Abstract
The current work studied the chemopreventive efficacy of orally administered chitosan coated solid-lipid nanoparticle (c-SLN) encapsulated aspirin (ASP), curcumin (CUR) and free sulforaphane (SFN), ACS-cSLN, in the LSL-Kras G12D/+; Pdx-1 Cre/+ transgenic mouse model of pancreatic ductal adenocarcinoma (PDAC). In vitro uptake study and intracellular localization of ODA-FITC labeled ASP and CUR c-SLNs were performed in Panc-1 and MIA PaCa-2 cells by fluorescence microscopy. LSL-Kras G12D/+; Pdx-1 Cre/+ transgenic mice (n = 30) were randomly divided into 5 groups. Treatment groups were orally gavaged with ACS c-SLNs in three doses: low (2 + 4.5 + 0.16 mg/kg), medium (20 + 45 + 1.6 mg/kg) and high (60 + 135 + 4.8 mg/kg), respectively. After 20 weeks of treatment, mice pancreas were harvested, stained with dye and scored according to various pancreatic intraepithelial neoplasms (PanIN) categories by an independent observer. In vitro, cellular uptake evaluated on Panc-1 and MIA PaCa-2 cells resulted in higher fluorescence intensities, indicating increased cellular uptake of ASP and CUR c-SLNs. For further evidence, the addition of lysoID (red fluorescence) demonstrated location and uptake of ASP and CUR c-SLNs into the lysosome. In vivo, treatment with ACS c-SLN for 20-weeks did not cause obvious adverse effects on growth and no statistically significant differences in body weight were observed between groups. However, the weight (mean ± SEM) of pancreas at the end of the study was higher in blank c-SLN group (223.6 ± 42.2 mg) compared to low (138.0 ± 26.0 mg; not significant [NS]), medium (145.0 ± 9.0 mg; NS), and high (133.8 ± 20.3 mg; NS) ACS c-SLN treated groups, demonstrating the efficacy of ACS c-SLN nanoformulations. The low, medium and high dose of ACS c-SLN combinations exhibited a reduction in tumor incidence (PanIN count) by 16.6% (P < 0.01), 66.8% (P < 0.01), and 83.4% (P < 0.01), respectively. These studies provide further proof for the use of an oral, low dose nanotechnology-based combinatorial regimen for the chemoprevention of PDAC.
Keywords: Chemoprevention, pancreatic ductal adenocarcinoma (PDAC), chitosan-solid lipid nanoparticles (c-SLNs), oncogenic KRas, transgenic mice
Introduction
Pancreatic cancer (PC) affects ~ 55,000 Americans each year resulting in ~ 44,000 deaths, 90% of which are a result of pancreatic ductal adenocarcinoma (PDAC) thus making it the fourth leading cause of cancer deaths in the US [1,2]. During 2012, a report from the Center for Disease Control and Prevention (CDC) stated, while cancer trends overall are in a state of decline in the US, the incidence and mortality from PDAC is increasing dramatically. Although the 5 year survival rate from this disease still remains at a dismal 8.5% [2] [https://www.cancer.org/cancer/pancreatic-cancer.html; https://seer.cancer.gov/statfacts/html/pancreas.html] despite over 20 years of research, there are signs of progress being reported particularly in less invasive surgery to resect neoplasms [3-5], novel drug combinations to improve survival [6-8], advances in radiation therapy [9], and the basic understanding of the fundamental genetics of PDAC [10-12]. Alternative strategies against PDAC include chemoprevention which aims to prevent the development or recurrence of precancerous lesions with the use of natural or synthetic agents that reverse, suppress, delay, or prevent carcinogenic progression to invasive disease [13]. Additionally, combination therapy with two or more chemopreventive agents appears to be a viable strategy allowing maximum efficacy at low drug concentrations [14-16].
Our group has been interested in the use of novel chemopreventive agents and their combinations towards PDAC prevention. We were the first group to report through our previous studies and publications that aspirin (ASP), curcumin (CUR) and sulforaphane (SFN) when combined together (ACS) elicit synergistic chemoprevention efficacy in PDAC [16-19].
To understand the mechanism by which ACS combination elicits the PDAC chemoprevention efficacy, a battery of in-vitro studies were performed on pancreatic cancer cells, MIA PaCa-2 and Panc-1. These studies included cell viability assay (MTS Assay), cell colony formation assay, flow cytometric analysis for apoptosis, NF-κB activation assay. and western blot analysis and the results were reported in our earlier publication [16]. In summary, the results indicated that the cell viability reduced significantly to ~ 70% (P < 0.001) with induction of cell apoptosis by ~ 51% (P < 0.001) when the cell lines were treated with low dose combination of ACS (ASP 1 mM, CUR 10 μM, and SFN 5 μM respectively). Further, it was also observed that the ACS induced cell death and apoptosis was accompanied with activation of caspase-3 and poly (ADP-ribose) polymerase (PARP) proteins and inhibition of the NF-κB DNA binding activity in both the cell lines. Mechanistically, the ACS combination was also observed to uniquely enhance the expression of phospho-extracellular signal-regulated kinase 1/2 (P-ERK1/2), c-Jun, p38 MAPK, and p53 proteins. Therefore it was concluded that the ACS combination elicits the chemoprevention efficacy by means of cell apoptosis induction and activation of ERK1/2 signaling pathway [16].
Over the past decade, we have also demonstrated the importance of delivering these agents using novel nanotechnology-based systems wherein high efficacy has been achieved at extremely low doses [17,18,20]. The method of drug delivery plays an important role in ensuring that drug reaches its target site to impose therapeutic effect. Novel modes of delivery methods using solid dispersions, microsphere and nanosphere technology have received wide attention as these have shown superior delivery efficiency and specificity compared to conventional dosage forms [21-25]. In this study, we plan to use a modified version of a novel nanotechnology-based drug delivery platform, previously developed and investigated in our laboratory [18]. Our initial studies demonstrated a significant decrease in dose, with comparable efficacy versus unmodified free drug combinations, when administered using solid lipid nanoparticles (SLNs) on pancreatic cancer cells. More recently, we reported the results of a 24-week chemopreventive study with the oral co-administration of the ACS combination regimen on the N-nitrosobis (2-oxopropyl) amine (BOP)-treated Syrian golden hamster model to suppress the progression of pancreatic intraepithelial neoplasms (PanIN) using unmodified (free drug) combinations of ACS, and nano-encapsulated (SLN) combinations of ACS [19,26]. Although SLN-based delivery systems have been available for many years [26,27], we are the first group to demonstrate its usage and efficacy in PDAC chemoprevention [17,18]. For the current study, we are introducing a “second generation” SLN delivery system incorporating chitosan, a biodegradable polysaccharide. Chitosan is a nontoxic and biocompatible polysaccharide derived from the shells of crustaceans, with proven in-vivo safety profiles [28]. Chitosan-SLN (c-SLN) is an effective hybrid nano-system by combining chitosan-based drug delivery systems with SLNs cancer chemoprevention. Chitosan-based nanoparticles exhibit a mucoadhesive feature because of their positive charge, thereby capable of prolonging their residence time in the negatively charged small intestine and allowing increased drug concentration at the site of absorption [29]. Moreover, chitosan can mediate the opening of tight junctions between epithelial cells reversibly, facilitating the paracellular transport of drug molecules, ultimately leading to improved bioavailability of the drugs [30]. c-SLNs are biodegradable, bioadhesive, have permeation enhancing properties and are easily scaled up for mass manufacture thus acting as promising vehicles for oral drug delivery with a wide range of pharmaceutical applications [31]. In summary, c-SLN combines the advantages of SLN with the biological properties of chitosan as an improved drug delivery vehicle for chemoprevention. Successful development of this delivery system will result in a paradigm shift in the area of chemoprevention. In our earlier studies, we have successfully developed and reported c-SLNs of ASP (size: 430 ± 51.1 nm, entrapment efficiency: 65 ± 4.06%) and c-SLNs of CUR (size: 440 ± 65.9 nm, entrapment efficiency: 72 ± 3.98%). Further, to ensure the safety of the proposed drug combinations, we performed an in-vivo acute (7-days), sub-acute (28-days), and sub-chronic (90-days) toxicity studies with the combination of ASP c-SLNs and CUR c-SLNs with free SFN. The studies did not show any signs of toxicity upon oral administration and hence were proven to be safe for long term use as proposed in case of PDAC chemoprevention [19].
