Abstract
Backgrounds and purpose: Robotic surgery has been applied in gastric carcinoma over a decade. Although a series of studies were performed to investigate the short-term outcomes of robot-assisted gastrectomy, few papers were in view of long-term outcomes. The current study was aimed to explore the oncological outcomes of robotic gastrectomy for gastric cancer patients. Methods: A total of 606 gastric cancer patients who underwent robot-assisted gastrectomy during March 2010 through March 2017, were enrolled in this research. The clinicopathologic characteristics, surgical procedures along with follow-up information and prognostic factors were recorded in detail. The disease-free survival and overall survival rates were tested by Kaplan-Meier analysis. Results: All the patients underwent the robotic surgery including 15 proximal gastrectomies, 403 distal gastrectomies, 169 total gastrectomies and 19 remnant gastrectomies. Fifiy-six (9.24%) patients were lost in the follow-up process (3-87 months, a media of 42 months). There were 119 recurrences observed, including 55 local recurrences, 51 peritoneal metastasis and 13 distant metastasis. The 3-year disease-free survival and overall survival were 73.60% and 74.24%, while the 5-year disease-free survivorship and overall survival rates were 68.73% and 69.33%. The 5-year overall survival rates grouped based on TNM stage were 96.58% for IA, 88.16% for IB, 87.03% for IIA, 80.62% fo IIB, 58.50% for IIIA, 48.62% for IIIB, 45.32% for IIIC and 17.03% for IV. Conclusion: Robot-assisted gastrectomy is a valuable procedure for gastric cancer patients. Beside its feasibility and safety, it reveals an acceptable long-term clinical outcome.
Keywords: Gastric cancer, robot-assisted gastrectomy, long-term outcomes, follow-up, survival
Introduction
The minimal invasive surgery (MIS) has been in use to treat malignant tumor since last three decades. Since the first laparoscopic-assisted gastrectomy (LAG) in 1994 [1], endoscopic surgery invades for gastric cancer treatment, the remarkable superiority compared to its open counterparts has been reported previously [2,3]. However, in terms basis of several limitations such as hand trembling, inadequate flexibility and two-dimensional planar imaging suppressed its further development, particularly for some operations needed precise manipulation [4].
To overcome the drawbacks of LAG, a novel method of robot system appeared that was recommended for gastric cancer surgery these years. Entailing a three-dimensional vision with high magnification, effective tremble filtering, and three flexible wristed instruments [5], this robot has access to provide a more accurate radical gastrectomy. This seems reasonable to achieve a positive surgical and oncological outcomes in gastric cancer patients, especially at terminal stages.
Recent accumulating evidence had identified the short-term outcomes of robotic-assisted gastrectomy (RAG) for gastric cancer [6,7]. The significantly decreased blood loss and the larger number of retrieved lymph nodes (LNs) from a more thoroughly lymphadenectomy compared to LAG, indicated an appreciable effect in tumor cure [8]. On the contrary, the extended operation time was also observed in few studies [9]. By using similar data from reported postoperative recovery information, RAG was regarded as a safe and feasible approach for partial and total gastrectomy. While the surgical outcomes assessed by previous researchers, few publications were focused on the long-term outcomes of RAG, the results remained uncertain and controversial. In order to clarify the issue, our monocenter reported the 7-year experience to investigate the oncological outcomes for RAG in gastric carcinoma.
Materials and methods
Patients characteristics
This retrospective study enrolled 606 patients who underwent RAG in our single minimal invasive center of Department of General Surgery & Center of Minimally Invasive Gastrointestinal Surgery, Southwest Hospital, Third Military Medical University, Chongqing, China, from March 2010 to March 2017. The study consisted of 15 proximal gastrectomy (PG), 403 distal gastrectomies (DG), 169 total gastrectomies (TG) and the other 19 remnant gastrectomy.
The patients combined with other primary cancer, synchronous malignant tumor in other organs or tissues, hepatic or renal failure and severe cardiovascular or respiratory diseases were excluded. Additionally, the patients who received pre-operative adjuvant radiotherapy or chemotherapy, and who underwent an emergency surgery because of obstruction, massive bleeding, ulcer erforation or other fatal complications also did not accord with our inclusion criteria.
