Abstract
Objectives: To investigate the positive effect of Th17 cells on the prognosis of patients with PTC and HT. Methods: The expression of nuclear specific marker RORγt of Th17 cells in fresh and paraffin thyroid tissues and serum specimens were analyzed. Flow cytometry was used to detect the formation rates of Th17 cells (CD3+CD8-IL-17A+/CD3+CD8-%) at different time points after co-culture of thyroid papillary carcinoma cell line (TPC-1 and K1) and umbilical cord blood initial T lymphocytes. The protein expression of RORγt in T lymphocytes after co-culture was detected. Preoperative serum levels of Th17 (IL-17) cytokines were measured. Results: The positive expression of RORγt in the tumor microenvironment of PTC patients with or without HT could inhibit the lymph node metastasis of the tumor. PTC cancer cells could induce initial T lymphocyte to differentiate into Th17 cells, and the K1 cell line with lymph node metastasis induced a higher proportion of RORγt protein than that in TPC-1 cell line without lymph node metastasis. In PTC patients with HT, serum IL-17 concentration was negatively correlated with lymph node metastasis in the central group. Conclusions: RORγt may play an anti-tumor role in reducing thyroid cell damage by reducing the thyroid autoimmune antibodies TPOAb and TGAb in the PTC population in Yunnan plateau region.
Keywords: Papillary thyroid carcinoma, Hashimoto thyroiditis, prognosis
Introduction
At the beginning of the 19th century, the Japanese surgeon Hashimoto first described the thyroid pathology with predominantly fibrosis and diffused lymphocytic infiltration, which was named Hashimoto thyroiditis (HT) [1]. Dailey et al. [2] first proposed in 1955 that the development of papillary thyroid carcinoma (PTC) evolved from the development of HT, which had been confirmed by many following studies. Recent reports indicated that PTC combined with HT was associated with better clinical prognosis and less aggressiveness, suggesting that autoimmunity was not only a risk factor for the evolution of thyroid cancer but has a protective effect on the further development of the disease [3]. BY Huang et al. [4] showed that patients with follicular carcinoma of PTC with HT had better clinical stage, lower relapse probability and lower mortality than patients with thyroid cancer without HT. The average tumor size, distant metastasis and recurrence probability of the former one were significantly lower than those of the latter one during the study. The probability of death was 0 during the follow-up of 20 years. Romaldini J et al. [5] also suggested that, as an immune response, lymphocyte infiltration might control tumor growth and reproduction, so HT chronic lymphocytic infiltration suggested a better prognosis of PTC, it is still controversial.
Recently discovered Th17 cells were T helper cell subsets that produce IL-17 cytokines which differ from Th1 or Th2 cells and had been described as a key factor in the development of inflammatory and autoimmune diseases and cancer [6-8]. Th17 cells stimulate or inhibit tumor growth in different tumor microenvironments and can play a dual anti-tumor immune role.
The role of Th17 cells in the tumor microenvironment of PTC patients with HT had not been investigated, and the purpose of this study was to investigate the reason for the good prognosis of PTC patients with HT from the perspective of Th17 cells.
Subjects and methods
Subjects
After approve of Ethical Committee of The First People’s Hospital of Yunnan Province, fresh tissue samples resected during thyroid tumor surgeries and serum samples before surgery from June 2013 to June 2014 in the Department of Breast and Thyroid Surgery, The First People’s Hospital of Yunnan Province were collected from patients without family history of tumor, without radiotherapy or chemotherapy at the time of collection, and without recent medication (such as anti-thyroid drugs, thyroxine, iodine drugs, glucocorticoids and sex hormones, etc.) and radioactive iodine treatment that might affect thyroid function. All patients were divided into three groups: thyroid papillary carcinoma (PTC) group, thyroid papillary carcinoma combined with Hashimoto’s thyroiditis (PTC-HT) group and Hashimoto’s thyroiditis (HT) group. The patients who were confirmed as PTC, PTC-HT and HT after surgery were collected from paraffin-embedded tissue sections in pathology room.
