Abstract
Due to low charge separation efficiency and poor stability, it is usually difficult for single-component photocatalysts such as graphitic carbon nitride (g-C3N4) and silver chromate (Ag2CrO4) to fulfill photocatalytic hydrogen production efficiently. Z-scheme charge transport mechanism that mimics the photosynthesis in nature is an effective way to solve the above problems. Inspired by photosynthesis, we report Ag2CrO4 nanoparticles-decorated g-C3N4 nanosheet as an efficient photocatalyst for hydrogen evolution reaction (HER) with methanol as sacrificial agent. The formation of Z-scheme g-C3N4/Ag2CrO4 nanosheets photocatalysts could inhibit the recombination of photogenerated electron-hole pairs, promote the generation of hydrogen by photosplitting of water. The experiment results indicate that g-C3N4/Ag2CrO4 nanocomposites present enhanced photocatalytic activity and stability in the H2 evolution of water splitting. And the nanocomposites g-C3N4/Ag2CrO4(23.1%) show the 14 times HER efficiency compared to that of bare g-C3N4.
Introduction
With fossil fuel reserves dwindling every day, there is an urgent need for clean and sustainable alternative energy sources. Hydrogen energy is an attractive alternative resource to fossil fuels due to its high energy density and environmentally friendly characteristics1–6. Many methods are used to produce hydrogen, which can be divided into two major categories based on the required raw materials and processes: reforming processes and splitting water processes. The former process is relatively mature and uses widely. However, it has some disadvantages such as high cost, large energy consumption, low efficiency, and complicated equipments and processes. Splitting water processes contains electrolytic water splitting, photocatalytic water splitting, bio-photocatalytic water splitting and thermochemical water splitting. Water electrolysis technology is relatively mature, with simple equipment, no pollution, and high product purity but large energy consumption; water biophotolysis process Bio-photocatalytic water splitting requires harsh reaction environments but has poor stability. There is no problem in the feasibility and high efficiency for thermochemical water splitting technology, but further research is needed to reduce costs and achieving efficient recycling. Among them, photocatalytic water splitting is a more efficient pathway to produce hydrogen gas and also does not generate any undesirable byproducts7–17. Since the discovery of the Honda-Fujishima effect in 197218, photocatalytic hydrogen production through water splitting has become a promising technology for utilizing renewable solar energy19–24. Development of efficient photocatalytic systems for hydrogen evolution via photoinduced water splitting is an active field of energy research. However, due to narrow absorption range, poor stability, low charge-separation efficiency and weak redox ability, it is often difficult for a single-component photocatalyst to fulfill this requirement. The heterostructure composed of two semiconductor catalysts can increase the catalytic efficiency25–29. Photosynthesis as a nature heterostructure system is widely studied due to its fast and efficient photocatalytic reaction.
In natural photosynthesis process, the Z-scheme photoreaction system is an important part of the plant photosynthesis, which involves two photochemical reactions and a series of intermediate enzymatic redox reactions. The electron transfer process is shown in Fig. 1a. After a series of reactions, the photogenerated electrons with high reducing ability and holes with high oxidizing ability are left in the LUMO of PS I and the HOMO of PS II, respectively. The transfer process of electrons in the figure constitutes a shape of letter Z, so it is called a Z-scheme30–32. This system was first proposed by Bard in 1979 after studying the photosynthesis of plants33. The Z-scheme photocatalytic system shows excellent redox ability in due to the different band gap of two semiconductor with broadening light absorption and the special charge transfer path ensuring high separation efficiency of photo-generated charges. Considering these unique advantages of the natural Z-scheme photocatalytic system, the artificial Z-scheme photocatalytic systems have been developed and used widely in photocatalytic field25,34–38. The artificial heterogeneous Z-scheme photocatalytic systems, mimicking the natural photosynthesis process, overcome the drawbacks of single-component photocatalysts and satisfy those aforementioned requirements.
Figure 1.
(a) Natural photosynthesis (the electrons in HOMO of PS II are excited to its LUMO under solar light; Then, the photogenerated electrons in LUMO of PS II are transferred to HOMO of PS I through the electron mediator. Further, the electrons in HOMO of PS I are excited to its LUMO. The transfer process of electrons in the figure constitutes a shape of letter Z, so it is called a Z-scheme) and (b) an artificial analogy composed of an organic semiconductor g-C3N4 (equivalent to PS I) and silver chromate (equivalent to PS II) (the electrons in HOMO of Ag2CrO4 are excited to its LUMO under visible light irradiation; Then, the electrons in HOMO of g-C3N4 are excited to its LUMO under visible light irradiation; the excited electrons of Ag2CrO4 and the holes of g-C3N4 recombine; as a result, the migration route of electrons is a Z-scheme).
For the first time, Martin39 reported that the robust organic semiconductor g-C3N4 can be integrated into a Z-scheme water splitting in a liquid system. While in this system, they used an electron acceptor/donor (NaI/IO3−) pair which they all absorb light to the different extent and occur back reactions. Therefore, the number of adsorbed photons by photocatalysts decreases and the effective number of photogenerated electrons and holes decrease sharply30. Mimicking the natural photosynthesis process, some all solid-state Z-scheme photocatalyst system based on g-C3N4 with other semiconductors were obtained and used, such as WO3, Ag3PO4, BiOX (X=Cl, Br, I), V2O5, etc40–44. A Z-scheme system of g-C3N4/WO3 was used to generate hydrogen and its separation mechanisms were also studied deeply40,45,46. He et al.41 reported that the Z-Scheme Ag3PO4/g-C3N4 composite was used to convert CO2 to fuel such as CO, methanol, methane and ethanol. Liu et al.44 synthetized a ternary composite photocatalyst g-C3N4/Ag3PO4/Ag2MoO4 for water splitting. BiOCl and BiOI were also compounded with g-C3N4 as high efficiency photocatalysts43,47. As a promising catalyst, Bi2WO6 was also combined with g-C3N4 to form a Z-scheme catalytic system48.
Silver chromate (Ag2CrO4) as a narrow band gap semiconductor with band gap of 1.80 eV has unique electronic structure, crystal structure49, excellent light sensitivity and high photocatalytic activity50,51. Hence, in recent years, some Ag2CrO4-based photocatalysts have been fabricated51–53. However, similar to other silver-based photocatalysts, the big aggregated particle size and easy photocorrosion properties seriously caused poor stability and restricted the photocatalytic performance of the Ag2CrO4 photocatalyst. The g-C3N4/Ag2CrO4 nanocomposites were synthesized and characterized, the dispersion and light stability of Ag2CrO4 were improved greatly. And their catalytic hydrogen evolution activity was evaluated when using methanol as the sacrificial electron donor under visible light. The as-obtained g-C3N4/Ag2CrO4 composites showed distinctly enhanced photo-catalytic activity than that of pure g-C3N4 nanosheets on the evolution of H2 under visible light irradiation. Compared to the two individuals, the enhancement of HER efficiency was mainly ascribed to the synergetic effect between Ag2CrO4 and g-C3N4 (as shown in Fig. 1b), effectively improved photogenerated electron-hole pairs separation efficiency via the direct Z-scheme charge transfer mechanism. Notably, the photocorrosion of Ag2CrO4 was efficiently hindered due to this synergistic effect. Furthermore, a possible direct Z-scheme mechanism for the enhanced photocatalytic activity of the g-C3N4/Ag2CrO4 composite was also discussed based on the relative band gap positions of these two semiconductors. The existence of the Z-scheme mechanism means strong redox ability and high transfer efficiency of photogenerated electron-hole pairs. This research will broaden the studies of Ag2CrO4 and g-C3N4 photocatalysts with excellent photocorrosion inhibition ability and high photocatalytic activity under visible light.
Results and Discussion
Phase structure and morphology analysis
In order to analyze the crystal structure of the prepared g-C3N4, g-C3N4/Ag2CrO4 composites, and Ag2CrO4, the XRD patterns were obtained (as shown in Fig. 2). Fig. 2a was the pattern of g-C3N4, two diffraction peaks at 27.6° and 13.0° were indexed to (002) and (100) planes of hexagonal g-C3N4 (JCPDS card No. 87–1526), corresponding to the graphite-like stacking and the in-plane structural repeating motifs of the conjugated aromatic units of g-C3N454. From Fig. 2b–f, the patterns correspond to the samples with an increasing Ag2CrO4 mass ratio to g-C3N4. For the g-C3N4/Ag2CrO4 composites, orthorhombic phase Ag2CrO4 and hexagonal phase g-C3N4 were both observed and no other impurity peaks were found, implying that g-C3N4 and Ag2CrO4 keep pure phase and no impurities formed in g-C3N4/Ag2CrO4 composites. Moreover, the peaks of Ag2CrO4 in g-C3N4/Ag2CrO4 composites were becoming clearer with the increasing of Ag2CrO4. And at the same time the peaks of g-C3N4 gradually decreased. Fig. 2g was the pattern of Ag2CrO4. It was clear to see that all diffraction peaks of the as-prepared Ag2CrO4 coincided well with the orthorhombic phase of Ag2CrO4 (JCPDS No. 26-0952).