Prior to operation, all the involved cases were diagnosed through gastrointestinal barium meal, gastrofiberscope examinations and then confirmed by histopathological studies. Then the pre-operative tests of routine chest X-ray, abdominal ultrasound, and upper abdominal CT examination were performed to estimate whether there was metastasis happened in other organs or tissues. At last, each case was performed with D1, D1+, D2, D2+ lymphadenectomy or palliative gastrectomy according to the Gastric Cancer Treatment Guidelines [10]. The postoperative intravenous chemotherapy consisted of routinely administered capecitabine and oxaliplatin, assisted by the oral medications of Cinobufacin and Tegafur Gimeracil Oteracil Potassium Capsule. The follow-up study period was 3 to 87 months with a median of 42.
Operational procedures
The operations were carried out under an induction of general plus epidural anesthesia involved in an endotracheal intubation with the selected patients positioned in the supine position with legs apart. Furthermore, a gastric tube and a catheter were also placed. The locations for each trocar in RAG was following the procedure reported earlier [11] (Figure 1). According to the Japanese Gastric Cancer Treatment Guidelines and the Japanese Classification of Gastric Carcinoma [12], all the operations were conducted by several experienced endoscopic surgeons. Concrete surgical procedures were also recorded in details by our published literature [11,13,14].
Figure 1.
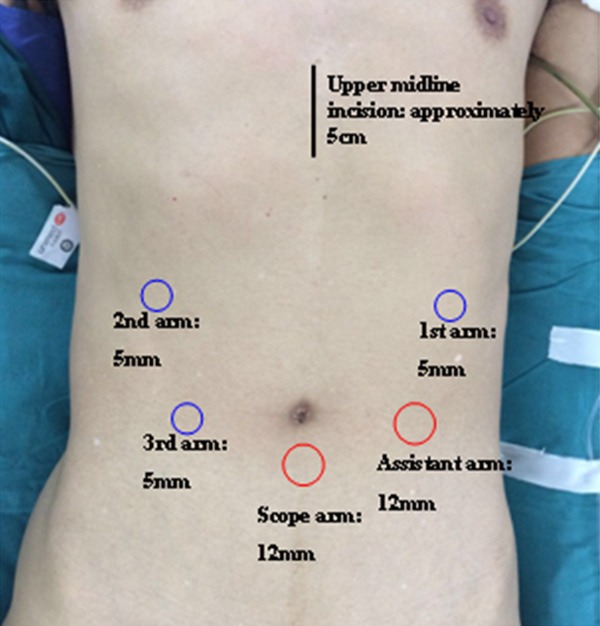
The placement and size for each trocar in RAG.
In brief, based on the TNM stages and tumor location, subtotal or total gastrectomy with D1, D1+, D2, D2+ LNs dissection or palliative gastrectomy using Billroth-I, Billroth-II, esophagogastric anastomosis or Roux-en-Y reconstruction were carried out for each case. The patient characteristics and surgical performance collected, tabulated and gone through the statistical analysis. The observation indices for subsequent studies of oncological outcomes include postoperative morbidity and the follow-up findings such as the incidence number of morbidities, recurrence rate, information of survival, death, and loss to follow-up, the overall and stratified prognosis from the survival analyses.
Statistical analysis
Data were expressed as the mean ± standard deviation. The univariate and multivariate analyses were applied to explore the risk factors associated with survival following RAG for the patients. Two-sided statistical tests were considered statistically significant (P<0.05). The cumulative survival time was calculated by using Kaplan-Meier method. All statistical analyses were performed by using SPSS 19.0 software package for Windows (SPSS, Inc., Chicago, IL).
Results
Backgrounds of patients
The clinicopathology characteristics were summarized in Table 1. With 445 males and 161 females, all the cases receiving RAG had a mean age of 56.79±10.50 years, an average Body Mass Index (BMI) was 22.21±2.94 kg/m2. Tumors from 103 patients located in the upper third of the stomach, 263 in the middle third, 221 in the lower third and the remaining 19 in the remnant stomach. On the basis of the 7th pathologic classification for gastric carcinoma from International Union Against Cancer (UICC), postoperative pathological results indicated the number of individuals with different TNM stage (IA, IB, IIA, IIB, IIIA, IIIB, IIIC and IV) were 99, 65, 32, 119, 75, 70, 115, 31, respectively. Forty-seven patients underwent a history of abdominal surgery and each of which had an effective recovery.
Table 1.
