Abstract
Tissue-engineered condyles provide a promising approach for end-stage osteoarthritis to reconstruct normal physiological structure and function of the temporomandibular joint (TMJ). However, lack of successful biological condyles in large animals restricts clinical translation. Scaffold-free cartilage cell sheets do not contain any polymeric material which potentially risks local nonspecific inflammatory reactions. In this study, we used cartilage cell sheets covering bone marrow mesenchymal stem cells-Polycaprolactone/Hydroxyapatite (BMSCs-PCL/HA) scaffolds (cell sheet group) transplanted subcutaneously and intramuscularly in mini-pigs. In contrast, autogenous chondrocytes were seeded on polyglycolic acid/ polylactic acid (PGA/PLA) scaffolds for 4 and 12 weeks in-vitro pre-cultivation. Then, they were used as a cartilage-phase composition covering BMSCs-PCL/HA scaffolds, then the entirety (biphase scaffold group) was transplanted subcutaneously into mini-pigs. After 12 weeks, the harvested samples were examined histologically. The cartilage layer was evaluated for thickness, glycosaminoglycan (GAG) quantitation, total collagen quantitation and Young’s modulus. The biphase scaffold group failed in regeneration, while the cell sheet group regenerated biological condyle with healthy osteochondral construct. The GAG quantitation, total collagen quantitation and Young’s modulus of regenerated cartilage was close to those of the natural condyle. Collectively, cartilage cell sheets combined with bone-phase composition had the potential to regenerate biological condylar.
Keywords: Cell sheet, osteochondral construct, regeneration, tissue-engineered condyle
Introduction
The end stage of temporomandibular joint osteoarthritis (TMJOA) is advanced damage of the articular surface, with organic destruction of osteochondral structures. Currently, there is no drug and surgery which can reverse this course. Joint replacement is the ultimate solution to relieve pain, dysfunction and malformation. However, there are many shortcomings in traditional autologous bone transplantations and artificial joint replacements [1,2]. Compared with traditional joint replacement methods, regenerated tissue-engineered osteochondral constructs may be able to reconstruct the condyle with biological structures and restore TMJ function [3].
In the entire field of biological condylar osteochondral construct regeneration, some progress has been made. A number of articles have reported successful regeneration of osteochondral structures of the condyle in nude mice. For example, Weng et al [4] used PGA/PLA inoculated with osteoblasts, covered by calcium alginate gel encapsulating chondrocytes in nude mice to regenerate mandibular condyles. Ding et al [5] used PGA/PLA inoculated with chondrocytes covering PCL/HA seeded with BMSCs, then implanted them subcutaneously in nude mice to regenerate the biological femoral head. However, the successful regeneration of entire joints has only been reported in immunodeficiency nude mice models. In large animal models with complete immune systems, regeneration research has not had a breakthrough, which severely limits the ability of tissue-engineered biological condyle to perform clinically.
Using nude mice as a carrier, the immune system is missing. In order to clarify the biosafety, in vivo inflammatory response, morphological maintenance and optimal implantation time of cells-scaffold compositions, implanting cells-scaffold compositions into a fully immunogenic animal model to evaluate their ectopic regeneration is indispensable to provide the basis for clinical grafting in situ. So far in large animal models, there has been no successful whole joint with osteochondral structure. The potential cause was the immune and inflammatory reactions. Degradation products of some biodegradable polymer materials, such as the commonly used PGA/PLA scaffolds, were compatible in nude mice, but caused inflammation, immune rejection and a series of adverse reactions, leading to failure of regeneration in large animal model [6]. In order to avoid such failures, we used cartilage cell sheets without materials to replace the chondrocytes-PGA/PLA composition as the cartilage repair phase, which covered bone phase cells-scaffold for implantation and succeeded in regenerating entire mandibular condyles with osteochondral structures in nude mice [7]. Whether the cartilage cell sheets can also effectively avoid the inflammatory reaction in large animals and successfully regenerate a biological condyle is not known.
This study was aimed to use cartilage cell sheets combined with a BMSCs-PCL/HA scaffold composition implanted into mini-pigs subcutaneously and intramuscularly to regenerate biological condyles. As a control group, chondrocytes-PGA/PLA scaffolds pre-cultivated in vitro for different time period combined with BMSCs-PCL/HA scaffold composition were implanted subcutaneously. Cartilage cell sheets and chondrocytes-PGA/PLA scaffolds were compared to demonstrate the difference in vivo response in mini-pigs.
Materials and methods
Cell acquisition, culture and proliferation
Chondrocytes were obtained from 10-month-old mini-pigs (Shanghai Jambo Biotechnology Co., Ltd.). Ketamine (8 mg/kg) was intramuscular injected into the mini-pigs and a 2.0 cm × 2.0 cm size of the auricle cartilage was removed and placed in 75% alcohol. After rinsing with 0.2% chloramphenicol, the skin, connective tissue, and perichondrium was removed and the cartilage was diced, rinsed and placed in 0.15% collagenase for 7-8 hours in a shaker at 37°C. The harvested cells were re-suspended with high-glucose Dulbecco’s modified Eagle’s medium (DMEM) and the chondrocytes were seeded on 100 mm petri dishes at a density of 2 × 104 cells/cm2. The medium was changed every 3-4 days. When the cells grew to 90%, the cell culture medium was removed and adherent cells were washed with phosphate buffered saline (PBS) and 1.5 ml trypsin was added to digest (including EDTA). The cells were observed under the light microscope and when they became spherical, 1.5 ml of culture medium was added to terminate the digestion. The cells were rinsed from the bottom of the dish and the suspension was collected and seeded on new petri dishes adding basic fibroblast growth factor (bFGF) at a ratio of 5 ng/ml. The passage 2 (P2) generation cells were used for inoculation of the material.
Bone mesenchymal stem cells (BMSCs) were taken from the ilium of the mini-pigs. After anesthesia and sterile preparation, a needle was introduced into the iliac bone to harvest about 8 ml of bone marrow aspirate. Thirty ml of low-glucose DMEM was added to the cell suspension, and the solution was centrifuged at 1800 r/min for 5 minutes. The floating fat and most of the supernatant was removed and the remaining solution was seeded on 100 mm Petri dishes and cultured in low glucose DMEM medium at a density of 2 × 104 cells/cm2. After 4 days of culture, changed medium containing 5 ng/mL of bFGF was added. When the cells reached 90% fusion state, they were passaged. The P2 BMSCs were used to seed on the scaffolds.
