Abstract
Postoperative fever is prevalent in many breast cancer patients. Some retrospective studies proposed that postoperative fever might also be considered as a rapid rough indicator for the poor prognosis of breast cancer patients. This study aims to explore the possible molecular mechanisms underlying the relapse of breast cancer patients with early postoperative fever. Our results indicated plasma levels of lncRNA MALAT1 were elevated in breast cancer patients with early postoperative fever and were associated with RFS. Lipopolysaccharide (LPS) was able to induce fever and systemic inflammatory responses in 4T1 xenograft mice, and promote lung metastasis. But after knocking down lncRNA MALAT1, the inflammatory responses and metastasis of lung were significantly reduced. Moreover, after knocking down lncRNA MALAT1 in the 4T1 cells, TNF-α level in the supernatants was sharply decreased, and the invasion and migration induced by LPS was also weakened. Cumulatively, our data indicates that MALAT1 is closely related to recurrence and metastasis of breast cancer patients with early postoperative fever.
Keywords: MALAT1, breast cancer, postoperative fever, recurrence, metastasis
Introduction
Breast cancer is the most common malignancy among women in developed countries, accounting for 27% of total cancer cases and 14% of total cancer-related deaths in 2012 [1]. Although delicate surgical ablation, effective chemotherapy and accurate radiotherapy may eradicate the visible primary tumor, metastasis and recurrence still occur frequently in some breast cancer patients, which eventually leads to the death [2]. Metastasis is not only regulated by intrinsic genetic changes in breast cancer cells but also depends upon tumor micro-environment [3]. Several studies have demonstrated that tumor-associated chronic inflammation is a hallmark of cancer and it also promotes disease progression and metastasis [4].
Postoperative fever refers to core temperature rises in patients after surgery. Early postoperative fever (>38°C in the first 72 h) is rarely caused by an infection and usually believed to be one of the host responses to surgery [5]. As a complex systemic defense reaction in the host, postoperative fever may result from the release of specific cytokines induced by infection, injury, surgery and general anesthetic trauma [6-8]. An early retrospective study in 1987 supported the association between postoperative fever and prognosis in 387 cases of breast cancer patients [9]. Lu J, et al also found that postoperative fever may contribute to relapse of node-negative breast cancer patients and could serve as a negative indicator of disease relapse [10].
However, the exact mechanism by which the postoperative fever leads to disease relapse and progression is remained unclear. Some relevant reports suggested that elevated expressions of pro-inflammatory cytokines, such as IL-6 and TNF-α, may exacerbate the pre-existing subclinical lesions and thus promote recurrence and death in patients with postoperative fever [11-13]. Interestingly, LncRNA metastasis-associated lung adenocarcinoma transcript 1 (MALAT1) was also identified as an important pro-inflammatory factor, which regulated hyperglycemia-induced inflammatory process in the endothelial cells [14]. And it was first recognized as a biomarker to predict metastasis and survival in early-stage non-small cell lung cancer [15]. More important, Mao YF et al observed that the MALAT1 upregulation plays an important role in breast cancer development, and serum MALAT1 levels may be of great value in diagnosis in breast cancer [16].
Therefore, the present study sought to evaluate whether MALAT1 promoted the relapse of patients with postoperative fever and to identify the underlying mechanisms involved.
Materials and methods
Patient population
Two hundred fifty-eight cases of consecutive patients with primary breast carcinoma admitted to the Third Hospital of Nanchang between June 15, 2014 to December 15, 2015 were included in the present study. Patients with initial distant metastases or systemic infectious diseases, systemic immunological disease, or serious liver or renal disease at the time of surgery were excluded from the study. Ethical approval was obtained from the Third Hospital of Nanchang, and informed consent was obtained from all patients prior to sample examination. The conventional clinicopathological data and treatment protocols were carefully documented. All patients were treated according to the guidelines or recommendations for the clinical diagnosis and treatment of breast cancer.
The body temperature was measured with an oral thermometer three times a day for each patient. Consistent with previous relevant reports [6,10,17], early postoperative fever was defined as one oral temperature measured at 38°C in the 3 days after surgery. According to the postoperative oral temperature, the patients were stratified into two groups as follows: the fever group and the non-fever group. The clinicopathological characteristics of both groups were listed in Table 1.
Table 1.
Summary of breast cancer patient’s characteristics and its association with postoperative fever
| Variable | Univariate analysis, n (%) | P value | |
|---|---|---|---|
|
| |||
| Fever | Non-Fever | ||
| (n = 61) | (n = 197) | ||
| Mean Age at Diagnosis (year, x ± SD) | 51.0±11.3 | 50.4±10.8 | 0.627 |
| Histologic type | |||
| DCIS/DCIS with microinvasive | 3 (10.7) | 25 (89.3) | 0.088 |
| Ductal invasive cancer | 51 (26.7) | 140 (73.3) | |
| Others invasive cancer | 7 (17.9) | 32 (82.1) | 0.251 |
| Type of Surgery | |||
| BCT | 3 (27.3) | 8 (72.7) | 0.772 |
| Mastectomy | 58 (23.5) | 189 (76.5) | |
| Axillary lymph node status | |||
| No | 32 (19.6) | 131 (80.4) | 0.047 |
| Yes | 29 (30.5) | 66 (69.5) | |
| No of positive lymph nodes | |||
| 1-3 | 16 (25.0) | 48 (75.0) | 0.093 |
| ≥4 | 13 (41.9) | 18 (58.1) | |
| ER Status | |||
| Positive | 37 (14.34) | 135 (52.33) | 0.254 |
| Negative | 24 (22.8) | 62 (77.2) | |
| PR Status | |||
| Positive | 34 (20.6) | 131 (79.4) | 0.126 |
| Negative | 27 (29.0) | 66 (71) | |
| HER-2 Status | |||
| Positive | 17 (27.9) | 44 (72.1) | 0.374 |
| Negative | 44 (22.3) | 153 (77.7) | |
| Ki-67 Index | |||
| ≥30% | 45 (27.3) | 120 (72.7) | 0.068 |
| <30% | 16 (17.2) | 77 (82.8) | |
| Relapse events | |||
| Yes | 6 (54.5) | 5 (45.5) | 0.014 |
| No | 55 (22.3) | 192 (77.7) | |
Clinical follow-up data were available for all the patients. Personal contact with the patient were carried out in the Third Hospital of Nanchang every 3 months during the first two years and every 6 months during the next three years. The follow-up period cut-off was June 15, 2017. Release-free survival (RFS) was defined as the time from the diagnosis of breast cancer to the first local, regional or distant recurrence.