Figure 2.
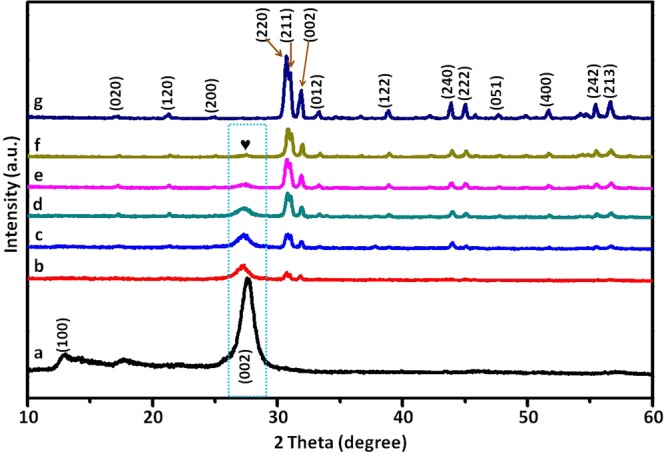
XRD patterns of (a) g-C3N4, (b) g-C3N4/Ag2CrO4(9.1%), (c) g-C3N4/Ag2CrO4(16.7%), (d) g-C3N4/Ag2CrO4(23.1%), (e) g-C3N4/Ag2CrO4(28.6%), (f) g-C3N4/Ag2CrO4(33.3%) and (g) Ag2CrO4.
In order to observe the morphology of the bare g-C3N4, Ag2CrO4 and the as-prepared g-C3N4/Ag2CrO4 composite, TEM was performed (see Fig. 3). The pure g-C3N4 sample possessed a very thin 2D layer structure (shown as Fig. 3a), which meant that the g-C3N4 was prepared successfully. As to the pristine Ag2CrO4 sample (shown in Fig. 3b), besides the obvious aggregations there was no special morphological features, and the sizes of Ag2CrO4 particles were between 140–300 nm and asymmetrical. The TEM image of the g-C3N4/Ag2CrO4 composite with the mass weight ratio 23.1% was showed in Fig. 3c. From the TEM picture, we could see that the Ag2CrO4 particles (labeled by red circles) with the size below 10 nm were uniformly embedded on g-C3N4. The adding of graphene-like g-C3N4 nanosheets made the Ag2CrO4 particles composites in the g-C3N4/Ag2CrO4(23.1%) closely enwrapped by g-C3N4 and much smaller than pure Ag2CrO4 nanoparticles. These results indicate that g-C3N4 nanosheets could not only serve as a graphene-like substrate to attach Ag2CrO4 nanoparticles on their surface, but also inhibit Ag2CrO4 nanoparticles aggregating into the big particles. Therefore, g-C3N4/Ag2CrO4 composite photocatalyst with closely interconnections between g-C3N4 and Ag2CrO4 nanoparticles were successfully synthesized, which would be beneficial for the transfer of the photogenerated electron-hole pairs. In Fig. 3d, obviously, the clear fringes spacing was ca. 0.28 nm, corresponding to the (211) lattice plane of orthorhombic phase Ag2CrO455,56. The composites with other different weight ratios (9.1%, 16.7%, 28.6%, and 33.3%) were shown in Fig. S1. We could see that when the ratio of the Ag2CrO4 below 23.1%, small Ag2CrO4 nanoparticles (below 10 nm) were uniformly embedded on g-C3N4 (labeled by red circles in Figs 3b, S1a and S1b). While the theoretical weight ratio was increased to 28.6%, Ag2CrO4 began to aggregate, the size of Ag2CrO4 grow to above 20 nm. When the theoretical ratio was 33.3%, the aggregation was very serious just like the pure Ag2CrO4.
Figure 3.
TEM images of (a) g-C3N4, (b) Ag2CrO4, (c) g-C3N4/Ag2CrO4(23.1%) (Ag2CrO4 were labeled by red circles), (d) HRTEM of g-C3N4/Ag2CrO4(23.1%).
Based on Figs 3 and S1, a possible formation mechanism of g-C3N4/Ag2CrO4 composites is proposed. Obviously, the structure of Ag2CrO4 ranged from nanoparticles (below 10 nm) to sub-microparticles (140–300 nm) with the decreasing of g-C3N4 mass fraction, which strongly confirmed that the g-C3N4 sheets played vital role in the formation of the nanojunction. Many investigations showed that large specific surface area and two-dimensional structure of g-C3N4 could provide a large scaffold for anchoring various substrates57,58. In addition, the surface of urea-derived graphitic g-C3N4 possessed positive charge with abundant alamino groups (C-NHx), which could provide a suitable environment for attracting negative charge particles via electrostatic attraction59,60. Accordingly, in our study, the CrO42− ions could adsorb onto g-C3N4 sheets via the electrostatic force in g-C3N4 suspension. The anchored CrO42− ions would form Ag2CrO4 nanocrystals in situ on the surface of g-C3N4 sheets during the reaction, then the tiny nanocrystal nucleus grows into the nanoparticles through oriented growth on the surface of g-C3N4 support61. Eventually, the Ag2CrO4 nanoparticles uniformly and tightly distribute onto the surface of g-C3N4 sheets (As shown in Figs 3c and S1a,b). However, with the increase of Ag2CrO4 mass ratio, the capacity of electrostatic attraction between g-C3N4 and CrO42− ions was decreased because the effective positive charge surface of g-C3N4 was decreasing. When the mass fraction of Ag2CrO4 was more than 28.6%, the excess of CrO42−−ions could not tightly adsorb on the surface of g-C3N4 sheets and would grow into Ag2CrO4 nanoparticles freely during the reaction progress. However, these free Ag2CrO4 nanocrystals possessed high specific surface energy, which would assemble spontaneously and form hierarchical sub-microparticles for reducing the interfacial energy52,62. As a result, part of Ag2CrO4 nanoparticles uniformly grew on the surface of g-C3N4 due to the strong electrostatic attraction between CrO42−− ions and C-NHx, whereas other Ag2CrO4 nanoparticles were densely self-assembled and formed 3D hierarchical structures which covered the g-C3N4 sheets (Fig. S1c). With the mass fraction of free Ag2CrO4 nanoparticles further increasing, the crystal growth of nanoparticles would cause the exfoliation of the g-C3N4 sheets (Fig. S1d)63,64. The Ag2CrO4 sub-microparticles could directly contacted with g-C3N4 sheets, which were shown in Fig. S1d. The pure Ag2CrO4 3D hierarchical structures was obtained with the absence of g-C3N4. It revealed that when the particles of Ag2CrO4 nanoparticles were not restricted by the functional alamino groups on the surface of g-C3N4 sheets, they would assemble spontaneously in a random way to form 3D sub-microparticles (shown as Fig. 3c)49. All in all, the results clearly confirmed that the mass ratio of g-C3N4 could be a key parameter for formation of g-C3N4/Ag2CrO4 nanocomposites. The positive charge and 2D structure of g-C3N4 sheets could provide a suitable environment for the growth of nanoparticles.
Component analysis
The elemental compositions of g-C3N4/Ag2CrO4(23.1%) nanocomposite were explored by EDS technique and the results were shown in Fig. 4. It could be seen that the g-C3N4/Ag2CrO4(23.1%) composite was composed of only C, N, Ag, Cr and O elements and no other element or impurity found, demonstrating that the existence of g-C3N4 and Ag2CrO4 in the as-fabricated g-C3N4/Ag2CrO4(23.1%) composite (shown as in Fig. 4a). Quantitative analysis of the g-C3N4/Ag2CrO4(23.1%) nanocomposite showed that weight percents of C, N, Ag, Cr and O elements were 29.4, 47.2, 15.0, 3.6, and 4.8%, respectively, which they were close to the theoretical percents of 30.1, 46.8, 15.0, 3.6 and 4.5%, respectively. To further investigate elemental composition and distribution uniformity, the elemental maps for g-C3N4/Ag2CrO4(23.1%) composite were displayed in Fig. 4c–g, which indicated that Ag, Cr and O elements were homogeneously distributed in the whole host of the g-C3N4/Ag2CrO4(23.1%) composite. Considering the Ag, Cr and O presented in the form of Ag2CrO4, these analyses demonstrated Ag2CrO4 tended to integrate with g-C3N4 nanosheets firmly and then formed hybrid structures. Except that, X-ray photoelectron spectroscopy and Fourier-transform infrared spectroscopy were also used to analyze the composition of nanomaterials (as shown in Figs S2 and S4).
Figure 4.