Fabrication of biphasic scaffold
PGA/PLA cartilage phase scaffolds were fabricated as described in the previous literature [8,9]. Female and the male molds of mandibular condyles were fabricated by 3D printing based on mini-pig mandibular condyle CT data. PLA-containing methylene chloride solution (0.5%) was added drop wise to solidify the non-woven PGA fibers. The procedure was repeated several times using a female mold and a male mold for compaction with PGA containing PLA solution inside to let methylene chloride volatilize. Then, the shape of the PGA/PLA scaffold formed (Figure 1D). The scaffolds were sterilized with ethylene oxide before use.
Figure 1.
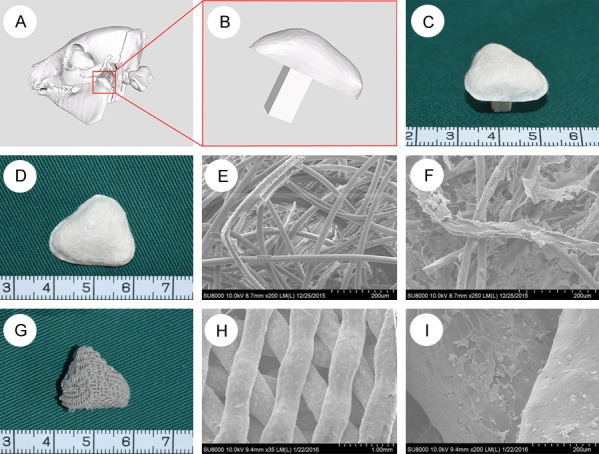
Fabrication of PGA/PLA and PLA/HA scaffold. Three-dimensional reconstruction of mandibular condyle by CT scanning mini-pig head (A). The red rectangular regions in (A) were magnified to better visualize the mandibular condyle (B). The combination of PGA/PLA and PLA/HA scaffold (C). The gross view of the cartilage-phase PGA/PLA scaffold (D). The scanning electron microscope (SEM) scan showed PGA fibers were bonded with PLA (E) and chondrocytes were attached well on the scaffold (F). The gross view of the bone-phase PCL/HA scaffold (G). The SEM scan showed interconnecting porosity of the scaffold and bone marrow stem cells (BMSCs) were attached well on the scaffold (H, I).
PCL/HA bone phase scaffolds were fabricated by using mixer (mixer 50EHT 3Z, Brabender, Germany) to mix polycaprolactone particles with nanoscale hydroxyapatite powder as described in previous literature [10], and 3D printed scaffolds were prepared according to the CT data (Figure 1A, 1B, 1G). The pore diameter was approximately 200-400 μm; the porosity was 54.6 ± 1.2%, and the scaffolds were sterilized by ethylene oxide [11].
Culture cells-scaffold compositions in vitro
The P2 generation chondrocytes were seeded onto the PGA/PLA scaffolds at a density of 100 × 106 cells/ml and placed in the incubator for 4-5 hours to allow them to adhere to the scaffold. The cells-scaffold composition was then incubated in chondrogenic induction medium for 4 or 12 weeks. The surface morphology and structure of the chondrocyte-PGA/PLA scaffold composition was evaluated by scanning electron microscopy (SEM) and histological staining.
The P2 generation BMSCs were seeded into the pores of the PCL/HA scaffold at a density of 20 × 106 cells/ml and placed in the incubator for 4-5 hours to allow the cells to adhere [12]. The cells-scaffold composition was then placed in osteogenic inducing medium to induce osteogenesis for 2-3 weeks, inducing BMSCs to differentiate into osteoblasts. The adhesion of BMSCs to PCL/HA scaffold was evaluated by SEM.
Fabrication of cartilage cell sheet
The P2 generation chondrocytes were seeded on a six-well plate and a six-well plate with transwell chamber at a concentration of 2.0 × 106 cells/cm2 and added to a conventional cell culture medium for static culture. On the second day, chondrogenic induction medium was added to the culture and the medium was changed daily. After a certain period of time (1, 2, 3, 4 weeks), cartilage cell sheets were evaluated by histological staining. The best cell sheet with maximum thickness and most glycosaminoglycan was used for the next transplantation experiment.
ALP, ARS staining and quantitative ALP activity
Alkaline phosphatase (ALP) staining was used to detect the osteogenic ability of BMSCs. BMSCs were seeded on a six-well plate at a density of 1 × 105 cells/well and subjected to alkaline phosphatase staining at day 0, 7 and 14 after osteogenic induction. The BMSCs on the six-well plates were fixed with 4% paraformaldehyde for 20 minutes, and the BCIP/NBT alkaline phosphatase color kit (Beyotime Institute of Biotechnology, China) was added and incubated for 30 minutes at 37°C. Alizarin red-S (ARS) staining was performed on days 0, 7 and 14 after osteogenic induction. Induced BMSCs were fixed and stained with alizarin red-S dye (Sigma-Aldrich) for 30 mins at 37°C as previously described [13].
ALP quantitation and ALP staining were performed at the same time. Cells were rinsed three times with PBS and lysed using RIPA lysis buffer (Beyotime). Then the liquid supernatant was collected to standard 96-well plate (BD Medical technology). Reagent from Alkaline Phosphatase Assay Kit (Beyotime) was added to 96-well plate according to manufacturer’s instruction. The absorption of 405 nm wave-length after 30 minutes incubation at 37°C indicated the quantitation of ALP activity.
Laboratory animal groups
Four 10-month-old mini-pigs (Shanghai Jambo Biotechnology Co., Ltd.) were used, one group for each. Group 1 (4 wk biphasic scaffold group, N = 8): the induced chondrocytes-PGA/PLA composition incubated for 4 weeks in vitro covered induced BMSCs-PCL/HA composition and sutured on scaffold edge by 5-0 silk thread to fix, then was implanted in mini-pigs subcutaneously. Group 2 (12 wk biphasic scaffold group, N = 8): The 12-weeks induced chondrocytes-PGA/PLA composition which was sutured with the induced BMSCs-PCL/HA composition at the same way above, was implanted in mini-pigs subcutaneously; Group 3 (cell sheet subcutaneous group, N = 8): cartilage cell sheets incubated 4 weeks was stacked into 2 layers and sutured on the BMSCs-PCL/HA compositions, then implanted in mini-pigs subcutaneously; group 4 (cell sheet intramuscular group, N = 8): implanted the grafts of group 3 into the mini-pigs’ muscle.