Measurement of serum IL-6 and TNF-α
For each patient, 5 mL of peripheral blood was collected within 12 hours after surgery. Then, the plasma samples were kept frozen at -80°C until biochemical assessment. The concentrations of cytokines in postoperative plasma samples and cell culture supernatants were determined by an ELISA Kit for human IL-6 and TNF-α (Elabscience Biotechnology Co. Ltd, China) according to the manufacturer’s instructions.
RNA isolation and qRT-PCR
Total RNA was extracted from plasma samples or 4T1 mammary carcinoma cells using TRI-zol® reagent according to the manufacturer’s instructions (Invitrogen, Paisley, UK) and was stored at -80°C until use. The expression of MALAT1 was determined by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) using the SYBR®Green (TaKaRa) dye detection method on an ABI StepOne PCR instrument. The primers were designed as follows: MALAT1 forward: 5’-CTTAAGCGCAGCGCCATTTT-3’; reverse: 5’-CCTCCAAAC CCCAAGACCAA-3’; GAPDH forward: 5’-AATCCCATCACCATCTTCCAG-3’; reverse: 5’-GAGCCCCAGCCTTCTCCAT-3’. The relative expression of MALAT1 was calculated by the qRT-PCR method using the GAPDH mRNA as an internal control.
Cell culture
The 4T1 mammary carcinoma cell line was purchased from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). 4T1 is a highly metastatic breast cancer cell line derived from a spontaneously arising BALB/c mammary tumor. The 4T1 mammary carcinoma cell line was maintained in RPMI-1640 medium supplemented with 10% FBS, 1% Glutamax-1 and 1% penicillin-streptomycin. Cells were routinely cultured in a humidified incubator with 5% CO2 at 37°C.
For Lipopolysaccharide (LPS) or IL-6 treatment, the cells were treated with LPS (0.5 µg/ml) or IL-6 (1 ng/ml or 10 ng/ml) after reaching 60-70% confluence in six-well plates. Both the LPS and IL-6 were dissolved in phosphate-buffered saline (PBS).
Cell transfection assays and plasmid preparation
Cell transfections were performed using the Lipofectamine 2000 kit (Invitrogen) according to the manufacturer’s instructions. To knockdown MALAT1, three siRNA oligonucleotides targeting MALAT1 and the negative control were purchased from GenePharma (Shanghai, China) and were transfected into 4T1 cells. The final MALAT1 and negative control (NC) siRNA concentrations for transfections were 50 nM.
The most effective sequence was selected by RT-qPCR and was used to construct a MALAT1-shRNA lentivirus (MALAT1-sh) (GenePharma, Shanghai, China). All siRNA sequences are listed in Table S1. After transfecting the vector into 4T1 cell, 1 μg/ml puromycin (Sigma-Aldrich) was added into the culture medium to obtain cell clones that stably express the shRNA. Then, the cells were cultured for an additional 48 hours before LPS treatment.
Transwell invasion assays
Transwell (24-well) chambers (Costar, Cambridge, MA, USA) were used to evaluate cell invasion. For the invasion assay, 1×105 4T1 cells of different groups were suspended in 200 ml serum-free medium and added into BD BioCoat Matrigel Invasion Chambers (a pore size of 8 mm; BD Biosciences). The lower chamber was filled with RPMI-1640 medium containing 10% FBS. The chamber was incubated for 24 h in 5% CO2 at 37°C. Finally, the attached cells in the lower section were stained with hematoxylin and eosin (H&E) and counted using light microscopy.
Western blot analysis
Cells were lysed in RIPA buffer containing fresh protease and phosphatase inhibitor cocktails (Sigma). Protein was separated by 10% SDS-PAGE and was transferred to PVDF membranes. The membranes were blocked in 2% skim milk and were incubated with anti-vimentin (1:1000, Abcam, USA) and anti-MMP-9 (1:1000, Wanlei, China) overnight at 4°C. Anti-GAPDH antibody (1:1000, Proteintech, USA) was used as an endogenous control and was incubated overnight at 4°C. Then, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibody (1:1000 dilution, Abcam, USA) for 2 h at room temperature. The relative band density was determined using the Tanon 5200 Multifunctional Imaging System (Beijing, China) with the ECL Western Blotting Substrate Kit (Millipore, Billerica, MA, USA).
Mouse model of LPS-induced sepsis
Female BALB/c mice (6-8 week old) were purchased from the Laboratory Animal Research Center of the Medical college of Nanchang University and were kept under sterile specific pathogen-free conditions. Then, the mice were separated into four groups with 6 mice of each. MLATA1-sh 4T1 cells and blank-vector 4T1 cells were pretreated with LPS (5 mg/kg) for 3 days before tumor cell inoculation, and the control group was treated with isometric phosphate-buffered saline (PBS). Approximately 1×105 cells suspended in 50:50 mixture of culture medium and matrigel (BD Bioscience) were injected into the coupled abdominal mammary glands of the BALB/c mice. After 3 weeks, the mice were sacrificed and the metastatic tumor nodules in the lungs were counted under a dissecting microscope. Before sacrifice, blood samples were collected for the IL-6 and TNF-α ELISA assay. The lungs from the different groups of mice were dissected and fixed in 4% paraformaldehyde for 48 hours, embedded in paraffin, and then were cut into slices with a thickness of 3-4 μm and stained with H&E staining. The slices were observed under a light microscope. Western blot assays were employed to detect the expression of MMP-9 and Vimentin in the fresh lung tissues of different groups of mice. All experiments were approved by the Third Hospital of Nanchang City Experimental Animal Committee.