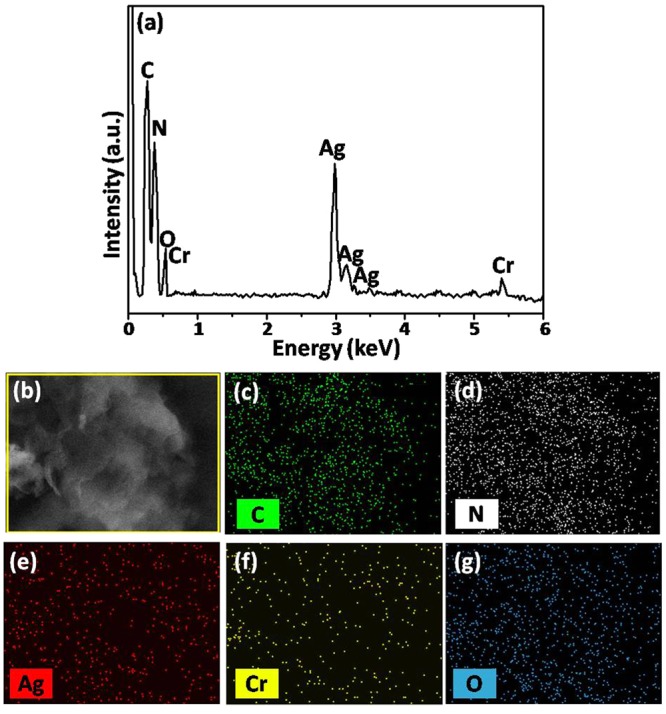
(a) EDS spectra for the g-C3N4/Ag2CrO4(23.1%), (b) SEM of g-C3N4/Ag2CrO4(23.1%), (c–g) EDS mapping for different elements of the g-C3N4/Ag2CrO4(23.1%) nanocomposite.
Optical properties of the g-C3N4/Ag2CrO4 composites
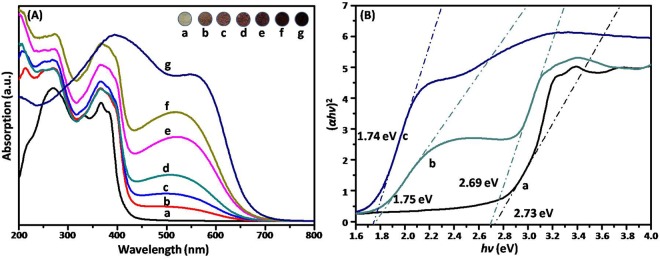
It was believed that remarkable absorption enhancement in the visible-light region was beneficial for improving photocatalytic activity. Optical absorption spectra of the prepared samples were provided by UV-vis DRS and the results in the range of 200–800 nm were shown in Fig. 5A. As can be seen, the absorption edge of the pure g-C3N4 and Ag2CrO4 located at approximately 454 nm and 712 nm, respectively. It was noteworthy that the individual Ag2CrO4 showed the photo-absorption property in the entire waveband, which suggested that an effective utilization of the solar source over Ag2CrO4 could be acquired. Interestingly, the absorption of composites was gradually strengthened in the visible region with increasing of Ag2CrO4 content. This phenomenon could be attributed to the difference between the band gap energies of bare g-C3N4 and Ag2CrO4. With more and more Ag2CrO4 nanoparticles with relative narrow band gap produced on the surface of g-C3N4, the band gap of the g-C3N4/Ag2CrO4 would decrease. Other groups also found the same trend when coupling the broader band gap semiconductors with other relatively narrow band gap semiconductors42,65. Therefore, introducing Ag2CrO4 into g-C3N4 might be favorable for photocatalytic reaction due to the enhancing of light absorbance. These results implied that the g-C3N4/Ag2CrO4 nanocomposite had the potential to be efficient visible light-driven photocatalyst. The UV-vis DRS spectra were also applied to calculate band gap energy (Eg) using Tauc’s equation:
| 1 |
Figure 5.
(A) UV–vis DRS (insert are the photos of samples) of (a) g-C3N4, (b) g-C3N4/Ag2CrO4(9.1%), (c) g-C3N4/Ag2CrO4(16.7%), (d) g-C3N4/Ag2CrO4(23.1%), (e) g-C3N4/Ag2CrO4(28.6%), (f) g-C3N4/Ag2CrO4(33.%) and (g) Ag2CrO4; (B) the band gap energies of (a) g-C3N4, (b) g-C3N4/Ag2CrO4(23.1%), and (c) Ag2CrO4.
In this equation, α, hν, and A were absorption coefficient, the photon energy, and proportionality constant, respectively66. The value of n was determined by the type of optical transition of a semiconductor (n = 1 for direct transition, and n = 4 for indirect transition), and the n values of g-C3N4 and Ag2CrO4 were 149,67. So according to the calculation ((αhν)2 = A2hν − A2Eg), the plot of (αhν)2 versus photon energy (hν) for g-C3N4, pure Ag2CrO4 and the as-prepared g-C3N4/Ag2CrO4(23.1%) composites were shown as Fig. 5B. The band gap energies of g-C3N4 and Ag2CrO4 were 2.73 eV and 1.74 eV, respectively. And the wavelength thresholds of the g-C3N4/Ag2CrO4(23.1%) composite were estimated at 461 and 708 nm, corresponding to the band gaps at 2.69 and 1.75 eV, respectively ascribed to g-C3N4 and Ag2CrO4. The reduced band gaps of the g-C3N4/Ag2CrO4(23.1%) composite and more response to the visible light were caused by the loaded Ag2CrO4 nanoparticles, thus more efficient utilization of solar energy could be achieved, and the improved photocatalytic activity of the g-C3N4/Ag2CrO4 composite could be anticipated.
Visible light photocatalytic activities and evaluation of stability
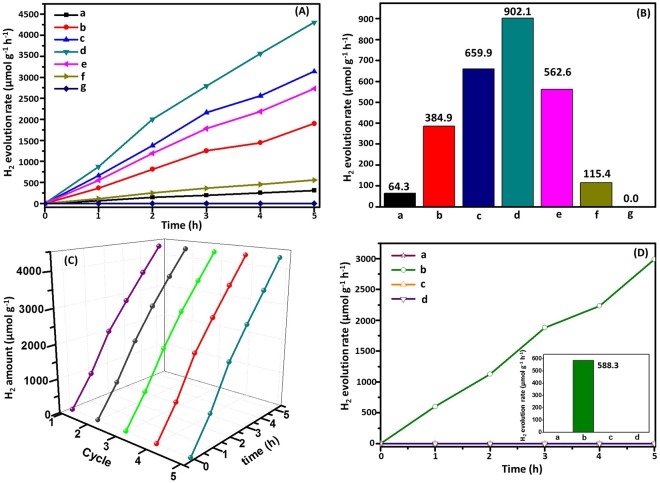
The photocatalytic activity of all samples was evaluated by monitoring the hydrogen evolution in the presence of a sacrificial reagent (methanol) under visible light illumination (λ ≥ 420 nm). Control experiments indicated that there was no appreciable H2 production in the absence of photocatalysts, light irradiation or H2O. According to the standard curve: y = 165550x, R2 = 0.9997 (as shown in Fig. S5), time-dependent (Fig. 6A) and average (Fig. 6B) photoinduced H2 evolution for different photocatalysts were observed and corresponding results were listed in Table S1. As illustrated, H2 production was not detected if pure Ag2CrO4 was used as a photocatalyst for the whole visible-light irradiation for 5 h; this suggested that Ag2CrO4 alone was not active for photocatalytic H2 generation. Pure g-C3N4 showed a poor photocatalytic activity under visible light (64.3 μmol g−1 h−1), owing to its limited light-harvesting efficiency and fast recombination of photogenerated electron-hole pairs. However, after the two semiconductors were combined, the evolution rate of g-C3N4/Ag2CrO4 was greatly enhanced and the largest rate as high as 902.1 μmol g−1 h−1 was attained by using g-C3N4/Ag2CrO4(23.1%) as photocatalyst, which was 14 times that of g-C3N4. Compared with other g-C3N4 based composite photocatalysts, the H2 production rate of g-C3N4/Ag2CrO4 was enhanced to a great extent, which could be ascribed to the effect of Ag2CrO4 (Table 1). In the scope of our literature survey, our efficiency of hydrogen production is very high. It is just lower compared to that of literature ref.6 and ref.15. Furthermore, it could also be clearly observed that the content of Ag2CrO4 exhibited a great influence on the H2 production rate. With its increasing, the H2 production rate rose firstly and then decreased. For bare g-C3N4, the H2 production rate was extremely low because of the deeply trapped electrons and fast recombination of electron-hole pairs; the photogenerated electrons were not transferred to catalytic sites and were unable to participate in H2 production68. As a compare, the pure g-C3N4 and g-C3N4/Ag2CrO4(23.1%) was conducted photogenerated hydrogen without Pt co-catalyst or methanol as electro donors. From Fig. 6D (the corresponding results were listed in Table S3), we could see that with methanol but not Pt, there was no observable hydrogen generation be detected using pure g-C3N4 as photocatalyst under visible light irradiation. And the hydrogen production efficiency of g-C3N4/Ag2CrO4(23.1%) was reduced. While without any electron donors such as methanol, no H2 is produced either using pure g-C3N4 or g-C3N4/Ag2CrO4(23.1%) as a catalyst.