Mini-pig subcutaneous and intramuscular implantation
All animal experiments were approved by the Shanghai Jiaotong University School of Medicine Experimental Animal Ethics Committee. All animals received humanitarian care and implement animal welfare measures according to the law of our country and the regulation of the standing committee. The animals were anesthetized with ketamine (8 mg/kg) intramuscular injection. The abdominal skin was prepared with iodophor and alcohol disinfectants. A scalpel was used to incise the skin. The subcutaneous tissues and abdominal muscle were separated, providing a space where the four groups of implants were implanted into four mini-pigs (Figure 4). The soft tissues were then closed in layers. The animals received intramuscular injections of one-million units/day penicillin for three consecutive days. After 12 weeks, the animals were euthanized by excessive CO2 anesthesia and the samples were taken out and fixed in 4% paraformaldehyde.
Figure 4.
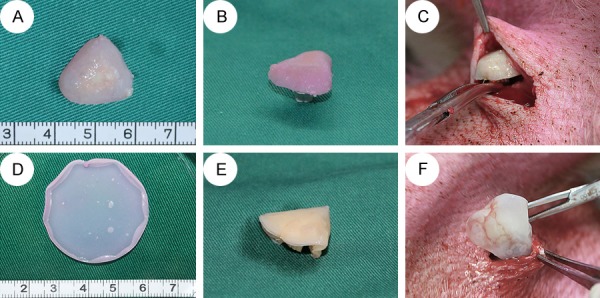
The transplantation of biphase scaffold group and cell sheet group. In biphase scaffold group, the Chondrocytes-PGA/PLA composition (A) covered with BMSCs-PCL/HA composition and sutured together (B). In cell sheet group, cartilage cell sheet (D) was cut to fit PCL/HA scaffold and was folded into two layers, sutured with BMSCs-PCL/HA composition together (E). Then, the grafts were implanted into the mini-pig (C, F).
Gross view
The specimens were observed to determine: 1) whether the constructs adhered to the surrounding tissue, 2) cartilage surface smoothness, and 3) size, shape of regenerative construct. They were then dissected sagittally to observe the interface of cartilage, subchondral tissue and so on.
Evaluation of histological and immunohistochemical staining
Specimens were fixed for more than 48 hours and then decalcified in 10% neutral EDTA. They underwent routine paraffin embedding and 6 μm thickness sections were obtained for staining with hematoxylin-eosin (HE), safranin-O/fast green (SO/FG), elastica van gieson (EVG), type I and type II collagen (monoclonal antibody, 1:75, Abcam, Cambridge, UK). The regenerated cartilage was evaluated according to the reference about the histological score of the International Society of Cartilage Repair [14]. To assess the cartilage regeneration precisely, three persons separately graded histological sections and. the mean value was taken [15]. In addition, the bone structure of regenerated bone was qualitatively evaluated by histological observation.
Biomechanical evaluation of regeneration cartilage in vivo
Specimens from group 3, group 4 and native condylar samples from mini-pigs were taken to conduct the tests. Continuous planar unconfined compressive tests were carried on at the speed of 1 mm per min and a strain-rate of 0.034 ± 0.0022/s at room temperature along the vertical direction until failure of sample (turning point occurred on the force-displacement curve). The biomechanical sensor could transmit the force-displacement curve and record it in real time. The compressive modulus was calculated by statistical analysis of the force-displacement curve [16]. The compressive strength was defined as the force when the sample fragmented.
Biochemical evaluation of regeneration cartilage in vivo
Specimens from group 3, group 4 and native condylar samples from mini-pigs were digested in 50 ug/ml papain solution (Sigma-Aldrich) at 65°C water bath. The digested solution was allowed to react with Alcian blue dye (Sigma-Aldrich). The intensity of color change was quantified immediately in a spectrophotometer by measuring absorbance at 600 nm. GAG contents of regenerated cartilage in subcutaneous and intramuscular sites, as well as native condylar cartilage were quantified by the Alcian Blue method [17]. DNA contents of the above three groups were quantified using a nucleic acid protein quantitation detector (Nanodrop 2000). Each sample was measure three times and the mean value was taken.
The total collagen contents of the above three groups were quantified by a hydroxyproline assay. The specimens were digested by alkaline hydrolysis. The hydroxyproline content was calculated by quantify the content of free hydroxyproline hydrolysates, which was assayed according to previously described methods [13]. The free hydroxyproline hydrolysates was converted to total collagen content according to the fixed ratio of collagen-to-hydroxyproline mass of 7.25 [18].
Statistical analysis
The results were expressed as mean ± standard deviation. SPSS 19.0 software (IBM, New York, USA) was used for statistical analysis. One-way ANOVA was used to compare the results. SNK (Student-Nweman-Keuls) = 0.05, P<0.05 indicates statistically significant.
Results
Results of cell-scaffold compositions and cartilage cell sheets in vitro
Chondrocytes adhered well on PGA/PLA scaffold (Figure 1F). The chondrocyte-PGA/PLA compositions were cultured in chondrogenesis solution for 4 weeks before fixation. Most of survived chondrocytes were detected in the histological staining slices, along with cartilage matrix and a large number of PGA fiber residues (Figure 2B-D). PGA fiber residues accounted for approximately 40% of the total tissue volume. After 12 weeks of in-vitro pre-cultivation, chondrocytes, typical cartilage lacunae and abundant chondrocyte extracellular matrix were found in the histological stained slices. PGA fibers were detected in tissue interstitium accounting for about 10% (Figure 2F-H).
Figure 2.
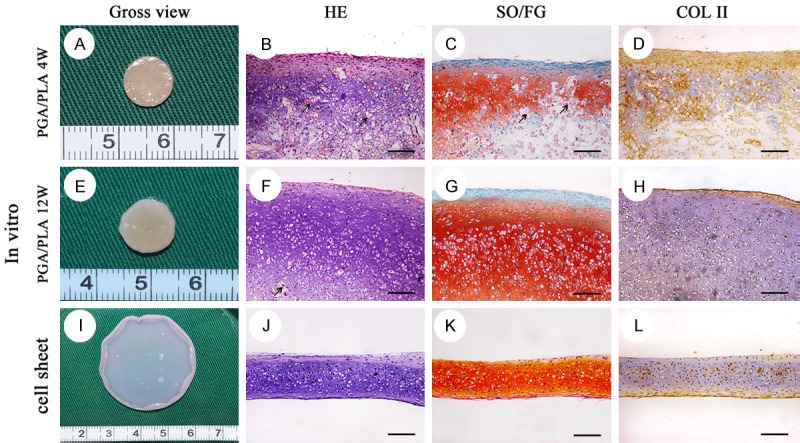
Gross view and histological staining of the in-vitro pre-cultivated cartilage cell sheet and chondrocytes-PGA/PLA composition. Gross view of chondrocytes-PGA/PLA composition in-vitro pre-cultivation for 4 w (A), 12 w (E) and cartilage cell sheet chondrogenic cultured for 3 w (I). The in-vitro pre-cultivated construct showed great many alive chondrocytes with typical lacunae (B, F, G; HE), strong positive staining of safranin O/fast green (C, G, K; SO/FG) and type II collagen (D, H, L; COL II). A number of PGA/PLA fiber still could be detected (black arrow) in histological staining section (B, C, F). Scaffold-free cartilage cell sheet was membrane without any foreign material residual in histological staining section (I-L). scale bar = 100 um.