Patients characteristics and surgical outcomes
| Variables | N (number=606) | Percentage (%) |
|---|---|---|
| Age (year) | 56.79±10.50 | |
| Gender | ||
| Male | 445 | 73.43 |
| Female | 161 | 26.57 |
| BMI | 22.21±2.94 | |
| Tumor location | ||
| Upper third | 103 | 16.99 |
| Middle third | 263 | 43.4 |
| Lower third | 221 | 36.48 |
| Remnant | 19 | 3.13 |
| TNM stage | ||
| IA | 99 | 16.34 |
| IB | 65 | 10.73 |
| IIA | 32 | 5.28 |
| IIB | 119 | 19.63 |
| IIIA | 75 | 12.38 |
| IIIB | 70 | 11.55 |
| IIIC | 115 | 18.98 |
| IV | 31 | 5.11 |
| Histologic grade | ||
| Well differentiated | 18 | 2.97 |
| Moderately differentiated | 149 | 24.59 |
| Poorly differentiated | 390 | 64.36 |
| Undifferentiated | 0 | 0 |
| Others | 49 | 8.08 |
| Past abdominal surgery | ||
| Yes | 47 | 7.76 |
| No | 559 | 92.24 |
| Type of resection | ||
| Proximal gastrectomy | 15 | 2.47 |
| Total gastrectomy | 169 | 27.89 |
| Distal gastrectomy | 403 | 66.5 |
| Remnant gastrectomy | 19 | 3.14 |
| Extent of lymph node dissection | ||
| D1 | 2 | 0.33 |
| D1+ | 2 | 0.33 |
| D2 | 532 | 87.79 |
| D2+ | 39 | 6.43 |
| Palliative | 31 | 5.12 |
| Reconstruction | ||
| Esophagogastrostomy | 15 | 2.47 |
| Billroth I | 5 | 0.82 |
| Billroth II | 382 | 63.04 |
| Billroth II+Braun | 11 | 1.82 |
| Roux-en-Y | 193 | 31.85 |
| Estimated blood loss (ml) | 190.49±164.11 | |
| Operating time (min) | 279.02±69.23 | |
| Number of retrieved lymph nodes | 33.09±12.18 | |
| Conversion to open laparotomy | 41 | 6.76 |
| Resection margin | ||
| Proximal | 4.78±1.32 | |
| Distal | 6.44±3.58 | |
| Mobidity | 67 | 11.06 |
| Postoperative pneumonia | 27 | 4.45 |
| Liquefaction of incision fat | 2 | 0.33 |
| Anastomosis leakage | 5 | 0.82 |
| Intro-abdominal infection | 8 | 1.32 |
| Duodenal stump leakage | 5 | 0.83 |
| Esophagus-jejunum anastomosis site stenosis | 2 | 0.33 |
| Septic shock | 2 | 0.33 |
| Incision infection | 4 | 0.66 |
| Intraperitoneal hemorrhage | 6 | 0.99 |
| Anastomotic stenosis | 4 | 0.66 |
| Intra-abdominal abscess | 2 | 0.33 |
| Anastomotic bleeding | 2 | 0.33 |
| Mortality | 4 | 0.66 |
Operative outcomes
Surgical results indicated that for the patients were 2 of them underwent a D1 LN dissection, 2 into D1+ LN dissection, 532 into a D2 LN dissection, 39 into D2+ LN dissection, and the rest 31 cases diagnosed at stage IV received palliative operations as a makeshift. There were 602 R0 resections but no R2 resection. Only 4 R1 resections were observed in current study based on fanal pathology examinations, including 3 positive proximal margins and 1 positive distal margin. Furthermore, these surgical procedures revealed a mean blood loss as 190.49±164.11 ml and a mean number of retrieved LNs of 33.09±12.18. There were 41 patients undergone conversions to open laparotomy due to different reasons and the rate was 6.76%, and the relative data were presented in Table 1.
Postoperative morbidity and mortality
A total of 67 postoperative complications were observed in this retrospective cohort, with an incidence rate of 11.06%. Additionally, the mortality rate was 0.66% (4 of 606 cases), 3 patients were presented with a duodenal stump leakage after a Billroch-II procedure, and then underwent reoperations of exploratory laparotomy. The other patient suffered from an anastomosis leakage, who also received a second surgical procedure to reconstruct the anastomosis. Regretfully, all of them died from a postoperative multiple organ failures within a month time.
Follow-up
During follow-up, results 56 patients were lost during this period of 3 to 87 months and the follow-up rate was 90.76% (Table 2). Within the long-term postoperative observation, 119 recurrences of gastric cancer were found, consisting of 55 with local recurrences, 51 with peritoneal metastasis and 13 with distant metastasis, indicating a recurrence rate of 19.64%. For the endpoint events, 482 cases were survived but 124 were dead out of which 116 were by the recurrences and 8 from other unknown factors.
Table 2.