Based on our extensive research on ACS combination for PDAC chemoprevention of over 5 years and the promising preliminary results obtained thus far, our next objective and the premise for these studies was to use nano-encapsulated c-SLN combinations of ASP and CUR mixed with free SFN to determine the efficacy of this therapeutic regimen on the LSL-Kras G12D/+; Pdx-1 Cre/+ transgenic mouse model of PDAC.
Materials and methods
Chemicals and reagents
ASP and CUR were purchased from Sigma-Aldrich (St. Louis, MO). Stearic acid, Poloxamer 188, and Chitosan were obtained from Spectrum Chemicals (Gardena, CA). Ethanol and Glacial Acetic Acid were obtained from Fisher Scientific (Houston, TX). Potassium Chloride, Potassium Phosphate Monobasic, and Tris Base were purchased from BDH Chemicals (Radnor, PA). Sodium Phosphate Dibasic was purchased from EMD Millipore (Billerica, MA). Sodium Chloride, Tween-20, and SFN were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Fluorescein isothiocyanate (FITC) and octadecylamine (ODA) were purchased from Sigma Aldrich USA (St. Louis, MO).
Chitosan-coated solid lipid nanoparticle (c-SLN) preparation
The following formulations were reproduced using an optimized formulation method as reported earlier [19]: (F1) Blank c-SLN; (F2) ASP c-SLN only; (F3) CUR c-SLN only; and (F4) free unmodified SFN only; and (F5) mixture of ASP and CUR c-SLNs with free SFN. F2 and F3 formulations were prepared in bulk amounts whereas F5 was prepared by blending known quantities of nanoparticles from F2, F3, and free-SFN. Blank nanoparticle formulations were prepared using stearic acid without any drugs, which served as the vehicle control. Briefly, stearic acid (1 g) was melted by heating at 70°C. The drug (250 mg) was suspended in ethanol (5 ml). The suspended drug solution was then added to the melted stearic acid. The poloxamer solution (50 ml, 2%, water phase) was heated to the same temperature as that of the oil phase and Oil/water emulsion was prepared using the modified hot homogenization technique (Figure 1). For this, the oil phase was then added to the water phase drop-wise under continuous high-speed (25,000 rpm) homogenization (Tissue Master 125, Omni International, Kennesaw, GA). The pre-emulsion was further sonicated for 30 s using an ultra-sonicator (Branson Sonifier 450, Los Angeles, CA). The resulting emulsion was passed through a high-pressure homogenizer (Nano DeBEE, BEE International, South Easton, MA) for 5 cycles, and kept on a stirrer for 2 h for solvent evaporation. The emulsion was then mixed with an equal volume of chitosan (0.1%) dissolved in glacial acetic acid (0.1%). The mixture of SLNs with chitosan was stirred for 2 h. The resulting c-SLNs were ultra-centrifuged and the pellet was collected. For the present study, mannitol was added as a cryoprotectant in the ratio of 1:1 (nanoparticles:cryoprotectant). For this, aqueous solution of mannitol (5 ml) was added to the pellet. The above solution was mixed well and frozen in a deep freezer at -80°C. The samples were then lyophilized in a freeze dryer (FreeZone 4.5 Plus, Labconco, Kansas City, MO) and coated with Eudragit L 100-55 dispersion (30%).
Figure 1.
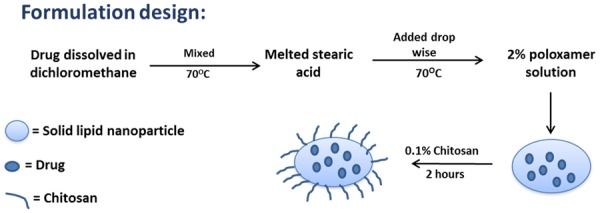
Schematic representation of the formulation design.
Preparation of ODA-FITC
In this study, the chemical conjugate of ODA-FITC was synthesized to incorporate into c-SLN for evaluating cellular uptake of c-SLN. The synthesis of ODA-FITC involves the reaction between amino group of ODA and isothiocyanate group of FITC. For the preparation, ODA (7 mg) was dissolved in N,N-dimethylformamide (DMF, 60 ml). The mixture was sonicated until the ODA was dissolved completely (Aqua Sonic® 75HT, VWR). FITC (20 mg) was dissolved in DMF (5 ml). The FITC and ODA solution was then mixed by shaking in a water bath (50°C) for 48 h. After the reaction, the mixture was cooled to room temperature, and then distilled water (20 ml) was added to the mixture to precipitate the ODA-FITC. The precipitate was collected by filtration using a Millipore filter (0.45 μm) and the precipitate washed twice with water (20 ml). The final product formed (ODA-FITC) was lyophilized and stored.
Preparation of ODA-FITC c-SLNs
Briefly, stearic acid (60 mg) and ODA-FITC (4.8 mg) were dissolved in ethanol (6 ml) by sonication in a water bath. The resultant organic solution was injected into of 70°C aqueous phase (60 ml) containing poloxamer (2%) under high sheer homogenization for 5 min. The mixture was then cooled to room temperature to obtain ODA-FITC labeled c-SLN dispersion. The dispersion formed was kept for continuous stirring (dark condition) for 2 h to precipitate c-SLN, and the precipitated c-SLN was then collected by centrifugation (@10,000 rpm, 10 min) and lyophilized.
Cellular uptake of fluorescent labeled ODA-FITC c-SLNs
The cellular uptake tests of the ASP and CUR loaded c-SLNs were performed in Panc-1 and MIA PaCa-2 cells by fluorescence microscope. Briefly, 1 × 105 cells were placed in each well of 8-well chamber slides and grown for 24 h in DMEM medium (200 µl) supplemented with FBS (10%), penicillin and streptomycin (1%) at 37°C in 5% CO2. Free ASP/CUR or ASP/CUR loaded fluorescent c-SLNs were added at a concentration of 20 µM each to the predesigned chamber slides and incubated for 30 min, 2 h and 24 h, respectively. After incubation, at each time point the growth media was removed, and the cells were washed three times with PBS for 5 min. The cells were fixed with formaldehyde (4%) and then washed 3X with PBS to remove excess formaldehyde and the dried slide is stained with mounting medium containing DAPI. Fluorescence microscope was used to observe the cellular uptake of ASP/CUR c-SLNs.
Intracellular localization of fluorescent c-SLNs
The intracellular localization of FITC (green) labeled ASP and CUR c-SLNs were performed in Panc-1 and MIA PaCa-2 cells by fluorescence microscope. Cells were cultured as described above. Free ASP/CUR or ASP/CUR loaded fluorescent c-SLNs were added at a concentration of 20 µM each to the predesigned chamber slides and incubated for 30 min, 2 h and 24 h, respectively. LysoID (2 μl, marker for lysosome labeling, red) was added to each chamber at 30 min prior to the end of each time point, and incubated for additional 30 min. Growth media was then removed, and the cells were washed (3X) with PBS for 5 min. The cells were fixed with formaldehyde (4%) and then washed (3X) with PBS to remove excess formaldehyde and the dried slide was stained with mounting medium containing DAPI (blue). Fluorescence microscope was used to detect the co-localization of lysosome and the labeled ASP/CUR nanoparticles.
