Abstract
The purpose of the current study was to investigate whether metformin can enhance the anti-cancer effect of cisplatin on epithelial ovarian cancer in vitro and in vivo. CCK-8 assays were performed to detect cell viability, and flow cytometry was performed to measure cell apoptosis rates. Transwell assays were used to detect the migration and invasion ability of ovarian cancer cells. Western blotting and qRT-PCR were performed to detect protein expression. Xenograft mouse models were constructed to clarify the treatment response in vivo. Metformin alone or cisplatin alone dose-dependently inhibited SKOV3 and Hey cell proliferation. The combination of these two drugs exerted a stronger inhibitory effect with a higher apoptosis rate than administration of either drug alone. Transwell assay results revealed that metformin promoted the inhibitory effect of cisplatin on ovarian cancer cell metastasis. Metformin and cisplatin co-treatment significantly inhibited N-cadherin and MMP-9 expression. The Western blotting results revealed that metformin and cisplatin co-treatment inhibited TGFβ1 expression and Smad2 and Smad3 phosphorylation. The in vivo study results were consistent with results from the in vitro study. Data from our study suggest that metformin enhanced the anti-tumour effect of cisplatin on epithelial ovarian cancer in vitro and in vivo, which provides more evidence supporting the use of metformin to treat epithelial ovarian cancer.
Keywords: TGFβ, metformin, epithelial ovarian cancer, cisplatin, metastasis
Introduction
As the most lethal gynaecological malignancy, an estimated 22440 new cases and 14080 deaths due to ovarian cancer were reported in America in 2016 [1]. Due to a lack of early symptoms, many patients are diagnosed at advanced stages. Currently, the standard treatment for ovarian cancer is radical surgery and platinum-based chemotherapy. Despite the development of novel chemotherapy drugs, the overall five-year survival rate of ovarian cancer patients remains approximately 40% [2]. Chemotherapy resistance is one of the leading causes of recurrence, and approximately 25% of patients experience recurrence within 6 months after the end of treatment [3]. Therefore, development of new, promising therapies for the treatment of ovarian cancer is an urgent need.
Metformin, a relatively safe, inexpensive and effective anti-diabetes mellitus drug, has been used clinically for several decades [4]. In 2005, Evans et al. reported that diabetic patients taking metformin had a lower risk of cancer than those who did not take metformin [5]. Since then, an increasing number of studies has reported the anti-cancer effect of metformin. Preclinically, metformin has been reported to inhibit the PI3K/AKT/mTOR signalling pathway, which plays a critical role in metabolism, proliferation and many other malignancy activities [6,7]. Verifying the published research, our previous study concluded that metformin induced ovarian cancer cell apoptosis by activating the p38 MAPK/JNK pathway [8]. Interestingly, metformin exerted its anti-cancer effect by directly promoting cancer cell apoptosis [9,10] and cancer cell cycle arrest at the G0/G1 stage [11,12]. Additionally, a low dose of metformin inhibited cancer stem cell self-renewal in ovarian cancer, breast cancer, liver cancer and bladder cancer [13-16]. In addition, metformin attenuated epithelial-mesenchymal transition (EMT), which is critical for cancer metastasis in prostate cancer and lung cancer [17,18]. In recent years, prospective clinical studies have indicated decreased Ki-67 expression in neoplasm tissue and reduced metastasis in patients diagnosed with breast cancer and endometrial cancer with metformin treatment [19-21]. In addition, combining metformin with chemotherapies has been attempted in various cancers. Lengyel E et al. treated ovarian cancer with metformin and paclitaxel in mouse models and found that metformin increased paclitaxel sensitivity [22]. In mouse xenografts generated with lung cancer and prostate cancer cell lines, metformin worked with a series of chemotherapeutic agents, including carboplatin, paclitaxel and doxorubicin, and had comparable effects on tumour regression and relapse prevention [23]. However, the effects of the metformin and cisplatin co-treatment on ovarian cancer proliferation, migration and invasion and the underlying mechanisms of these effects remain unclear.
On the basis of findings from previous preclinical and clinical studies, we hypothesized that metformin and cisplatin co-treatment exerts a powerful influence on ovarian cancer. In the present study, we aimed to explore whether metformin can enhance cisplatin cytotoxicity in epithelial ovarian cancer in vitro and in vivo. The results from our study revealed that metformin effectively boosted the inhibitory effects of cisplatin on tumour proliferation and metastasis.
Materials and methods
Cell lines and reagents
SKOV3 and Hey cell lines were purchased from the American Type Culture Collection (ATCC). The SKOV3 and Hey cells were cultured in RPMI 1640 medium supplemented with 10% foetal bovine serum (FBS) (Gibco, Life Technologies, USA), 1% penicillin, 1% streptomycin and 1% amphotericin B at 37°C with 5% CO2. Cisplatin and metformin were purchased from Sigma-Aldrich (St. Louis, USA) and dissolved in phosphate-buffered saline (PBS). Compound C was purchased from MedChem Express (Shanghai, China).
Cck-8 assay
Cell viability was detected by a CCK-8 assay kit (Dojindo, Japan). Briefly, cells were seeded into 96-well plates at a density of 3000 cells/well and cultured for 24 hours. After starvation with FBS-free RPMI 1640 medium for 12 hours, the cells were treated with cisplatin or metformin for the indicated times. CCK-8 reagent (10 µl per well) and RPMI 1640 (100 µl per well) were added to the 96-well plates, and the cells were incubated for 30 min at 37°C. The absorbance values were measured at 450 nm, and cell viability was calculated. All experiments were repeated in triplicate.
Flow cytometry assay
The rate of cell apoptosis was measured by an Annexin V-FITC apoptosis detection kit (BD, USA). Briefly, cells were starved for 12 hours with FBS-free medium and treated with cisplatin or metformin after culturing in 6-well plates for 24 hours. The cells were resuspended in 1 × binding buffer after being washed twice with cold PBS. Then, 5 µl of PI and 5 µl of FITC were added and incubated for 15 min at room temperature in the dark. The apoptotic cells were detected by flow cytometry. FlowJo 7.6 (USA) was used to analyse the flow cytometry data. Each experiment was conducted in triplicate.