Follow-up results of the patients underwent RAG
| Cases (n=606) | Percentage (%) | |
|---|---|---|
| Total | 606 | 100 |
| Survival | 482 | 79.53 |
| Death | 124 | 20.46 |
| Gastric cancer-related | 116 | 19.14 |
| Other factors | 8 | 1.32 |
| Loss to follow-up | 56 | 9.24 |
| Recurrence | 119 | 19.64 |
| Local recurrence | 55 | 9.76 |
| Peritoneal metastasis | 51 | 8.42 |
| Distant metastasis | 13 | 2.15 |
Prognostic factors and survival
According to the findings from univariate analysis to explore the potential prognostic factors (Table 3), significant discrepancies of overall survival were observed in these indexes of age (P=0.001), BMI (P<0.001), TMN stage (P<0.001), type of resection (P<0.001), extent of LN dissection (P<0.001) and estimated blood loss (P<0.001). However, there were no significant differences for sex, retrieved LNs, the past abdominal surgery, operation time and resection margins. Subsequently, multivariate analysis showed that the significantly different results existing in age (P=0.003), BMI (P=0.009) and TMN stage (P<0.001) (Table 3). This strong evidence trend to testify these three indexes as independent prognostic factors.
Table 3.
Prognosic factors for overall survival of the patients tested by univariate and multivariate analyses
| Factors | Univariate | Multivariate |
|---|---|---|
| P-value | P-value | |
| Age | 0.001 | 0.003 |
| Sex | 0.412 | |
| BMI | <0.001 | 0.009 |
| TNM stage | <0.001 | <0.001 |
| Retrieved lymph nodes | 0.315 | |
| Type of resection | <0.001 | 0.140 |
| Extent of lymph node dissection | <0.001 | 0.101 |
| Past abdominal surgery | 0.132 | |
| Estimated blood loss (ml) | <0.001 | 0.535 |
| Operation time | 0.081 | |
| Resection margin | ||
| Proximal | 0.894 | |
| Distal | 0.724 |
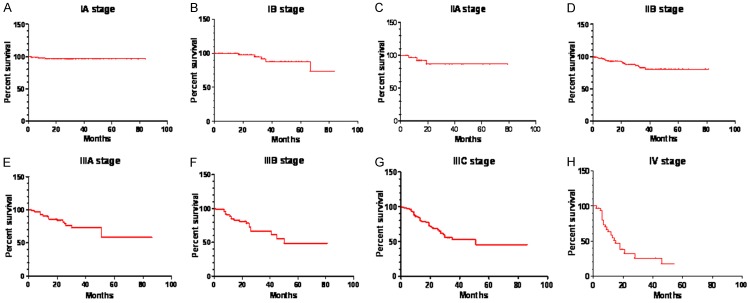
In regard to the survival analysis, our Kaplan-Meier curves indicated a 73.60% and 68.73% for 3-year disease-free survival (DFS) rate and 5-year DFS rate, respectively. Similarly, 3-year overall survival (OS) was 74.24% and the 5-year OS was 69.33% (Table 4). When stratified analyses were subgrouped based on the TNM stage, we found that the 5-year OS for each stage were IA (96.58%), IB (88.16%), IIA (87.03%), IIB (80.62%), IIIA (58.50%), IIIB (48.62%), IIIC (45.32%) and IV (17.03%) (Table 4; Figure 2A-H). Moreover, as the subgroup were classified by some other clinical data to further explore the prognosis, significant data was recorded in the analyses according to age (P=0.0008, Figure 3A), type of resection (P<0.0001, Figure 3B) and BMI (P=0.00148, Figure 3C).
Table 4.
The data of DFS/OS and the OS of tumor subgroups based on TNM stage
| Survival | ||||
|---|---|---|---|---|
|
| ||||
| 1 y | 3 y | 5 y | ||
| DFS/OS (%) | DFS (%) | 89.56 | 73.60 | 68.73 |
| OS (%) | 89.56 | 74.24 | 69.33 | |
| OS (%) based on TNM stage | IA | 96.58 | 96.58 | 96.58 |
| IB | 100 | 91.84 | 88.16 | |
| IIA | 92.46 | 87.03 | 87.03 | |
| IIB | 92.75 | 82.74 | 80.62 | |
| IIIA | 91.15 | 73.12 | 58.5 | |
| IIIB | 84.97 | 66.49 | 48.62 | |
| IIIC | 84.04 | 52.87 | 45.32 | |
| IV | 58.95 | 25.54 | 17.03 | |
Figure 2.
Cumulative 5-year overall survival conducted by Kaplan-Meier analysis. A. Stage I patients. B. Stage IB patients. C. Stage IIA patients. D. Stage IIB patients. E. Stage IIIA patients. F. Stage IIIB patients. G. Stage IIIC patients. H. Stage IV patients.