Figure 6.
(A) Time courses of photocatalytic H2 (B) the average rate of H2 of (a) g-C3N4, (b) g-C3N4/Ag2CrO4(9.1%), (c) g-C3N4/Ag2CrO4(16.7%), (d) g-C3N4/Ag2CrO4(23.1%), (e) g-C3N4/Ag2CrO4(28.6%), (f) g-C3N4/Ag2CrO4(33.3%) and (g) Ag2CrO4 under visible light irradiation; (C) Recyclability of the g-C3N4/Ag2CrO4(23.1%) in five successive experiments for the H2 evolution under visible light irradiation; (D) Time courses of photocatalytic H2 (insert: the average rate of H2) of (a) g-C3N4 with methanol without Pt, (b) g-C3N4/Ag2CrO4(23.1%) with methanol without Pt, (c) g-C3N4 without methanol with Pt, and (d) g-C3N4/Ag2CrO4(23.1%) without methanol with Pt.
Table 1.
Comparison of the photocatalytic H2 production rate reported in the literatures with Z-scheme g-C3N4/Ag2CrO4 in our work with methanol as sacrificial agent under visible light irradiation.
| Sample | Efficiency (μmol h−1 g−1) | Co-catalyst (Pt) | Light source | Refrence |
|---|---|---|---|---|
| g-C3N4/InVO4 | 212 | 0.6% | >420 nm | ref.5 |
| g-C3N4/NiFe-LDH | 24800 | No | ≥420 nm | ref.6 |
| GCN/NT NFs | 8931.3 | A certain amount | simulated solar light | ref.15 |
| CdS/Au/g-C3N4 | 19 | No | >420 nm | ref.16 |
| Fe2(MoO4)3/g-C3N4 | 0.18 | No | >420 nm | ref.17 |
| Au/SnO2/g-C3N4 | 770 | No | >400 nm | ref.21 |
| Au/PtO/g-C3N4 | 16.9 | No | >400 nm | 18222 |
| TiO2/g-C3N4 | 74.7 | 0.5% | >400 nm | ref.23 |
| MoS2/g-C3N4 | 231 | 1% | >400 nm | ref.24 |
| g-C3N4/Au/P25 | 259 | No | simulated solar light | ref.25 |
| Fe/P-g-C3N4 | 150.6 | No | >400 nm | ref.26 |
| g-C3N4/WS2 | 101 | No | ≥420 nm | ref.27 |
| Ag2S/g-C3N4 | 200 | No | =420 nm | ref.28 |
| g-C3N4/TiO2 | 559.7 | No | Full light | ref.29 |
| g-C3N4/Ag2CrO4 | 902.1 | 0.6% | ≥420 nm | Our work |
To evaluate the stability and reusability of the g-C3N4/Ag2CrO4 nanocomposites, a recycling test of the g-C3N4/Ag2CrO4(23.1%) (5 h) was performed and the corresponding results were displayed in Fig. 6C and the corresponding results were listed in Table S2. The amount of H2 produced increased steadily with an extension in the reaction time and no significant deactivation was observed after 5 cycles, indicating that the g-C3N4/Ag2CrO4 nanocomposites had high stability in the photocatalytic H2 evolution. Furthermore, the XRD of g-C3N4/Ag2CrO4(23.1%) before and after H2 production were shown as Fig. S6. It was seen that the peaks of the catalyst were similar, and the morphology was steady. Furthermore, there were no new peaks according to Pt appeared due to its low content. And the TEM of g-C3N4/Ag2CrO4(23.1%) after five cycles also indicated that Ag2CrO4 nanoparticless were still anchored on the surface of g-C3N4 (Fig. S7), suggesting a good stability of inherent structure for g-C3N4 and Ag2CrO4 nanoparticles. Such high stability might result from the formation of the heterostructure between g-C3N4 and Ag2CrO4.
Charge transfer properties
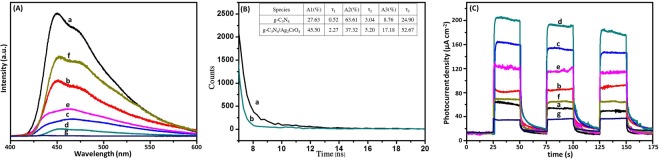
As shown in Fig. 6, the Z-scheme nanocomposite formed between g-C3N4 and Ag2CrO4 dramatically enhanced the photocatalytic performance under visible-light irradiation. To explore the effect of silver chromate on separation efficiency of the photogenerated electron-hole pairs on the g-C3N4, PL spectra for the g-C3N4, g-C3N4/Ag2CrO4 and Ag2CrO4 samples were provided in the range of 400–600 nm and the results were displayed in Fig. 7A. Since PL emission spectra derived from the recombination of free carriers, it was generally accepted that the PL spectrum with low intensity indicated efficiently separation of the charge carriers, leading to participation of more electrons and holes in the oxidation and reduction reactions. It could be observed that all of the samples had similar spectra in this range. It was clear to see that the bare g-C3N4 had a wide and strong peak around 450 nm. However, there was a considerable decrease in the intensity of the PL spectrum for the g-C3N4/Ag2CrO4 nanocomposites compared to that of the pure g-C3N4. Hence, the photogenerated electron-hole pairs could migrate easily between g-C3N4 and Ag2CrO4, due to the matching band potentials30,69. It was noted that the g-C3N4/Ag2CrO4(23.1%) sample showed the weakest emission intensity, meaning that it had the lowest recombination rate of the photogenerated charge carriers and the highest photocatalytic activity compared with other composites. Additionally, it was worth noting that the peaks density of PL first declined, then increased along with the further increasing content of Ag2CrO4. That was excess Ag2CrO4 species increased the recombination rate of photogenerated charge carriers, leading to inferior photocatalytic efficiency, arising from the excess Ag2CrO4 species might agglomerate seriously leading to decrease of the combinations between counterparts of the nanocomposite and acted as a recombination center and thereby reducing the efficiency of charge separation70–72. The time-resolved photoluminescence spectra for g-C3N4 and g-C3N4/Ag2CrO4(23.1%) were tested and shown in Fig. 7B. It could be clearly observed that g-C3N4/Ag2CrO4(23.1%) showed longer decay time value compared to pure g-C3N4 which could be ascribed to the effective charge transfer across the interface of g-C3N4 and Ag2CrO473.
Figure 7.
(A) Photoluminescence spectra of (a) g-C3N4, (b) g-C3N4/Ag2CrO4(9.1%), (c) g-C3N4/Ag2CrO4(16.7%), (d) g-C3N4/Ag2CrO4(23.1%), (e) g-C3N4/Ag2CrO4(28.6%), (f) g-C3N4/Ag2CrO4(33.3%) and (g) Ag2CrO4; (B) Time-resolved photoluminescence spectra for (a) g-C3N4, (b) g-C3N4/Ag2CrO4(23.1%); (C) it of (a) g-C3N4, (b) g-C3N4/Ag2CrO4(9.1%), (c) g-C3N4/Ag2CrO4(16.7%), (d) g-C3N4/Ag2CrO4(23.1%), (e) g-C3N4/Ag2CrO4(28.6%), (f) g-C3N4/Ag2CrO4(33.3%) and (g) Ag2CrO4.
Consequently, we recorded the transient photocurrent responses of the pure g-C3N4, g-C3N4/Ag2CrO4 nanocomposite and Ag2CrO4 under dark conditions and visible light irradiation. Fig. 7C showed a comparison of the photocurrent-time curves for photocatalysts, with three on-off intermittent irradiation cycles. It was widely considered as the most efficient evidence for explaining the electrons and holes separation in the composite photocatalysts38,74,75. Generally, the corresponding relationship was recognized as follows: the higher photocurrent implied the higher electrons-holes separation efficiency, thus leading to the higher photocatalytic activity. It was clear that the photocurrent densities rapidly decreased to zero as soon as the lamp was turned off, and that the photocurrent densities maintained stable values when the lamp was turned on, indicating a rapid photocurrent response to the on-off intermittent irradiation. It could be obviously seen that the photocurrent responses of the g-C3N4/Ag2CrO4 nanocomposites were increased significantly in comparison with that of pure g-C3N4, indicating a higher separation and transfer efficiency of the photogenerated electron-hole pairs under visible light irradiation, and hence higher photocatalytic activity. Additionally, it was worth noting that the photocurrent density first increased, then declined along with the increasing content of Ag2CrO4, which was in well accordance with the above PL results. This provided further evidence to support the above PL results. Hence, the abovementioned results obviously confirmed the superior charge transfer and recombination inhibition in the g-C3N4/Ag2CrO4 composites in comparison with only g-C3N4, demonstrating that the introduction of Ag2CrO4 could effectively enhance the separation and transfer efficiency of photogenerated electron-hole pairs of g-C3N4, which could improve the photocatalytic performance.