After a certain period of time (1, 2, 3, 4 weeks) in culture and chondrogenesis induction, chondrocytes formed a complete cell sheet with certain mechanical stability and were easily lifted from the culture dish. The cartilage cell sheets were a solid, opaque and opal film that was smooth and easy to bend or fold. It was enough to withstand handling with tweezers. A large number of chondrocytes, significant cartilage lacunae, and abundant cartilage matrices were observed from histological staining (HE) (Figures 2I-L, 3). Large amount of cartilage matrix supported the cell sheet morphology. The middle did not contain any foreign material, and the cartilage cell sheet was continuous, homogeneous without any gaps.
Figure 3.
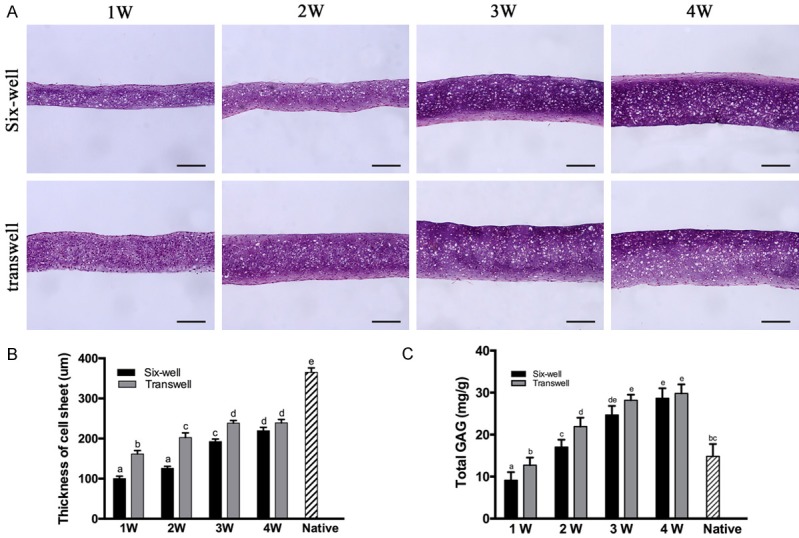
Histological and quantitative evaluation of in-vitro cultured cartilage cell sheet in six-well plate and transwell plate in different time. Quantitative thickness and total Glycosaminoglycan (GAG) of cell sheet in-vitro pre-cultivation at the time of 1, 2, 3, 4 week (A). The Quantification of the thickness (B) and total GAG content (C). As extend in-vitro culture time, the thickness and total GAG content of cartilage cell sheet increased significantly until 3rd week. Between normal six-well group and transwell group, there was significant difference about thickness and total GAG content at the time of 1, 2, 3 week. Columns with different letters indicate statistical significance. scale bar = 100 μm.
Pre-cultivation of cartilage cell sheet in vitro
With the increase of in vitro culture time (1 week-3 weeks), the thickness of the two groups of cartilage cell sheets increased, and there was a significant difference in the content of extracellular, like matrix gradually increased, the staining deepened, the entire cartilage became denser. However, from the third week to the fourth week, the thickness of the cell sheets increased insignificantly. After 4 weeks cultured in common six-well plate in vitro, the thickness of the cartilage cell sheet remained at 219.61 ± 8.09 μm, and the final thickness of transwell chamber-cultured cartilage cell sheet remained at 239.14 ± 8.27 μm (Figure 3A). The average thickness of the condylar cartilage layer was 363.06 ± 12.80 μm. Two layers of cell sheets were needed to reach the thickness of the natural condylar cartilage layer.
As the in vitro culture time increased to 4 weeks, the GAG content of the two groups of cartilage patches gradually increased. At each time period, the GAG content of transwell chamber-cultured chondrocytes was greater than that of the normal six-well plate, but no significant difference was observed between the two groups at the time of 4-weeks. After cultured in vitro for 4 weeks, the GAG content of cartilage cell sheets cultured in common six-well plate was 28.649 ± 2.40 mg/g, and the GAG content of cartilage cell sheets in transwell chamber was 29.802 ± 2.33 mg/g. The natural TMJ condylar cartilage of mini-pig was fibrocartilaginous with a GAG content of 14.836 ± 2.92 mg/g (Figure 3B).
Alkaline phosphatase and alizarin red-S staining results
BMSCs were cultured in osteogenic induction medium for 14 days. As the induced time increased, the staining of the alkaline phosphatase (ALP) was deeper-colored, indicating the activity level was higher, and reached a peak at 14 days (Figure 5). The alizarin red-S staining (ARS) showed the mineralization level of the extracellular matrix, and the trend of ARS staining was consistent with that of ALP analysis.
Figure 5.
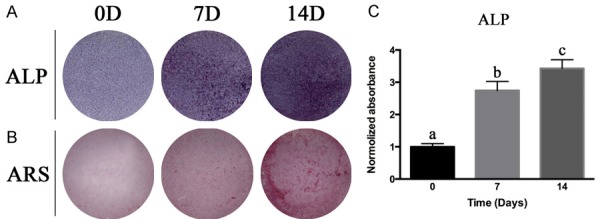
The osteogenic induction of BMSCs. BMSCs were cultured in osteogenic induction medium up to 14 days. Alkaline phosphatase (ALP) staining (A) and semi-quantitative analysis of ALP (C) at day 0, day 7 and day 14 showed increased ALP activity as in-vitro osteogenic induction time extended. Alizarin red staining (ARS) (B) at day 0, day 7 and day 14 revealed that extracellular matrix calcium deposition accumulated when lengthened osteogenic induction in vitro. Columns with different letters indicate statistical significance.