Figure 3.
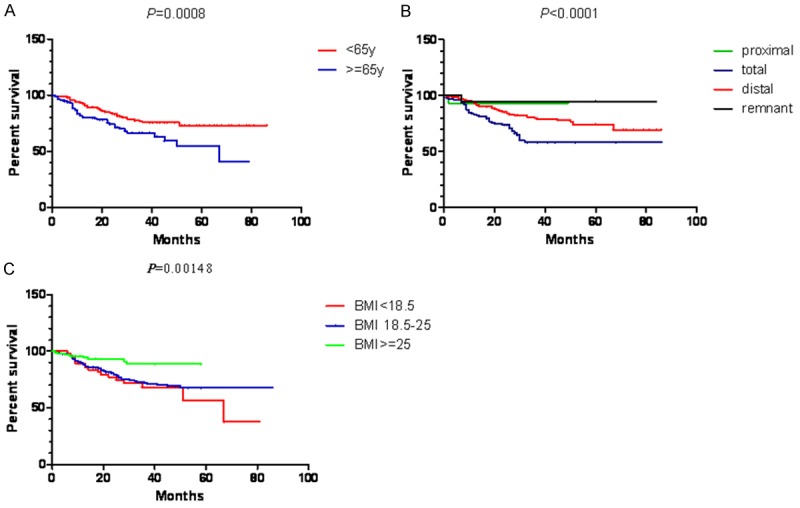
The results of cumulative 5-year overall survival for subgroup analyses. A. Subgroup analysis by the age (<65 y or ≥65 y). B. Subgroup analysis by the types of resection. C. Subgroup analysis by the BMI.
Discussion
It is well established that robot surgery was widely regarded as a novel therapeutic strategy for gastric cancer patients in recent years [15,16]. At higher magnification clearer images eliminated tremor and the flexible artificial endowrist, the robot allows a more precise procedure over laparoscopic or conditional open surgery in radical gastrectomy, which became a great constituent of MIS and offset the deficiencies from the laparoscopic-assisted counterparts.
Since Hashizume et al. firstly reported the RAG in 2003 [17], a number of investigations and meta-analyses were performed to evaluate the clinical efficacy of this novel surgical approach [18-20]. In addition, the comparable data focused on postoperative recovery such as time to first liquid diet, time to first ground activity and postoperative hospital stay, were also published recently to identify the effective short-outcomes provided by RAG. However, literature seldom reported long-term outcomes-related studies. Although a west center has offered its primary work in regard to the oncological results of RAG in gastric cancer patients [21], the enrolled number of cases seems a little small. The debates on long-term outcomes by RAG continuously existed. With a large-scale sample size in the present study, we would further explore the long-term outcomes of RAG for gastric cancer treatment.
This report showed the mean number of retrieved LNs was 33.09, along with the results of the previous robotic, laparoscopic and open gastrectomy [6,22] indicating an adequate LNs dissection of radical lymphadenectomy. The total postoperative morbidity rate was 11.06%, although in line with the related but comparative reports mentioned morbidity rate was between 5.2% and 11.58% [23-25]. Meanwhile, it is less (15.9%) than the incidence of LAG and the rate of 12.2% in the RAG-related studies [21,26]. In view of the local complications, a Japanese research group conducted a retrospective cohort study that displayed systematical results for LAG, which were approximately comparable to morbidity rate of 0.33% in both of anastomotic bleeding and anastomotic stenosis, 0.66% in wound infection, and 1.15% in intro-abdominal infection. But our 0.82% incidence for anastomosis leakage was significantly lower as reported by the Japanese group provided an association rate of 2.5% [27]. For the systemic complications, 4.45% rate for pneumonia was reported herein, however, no pulmonary embolism and edema were observed, which might benefit from the postoperative early mobilization according to the enhanced recovery following surgery (ERAS). Moreover, focused on the decreased morbidity rates, we suggested that with a long period of training and practice, professional and experienced surgeons were reasonable to contribute to preventing the occurrence of postoperative troubles especially serious complications.
