Abstract
Cardiopulmonary bypass (CPB) induces cytokine production and causes postoperative monocytic inflammatory responses, which are associated with patient outcomes. In fact, monocytes regulate immunity through dynamic networks of survival and cellular apoptosis as well as thrombomodulin (TM)-associated differenciiation. Whether CPB affects the plasma level of eotaxin-2, a potent chemoattractant, or stimulates monocyte apoptosis among patients undergoing elective coronary artery bypass graft (CABG) surgery is also unknown. Thus, we aimed to investigate this subject and explored the feasible roles of TM in the phenomena. Firstly, clinical data showed that after CABG surgery, patients with lower plasma eotaxin-2 levels and higher TM expression levels exhibited reduced monocytic apoptosis, compared with that in patients with lower TM expression levels. Subsequently, to explore the hypothesis that eotaxin-2 induces monocytic apoptosis mediation by TM expression, we used in vitro monocytic THP-1 cells. The results indicated that treatment of THP-1 cells with eotaxin-2 markedly increased apoptosis. Knockdown of TM significantly increased, and overexpression of TM significantly reversed eotaxin-2-induced monocyte apoptosis, which was compared with that of only eotaxin-2-treated THP-1 cells. TM may regulate mitochondria-mediated apoptosis by its PI3K/Akt axis signaling pathway, which acts as an extinguisher for p53 and BAX activation, as well as limit further downstream release of cytochrome c and cleavage of caspases 8 and 3; we suggest that TM interacts with the cofilin cytoskeleton, which further supports a role for TM in eotaxin-induced THP-1 cell apoptosis. Based on clinical observation and in vitro study, we conclude that TM expression on monocytes is associated with their apoptosis. The above mechanisms may be relevant to clinical phenomena in which patients exhibiting more monocytic apoptosis are complicated by higher plasma levels of eotaxin-2 and lower TM expression on monocytes after CABG surgery.
Keywords: Eotaxin-2, thrombomodulin, apoptosis
Introduction
Cardiopulmonary bypass (CPB) is still an indispensable technology that is widely used in cardiac surgery. However, CPB causes postoperative inflammatory responses that involve the expression of cytokines [1-7]. Therefore, cardiac surgeons, pharmacologists, and scientists have devoted their efforts to developing better surgical processes, drugs, and CPB materials to regulate postoperative inflammatory responses as appropriate. With the exception of granulocytes, monocytic function is associated with the inflammatory and coagulation physiology in patients undergoing cardiac surgery [8]. CPB alters the inflammatory capacity of monocytes [6]. CPB may activate monocytes and trigger the secretion of proinflammatory cytokines [9], which allows monocyte binding to complement and subsequent adherence of fibrinogen to the endothelium [10]. Additionally, monocytes can also influence the coagulation cascade directly during CPB, mediated by increases in tissue factor expression [11] and factor X activation [10]. Dysfunction of monocytes may cause the pathogenesis of myocardial ischemia and stunning in systemic inflammation after cardiac surgery [12]. Since monocytes play an essential role in regulation inflammation after cardiac surgery, their apoptosis is a key mechanism in this process that engineers and regulates host immune responses during systemic inflammation [13]. Maintaining adequate function of monocytes is essential to maintaining good recovery in the clinic after cardiac surgery [14,15]. Sequelae from cardiac surgery with CPB may induce the apoptosis of monocytes, which is associated with the acute phase of several postoperative complications [16]; thus, it is necessary to understand the occurrence, mechanism, and regulation of monocytic apoptosis after surgery.
Thrombomodulin (TM) is expressed constitutively on the surfaces of monocytes and macrophages [17], which include a single-chain transmembrane glycoprotein with five distinct domains, including a C-type lectin-like domain, six sequential epidermal growth factor-like peptides, a serine/threonine-rich domain, a transmembrane domain and a cytoplasmic tail [18]. Previously, we showed that IL-6 induces monocytic cell migration in vitro and inflammatory responses [8,10,19] in patients receiving coronary artery bypass graft (CABG) surgery [20] that are regulated by TM via its domain 5 (cytoplasmic tail) co-localized with the cytoskeleton, F-actin and intersectin I [21]. Additionally, TM also exhibits procoagulant activity and adhesion molecules expressing microparticles [22], which may be a key regulator of monocyte-related coagulation reactions. Recently, evidence has also demonstrated that TM regulates monocyte differentiation via PKCδ and ERK1/2 pathways in vitro and in atherogenesis [23]. Decreased TM expression/function may yield inflammatory responses [24], as well as coagulopathy after cardiac surgery [25,26]; in contrast, the increasing production of TM may prevent the morbidity of allografts, which results from anti-coagulant and anti-inflammatory effects of TM [27]. Since TM plays critical roles in monocytic function and TM function is also altered by several pathophysiological and biological factors in monocytes [24,28], it is necessary to thoroughly elucidate the roles of TM in monocytes.
Evidence has proved that eotaxin-2 is a potent chemoattractant that is encoded by the chemokine (C-C motif) ligand 24 gene on chromosome 7 in humans [29]. Eotaxin-2 is produced by activated monocytes and T lymphocytes, which attracts lymphocytes, basophils, eosinophils, and monocytes in inflammation [30]. Additionally, the results from respiratory epithelial cells, bronchial smooth muscle cells, vascular endothelial cells, fibroblasts, helper T cells, etc., also express C-C chemokine receptor-3, a receptor for eotaxin-2 [31,32], and respond to eotaxin-2 stimulation [33,34]. Monocyte-derived eotaxin-2 and macrophage-derived eotaxin-2 are differentially regulated and are implicated in innate and adaptive immunity, respectively [35]. A previous report has noted that cardiac surgery increases the plasma level of eotaxin-2 in patients. Our preliminary analysis also demonstrated that CPB may induce the production of eotxin-2, which we speculate may be due to the process of immune-induced environmental changes in physiology, although the impact on the recovery period after cardiac surgery needs to be elucidated. Furthermore, the function/survival of monocytic cells is related to inflammation and immunosuppressive situation in patients who are undergoing cardiac surgery. In the past, our research showed that TM expression by monocytes is related to their differentiation and migration, which is also related to the outcome after cardiac surgery. Given that cell-mediated apoptosis is also one of the factors that affect the function of monocytes. Therefore, we aimed this study to explore the impact of eotaxin-2 on apoptosis in monocytes during cardiac surgery and to determine whether TM plays an important role in this process.
Materials and methods
Clinical study
Ethics and patient collection
The ethics committee of our institution approved this study. Written informed consent was obtained from 18 patients undergoing elective CABG surgery. Patients were excluded from the study if they had undergone previous isolated cardiac surgery, had experienced a reduced cardiac ejection fraction (less than 50%), had a history of cardiogenic shock, had used an intra-aortic balloon pump (IABP), had received extracorporeal membrane oxygenation (ECMO), or had been placed on a respiratory ventilator. Patients with rheumatoid arthritis, asthma, chronic bronchitis, cancer, or autoimmune disease and patients receiving steroidal or nonsteroidal anti-inflammatory drug therapies were also excluded. Aspirin was discontinued in all patients 7 days before the operation.
Conventional CPB technique and CABG surgery
Before the operation, radial artery catheterization was performed, and a Swan-Ganz standard thermodilution pulmonary artery catheter (Abbott Laboratories, Abbott Park, IL, USA) was inserted through the internal jugular vein. Anesthesia for all patients was induced with thiopental and maintained with isoflurane in oxygen, fentanyl, and pancuronium. All patients underwent median sternotomy CABG. The CPB was performed with standard cannulation and a Capiox SX 18 extracorporeal membrane oxygenator (Terumo, Aliso Viejo, CA, USA). Porcine heparin was administered as an anticoagulant before cannulation. A nonpulsatile Terumo Sarns 9000 roller pump was used for all patients. The invasive blood pressure was monitored with an arterial catheter during the surgical procedure. The mean arterial pressure of all patients placed on CPB was maintained at approximately 70 mmHg with pharmacological treatment and/or heart-lung machine/mechanical support. During the surgical process, the patients were cooled to a 28°C to 30°C body temperature, and cardiac arrest was induced with warm blood cardioplegia (the ratio of cardioplegia solution to blood was 1:4; 15 mL/kg body weight), which was delivered antegradely and retrogradely every 20 minutes. The saphenous vein and intermittent anastomosis of the graft were used for revascularization. On discontinuation of the CPB circuit, heparin was neutralized with protamine sulfate. No antifibrinolytic drugs (such as aprotinin) were used in these patients.
Plasma extraction and enzyme-linked immunosorbent assay
Blood was obtained from an indwelling arterial catheter and was taken preincision and 24 hours after the end of the operation (the end of CPB). All blood samples were collected into tubes containing 3.8% sodium citrate. Plasma was separated from fresh blood by centrifugation and stored at -80°C until used. Enzyme-linked immunosorbent assays (ELISAs) were performed to determine the plasma concentration of eotaxin-2 according to the manufacturer’s protocol (Thermo Fisher Scientific Co., Waltham, MA, USA). The absorbance at 450 nm was determined using a microplate reader.