Healthy neonatal heparinized cord blood samples were collected from March to August 2014 in obstetric surgery room of Yunnan First People’s Hospital from pregnant women aged 22-30 years old without infectious diseases or blood diseases who were caesarean. Inclusion criteria included normal pregnancy without HIV, hepatitis B virus and hepatitis C virus. Exclusion criteria included preeclampsia, fever, acute infection, diabetes and/or chronic disease. All samples in this study were obtained with informed consent from patients and approvement by ethics committee of Yunnan First People’s Hospital.
Two thyroid papillary carcinoma cell lines, TPC-1 [9] (human papillary thyroid carcinoma), K1 [10] (human papillary thyroid carcinoma), were selected as the experimental group, and Human thyroid follicular epithelium cell line Nthy-ori 3-1 [11] as the control group, which were all purchased from Guangzhou Jennio Biotechnology Co., Ltd.
Methods
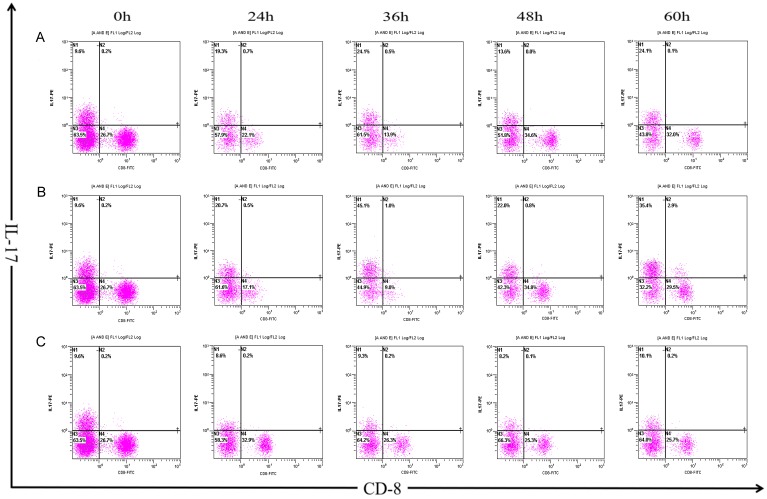
Surgically resected human thyroid fresh tissues, paraffin tissues, and serum samples (cancer tissues from patients PTC with or without HT, and thyroid tissues from patients with pathological diagnosis of HT) were collected. Envision immunohistochemical staining and real-time fluorescence quantitative PCR (RTPCR) were used to analyze the expression of nuclear specific marker RORγt of Th17 cells. Transwell chambers were used to simulate the environment coaffected by initial cord blood T lymphocytes and thyroid papillary carcinoma cells (TPC-1 and K1), and to compare it with human normal thyroid follicular epithelial cell line Nthy-ori 3-1 (Figure 1). Flow cytometry was used to detect the expression of Th17 cells (CD3+CD8-IL-17A+/CD3+CD8-%) at 0 h, 24 h, 36 h, 48 h and 60 h after co-treatment with papillary thyroid carcinoma cell lines (TPC-1 and K1) and umbilical cord blood primary T lymphocytes: the lymphocyte population was first localized with CD45+ and lateral scatter angles (SSC) (Figure 2A) followed by CD4+ gating (Figure 2B) followed by CD3+CD8- dual gating in CD4+ group (Figure 2C), and finally the percentages of CD3+CD8-IL-17A+/CD3+CD8-% were analyzed (Figure 2D); T-lymphocytes after co-administration were detected by Western blot In RORγt protein expression. Cytometric Bead Array (CBA) was used to detect the expression of Th17 (IL-17) cytokines in preoperative serum of patients.
Figure 1.
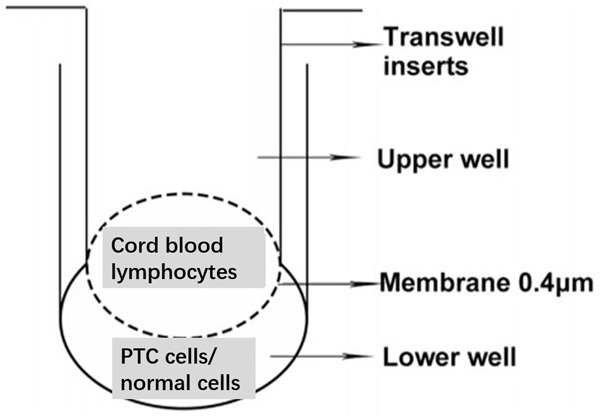
Schematic diagram of transwell chamber isolation co-culture.