Mechanism of enhanced photoactivity
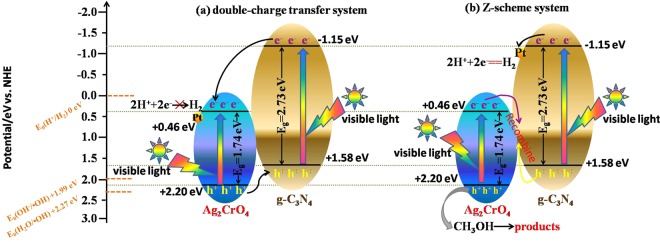
As far as bare g-C3N4 was concerned, normally, the photogenerated electron-hole pairs quickly recombined and only a fraction of them participated in water splitting reaction, resulting in low photocatalytic activity5,6,15,21. Based on the above analysis of the experiment and characterization results, the possible photocatalytic reaction mechanism and electron transfer processes of the g-C3N4/Ag2CrO4 nanocomposites were proposed (Fig. 8). The conduction band potential (ECB) and valence band potential (EVB) of the semiconductors could be calculated by the following empirical equations76:
| 2 |
| 3 |
where X was the absolute electronegativity of the semiconductor, which is the geometric mean of the electronegativity of the constituent atoms, and the values of the X for g-C3N4 and Ag2CrO4 are 4.72 eV and 5.83 eV, respectively52,77. Ee was the energy of free electrons on the hydrogen scale (∼4.50 eV vs NHE). Therefore, the ECB potentials of g-C3N4 and Ag2CrO4 were −1.15 eV and +0.46 eV, and corresponding EVB potentials could be estimated to be +1.58 eV and +2.20 eV, respectively. The values of VB for g-C3N4 and Ag2CrO4 were also obtained by XPS and the results for them were 1.60 eV and 2.20 eV respectively (shown as Fig. S3), which were similar with the results of calculation ones. Under visible light irradiation, Ag2CrO4 and g-C3N4 were both excited and yield electron-hole pairs. According to the double-charge transfer theory (as shown in Fig. 8a)78–80, the photo-generated electrons of g-C3N4 would migrate to the conduction band (CB) of Ag2CrO4 and focused on the Pt nanoparticales, then the holes of Ag2CrO4 would transfer to the valence band (VB) of g-C3N4, because the VB and CB potentials of Ag2CrO4 were both lower than those of g-C3N4. However, if the composites followed the double charge transfer theory, the electrons in the CB of Ag2CrO4 could not reduce H+ to generate H• due to the CB potential of Ag2CrO4 (0.46 eV/vs. NHE) was higher than E0 (H+/H2) (0 eV/vs. NHE). Therefore, a Z-scheme mechanism was proposed based on the above analysis. As illustrated in Fig. 8b, the photo-generated electrons on the CB of Ag2CrO4 could easily transfer to the VB of g-C3N4 and reacted with the holes of g-C3N4, effectively inhibiting the electron-hole pairs recombination in both Ag2CrO4 and g-C3N4, prolonging the lifetime of the photogenerated electrons on the CB of g-C3N4 and the photogenerated holes on the VB of Ag2CrO4, so enhancing the interfacial charge transfer. Furthermore, Pt as co-catalyst could accept and transfer electrons and functioning as an effective hydrogen evolution promoter for g-C3N4. In such a way, the photogenerated electrons and holes were efficiently separated by the Z-scheme charge transfer; it could be further confirmed by the PL spectra and photocurrent analysis. Subsequently, the photogenerated electrons left at the CB of g-C3N4 which have more negative potential than the standard redox potential of H+/H2 (0 eV vs. NHE) could reduce H+ to yield H• which was the source to produce hydrogen. Holes stored in the VB of Ag2CrO4 could directly react with sacrificial agent (methanol).
Figure 8.
Schematic representation of the charge generation, migration and hydrogen production mechanism of Z-scheme g-C3N4/Ag2CrO4 nanocomposites.
Conclusion
In summary, an efficient visible-light-driven bio-inspired Z-scheme g-C3N4/Ag2CrO4 heterostructure nanocomposite had been successfully fabricated via a facile chemical precipitation method and was applied in the photocatalytic H2 generation. The obtained g-C3N4/Ag2CrO4 photocatalysts exhibited excellent hydrogen evolution efficiency in comparing with the individual Ag2CrO4 and g-C3N4 under visible light irradiation (λ ≥ 420 nm). The composite with the optimal mass ratio of 23.1% exhibited the highest photocatalytic activity, which could be achieved 902.1 μmol g−1 h−1. The enhanced photocatalytic activityis ascribed to the formation of the Z-scheme g-C3N4/Ag2CrO4 heterostructures which possessed higher separation and transfer efficiencies of the photogenerated electron-hole pairs. Moreover, owing to the firmly combination between Ag2CrO4 and g-C3N4 in heterostructures, the photocorrosion of Ag2CrO4 nanoparticles was strongly suppressed. This study might help to understand the mechanism of the g-C3N4/silver composites and provided a new insight to the design of the Z-scheme heterostructures.
Experimental Section
Preparation
All reagents (except the urea was chemical grade) were analytical grade and used without further purification. The product of bulk g-C3N4 was obtained by a simple calcination method81. Typically, the precursor urea (10 g) was calcined at 600 °C for 4 h with a ramp rate of 5 °C/min in a covered alumina crucible in order to prevent sublimation of urea and kept the calcinations took place in a static air atmosphere. The obtained light-yellow powder was washed and dried at 50 °C in a vacuum oven. After that the light-yellow products were milled into powder in an agate mortar for further experiments.
The g-C3N4/Ag2CrO4 composites were prepared by an in-situ chemical precipitation method under room temperature. Typically, 100 mg as-prepared g-C3N4 samples were immersed into 40 mL ultra-pure water and ultrasonically dispersed for 15 min. After that, 17.6 mg of K2CrO4 was added to the suspension under stirring at room temperature for 1 h, and then was treated using ultrasonic treatment for 1 h to ensure that CrO42− was fully adsorbed on the surface of g-C3N4 sheets. Afterwards, an aqueous solution of AgNO3 (30.8 mg in 20 mL of water) was drop wise added to the suspension under stirring. After stirring for another 2 h, the precipitate was collected by centrifugation and washed with ethanol and water for several times, and dried at 60 °C for 12 h under vacuum condition. Finally, the g-C3N4/Ag2CrO4 composite with a theoretical weight ratio of Ag2CrO4 to g-C3N4 at 30:100 was prepared and named as g-C3N4/Ag2CrO4(23.1%). The g-C3N4/Ag2CrO4 composites with different mass ratios were fabricated by changing the addition amount of AgNO3 and K2CrO4 solution. The samples were marked as g-C3N4/Ag2CrO4(9.1%), g-C3N4/Ag2CrO4(16.7%), g-C3N4/Ag2CrO4(28.6%), and g-C3N4/Ag2CrO4(33.3%). The Ag2CrO4 was synthesized by the similar method but without g-C3N4.
Characterization
The phase structures were analyzed by X-ray diffraction (XRD) with a powder diffractometer (XRD-6000, Shimadzu, Japan). The topographies were observed by using transmission electron microscopy (TEM, Tecnai G2 20 S-TWIN, FEI, America). Energy dispersive spectroscopy (EDS) was obtained from a scanning electron microscopy (JSM 7500F, Japan Electron Optics Laboratory Co., Ltd., Japan) equipped with EDS attachment (INCA Energy 250, Oxford, America). Chemical compositions of the samples were also analyzed using X-ray photoelectron spectroscopy (XPS, ESCALAB 250Xi, Thermo Fisher, America). Uv-visible diffuse reflectance spectra (UV-vis DRS) were collected on a spectrophotometer (UV-3600, Shimadzu, Japan). Fourier-transform infrared (FT-IR) spectra were obtained on a FT-IR spectrophotometer (Nicolet iS10 IR, Thermo Scientific, America). Photoluminescence (PL) spectra were recorded on a fluorescence spectrometer (F-4500, Hitachi, Japan) with photomultiplier tube voltage of 400 V and scanning speed of 240 nm/min. The photocurrent experiments were performed on an electrochemical workstation (CHI 660D, Shanghai Chen Hua Instrument Co., Ltd., China) in a standard three-electrode system with Pt plate and Ag/AgCl (saturated KCl) electrode as counter electrode and reference electrode, respectively. A solar simulator illumination (CXE-350, Beijing Aodite Photoelectronic Technology Co., Ltd., China) at intensity of 100 mW cm−2 was used. The working electrode was prepared as follows: 10 mg powder was dispersed ultrasonically in 0.5 mL of mixture solution (water: ethanol: Nafion (5%) = 230:250:20). Then, 20 μL of the resulting colloidal dispersion (20 mg/mL) was drop-cast onto a piece of ITO with a fixed area of 1 cm × 1 cm, and the electrodes were dried under ambient for 4 h.