About 124 deaths were observed in our study during the follow-up period, the death rate (20.46%) can be comparable to previous studies [26,28], while 56 patients were lost in the follow up process, 119 recurrences existed in the cohort and the majors were indicated to be local recurrence (9.76%) and peritoneal metastasis (8.42%), but not distant metastasis (2.15%). Despite the similar recurrence rate to the previously reported data [26,29], RAG may reveal enough restriction against distant metastasis for gastric cancer. For the purpose to determine the prognosis factors that impaired survival, we applied univariate and multivariate analyses to find the age, BMI and TNM stages as the independent prognosis factors for gastric cancer patients who underwent the radical RAG. As the TNM stage was confirmed to be involved in the prognosis, the effect of age was considered of controversial. Here we suggested that this finding might due to a more fragile host-defense condition in elderly gastric cancer patients. The rest factor of BMI made us believed that excellent nutritional status might necessary for malignancy patients to bear surgical procedures and adjuvant chemotherapies. However, a poor physical condition possibly had an adverse effect on postoperative recovery.
While following the survival analysis results in the current study, it was observed that both the 3-year DFS and OS survival rate were similar to previous reports [11] but slightly lower than the data from the Japanese group [30]. Considering the potential of the work, it was supposed that with rigorous screening for gastric cancer, the proportion of early-stage patients must be larger in Japan as strong evidences to provide a better prognosis. Whereas some investigations showed comparable data both for 5-year DFS and OS in LAG [22,31], better results were highlighted by a South Korean group [32], which might because that the group consisted of dozens of stage-IV patients enrolled in our study had decreased overall survival. The stratified analysis clarified by the TMN stage, but we failed to find any remarkable discrepancies for stage I and II patients herein and several previous reports for postoperative survival [33-35]. However, our 5-year OS in the stage III was appropriately higher than some other series for gastrectomy, particularly in the IIIC stage patients [36,37]. It is being suggested that due to the precise surgical procedures conducted by the robot system, the D2 or D2+ lymphadenectomy with a low positive resection margin rate was reasonably realistic, and the results indicated various advantages from RAG mainly helpful for the treatment of advanced gastric cancer. Furthermore, results for BMI and age based on the Kaplan-Meier analysis were in line with the former multivariate analysis reports. During the long follow-up period, 56 cases were lost so that we were not able to achieve their accurate survival status, this unavoidable limitation should be noticed in future studies.
Conclusion
In conclusion, to discuss the long-term outcomes of RAG for gastric cancer patients, we conducted this large-scale retrospective study. The interesting results including postoperative morbidity rate, the recurrence, and the 5-year DFS and OS, were to be acceptable considering the data recorded by previous studies on LAG or traditional open gastrectomy. Moreover, its potential benefit for stage III gastric carcinoma observed in the present oncological outcomes may recommend RAG as a novel and important approach for advanced gastric cancer therapy.
Acknowledgements
The authors thank Yingjie Wan, Xiao Luo and Yan Wen for collecting data and information of the patients for us.
Disclosure of conflict of interest
None.
References
- 1.Kitano S, Iso Y, Moriyama M, Sugimachi K. Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc. 1994;4:146–148. [PubMed] [Google Scholar]
- 2.Chen XZ, Wen L, Rui YY, Liu CX, Zhao QC, Zhou ZG, Hu JK. Long-term survival outcomes of laparoscopic versus open gastrectomy for gastric cancer: a systematic review and metaanalysis. Medicine (Baltimore) 2015;94:e454. doi: 10.1097/MD.0000000000000454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips JD, Nagle AP, Soper NJ. Laparoscopic gastrectomy for cancer. Surg Oncol Clin N Am. 2013;22:39–57. v–vi. doi: 10.1016/j.soc.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 4.Kuper MA, Eisner F, Konigsrainer A, Glatzle J. Laparoscopic surgery for benign and malign diseases of the digestive system: indications, limitations, and evidence. World J Gastroenterol. 2014;20:4883–4891. doi: 10.3748/wjg.v20.i17.4883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bamboat ZM, Strong VE. Minimally invasive surgery for gastric cancer. J Surg Oncol. 2013;107:271–276. doi: 10.1002/jso.23237. [DOI] [PubMed] [Google Scholar]