Figure 2.
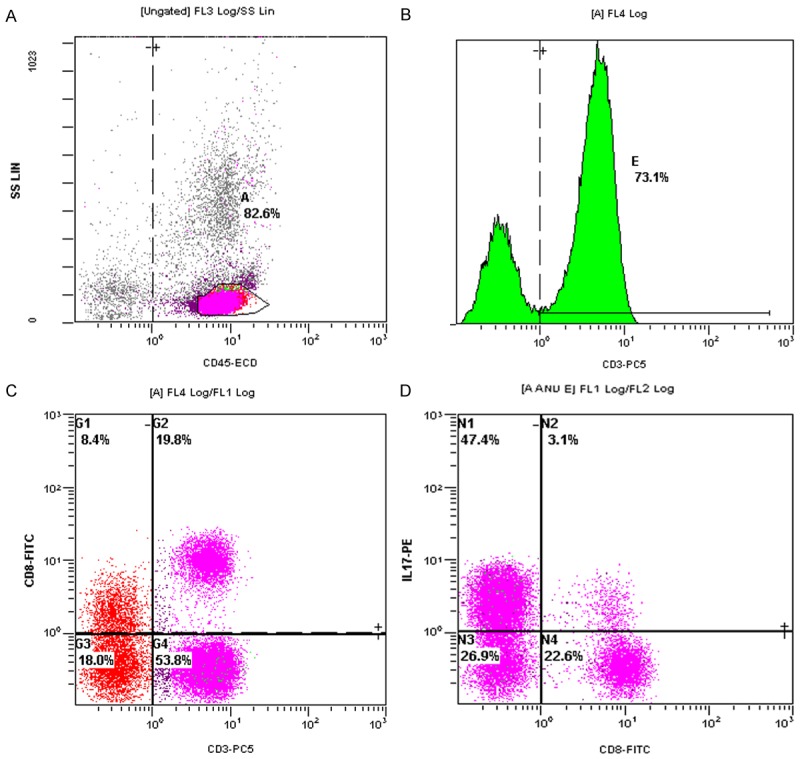
Th17 (CD3+CD8-IL-17+) lymphocyte in umbilical cord blood flow cytometry gate (A: CD45+ lymphocyte in umbilical cord blood; B: CD3+ lymphocyte; C: CD3+CD8-IL-17+ lymphocyte; D: Th17 (CD3+CD8-IL-17+) lymphocyte).
Fresh thyroid tumor specimens were obtained from 10 patients who underwent thyroidectomy. Among them, 6 were in PTC group and 4 were in PTC/HT group. All tissue specimens were placed in liquid nitrogen within 30 minutes after isolation, and then stored at -80°C and confirmed by pathological examination. The gene chip used is the human HT-12 V4 gene expression profile chip of Illumina company of America, which is provided by Shanghai Crystal Energy Biochip Co., Ltd. The microarray covers 47,000 transcripts of the human genome and represents 25,000 distinct genes. We used the human HT-12 V4 gene expression profiling chip of Illumina Company and real-time fluorescence quantitative PCR (qPCR) to select differentially expressed mRNA. We screened the differentially expressed genes from the above three groups of microarray data, and screened the differentially expressed genes by the RVM model modified F test (MultiClass Dif). Through functional analysis, we obtained the significant, reliable and targeted gene function (GO-Analysis); constructed the Immuno-related co-expression network of the significantly different genes, and found the most powerful gene (Dynamic-Gene-Network) through network analysis.
Statistical analysis
The original data was formed into an Excel table to establish a database. SPSS17.0 software was used for statistical analysis. The measurement data were expressed as x ± s. The t test was used to compare the mean of the two groups. One-way ANOVA was used to compare the mean of the three groups. S-N-K test was used for pairwise comparisons between groups. Counting data was shown by rate, and analyzed by χ2 test. Spearman test was used to analyze the relationship between the factors. P < 0.05 was considered to be statistically significant. The protein bands were analyzed by Image J software (Version 1.38).