Monocyte extraction and flow cytometry
Total mononuclear cells were isolated from total blood cells by density gradient centrifugation with Histopaque-1077 (Sigma-Aldrich, St. Louis, MO, USA) and analyzed immediately [36]. Fluorescein isothiocyanate (FITC)-conjugated mouse anti-hCD45 antibodies (BioLegend, San Diego, CA, USA) and phycoerythrin (PE)-conjugated mouse anti-TM antibodies (BioLegend, San Diego, CA, USA) were added and incubated in the dark for 20 minutes. The cells were then fixed with 1% paraformaldehyde. The CD45-positive cells were separated into forward-scatter and side-scatter fractions by flow cytometry (Becton Dickinson, San Jose, CA, USA), and lymphocytes, granulocytes, and monocytes were included. Ten thousand monocytes were gated for the analysis of membrane TM expression. Additionally, an Annexin V/Propidium Iodide Staining Kit (eBioscience, San Diego, CA, USA) was used to detect the cellular apoptosis.
In vitro study
Purification of recombinant eotaxin-2 protein
The open reading frame of eotaxin-2 (CCL24) was originally PCR-amplified using THP-1 cell cDNA as a template, 0.1 mM dNTPs, 0.2 mM each of gene-specific primers and 1 U Pfu DNA polymerase (Promega, Madison, WI, USA) with the following program: one cycle of 95°C for 5 minutes; 35 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds; 1 cycle of 60°C for 30 seconds and 72°C for 10 minutes; and a final incubation at 72°C for 10 minutes with 1 U Taq DNA polymerase. The eotaxin-2-specific forward and reverse primers used in the PCR reaction were as follows: Pr-CCL24-BamHI-F2: 5’aag gat cca agt ggt cat ccc ctc tcc ctg ct 3’ and Pr-CCL24-R1: 5’ ccc tcg agt tag cag gtg gtt tgg ttg cca g 3’. The amplified eotaxin-2 (CCL24) fragment was then cloned into the pCR2.1-TOPO vector (Invitrogen, Carlsbad, CA, USA) and subsequently cloned in-frame into the BamHI and XhoI sites of the pGEX-5X-1 expression vector (GE Healthcare Biosciences, Pittsburgh, PA, USA) for expression in E. coli (DH5a). For purification of recombinant eotaxin-2 protein, BL21 cells were transformed with the pGEX-5X-1-eotaxin-2 expression vector, and the recombinant eotaxin-2 protein was purified. Briefly, BL21 (DE3) pLysS cells (RBC Bioscience, New Taipei City, Taiwan) containing the plasmid pGEX-5X-1-eotaxin-2 were grown overnight at 37°C in 50 mL of LB medium supplemented with 100 µg/mL ampicillin. Then, 50 mL of overnight culture was transferred into 1000 mL of LB medium and grown at 16°C to an A600 of 0.6-0.8 (approximately 2 hours). Expression of the fusion protein was then induced by adding IPTG to a final concentration of 1 mM at 16°C for 6 hours. The bacteria were pelleted by centrifugation for 10 minutes at 8000 rpm, and recombinant GroEL was extracted under native conditions using the GST Gene Fusion System according to the manufacturer’s instructions (GE Healthcare Biosciences, Pittsburgh, PA, USA). Finally, the recombinant eotaxin-2 protein was purified with an elution buffer containing 50 mM Tris-HCl and 10 mM reduced glutathione (pH 8.0). The quantity of recombinant eotaxin-2 protein was measured using the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA, USA). The fusion protein was detected by SDS gel electrophoresis and identified by immunoblotting with a GST antibody (GE Healthcare Biosciences, Pittsburgh, PA, USA). The endotoxin levels in the recombinant eotaxin-2 protein preparation were measured using a Limulus Amebocyte Lysate Kit from Cambrex, Inc., in the USA. The LPS levels were below 1 pg/mL.
Cell culture
THP-1 cells, a human promyelomonocytic cell line, were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and grown in RPMI 1640 medium with 2 mM L-glutamate, 4.5 g/L glucose, 10 mmol/L HEPES, 1.0 mmol/L sodium pyruvate, 10% fetal bovine serum, and 1% antibiotic-antimycotic mixture. The cell density was maintained between 5 × 104 and 8 × 105 viable cells/mL, and the medium was refreshed every 2-3 days.
Construction and expression of HA-tagged full-length TM expression vectors
The pCDNA3.1/V5-His-TM plasmid containing a segment of the TM open reading frame was a gift from Professor Yu-Jia Chang, Taipei Medical University, Taiwan. For the construction of the influenza hemagglutinin (HA) epitope-tagged full-length TM expression plasmid (HA-TM FL), the pCDNA3.1/V5-His-TM plasmid was first digested with SacI and XhoI enzymes, filled in with Klenow, and subsequently cloned in-frame into the SmaI sites of the pXJN-HA vector, which contains the chick β-globin intron downstream (3’) of the CMV promoter. THP-1 cells were transfected with HA-TM FL plasmids via electroporation using a Neon transfection system (Invitrogen, Carlsbad, CA, USA), according to the manufacturer’s instructions. A total of 106 cells were transfected with 2 μg of HA-TM FL plasmid. The cells were analyzed for overexpression 24 hours after electroporation.
Knockdown of gene expression by RNA interference
The knockdown of TM gene expression was performed by siRNA transfection. Cells (3 × 106) were suspended in 2.5 mL of serum-free medium, and 25 mM of TM siRNA duplexes (Invitrogen Catalog THBD-HSS110719, 110721, 186320, Carlsbad, CA, USA) were transfected according to the manufacturer’s instructions. Silencer Validated siRNA (negative control siRNA, Santa Cruz Catalog sc-44230, Dallas, TX, USA) was used to validate the knockdown. The cells were seeded into six-well plates immediately following transfection for further experiments 24 hours after transfection. Following knockdown of gene expression by RNA interference, TM expression was analyzed by flow cytometry and confocal microscopy.
Detection of apoptosis using fluorescence microscopy and flow cytometry
THP-1 cells (105) were rinsed with PBS, fixed in 4% paraformaldehyde, and spun onto glass slides using a Shandon Cytospin 4 Cytocentrifuge (Thermo Scientific, Pittsburgh, PA, USA). The slides were stained using an Annexin V-conjugated Alexa Fluor® 488 Assay kit (Thermo Fisher Scientific Co., Waltham, MA USA), and nuclei were identified using Hoechst 33258 (Sigma, St. Louis, MO, USA). Images were obtained using an Axio Imager A1 microscope (Carl Zeiss Micro Imaging Inc., Thornwood, NY, USA). Additionally, the fixed THP-1 cells were also stained using the BD Annexin V-FITC Assay on the BD FACSVerseTM System and analyzed using flow cytometry on a FACSCaliburTM platform (BD Biosciences, Franklin Lakes, NJ, USA).
Western blot analysis
We placed 3 × 106 cells into a 10 cm dish for western blot analysis. We collected the suspended and attached THP-1 cells, and the total cell lysates or mitochondrial protein lysates were extracted. Protein concentrations were determined using a Bio-Rad Protein Assay Kit (Bio-Rad Inc., CA, USA), with BSA used as the standard. For each blot, approximately 50 μg of total protein was fractionated by SDS-PAGE and transferred to a PVDF (polyvinylidene difluoride) membrane. The membranes were separately probed with rabbit anti-human bcl-2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-BAX (Cell Signaling, Danvers, CA, USA), rabbit anti-p53 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-phosphorylated Akt (Merck-Millipore, Temecula, CA, USA), mouse anti-total Akt (Merck-Millipore, Temecula, CA, USA), rabbit anti-caspase 3 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rabbit anti-caspase 8 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), mouse anti-human cytochrome c (BD Pharmingen, San Jose, CA, USA), and rabbit anti-Cofilin (Abcam, Cambridge, MA, USA) antibodies. The membranes were then incubated with specific horseradish peroxidase (HRP)-conjugated secondary antibodies. The proteins were visualized using an enhanced chemiluminescence (ECL) detection kit (Amersham Biosciences, Piscataway, NJ, USA). Mouse anti-Actin anti-bodies (Chemicon-Millipore, Temecula, CA, USA) and rabbit anti-human/mouse GRP94 (Abcam, Cambridge, MA, USA) were used as the loading controls.
Detection of the mitochondrial potential for assessing apoptosis using image cytometry
Loss of the mitochondrial membrane potential is known to precede apoptosis; therefore, the lipophilic dye 5, 5, 6, 6-tetrachloro-1, 1, 3, 3-tetraethylbenzimidazol-carbocyanine iodide (JC-1), which can display membrane potential-dependent accumulation in the mitochondrial was used to evaluate the mitochondrial potential. THP-1 cells (106) were rinsed with PBS, a solution of JC-1 (final concentration: 2.5 µg/ml) was added to the cells, and the mixtures were incubated 10 minutes at 37°C. Then, the supernatant was completely removed without disturbing the cell pellet and washed 3 times using PBS. Finally, the cell pellet was resuspended by pipetting in 0.25 mL PBS containing 1 µg/ml DAPI and analyzed immediately using a NucleoCounter® NC3000TM Cell Analyzer (ChemoMetec A/S, Davis, CA, USA).