- 6.Patriti A, Ceccarelli G, Bellochi R, Bartoli A, Spaziani A, Di Zitti L, Casciola L. Robot-assisted laparoscopic total and partial gastric resection with D2 lymph node dissection for adenocarcinoma. Surg Endosc. 2008;22:2753–2760. doi: 10.1007/s00464-008-0129-0. [DOI] [PubMed] [Google Scholar]
- 7.Xiong B, Ma L, Zhang C. Robotic versus laparoscopic gastrectomy for gastric cancer: a meta-analysis of short outcomes. Surg Oncol. 2012;21:274–280. doi: 10.1016/j.suronc.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 8.Shen W, Xi H, Wei B, Cui J, Bian S, Zhang K, Wang N, Huang X, Chen L. Robotic versus laparoscopic gastrectomy for gastric cancer: comparison of short-term surgical outcomes. Surg Endosc. 2016;30:574–580. doi: 10.1007/s00464-015-4241-7. [DOI] [PubMed] [Google Scholar]
- 9.Kakiashvili I, Brauner E, Yshai OB, Almog R, Beny A, Kluger Y. 2279 Robotic versus laparoscopic versus open gastrectomy for gastric cancer. European Journal of Cancer. 2015;51:S425–S426. [Google Scholar]
- 10.Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2010 (ver. 3) Gastric Cancer. 2011;14:113–123. doi: 10.1007/s10120-011-0042-4. [DOI] [PubMed] [Google Scholar]
- 11.Junfeng Z, Yan S, Bo T, Yingxue H, Dongzhu Z, Yongliang Z, Feng Q, Peiwu Y. Robotic gastrectomy versus laparoscopic gastrectomy for gastric cancer: comparison of surgical performance and short-term outcomes. Surg Endosc. 2014;28:1779–1787. doi: 10.1007/s00464-013-3385-6. [DOI] [PubMed] [Google Scholar]
- 12.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5. [DOI] [PubMed] [Google Scholar]
- 13.Zhao Y, Yu P, Hao Y, Qian F, Tang B, Shi Y, Luo H, Zhang Y. Comparison of outcomes for laparoscopically assisted and open radical distal gastrectomy with lymphadenectomy for advanced gastric cancer. Surg Endosc. 2011;25:2960–2966. doi: 10.1007/s00464-011-1652-y. [DOI] [PubMed] [Google Scholar]
- 14.Bo T, Peiwu Y, Feng Q, Yongliang Z, Yan S, Yingxue H, Huaxing L. Laparoscopy-assisted vs. open total gastrectomy for advanced gastric cancer: long-term outcomes and technical aspects of a case-control study. J Gastrointest Surg. 2013;17:1202–1208. doi: 10.1007/s11605-013-2218-1. [DOI] [PubMed] [Google Scholar]
- 15.Mihmanli M, Ilhan E, Idiz UO, Alemdar A, Demir U. Recent developments and innovations in gastric cancer. World J Gastroenterol. 2016;22:4307–4320. doi: 10.3748/wjg.v22.i17.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diana M, Marescaux J. Robotic surgery. Br J Surg. 2015;102:e15–28. doi: 10.1002/bjs.9711. [DOI] [PubMed] [Google Scholar]
- 17.Hashizume M, Sugimachi K. Robot-assisted gastric surgery. Surg Clin North Am. 2003;83:1429–1444. doi: 10.1016/S0039-6109(03)00158-0. [DOI] [PubMed] [Google Scholar]
- 18.Kim HI, Han SU, Yang HK, Kim YW, Lee HJ, Ryu KW, Park JM, An JY, Kim MC, Park S, Song KY, Oh SJ, Kong SH, Suh BJ, Yang DH, Ha TK, Kim YN, Hyung WJ. Multicenter prospective comparative study of robotic versus laparoscopic gastrectomy for gastric adenocarcinoma. Ann Surg. 2016;263:103–109. doi: 10.1097/SLA.0000000000001249. [DOI] [PubMed] [Google Scholar]
- 19.Park JY, Eom BW, Jo MJ, Yoon HM, Ryu KW, Kim YW, Nam BH, Lee JH. Health-related quality of life after robot-assisted distal gastrectomy in early gastric cancer. World J Surg. 2014;38:1112–1120. doi: 10.1007/s00268-013-2390-1. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Wang G, He J, Wu F, Ren S. Robotic gastrectomy versus open gastrectomy in the treatment of gastric cancer. J Cancer Res Clin Oncol. 2017;143:105–114. doi: 10.1007/s00432-016-2240-2. [DOI] [PubMed] [Google Scholar]