Results
RORγt expression differences in tumor microenvironment between PTC patients with or without HT and benign thyroid nodules
No positive expression of RORγt was found in nodular goiter. RORγt was mainly expressed in the nucleus of infiltrating lymphocytes in tumor tissues. The number of RORγt+ lymphocytes infiltration in the tumor tissue of 39 PTC patients ranged from 0 to 129 per HPF, with an average of 62 per HPF (Figure 3A, 3B), with 21 cases in the low-expression group and 18 cases in the high-expression group. The number of RORγt+ lymphocyte infiltration in 46 PTC/HT patients ranged from 0 to 113 per HPF, with an average of 56 per HPF (Figure 3C, 3D), 26 cases in the low-expression group and 30 cases in the high-expression group.
Figure 3.
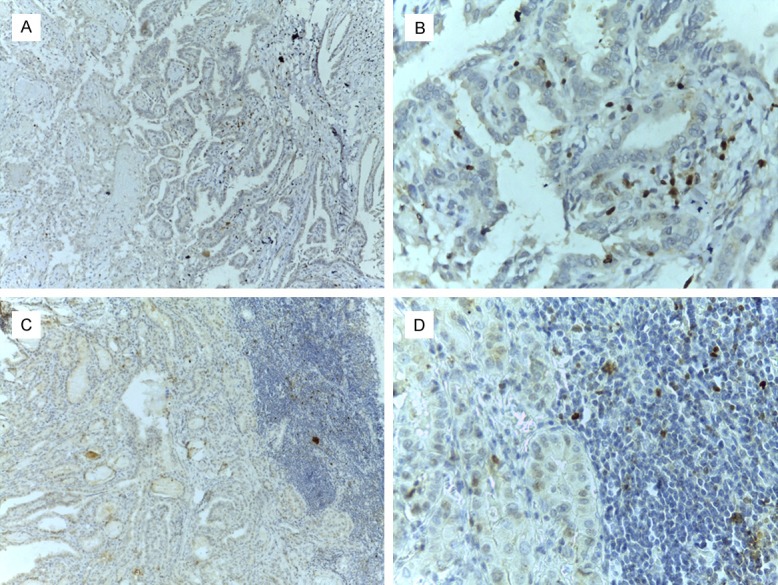
Expression of RORγt in thyroid papillary carcinoma with or without Hashimoto’s thyroiditis microenvironment (A: PTC alone ×100; B: PTC alone ×400; C: PTC with HT ×100; D: PTC with HT ×400).
Relationship of RORγt expression and clinical pathological features in PTC microenvironment
The rate of lymph node metastasis in RORγt low expression group was significantly higher than that in high expression group (71.4% vs. 33.3%, P = 0.017). The expression level of RORγt in tumor microenvironment of PTC/HT patients was not related to age and gender (P > 0.05) (Table 1).
Table 1.
Relationship of RORγt expression and clinical pathological features in PTC microenvironment
| Clinical Features | Low Expression (%) | High Expression (%) | Quantity | P | |
|---|---|---|---|---|---|
| n = 21 | n = 18 | ||||
| Age | < 45 | 8 (38.1) | 6 (33.3) | 0.10 | 0.757 |
| ≥ 45 | 13 (61.9) | 12 (66.7) | |||
| Gender | Male | 3 (14.3) | 3 (16.7) | 0.04 | 0.837 |
| Female | 18 (85.7) | 15 (83.3) | |||
| Lymph node metastasis | No | 6 (28.6) | 12 (66.7) | 5.66 | 0.017* |
| Yes | 15 (71.4) | 6 (33.3) | |||
| Tumor Diameter | 1.94 ± 0.81 | 1.73 ± 0.75 | 0.83 | 0.410 | |
P < 0.05.
Relationship of RORγt expression and clinical pathological features in PTC/HT microenvironment
The rate of lymph node metastasis in RORγt low expression group was significantly higher than that in high expression group (69.2% vs. 30.0%, P = 0.008). The expression level of RORγt in tumor microenvironment of PTC/HT patients was not related to age and gender (P > 0.05) (Table 2).
Table 2.