Immunoprecipitation
THP-1 cells were transfected with either TM siRNA or NC siRNA and lysed in buffer containing 150 mM NaCl, 50 mM Tris-HCl (pH 7.4), 1% NP-40, and 1 × protease inhibitor cocktail (Sigma, St. Louis, MO, USA). Lysed cells were placed on ice for 30 minutes and then centrifuged at 10,000 × g for 30 minutes to remove cell debris. Approximately 500 μg of protein extract was precleaned with 20 mL of a 50% protein A/G suspension mixture (protein A: Bio-Rad, CA, USA; protein G: Amersham, IL, USA). The pre-cleaned lysates were immunoreacted with rabbit anti-human TM (OriGene Technologies, Rockville, MD, USA), mouse anti-human cofilin antibodies (Abcam, Cambridge, MA, USA) or rabbit IgG (Sigma, St. Louis, MO, USA) at 4°C for 16 hours and then immunoprecipitated by adding 50 mL of a 50% protein A/G sepharose mixture at 4°C for 1.5 hours. The beads were washed three times with ice-cold lysis buffer, and the bound proteins were eluted with 2 × SDS-PAGE loading buffer and subjected to western blot analysis.
Statistical analyses
Values are expressed as the means ± SD. Statistical evaluations were performed using Student’s t-tests and a one- or two-way ANOVA followed by Dunnett’s test. A p value of <0.05 was considered significant.
Results
Greater monocytic apoptosis in patients was complicated by lower TM expression after surgery
To determine whether expression of TM was associated with monocytic apoptosis in patients who underwent CABG surgery, we used flow cytometry to analyze the annexin V and TM expression. As shown in Figure 1A, the interesting results demonstrated a tendency for greater monocytic apoptosis in patients to be complicated by lower TM expression 24 hours after surgery. Then, we divided the patients with more than a 2-fold increase in annexin V into group 1 (11 patients) and the other patients into group 2 (7 patients). As shown in Figure 1B, the patients exhibited 126.3 ± 14.1% and 302.6 ± 42.3% of TM expression before surgery in group 1 and group 2, respectively. In contrast, monocytic annexin V increased to 267.5 ± 23.9% of the preoperative level in group 1 and 114.2 ± 83.9% of the preoperative level in group 2. The monocytic TM decreased and the apoptosis increased markedly in group 1 and was significantly different from that of group 2. Additionally, eotaxin was significantly increased during the process of cardiac surgery [3]; therefore, we decided to explore the effects of eotaxin-2 on monocytic TM and annexin V expression. ELISA was used to analyze the plasma eotaxin-2 concentrations in the two groups of patients. Before surgery, the plasma levels of eotaxin-2 were 124.3 ± 20.0 pg/mL and 126.3 ± 19.7 pg/mL in groups 1 and 2, respectively. However, the eotaxin-2 level reached 187.5 ± 14.9 pg/mL in group 1, which was significantly different from 135.5 ± 15.7 pg/mL in group 2 24 hours after surgery. To rule out the impact of patient demographic characteristics and perioperative surgical characteristics on monocytic TM expression and apoptosis, we collected demographic data and perioperative surgical characteristics, which are shown in Tables 1 and 2. The characteristics did not differ significantly across the two groups. These results indicated that patients with higher TM expression presented fewer apoptotic monocytes than did patients with lower TM expression after surgery. We also predicted that the increased plasma eotaxin-2 level would lead to declined TM expression and, thus, increased apoptosis.
Figure 1.
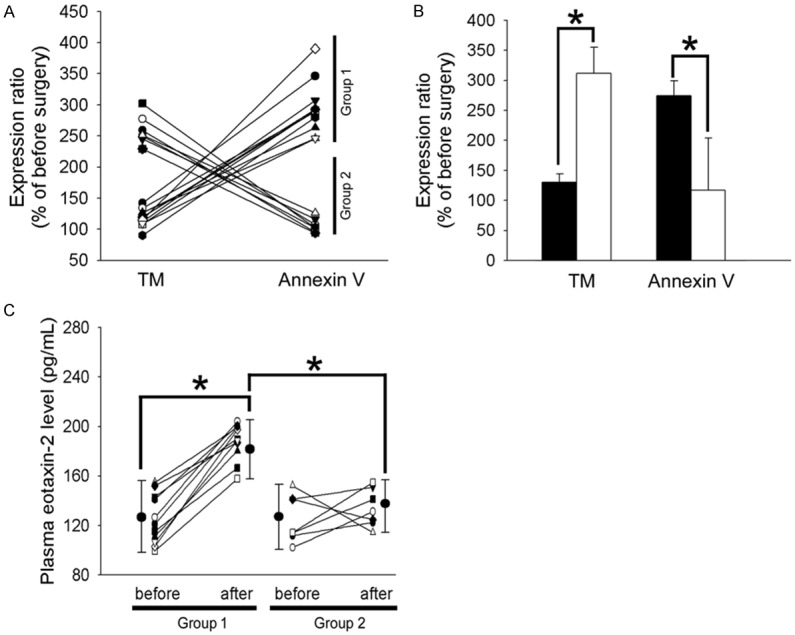
Patients have lower monocytic TM expression in combination with higher monocytic apoptosis and plasma eotaxin-2 level after surgery. A. Blood samples were collected at preincision and 24 hours after the commencement of CPB. Membrane TM expression and annexin V expression on monocytes were analyzed with flow cytometry. B. According to the tendency toward TM expression and the apoptosis situation, we distributed the patients with more than a 2-fold increase in annexin V into group 1 (11 patients) and the others into group 2 (7 patients). The bar graph shows the expression ratio (% before surgery) of TM and annexin V in group 1 (■) and group 2 (□). C. Changes in plasma concentrations of eotaxin-2 in patients who had undergone CABG. Data are presented as the means ± SEM; *P<0.05 was considered significant.
Table 1.
Demographic characteristics before surgery in elective CABG patients
| Group 1 (n=11) | Group 2 (n=7) | |
|---|---|---|
| Gender (Male/Female) | 5/6 | 4/3 |
| Age (years) | 67.3 ± 12.2 | 64.7 ± 14.2 |
| Body weight (kg) | 65.3 ± 9.8 | 64.9 ± 10.3 |
| Body height (cm) | 155.7 ± 10.2 | 159.2 ± 16.4 |
| Hypertension (n, %) | 10, 90.9% | 6, 85.7% |
| Smoke (n, %) | 3, 27.3% | 1, 14.3% |
| Hypercholesterolemia (n, %) | 8, 72.7% | 5, 71.4% |
| Diabetes mellitus (n, %) | 7, 63.6% | 5, 71.4% |
| Peripheral vascular disease (n, %) | 4, 36.4% | 2, 28.6% |
| COPD (n, %) | 5, 45.5% | 3, 42.9% |
| Eection fraction (%) | 60 ± 8% | 61 ± 7% |
CABG, coronary artery bypass grafting; COPD, chronic obstructive pulmonary disease.; Values are mean ± SD.
Table 2.
Perioperative characteristics in elective CABG patients
| Group 1 (n=11) | Group 2 (n=7) | |
|---|---|---|
| Minimal esophageal temperature | 29.1 ± 1.1°C | 29.5 ± 1.2°C |
| Heparin (unit) | 20500 ± 4931 | 19980 ± 5632 |
| CPB time (minutes) | 108.5 ± 48.7 | 107.3 ± 51.5 |
| Aortic clamping time (minutes) | 71.2 ± 15.1 | 69.2 ± 14.3 |
| pRBC transfusion (unit) | 3.2 ± 2.2 | 3.9 ± 2.4 |
| Drainage loss (mL) | 813.6 ± 120.9 | 796.1 ± 142.3 |
| Number of grafts | 3.5 | 3.2 |
| Mortality | 0 | 0 |
CPB, cardiopulmonary bypass; pRBC, packed red blood cells; Values are mean ± SD.
TM was associated with eotaxin-2-induced THP-1 cell apoptosis
To explore the hypothesis that eotaxin-2 induces monocytic apoptosis mediated by TM expression, we used fluorescent microscopy and flow cytometry for an in vitro investigation of monocytic THP-1 cells. We first determined the expression of annexin V/PI in eotaxin-2-induced THP-1 cells. The data indicated that treatment of THP-1 cells with 10 ng/mL eotaxin-2 for 24 hours markedly increased the intensity of annexin V/PI (Figure 2A). To verify that TM contributed to the effects of eotaxin-2-induced THP-1 cell apoptosis, we transfected THP-1 cells with either TM siRNA for knockdown or an HA-TM FL plasmid for overexpression for 24 hours, followed by eotaxin-2 treatment for 24 hours. The results showed that knockdown of TM significantly increased eotaxin-2-induced monocytic apoptosis compared with that in THP-1 cells receiving only eotaxin-2 treatment. In contrast, overexpression of TM significantly reversed the apoptosis situation when followed by eotaxin-2 treatment in THP-1 cells. Similarly, flow cytometry was used to confirm the immunofluorescence data. As shown in Figure 2B and 2C, the cell surface annexin/PI expression was strongly induced by 203.0 ± 30.1% of the control level after the cells were exposed to eotaxin-2 to induce apoptosis. The siRNA knockdown of TM markedly deteriorated eotaxin-2-induced apoptosis by 350.7 ± 20.4% of the control level relative to the eotaxin-2-treated group. Conversely, the levels of annexin/PI expression were significantly decreased by 109.9 ± 15.7% of the control levels when THP-1 cells were transfected with the HA-TM FL plasmid followed by eotaxin-2 stimulation. On the other hand, transfection of THP-1 cells with NC siRNA or a mock plasmid did not affect the annexin V/PI expression. The data indicated that TM played a role in eotaxin-2-induced monocytic THP-1 cell apoptosis.