Statistical analysis
Statistical differences were determined by 2-tailed t-test, one-way ANOVA coupled with a Tukey’s post hoc test or two-way ANOVA coupled with a Bonferroni post hoc test using the GraphPad Prism 6 program (GraphPad Software, La Jolla, CA, USA). Statistical significance was analyzed using Statistical Package for the Social Sciences (SPSS) version 19 for Windows (SPSS Inc., Chicago, IL). The Chi-square test was used to analyze the association of postoperative fever with the clinicopathological features of the tumors. Disease free survival (DFS) was calculated from the date of initial diagnosis to the date of recurrence or death. Cumulative DFS probabilities were calculated according to the Kaplan-Meier method and statistical significance was analyzed by the log-rank test. P≤0.05 represents a statistically significant difference, and all reported P values were two-sided.
Results
LncRNA MALAT1 elevated in breast cancer patients with postoperative fever and was associated with the RFS
The correlation between fever and tumor recurrence has attracted particular attention recently. The inflammatory mediators were supposed to mediate the disease relapse regulated by fever. To confirm this hypothesis, we analyzed the co-relationships between the pro-inflammatory factors and fever or tumor recurrence in 258 breast patients. The results showed that the plasma IL-6, TNF-α and MALAT1 levels in the fever group were significantly higher than those in the non-fever group (P = 0.0154, <0.001, <0.001) (Figure 1A, 1C and 1E).
Figure 1.
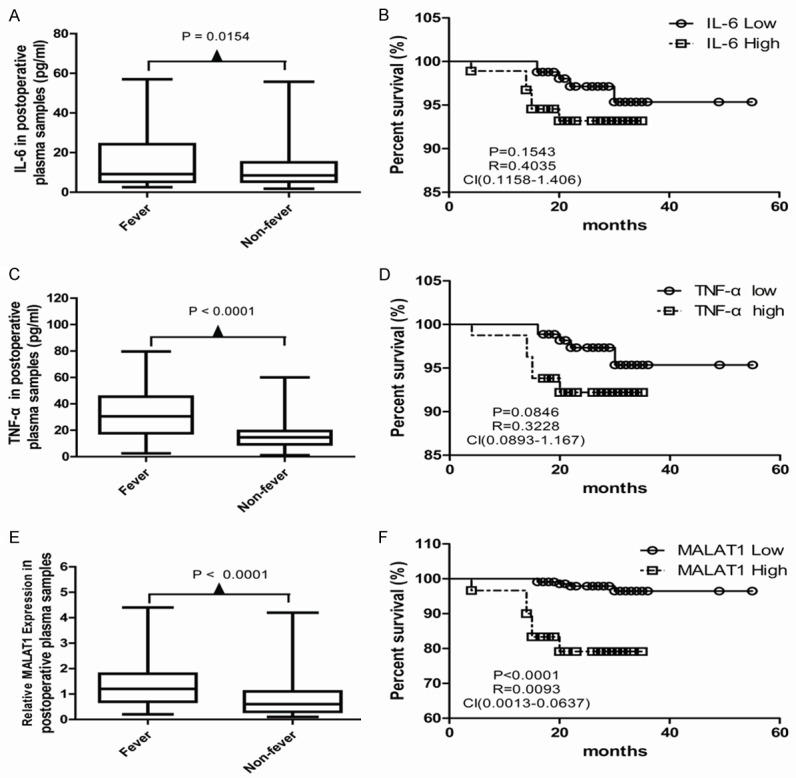
The association among the pro-inflammatory cytokines, postoperative fever and the prognosis of breast cancer patients. Plasma IL-6 (A), TNF-α (C) and MALAT1 (E) levels in the fever group were significantly higher than those in the non-fever group (P = 0.0154, <0.001, <0.001). Kaplan-Meier analysis of the RFS of the patients with low IL-6 expression and high IL-6 expression, (B) with low TNF-α expression and high TNF-α expression (D), and with high MALAT1 expression and low MALAT1 expression (F) in 258 primary breast cancer patients (Log-rank test P = 0.1543, 0.0846, and P<0.001, respectively).
In the univariate survival analysis, we found high expression of MALAT1 was associated poor RFS survival in 258 primary breast cancer patients (Figure 1F), but similar results of the IL-6 and TNF-α expression were not observed (Figure 1B and 1D). The short-term RFS rates for the MALAT1 high expression group and low expression group were 79.17% and 96.47%, respectively. Low MALAT1 expression predicted favorable short-term RFS in our cohort (HR = 0.0093, 95% CI: 0.0013-0.0637, P<0.001) (Figure 1F).
LPS promoted the expression of MALAT1 and induced the invasion and migration of 4T1 cells
Lipopolysaccharide (LPS) is capable of inducing significant systemic inflammation and severe sepsis. We observed that IL-6, TNF-α and MALAT1 were elevated in the supernatants of 4T1 cells treated with LPS (Figure 2A-C). In addition, wound healing assay was employed to assess the 4T1 cell migration and Transwell assay was employed to assess 4T1 cell invasion. Compared with control, LPS treatment obviously increased 4T1 cell migration (Figure 2D) and invasion (Figure 2E).
Figure 2.