Gross view of regenerated biological condyle
After 12 weeks of implantation in the mini-pig model, as shown in Figure 6, group 1 (4 wk biphasic scaffold group) samples adhered with the surrounding connective tissue. It was difficult to completely separate the regenerative tissue from the native tissue. The surface and deeper sections of the regenerative tissue was filled with yellow chaotic mixed tissue (Figure 6A-C). In group 2 (12 wk biphasic scaffold group) cartilage phase and bone phase regenerative tissues were filled with earth yellow dense tissue, a rough surface, showing that bone phase and cartilage phase regenerative tissue can still be combined (Figure 6D-F). In group 3 (cell sheet subcutaneous group) and group 4 (cell sheet intramuscular group), the boundary between harvested samples and surrounding tissues was clear, easy to peel, and the samples maintained their original shape and size (13 mm × 12 mm × 8 mm). The surface was covered with a smooth, continuous milky white cartilage-like tissue. The thickness of the cartilage layer was approximately 1.0 mm. The subchondral PCL/HA scaffold was filled with white stiff tissue like cancellous bone (Figure 6G-L).
Figure 6.
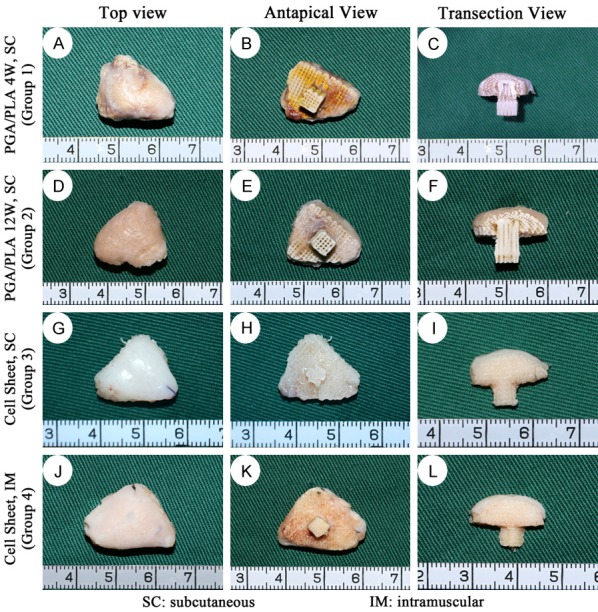
Gross view of the harvested in-vivo regenerated biological condyle from mini-pig in different groups. The top, antapical and transection view of group 1 showed that the regenerative tissue was unevenly chaotic in cartilage phase and bone phase (A-C); Those of group 2 showed yellow dense connective tissue with rough surface (D-F); In group 3 and group 4, the upper layer was presented as smooth, continuous milky white cartilage-like tissue. The PCL/HA scaffold was filled with white stiff tissue (G-L).
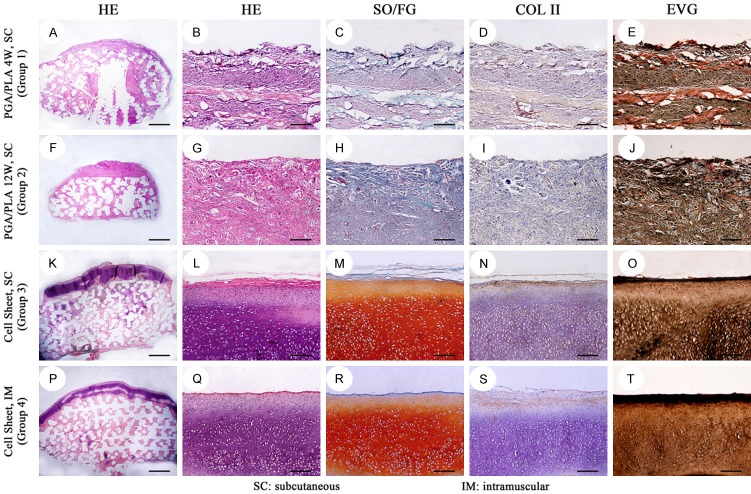
Histological evaluation of the regenerated biological condyle
Histologically, inflammatory granulation tissue, visible lymphocyte aggregation, partial plasma cell differentiation, foreign body giant cell response, PGA fiber residues and blood vessels were observed on the cartilage phase and bone phase regenerative constructs in groups 1 and 2. No visible cartilage tissue or bone tissue was noted (Figures 7, 8 and 9).
Figure 7.
Histological and immunohistochemical staining of the in-vivo regenerated cartilage-phase construct in different groups. In group 1 (A-E), confused loose connective tissue was detected. In group 2 (F-J), the regenerated construct displayed disorderly dense connective tissue with uncertain structure. Group 3 and group 4 showed alive chondrocytes, typical lacunae (K, L, P, Q; HE) with strong positive staining of safranin O/fast green (M, R; SO/FG), type II collagen (N, S; COL II) and elastic fibre (O, T; EVG). Scale bar = 1000 um (A, F, K, P), scale bar = 100 um (B-E, G-J, L-O, Q-T).
Figure 8.
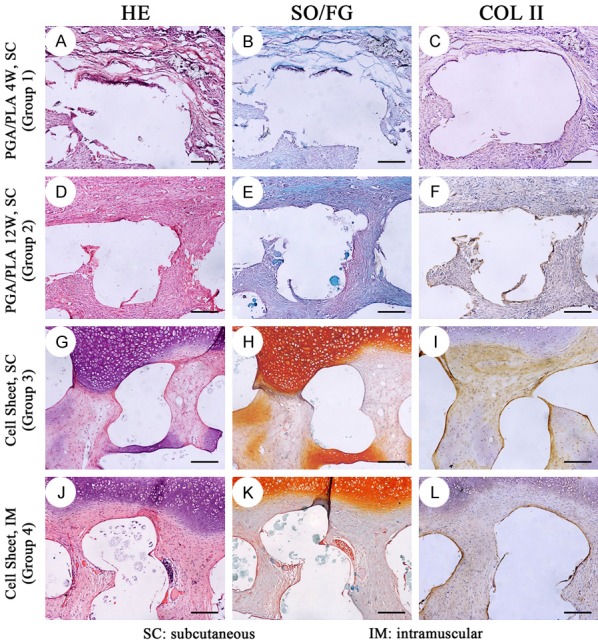
Histological and immunohistochemical staining of the in-vivo regenerated osteochondral interface in different groups. In group 1 (A-C) and group 2 (D-F), the regenerative tissue in bone-phase was similar with those in cartilage-phase in Figure 7, which was disorderly connective tissue with nonspecific structure. In group 3 (G-I) and group 4 (J-L), the regenerative cartilage was well integrated with the regenerated subchondral tissue. The subchondral tissue was cartilage, vessels and connective tissue. The white empty area was PCL/HA scaffold which was dissolved during the paraffin embedding process. scale bar = 100 um.