Transwell assay
Cell migration and invasion were detected with Transwell assays. For the migration assay, cells were resuspended in 200 µl of FBS-free medium after being starved for 12 hours and were plated into the upper chambers (BD, USA) of 24-well plates at a density of 1 × 105 cells. RPMI 1640 (600 µl) supplemented with 20% FBS was added to the lower chambers. After being treated with the indicated drugs for 24 hours, the cells were fixed in 4% paraformaldehyde, stained with 1% crystal violet and rinsed in PBS three times. The migrated cells were counted under an inverted microscope. For the invasion assay, 50 µl of Matrigel (BD, USA) was pre-applied to the upper chambers, and the starved cells were plated. Similar to the migration assay, a total of 600 µl of medium containing 20% FBS was added into the lower chambers. The cells were incubated with the indicated drugs for 48 hours and then fixed and stained as described in the migration assay. Cells in the upper chambers were gently wiped with cotton swabs. An inverted microscope was used to detect the invading cells. Five visual fields were randomly selected for each chamber. All experiments were repeated in triplicate.
Quantitative real-time PCR
Total RNA was extracted with TRIzol (Invitrogen, USA) and reverse transcribed into cDNA using PrimeScript RT Master Mix (Takara, USA). qRT-PCR was performed with the 7900HT Fast Real-Time PCR System (Thermo Fisher, USA). The primers for N-cadherin, MMP-9 and GAPDH were synthesized by BioTNT (Guantai, China). The primers were as follows: N-cadherin, forward 5’-CTATGAGTGGAA CAGGAACG-3’ and reverse 5’-GGTCTGGAGTTTCGCAAGTC-3’; MMP-9, forward 5’-CGAACTTTGACAGCGACAAG-3’ and reverse 5’-TTCAGGGCGAGGACCATA G-3’; and GAPDH, forward 5’-TGACTTCAACAGCGACACCCA-3’ and reverse 5’-CACCCTGTTGCTGTAGCCAAA-3’. GAPDH was used as a control. The relative mRNA levels were calculated with the 2-ΔΔCT method.
Western blotting analysis
Cells were harvested and lysed with RIPA (Beyotime, China) supplemented with phenylmethyl sulfonyl fluoride (PMSF) (Beyotime, China) and phosphatase inhibitors. The protein concentration was quantified with a BCA kit (Beyotime, China), and 30 µg of protein was loaded onto SDS-PAGE gels for electrophoresis and transferred to PVDF membranes. The membranes were blocked in 5% fat-free milk for one hour at room temperature and incubated in primary antibodies at 4°C overnight. Then, the membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:4000) (Cell Signaling Technology, USA) for one hour at room temperature and detected with Immobilon Western substrate (Millipore, USA). The anti-N-cadherin, anti-MMP-9, anti-TGFβ-1, anti-P-Smad2, anti-P-Smad3, anti-Smad2 and anti-Smad3 antibodies were purchased from Abcam (USA). The anti-Bcl-XL, anti-Bcl-2, anti-Bax, anti-cleaved caspase-3, anti-cleaved PARP, anti-cleaved caspase-9, anti-P-AMPKα, anti-AMPKα and anti-GAPDH antibodies were purchased from Cell Signaling Technology (USA). All primary antibodies were monoclonal and applied at a dilution of 1:1000. The relative protein expression levels were normalized to the GAPDH level. All experiments were repeated three times.
Xenograft mouse experiment
A total of twenty-four 5-week-old female nude BALB/c mice were purchased from Slac Laboratory Animal Corporation (China), and all animal experiments were approved by the Animal Care and Research Committee of Fudan University. SKOV3 cells were resuspended in 200 µl of RPMI 1640 at a concentration of 2 × 107 cells/ml and injected subcutaneously into the flank of nude mice. The mice were randomly divided into four groups (n=6) when the tumour size increased to 50 mm3. The four groups were as follows: Group A (the control group), Group B (the metformin group), Group C (the cisplatin group) and Group D (the metformin and cisplatin co-treatment group). The mice in the control group were given PBS through intraperitoneal injection and oral gavage. Metformin (200 mg/kg) was given by oral gavage for 21 consecutive days. Cisplatin (4 mg/kg) was injected intraperitoneally every six days for three weeks. A Vernier calliper was used to measure the tumour size every three days. The tumour volume (mm3) was calculated as follows: 0.5 × length × width2. All mice were sacrificed after 21 days. Tumours were gently resected and fixed in paraformaldehyde.
Immunohistochemistry
The fixed tumour tissue was embedded in paraffin and sliced into 5-µm-thick sections. After deparaffinization and dehydration, the slices underwent antigen retrieval at 95°C for 30 min and were blocked with goat serum for one hour. Then, the slices were incubated with primary antibodies at 4°C overnight and with HRP-conjugated secondary antibodies at 37°C for 45 min. DAB (Jiehao Biotechnology, China) was used for colour development. The slides were examined under the microscope, and the representative images were captured. For each slide, five visual fields were randomly selected at × 400. The anti-Ki-67 and anti-cleaved caspase-3 antibodies were purchased from Cell Signaling Technology (USA). The anti-N-cadherin, anti-MMP-9, anti-P-Smad2 and anti-P-Smad3 antibodies were purchased from Abcam (USA). Image-Pro Plus 6.0 (Media Cybernetics, USA) was used to analyse the captured images.
Statistical analysis
Quantitative data are expressed as the means ± SD. SPSS 16.0 (IBM, USA) was used to analyse the data. One-way ANOVA was used to compare the means among the four groups. P<0.05 was considered statistically significant.