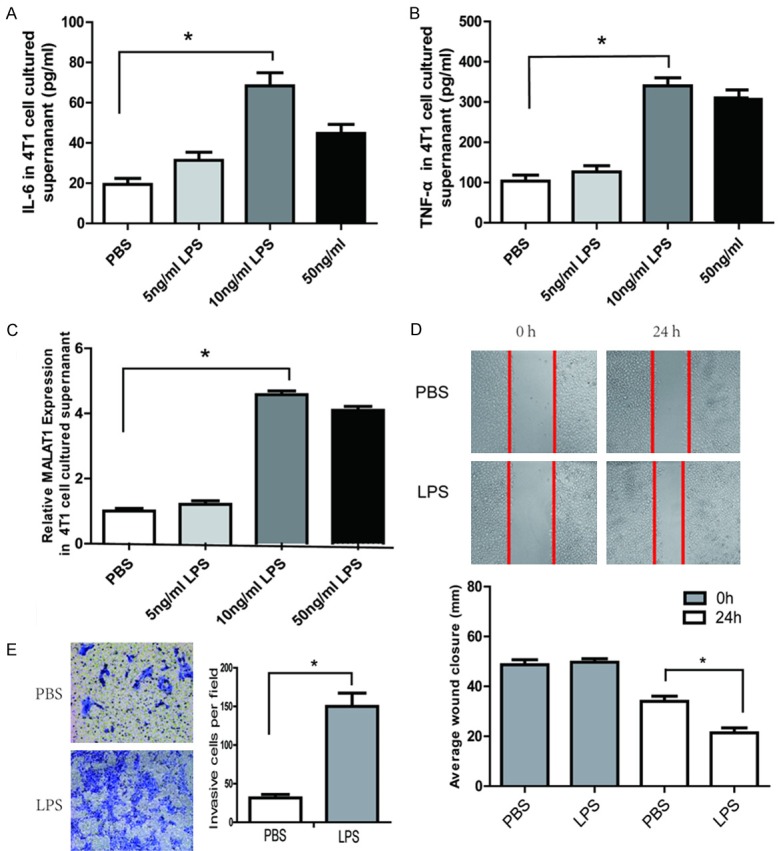
LPS induced IL-6, TNF-α and MALAT1 expression and promoted invasion and migration of breast cancer cells. ELISA assay of IL-6 (A) and TNF-α (B) levels in the cultured supernatant of 4T1 cells after treatment with LPS or PBS. (C) Q-PCR analysis of MALAT1 expression in 4T1 cells after treatment with LPS or PBS. (D) Effects of LPS on the migration of 4T1 cells evaluated by the scratch wound healing assay. Images were taken at 0 and 24 h after wound formation, and the wound surface areas were measured using Image J software and normalized to the initial state. Representative phase-contrast images of 4T1 cells migrating into the wounded area were shown. (E) Effects of LPS on the invasion of 4T1 cells evaluated by Transwell migration assay. Representative images of the migrating cells on the bottom of the Millicell membranes (pore size 8 μm) was shown. All data are presented as the mean ± SEM from three independent experiments (n = 3). *P<0.05.
MALAT1 regulated LPS-induced migration and invasion of 4T1 cells
LPS was found to be able to induce breast cancer cell invasion and metastasis via regulating NF-κB and the Erk pathway [18]. MALAT1 may regulate the expression of inflammatory mediators in endothelial cells, including TNF-α and IL-6 [15] and induce the migration and invasion of human breast cancer cells [19]. To investigate the role of MALAT1 in LPS-induced breast cancer cell invasion and metastasis, MALAT1-shRNA lentivirus was transfected into the 4T1 cell line, and subsequently, qPCR assays revealed that MALAT1 expression was significantly reduced (Figure 3A). Our results showed that the LPS-induced IL-6 expression in the MALAT1-shRNA group was not different from that in the empty vector group (Figure 3B), but the TNF-α expression was sharply decreased (P<0.01) (Figure 3C). Wound healing assays and Transwell assays also indicated that the cell migration and invasion induced by LPS decreased after knocking down MALAT1 expression (Figure 3D-G).
Figure 3.
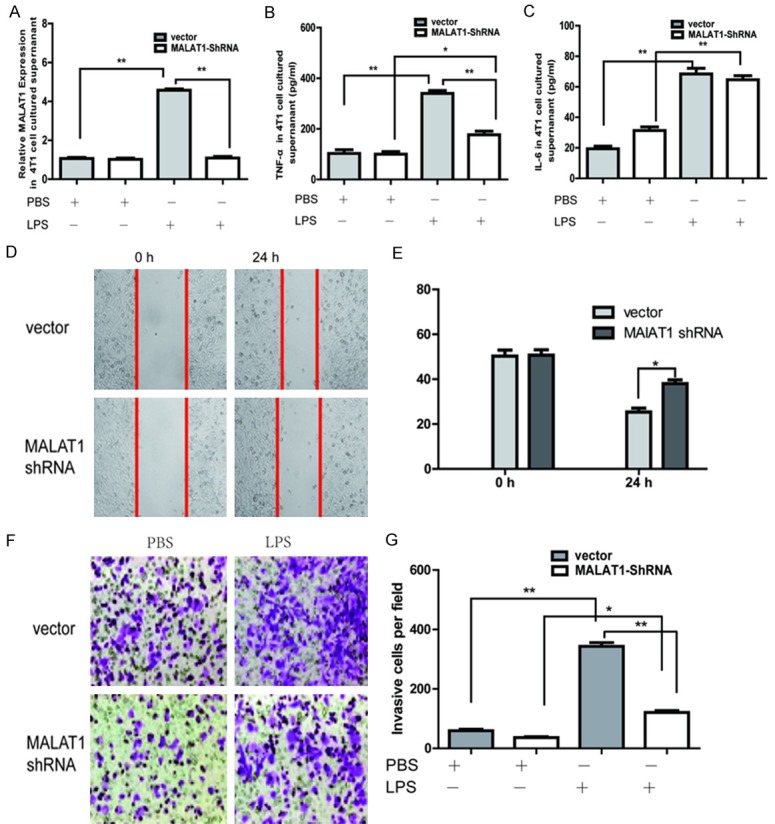
MALAT1 regulated the LPS-induced invasion and migration of 4T1 cells. The effect of LPS treatment on MALAT1 expression (A) and the TNF-α level (B) in the MALAT1-shRNA group was significantly reduced compared with the empty vector group. (C) The IL-6 level were almost the same in the MALAT1-shRNA group and the empty vector group after LPS treatment. (D, E) In the wound healing assay, the cell motility of the MALAT1-shRNA group induced by LPS was decreased compared with that of the empty vector group. (F, G) Transwell assay indicated that the cell migration of the MALAT1-shRNA group induced by LPS decreased compared with that of the empty vector group. All data are presented as the mean ± SEM from three independent experiments (n = 3). *P<0.05, **P<0.01.