Figure 2.
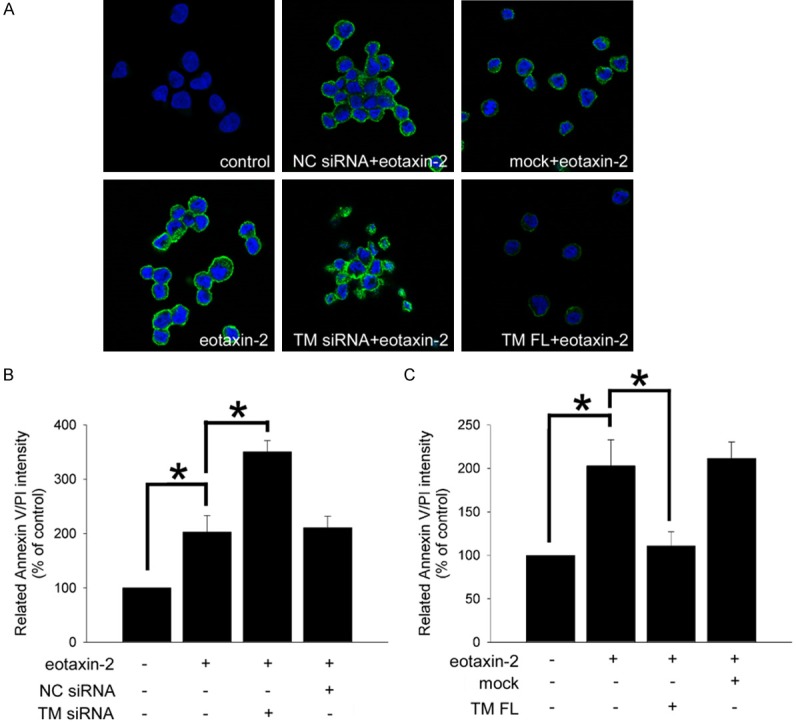
TM plays a role in eotaxin-2-induced monocytic THP-1 cell apoptosis. THP-1 cells were transfected with TM siRNA or HA-TM FL plasmid for 24 hours followed by 10 ng/mL eotaxin-2 stimulation for 24 hours. The expression of annexin V/PI expression on THP-1 cells was analyzed with fluorescence microscopy (A) and flow cytometry (B and C). Silencer validated siRNA (NC siRNA) and mock plasmid were used to validate the knockdown and overexpression, respectively. Five independent experiments were performed (n=5), and representative immunofluorescence images are shown. Data are presented as the means ± SEM; *P<0.05 was considered significant.
TM modulates apoptosis-related protein expression via PI3K/Akt axis signaling, which reduces apoptosis in eotaxin-2-stimulated THP-1 cells
Apoptosis is a process of programmed cell death that is generally characterized by various protein-dependent biochemical mechanisms. The expression of pro-apoptotic protein BAX and anti-apoptotic protein BCL-2 was analyzed using western blotting. As shown in Figure 3A, inducing apoptosis with eotaxin-2 markedly inhibited Bcl-2 and increased BAX relative to the levels in the untreated group. To determine whether TM contributed to these effects, we knocked down TM expression using TM siRNA prior to eotaxin-2 treatment. The results showed that TM knockdown significantly increased BAX and inhibited Bcl-2 expression in the eotaxin-2 treatment group compared with the only-eotaxin-2 treatment group. On the other hand, when followed by eotaxin-2 treatment, overexpression of TM in THP-1 cells markedly decreased the BAX expression and slightly increased the Bcl-2 expression relative to the expression levels in the eotaxin-2 treatment group (Figure 3B). Bcl-2 and BAX are transcriptional targets for the p53, a tumor suppressor protein that induces apoptosis [37]. Additionally, PI3K/Akt signaling is an important pathway for regulating the apoptosis mediated by Bcl-2 and BAX expression. Therefore, Figure 3C demonstrates that the level of p53 was decreased and the phosphorylation/activation of Akt was increased when cells were transfected with HA-TM FL followed by eotaxin-2 treatment, suggesting that TM contributed to the effects of eotaxin-2-regulated p53 and Akt expression in THP-1 cells. Pretreatment with the PI3K inhibitor LY294002 for 1 hour significantly ameliorated the positive effects of HA-TM FL transfection in eotaxin-2-treated THP-1 cells (Figure 3D). The above results indicate that TM modulates apoptosis-related protein expression via PI3K/Akt axis signaling, which involves apoptosis in eotaxin-2-stimulated THP-1 cells.
Figure 3.
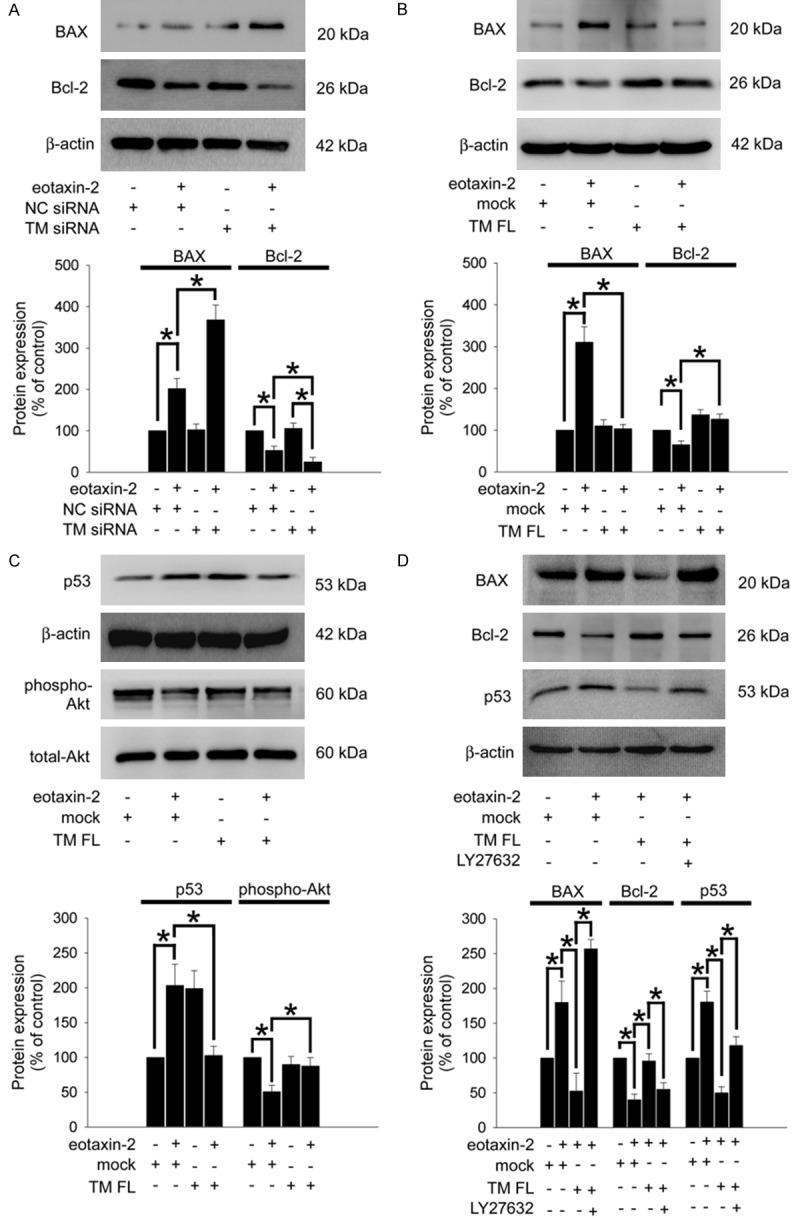
TM modulates BAX and Bcl-2 expression via PI3K/Akt axis signaling in eotaxin-2-stimulated THP-1 cells. THP-1 cells were transfected with TM siRNA or HA-TM FL plasmid followed by eotaxin-2 stimulation for 18 hours (A and B) or 6 hours (C). The expression of Bcl-2, BAX, and p53 and the phosphorylation of Akt on THP-1 cells were analyzed using western blot analysis. (D) THP-1 cells were transfected with HA-TM FL plasmid for 24 hours followed by 10 mM LY294002 for 1 hour then 10 ng/mL eotaxin-2 stimulation for 18 hours (for BAX and Bcl-2) or 6 hours (for p53). BAX, Bcl-2, and p53 were analyzed using western blot analysis. The total-Akt and β-actin were as loading controls. Silencer validated siRNA (NC siRNA) and mock plasmid were used to validate the knockdown and overexpression, respectively. The amount of protein expression was quantified using densitometry and is presented as a bar graph. The data are presented as the mean ± SEM (n=3), and *P<0.05 was considered significant.