Results
Metformin promotes the inhibitory effect of cisplatin on epithelial ovarian cancer cell proliferation by inducing apoptosis
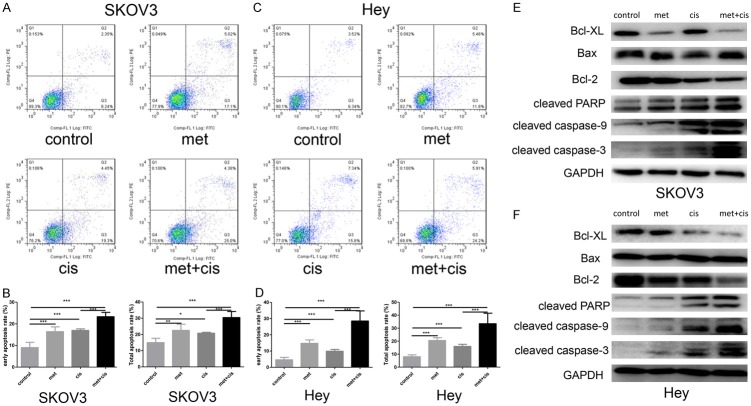
To detect cell viability after metformin and/or cisplatin treatment, the CCK-8 assay was performed. The results revealed that both metformin and cisplatin dose- and time-dependently inhibited SKOV3 and Hey cell proliferation (Figure 1A-D). To clarify whether metformin can enhance the inhibitory effect exerted by cisplatin, low doses of metformin (5 mM) combined with cisplatin at different doses were added to the SKOV3 and Hey cells. Surprisingly, compared with cisplatin treatment alone, combination treatment with the two drugs significantly attenuated cell proliferation (P<0.001) (Figure 1E, 1F). To understand the underlying mechanisms by which metformin inhibited proliferation, flow cytometry was performed to examine the apoptosis rate. Data from our study indicated that compared with metformin or cisplatin alone, metformin and cisplatin co-treatment dramatically increased the apoptosis rate (Figure 2A-D). Western blot analysis was used to detect apoptosis-related proteins. The metformin and cisplatin co-treatment substantially increased the level of pro-apoptotic proteins cleaved caspase-3, cleaved PARP and cleaved caspase-9 and decreased the level of anti-apoptotic proteins Bcl-2, Bcl-XL and Bcl-XL/Bax (Figure 2E, 2F). In summary, we concluded that metformin promoted the inhibitory effect of cisplatin on epithelial ovarian cancer cell proliferation by inducing apoptosis.
Figure 1.
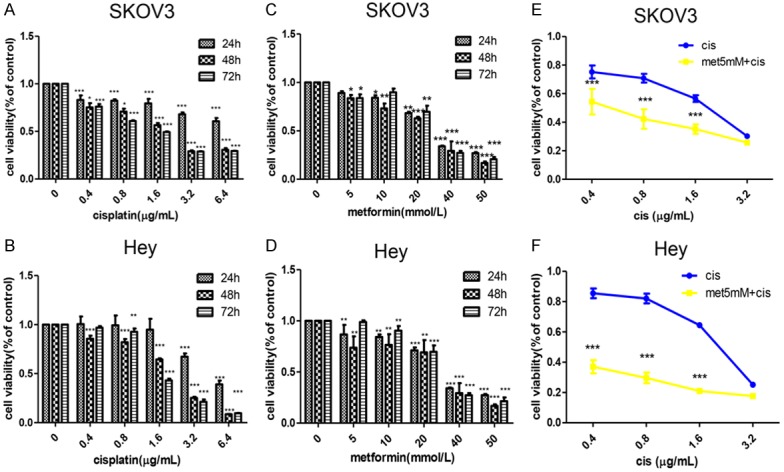
Metformin enhances the inhibitory effect of cisplatin on proliferation in vitro. A-D. SKOV3 and Hey cells were treated with metformin (0 mmol/L, 5 mmol/L, 10 mmol/L, 20 mmol/L, 40 mmol/L, 50 mmol/L) or cisplatin (0 µg/mL, 0.4 µg/mL, 0.8 µg/mL, 1.6 µg/mL, 3.2 µg/mL, 6.4 µg/mL) alone for 24, 48 and 72 hours. The CCK-8 assay was performed to detect cell viability. E, F. SKOV3 and Hey cells were treated with metformin (5 mmol/L) combined with cisplatin (0.4 µg/mL, 0.8 µg/mL, 1.6 µg/mL, 3.2 µg/mL) for 48 hours. The CCK-8 assay was used to detect cell viability. *, P<0.05, **, P<0.01, and ***, P<0.001.
Figure 2.
Metformin promotes cisplatin-induced cell apoptosis in vitro. A-D. SKOV3 and Hey cells were divided into four groups: the control group, metformin group (20 mM), cisplatin group (1.6 µg/ml) and metformin + cisplatin group (20 mM metformin + 1.6 µg/ml cisplatin). Flow cytometric analysis was used to detect the apoptosis rate in different groups after 48 hours of treatment. E, F. Western blotting was performed to detect apoptosis-related protein expression in different groups after 48 hours of treatment. GAPDH was used as a loading control. *, P<0.05, **, P<0.01, and ***, P<0.001.