Moreover, qPCR and Western blot assay were applied to evaluate the mRNA and protein expression of MMP-9 and vimentin in the 4T1 cells. We found LPS promoted MMP-9 and vimentin expression, whereas these effects were decreased after MALAT1 knockdown (Figure 4A-C). These results together suggested that MALAT1 regulated the process of LPS-induced breast cancer cell migration and invasion.
Figure 4.
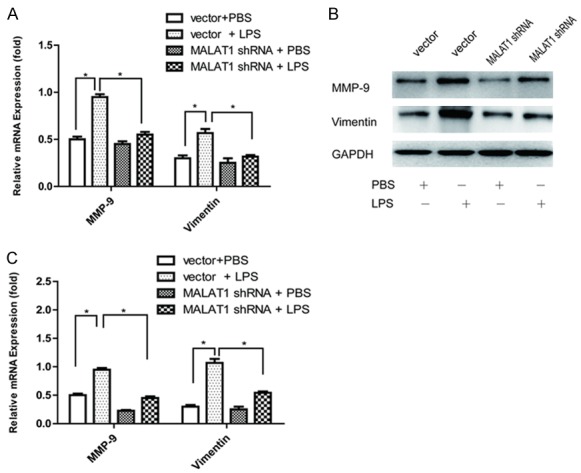
LPS-induced MMP-9 and vimentin expression in the 4T1 cells was regulated by MALAT1. A. The mRNA level of MMP-9 and vimentin in the different groups. B. The protein expression of MMP-9 and vimentin in the different groups, which was evaluated using Western blot assay. C. Quantification used a densitometry analysis. Mean + SD, n = 3, *P<0.05 vs. GAPDH.
LPS induced pulmonary inflammation and promoted metastasis, and this effect was inhibited after MALAT1 knockdown
As reported by Jiang et al [20], LPS induced systemic inflammation in BALB/c mice. In the present study, IL-6 and TNF-α levels detected by ELISA in the plasma samples of the LPS-treated mice were found to increase compared with the PBS-treated mice (Figure 5A and 5B). Similar observation was also shown in the MALAT1 expression in the mice plasma samples (Figure 5C). There was a significant increase in edema and the lung wet/dry ratio in LPS-treated mice compared with PBS-treated mice (Figure 5D and 5E). In the H&E staining, all the lungs in LPS-treated mice were found inflammatory cell infiltration characterized by prominent neutrophilic and granulocytic cells compared with PBS-treated mice. Interestingly, the LPS-induced inflammation response including IL-6, TNF-α and MALAT1 expression in the plasma samples as well as the pulmonary inflammation in the H&E staining in the MALAT1-shRNA xenograft mouse were inhibited when compared with that in the empty-vector counterparts (Figure 5A-E).
Figure 5.
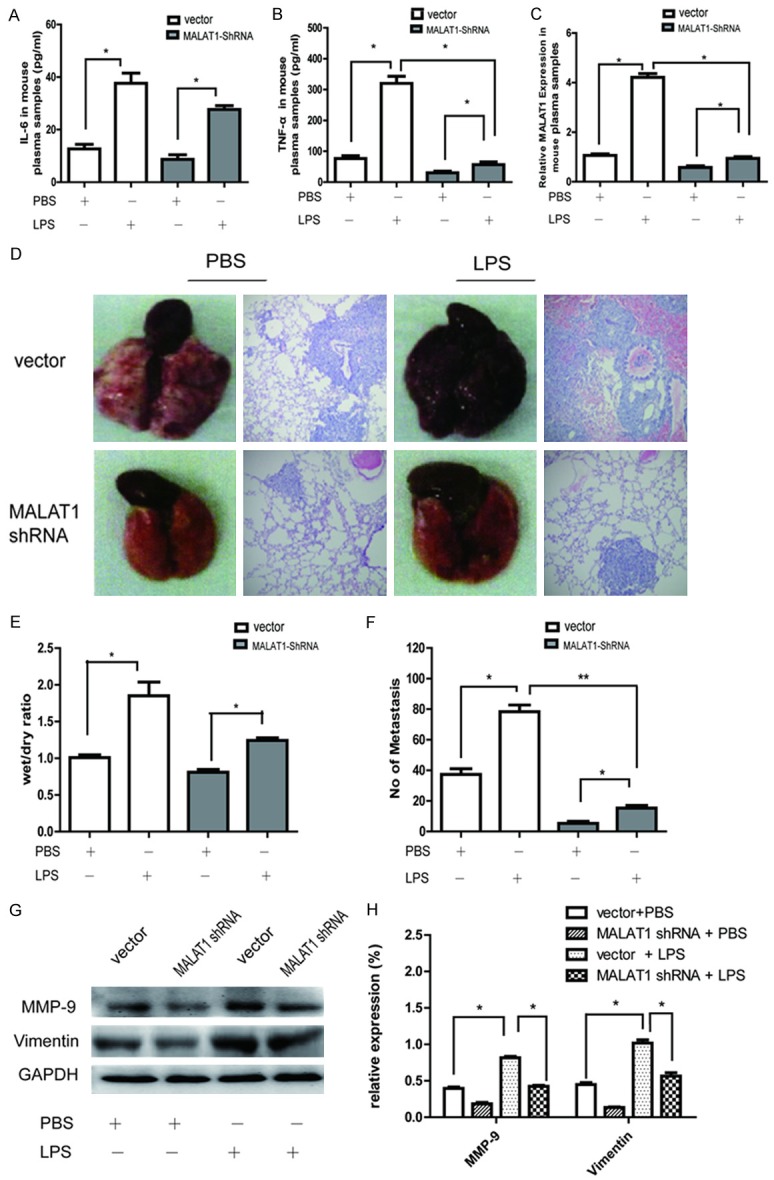
LPS induced pulmonary inflammation and promoted metastasis, while knocking down MALAT1 reduced the effect in vivo. The xenograft mice were divided into four groups including PBS + vector group, PBS + MALAT1-shRNA group, LPS + vector group and LPS + MALAT1-shRNA group. (A, B) ELISA assay of the plasma IL-6 (A) and TNF-α (B) levels in the different xenograft mice. (C) Q-PCR analysis of MALAT1 expression in the plasma samples of the different xenograft mice. lung morphology and H&E staining of the lung in different xenograft mice. (D) The edema and lung wet/dry ratio, inflammatory cellular infiltrates and the number of the lung metastasis were facilitated by LPS, but the inducing effect was decreased in the MALAT1-shRNA group compared to the empty vector group. (E) The quantitative analysis of edema and the lung wet/dry ratio in the different groups. (F) The quantitative analysis of the lung metastasis in the different groups. All data are presented as the mean ± SEM (n = 3). *P<0.05, **P<0.01 compared with control (empty vector). (G) The difference in the expression of MMP-9 and vimentin in the fresh lung tissues of different groups. (H) Quantification used a densitometry analysis. Mean + SD, n = 3, *P<0.05 vs. GAPDH.
In addition, the number and size of the lung metastases significantly increased in LPS-treated mice than in PBS-treated mice (Figure 5D and 5F). The different expression of MMP-9 and vimentin in the fresh lung tissues between LPS-treated mice and PBS-treated mice further support the hypothesis that LPS promoted the breast cancer lung metastases in vivo (Figure 5G and 5H). More specifically, the LPS-induced lung metastasis and EMT-related protein (MMP-9 and Vimentin) expression were inhibited after MALAT1 knocked down. Thus, MALAT1 is a key regulator of LPS-induced pulmonary inflammation and metastasis in xenografted model.