Figure 9.
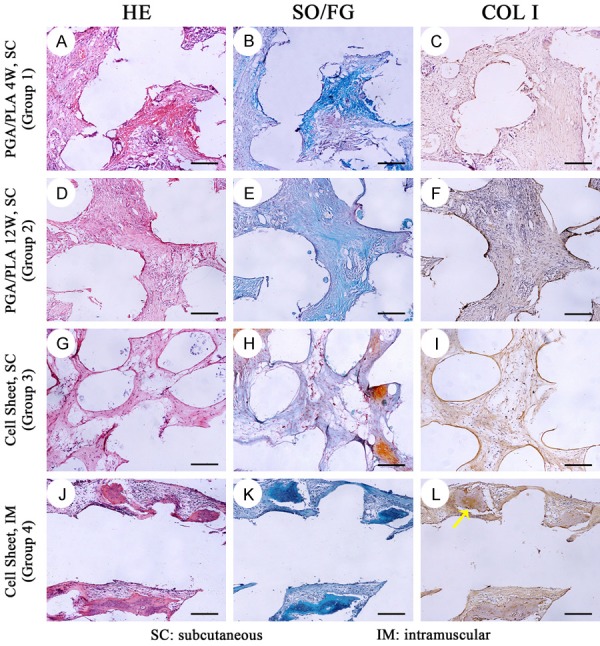
Histological and immunohistochemical staining of the in-vivo regenerated bone-phase construct in different groups. In group 1 (A-C) and group 2 (D-F), disorderly connective tissue with nonspecific structure was observed. In group 3 (G-I), connective tissue was detected with rare bone formation. In group 4, new bone formation (J-L) with trabecular structure and typical osteocytes was detected. Positive immunohistochemical localization of type I collagen (COL I) was shown in osseous component (yellow arrow, L). scale bar = 100 um.
In groups 3 and 4, two layers of cartilage cell sheets fused into one layer of cartilage construct, forming a continuous, homogeneous, single, bloodless healthy cartilage in which a large number of chondrocytes, typical cartilage lacunae and rich cartilage matrix were detected. Each cartilage lacuna was triangular, containing a single internal chondrocyte or two, and collagen-like fibers staggered arrangement around lacuna. The cartilage tissue did not contain any foreign material or foreign body reaction, and did not produce any immune evidence of rejection. Safranin-O/fast green staining showed a typical red cartilage matrix and immunohistochemical staining of type II collagen was positive (Figure 7).
In group 3 and group 4, the cartilage phase component was closely connected with the bone phase component. In the middle of the bone phase scaffold, scattered new bone formation distant from the cartilage layer was observed. The periphery of the new formed bone was surrounded by osteoblast-like cells. Bone cells were present in the bone tissue and the vascular and mesenchymal tissues were around bone tissue (Figure 9). In addition to bone, connective tissue and some cartilage tissue formation were also seen in microchannels of the PCL/HA scaffold. Chondrocytes were found near the cartilage tissue which was on outer layer of the scaffold (Figure 8).
Biomechanical and biochemical evaluations of regenerated biological condyles
In general, after 12 weeks post-operation, biological condyles of group 4 showed higher cartilage ECM contents (GAG, total collagen) and better compressive modulus compared with those of group 3. However, DNA content of two experimental groups presented a contradictory result (Figure 10, P<0.05). When compared with native condylar cartilage, two experimental groups showed significant lower DNA content, total collagen, worse visual score and weaker biomechanical properties (compressive modulus and compressive strength), but higher total GAG (Figure 10, P<0.05). No significant differences were observed between groups 3 and 4 in visual assessment and compressive strength. These results indicated that the regenerated cartilage layer in group 4 had somewhat better quality than that in group 3. The biomechanical and biochemical evaluation of the two experimental groups was approaching that of native condylar cartilage.
Figure 10.
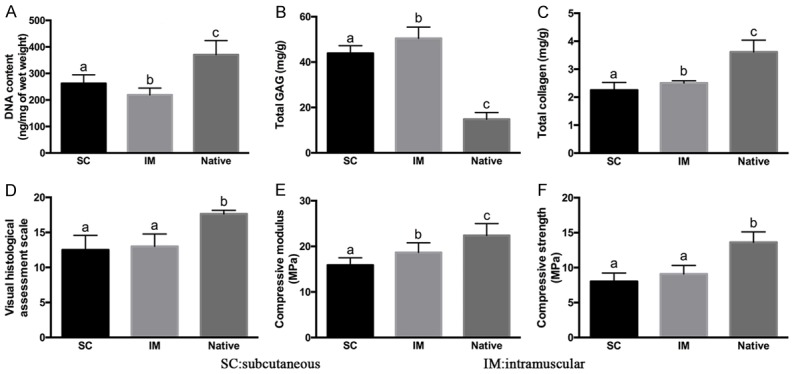
Biochemical and biomechanical evaluation of in-vivo regenerated tissue. Compared with group SC and group IM, the native group showed higher contents of DNA content (A), total collagen (C), as well as increased visual scale (D), compressive modulus (E) and compressive strength (F), but lower GAG content (B). Between the two regenerated groups, cell sheet intramuscular group displayed higher GAG, total collagen, compressive modulus and compressive strength, but lower DNA content than the SC group. Columns with different letters indicate statistical significance.
Discussion
Although great progress had been made in regeneration of the osteochondral construct in nude mice, so far, no literature has reported the successful reconstruction of the entire biological condylar osteochondral construct in large animals with complete immune systems. In this study, cartilage cell sheets were used to replace the PGA/PLA scaffold as the cartilage repair layer which covered the bone phase BMSCs-PCL/HA scaffold and successfully rege- nerated biological condyles in large animals. Cartilage cell sheet technology solved the inflammation and immune response shortcomings caused by previous scaffolds. This finding has important clinical significance and can partially predict the results of using such materials in humans. Our results provide possibilities for future regenerative transplantation of a biological condyle in situ.