Relationship of RORγt expression and clinical pathological features in PTC/HT microenvironment
| Clinical Features | Low Expression (%) | High Expression (%) | Quantity | P | |
|---|---|---|---|---|---|
| n = 26 | n = 20 | ||||
| Age | < 45 | 16 (61.5) | 9 (45.0) | 1.25 | 0.264 |
| ≥ 45 | 10 (38.5) | 11 (55.0) | |||
| Gender | Male | 6 (23.1) | 4 (20.0) | 0.06 | 0.802 |
| Female | 20 (76.9) | 16 (80.0) | |||
| Lymph node metastasis | No | 8 (30.8) | 14 (70.0) | 6.97 | 0.008* |
| Yes | 18 (69.2) | 6 (30.0) | |||
| Tumor Diameter | 1.86 ± 1.02 | 1.59 ± 0.65 | 1.08 | 0.28 | |
P < 0.05.
Expression difference of RORγt between PTC combined with HT and PTC alone in tumor microenvironment
It was observed that patients with PTC/HT had a lower rate of RORγt high expression than patients with PTC alone, but the difference was not statistically significant (43.5% vs. 46.2%, P = 0.805) (Table 3).
Table 3.
Expression difference of RORγt between PTC combined with HT and PTC alone in tumor microenvironment
| Group | Low Expression | High Expression | Quantity | P |
|---|---|---|---|---|
| (%) (n = 47) | (%) (n = 38) | |||
| PTC/HT | 26 (56.5) | 20 (43.5) | 0.06 | 0.805 |
| PTC | 21 (53.8) | 18 (46.2) |
Correlation of RORγt expression and clinical pathological features between PTC combined with HT and PTC alone in tumor microenvironment
Both group showed negative correlation (P = 0.024, P = 0.046) between RORγt and lymph node metastasis, and the differences were statistically significant (Table 4).
Table 4.
Correlation of RORγt expression and clinical pathological features between PTC combined with HT and PTC alone in tumor microenvironment
P < 0.05.
RORγt PTC with or without HT patients and thyroid function correlation analysis
The RORγt showed a negative correlation (P = 0.003, 0.027) with TPOAb and TGAb in patients with PTC only with statistical significance. The RORγt showed a negative correlation (P = 0.001) with TGAb in patients with PTC only with statistical significance (Table 5).
Table 5.
Correlation of RORγt expression and thyroid function between PTC combined with HT and PTC in tumor microenvironment
| TG | TGAb | TPOAb | |
|---|---|---|---|
| PTC/HT (RORγt) | -0.049 (0.848) | -0.686 (0.001)* | -0.239 (0.272) |
| PTC (RORγt) | -0.186 (0.507) | -0.553 (0.003)* | -0.551 (0.027)* |
P < 0.05.
RORγt mRNA expression abundance in thyroid cancer tissue
The results of Real-Time PCR showed that the expression abundance of 2-ΔΔCT of RORγt mRNA was (1.39 ± 2.37) * 10-3 and (6.55 ± 7.39) * 10-3 respectively in PTC group and PTC/HT group. The expression of RORγt in PTC/HT group was significantly higher than PTC group (F = 2.62, P = 0.01, Figure 4).
Figure 4.
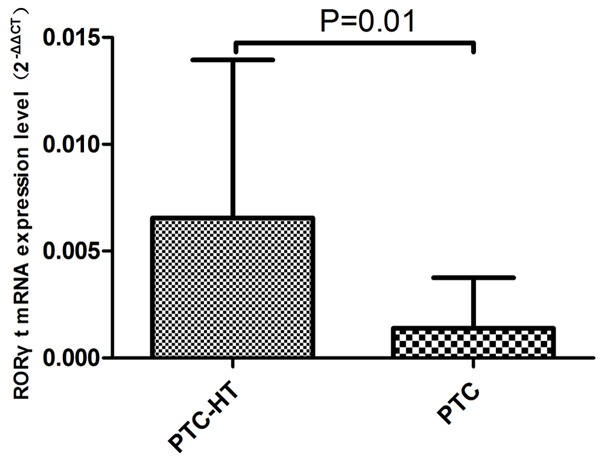
mRNA expressive abundance of RORγt gene in cancer tissue associated with PTC and PTC/HT patients (NS: No statistical significance).