Metformin promotes the inhibitory effect of cisplatin on ovarian cancer cell metastasis
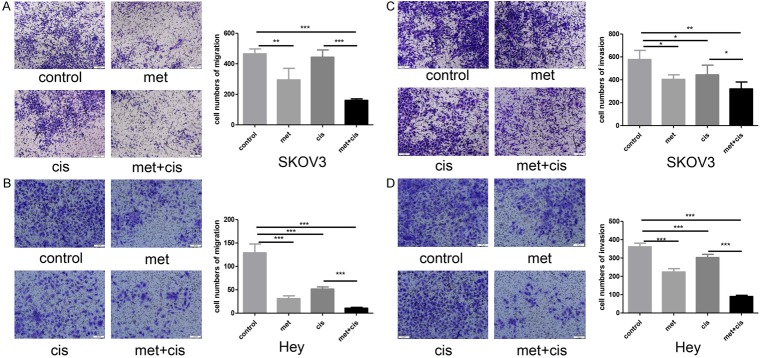
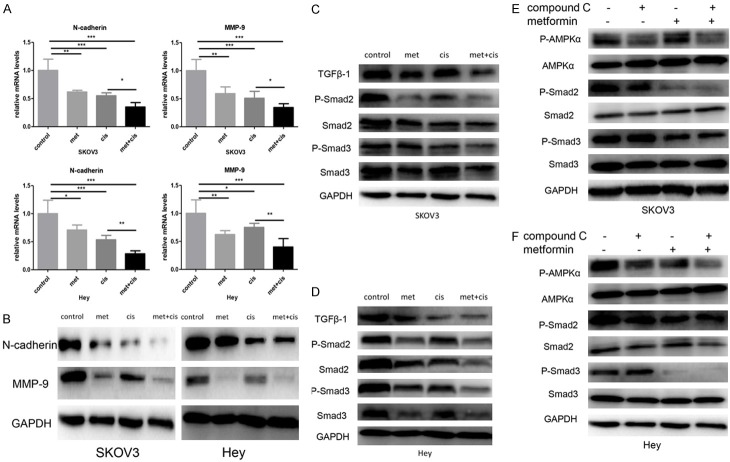
To investigate the influence of metformin on ovarian cancer cell migration and invasion, a Transwell experiment was performed. Our results revealed that cisplatin slightly inhibited the migration and invasion of SKOV3 and Hey cells, which was enhanced by metformin treatment (Figure 3). EMT plays a critical role in ovarian cancer cell metastasis. qRT-PCR and Western blotting were performed to detect the expression of EMT-related proteins. The results revealed that N-cadherin and MMP-9 expression levels were significantly inhibited transcriptionally and translationally by the drug combination in both SKOV3 and Hey cells (Figure 4A, 4B).
Figure 3.
Metformin enhances the inhibitory effect of cisplatin on cell migration and invasion. A, B. SKOV3 and Hey cells were divided into four groups: the control group, metformin group (20 mM), cisplatin group (1.6 µg/ml) and metformin + cisplatin group (20 mM metformin + 1.6 µg/ml cisplatin). Transwell assays were used to measure cell migration after 24 hours of treatment. C, D. SKOV3 and Hey cells were divided into four groups: the control group, metformin group (20 mM), cisplatin group (1.6 µg/ml) and metformin + cisplatin group (20 mM metformin + 1.6 µg/ml cisplatin). Transwell assays were used to measure cell migration after 48 hours of treatment. *, P<0.05, **, P<0.01, and ***, P<0.001.
Figure 4.
Metformin inhibited EMT via the TGFβ signalling pathway. A. SKOV3 and Hey cells were divided into four groups: the control group, metformin group (20 mM), cisplatin group (1.6 µg/ml) and metformin + cisplatin group (20 mM metformin + 1.6 µg/ml cisplatin). qRT-PCR was performed to detect the mRNAs levels of N-cadherin and MMP-9 after 48 hours of treatment. B. SKOV3 and Hey cells were divided into four groups: the control group, metformin group (20 mM), cisplatin group (1.6 µg/ml) and metformin + cisplatin group (20 mM metformin + 1.6 µg/ml cisplatin). Western blotting was performed to detect the protein levels of N-cadherin and MMP-9 after 48 hours of treatment. C, D. SKOV3 and Hey cells were divided into four groups: the control group, metformin group (20 mM), cisplatin group (1.6 µg/ml) and metformin + cisplatin group (20 mM metformin + 1.6 µg/ml cisplatin). Western blotting was performed to detect the protein levels of TGFβ1, P-Smad2, P-Smad3, Smad2 and Smad3 after 48 hours of treatment. E, F. Compound C (10 µM) was added to SKOV3 and Hey cells that were pre-treated with metformin (20 mM) for two hours. Western blotting was performed to detect the protein levels of P-AMPKα, AMPKα, P-Smad2, P-Smad3, Smad2 and Smad3 after 48 hours of treatment. GAPDH was used as a loading control. *, P<0.05, **, P<0.01, and ***, P<0.001.
Metformin inhibited ovarian cancer metastasis via the TGFβ-1 pathway
To investigate the mechanisms by which metformin inhibited metastasis, we focused on the TGFβ-1 pathway, which is a well-documented modulatory signalling pathway for ovarian cancer metastasis. The Western blotting results revealed that metformin alone significantly inhibited TGFβ-1, P-Smad2 and P-Smad3 expression, which was strengthened by the metformin and cisplatin co-treatment (Figure 4C, 4D). To investigate whether the impact of metformin on the TGFβ-mediated tumour signalling pathway is AMPK dependent, compound C and/or metformin were added to the ovarian cancer cell lines. The results revealed that AMPK inhibition by compound C did not relieve the inhibitory effect of metformin on the TGFβ signalling pathway (Figure 4E, 4F). We thus concluded that metformin impacts the TGFβ pathway in an AMPK-independent manner in ovarian cancer.