Photocatalytic activity of H2 evolution
Photocatalytic H2-evolution experiments were performed in a gas-closed circulation system (Labsolar-6A, Beijing Perfectlight Technology Co., Ltd., China) with a top-irradiation quartz vessel. In a typical experiment, 50 mg of the as-prepared photocatalyst was dispersed in 100 mL mixed solution containing 75 mL water and 25 mL methanol (with 10 mM NaHCO3). 0.6% of co-catalyst Pt was deposited onto the surface of the photocatalyst by in-site photo-deposition when a certain amount of H2PtCl6 solution was added. Before irradiation, the air in the system was removed by a vacuum pump. A 300 W Xe lamp (light intensity: 200 mW cm−2, Microsolar300, Beijing Perfectlight Technology Co., Ltd., China) with a 420 nm cut off filter was used as the visible light source. The produced hydrogen was in situ detected periodically using an online gas chromatograph (GC7900, Tech-comp Shanghai Co., Ltd., China) with argon as carried gas.
Electronic supplementary material
Acknowledgements
This work was supported by the National Natural Science Foundation of China [grant numbers 21641006], the International Science and Technology Cooperation Program of China [grant number 2014DFA52820], the National Key Research and Development Program of China [2017YFA0206902] and the National Basic Research Program [grant number 2012CB720904].
Author Contributions
Y.C. and J.Z. supervised the project. Y.C. designed and carried out all experiments. B.L. and Q.Q. helped with the experiments in electrochemical test, and with the SEM measurements. H.C. helped with the TEM measurements. K.W. and Z.L. helped with the analysis of the experimental results. Y.C. wrote the manuscript with important input from all authors.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Jin Zhai, Email: zhaijin@buaa.edu.cn.
Kefeng Wang, Email: wangkf2007@163.com.
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-018-34287-w.
References
- 1.Schlapbach L, Züttel A. Hydrogen-storage materials for mobile applications. Nature. 2001;415:353–358. doi: 10.1038/35104634. [DOI] [PubMed] [Google Scholar]
- 2.Zhu QL, Xu Q. Liquid organic and inorganic chemical hydrides for high-capacity hydrogen storage. Energ. Environ. Sci. 2015;8:478–512. doi: 10.1039/C4EE03690E. [DOI] [Google Scholar]
- 3.Edwards PP, Kuznetsov VL, David WIF, Brandon NP. Hydrogen and fuel cells: towards a sustainable energy future. Energ. Policy. 2008;36:4356–4362. doi: 10.1016/j.enpol.2008.09.036. [DOI] [Google Scholar]
- 4.Satyapal S, Petrovic J, Read C, Thomas G, Ordaz G. The U.S. department of energy’s national hydrogen storage project: progress towards meeting hydrogen-powered vehicle requirements. Catal. Today. 2007;120:246–256. doi: 10.1016/j.cattod.2006.09.022. [DOI] [Google Scholar]
- 5.Hu B, et al. Hydrothermal synthesis g-C3N4/Nano-InVO4 nanocomposites and enhanced photocatalytic activity for hydrogen production under visible light irradiation. ACS Appl. Mater. Inter. 2015;7:18247–1825. doi: 10.1021/acsami.5b05715. [DOI] [PubMed] [Google Scholar]
- 6.Nayak S, Mohapatra L, Parida K. Visible light-driven novel g-C3N4/NiFe-LDH composite photocatalyst with enhanced photocatalytic activity towards water oxidation and reduction reaction. J. Mater. Chem. A. 2015;3:18622–18635. doi: 10.1039/C5TA05002B. [DOI] [Google Scholar]
- 7.Cook TR, et al. Solar energy supply and storage for the legacy and nonlegacy worlds. Chem. Rev. 2010;110:6474–6502. doi: 10.1021/cr100246c. [DOI] [PubMed] [Google Scholar]
- 8.Lewis NS, Nocera DG. Powering the planet: Chemical challenges in solar energy utilization. PNAS. 2006;103:15729–15735. doi: 10.1073/pnas.0603395103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gray HB. Powering the planet with solar fuel. Nat. Chem. 2009;1:112–112. doi: 10.1038/nchem.206. [DOI] [PubMed] [Google Scholar]
- 10.Turner JA. Sustainable hydrogen production. Science. 2004;305:972–974. doi: 10.1126/science.1103197. [DOI] [PubMed] [Google Scholar]
- 11.Hou YD, et al. Bioinspired molecular co-catalysts bonded to a silicon photocathode for solar hydrogen evolution. Nat. Mater. 2011;10:434–438. doi: 10.1038/nmat3008. [DOI] [PubMed] [Google Scholar]
- 12.Laursen AB, Kegnæs S, Dahl S, Chorkendorff I. Molybdenum sulfides-efficient and viable materials for electro-and photoelectrocatalytic hydrogen evolution. Energ. Environ. Sci. 2012;5:5577–5591. doi: 10.1039/c2ee02618j. [DOI] [Google Scholar]
- 13.Morales-Guio CG, Stern LA, Hu XL. Nanostructured hydrotreating catalysts for electrochemical hydrogen evolution. Chem. Soc. Rev. 2014;43:6555–6569. doi: 10.1039/C3CS60468C. [DOI] [PubMed] [Google Scholar]
- 14.Faber MS, Jin S. Earth-abundant inorganic electrocatalysts and their nanostructures for energy conversion applications. Energ. Environ. Sci. 2014;7:3519–3542. doi: 10.1039/C4EE01760A. [DOI] [Google Scholar]
- 15.Han C, et al. In situ synthesis of graphitic-C3N4 nanosheet hybridized N-doped TiO2 nanofibers for efficient photocatalytic H2 production and degradation. Nano Res. 2014;8:1199–1209. doi: 10.1007/s12274-014-0600-2. [DOI] [Google Scholar]
- 16.Ding XL, et al. Enhanced photocatalytic H2 evolution over CdS/Au/g-C3N4 composite photocatalyst under visible-light irradiation. APL Mater. 2015;3:104410. doi: 10.1063/1.4926935. [DOI] [Google Scholar]
- 17.Yu JX, et al. Novel Fe2(MoO4)3/g-C3N4 heterojunction for efficient contaminant removal and hydrogen production under visible light irradiation. Sol. Energy. 2016;139:355–364. doi: 10.1016/j.solener.2016.10.014. [DOI] [Google Scholar]
- 18.Fujishima A, Honda K. Electrochemical photolysis of water at a semiconductor electrode. Nature. 1972;238:37–38. doi: 10.1038/238037a0. [DOI] [PubMed] [Google Scholar]
- 19.Tachibana Y, Vayssieres L, Durrant JR. Artificial photosynthesis for solar water-splitting. Nat. Photonics. 2012;6:511–518. doi: 10.1038/nphoton.2012.175. [DOI] [Google Scholar]
- 20.Hisatomi T, Kubota J, Domen K. Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting. Chem. Soc. Rev. 2014;43:7520–7535. doi: 10.1039/C3CS60378D. [DOI] [PubMed] [Google Scholar]
- 21.Zada A, et al. Exceptional visible-light-driven cocatalyst-free photocatalytic activity of g-C3N4 by well designed nanocomposites with plasmonic Au and SnO2. Adv. Energy Mater. 2016;6:1601190. doi: 10.1002/aenm.201601190. [DOI] [Google Scholar]
- 22.Jiang J, Yu JG, Cao SW. Au/PtO nanoparticle-modified g-C3N4 for plasmon-enhanced photocatalytic hydrogen evolution under visible light. J. Colloi Inter. Sci. 2016;461:56–63. doi: 10.1016/j.jcis.2015.08.076. [DOI] [PubMed] [Google Scholar]
- 23.Yan HJ, Yang HX. TiO2-g-C3N4 composite materials for photocatalytic H2 evolution under visible light irradiation. J. Alloy. Compd. 2011;509:L26–L29. doi: 10.1016/j.jallcom.2010.09.201. [DOI] [Google Scholar]