Flow cytometry used to detect the formation rates of Th17 cells after co-treatment of Thyroid papillary carcinoma cell line (TPC-1 and K1) and umbilical cord blood initial T lymphocytes
The proportion of CD3+CD8-IL-17A+Th17 cells in the initial T lymphocytes of cord blood was 7.26 ± 2.14%. After Thyroid Papillary Carcinoma Cell Line TPC-1 co-treatment with umbilical cord blood initial T lymphocytes for 36 h, the Th17 ratio reached 24.00 ± 1.68%, higher than those induced at 24 h, 48 h and 60 h. The difference was statistically significant (P < 0.001). The co-treatment of normal thyroid follicular epithelial cell line with umbilical cord blood initial T lymphocytes in the control group could also induce the differentiation into Th17 cells. With the time going on, the ratio of Th17 cells was gradually increasing, reaching 10.70 ± 1.45% at 36 h. It was lower than that after co-treatment with TPC-1 (P < 0.001) (Figures 5 and 6).
Figure 5.
The percentage of Th17 cells induced by co-culture for 24 h, 36 h, 48 h and 60 h after isolation with Umbilical cord blood initial T lymphocytes and papillary thyroid carcinoma cell lines (A: TPC-1 and B: K1), normal thyroid follicular epithelial cell lines (C: Nthy ori 3-1).
Figure 6.
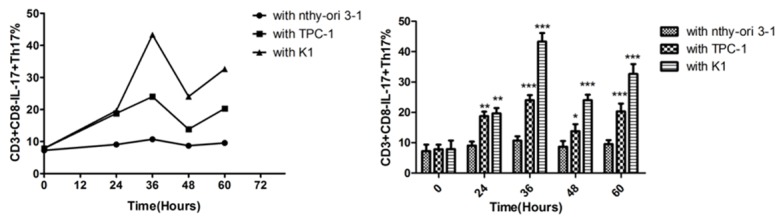
Flow cytometry detection of the proportion change trend of inductive formation CD3+CD8-IL-17+Th17 cells after isolation co culture of cord blood initial T cells combined with papillary thyroid cancer cell lineage (TPC-1 and K1) and normal thyroid follicular epithelial cell line (Nthy ori 3-1) treated for 24 h, 36 h, 48 h, 60 h. The results of co-culture with Nthy ori 3-1 were 7.26 ± 2.14 in 0 h, 9.07 ± 1.33 in 24 h, 10.70 ± 1.45 in 36 h, 8.70 ± 1.87 in 48 h, 9.57 ± 1.29 in 60 h. The results of co-culture with TPC-1 were 7.85 ± 1.50 in 0 h, 18.77 ± 1.45 in 24 h, 24.00 ± 1.68 in 36 h, 13.83 ± 2.25 in 48 h, 9.57 ± 1.29 in 60 h. The results of co-culture with K1 were 7.88 ± 2.84 in 0 h, 19.63 ± 1.81 in 24 h, 43.30 ± 2.86 in 36 h, 24.03 ± 1.78 in 48 h, 32.63 ± 3.25 in 60 h. Symbol *indicate significance at the 0.05 level, symbol **indicate significance at the 0.01 level, symbol ***indicate significance at the 0.001 level.
After co-treating with Thyroid papillary carcinoma cell line K1, initial cord blood T lymphocytes could induce the initial differentiation of CD4+ T lymphocytes into CD3+CD8-IL-17A+Th17 cells, which over time increased to 43.30 ± 0.45% at 36 h that was significantly higher than that at 24 h, 48 h and 60 h (P < 0.001). Th17 cells generated by initial T lymphocytes after co-treatment with K1 at 36 h were significantly higher than the control group at 36 h and also significantly higher than Th17 cells induced by TPC-1 at 36 h (P < 0.05). The differences were statistically significant (P < 0.001) (Figures 5 and 6).
Western blot semi-quantitative detection of cord blood lymphocytes and PTC cell lines co-cultured lymphocytes RORγt protein expression
After induced by co-culture with isolated papillary thyroid carcinoma cell lines TPC-1 and K1, the cord blood initial lymphocytes expressed low level of RORγt protein, which increased significantly with the culture time with a volatile growth. At 36 h, the percentage of induced RORγt protein reached the top, and the proportion of RORγt protein induced by K1 cell line with lymph node metastasis was higher than that induced by TPC-1 cell line without lymph node metastasis. Normal thyroid follicular epithelial cells could also induce cord blood T cells to express RORγt protein, but the expression level was stable without significant fluctuations (Figure 7).
Figure 7.