Metformin enhanced the inhibitory effect of cisplatin on ovarian cancer in vivo
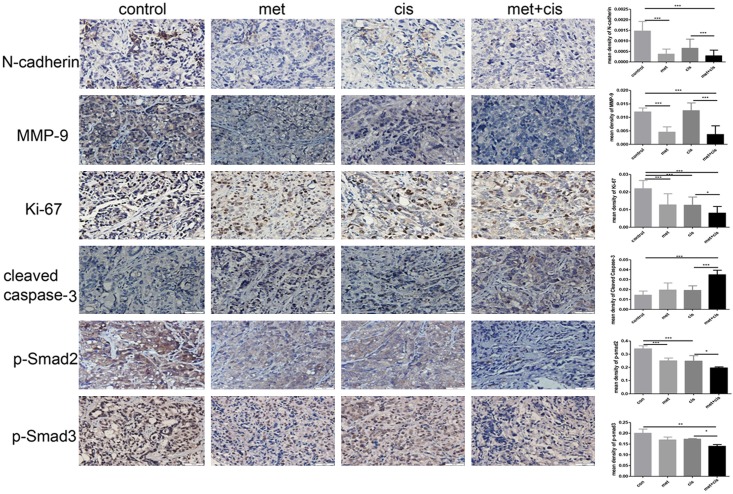
To investigate whether metformin can exert similar effects in vivo, we established xenograft mouse models with SKOV3 cells. By monitoring the tumour growth, we found that the tumour volume in the control group increased rapidly, while the increase in tumour volume in the drug combination group was significantly slower than that in the control group (Figure 5A). For mouse body weight, we did not detect any significant differences among the four groups (P>0.05) (Figure 5B). At the time of sacrifice, we resected and weighted the tumours. Tumours in the drug combination group were smaller and lighter than those in the metformin or cisplatin only groups (P<0.05) (Figure 5C, 5D). Immunohistochemistry results revealed that the metformin and cisplatin co-treatment dramatically reduced Ki-67 expression and increased cleaved caspase-3 expression (Figure 6). Thus, the metformin and cisplatin co-treatment significantly inhibited ovarian cancer growth in vivo. In addition, N-cadherin and MMP-9 expression was substantially inhibited by the metformin and cisplatin co-treatment in the mouse models, which was consistent with the in vitro results (Figure 6). In accordance with the results from the in vitro experiment, the metformin and cisplatin co-treatment significantly inhibited TGFβ pathway activation, which was manifested as reduced P-Smad2 and P-Smad3 levels (Figure 6). For these reasons, we concluded that metformin enhanced the tumour suppression effect of cisplatin in vivo.
Figure 5.
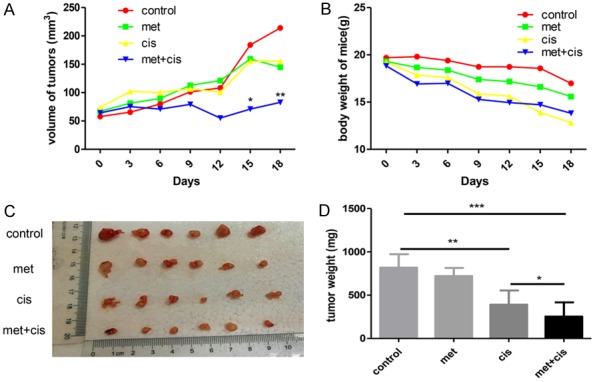
Metformin enhanced cisplatin cytotoxicity in ovarian cancer in vivo. Twenty-four nude mice with xenograft tumours were randomly divided into four groups: the control group (PBS, oral gavage and intraperitoneally), metformin group (200 mg/kg, oral gavage), cisplatin group (4 mg/kg, intraperitoneally) and metformin + cisplatin group (200 mg/kg metformin + 4 mg/kg cisplatin). A. Tumour volume was measured and calculated every three days. B. The mice were weighed every three days. C, D. The tumours were resected and weighed after the mice were sacrificed. *, P<0.05, **, P<0.01, and ***, P<0.001.
Figure 6.
Combination therapy with metformin and cisplatin inhibited ovarian cancer proliferation and EMT in vivo through the TGFβ signalling pathway. Representative (×400) staining showing N-cadherin, MMP-9, Ki-67, cleaved caspase-3, P-Smad2 and P-Smad3 expression in tumour tissues excised from mice at 3 weeks. *, P<0.05 and ***, P<0.001.
Discussion
The TGFβ signalling pathway is a critical player in various biological functions, including stem cell renewal, immune system suppression, wound repair and neoplasm progression [24]. As a key modulator of cancer progression, TGFβ has been investigated in various kinds of tumours and is considered pleiotropic, coordinated and contextual [25]. TGFβ enrichment in the tumour microenvironment has been verified in various tumours [26]. TGFβ binds to its membrane receptors, which phosphorylate Smad2 and Smad3. Phosphorylated Smad2 and Smad3 combine with Smad4 to form a complex and are shuttled to the nucleus to activate transcription factors, such as p21 [27]. EMT is a typical pathological feature in neoplasia, and TGFβ-induced EMT has been reported in several tumour cell lines, including breast cancer and skin carcinomas [28,29]. In mesenchymal stem-like/claudin-low breast cancer, metformin attenuated the activation of Smad2 and Smad3 induced by TGFβ and effectively inhibited breast cancer cell proliferation, migration and motility [30]. Similar findings have been confirmed in prostate cancer, in which metformin inhibited the increase in N-cadherin and Vimentin expression and decrease in E-cadherin and β-catenin expression induced by TGFβ [18].
Apart from the aforementioned studies, several other preclinical and clinical studies have demonstrated that metformin attenuates the growth and progression of multiple tumours and is beneficial to patients. Under glucose-deprivation conditions, metformin synergistically enhanced cisplatin cytotoxicity in oesophageal squamous cancer cells by deregulating AKT and AMPK [31]. In human nasopharyngeal cancer, the combination of metformin and cisplatin significantly downregulated MMP-9 expression and upregulated E-cadherin expression; therefore, the combination therapy inhibited nasopharyngeal cancer cell metastasis [32]. Similar tumour-inhibitory effects have also been found in lung cancer, gastric cancer, colon cancer and endometrial cancer [33-36]. In ovarian cancers, a number of studies has reported that metformin enhanced the anti-cancer activity of cisplatin or paclitaxel in vitro and in vivo. In 2011, Ramandeep Rattan et al. constructed intraperitoneal ovarian cancer models and found that metformin effectively inhibited the proliferation and migration of ovarian cancer [37]. Additionally, the researchers demonstrated that metformin exerted anti-tumour effects through both AMPK-dependent and AMPK-independent pathways [38]. Another recent study indicated that metformin combined with cisplatin inhibited cell viability and induced apoptosis in human ovarian cancer cells by inactivating ERK 1/2 [39]. In addition, several retrospective and prospective studies have demonstrated the benefits of metformin on cancer patient survival. A total of 102 patients with newly diagnosed endometrial cancer were divided into the control group and metformin group. Metformin substantially decreased the level of insulin and insulin-like growth factor-1 (IGF-1) and the number of metastatic patients [19]. A retrospective study revealed that obese patients with type I endometrial cancer who received metformin treatment had a recurrence rate of 7.8% versus 15.3% in the control patients [40]. A case-control study consisting of 215 cases indicated that metformin intake improved ovarian cancer patient survival [41].