Breeding and genotyping analysis
Breeder pairs of LSL-Kras G12D/+ and Pdx-1 Cre/+ mice in the C57BL/6 background were obtained from the NCI mouse repository. Animals were housed in ventilated cages under standardized conditions (24°C, 60% humidity, 12 h light/12 h dark cycle, 20 air changes/h). LSL-Kras G12D/+ and Pdx-1 Cre/+ mice were cross bred and the offspring of LSL-Kras G12D/+; Pdx-1 Cre/+ were generated. The genotype of each pup was confirmed by tail DNA extraction and polymerase chain reaction (PCR) as described. Briefly, genomic DNA was extracted from tail tissue samples using the mini-prep kit (Invitrogen). PCR was performed for Kras and Cre genes using the following conditions: denaturation at 95°C for 5 min, followed by 35 cycles at 95°C for 1 min, 60°C for 1 min and 72°C for 1 min. Oligonucleotide primer sequences used were as follows: For genotyping Cre (PCR product size 650-base pairs (bp)): Pdx-F: 5’-CTGGACTACATCTTGAGTTGC-3’; Pdx-R: 5’-GGTGTACGGTCAGTAAATTTG-3’. This strain contains the Pxd-1 promoter element controlling expression of Cre recombinase in the pancreas. The strategy of this genotyping method is to anchor the sense primer in the Pdx promoter, and the antisense primer in the Cre gene. This will produce a more specific PCR product than just assaying for Cre with generic Cre primers.
For genotyping Kras: Kras-y117: 5’-CTAGCCACCATGGCTTGAGT-3’; Kras-y118: 5’-ATGTCTTTCCCCAGCACAGT-3’; Kras-y1116: TCCGAATTCAGTGACTACAGATG-3’. For Kras wildtype, y117/y118 primer pairs generated a PCR product of 450-bp. For Kras mutant (LOX), y117/y116 primer pairs generated a PCR product of 327-bp.
In vivo chemopreventive efficacy studies
The study was conducted using LSL-Kras G12D/+; Pdx-1 Cre/+ mice with an average body weight of ~ 20 g, and all protocols were approved by the Western University of Health Sciences Institutional Animal Care and Use Committee and conformed to the “Principles of Laboratory Animal Care”. Mice were observed daily for any signs of illness and weighed weekly throughout the experimental period. Six-week old transgenic mice were randomly divided into 5 groups (3 male + 3 female/group; total 30) and the treatment plan is summarized in Table 1. Group 1 (G1) served as a saline control. Group 2 (G2; c-SLN vehicle control) served as overall control group. The latter control group received blank c-SLNs (drug free) to ensure that the nanoparticle contents do not interfere with the end point analysis. The remaining groups received three different doses [low (G3), medium (G4), and high (G5)] of ACS cSLNs chemopreventive combination regimen daily via oral gavage. Therapy was continued every 24 h for the duration of 20 weeks. The highest dose of nano-ASP, nano-CUR and SFN used for these experiments were considerably less than published literature based on animal studies of free ASP, CUR and SFN. Previous studies conducted in our laboratory demonstrated that the nanoparticle-encapsulated drugs could be dosed at 1/10 of free drugs with comparable efficacy [17]. The highest dose selected for ASP, CUR and SFN regimen was 60, 135 and 4.8 mg/kg, respectively, which was determined based on current evidence in literature. The medium and low doses were calculated as 1/3 and 1/30 of the high dose of chemopreventive agents, respectively. The pancreas, brain, heart, kidney, and liver were harvested and weighed. The pancreas was washed with saline and prepared for histopathological evaluation to identify PanIN lesions.
Table 1.
Treatment plan for the ACS c-SLNs in vivo chemoprevention study in the LSL-KrasG12D/+; Pdx-1Cre/+ PDAC mouse model
| Group (G) (3 Male + 3 Females/Group) | Treatment Plan (Oral Gavage) | Dose mg/kg (ASP + CUR + SFN) | |
|---|---|---|---|
| G1 | Saline control | Saline | 0 |
| G2 | c-SLN vehicle control | Blank c-SLNs (drug free) | 0 |
| G3 | Low dose of ACS cSLNs | ASP c-SLNs + CUR c-SLNs + SFN | 2 + 4.5 + 0.16 |
| G4 | Medium dose of ACS cSLNs | ASP c-SLNs + CUR c-SLNs + SFN | 20 + 45 + 1.6 |
| G5 | High dose of ACS cSLNs | ASP c-SLNs + CUR c-SLNs + SFN | 60 + 135 + 4.8 |
Histological examination
All organs of the thoracic and abdominal cavities were carefully examined in situ macroscopically. The pancreas were fixed in phosphate-buffered formalin (10%) for 24 h. The formalin-fixed pancreas were processed for routine histology and 5-µm thick sections were cut and stained with H&E. An independent consultant expert blinded to samples evaluated the sections of the pancreas and scored them according to PanIN criteria within the following categories: PanIN1, PanIN2, PanIN3, and carcinoma [32]. Tumor incidence (percentage of mice with early signs of PanINs) was calculated based on these scores.
Statistical analysis
The data were expressed as mean ± SEM. Differences between treatment and blank c-SLN treated vehicle control were analyzed by ANOVA followed by Dunnett’s multiple comparison test using Graph pad prism software. P ≤ 0.05 was considered statistically significant.
Results
The c-SLN formulations were successfully reproduced and exhibited similar physicochemical characteristics as reported earlier [19] which were investigated for further studies as follows.
Cellular uptake of fluorescent c-SLNs
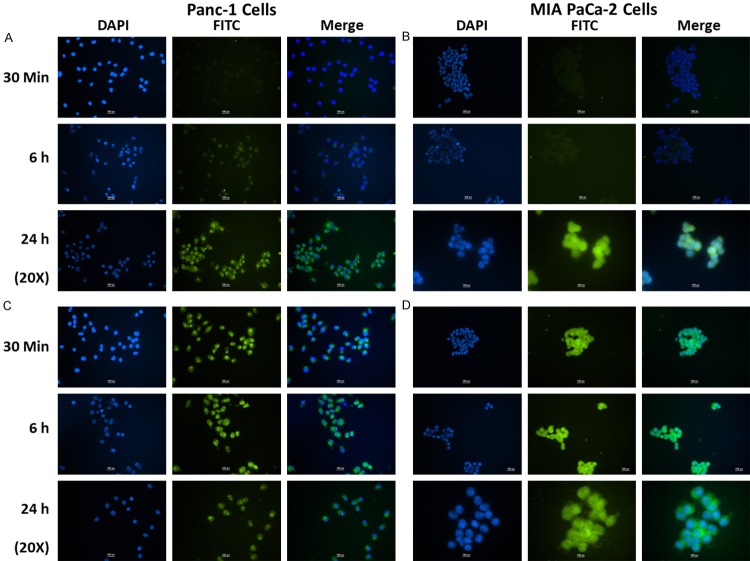
Cellular uptake of ASP c-SLN and CUR c-SLNs into Panc-1 and MIA Paca-2 cells were studied using fluorescence microscopy. Panc-1 and MIA PaCa-2 cancer cells were exposed to ASP and CUR loaded fluorescence c-SLNs for 30 min, 6 h, and 24 h, respectively. Upon FITC labeled c-SLN incubation, Panc1 and MIA PaCa-2 cells showed a green fluorescence caused by FITC around the nucleus within the cytoplasm. Figure 2A and 2B showed that the cellular fluorescent intensities accumulated from 30 min to 24 h in ASP c-SLN loaded nanoparticle-treated groups, suggesting that particle uptake of c-SLN was a time-dependent process. With FITC loaded ASP c-SLNs, the fluorescence intensity increased after 6 h and it remained almost steady even at 24 h, suggesting the sustained intracellular release and retention of encapsulated ASP inside the cells. Figure 2C and 2D showed FITC loaded CUR c-SLNs in Panc-1 and MIA PaCa-2 cells, respectively. The green fluorescence inside the cells was already marked after 30 min of incubation, and it remained almost steady even at 24 h, suggesting sustained intracellular uptake and release of CUR. Overall, we conclude that the cellular uptake of ASP and CUR in chitosan-coated nanoparticles accumulated over a 24 h period.