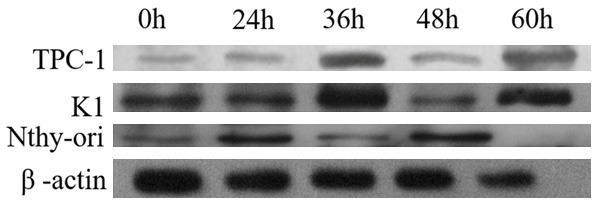
Flow cytometry detection of the proportion change trend of inductive formation Th17 cells after isolation co culture of cord blood initial T cells combined with papillary thyroid cancer cell lineage (TPC-1 and K1) and normal thyroid follicular epithelial cell line (Nthy ori 3-1) treated for 24 h, 36 h, 48 h, 60 h.
Image J gray-scale scanning was used to obtain the gray value of each target protein band to calculate the relative expression level of RORγt protein. The calculation formula was as follows: the relative expression level of targeted protein = (grayscale target protein-gray value background)/(gray value β-actin-gray value background) (Table 3). The relative expression levels of RORγt protein in T lymphocytes at different time points of co-cultivation could be obtained with graphing with T lymphocytes as the abscissa and the relative expression levels of targeted protein as the ordinate and the level of the lowest expression targeted protein at 0h as 1 (Figure 7).
Peripheral serum IL-17 was negatively correlated with lymph node metastasis in patients with PTC/HT
Compared with the control group, the expression level of IL-17a in HT group was higher (P < 0.05). Compared with HT group, the IL-17 expression level in peripheral blood in PTC group was significantly higher (P < 0.05). The concentration of IL-17a was positively correlated with the local invasion, the difference was statistically significant (P < 0.05) (Figure 8). In PTC/HT group, there was a negative correlation between IL-17 concentration and the central group lymph node metastasis (P < 0.05) (Table 6).
Figure 8.
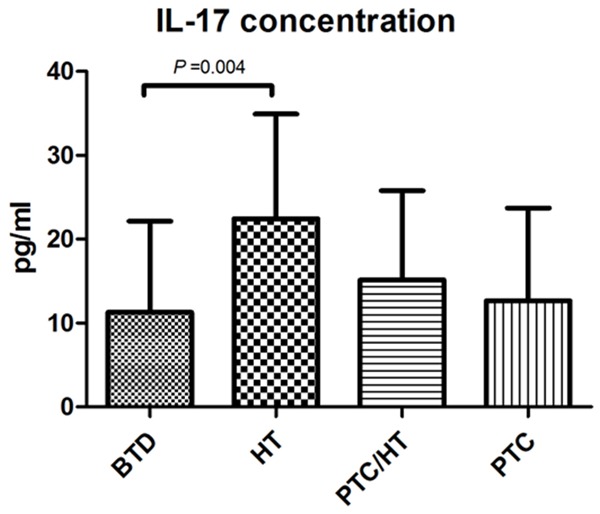
Expression level of IL-17 in peripheral blood serum of patients in benign tumor control (BTD) group 11.28 ± 10.86 pg/ml, Hashimoto’s thyroiditis (HT) group 22.45 ± 12.47 pg/ml, thyroid papillary carcinoma with Hashimoto’s Thyroiditis (PTC/HT) group 15.16 ± 10.61 pg/ml and papillary thyroid carcinoma (PTC) group 12.64 ± 11.07 pg/ml.
Table 6.
Correlation between Th17 cytokines and clinicopathological parameters in PTC group and PTC/HT group
| Group | Th17 | |
|---|---|---|
|
| ||
| IL-17 | ||
| PTC | Tumor Stage | 0.06 |
| TNM Stage | 0.32 | |
| Central Metastasis | 0.38 | |
| Peripheral Metastasis | -0.01 | |
| Regional Invasion | 0.47* (0.02) | |
| PTC/HT | Tumor Stage | -0.24 |
| TNM Stage | -0.36 | |
| Central Metastasis | -0.51* (0.04) | |
| Peripheral Metastasis | -0.45 | |
P < 0.05.
GO enrichment analysis showed that in the evolution process from HT to PT, RORγt gene was involved in five differential genes significant functions, including Protein phosphorylation, Gene expression, Transcription initiation from RNA polymerase II promoter, Lymph node development, T-helper 17 cell differentiation (Table 7). Among them, GO 0048535 (lymph node formation and metastasis) significantly enriched five genes including LTA, LTB, CXCL13, IKZF1, RORC, CXCR5. According to Dynamic calculation, RORγt was positively correlated with CAV1 (Interaction = 0.93) (Figure 9).