In the past, there was no report about the successful construction of the entire articular osteochondral construct in large animal model, possibly due to the inflammation and the immune response of the scaffold materials, as the inflammatory reaction of the scaffolds could seriously interfere with the cartilage and bone regeneration. However, with the prolongation of the culture time in vitro, the scaffold materials will be degraded slowly and the volume of cartilage will increase gradually [19]. With this understanding, the chondrocytes-PGA/PLA scaffold composition was cultured in vitro for up to 12 weeks in order to degrade the PGA/PLA scaffold to the maximum extent. From the histological sections, it was observed that PGA/PLA fibers had been largely degraded and mature cartilage tissue was added to fill the degrading scaffolds and pores. However, long-term cultured chondrocytes-PGA/PLA scaffold compositions still failed to regenerate in mini-pigs, mainly due to the randomness and incompleteness of PGA/PLA degradation, and the residues of PGA/PLA scaffolds and their acidic degradation may lead to significant local non-specific inflammation and foreign body giant cell responses in mini-pigs [20]. In contrast, with the same culture and transplantation method, the cell sheet group without any scaffold material can successfully regenerate the biological condyle. This indicates that the inflammatory response produced by the residual scaffold material had a decisive impact on the regenerative results. Inflammation erodes constructed cartilage and affects the growth, differentiation, proliferation and secretion of BMSCs in bone scaffolds. These reactions lead to tissue fibrosis and excessive formation of inflammatory granulation tissue, resulting in destructive damage on the early stages of cartilage and bone formation and the failure of biological condylar regeneration.
The use of scaffold-free cartilage cell sheets combined with the BMSCs-PCL/HA bone scaffolds regenerated complete, continuous, homogeneous and healthy cartilage layers in the subcutaneous and muscle tissues of mini-pigs. Histologically, a triangular cartilage lacuna containing a single chondrocyte can be observed, occasionally, double cells were arranged side by side, surrounded by rich matrix and other mature cartilage tissue. No pathological reactions such as significant inflammation, aggregation of lymphocytes, or foreign body giant cell responses were observed. At the same time, the use of ultra-high density culture of chondrocytes to maintain the phenotypic stability of chondrocytes is superior to low density cultured chondrocyte [12,21]. Chondrocyte hypertrophy and dedifferentiation were not shown in both in vitro and post-implantation histological staining. After 12 weeks of growth in vivo, the regenerative bone phase component tightly integrated with the cartilage cell sheet with no intervening gaps. Within the micropores of the bone phase, new cancellous bone formation could be observed and the osteochondral interface transition was normal. The reasons that cartilage cell sheets sutured on BMSCs-PCL/HA scaffolds could construct the biological condyles in the large animals are as follows: 1, the scaffolds which may cause inflammation were not used to construct the condyles; 2, the seeded cells were derived from autologous chondrocytes and bone marrow mesenchymal stem cells; 3, the culture process did not contain any foreign antigens; 4, in vitro construction prior to implantation ensured the thickness of the cartilage necessary to maintain the shape of the biological condyle.
Quantitative analysis showed that the cartilage cell sheets further grew into mature cartilage in vivo. GAG content and total collagen content were significantly increased, nearly consistent with the content of condylar cartilage, and the total DNA decreased significantly, indicating that the number of chondrocytes decreased. The mature cartilage and extracellular matrix (glycosaminoglycans and collagen, etc.) occupied the vast majority of volume (chondrocytes only accounted for 5% of the cartilage). However, there were some differences between regenerated cartilage and natural condylar cartilage. Auricular cartilage and condylar cartilage belong to two different types of cartilage, and some differences exist in their regenerative and growth patterns. Elastic protein staining showed that the regenerated cartilage was still a kind of auricular cartilage derived from elastic cartilage, while condylar cartilage was a fibrous cartilage. With our current technology, the auricular cartilage is easier to obtain and the donor trauma is minor. Additionally, it does not affect donor function and aesthetics, and also can meet the functional requirements of condylar cartilage.
We also observed cartilage-like tissue formation derived from BMSCs between micropores of the bone scaffolds in groups 3 and 4, and the quantity and quality of the new bone were not ideal. The reason may be due to the several factors. First, auricular cartilage cells have a strong ability to induce and maintain cartilage. It has been previously reported that when transplanted with mature chondrocytes, BMSCs can form cartilage like tissue in the subcutaneous environment [22]. A series of soluble factors secreted by mature chondrocytes act on the BMSCs by means of paracrine signal and coordinately promote BMSCs chondrogenic differentiation in vivo ectopic growth [23]; Second, auricular cartilage cells secrete angiostatin, which inhibits osteoblast vascularization and bone regeneration. Third, subcutaneous and intramuscular regeneration is ectopic osteogenesis, which is not the best micro-environment for bone tissue regeneration. Fourth, lack of using osteogenic cytokines and optimizing cells-seeded density. These factors eventually led to a significant effect on osteogenesis. In the follow-up study, we plan to improve the quality and quantity of bone formation by improving the design of the scaffold material and the numbers of seeded cells.
Conclusion
The cell sheet groups could successfully regenerate biological condyles with osteochondral construct in mini-pig model, better than the biphase scaffold group results. The results of cartilage regeneration in intramuscular sites were better than those in subcutaneous sites in the mini-pig model.
Acknowledgements
This study was supported by grants from the National Natural Science Foundation of China (81472117), the Fund of Medicine and Engineering Interdisciplinary Program of Shanghai Jiao Tong University (YG2014MS05), the Science and Technology Commission of Shanghai Municipality Science Research Project (14DZ2294300, 15140902500, 17441900300), Combined Medicine and Engineering Project of Shanghai Jiao Tong University (YG2015QN04), Shanghai Shen Kang Medical Development Fund (16CR3045A), The Nature Key Research and Development Program of China (2017YFC1103900).
Disclosure of conflict of interest
None.