Figure 2.
Immunocytochemical analysis of cellular uptake of (A) FITC labeled aspirin (ASP) c-SLN in Panc-1 cells, (B) FITC labeled ASP c-SLN in MIA PaCa-2 cells, (C) FITC labeled curcumin (CUR) c-SLN in Panc-1 cells, and (D) FITC labeled CUR c-SLN in MIA PaCa-2 cells at 30 min, 6 h, and 24 h. DAPI (blue color) shows nuclear staining, and FITC labeled ASP and CUR c-SLNs (green color) shows uptake of ASP and CUR c-SLN inside the cells (Scale bar - 100 px (1.17 μm/px)). [http://www.techinst.com/userfiles/files/How%20To%20Calibrate%200020.pdf].
Intracellular co-localization study of FITC loaded c-SLN with lysoID
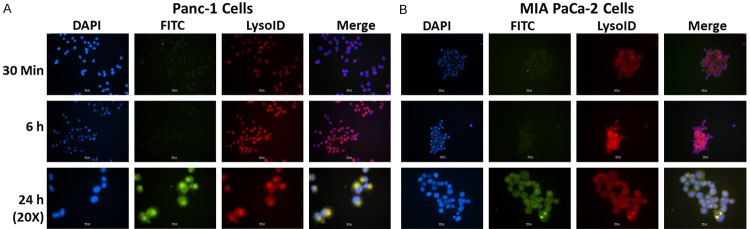
Co-localization study of FITC labeled ASP and CUR c-SLNs with lysosome was evaluated by fluorescent microscopy by incubating FITC labeled c-SLNs (Green color) with lysosomal marker lysoID (Red color). Figure 3A and 3B showed co-localization of red fluorescence from lysoID with green fluorescence from FITC labeled ASP c-SLNs in Panc-1 and MIA PaCa-2 cells, respectively. The co-localization was clearly evidenced by yellow color fluorescence (Green and red color overlay) in merged image. The green fluorescence inside the cells was increased after 6 h of incubation and showed clear evidence of co-localization in lysosomes at 24 h.
Figure 3.
Immunocytochemical analysis of intracellular localization of FITC labeled aspirin (ASP) c-SLN in (A) Panc-1 cells, and (B) MIA PaCa-2 cells at 30 min, 6 h, and 24 h. DAPI (blue color) shows nuclear staining; FITC labeled ASP c-SLN (green color) shows uptake of ASP c-SLN; and LysoID (marker for lysosome labeling, red color) shows lysosomes in the cytoplasm; Co-localization (yellow color) of red fluorescence from LysoID with green fluorescence from c-SLNs suggests that ASP c-SLNs accumulate in lysosomes (Scale bar - 100 px (1.17 µm/px)). [http://www.techinst.com/userfiles/files/How%20To%20Calibrate%200020.pdf].
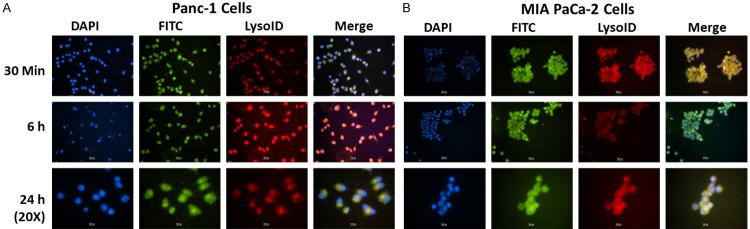
Figure 4A and 4B showed co-localization of FITC labeled CUR c-SLNs in Panc-1 and MIA PaCa-2 cells, respectively. The merged images of incubation of CUR c-SLNs with lysoID revealed enhanced co-localization after 30 min and remained steady even at 24 h. Figures 3 and 4 confirmed an increased accumulation of ASP and CUR c-SLNs in the lysosomes.
Figure 4.
Immunocytochemical analysis of intracellular localization of FITC labeled curcumin (CUR) c-SLN in (A) Panc-1 cells, and (B) MIA PaCa-2 cells at 30 min, 6 h, and 24 h. DAPI (blue color) shows nuclear staining; FITC labelled CUR c-SLN (green color) shows uptake of CUR c-SLN; and LysoID shows lysosomes in the cytoplasm; Co-localization (yellow color) of red fluorescence from LysoID with green fluorescence from c-SLNs suggest that CUR c-SLN accumulate in lysosomes (Scale bar - 100 px (1.17 µm/px)). [http://www.techinst.com/userfiles/files/How%20To%20Calibrate%200020.pdf].
In vivo chemoprevention study in LSL-KrasG12D/+; Pdx-1Cre/+ PDAC model
No sign of weight loss was observed in the mice during the 20-week study period. Indeed, the body weight transition curves of all the groups showed a steady gain from start to the end of the study period (Figure 5A). Notably, treatment with ACS c-SLN for 20-weeks did not cause any adverse effects on growth. Additionally, no statistical difference was found between the control group and ACS combination treated groups as determined by one-way ANOVA followed by Dunnett’s multiple comparison test post hoc analysis during the 20-week study (Figure 5B).
Figure 5.
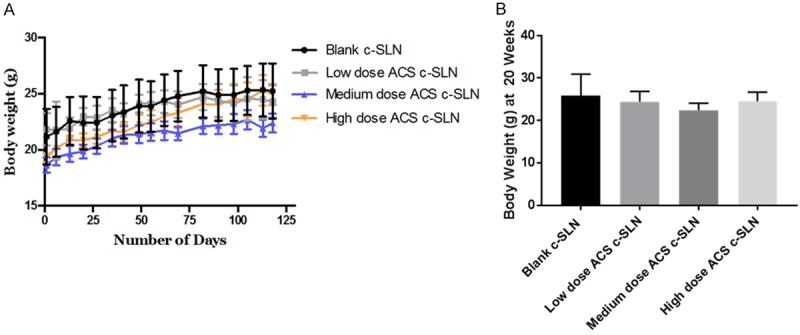
Effect of ACS c-SLN combination treatment on body weight, pancreatic organ weight, and tumor incidence. A. Body weight changes of LSL-Kras G12D/+; Pdx-1 Cre/+ transgenic mice during 20 weeks of ACS c-SLN treatment. B. Body weight changes of LSL-Kras G12D/+; Pdx-1 Cre/+ transgenic mice at the end of 20 weeks of ACS c-SLN treatment.
The average weight (mg) (mean ± SEM) of pancreas at the end of the study was higher in blank c-SLN group (223.6 mg ± 42.2; Not significant [NS]) compared to low (138.0 mg ± 26.0; NS), medium (145.0 mg ± 9.0; NS), and high (133.8 mg ± 20.3; NS) ACS c-SLN treatment groups, respectively, suggesting PDAC tumor progression in blank c-SLN group. The statistical difference between blank c-SLN group and ACS combination-treated groups was determined by one-way ANOVA followed by Dunnett’s multiple comparison test post hoc analysis (Figure 6A).
Figure 6.