Recently, a series of novel anti-cancer metformin mechanisms have been described. It was reported that metformin can inhibit tumours by epigenetically modulating gene expression. SUV39H1 (a histone methyltransferase of H3 Lys9) overexpression promotes prostate cancer cell migration. However, SUV39H1 expression was reduced by metformin in prostate cancer cells in a time-dependent manner by disturbing the integrin-FAK (focal adhesion kinase) signalling pathway [42]. Zhong T et al. reported that metformin induced genome-wide alterations in DNA methylation by regulating S-adenosylhomocysteine hydrolase (SAHH) activity [43]. These researchers conducted the study with an endometrial cancer cell line and a breast cancer cell line and found that metformin treatment induced the hypermethylation of tumour-promoting genes and inhibited tumour proliferation. In summary, the comprehensive anti-cancer mechanisms of metformin remain elusive.
Herein, we developed in vitro and in vivo studies. Consistent with previous studies, our results revealed that the combination of metformin and cisplatin inhibited the proliferation and promoted the apoptosis of epithelial ovarian cancer cells. In addition, we found that the combination therapy significantly decreased N-cadherin and MMP-9 expression in vitro and in vivo, which indicated that metformin can inhibit EMT in epithelial ovarian cancer. To our knowledge, this was the first study to demonstrate that metformin inhibits epithelial ovarian cancer metastasis via the TGFβ signalling pathway (Figure 7). By using xenograft mouse models with epithelial ovarian cancer cells, we indicated that metformin enhanced cisplatin cytotoxicity in vivo. However, our study still has some limitations. For example, we constructed ovarian cancer cell xenograft mouse models subcutaneously rather than intraperitoneally. Therefore, further studies are needed to investigate whether the metformin and cisplatin co-treatment can inhibit metastasis and ascites formation in intraperitoneal models. Additionally, platinum-resistant ovarian cancer cell lines should also be investigated to better determine if metformin can restrict the growth of platinum-resistant ovarian cancer. Furthermore, additional studies are needed to explore the novel mechanisms underlying the anti-tumour effect of metformin.
Figure 7.
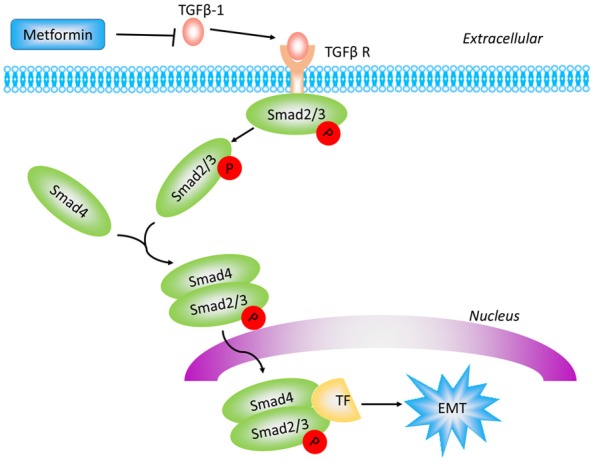
Schematic diagram of the proposed mechanism by which metformin enhances the inhibitory effect of cisplatin on epithelial ovarian cancer metastasis. Metformin decreased the level of TGFβ-1, the subsequent Smad2/3 phosphorylation and EMT.
Collectively, our results revealed that metformin alone or combined with cisplatin attenuated epithelial ovarian cancer growth and metastasis in vitro and in vivo. We propose that metformin could be used as adjunct therapy in the treatment of epithelial ovarian cancer.
Acknowledgements
This work was supported by the Shanghai Science and Technology Department Funds (No. 16411963600) to Hong Sun.
Disclosure of conflict of interest
None.
Abbreviations
- qRT-PCR
quantitative reverse transcription polymerase chain reaction
- MMP-9
matrix metalloproteinase-9
- TGFβ1
transforming groth factor beta 1
- PI3K
phosphatidylinositol-3-kinases
- mTOR
mammalian target of rapamycin
- MAPK
mitogen-activated protein kinase
- JNK
c-Jun N-terminal kinases
- AMPK
AMP-activated protein kinase
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 3.Gasparri ML, Bardhi E, Ruscito I, Papadia A, Farooqi AA, Marchetti C, Bogani G, Ceccacci I, Mueller MD, Benedetti PP. PI3K/AKT/mTOR pathway in ovarian cancer treatment: are we on the right track? Geburtshilfe Frauenheilkd. 2017;77:1095–1103. doi: 10.1055/s-0043-118907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wei M, Liu Y, Wang C, Yu C, Li D, Zhou W, Zhang ZJ. The need for differentiating diabetes-specific mortality from total mortality when comparing metformin with insulin regarding cancer survival. Acta Diabetol. 2017;54:219–220. doi: 10.1007/s00592-016-0933-2. [DOI] [PubMed] [Google Scholar]
- 5.Evans JM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330:1304–1305. doi: 10.1136/bmj.38415.708634.F7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han B, Cui H, Kang L, Zhang X, Jin Z, Lu L, Fan Z. Metformin inhibits thyroid cancer cell growth, migration, and EMT through the mTOR pathway. Tumour Biol. 2015;36:6295–6304. doi: 10.1007/s13277-015-3315-4. [DOI] [PubMed] [Google Scholar]
- 7.Honjo S, Ajani JA, Scott AW, Chen Q, Skinner HD, Stroehlein J, Johnson RL, Song S. Metformin sensitizes chemotherapy by targeting cancer stem cells and the mTOR pathway in esophageal cancer. Int J Oncol. 2014;45:567–574. doi: 10.3892/ijo.2014.2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhu J, Zheng Y, Zhang H, Sun H. Targeting cancer cell metabolism: the combination of metformin and 2-Deoxyglucose regulates apoptosis in ovarian cancer cells via p38 MAPK/JNK signaling pathway. Am J Transl Res. 2016;8:4812–4821. [PMC free article] [PubMed] [Google Scholar]