- 24.Ge L, Han CC, Xiao XL, Guo LL. Synthesis and characterization of composite visible light active photocatalysts MoS2-g-C3N4 with enhanced hydrogen evolution activity. Int. J. Hydrogen Energ. 2013;38:6960–6969. doi: 10.1016/j.ijhydene.2013.04.006. [DOI] [Google Scholar]
- 25.Zhao WR, et al. Enhanced photocatalytic activity of all-solid-state g-C3N4/Au/P25 Z-scheme system for visible-light-driven H2 evolution. Int. J. Hydrogen Energ. 2016;41:6277–6287. doi: 10.1016/j.ijhydene.2016.02.148. [DOI] [Google Scholar]
- 26.Hu SZ, et al. Enhanced visible light photocatalytic performance of g-C3N4 photocatalysts co-doped with iron and phosphorus. Appl. Surf. Sci. 2014;311:164–171. doi: 10.1016/j.apsusc.2014.05.036. [DOI] [Google Scholar]
- 27.Akple MS, et al. Enhanced visible light photocatalytic H2-production of g-C3N4/WS2 composite heterostructures. Appl. Surf. Sci. 2015;358:196–203. doi: 10.1016/j.apsusc.2015.08.250. [DOI] [Google Scholar]
- 28.Jiang DL, Chen LL, Xie JM, Chen M. Ag2S/g-C3N4 composite photocatalysts for efficient Pt-free hydrogen production. The co-catalyst function of Ag/Ag2S formed by simultaneous photodeposition. Dalton T. 2014;43:4878–4885. doi: 10.1039/C3DT53526F. [DOI] [PubMed] [Google Scholar]
- 29.Wang JX, Huang J, Xie HL, Qu AL. Synthesis of g-C3N4/TiO2 with enhanced photocatalytic activity for H2 evolution by a simple method. Int. J. Hydrogen Energ. 2014;39:6354–6363. doi: 10.1016/j.ijhydene.2014.02.020. [DOI] [Google Scholar]
- 30.Zhou P, Yu JG, Jaroniec M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014;26:4920–4935. doi: 10.1002/adma.201400288. [DOI] [PubMed] [Google Scholar]
- 31.Bard AJ, Fox MA. Artificial photosynthesis: solar splitting of water to hydrogen and oxygen. Chem. Res. 1995;28:141–145. doi: 10.1021/ar00051a007. [DOI] [Google Scholar]
- 32.Kothe T, et al. Combination of a photosystem 1-based photocathode and a photosystem 2-based photoanode to a Z-scheme mimic for biophotovoltaic applications. Angew. Chem. Int. Edit. 2013;52:14233–14236. doi: 10.1002/anie.201303671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bard AJ. Photoelectrochemistry and heterogeneous photocatalysis at semiconductors. J. Photochem. 1979;10:59–75. doi: 10.1016/0047-2670(79)80037-4. [DOI] [Google Scholar]
- 34.Hagiwara H, Inoue T, Ida S, Ishihara T. Long-time charge separation in porphyrin/KTa(Zr)O3 as water splitting photocatalyst. Phys. Chem. Chem. Phys. 2011;13:18031–18037. doi: 10.1039/c1cp22425e. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki Y, Nemoto H, Saito K, Kudo A. Solar water splitting using powdered photocatalysts driven by Z-schematic interparticle electron transfer without an electron mediator. J. Phys. Chem. C. 2009;113:17536–17542. doi: 10.1021/jp907128k. [DOI] [Google Scholar]
- 36.Liu C, Tang JY, Chen HM, Liu B, Yang PD. A fully integrated nanosystem of semiconductor nanowires for direct solar water splitting. Nano Lett. 2013;13:2989–2992. doi: 10.1021/nl401615t. [DOI] [PubMed] [Google Scholar]
- 37.Yu JG, Wang SH, Low JX, Xiao W. Enhanced photocatalytic performance of direct Z-scheme g-C3N4-TiO2 photocatalysts for the decomposition of formaldehyde in air. Phys. Chem. Chem. Phys. 2013;15:16883–16890. doi: 10.1039/c3cp53131g. [DOI] [PubMed] [Google Scholar]
- 38.Ye R, et al. Fabrication of CoTiO3/g-C3N4 hybrid photocatalysts with enhanced H2evolution: Z-scheme photocatalytic mechanism insight. ACS Appl. Mater. Inter. 2016;8:13879–13889. doi: 10.1021/acsami.6b01850. [DOI] [PubMed] [Google Scholar]
- 39.Martin DJ, Reardon PJT, Moniz SJA, Tang JW. Visible light-driven pure water splitting by a nature-inspired organic semiconductor-based system. J. Am. Chem. Soc. 2014;136:12568–12571. doi: 10.1021/ja506386e. [DOI] [PubMed] [Google Scholar]
- 40.Katsumata H, Tachi Y, Suzuki T, Kaneco S. Z-scheme photocatalytic hydrogen production over WO3/g-C3N4 composite photocatalysts. RSC Adv. 2014;4:21405–21409. doi: 10.1039/C4RA02511C. [DOI] [Google Scholar]
- 41.He Y, Zhang L, Teng B, Fan M. New application of Z-Scheme Ag3PO4/g-C3N4 composite in converting CO2 to fuel. Environ. Sci. Technol. 2015;49:649–656. doi: 10.1021/es5046309. [DOI] [PubMed] [Google Scholar]
- 42.Hong Y, et al. In-situ synthesis of direct solid-state Z-scheme V2O5/g-C3N4 heterojunctions with enhanced visible light efficiency in photocatalytic degradation of pollutants. Appl. Catal. B-Environ. 2016;180:663–673. doi: 10.1016/j.apcatb.2015.06.057. [DOI] [Google Scholar]
- 43.Bai Y, Wang PQ, Liu JY, Liu XJ. Enhanced photocatalytic performance of direct Z-scheme BiOCl-g-C3N4 photocatalysts. RSC Adv. 2014;4:19456. doi: 10.1039/c4ra01629g. [DOI] [Google Scholar]
- 44.Liu W, Shen J, Yang X, Liu Q, Tang H. Dual Z-scheme g-C3N4/Ag3PO4/Ag2MoO4 ternary composite photocatalyst for solar oxygen evolution from water splitting. Appl. Surf. Sci. 2018;456:369–378. doi: 10.1016/j.apsusc.2018.06.156. [DOI] [Google Scholar]
- 45.Chen S, Hu Y, Meng S, Fu X. Study on the separation mechanisms of photogenerated electrons and holes for composite photocatalysts g-C3N4-WO3. Appl. Catal. B-Environ. 2014;150-151:564–573. doi: 10.1016/j.apcatb.2013.12.053. [DOI] [Google Scholar]
- 46.Yu W, et al. Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production. Appl. Catal. B-Environ. 2017;219:693–704. doi: 10.1016/j.apcatb.2017.08.018. [DOI] [Google Scholar]
- 47.Wang JC, et al. Indirect Z-Scheme BiOI/g-C3N4 photocatalysts with enhanced photoreduction CO2 activity under visible light irradiation. ACS Appl. Mater. Interfaces. 2016;8:3765–3775. doi: 10.1021/acsami.5b09901. [DOI] [PubMed] [Google Scholar]
- 48.Li M, et al. Highly selective CO2 photoreduction to CO over g-C3N4/Bi2WO6 composites under visible light. J. Mater. Chem. A. 2015;3:5189–5196. doi: 10.1039/C4TA06295G. [DOI] [Google Scholar]
- 49.Xu DF, Cao SW, Zhang JF, Cheng B, Yu JG. Effects of the preparation method on the structure and the visible-light photocatalytic activity of Ag2CrO4. Beilstein J. Nanotech. 2014;5:658–666. doi: 10.3762/bjnano.5.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu D, et al. Photocatalytic activity of Ag2MO4 (M=Cr, Mo, W) photocatalysts. J. Mater. Chem. A. 2015;3:20153–20166. doi: 10.1039/C5TA05248C. [DOI] [Google Scholar]
- 51.Feizpoor S, Habibi-Yangjeh A, Vadivel S. Novel TiO2/Ag2CrO4 nanocomposites: Efficient visible-light-driven photocatalysts with n-n heterojunctions. J. Photoch. and Photobio. A-Chem. 2017;341:57–68. doi: 10.1016/j.jphotochem.2017.03.028. [DOI] [Google Scholar]
- 52.Xu D, Cheng B, Cao S, Yu J. Enhanced photocatalytic activity and stability of Z-scheme Ag2CrO4-GO composite photocatalysts for organic pollutant degradation. Appl. Cataly. B-Environ. 2015;164:380–388. doi: 10.1016/j.apcatb.2014.09.051. [DOI] [Google Scholar]
- 53.Pirhashemi M, Habibi-Yangjeh A. Novel ZnO/Ag2CrO4 nanocomposites with n-n heterojunctions as excellent photocatalysts for degradation of different pollutants under visible light. J. Mater. Sci.-Mater. El. 2016;27:4098–4108. doi: 10.1007/s10854-015-4269-4. [DOI] [Google Scholar]