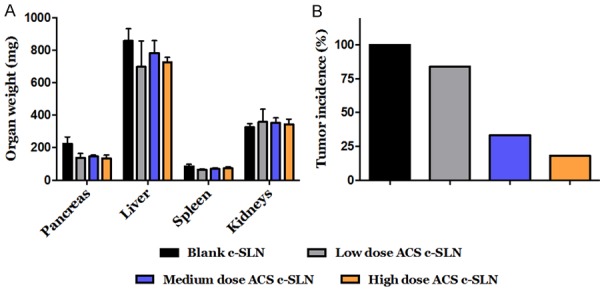
Effect of ACS c-SLN combination treatment on body weight, pancreatic organ weight, and tumor incidence. A. Average organ weight at the end of ACS c-SLN treatment. B. Effect of ACS c-SLN combination treatment on pancreatic tumor incidence. Tumor incidence is determined by percentage of mice with early signs of PanINs. The statistical significance was determined by one-way ANOVA followed by Dunnett’s multiple comparison test. Data are shown as Mean ± SEM.
ACS c-SLN regimen significantly reduces tumor incidence in LSL-KrasG12D/+; Pdx-1Cre/+ mice
The chemopreventive efficacy of the ACS c-SLN combination regimen was evaluated based on tumor incidence. The histological analysis determined that (Figure 6B) the blank c-SLN treated group (G2) exhibited tumor incidence of 100% (percentage of mice with early signs of PanINs); whereas the low dose (G3) treatment group had an incidence of 83.4% (P > 0.05; NS); the medium (G4) and high dose (G5) ACS groups showed significantly low incidence of 33.2% (60.2% reduction; P < 0.05) and 16.6% (80.1% reduction; P < 0.05) respectively, as determined by histological analysis. These results show that the medium and high doses of ACS combination regimens were effective in reducing cancer incidence when compared to the blank c-SLN vehicle control group.
ACS c-SLN regimen delays the progression of PanINs into adenocarcinoma in LSL-KrasG12D/+/Pdx-1Cre/+ mice model
The isolated pancreatic tissues were subjected to H&E staining and different grades of PanINs were counted upon histological examination based on an established classification system for pancreatic duct lesions. PanIN1s are flat epithelial lesions composed of tall columnar cells with basally located nuclei or a papillary pseudo-stratified architecture. Further progression of PanIN1s results in the formation of PanIN2s, flat or papillary mucinous epithelial lesions with nuclear abnormalities, which may include some loss of polarity, nuclear crowding, and enlarged nuclei. Finally, PanIN3s, resulting from PanIN2 lesions are identified as papillary or micro-papillary structures characterized with high-grade dysplasia and loss of nuclear polarity, indicating development of PC. Based on the above criteria and upon histological examination of H&E stained pancreatic tissues, PanINs were enumerated in the pancreatic tissues in different treatment groups (Figure 7A-D).
Figure 7.
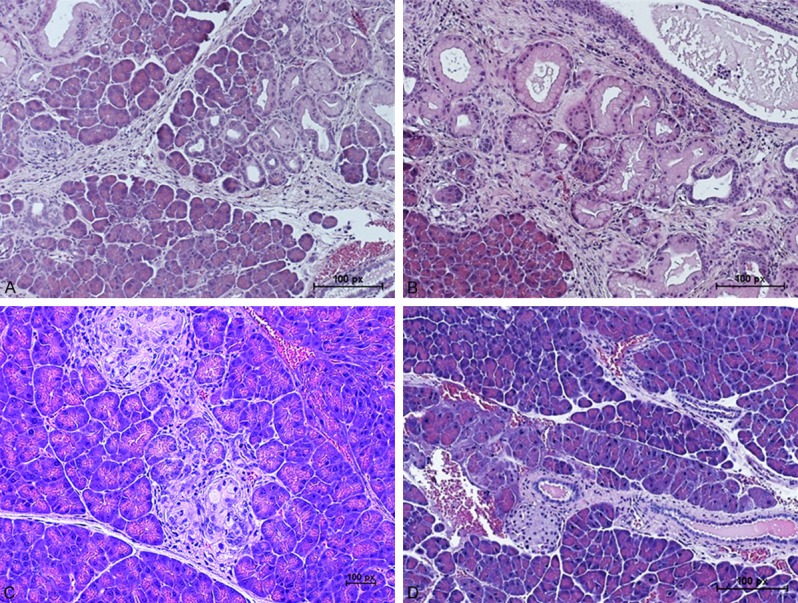
Hematoxylin and eosin (H&E) staining of pancreatic tissues from ACS c-SLN treated LSL-Kras G12D/+; Pdx-1 Cre/+ transgenic mice (A) Blank c-SLN vehicle control group, (B) Low dose ACS c-SLN treated group, (C) Medium dose ACS c-SLN treated group, and (D) High dose ACS c-SLN treated group. (Scale bar - 100 px (1.17 µm/px)). [http://www.techinst.com/userfiles/files/How%20To%20Calibrate%200020.pdf].
The blank c-SLN vehicle control group showed intralobular ducts lined with high cuboidal to low columnar and mostly basal nuclei of either flatted or hyperatrophied in shape. Large amount of mucus granules filled the apical portion of these cells (Figure 7A). The low and medium ACS c-SLN treated tissues showed few scattered proliferating intralobular ducts and pyramidal to low cuboidal epithelium lining the ducts starting to invade the exocrine portion of the pancreas. Nuclei were large rounded to flattened and basally located. The interlobular ducts were very organized with regular type of epithelium. No lymphocytic infiltration outside or inside the pancreas was seen (Figure 7B and 7C). The high dose ACS c-SLN treated tissues look normal histologically for both endocrine and exocrine portions with the exception of congestion of blood vessels. Aggregates of lymphocytes, macrophages and plasma cells were detected at the capsule among and inside the adipose tissue around the visceral surface of the pancreas. All ducts appeared normal (Figure 7D).
Discussion
Pancreatic ductal adenocarcinoma (PDAC) affects over 40,000 Americans each year. It is expected to increase by more than 55% over next 20 years to become the second leading cause of cancer deaths after lung cancer [33]. The survival rate still remains at a dismal 8% despite decades of research [34-36], bringing to the forefront the unmet need to find alternative means to fight against PDAC. An alternative strategy that has emerged in recent years is the chemoprevention aiming to prevent the development or recurrence of precancerous lesions known as pancreatic intraepithelial neoplasms (PanINs) with the use of natural or synthetic agents that reverse, suppress, delay, or prevent carcinogenic progression to invasive disease [13]. Additionally, combination therapy with two or more chemopreventive agents is a viable strategy, allowing maximum efficacy at low drug concentrations [14,15]. A recent report from the National Cancer Institute (NCI) highlighted the urgent need for developing chemopreventive strategies to reduce the significant mortality and morbidity resulting from this disease [37].
Our group has been actively studying the novel chemopreventive agents and their combinations for PDAC prevention. Recently, we demonstrated the use of a combination of solid lipid nanoparticle (SLN)-based delivery system to deliver aspirin (A) and curcumin (C) with free sulforaphane (S) to achieve high efficacy at extremely low doses [16-18,20]. Nanoparticle-based drug delivery has emerged as a promising therapeutic or chemopreventive approach [38-40]. Using these novel combined ACS SLN regimens, we previously reported the successful results of a 24-week chemopreventive study with the oral administration of the ACS combination regimen on the N-nitrosobis (2-oxopropyl) amine (BOP)-treated Syrian golden hamster model to suppress the progression of PanINs using unmodified (free drug) and nano-encapsulated combinations of ACS. High dose ACS SLN in BOP carcinogen induced PC in hamster reduced tumor incidence by 75% when using 10X lower doses of nano-encapsulated regimens of ACS versus 68.7% reduction in unmodified combinations. Also, tumor apoptotic indices increased with our modified regimens, compared with the BOP-treated vehicle control. With our 2nd generation of c-SLN formulation in LSL-Kras G12D/+; Pdx-1 Cre/+ mice, the low, medium and high dose of ACS c-SLN combinations exhibited a reduction in tumor incidence by 16.6% (P > 0.05), 33.2% (P < 0.01), and 83.4% (P < 0.01), respectively.