- 21.Coratti A, Fernandes E, Lombardi A, Di Marino M, Annecchiarico M, Felicioni L, Giulianotti PC. Robot-assisted surgery for gastric carcinoma: five years follow-up and beyond: a single western center experience and long-term oncological outcomes. Eur J Surg Oncol. 2015;41:1106–1113. doi: 10.1016/j.ejso.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 22.Hao Y, Yu P, Qian F, Zhao Y, Shi Y, Tang B, Zeng D, Zhang C. Comparison of laparoscopyassisted and open radical gastrectomy for advanced gastric cancer: a retrospective study in a single minimally invasive surgery center. Medicine (Baltimore) 2016;95:e3936. doi: 10.1097/MD.0000000000003936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang SY, Roh KH, Kim YN, Cho M, Lim SH, Son T, Hyung WJ, Kim HI. Surgical outcomes after open, laparoscopic, and robotic gastrectomy for gastric cancer. Ann Surg Oncol. 2017;24:1770–1777. doi: 10.1245/s10434-017-5851-1. [DOI] [PubMed] [Google Scholar]
- 24.Wang G, Jiang Z, Zhao J, Liu J, Zhang S, Zhao K, Feng X, Li J. Assessing the safety and efficacy of full robotic gastrectomy with intracorporeal robot-sewn anastomosis for gastric cancer: a randomized clinical trial. J Surg Oncol. 2016;113:397–404. doi: 10.1002/jso.24146. [DOI] [PubMed] [Google Scholar]
- 25.Huang KH, Lan YT, Fang WL, Chen JH, Lo SS, Li AF, Chiou SH, Wu CW, Shyr YM. Comparison of the operative outcomes and learning curves between laparoscopic and robotic gastrectomy for gastric cancer. PLoS One. 2014;9:e111499. doi: 10.1371/journal.pone.0111499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park DJ, Han SU, Hyung WJ, Kim MC, Kim W, Ryu SY, Ryu SW, Song KY, Lee HJ, Cho GS, Kim HH Korean Laparoscopic Gastrointestinal Surgery Study (KLASS) Group. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: a large-scale multicenter retrospective study. Surg Endosc. 2012;26:1548–1553. doi: 10.1007/s00464-011-2065-7. [DOI] [PubMed] [Google Scholar]
- 27.Suda K, Man IM, Ishida Y, Kawamura Y, Satoh S, Uyama I. Potential advantages of robotic radical gastrectomy for gastric adenocarcinoma in comparison with conventional laparoscopic approach: a single institutional retrospective comparative cohort study. Surg Endosc. 2015;29:673–685. doi: 10.1007/s00464-014-3718-0. [DOI] [PubMed] [Google Scholar]
- 28.Chen K, Mou Y, Xu X, Cai J, Wu D, Pan Y, Zhang R. Short-term surgical and long-term survival outcomes after laparoscopic distal gastrectomy with D 2 lymphadenectomy for gastric cancer. BMC Gastroenterology. 2014;14:41–41. doi: 10.1186/1471-230X-14-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Eom BW, Kim YW, Lee SE, Ryu KW, Lee JH, Yoon HM, Cho SJ, Kook MC, Kim SJ. Survival and surgical outcomes after laparoscopyassisted total gastrectomy for gastric cancer: case-control study. Surg Endosc. 2012;26:3273–3281. doi: 10.1007/s00464-012-2338-9. [DOI] [PubMed] [Google Scholar]
- 30.Nakauchi M, Suda K, Susumu S, Kadoya S, Inaba K, Ishida Y, Uyama I. Comparison of the long-term outcomes of robotic radical gastrectomy for gastric cancer and conventional laparoscopic approach: a single institutional retrospective cohort study. Surg Endosc. 2016;30:5444–5452. doi: 10.1007/s00464-016-4904-z. [DOI] [PubMed] [Google Scholar]
- 31.Nashimoto A, Akazawa K, Isobe Y, Miyashiro I, Katai H, Kodera Y, Tsujitani S, Seto Y, Furukawa H, Oda I, Ono H, Tanabe S, Kaminishi M. Gastric cancer treated in 2002 in Japan: 2009 annual report of the JGCA nationwide registry. Gastric Cancer. 2013;16:1–27. doi: 10.1007/s10120-012-0163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee J, Kim W. Long-term outcomes after laparoscopy-assisted gastrectomy for advanced gastric cancer: analysis of consecutive 106 experiences. J Surg Oncol. 2009;100:693–698. doi: 10.1002/jso.21400. [DOI] [PubMed] [Google Scholar]
- 33.Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C. Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg. 2005;241:232–237. doi: 10.1097/01.sla.0000151892.35922.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N. Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg. 2010;211:33–40. doi: 10.1016/j.jamcollsurg.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Hosono S, Arimoto Y, Ohtani H, Kanamiya Y. Meta-analysis of short-term outcomes after laparoscopy-assisted distal gastrectomy. World J Gastroenterol. 2006;12:7676–7683. doi: 10.3748/wjg.v12.i47.7676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rausei S, Dionigi G, Boni L. Evaluation of the seventh american joint committee on cancer/international union against cancer classification of gastric adenocarcinoma in comparison with the sixth classification. Cancer. 2011;117:2823–2824. doi: 10.1002/cncr.25801. author reply 2824. [DOI] [PubMed] [Google Scholar]
- 37.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]