- 54.Dong F, et al. In situ construction of g-C3N4/g-C3N4 metal-Free heterojunction for enhanced visible-light photocatalysis. ACS Appl. Mater. Inter. 2013;5:11392–11401. doi: 10.1021/am403653a. [DOI] [PubMed] [Google Scholar]
- 55.Luo J, et al. Facile fabrication and enhanced visible-light photocatalytic activity of In2O3/Ag2CrO4 composites. RSC Adv. 2016;6:52627–52635. doi: 10.1039/C6RA09564J. [DOI] [Google Scholar]
- 56.Luo J, et al. Enhanced photodegradation activity of methyl orange over Ag2CrO4/SnS2 composites under visible light irradiation. Mater. Res. Bull. 2016;77:291–299. doi: 10.1016/j.materresbull.2016.02.005. [DOI] [Google Scholar]
- 57.Sun L, et al. Enhanced visible-light photocatalytic activity of g-C3N4–ZnWO4 by fabricating a heterojunction: investigation based on experimental and theoretical studies. J. Mater. Chem. 2012;22:23428–23438. doi: 10.1039/c2jm34965e. [DOI] [Google Scholar]
- 58.Li H, et al. Synthesis and characterization of g-C3N4/Bi2MoO6 heterojunctions with enhanced visible light photocatalytic activity. Appl. Catal. B-Environ. 2014;160-161:89–97. doi: 10.1016/j.apcatb.2014.05.019. [DOI] [Google Scholar]
- 59.Faisal M, et al. Synthesis of highly dispersed silver doped g-C3N4 nanocomposites with enhanced visible-light photocatalytic activity. Mater. Design. 2016;98:223–230. doi: 10.1016/j.matdes.2016.03.019. [DOI] [Google Scholar]
- 60.Yu Q, Guo S, Li X, Zhang M. Template free fabrication of porous g-C3N4/graphene hybrid with enhanced photocatalytic capability under visible light. Mater. Technol. 2014;29:172–178. doi: 10.1179/1753555714Y.0000000126. [DOI] [Google Scholar]
- 61.Fu Y, et al. Copper ferrite-graphene hybrid: a multifunctional heteroarchitecture for photocatalysis and energy storage. Ind. Eng. Chem. Res. 2012;51:11700–11709. doi: 10.1021/ie301347j. [DOI] [Google Scholar]
- 62.Ouyang SX, et al. Correlation of crystal structures, electronic structures, and photocatalytic properties in a series of Ag-based oxides: AgAlO2, AgCrO2, and Ag2CrO4. J. Phys. Chem. C. 2008;112:3134–3141. doi: 10.1021/jp077127w. [DOI] [Google Scholar]
- 63.Fu Y, Wang X. Magnetically Separable ZnFe2O4-graphene catalyst and its high photocatalytic performance under visible light irradiation. Ind. Eng. Chem. Res. 2011;50:7210–7218. doi: 10.1021/ie200162a. [DOI] [Google Scholar]
- 64.Fu Y, Xiong P, Chen H, Sun X, Wang X. High photocatalytic activity of magnetically separable manganese ferrite−graphene heteroarchitectures. Ind. Eng. Chem. Res. 2012;51:725–731. doi: 10.1021/ie2026212. [DOI] [Google Scholar]
- 65.Ge L, et al. Synthesis and efficient visible light photocatalytic hydrogen evolution of polymeric g-C3N4 coupled with CdS quantum dots. J. Phys. Chem. C. 2012;116:13708–13714. doi: 10.1021/jp3041692. [DOI] [Google Scholar]
- 66.Li XK, Ye JH. Photocatalytic degradation of rhodamine B over Pb3Nb4O13/fumed SiO2 composite under visible light irradiation. J. Phys. Chem. C. 2007;111:13109–13116. doi: 10.1021/jp072752m. [DOI] [Google Scholar]
- 67.Liu Y, et al. Novel visible light-induced g-C3N4-Sb2S3/Sb4O5Cl2 composite photocatalysts for efficient degradation of methyl orange. Catal. Commun. 2015;70:17–20. doi: 10.1016/j.catcom.2015.07.015. [DOI] [Google Scholar]
- 68.Godin R, Wang Y, Zwijnenburg MA, Tang JW, Durrant JR. Time-resolved spectroscopic investigation of charge trapping in carbon nitrides photocatalysts for hydrogen generation. J. Am. Chem. Soc. 2017;139:5216–5224. doi: 10.1021/jacs.7b01547. [DOI] [PubMed] [Google Scholar]
- 69.Deng Y, et al. Facile fabrication of a direct Z-scheme Ag2CrO4/g-C3N4 photocatalystwith enhanced visible light photocatalytic activity. J. Mol. Catal. A-Chem. 2016;421:209–221. doi: 10.1016/j.molcata.2016.05.024. [DOI] [Google Scholar]
- 70.Pirhashemi M, Habibi-Yangjeh A. Ultrasonic-assisted preparation of plasmonic ZnO/Ag/Ag2WO4 nanocomposites with high visible-light photocatalytic performance for degradation of organic pollutants. J. Colloid Interf. Sci. 2017;491:216–229. doi: 10.1016/j.jcis.2016.12.044. [DOI] [PubMed] [Google Scholar]
- 71.Akhundi A, Habibi-Yangjeh A. High performance magnetically recoverable g-C3N4/Fe3O4/Ag/Ag2SO3 plasmonic photocatalyst for enhanced photocatalytic degradation of water pollutants. Adv. Powder Technol. 2017;28:565–574. doi: 10.1016/j.apt.2016.10.025. [DOI] [Google Scholar]
- 72.Shekofteh-Gohari M, Habibi-Yangjeh A. Fe3O4/ZnO/CoWO4 nanocomposites: Novel magnetically separable visiblelight-driven photocatalysts with enhanced activity in degradation of different dye pollutants. Ceram. Int. 2017;43:3063–3071. doi: 10.1016/j.ceramint.2016.11.115. [DOI] [Google Scholar]
- 73.Padhi DK, Parida K, Singh SK. Facile fabrication Of RGO/N-GZ mixed oxide nanocomposite for efficient hydrogen production under visible Light. J. Phys. Chem. C. 2015;119:6634–6646. doi: 10.1021/acs.jpcc.5b00311. [DOI] [Google Scholar]
- 74.Dai K, Lu LH, Liang CH, Liu Q, Zhu GP. Heterojunction of facet coupled g-C3N4/surface-fluorinated TiO2 nanosheets for organic pollutants degradation under visible LED lightirradiation. Appl. Catal. B-Environ. 2014;156–157:331–340. doi: 10.1016/j.apcatb.2014.03.039. [DOI] [Google Scholar]
- 75.Dai K, et al. Plasmonic TiO2/AgBr/Ag ternary composite nanosphere with heterojunction structure for advanced visible light photocatalyst. Appl. Surf. Sci. 2014;314:864–871. doi: 10.1016/j.apsusc.2014.06.183. [DOI] [Google Scholar]
- 76.Chen F, et al. Enhanced visible light photocatalytic activity and mechanism of ZnSn(OH)(6) nanocubes modified with AgI nanoparticles. Catal. Commun. 2016;73:1–6. doi: 10.1016/j.catcom.2015.10.003. [DOI] [Google Scholar]
- 77.Huang LY, et al. Synthesis and characterization of g-C3N4/MoO3 photocatalyst withimproved visible-light photoactivity. Appl. Sur. Sci. 2013;283:25–32. doi: 10.1016/j.apsusc.2013.05.106. [DOI] [Google Scholar]
- 78.Xu H, et al. Novel visible-light-driven AgX/graphite-like C3N4 (X = Br, I) hybrid materials with synergistic photocatalytic activity. Appl. Catal. B-Environ. 2013;129:182–193. doi: 10.1016/j.apcatb.2012.08.015. [DOI] [Google Scholar]
- 79.Yue D, et al. Enhancement of visible photocatalytic performances of a Bi2MoO6-BiOCl nanocomposite with plate-on-plate heterojunction structure. Phys. Chem. Chem. Phys. 2014;16:26314–26321. doi: 10.1039/C4CP03865G. [DOI] [PubMed] [Google Scholar]
- 80.Wang XX, Chen J, Guan XJ, Guo LJ. Enhanced efficiency and stability for visible light driven water splitting hydrogen production over Cd0.5Zn0.5S/g-C3N4 composite photocatalyst. Int. J. Hydrogen Energ. 2015;40:7546–7552. doi: 10.1016/j.ijhydene.2014.11.055. [DOI] [Google Scholar]
- 81.Hong JD, Zhang W, Wang YB, Zhou TH, Xu R. Photocatalytic reduction of carbon dioxide over self-assembled carbon nitride and layered double hydroxide: the role of carbon dioxide enrichment. ChemCatChem. 2014;6:2315–2321. doi: 10.1002/cctc.201402195. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.