Aspirin, a non-steroidal anti-inflammatory drug (NSAID) is one of the best-studied chemopreventive agents for many cancers, including PC and has been demonstrated to modulate the NF-κB inflammatory pathway [16,41-44]. Reduced risk of PC has been demonstrated for both low dose (81-100 mg) and regular dose (325 mg) aspirin based on increasing timespan of use [43,44]. Our previous studies conducted demonstrated chemopreventive efficacy of aspirin SLN regimens at much lower doses (18.2 mg and 63.7 mg) when combined with curcumin and sulforaphane [17]. Curcumin, the active ingredient in turmeric, has recently received considerable attention due to its pronounced anti-inflammatory, anti-oxidative and anti-carcinogenic activities [45-50]. However, in humans, orally administered curcumin is poorly absorbed, rapidly metabolized and eliminated, limiting its therapeutic potential. For PC treatment, combining curcumin with anticancer drugs, such as gemcitabine, appear to be safe and well tolerated [51,52]. In addition, registered phase I/II clinical trials to investigate the effectiveness of curcumin alone or with first-line treatment in patients with breast, prostate, pancreatic, lung, or colorectal cancer are ongoing.
Results from previous studies showed that the uptake of SLNs from the intestinal lymphatic system bypassed first pass metabolism in the liver, thus increasing circulation time, reducing dosage and ensuring high bioavailability of the drugs [53-56]. It has been demonstrated that SLNs are ingested as intact particles into the lymph after duodenal administration to fed rats [57,58] to avoid the degradation of the lipid nanoparticles (exposing the free drug) in lymphatic system [54,59]. The oral delivery of pancreas-targeted nanoparticles remains unpredictable, as the pancreas is a difficult organ to reach via the oral route. The premise of this study is to use combinations of chemopreventive agents (ACS) encapsulated in chitosan coated SLNs for the synergistic chemoprevention of PDAC. Incorporating chitosan, a biodegradable polysaccharide, will optimize the formulation parameters and efficacy of the SLN delivery system. Chitosan is a nontoxic and biocompatible polysaccharide derived from the shells of crustaceans, with proven in vivo safety profiles [28]. Combined chitosan-based drug delivery systems [23] with SLNs provide us with an effective hybrid nano-system (chitosan-SLN or c-SLN) for cancer chemoprevention. Chitosan-based nanoparticles exhibit a mucoadhesive feature because of their positive charge, prolonging their residence time in the negatively charged small intestine [29], increasing the drug concentration at the site of absorption. Moreover, chitosan can mediate the opening of tight junctions between epithelial cells reversibly, facilitating the paracellular transport of drug molecules ultimately leading to improved bioavailability of the drugs [30]. c-SLNs have permeation enhancing properties and are easily scaled up for mass manufacture thus acting as promising vehicles for oral drug delivery with a wide range of pharmaceutical applications [31,60,61]. Drug-loaded c-SLNs will be coated with Eudragit L-100 polymer to avoid drug release in the stomach [62]; instead, dissolving in the small intestine and releasing c-SLNs in the duodenal region, which would subsequently be taken into the lymphatic circulation.
Histopathological findings support the study hypothesis as we observed normal histo-architecture of both exocrine and endocrine portions of the pancreatic tissue in high dose treated group. Congestions of the blood vessels is a consequence of method of scarifying the experimental animals. Only in high dose group, inflammatory cells were observed within the adipose and peri-pancreatic tissue. Inflammatory cells were reported in the peri-pancreatic tissue in patients with different grades of PanIN linked to poor survival rates in non-neoplastic pancreatic tissue [63].
In conclusion, the combined release of ACS from c-SLNs will ensure a sustained, sub-toxic presence of these chemopreventive agents to allow significant chemopreventive effect. Due to the enhanced bioavailability, the dosage of these chemopreventive agents are significantly reduced, thus immediately reducing the potential for severe side effects. Additionally, our results provided strong proof-of-concept of the potential of chemoprevention using ACS c-SLN therapy regimens to suppress or delay the progression of PDAC.
Acknowledgements
This work was supported by National Institutes of Health R15 grant (1R15CA182834-01A1; SP).
Disclosure of conflict of interest
None.
References
- 1.Desai P, Ann D, Wang J, Prabhu S. Pancreatic cancer: recent advances in nano-formulation based therapies. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2018 [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 3.Huebner M, Kendrick M, Reid-Lombardo KM, Que F, Therneau T, Qin R, Donohue J, Nagorney D, Farnell M, Sarr M. Number of lymph nodes evaluated: prognostic value in pancreatic adenocarcinoma. J Gastrointest Surg. 2012;16:920–926. doi: 10.1007/s11605-012-1853-2. [DOI] [PubMed] [Google Scholar]
- 4.Venkat R, Edil BH, Schulick RD, Lidor AO, Makary MA, Wolfgang CL. Laparoscopic distal pancreatectomy is associated with significantly less overall morbidity compared to the open technique: a systematic review and meta-analysis. Ann Surg. 2012;255:1048–1059. doi: 10.1097/SLA.0b013e318251ee09. [DOI] [PubMed] [Google Scholar]
- 5.Wolfgang CL, Herman JM, Laheru DA, Klein AP, Erdek MA, Fishman EK, Hruban RH. Recent progress in pancreatic cancer. CA Cancer J Clin. 2013;63:318–348. doi: 10.3322/caac.21190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Paulson AS, Tran Cao HS, Tempero MA, Lowy AM. Therapeutic advances in pancreatic cancer. Gastroenterology. 2013;144:1316–1326. doi: 10.1053/j.gastro.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 7.Picozzi VJ, Abrams RA, Decker PA, Traverso W, O’Reilly EM, Greeno E, Martin RC, Wilfong LS, Rothenberg ML, Posner MC, Pisters PW American College of Surgeons Oncology Group. Multicenter phase II trial of adjuvant therapy for resected pancreatic cancer using cisplatin, 5-fluorouracil, and interferon-alfa-2b-based chemoradiation: ACOSOG Trial Z05031. Ann Oncol. 2011;22:348–354. doi: 10.1093/annonc/mdq384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schmidt J, Abel U, Debus J, Harig S, Hoffmann K, Herrmann T, Bartsch D, Klein J, Mansmann U, Jager D, Capussotti L, Kunz R, Buchler MW. Open-label, multicenter, randomized phase III trial of adjuvant chemoradiation plus interferon Alfa-2b versus fluorouracil and folinic acid for patients with resected pancreatic adenocarcinoma. J. Clin. Oncol. 2012;30:4077–4083. doi: 10.1200/JCO.2011.38.2960. [DOI] [PubMed] [Google Scholar]