Discussion
Fever is prevalent in many postoperative patients, with an incidence ranging from 13 to 39%. Infectious factors should be considered for fever presenting later than 48 hours after surgery, whereas early postoperative fever is usually deemed as the host response to surgery [21,22]. Interestingly, some retrospective studies proposed that postoperative fever might also serve as a rough indicator for the poor prognosis of breast cancer patients [9,10]. To investigate the association between postoperative fever and short-term relapse, 258 newly admitted breast cancer patients who underwent surgical treatment in the Third hospital of Nanchang City were enrolled in this study during June 15, 2014 to December 15, 2015. Our results showed that the short-term relapse-free survival rate of the fever group and the non-fever group were 90.2% and 97.5%, respectively, and the difference between them was statistical significant (P = 0.0149), which was shown in Supplementary Data.
It is well known that postoperative fever refers to a rise in the core temperature, which is most commonly caused by inflammatory changes due to the release of pyrogenic cytokines, such as IL-6 and TNF-α [23]. These cytokines may be induced by infection, injury, surgery, general anesthesia trauma or be secreted by the tumor itself. Previous studies have elaborated on the independent negative impact on prognosis of high levels of IL-6 or TNF-α in the serum of patients with breast cancer [24]. Our study also found that the expression of plasma IL-6 or TNF-α in postoperative fever patients with breast cancer increased, but these high expression of the two pre-inflammatory factors described above was not associated with the adverse prognosis of breast cancer patients. In other words, IL-6 or TNF-α were not the root of the postoperative fever that was associated with the poor prognosis of breast cancer patients, and maybe some other cytokines were involved in this process. Recently, the long noncoding RNA MALAT1 was also considered a pro-inflammatory factor, which regulated the lipopolysaccharide-induced inflammatory response [25] and EMT process of breast cancer cells [16,26]. Interestingly, our study indicated that there was a difference in the expression of plasma MALAT1 between the fever group and the non-Fever group. High MALAT1 expression predicted the adverse short-term DFS of these patients.
Lipopolysaccharide (LPS), which is in the outer cell membrane of Gram-negative bacteria, can induce significant systemic inflammation and several pro-inflammatory cytokines secretion, including IL-6, tumor necrosis alpha (TNF-α) etc. [27]. Our study found that the expression of IL-6 and TNF-α in the supernatants of 4T1 cells was elevated in the LPS-treated group compared to the control group, which was detected by ELISA assay. Similarly, our qRT-PCR assay indicated that the expression of MALAT1 was also increased after LPS treatment. Thus, LPS was believed to promote the expression of IL-6, TNF-α and MALAT1 in the 4T1 cells. However, after knocking down the expression of MALAT1 in 4T1 cells, the TNF-α expression was dramatically decreased while the IL-6 expression remain unchanged. Recently, Zhang et al reported that IL-6-induced lncRNA MALAT1 enhances TNF-α expression in LPS-induced septic cardiomyocytes [28]. Puthanveetil et al also found that the lncRNA MALAT1 regulates the glucose-induced up-regulation of the inflammatory mediators IL-6 and TNF-α through the activation of SAA3 [15]. Our research herein may provide a new mechanism by which lncRNA MALAT1 participated in the regulation of the LPS-induced inflammatory response in breast cancer cells.