- 9.Gao ZY, Liu Z, Bi MH, Zhang JJ, Han ZQ, Han X, Wang HY, Sun GP, Liu H. Metformin induces apoptosis via a mitochondria-mediated pathway in human breast cancer cells. Exp Ther Med. 2016;11:1700–1706. doi: 10.3892/etm.2016.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nazim UM, Moon JH, Lee JH, Lee YJ, Seol JW, Eo SK, Lee JH, Park SY. Activation of autophagy flux by metformin downregulates cellular FLICE-like inhibitory protein and enhances TRAIL-induced apoptosis. Oncotarget. 2016;7:23468–23481. doi: 10.18632/oncotarget.8048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gwak H, Kim Y, An H, Dhanasekaran DN, Song YS. Metformin induces degradation of cyclin D1 via AMPK/GSK3beta axis in ovarian cancer. Mol Carcinog. 2016;56:349–358. doi: 10.1002/mc.22498. [DOI] [PubMed] [Google Scholar]
- 12.Kato K, Iwama H, Yamashita T, Kobayashi K, Fujihara S, Fujimori T, Kamada H, Kobara H, Masaki T. The anti-diabetic drug metformin inhibits pancreatic cancer cell proliferation in vitro and in vivo: study of the microRNAs associated with the antitumor effect of metformin. Oncol Rep. 2016;35:1582–1592. doi: 10.3892/or.2015.4496. [DOI] [PubMed] [Google Scholar]
- 13.Zhu P, Davis M, Blackwelder AJ, Bachman N, Liu B, Edgerton S, Williams LL, Thor AD, Yang X. Metformin selectively targets tumorinitiating cells in ErbB2-overexpressing breast cancer models. Cancer Prev Res (Phila) 2014;7:199–210. doi: 10.1158/1940-6207.CAPR-13-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang R, Zhang P, Wang H, Hou D, Li W, Xiao G, Li C. Inhibitory effects of metformin at low concentration on epithelial-mesenchymal transition of CD44(+)CD117(+) ovarian cancer stem cells. Stem Cell Res Ther. 2015;6:262. doi: 10.1186/s13287-015-0249-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen C, Ka SO, Kim SJ, Kim JH, Park BH, Park JH. Metformin and AICAR regulate NANOG expression via the JNK pathway in HepG2 cells independently of AMPK. Tumour Biol. 2016;37:11199–11208. doi: 10.1007/s13277-016-5007-0. [DOI] [PubMed] [Google Scholar]
- 16.Liu Q, Yuan W, Tong D, Liu G, Lan W, Zhang D, Xiao H, Zhang Y, Huang Z, Yang J, Zhang J, Jiang J. Metformin represses bladder cancer progression by inhibiting stem cell repopulation via COX2/PGE2/STAT3 axis. Oncotarget. 2016;7:28235–28246. doi: 10.18632/oncotarget.8595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banerjee P, Surendran H, Chowdhury DR, Prabhakar K, Pal R. Metformin mediated reversal of epithelial to mesenchymal transition is triggered by epigenetic changes in E-cadherin promoter. J Mol Med (Berl) 2016;94:1397–1409. doi: 10.1007/s00109-016-1455-7. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Shen C, Wang L, Ma Q, Xia P, Qi M, Yang M, Han B. Metformin inhibits epithelial-mesenchymal transition in prostate cancer cells: involvement of the tumor suppressor mi-R30a and its target gene SOX4. Biochem Biophys Res Commun. 2014;452:746–752. doi: 10.1016/j.bbrc.2014.08.154. [DOI] [PubMed] [Google Scholar]
- 19.El-Haggar SM, El-Shitany NA, Mostafa MF, El-Bassiouny NA. Metformin may protect nondiabetic breast cancer women from metastasis. Clin Exp Metastasis. 2016;33:339–357. doi: 10.1007/s10585-016-9782-1. [DOI] [PubMed] [Google Scholar]
- 20.Hadad SM, Coates P, Jordan LB, Dowling RJ, Chang MC, Done SJ, Purdie CA, Goodwin PJ, Stambolic V, Moulder-Thompson S, Thompson AM. Evidence for biological effects of metformin in operable breast cancer: biomarker analysis in a pre-operative window of opportunity randomized trial. Breast Cancer Res Treat. 2015;150:149–155. doi: 10.1007/s10549-015-3307-5. [DOI] [PubMed] [Google Scholar]
- 21.Schuler KM, Rambally BS, DiFurio MJ, Sampey BP, Gehrig PA, Makowski L, Bae-Jump VL. Antiproliferative and metabolic effects of metformin in a preoperative window clinical trial for endometrial cancer. Cancer Med. 2015;4:161–173. doi: 10.1002/cam4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lengyel E, Litchfield LM, Mitra AK, Nieman KM, Mukherjee A, Zhang Y, Johnson A, Bradaric M, Lee W, Romero IL. Metformin inhibits ovarian cancer growth and increases sensitivity to paclitaxel in mouse models. Am J Obstet Gynecol. 2015;212:471–479. doi: 10.1016/j.ajog.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iliopoulos D, Hirsch HA, Struhl K. Metformin decreases the dose of chemotherapy for prolonging tumor remission in mouse xenografts involving multiple cancer cell types. Cancer Res. 2011;71:3196–3201. doi: 10.1158/0008-5472.CAN-10-3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Massague J. TGFbeta signalling in context. Nat Rev Mol Cell Biol. 2012;13:616–630. doi: 10.1038/nrm3434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shi Y, Massague J. Mechanisms of TGFbeta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 26.Xie W, Mertens JC, Reiss DJ, Rimm DL, Camp RL, Haffty BG, Reiss M. Alterations of Smad signaling in human breast carcinoma are associated with poor outcome: a tissue microarray study. Cancer Res. 2002;62:497–505. [PubMed] [Google Scholar]