- 9.Lutz ST, Jones J, Chow E. Role of radiation therapy in palliative care of the patient with cancer. J. Clin. Oncol. 2014;32:2913–2919. doi: 10.1200/JCO.2014.55.1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jones S, Hruban RH, Kamiyama M, Borges M, Zhang X, Parsons DW, Lin JC, Palmisano E, Brune K, Jaffee EM, Iacobuzio-Donahue CA, Maitra A, Parmigiani G, Kern SE, Velculescu VE, Kinzler KW, Vogelstein B, Eshleman JR, Goggins M, Klein AP. Exomic sequencing identifies PALB2 as a pancreatic cancer susceptibility gene. Science. 2009;324:217. doi: 10.1126/science.1171202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lowery MA, Kelsen DP, Stadler ZK, Yu KH, Janjigian YY, Ludwig E, D’Adamo DR, Salo-Mullen E, Robson ME, Allen PJ, Kurtz RC, O’Reilly EM. An emerging entity: pancreatic adenocarcinoma associated with a known BRCA mutation: clinical descriptors, treatment implications, and future directions. Oncologist. 2011;16:1397–1402. doi: 10.1634/theoncologist.2011-0185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McWilliams RR, Wieben ED, Rabe KG, Pedersen KS, Wu Y, Sicotte H, Petersen GM. Prevalence of CDKN2A mutations in pancreatic cancer patients: implications for genetic counseling. Eur J Hum Genet. 2011;19:472–478. doi: 10.1038/ejhg.2010.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Serrano D, Lazzeroni M, Decensi A. Chemoprevention of colorectal cancer: an update. Tech Coloproctol. 2004;8(Suppl 2):s248–252. doi: 10.1007/s10151-004-0170-5. [DOI] [PubMed] [Google Scholar]
- 14.Chaudhary A, Wang J, Prabhu S. Development and validation of a high-performance liquid chromatography method for the simultaneous determination of aspirin and folic acid from nano-particulate systems. Biomed Chromatogr. 2010;24:919–925. doi: 10.1002/bmc.1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reddy BS. Studies with the azoxymethane-rat preclinical model for assessing colon tumor development and chemoprevention. Environ Mol Mutagen. 2004;44:26–35. doi: 10.1002/em.20026. [DOI] [PubMed] [Google Scholar]
- 16.Thakkar A, Sutaria D, Grandhi BK, Wang J, Prabhu S. The molecular mechanism of action of aspirin, curcumin and sulforaphane combinations in the chemoprevention of pancreatic cancer. Oncol Rep. 2013;29:1671–1677. doi: 10.3892/or.2013.2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grandhi BK, Thakkar A, Wang J, Prabhu S. A novel combinatorial nanotechnology-based oral chemopreventive regimen demonstrates significant suppression of pancreatic cancer neoplastic lesions. Cancer Prev Res (Phila) 2013;6:1015–1025. doi: 10.1158/1940-6207.CAPR-13-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sutaria D, Grandhi BK, Thakkar A, Wang J, Prabhu S. Chemoprevention of pancreatic cancer using solid-lipid nanoparticulate delivery of a novel aspirin, curcumin and sulforaphane drug combination regimen. Int J Oncol. 2012;41:2260–2268. doi: 10.3892/ijo.2012.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thakkar A, Chenreddy S, Thio A, Khamas W, Wang J, Prabhu S. Preclinical systemic toxicity evaluation of chitosan-solid lipid nanoparticle-encapsulated aspirin and curcumin in combination with free sulforaphane in BALB/c mice. Int J Nanomedicine. 2016;11:3265–3276. doi: 10.2147/IJN.S106736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaudhary A, Sutaria D, Huang Y, Wang J, Prabhu S. Chemoprevention of colon cancer in a rat carcinogenesis model using a novel nanotechnology-based combined treatment system. Cancer Prev Res (Phila) 2011;4:1655–1664. doi: 10.1158/1940-6207.CAPR-11-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawashima Y. Panoparticulate systems for improved drug delivery. Adv Drug Deliv Rev. 2001;47:1–2. doi: 10.1016/s0169-409x(00)00117-4. [DOI] [PubMed] [Google Scholar]
- 22.Prabhu S, Brocks DR, Betageri GV. Enhancement of dissolution of ethopropazine using solid dispersions prepared with phospholipid and/or polyethylene glycol. Drug Dev Ind Pharm. 2001;27:413–418. doi: 10.1081/ddc-100104316. [DOI] [PubMed] [Google Scholar]
- 23.Prabhu S, Kanthamneni N, Ma C. Novel combinations of rate-controlling polymers for the release of leuprolide acetate in the colon. Drug Deliv. 2008;15:119–125. doi: 10.1080/10717540801905157. [DOI] [PubMed] [Google Scholar]
- 24.Prabhu S, Ortega M, Ma C. Novel lipid-based formulations enhancing the in vitro dissolution and permeability characteristics of a poorly water-soluble model drug, piroxicam. Int J Pharm. 2005;301:209–216. doi: 10.1016/j.ijpharm.2005.05.032. [DOI] [PubMed] [Google Scholar]
- 25.Prabhu S, Sullivan JL, Betageri GV. Comparative assessment of in vitro release kinetics of calcitonin polypeptide from biodegradable microspheres. Drug Deliv. 2002;9:195–198. doi: 10.1080/15227950290097633. [DOI] [PubMed] [Google Scholar]
- 26.Dasgupta S, Mazumder B, Ghosh SK, Kaurav SS. Solid lipid nanoparticles (SLNs) for topical delivery of aceclofenac by using xanthan gum: ex vivo and in vivo evaluation. Curr Drug Deliv. 2012 [Epub ahead of print] [PubMed] [Google Scholar]
- 27.Wang M, Qin L, Li K, Zhu R, Wang W, Wang S. The improvement of the anticancer effect of a novel compound benzoic acid, 2-hydroxy-, 2-D-ribofuranosylhydrazide (BHR) loaded in solid lipid nanoparticles. AAPS PharmSciTech. 2012;13:1348–1354. doi: 10.1208/s12249-012-9862-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roy K, Mao HQ, Huang SK, Leong KW. Oral gene delivery with chitosan--DNA nanoparticles generates immunologic protection in a murine model of peanut allergy. Nat Med. 1999;5:387–391. doi: 10.1038/7385. [DOI] [PubMed] [Google Scholar]
- 29.Sakloetsakun D, Perera G, Hombach J, Millotti G, Bernkop-Schnurch A. The impact of vehicles on the mucoadhesive properties of orally administrated nanoparticles: a case study with chitosan-4-thiobutylamidine conjugate. AAPS PharmSciTech. 2010;11:1185–1192. doi: 10.1208/s12249-010-9479-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fonte P, Andrade F, Araujo F, Andrade C, Neves J, Sarmento B. Chitosan-coated solid lipid nanoparticles for insulin delivery. Methods Enzymol. 2012;508:295–314. doi: 10.1016/B978-0-12-391860-4.00015-X. [DOI] [PubMed] [Google Scholar]
- 31.Sogias IA, Williams AC, Khutoryanskiy VV. Why is chitosan mucoadhesive? Biomacromolecules. 2008;9:1837–1842. doi: 10.1021/bm800276d. [DOI] [PubMed] [Google Scholar]
- 32.Haugk B. Pancreatic intraepithelial neoplasia-can we detect early pancreatic cancer? Histopathology. 2010;57:503–514. doi: 10.1111/j.1365-2559.2010.03610.x. [DOI] [PubMed] [Google Scholar]
- 33.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 34.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 35.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 36.Zhang J, Dhakal I, Yan H, Phillips M, Kesteloot H, Registries SC. Trends in pancreatic cancer incidence in nine SEER Cancer Registries, 1973-2002. Ann Oncol. 2007;18:1268–1279. doi: 10.1093/annonc/mdm123. [DOI] [PubMed] [Google Scholar]