LPS is also the primary ligand for TLR4, which can induce the inflammation-associated invasion and metastasis of breast cancer cells [29,30]. Our data indicated that treating 4T1 cells with LPS could increase cell migration and invasion, which can be blocked by MALAT1 knockdown. Recent report demonstrated that the long noncoding RNA MALAT1 regulates the LPS-induced inflammatory response through its interaction with NF-κB [31]. It has been shown that NF-κB, a key protein regulating the immune and inflammatory process, also plays an important role in regulating the EMT process [32]. Notably, matrix metalloproteinase 9 (MMP9), which is directly regulated by NF-κB, is a proteolytic enzyme which particularly plays a role in the early steps of cancer cell invasion [33,34]. Moreover, NF-κB is one of the transcription factors that binds to the promoter of vimentin which is overexpressed during the epithelial-mesenchymal transition (EMT) process [35,36]. Our data also indicated that LPS promoted MMP-9 and vimentin expression, whereas these effects were inhibited after knocking down MALAT1 expression. Therefore, MALAT1 is believed to induce the migration and invasion of human breast cancer cells [19], and may also contribute to the postoperative fever induced recurrence in patients.
In conclusion, early postoperative fever was an independent risk factor for the prognosis of breast cancer patients. Specific inflammatory cytokines released from the breast cancer patients induced a systemic inflammatory response and postoperative fever. As an important pre-inflammatory factor, MALAT1 is involved in the regulation of the systemic inflammatory response and promotes the invasion and metastasis of breast cancer. And MALAT1 is believed to engage in the recurrence, metastasis and postoperative fever in breast cancer patients.
Acknowledgements
This work was supported by the Natural Science Foundation of Jiangxi Province (Contract grant number: 2014ZBAB205003), the National Natural Science Foundation of China (Contract grant numbers: 81260389).
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Steeg PS. Tumor metastasis: mechanistic insights and clinical challenges. Nat Med. 2006;12:895–904. doi: 10.1038/nm1469. [DOI] [PubMed] [Google Scholar]
- 3.Liu Y, Cao X. Characteristics and significance of the pre-metastatic niche. Cancer Cell. 2016;30:668–681. doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Suarez-Carmona M, Lesage J, Cataldo D, Gilles C. EMT and inflammation: inseparable actors of cancer progression. Mol Oncol. 2017;11:805–823. doi: 10.1002/1878-0261.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lesperance R, Lehman R, Lesperance K, Cronk D, Martin M. Early postoperative fever and the “routine” fever work-up: results of a prospective study. J Surg Res. 2011;171:245–50. doi: 10.1016/j.jss.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell JD, Grocott HP, Phillips-Bute B, Mathew JP, Newman MF, Bar-Yosef S. Cytokine secretion after cardiac surgery and its relationship to postoperative fever. Cytokine. 2007;38:37–42. doi: 10.1016/j.cyto.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 7.Kumar S, Mehta Y, Vats M, Chand R, Kapoor P, Trehan N. An observational study to know the association of leukocytosis and fever with infection in post cardiac surgery patients. Indian Heart J. 2007;59:316–22. [PubMed] [Google Scholar]
- 8.McCarter MD, Mack VE, Daly JM, Naama HA, Calvano SE. Trauma-induced alterations in macrophage function. Surgery. 1998;123:96–101. [PubMed] [Google Scholar]
- 9.Teucher G, Schindler AE. Postoperative fever and prognosis in breast cancer. Arch Geschwulstforsch. 1987;57:309–17. [PubMed] [Google Scholar]
- 10.Yan T, Yin W, Zhou L, Jiang Y, Shen Z, Shao Z, Lu J. Postoperative fever: the potential relationship with prognosis in node negative breast cancer patients. PLoS One. 2010;5:e15903. doi: 10.1371/journal.pone.0015903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MY, Oskarsson T, Acharyya S, Nguyen DX, Zhang XH, Norton L, Massagué J. Tumor selfseeding by circulating cancer cells. Cell. 2009;139:1315–26. doi: 10.1016/j.cell.2009.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang L, Miyahira AK, Simons DL, Lu X, Chang AY, Wang C, Suni MA, Maino VC, Dirbas FM, Yim J, Waisman J, Lee PP. IL6 signaling in peripheral blood t cells predicts clinical outcome in breast cancer. Cancer Res. 2017;77:1119–1126. doi: 10.1158/0008-5472.CAN-16-1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korobeinikova E, Myrzaliyeva D, Ugenskiene R, Raulinaityte D, Gedminaite J, Smigelskas K, Juozaityte E. The prognostic value of IL10 and TNF alpha functional polymorphisms in premenopausal early-stage breast cancer patients. BMC Genet. 2015;16:70. doi: 10.1186/s12863-015-0234-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Puthanveetil P, Chen S, Feng B, Gautam A, Chakrabarti S. Long non-coding RNA MALAT1 regulates hyperglycaemia induced inflammatory process in the endothelial cells. J Cell Mol Med. 2015;19:1418–25. doi: 10.1111/jcmm.12576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ji P, Diederichs S, Wang W, Böing S, Metzger R, Schneider PM, Tidow N, Brandt B, Buerger H, Bulk E, Thomas M, Berdel WE, Serve H, Müller-Tidow C. MALAT-1, a novel noncoding RNA, and thymosin beta4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene. 2003;22:8031–41. doi: 10.1038/sj.onc.1206928. [DOI] [PubMed] [Google Scholar]