TM regulates mitochondria-mediated apoptosis in eotaxin-2-stimulated THP-1 cells
According to previous evidence, Bcl-2 and BAX regulate mitochondria-mediated apoptosis [38]. Collapse of the mitochondrial membrane potential is an initial phenomenon during the cellular apoptosis process [39]. The analysis of JC-1 aggregation using image cytometry (Figure 4A showed that the mitochondria membrane potentials were strongly decreased by 62.5 ± 10.4% of the control level after the cells were exposed to eotaxin-2 for 6 hours. The siRNA knockdown of TM markedly deteriorated the eotaxin-2-decreased mitochondria membrane potential by 40.1 ± 7.1% of the control level relative to the eotaxin-2-treated group. Conversely, the levels of the mitochondria membrane potential were significantly reversed by 110.6 ± 16.4% of the control levels when THP-1 cells were transfected with the HA-TM FL plasmid followed by eotaxin-2 stimulation (Figure 4B). During mitochondria-mediated apoptosis, the stimulation induces cytochrome c release from mitochondria, and then caspase 9 and caspase 3 are cleaved/activated and accumulate rapidly. Indeed, Figure 4C shows that induction with eotaxin-2 for 6 hours markedly increased cytochrome c release and cleaved caspase 3 and 8 ac-cumulation relative to the levels in the untreated group. Even though TM knockdown significantly increased cytochrome c release in the control group, TM knockdown severely increased cytochrome c release and cleaved caspase 3 and 8 accumulation in the eotaxin-2-treatment group compared with the control, only-eotaxin-2 treatment, and only-TM-knockdown groups. On the other hand, overexpression of TM in THP-1 cells markedly decreased the cytochrome c release and cleaved caspase 3 and 8 accumulation followed by eotaxin-2 treatment relative to the eotaxin-2 treatment group (Figure 4D). The above results show that TM regulates mitochondria-mediated apoptosis in eotaxin-2-stimulated THP-1 cells.
Figure 4.
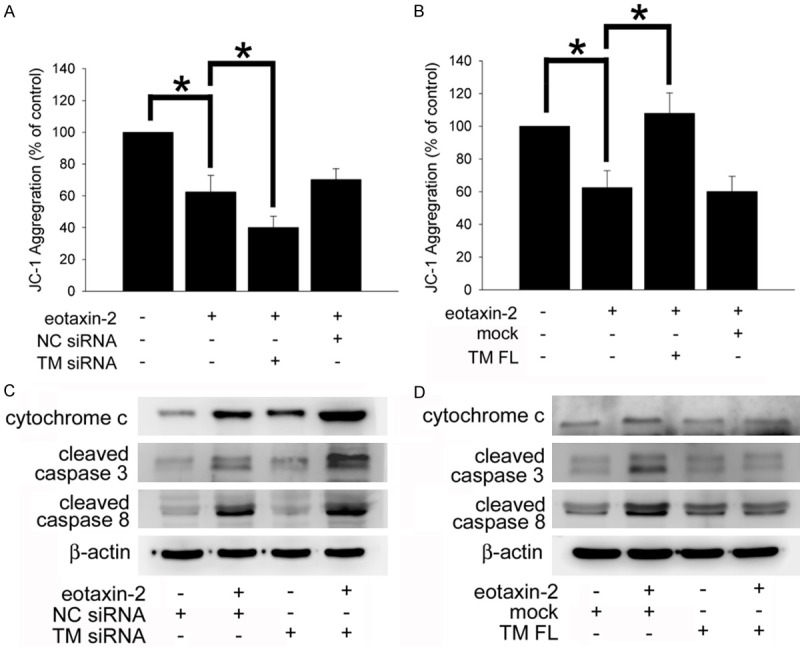
TM modulates mitochondria-mediated apoptosis in eotaxin-2- stimulated THP-1 cells. A and B. THP-1 cells were transfected with TM siRNA or HA-TM FL plasmid followed by eotaxin-2 stimulation for 6 hours. The mitochondria membrane potentials were analyzed using image cytometry. The percentage of JC-1 aggregation in experimental groups relative to the control group represents the decline in the mitochondria membrane potential. The data are presented as the mean ± SEM (n=5), and *P<0.05 was considered significant. C and D. THP-1 cells were transfected with TM siRNA or the HA-TM FL plasmid followed by eotaxin-2 stimulation for 8 hours. The release of cytochrome c and cleaved/activated of caspase 3 and 8 on THP-1 cells were analyzed using western blot analysis. Silencer validated siRNA (NC siRNA) and mock plasmid were used to validate the knockdown and overexpression, respectively. β-actin was employed as a loading control.
TM interacts with cofilin, which may regulate eotaxin-induced THP-1 cell apoptosis
Increasing evidence has revealed that mitochondrial translocation and interaction with cofilin is an early step during the apoptosis process [40,41]. Hence, we hypothesized that TM may act as a scaffold for cofilin docking. We first determined whether cofilin expression was responsible for the function of TM in eotaxin-2-induced THP-1 cells. Western blotting showed that induction with eotaxin-2 markedly inhibited the phosphorylation of cofilin in the cytosolic fraction relative to the levels in the untreated group. The level of cofilin phosphorylation was significantly reversed when THP-1 cells were transfected with the HA-TM FL plasmid followed by eotaxin-2 stimulation (Figure 5A). Conversely, the levels of cofilin in the mitochondria fraction was significantly increased when THP-1 cells were treated with eotaxin-2, and transfection with the HA-TM FL plasmid reversed the phenomenon, followed by eotaxin-2 stimulation (Figure 5B). A co-IP assay was used to verify the interaction between TM and cofilin in eotaxin-2-stimulated THP-1 cells. Western blot analysis showed that no cofilin signal was observed in the IgG IP control (Figure 5C, upper lane 2), whereas cofilin was detectable in the anti-TM-antibody immunoprecipitated fraction derived from naïve THP-1 cells (Figure 5C, upper lane 3). Additionally, the interaction of TM and cofilin was decreased by TM siRNA (Figure 5C, upper lane 4), indicating that there was an interaction between TM and cofilin in THP-1 cells. To further confirm the interaction between TM and cofilin, immunoprecipitation using goat anti-cofilin antibody was also performed in non-transfected THP-1 cells (Figure 5C, upper lane 4). To confirm the validity, we collected data, which indicated that the co-immunoprecipitated TM was clearly present in the anti-cofilin antibody immunoprecipitate (Figure 5C, lower). These results indicate that cofilin interacts with TM. We therefore suggest that TM acts as a scaffold for cofilin docking, which regulates the mitochondria-mediated downstream signaling pathway.
Figure 5.
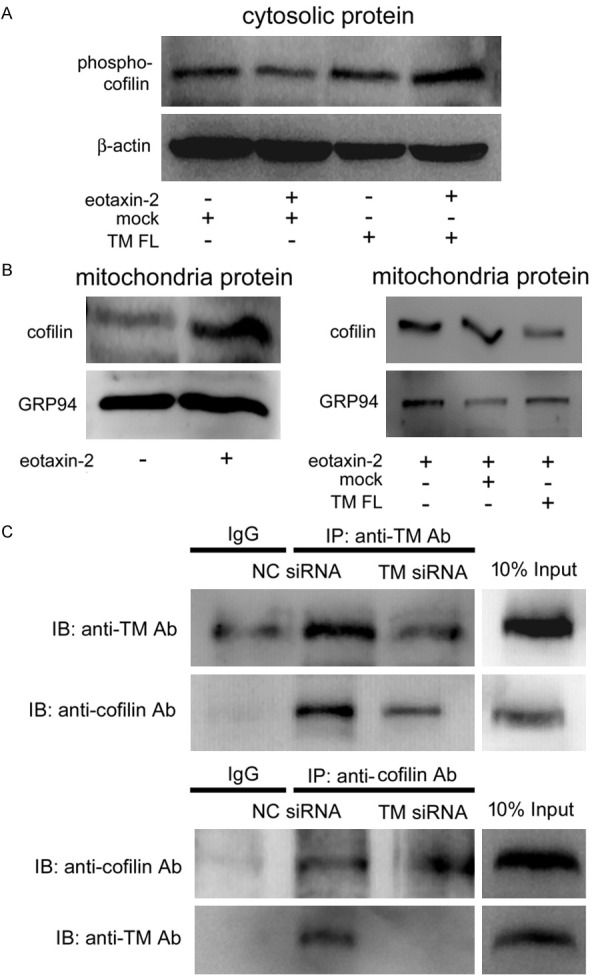
TM may act as a scaffold for cofilin docking, which modulates mitochondria-mediated apoptosis in THP-1 cells. THP-1 cells were transfected with HA-TM FL plasmid for 24 hours followed by 10 ng/mL eotaxin-2 stimulation for 12 hours. A. The total cell lysates were purified and analyzed for the activation (phosphorylation) of cofilin using western blot analysis. b-actin was used as a loading control. B. The mitochondrial fractions were extracted and analyzed for the cofilin using western blot analysis. GRP94 was used as a loading control. C. Total cell lysates of THP-1 cells were immunoprecipitated using a rabbit IgG control, an anti-TM antibody, or anti-cofilin antibody followed by immunoblotting with antibodies against TM, or cofilin, as indicated. Ten-percent inputs were used to identify the specificities of the antibodies.