- 37.Miller MS, Allen P, Brentnall TA, Goggins M, Hruban RH, Petersen GM, Rao CV, Whitcomb DC, Brand RE, Chari ST, Klein AP, Lubman DM, Rhim AD, Simeone DM, Wolpin BM, Umar A, Srivastava S, Steele VE, Rinaudo JA. Pancreatic cancer chemoprevention translational workshop: meeting report. Pancreas. 2016;45:1080–1091. doi: 10.1097/MPA.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cucinotto I, Fiorillo L, Gualtieri S, Arbitrio M, Ciliberto D, Staropoli N, Grimaldi A, Luce A, Tassone P, Caraglia M, Tagliaferri P. Nanoparticle albumin bound Paclitaxel in the treatment of human cancer: nanodelivery reaches prime-time? J Drug Deliv. 2013;2013:905091. doi: 10.1155/2013/905091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SW, Yun MH, Jeong SW, In CH, Kim JY, Seo MH, Pai CM, Kim SO. Development of docetaxel-loaded intravenous formulation, Nanoxel-PM™ using polymer-based delivery system. J Control Release. 2011;155:262–271. doi: 10.1016/j.jconrel.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 40.Marra M, Salzano G, Leonetti C, Tassone P, Scarsella M, Zappavigna S, Calimeri T, Franco R, Liguori G, Cigliana G, Ascani R, La Rotonda MI, Abbruzzese A, Tagliaferri P, Caraglia M, De Rosa G. Nanotechnologies to use bisphosphonates as potent anticancer agents: the effects of zoledronic acid encapsulated into liposomes. Nanomedicine. 2011;7:955–964. doi: 10.1016/j.nano.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Bonifazi M, Gallus S, Bosetti C, Polesel J, Serraino D, Talamini R, Negri E, La Vecchia C. Aspirin use and pancreatic cancer risk. Eur J Cancer Prev. 2010;19:352–354. doi: 10.1097/CEJ.0b013e32833b48a4. [DOI] [PubMed] [Google Scholar]
- 42.Cui XJ, He Q, Zhang JM, Fan HJ, Wen ZF, Qin YR. High-dose aspirin consumption contributes to decreased risk for pancreatic cancer in a systematic review and meta-analysis. Pancreas. 2014;43:135–140. doi: 10.1097/MPA.0b013e3182a8d41f. [DOI] [PubMed] [Google Scholar]
- 43.Streicher SA, Yu H, Lu L, Kidd MS, Risch HA. Case-control study of aspirin use and risk of pancreatic cancer. Cancer Epidemiol Biomarkers Prev. 2014;23:1254–1263. doi: 10.1158/1055-9965.EPI-13-1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang YP, Wan YD, Sun YL, Li J, Zhu RT. Aspirin might reduce the incidence of pancreatic cancer: a meta-analysis of observational studies. Sci Rep. 2015;5:15460. doi: 10.1038/srep15460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bar-Sela G, Epelbaum R, Schaffer M. Curcumin as an anti-cancer agent: review of the gap between basic and clinical applications. Curr Med Chem. 2010;17:190–197. doi: 10.2174/092986710790149738. [DOI] [PubMed] [Google Scholar]
- 46.Johnson JJ, Mukhtar H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007;255:170–181. doi: 10.1016/j.canlet.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Kuo ML, Huang TS, Lin JK. Curcumin, an antioxidant and anti-tumor promoter, induces apoptosis in human leukemia cells. Biochim Biophys Acta. 1996;1317:95–100. doi: 10.1016/s0925-4439(96)00032-4. [DOI] [PubMed] [Google Scholar]
- 48.Park W, Amin AR, Chen ZG, Shin DM. New perspectives of curcumin in cancer prevention. Cancer Prev Res (Phila) 2013;6:387–400. doi: 10.1158/1940-6207.CAPR-12-0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sareen R, Jain N, Pandit V. Curcumin: a boon to colonic diseases. Curr Drug Targets. 2013;14:1210–1218. doi: 10.2174/13894501113149990168. [DOI] [PubMed] [Google Scholar]
- 50.Zhou DH, Wang X, Yang M, Shi X, Huang W, Feng Q. Combination of low concentration of (-)-epigallocatechin gallate (EGCG) and curcumin strongly suppresses the growth of non-small cell lung cancer in vitro and in vivo through causing cell cycle arrest. Int J Mol Sci. 2013;14:12023–12036. doi: 10.3390/ijms140612023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Epelbaum R, Schaffer M, Vizel B, Badmaev V, Bar-Sela G. Curcumin and gemcitabine in patients with advanced pancreatic cancer. Nutr Cancer. 2010;62:1137–1141. doi: 10.1080/01635581.2010.513802. [DOI] [PubMed] [Google Scholar]
- 52.Kanai M, Yoshimura K, Asada M, Imaizumi A, Suzuki C, Matsumoto S, Nishimura T, Mori Y, Masui T, Kawaguchi Y, Yanagihara K, Yazumi S, Chiba T, Guha S, Aggarwal BB. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother Pharmacol. 2011;68:157–164. doi: 10.1007/s00280-010-1470-2. [DOI] [PubMed] [Google Scholar]
- 53.Hashida N, Murakami M, Yoshikawa H, Takada K, Muranishi S. Intestinal absorption of carboxyfluorescein entrapped in liposomes in comparison with its administration with lipid-surfactant mixed micelles. J Pharmacobiodyn. 1984;7:195–203. doi: 10.1248/bpb1978.7.195. [DOI] [PubMed] [Google Scholar]
- 54.Ling SS, Magosso E, Khan NA, Yuen KH, Barker SA. Enhanced oral bioavailability and intestinal lymphatic transport of a hydrophilic drug using liposomes. Drug Dev Ind Pharm. 2006;32:335–345. doi: 10.1080/03639040500519102. [DOI] [PubMed] [Google Scholar]
- 55.Sanjula B, Shah FM, Javed A, Alka A. Effect of poloxamer 188 on lymphatic uptake of carvedilol-loaded solid lipid nanoparticles for bioavailability enhancement. J Drug Target. 2009;17:249–256. doi: 10.1080/10611860902718672. [DOI] [PubMed] [Google Scholar]
- 56.Yuan H, Chen J, Du YZ, Hu FQ, Zeng S, Zhao HL. Studies on oral absorption of stearic acid SLN by a novel fluorometric method. Colloids Surf B Biointerfaces. 2007;58:157–164. doi: 10.1016/j.colsurfb.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 57.Bargoni A, Cavalli R, Caputo O, Fundaro A, Gasco MR, Zara GP. Solid lipid nanoparticles in lymph and plasma after duodenal administration to rats. Pharm Res. 1998;15:745–750. doi: 10.1023/a:1011975120776. [DOI] [PubMed] [Google Scholar]
- 58.Cavalli R, Bargoni A, Podio V, Muntoni E, Zara GP, Gasco MR. Duodenal administration of solid lipid nanoparticles loaded with different percentages of tobramycin. J Pharm Sci. 2003;92:1085–1094. doi: 10.1002/jps.10368. [DOI] [PubMed] [Google Scholar]
- 59.Paliwal R, Rai S, Vaidya B, Khatri K, Goyal AK, Mishra N, Mehta A, Vyas SP. Effect of lipid core material on characteristics of solid lipid nanoparticles designed for oral lymphatic delivery. Nanomedicine. 2009;5:184–191. doi: 10.1016/j.nano.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 60.Luo Y, Teng Z, Li Y, Wang Q. Solid lipid nanoparticles for oral drug delivery: chitosan coating improves stability, controlled delivery, mucoadhesion and cellular uptake. Carbohydr Polym. 2015;122:221–229. doi: 10.1016/j.carbpol.2014.12.084. [DOI] [PubMed] [Google Scholar]
- 61.Nair R, Kumar AC, Priya VK, Yadav CM, Raju PY. Formulation and evaluation of chitosan solid lipid nanoparticles of carbamazepine. Lipids Health Dis. 2012;11:72. doi: 10.1186/1476-511X-11-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cole ET, Scott RA, Connor AL, Wilding IR, Petereit HU, Schminke C, Beckert T, Cade D. Enteric coated HPMC capsules designed to achieve intestinal targeting. Int J Pharm. 2002;231:83–95. doi: 10.1016/s0378-5173(01)00871-7. [DOI] [PubMed] [Google Scholar]
- 63.Chatterjee D, Katz MH, Rashid A, Estrella JS, Wang H, Varadhachary GR, Wolff RA, Lee JE, Pisters PW, Abbruzzese JL, Fleming JB, Wang H. Pancreatic intraepithelial neoplasia and histological changes in non-neoplastic pancreas associated with neoadjuvant therapy in patients with pancreatic ductal adenocarcinoma. Histopathology. 2013;63:841–851. doi: 10.1111/his.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]