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ou WJ, Mulik RS, Anwar A, McDonald JG, He XS, Corbin IR. Low-density lipoprotein docosahexaenoic acid nanoparticles induce ferroptotic cell death in hepatocellular carcinoma. Free Radic Biol Med. 2017;112:597–607. doi: 10.1016/j.freeradbiomed.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yue LD, Wang JL, Dai ZC, Hu ZF, Chen X, Qi YF, Zheng XW, Yu DX. pH-Responsive, self-sacrificial nanotheranostic agent for potential in vivo and in vitro dual modal MRI/CT imaging, real-time, and in situ monitoring of cancer therapy. Bioconjug Chem. 2017;28:400–409. doi: 10.1021/acs.bioconjchem.6b00562. [DOI] [PubMed] [Google Scholar]
- 66.Guo XM, Li W, Luo LH, Wang ZH, Li QP, Kong FF, Zhang HB, Yang J, Zhu CQ, Du YZ, You J. External magnetic field-enhanced chemo-photothermal combination tumor therapy via iron oxide nanoparticles. ACS Appl Mater Interfaces. 2017;9:16581–16593. doi: 10.1021/acsami.6b16513. [DOI] [PubMed] [Google Scholar]
- 67.Gao LZ, Zhuang J, Nie L, Zhang JB, Zhang Y, Gu N, Wang TH, Feng J, Yang DL, Perrett S, Yan X. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat Nanotechnol. 2007;2:577–583. doi: 10.1038/nnano.2007.260. [DOI] [PubMed] [Google Scholar]
- 68.Wei H, Wang EK. Nanomaterials with enzyme-like characteristics (nanozymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060–6093. doi: 10.1039/c3cs35486e. [DOI] [PubMed] [Google Scholar]
- 69.Tapeinos C, Pandit A. Physical, chemical, and biological structures based on ros-sensitive moieties that are able to respond to oxidative microenvironments. Advanced Materials. 2016;28:5553–5585. doi: 10.1002/adma.201505376. [DOI] [PubMed] [Google Scholar]
- 70.Huo MF, Wang LY, Chen Y, Shi JL. Tumor-selective catalytic nanomedicine by nanocatalyst delivery. Nat Commun. 2017;8:357. doi: 10.1038/s41467-017-00424-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zakharchenko A, Guz N, Laradji AM, Katz E, Minko S. Magnetic field remotely controlled selective biocatalysis. Nature Catalysis. 2018;1:73–81. [Google Scholar]
- 72.Flanagan SW, Moseley PL, Buettner GR. Increased flux of free radicals in cells subjected to hyperthermia: detection by electron paramagnetic resonance spin trapping. FEBS Lett. 1998;431:285–286. doi: 10.1016/s0014-5793(98)00779-0. [DOI] [PubMed] [Google Scholar]
- 73.Ludwig R, Teran FJ, Teichgraeber U, Hilger I. Nanoparticle-based hyperthermia distinctly impacts production of ROS, expression of Ki-67, TOP2A, and TPX2, and induction of apoptosis in pancreatic cancer. Int J Nanomedicine. 2017;12:1009–1018. doi: 10.2147/IJN.S108577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ghodbane S, Lahbib A, Sakly M, Abdelmelek H. Bioeffects of static magnetic fields: oxidative stress, genotoxic effects, and cancer studies. Biomed Res Int. 2013;2013:602987. doi: 10.1155/2013/602987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spyridopoulou K, Makridis A, Maniotis N, Karypidou N, Myrovali E, Samaras T, Angelakeris M, Chlichlia K, Kalogirou O. Effect of low frequency magnetic fields on the growth of MNP-treated HT29 colon cancer cells. Nanotechnology. 2018;29:175101. doi: 10.1088/1361-6528/aaaea9. [DOI] [PubMed] [Google Scholar]
- 76.Verdom BH, Abdolmaleki P, Behmanesh M. The static magnetic field remotely boosts the efficiency of doxorubicin through modulating ROS behaviors. Sci Rep. 2018;8:990. doi: 10.1038/s41598-018-19247-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Angeli JPF, Schneider M, Proneth B, Tyurina YY, Tyurin VA, Hammond VJ, Herbach N, Aichler M, Walch A, Eggenhofer E, Basavarajappa D, Radmark O, Kobayashi S, Seibt T, Beck H, Neff F, Esposito I, Wanke R, Forster H, Yefremova O, Heinrichmeyer M, Bornkamm GW, Geissler EK, Thomas SB, Stockwell BR, O’Donnell VB, Kagan VE, Schick JA, Conrad M. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16:1180–91. doi: 10.1038/ncb3064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, Brown LM, Girotti AW, Cornish VW, Schreiber SL, Stockwell BR. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Viswanathan VS, Ryan MJ, Dhruv HD, Gill S, Eichhoff OM, Seashore-Ludlow B, Kaffenberger SD, Eaton JK, Shimada K, Aguirre AJ, Viswanathan SR, Chattopadhyay S, Tamayo P, Yang WS, Rees MG, Chen SX, Boskovic ZV, Javaid S, Huang C, Wu XY, Tseng YY, Roider EM, Gao D, Cleary JM, Wolpin BM, Mesirov JP, Haber DA, Engelman JA, Boehm JS, Kotz JD, Hon CS, Chen Y, Hahn WC, Levesque MP, Doench JG, Berens ME, Shamji AF, Clemons PA, Stockwell BR, Schreiber SL. Dependency of a therapy-resistant state of cancer cells on a lipid peroxidase pathway. Nature. 2017;547:453–457. doi: 10.1038/nature23007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kerins MJ, Ooi A. The roles of NRF2 in modulating cellular iron homeostasis. Antioxid Redox Signal. 2017 doi: 10.1089/ars.2017.7176. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shimada K, Skouta R, Kaplan A, Yang WS, Hayano M, Dixon SJ, Brown LM, Valenzuela CA, Wolpaw AJ, Stockwell BR. Global survey of cell death mechanisms reveals metabolic regulation of ferroptosis. Nat Chem Biol. 2016;12:497–503. doi: 10.1038/nchembio.2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Torti SV, Torti FM. Iron and cancer: more ore to be mined. Nat Rev Cancer. 2013;13:342–355. doi: 10.1038/nrc3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hentze MW, Muckenthaler MU, Andrews NC. Balancing acts: molecular control of mammalian iron metabolism. Cell. 2004;117:285–297. doi: 10.1016/s0092-8674(04)00343-5. [DOI] [PubMed] [Google Scholar]
- 84.Zhang SP, Chen Y, Guo WL, Yuan L, Zhang DQ, Xu Y, Nemeth E, Ganz T, Liu SJ. Disordered hepcidin-ferroportin signaling promotes breast cancer growth. Cell Signal. 2014;26:2539–2550. doi: 10.1016/j.cellsig.2014.07.029. [DOI] [PubMed] [Google Scholar]