Discussion
The schematic shown in Figure 6 summarizes our current results in vitro. We demonstrated for the first time that TM might decrease cell apoptosis in eotaxin-2-stimulated monocytic cells. In fact, TM may regulate mitochondria-mediated apoptosis by the PI3K/Akt axis signaling pathway, which acts as an extinguisher of p53 and BAX activation and limits further downstream release of cytochrome c and cle-avage of caspase 8 and 3. Furthermore, we suggest that TM interacts with the cofilin cytoskeleton, which further supports a role of TM in cell apoptosis. These above mechanisms may be relevant to the clinical situations in which patients with more monocytic apoptosis were complicated by lower TM expression after CABG surgery.
Figure 6.
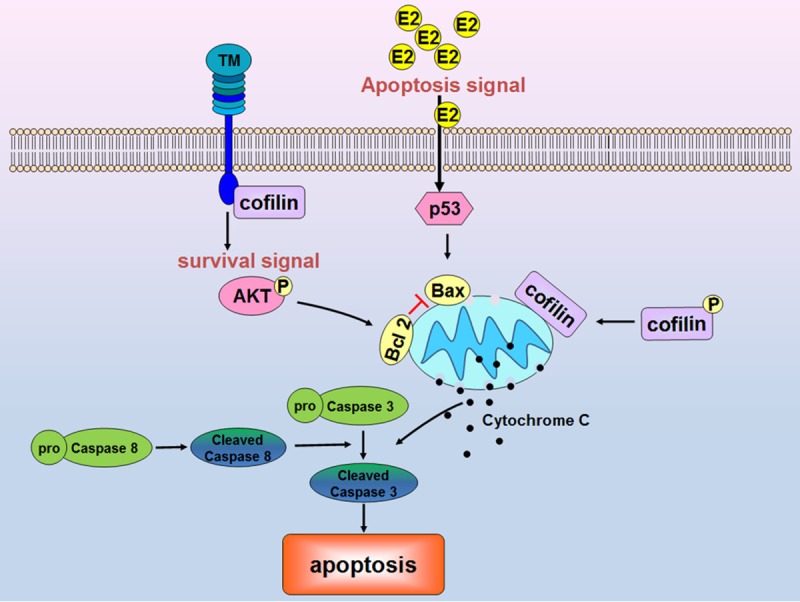
The mechanism by which TM contributes to eotaxin-2-mediated apoptosis in monocytic cells. TM may regulate cell apoptosis via the PI3K/Akt axis signaling pathway, which acts as an extinguisher for p53 and BAX activation, as well as further downstream cytochrome c release and caspase 8 and 3 cleavage. The interaction between TM and cofilin further supported a role for TM in cell apoptosis.
Eotaxin-2 regulates lymphocyte activation. CD16-postive macrophage express eotaxin-2 and then activate T cells during HIV infections [42]; eotaxin-2 is involved in lentiviral protein-induced CD4-postive lymphocyte activation [43]; a high concentration of eotaxin-2 triggers T cell migration and is associated with metastatic tumors of colorectal cancer [44]; and eotaxin-induced thymocyte activation may have important physiological implications for lymphocyte mobilization within and from this lymphoid organ [45]. Eotaxin-2 expression in the epithelium may be associated with asthma onset, control, and severity, resulting from its contribution to eosinophil migration [46]. Patients with a high plasma level of eotaxin-2 show a positive correlation with susceptibility to severe asthma, which may be associated with the +1272 A to G polymorphism and ht2 as well as ht6 haplotypes in the eotaxin-2 gene [47]. Interestingly, anti-eotaxin-2 anti-bodies provide efficacious protection in experimental atherosclerosis and arthritis [30,48]. However, an analysis from the Taiwan National Health Insurance Research Database has shown that major complications and mortality are significantly increased in patients with asthmatic disease who undergo major inpatient surgeries [49]. Cardiac surgery may result in a severe attack of asthma or other complications during or immediately after cardiac surgery. Similarly to previous investigators [3], we found that the level of eotaxin-2 significantly increased in some CPB patients and predicted that the increase in eotaxin-2 may be associated with post-surgical complications/early outcomes in the hospital, although we did not analyze the phenomena in this clinical study. On the other hand, the results indicated that eotaxin-2-induced monocytic apoptosis may be associated with axis of immunity after CPB. Additionally, why eotaxin-2 did not increase in some patients after cardiac surgery remains to be further analyzed. Eotaxin-2 is also secreted from epithelial cells, eosinophils, monocytes, and macrophages. We observed that plasma levels of eotaxin-2 were increased in some patients who underwent CPB, although we did not identify the source organs and tissues. Undoubtedly, since cardiac surgery increases plasma eotaxin-2 levels and eotaxin-2 may increase the risk of postoperative complications, it is essential to explore the effects on monocytic function, and eotaxin-2 may be a potential therapeutic target for preventing and treating postsurgical complications.
Inflammatory responses are necessary for an organism to resist external stressors such as bacterial/viral infections and trauma and to repair and restore damaged tissue/cellular functions. Many types of cells are involved in inflammatory responses, including granulocytes, lymphocytes, and monocytes. For monocytes, the number and physiological status of the cells need to be maintained in a specific and appropriate manner so that the inflammatory reaction can be accomplished correctly and completely [50]; that is, after appropriate inflammatory responses are launched and the stress is eliminated, the monocytes should stop their activity smoothly so as not to cause excessive inflammation and secondary damage to the tissue/organism [51]. Under normal and theoretical conditions, cellular apoptosis is one of the ways to regulate and stop the inflammatory response in monocytes [52]. The timing of this braking mechanism is the key factor in determining the appropriateness of the inflammatory response; therefore, many diseases, including neurodegenerative diseases, ischemic damage, immune deficiency, and cancers, are due to inappropriate apoptosis [50], and the inflammatory response cannot be properly terminated [13]. Previously, we had demonstrated that TM regulates monocytic migration and differentiation, which is associated with early outcomes in cardiac surgery patients during hospitalization [20,21]. Additionally, TM gene C1418T polymorphism is associated with the development of coronary allograft vasculopathy [53] which may result from chronic systemic inflammation and monocytic activity. In this study, we also found that patients with less increase in cellular TM at 24 hours after CABG surgery had an increased apoptotic status in monocytes (probably due to an increased level of plasma eotaxin-2). Based on these results, we predict that CPB can cause disorder of the inflammatory dynamical mechanism of monocytes. Which of the factors (cardioplegia solution infusion, foreign material attachment, ischemia and reperfusion) is the main stimulus still needs to be elucidated. There was no significant difference in preoperative demographic characteristics and perioperative characteristics between patients in groups 1 and 2. However, the same surgical approach and the single-surgeon operation still caused differences in the plasma levels of eotaxin-2; we are curious as to the distinguishing factors that cause some patients to retain their sensitivity to CPB and are currently studying this issue.
The switching on of inflammation is an immune system process that is necessary for the organism to repair damaged tissues and resist the pressure of foreign pathogens and antigens. In this process, the monocyte is a central component that plays critical roles. In the absence of immune stress, monocytes generally circulate in the bloodstream for 1-2 days and expire via the activation of spontaneous apoptosis [54]. During inflammation, the monocyte life span is prolonged by intracellular mechanical inhibition of the spontaneous apoptosis [55], which provides enough time for differentiation and migration as well as relevant inflammatory responses. However, previous studies have also shown that after the inflammatory response has started and the proper effects have occurred, the relevant monocytes involved in the inflammation should stop their activity via cellular apoptosis, which results in the return of the physiological state to a well-balanced origin [56,57]. The inflammatory response is similar to a double-edged sword in that over-response as well as insufficient action will cause serious disturbances of cellular physiological functions. However, it is not easy to achieve this goal with precision to maintain an appropriate, i.e., not excessive or insufficient, inflammatory response in the organism. Pleiotropic stress always undermines the ability of the immune system to initiate immune cell activity and to terminate the inflammatory responses of immune cells in a timely manner. We had demonstrated that patients undergoing CABG surgery who have increased blood levels of TNF-α may exhibit decreased TM expression in monocytes and, hence, a poor early outcome after cardiac surgery [20]; we interpret this situation to mean that decreased TM in monocytes after CABG surgery results in an unfit inflammatory state. Additionally, TM directly colocalizes and interacts with highly expressed PKCδ in CD68-positive infiltrated macrophages in human atherosclerotic plaques, indicating that the coordination between TM and PKCδ in macrophages participates in atherogenesis [23]. In this study, eotaxin-2 induced by CABG surgery promoted the decrease of TM in monocytes, subsequently resulting in apoptosis (although we did not confirm the majority source of plasma eotaxin-2). The decrease in TM caused by CABG surgery results in increased monocytic migration and apoptosis, and TM modulates monocytic differentiation and infiltration into the arterial wall during atherogenesis, which can be considered a result of the change in immune capacity. This phenomenon causes a disorder of inflammatory patterns and disturbances of the inflammatory process so that immune cells cannot respond properly at appropriate times to the pressure. How to control monocytic migration, apoptosis, differentiation and inflammatory responses in an appropriate state through regulating TM is the principal research subject for the near future.