- 16.Miao Y, Fan R, Chen L, Qian H. Clinical significance of long non-coding RNA MALAT1 expression in tissue and serum of breast cancer. Ann Clin Lab Sci. 2016;46:418–24. [PubMed] [Google Scholar]
- 17.Marcy SM, Kohl KS, Dagan R, Nalin D, Blum M, Jones MC, Hansen J, Labadie J, Lee L, Martin BL, O’Brien K, Rothstein E, Vermeer P Brighton Collaboration Fever Working Group. Fever as an adverse event following immunization: case definition and guidelines of data collection, analysis, and presentation. Vaccine. 2004;22:551–556. doi: 10.1016/j.vaccine.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Liu X, Zhao W, Wang W, Lin S, Yang L. Puerarin suppresses LPS-induced breast cancer cell migration, invasion and adhesion by blockageNF-κB and Erk pathway. Biomed Pharmacother. 2017;92:429–436. doi: 10.1016/j.biopha.2017.05.102. [DOI] [PubMed] [Google Scholar]
- 19.Chou J, Wang B, Zheng T, Li X, Zheng L, Hu J, Zhang Y, Xing Y, Xi T. MALAT1 induced migration and invasion of human breast cancer cells by competitively binding miR-1 with cdc42. Biochem Biophys Res Commun. 2016;472:262–9. doi: 10.1016/j.bbrc.2016.02.102. [DOI] [PubMed] [Google Scholar]
- 20.Jiang M, Xu X, Bi Y, Xu J, Qin C, Han M. Systemic inflammation promotes lung metastasis via E-selectin upregulation in mouse breast cancer model. Cancer Biol Ther. 2014;15:789–96. doi: 10.4161/cbt.28552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dellinger EP. Should we measure body temperature for patients who have recently undergone surgery? Clin Infect Dis. 2005;40:1411–1412. doi: 10.1086/429629. [DOI] [PubMed] [Google Scholar]
- 22.Jorgensen FS, Sorensen CG, Kjaergaard J. Postoperative fever after major abdominal surgery. Ann Chir Gynaecol. 1988;77:47–50. [PubMed] [Google Scholar]
- 23.Narayan M, Medinilla SP. Fever in the postoperative patient. Emerg Med Clin North Am. 2013;31:1045–58. doi: 10.1016/j.emc.2013.07.011. [DOI] [PubMed] [Google Scholar]
- 24.Tripsianis G, Papadopoulou E, Anagnostopoulos K, Botaitis S, Katotomichelakis M, Romanidis K, Kontomanolis E, Tentes I, Kortsaris A. Coexpression of IL-6 and TNF-α: prognostic significance on breast cancer outcome. Neoplasma. 2014;61:205–12. doi: 10.4149/neo_2014_026. [DOI] [PubMed] [Google Scholar]
- 25.Zhao Y, Kong X, Li X, Yan S, Yuan C, Hu W, Yang Q. Metadherin mediates lipopolysaccharideinduced migration and invasion of breast cancer cells. PLoS One. 2011;6:e29363. doi: 10.1371/journal.pone.0029363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang NS, Chi YY, Xue JY, Liu MY, Huang S, Mo M, Zhou SL, Wu J. Long non-coding RNA metastasis associated in lung adenocarcinoma transcript 1 (MALAT1) interacts with estrogen receptor and predicted poor survival in breast cancer. Oncotarget. 2016;7:37957–37965. doi: 10.18632/oncotarget.9364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKallip RJ, Ban H, Uchakina ON. Treatment with the hyaluronic Acid synthesis inhibitor 4-methylumbelliferone suppresses LPS-induced lung inflammation. Inflammation. 2015;38:1250–1259. doi: 10.1007/s10753-014-0092-y. [DOI] [PubMed] [Google Scholar]
- 28.Zhuang YT, Xu DY, Wang GY, Sun JL, Huang Y, Wang SZ. IL-6 induced lncRNA MALAT1 enhances TNF-α expression in LPS-induced septic cardiomyocytes via activation of SAA3. Eur Rev Med Pharmacol Sci. 2017;21:302–309. [PubMed] [Google Scholar]
- 29.Li J, Yin J, Shen W, Gao R, Liu Y, Chen Y, Li X, Liu C, Xiang R, Luo N. TLR4 promotes breast cancer metastasis via Akt/GSK3β/β-Catenin pathway upon LPS stimulation. Anat Rec (Hoboken) 2017;300:1219–1229. doi: 10.1002/ar.23590. [DOI] [PubMed] [Google Scholar]
- 30.Yang H, Wang B, Wang T, Xu L, He C, Wen H, Yan J, Su H, Zhu X. Toll-like receptor 4 prompts human breast cancer cells invasiveness via lipopolysaccharide stimulation and is overexpressed in patients with lymph node metastasis. PLoS One. 2014;9:e109980. doi: 10.1371/journal.pone.0109980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhao G, Su Z, Song D, Mao Y, Mao X. The long noncoding RNA MALAT1 regulates the lipopolysaccharide-induced inflammatory response through its interaction with NF-κB. FEBS Lett. 2016;590:2884–95. doi: 10.1002/1873-3468.12315. [DOI] [PubMed] [Google Scholar]
- 32.Min C, Eddy SF, Sherr DH, Sonenshein GE. NFkappaB and epithelial to mesenchymal transition of cancer. J Cell Biochem. 2008;104:733–744. doi: 10.1002/jcb.21695. [DOI] [PubMed] [Google Scholar]
- 33.Ricca A, Biroccio A, Del Bufalo D, Mackay AR, Santoni A, Cippitelli M. bcl-2 over-expression enhances NF-kappaB activity and induces mmp-9 transcription in human MCF7(ADR) breast-cancer cells. Int J Cancer. 2000;86:188–196. doi: 10.1002/(sici)1097-0215(20000415)86:2<188::aid-ijc7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 34.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 35.Satelli A, Li S. Vimentin in cancer and its potential as a molecular target for cancer therapy. Cell Mol Life Sci. 2011;68:3033–46. doi: 10.1007/s00018-011-0735-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vuoriluoto K, Haugen H, Kiviluoto S, Mpindi JP, Nevo J, Gjerdrum C, Tiron C, Lorens JB, Ivaska J. Vimentin regulates EMT induction by slug and oncogenic H-Ras and migration by governing Axl expression in breast cancer. Oncogene. 2011;30:1436–48. doi: 10.1038/onc.2010.509. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