References
- 1.Khadka A, Hu J. Autogenous grafts for condylar reconstruction in treatment of TMJ ankylosis: current concepts and considerations for the future. Int J Oral Maxillofac Surg. 2012;41:94–102. doi: 10.1016/j.ijom.2011.10.018. [DOI] [PubMed] [Google Scholar]
- 2.Ren PG, Irani A, Huang Z, Ma T, Biswal S, Goodman SB. Continuous infusion of UHMWPE particles induces increased bone macrophages and osteolysis. Clin Orthop Relat Res. 2011;469:113–122. doi: 10.1007/s11999-010-1645-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rahaman MN, Mao JJ. Stem cell-based composite tissue constructs for regenerative medicine. Biotechnol Bioeng. 2005;91:261–284. doi: 10.1002/bit.20292. [DOI] [PubMed] [Google Scholar]
- 4.Weng Y, Cao Y, Silva CA, Vacanti MP, Vacanti CA. Tissue-engineered composites of bone and cartilage for mandible condylar reconstruction. J Oral Maxillofac Surg. 2001;59:185–190. doi: 10.1053/joms.2001.20491. [DOI] [PubMed] [Google Scholar]
- 5.Ding C, Qiao Z, Jiang W, Li H, Wei J, Zhou G, Dai K. Regeneration of a goat femoral head using a tissue-specific, biphasic scaffold fabricated with CAD/CAM technology. Biomaterials. 2013;34:6706–6716. doi: 10.1016/j.biomaterials.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 6.Ylinen P, Suuronen R, Taurio R, Tormala P, Rokkanen P. Use of hydroxylapatite/polymercomposite in facial bone augmentation. An experimental study. Int J Oral Maxillofac Surg. 2002;31:405–409. doi: 10.1054/ijom.2002.0252. [DOI] [PubMed] [Google Scholar]
- 7.Wang F, Hu Y, He D, Zhou G, Yang X, Ellis E. Regeneration of subcutaneous tissue-engineered mandibular condyle in nude mice. J Craniomaxillofac Surg. 2017;45:855–861. doi: 10.1016/j.jcms.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 8.Liu K, Zhou G, Liu W, Zhang W, Cui L, Liu X, Liu T, Cao Y. The dependence of in vivo stable ectopic chondrogenesis by human mesenchymal stem cells on chondrogenic differentiation in vitro. Biomaterials. 2008;29:2183–2192. doi: 10.1016/j.biomaterials.2008.01.021. [DOI] [PubMed] [Google Scholar]
- 9.He A, Liu L, Luo X, Liu Y, Liu Y, Liu F, Wang X, Zhang Z, Zhang W, Liu W, Cao Y, Zhou G. Repair of osteochondral defects with in vitro engineered cartilage based on autologous bone marrow stromal cells in a swine model. Sci Rep. 2017;7:40489. doi: 10.1038/srep40489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jiang W, Shi J, Li W, Sun K. Three dimensional melt-deposition of polycaprolactone/bio-derived hydroxyapatite composite into scaffold for bone repair. J Biomater Sci Polym Ed. 2013;24:539–550. doi: 10.1080/09205063.2012.698894. [DOI] [PubMed] [Google Scholar]
- 11.Jiang W, Shi J, Li W, Sun K. Morphology, wettability, and mechanical properties of polycaprolactone/hydroxyapatite composite scaffolds with interconnected pore structures fabricated by a mini-deposition system. Polymer Engineering & Science. 2012;52:2396–2402. [Google Scholar]
- 12.Schulze-Tanzil G, de Souza P, Villegas Castrejon H, John T, Merker H, Scheid A, Shakibaei M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002;308:371–379. doi: 10.1007/s00441-002-0562-7. [DOI] [PubMed] [Google Scholar]
- 13.Xie Q, Wang Z, Bi X, Zhou H, Wang Y, Gu P, Fan X. Effects of miR-31 on the osteogenesis of human mesenchymal stem cells. Biochem Biophys Res Commun. 2014;446:98–104. doi: 10.1016/j.bbrc.2014.02.058. [DOI] [PubMed] [Google Scholar]
- 14.Mainil-Varlet P, Aigner T, Brittberg M, Bullough P, Hollander A, Hunziker E, Kandel R, Nehrer S, Pritzker K, Roberts S, Stauffer E International Cartilage Repair Society. Histological assessment of cartilage repair: a report by the histology endpoint committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85-A(Suppl 2):45–57. [PubMed] [Google Scholar]
- 15.Wakitani S, Goto T, Pineda SJ, Young RG, Mansour JM, Caplan AI, Goldberg VM. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am. 1994;76:579–592. doi: 10.2106/00004623-199404000-00013. [DOI] [PubMed] [Google Scholar]
- 16.Yan D, Zhou G, Zhou X, Liu W, Zhang WJ, Luo X, Zhang L, Jiang T, Cui L, Cao Y. The impact of low levels of collagen IX and pyridinoline on the mechanical properties of in vitro engineered cartilage. Biomaterials. 2009;30:814–821. doi: 10.1016/j.biomaterials.2008.10.042. [DOI] [PubMed] [Google Scholar]
- 17.Enobakhare B, Bader D, Lee D. Quantification of sulfated glycosaminoglycans in chondrocyte/alginate cultures, by use of 1,9-dimethylmethylene blue. Anal Biochem. 1996;243:189–191. doi: 10.1006/abio.1996.0502. [DOI] [PubMed] [Google Scholar]
- 18.Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996;29:225–229. doi: 10.1016/0009-9120(96)00003-6. [DOI] [PubMed] [Google Scholar]
- 19.Liu Y, Li D, Yin Z, Luo X, Liu W, Zhang W, Zhang Z, Cao Y, Liu Y, Zhou G. Prolonged in vitro precultivation alleviates post-implantation inflammation and promotes stable subcutaneous cartilage formation in a goat model. Biomed Mater. 2016;12:015006. doi: 10.1088/1748-605X/12/1/015006. [DOI] [PubMed] [Google Scholar]
- 20.Cao Y, Rodriguez A, Vacanti M, Ibarra C, Arevalo C, Vacanti C. Comparative study of the use of poly(glycolic acid), calcium alginate and pluronics in the engineering of autologous porcine cartilage. J Biomater Sci Polym Ed. 1998;9:475–487. doi: 10.1163/156856298x00578. [DOI] [PubMed] [Google Scholar]
- 21.Watt FM. Effect of seeding density on stability of the differentiated phenotype of pig articular chondrocytes in culture. J Cell Sci. 1988;89:373–378. doi: 10.1242/jcs.89.3.373. [DOI] [PubMed] [Google Scholar]
- 22.Li D, Zhu L, Liu Y, Yin Z, Liu Y, Liu F, He A, Feng S, Zhang Y, Zhang Z, Zhang W, Liu W, Cao Y, Zhou G. Stable subcutaneous cartilage regeneration of bone marrow stromal cells directed by chondrocyte sheet. Acta Biomater. 2017;54:321–332. doi: 10.1016/j.actbio.2017.03.031. [DOI] [PubMed] [Google Scholar]
- 23.Liu X, Sun H, Yan D, Zhang L, Lv X, Liu T, Zhang W, Liu W, Cao Y, Zhou G. In vivo ectopic chondrogenesis of BMSCs directed by mature chondrocytes. Biomaterials. 2010;31:9406–9414. doi: 10.1016/j.biomaterials.2010.08.052. [DOI] [PubMed] [Google Scholar]