In conclusion, the increased eotaxin-2 after CPB may induce monocytic apoptosis which is associated with TM expression. TM may regulate mitochondria-mediated apoptosis by its PI3K/Akt axis signaling pathway, which acts as an extinguisher for p53 and BAX activation, as well as limit further downstream release of cytochrome c and cleavage of caspases 8 and 3; these data strongly suggest that TM expression participates in eotaxin-2-induced monocyte apoptosis, and the present study provides a novel view of the role of TM in monocyte apoptosis during the development and regulation of inflammation after cardiac surgery.
Acknowledgements
This work was supported by National Ministry of Science and Technology (MOST 106-2320-B-038-037 and MOST 107-2314-B-016-062), Tri-service General Hospital (TSGH-C105-007-S01, TSGH-C106-005-007-S01, and TSGH-C107-005-005-007-S01), and Taipei Medical University Hospital (107TMU-TMUH-22) Taipei, Taiwan.
Disclosure of conflict of interest
None.
References
- 1.Kawamura T, Wakusawa R, Okada K, Inada S. Elevation of cytokines during open heart surgery with cardiopulmonary bypass: participation of interleukin 8 and 6 in reperfusion injury. Can J Anaesth. 1993;40:1016–1021. doi: 10.1007/BF03009470. [DOI] [PubMed] [Google Scholar]
- 2.Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 3.Castellheim A, Hoel TN, Videm V, Fosse E, Pharo A, Svennevig JL, Fiane AE, Mollnes TE. Biomarker profile in off-pump and on-pump coronary artery bypass grafting surgery in low-risk patients. Ann Thorac Surg. 2008;85:1994–2002. doi: 10.1016/j.athoracsur.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 4.Raja SG, Berg GA. Impact of off-pump coronary artery bypass surgery on systemic inflammation: current best available evidence. J Card Surg. 2007;22:445–455. doi: 10.1111/j.1540-8191.2007.00447.x. [DOI] [PubMed] [Google Scholar]
- 5.brana S, Parri MS, De Filippis R, Gianetti J, Clerico A. Monitoring of monocyte functional state after extracorporeal circulation: a flow cytometry study. Cytometry B Clin Cytom. 2004;58:17–24. doi: 10.1002/cyto.b.10061. [DOI] [PubMed] [Google Scholar]
- 6.Hadley JS, Wang JE, Michaels LC, Dempsey CM, Foster SJ, Thiemermann C, Hinds CJ. Alterations in inflammatory capacity and tlr expression on monocytes and neutrophils after cardiopulmonary bypass. Shock. 2007;27:466–473. doi: 10.1097/01.shk.0000245033.69977.c5. [DOI] [PubMed] [Google Scholar]
- 7.Sato K, Li J, Metais C, Bianchi C, Sellke F. Increased pulmonary vascular contraction to serotonin after cardiopulmonary bypass: role of cyclooxygenase. J Surg Res. 2000;90:138–143. doi: 10.1006/jsre.2000.5869. [DOI] [PubMed] [Google Scholar]
- 8.Laffey JG, Boylan JF, Cheng DC. The systemic inflammatory response to cardiac surgery: implications for the anesthesiologist. Anesthesiology. 2002;97:215–252. doi: 10.1097/00000542-200207000-00030. [DOI] [PubMed] [Google Scholar]
- 9.Takala AJ, Jousela IT, Takkunen OS, Jansson SE, Kyosola KT, Olkkola KT, Leirisalo-Repo M, Repo H. Time course of beta 2-integrin cd11b/cd18 (mac-1, alpha m beta 2) upregulation on neutrophils and monocytes after coronary artery bypass grafting. Cd11b upregulation after cabg surgery. Scand J Thorac Cardiovasc Surg. 1996;30:141–148. doi: 10.3109/14017439609107259. [DOI] [PubMed] [Google Scholar]
- 10.Parratt R, Hunt BJ. Direct activation of factor x by monocytes occurs during cardiopulmonary bypass. Br J Haematol. 1998;101:40–46. doi: 10.1046/j.1365-2141.1998.00674.x. [DOI] [PubMed] [Google Scholar]
- 11.Fan ST, Edgington TS. Coupling of the adhesive receptor cd11b/cd18 to functional enhancement of effector macrophage tissue factor response. J Clin Invest. 1991;87:50–57. doi: 10.1172/JCI115000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anselmi A, Abbate A, Girola F, Nasso G, Biondi-Zoccai GG, Possati G, Gaudino M. Myocardial ischemia, stunning, inflammation, and apoptosis during cardiac surgery: a review of evidence. Eur J Cardiothorac Surg. 2004;25:304–311. doi: 10.1016/j.ejcts.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Parihar A, Eubank TD, Doseff AI. Monocytes and macrophages regulate immunity through dynamic networks of survival and cell death. J Innate Immun. 2010;2:204–215. doi: 10.1159/000296507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinbroum AA, Biderman P, Soffer D, Klausner JM, Szold O. Reliability of cardiac output calculation by the fick principle and central venous oxygen saturation in emergency conditions. J Clin Monit Comput. 2008;22:361–366. doi: 10.1007/s10877-008-9143-y. [DOI] [PubMed] [Google Scholar]
- 15.Hiesmayr MJ, Spittler A, Lassnigg A, Berger R, Laufer G, Kocher A, Artemiou O, Boltz-Nitulescu G, Roth E. Alterations in the number of circulating leucocytes, phenotype of monocyte and cytokine production in patients undergoing cardiothoracic surgery. Clin Exp Immunol. 1999;115:315–323. doi: 10.1046/j.1365-2249.1999.00801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Paparella D, Yau TM, Young E. Cardiopulmonary bypass induced inflammation: pathophysiology and treatment. An update. Eur J Cardiothorac Surg. 2002;21:232–244. doi: 10.1016/s1010-7940(01)01099-5. [DOI] [PubMed] [Google Scholar]
- 17.Hirose H, Ohishi A, Nakamura H, Sugiura H, Umezawa A, Hosoda Y. Fatal splenic rupture in anabolic steroid-induced peliosis in a patient with myelodysplastic syndrome. Br J Haematol. 1991;78:128–129. doi: 10.1111/j.1365-2141.1991.tb04398.x. [DOI] [PubMed] [Google Scholar]
- 18.Wu HL, Lin CI, Huang YL, Chen PS, Kuo CH, Chen MS, Wu GC, Shi GY, Yang HY, Lee H. Lysophosphatidic acid stimulates thrombomodulin lectin-like domain shedding in human endothelial cells. Biochem Biophys Res Commun. 2008;367:162–168. doi: 10.1016/j.bbrc.2007.12.135. [DOI] [PubMed] [Google Scholar]
- 19.Weerasinghe A, Athanasiou T, Philippidis P, Day J, Mandal K, Warren O, Anderson J, Taylor K. Platelet-monocyte pro-coagulant interactions in on-pump coronary surgery. Eur J Cardiothorac Surg. 2006;29:312–318. doi: 10.1016/j.ejcts.2005.11.036. [DOI] [PubMed] [Google Scholar]
- 20.Tsai CS, Tsai YT, Lin CY, Lin TC, Huang GS, Hong GJ, Lin FY. Expression of thrombomodulin on monocytes is associated with early outcomes in patients with coronary artery bypass graft surgery. Shock. 2010;34:31–39. doi: 10.1097/SHK.0b013e3181d494c4. [DOI] [PubMed] [Google Scholar]
- 21.Lin YW, Huang CY, Shih CM, Chang WL, Shyue SK, Tsai YT, Lin CY, Lee CY, Chang YJ, Chang NC, Lin FY, Tsai CS. The c-terminal domain of thrombomodulin regulates monocytes migration with interleukin-6-stimulation. Euro J Inflam. 2014;12:27–39. [Google Scholar]
- 22.Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwege V, Hedman H, Freyssinet JM. Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 1994;153:3245–3255. [PubMed] [Google Scholar]
- 23.Tsai CS, Lin YW, Huang CY, Shih CM, Tsai YT, Tsao NW, Lin CS, Shih CC, Jeng H, Lin FY. Thrombomodulin regulates monocye differentiation via pkcdelta and erk1/2 pathway in vitro and in atherosclerotic artery. Sci Rep. 2016;6:38421. doi: 10.1038/srep38421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim HK, Kim JE, Chung J, Kim YT, Kang SH, Han KS, Cho HI. Lipopolysaccharide down-regulates the thrombomodulin expression of peripheral blood monocytes: effect of serum on thrombomodulin expression in the thp-1 monocytic cell line. Blood Coagul Fibrinolysis. 2007;18:157–164. doi: 10.1097/MBC.0b013e32801481cb. [DOI] [PubMed] [Google Scholar]
- 25.Van de Wouwer M, Collen D, Conway EM. Thrombomodulin-protein c-epcr system: integrated to regulate coagulation and inflammation. Arterioscler Thromb Vasc Biol. 2004;24:1374–1383. doi: 10.1161/01.ATV.0000134298.25489.92. [DOI] [PubMed] [Google Scholar]