- 27.Seoane J, Le HV, Shen L, Anderson SA, Massague J. Integration of Smad and forkhead pathways in the control of neuroepithelial and glioblastoma cell proliferation. Cell. 2004;117:211–223. doi: 10.1016/s0092-8674(04)00298-3. [DOI] [PubMed] [Google Scholar]
- 28.Derynck R, Akhurst RJ. Differentiation plasticity regulated by TGF-beta family proteins in development and disease. Nat Cell Biol. 2007;9:1000–1004. doi: 10.1038/ncb434. [DOI] [PubMed] [Google Scholar]
- 29.Shipitsin M, Campbell LL, Argani P, Weremowicz S, Bloushtain-Qimron N, Yao J, Nikolskaya T, Serebryiskaya T, Beroukhim R, Hu M, Halushka MK, Sukumar S, Parker LM, Anderson KS, Harris LN, Garber JE, Richardson AL, Schnitt SJ, Nikolsky Y, Gelman RS, Polyak K. Molecular definition of breast tumor heterogeneity. Cancer Cell. 2007;11:259–273. doi: 10.1016/j.ccr.2007.01.013. [DOI] [PubMed] [Google Scholar]
- 30.Wahdan-Alaswad R, Harrell JC, Fan Z, Edgerton SM, Liu B, Thor AD. Metformin attenuates transforming growth factor beta (TGF-beta) mediated oncogenesis in mesenchymal stem-like/claudin-low triple negative breast cancer. Cell Cycle. 2016;15:1046–1059. doi: 10.1080/15384101.2016.1152432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu H, Bian X, Gu D, He X. Metformin synergistically enhances cisplatin-induced cytotoxicity in esophageal squamous cancer cells under glucose-deprivation conditions. Biomed Res Int. 2016;2016:8678634. doi: 10.1155/2016/8678634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun XJ, Zhang P, Li HH, Jiang ZW, Jiang CC, Liu H. Cisplatin combined with metformin inhibits migration and invasion of human nasopharyngeal carcinoma cells by regulating Ecadherin and MMP-9. Asian Pac J Cancer Prev. 2014;15:4019–4023. doi: 10.7314/apjcp.2014.15.9.4019. [DOI] [PubMed] [Google Scholar]
- 33.Laskov I, Abou-Nader P, Amin O, Philip CA, Beauchamp MC, Yasmeen A, Gotlieb WH. Metformin increases e-cadherin in tumors of diabetic patients with endometrial cancer and suppresses epithelial-mesenchymal transition in endometrial cancer cell lines. Int J Gynecol Cancer. 2016;26:1213–1221. doi: 10.1097/IGC.0000000000000761. [DOI] [PubMed] [Google Scholar]
- 34.Li L, Wang Y, Peng T, Zhang K, Lin C, Han R, Lu C, He Y. Metformin restores crizotinib sensitivity in crizotinib-resistant human lung cancer cells through inhibition of IGF1-R signaling pathway. Oncotarget. 2016;7:34442–34452. doi: 10.18632/oncotarget.9120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu G, Fang W, Xia T, Chen Y, Gao Y, Jiao X, Huang S, Wang J, Li Z, Xie K. Metformin potentiates rapamycin and cisplatin in gastric cancer in mice. Oncotarget. 2015;6:12748–12762. doi: 10.18632/oncotarget.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saber MM, Galal MA, Ain-Shoka AA, Shouman SA. Combination of metformin and 5-aminosalicylic acid cooperates to decrease proliferation and induce apoptosis in colorectal cancer cell lines. BMC Cancer. 2016;16:126. doi: 10.1186/s12885-016-2157-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rattan R, Graham RP, Maguire JL, Giri S, Shridhar V. Metformin suppresses ovarian cancer growth and metastasis with enhancement of cisplatin cytotoxicity in vivo. Neoplasia. 2011;13:130–483. doi: 10.1593/neo.11148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rattan R, Giri S, Hartmann LC, Shridhar V. Metformin attenuates ovarian cancer cell growth in an AMP-kinase dispensable manner. J Cell Mol Med. 2011;15:166–178. doi: 10.1111/j.1582-4934.2009.00954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dang JH, Jin ZJ, Liu XJ, Hu D, Wang J, Luo Y, Li LL. Metformin in combination with cisplatin inhibits cell viability and induces apoptosis of human ovarian cancer cells by inactivating ERK 1/2. Oncol Lett. 2017;14:7557–7564. doi: 10.3892/ol.2017.7176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hall C, Stone RL, Gehlot A, Zorn KK, Burnett AF. Use of metformin in obese women with type I endometrial cancer is associated with a reduced incidence of cancer recurrence. Int J Gynecol Cancer. 2016;26:313–317. doi: 10.1097/IGC.0000000000000603. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S, Meuter A, Thapa P, Langstraat C, Giri S, Chien J, Rattan R, Cliby W, Shridhar V. Metformin intake is associated with better survival in ovarian cancer: a case-control study. Cancer. 2013;119:555–562. doi: 10.1002/cncr.27706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu T, Wang C, Yang J, Guo Y, Wu Y, Li X. Metformin inhibits SUV39H1-mediated migration of prostate cancer cells. Oncogenesis. 2017;6:e324. doi: 10.1038/oncsis.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhong T, Men Y, Lu L, Geng T, Zhou J, Mitsuhashi A, Shozu M, Maihle NJ, Carmichael GG, Taylor HS, Huang Y. Metformin alters DNA methylation genome-wide via the H19/SAHH axis. Oncogene. 2017;36:2345–2354. doi: 10.1038/onc.2016.391. [DOI] [PMC free article] [PubMed] [Google Scholar]