- 26.Jaggers J, Lawson JH. Coagulopathy and inflammation in neonatal heart surgery: mechanisms and strategies. Ann Thorac Surg. 2006;81:S2360–2366. doi: 10.1016/j.athoracsur.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 27.Crikis S, Zhang XM, Dezfouli S, Dwyer KM, Murray-Segal LM, Salvaris E, Selan C, Robson SC, Nandurkar HH, Cowan PJ, d’Apice AJ. Anti-inflammatory and anticoagulant effects of transgenic expression of human thrombomodulin in mice. Am J Transplant. 2010;10:242–250. doi: 10.1111/j.1600-6143.2009.02939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Grey ST, Hancock WW. A physiologic anti-inflammatory pathway based on thrombomodulin expression and generation of activated protein c by human mononuclear phagocytes. J Immunol. 1996;156:2256–2263. [PubMed] [Google Scholar]
- 29.Patel VP, Kreider BL, Li Y, Li H, Leung K, Salcedo T, Nardelli B, Pippalla V, Gentz S, Thotakura R, Parmelee D, Gentz R, Garotta G. Molecular and functional characterization of two novel human c-c chemokines as inhibitors of two distinct classes of myeloid progenitors. J Exp Med. 1997;185:1163–1172. doi: 10.1084/jem.185.7.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ablin JN, Entin-Meer M, Aloush V, Oren S, Elkayam O, George J, Barshack I. Protective effect of eotaxin-2 inhibition in adjuvant-induced arthritis. Clin Exp Immunol. 2010;161:276–283. doi: 10.1111/j.1365-2249.2010.04172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Paulis A, Annunziato F, Di Gioia L, Romagnani S, Carfora M, Beltrame C, Marone G, Romagnani P. Expression of the chemokine receptor ccr3 on human mast cells. Int Arch Allergy Immunol. 2001;124:146–150. doi: 10.1159/000053694. [DOI] [PubMed] [Google Scholar]
- 32.Sallusto F, Mackay CR, Lanzavecchia A. Selective expression of the eotaxin receptor CCR3 by human T helper 2 cells. Science. 1997;277:2005–2007. doi: 10.1126/science.277.5334.2005. [DOI] [PubMed] [Google Scholar]
- 33.Forssmann U, Uguccioni M, Loetscher P, Dahinden CA, Langen H, Thelen M, Baggiolini M. Eotaxin-2, a novel cc chemokine that is selective for the chemokine receptor ccr3, and acts like eotaxin on human eosinophil and basophil leukocytes. J Exp Med. 1997;185:2171–2176. doi: 10.1084/jem.185.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Menzies-Gow A, Ying S, Sabroe I, Stubbs VL, Soler D, Williams TJ, Kay AB. Eotaxin (ccl11) and eotaxin-2 (ccl24) induce recruitment of eosinophils, basophils, neutrophils, and macrophages as well as features of early- and latephase allergic reactions following cutaneous injection in human atopic and nonatopic volunteers. J Immunol. 2002;169:2712–2718. doi: 10.4049/jimmunol.169.5.2712. [DOI] [PubMed] [Google Scholar]
- 35.Watanabe K, Jose PJ, Rankin SM. Eotaxin-2 generation is differentially regulated by lipopolysaccharide and il-4 in monocytes and macrophages. J Immunol. 2002;168:1911–1918. doi: 10.4049/jimmunol.168.4.1911. [DOI] [PubMed] [Google Scholar]
- 36.Chen YH, Lin SJ, Lin FY, Wu TC, Tsao CR, Huang PH, Liu PL, Chen YL, Chen JW. High glucose impairs early and late endothelial progenitor cells by modifying nitric oxide-related but not oxidative stress-mediated mechanisms. Diabetes. 2007;56:1559–1568. doi: 10.2337/db06-1103. [DOI] [PubMed] [Google Scholar]
- 37.Basu A, Haldar S. The relationship between bci2, bax and p53: consequences for cell cycle progression and cell death. Mol Hum Reprod. 1998;4:1099–1109. doi: 10.1093/molehr/4.12.1099. [DOI] [PubMed] [Google Scholar]
- 38.Hockenbery D, Nunez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 39.Gottlieb E, Armour SM, Harris MH, Thompson CB. Mitochondrial membrane potential regulates matrix configuration and cytochrome c release during apoptosis. Cell Death Differ. 2003;10:709–717. doi: 10.1038/sj.cdd.4401231. [DOI] [PubMed] [Google Scholar]
- 40.Chua BT, Volbracht C, Tan KO, Li R, Yu VC, Li P. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol. 2003;5:1083–1089. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- 41.Li G, Zhou J, Budhraja A, Hu X, Chen Y, Cheng Q, Liu L, Zhou T, Li P, Liu E, Gao N. Mitochondrial translocation and interaction of cofilin and drp1 are required for erucin-induced mitochondrial fission and apoptosis. Oncotarget. 2015;6:1834–1849. doi: 10.18632/oncotarget.2795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ancuta P, Autissier P, Wurcel A, Zaman T, Stone D, Gabuzda D. Cd16+ monocyte-derived macrophages activate resting t cells for hiv infection by producing ccr3 and ccr4 ligands. J Immunol. 2006;176:5760–5771. doi: 10.4049/jimmunol.176.10.5760. [DOI] [PubMed] [Google Scholar]
- 43.Fiorucci G, Olivetta E, Chiantore MV, Federico M. Microarray analysis reveals ccl24/eotaxin-2 as an effector of the pathogenetic effects induced by hiv-1 nef. Curr Drug Discov Technol. 2007;4:12–23. doi: 10.2174/157016307781115502. [DOI] [PubMed] [Google Scholar]
- 44.Cheadle EJ, Riyad K, Subar D, Rothwell DG, Ashton G, Batha H, Sherlock DJ, Hawkins RE, Gilham DE. Eotaxin-2 and colorectal cancer: a potential target for immune therapy. Clin Cancer Res. 2007;13:5719–5728. doi: 10.1158/1078-0432.CCR-07-1145. [DOI] [PubMed] [Google Scholar]
- 45.Franz-Bacon K, Dairaghi DJ, Boehme SA, Sullivan SK, Schall TJ, Conlon PJ, Taylor N, Bacon KB. Human thymocytes express ccr-3 and are activated by eotaxin. Blood. 1999;93:3233–3240. [PubMed] [Google Scholar]
- 46.Coleman JM, Naik C, Holguin F, Ray A, Ray P, Trudeau JB, Wenzel SE. Epithelial eotaxin-2 and eotaxin-3 expression: relation to asthma severity, luminal eosinophilia and age at onset. Thorax. 2012;67:1061–1066. doi: 10.1136/thoraxjnl-2012-201634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Min JW, Lee JH, Park CS, Chang HS, Rhim TY, Park SW, Jang AS, Shin HD. Association of eotaxin-2 gene polymorphisms with plasma eotaxin-2 concentration. J Hum Genet. 2005;50:118–123. doi: 10.1007/s10038-005-0230-3. [DOI] [PubMed] [Google Scholar]
- 48.Adi M, Arnon A, Michai EM, Gad K, Jacob G. Anti eotaxin-2 antibodies attenuate the initiation and progression of experimental atherosclerosis. World J Cardiovas Dis. 2013;3:339–346. [Google Scholar]
- 49.Lin CS, Chang CC, Yeh CC, Chung CL, Chen TL, Liao CC. Postoperative adverse outcomes in patients with asthma: a nationwide population-based cohort study. Medicine (Baltimore) 2016;95:e2548. doi: 10.1097/MD.0000000000002548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kolb JP, Oguin TH 3rd, Oberst A, Martinez J. Programmed cell death and inflammation: winter is coming. Trends Immunol. 2017;38:705–718. doi: 10.1016/j.it.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen C, Zhao D, Fang S, Chen Q, Cheng B, Fang X, Shu Q. Trim22-mediated apoptosis is associated with bak oligomerization in monocytes. Sci Rep. 2017;7:39961. doi: 10.1038/srep39961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tsai CS, Lin YW, Shih CC, Chen YH, Lin CY, Tsai YT, Lee CY, Shih CM, Huang CY, Chung HH, Lin FY. Thrombomodulin gene polymorphism (c1418t) is associated with the development of coronary allograft vasculopathy. Euro J Inflam. 2013;11:685–696. [Google Scholar]
- 54.Fahy RJ, Doseff AI, Wewers MD. Spontaneous human monocyte apoptosis utilizes a caspase-3-dependent pathway that is blocked by endotoxin and is independent of caspase-1. J Immunol. 1999;163:1755–1762. [PubMed] [Google Scholar]
- 55.Savill J. Apoptosis in resolution of inflammation. J Leukoc Biol. 1997;61:375–380. doi: 10.1002/jlb.61.4.375. [DOI] [PubMed] [Google Scholar]
- 56.Giamarellos-Bourboulis EJ, Routsi C, Plachouras D, Markaki V, Raftogiannis M, Zervakis D, Koussoulas V, Orfanos S, Kotanidou A, Armaganidis A, Roussos C, Giamarellou H. Early apoptosis of blood monocytes in the septic host: is it a mechanism of protection in the event of septic shock? Crit Care. 2006;10:R76. doi: 10.1186/cc4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Antonopoulou A, Raftogiannis M, Giamarellos-Bourboulis EJ, Koutoukas P, Sabracos L, Mouktaroudi M, Adamis T, Tzepi I, Giamarellou H, Douzinas EE. Early apoptosis of blood monocytes is a determinant of survival in experimental sepsis by multi-drug-resistant pseudomonas aeruginosa. Clin Exp Immunol. 2007;149:103–108. doi: 10.1111/j.1365-2249.2007.03392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