Table 7.
GO functional enrichment analysis of RORγt participates in PTC combined or uncombined HT differentially expressed genes
| Gene name | Go id | Go name | Enrichment | P-value | FDR |
|---|---|---|---|---|---|
| RORγt | GO:0006468 | Protein phosphorylation | 2.945878836 | 1.34821E-19 | 4.32145E-17 |
| RORγt | GO:0010467 | Gene expression | 2.183872161 | 3.27715E-16 | 9.26854E-14 |
| RORγt | GO:0006367 | Transcription initiation from RNA polymerase II promoter | 2.391106406 | 1.71743E-06 | 7.86418E-05 |
| RORγt | GO:0048535 | Lymph node development | 6.946793349 | 0.000255764 | 0.005465384 |
| RORγt | GO:0072539 | T-helper 17 cell differentiation | 11.57798892 | 0.029832455 | 0.196485541 |
Figure 9.
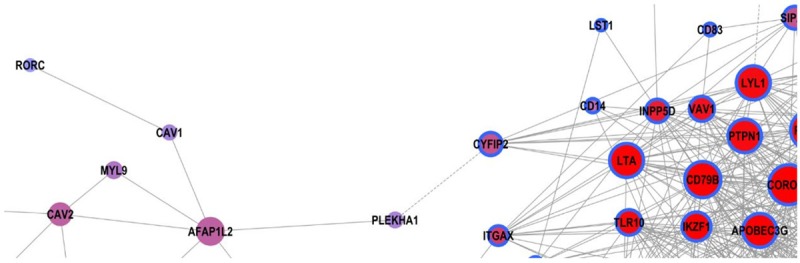
Dynamic-Gene Net of differential Immune-related gene (RORγt gene was positively correlated with CAV1, the interaction coefficient = 0.93. The interaction coefficient is the degree of co-expression of two genes).
Discussion
It has been widely reported that the immune system suppresses the growth of tumor cells through immune surveillance [12,13], and tumor infiltrating lymphocytes (TIL) representing host immune responses are directly involved in the micro invasive state playing an important role in the tumor microenvironment. In TIL, CD4+ T helper T lymphocytes are generally considered to be important players in tumor immunity and have a significant impact on tumor progression [14]. They play important roles in the immune response depending on different cytokines and transcription factors differentiating into four kinds of immune subtypes (Th17, Treg, Th1, Th2) [15]. It has been reported that the infiltration of lymphocytes is associated with the prognosis of PTC [16,17]. The more TIL infiltrates in the PTC tumor microenvironment, the better the prognosis may be. There were few studies on the relationship between special cell subsets and PTC and HT. The relationship between T lymphocyte subsets infiltration and PTC immune regulation and clinic pathological parameters was investigated to clarify the role of Th17 helper CD4+ T lymphocytes in HT-PTC evolution in PTC with or without HT tumor microenvironment due to the complicated phenotype and function of TIL.
It is known that thyroid autoimmune inflammation is associated with a high incidence of PTC [18,19], among which there are only 10-15% patients that cannot be cured by surgery and iodine therapy [20]. PTC-associated chronic lymphocytic thyroiditis (LT) is related with intratumoral infiltration of lymphocytes and macrophage banks, suggesting that it can modulate the tumor microenvironment to increase anti-tumor immune response. In fact, it has been found that the better prognosis of PTC patients with LT is correlated directly with tumor-associated macrophages (TAMs) infiltrating the tumor [16,21]. Tumor infiltrating lymphocytes, especially Foxp3+ Treg cells in the tumor microenvironment, promote disease progression and increase the chance of invasion and metastasis [22], while RORγt+ Th17 cells in the tumor microenvironment are tumor suppressing. Romaldini et al. [5] found that patients lacking TIL are more susceptible to multifocal tumor lesions. There was a high rate of recurrence in patients without TIL infiltration and multivariate model analysis confirmed that the presence of TIL was significantly associated with good prognosis. These studies suggested that autoimmune antibodies play protective roles in the outcome of patients with differentiated thyroid cancer [23]. It was found in our study that PTC patients with or without HT were infiltrated with a large number of Th17 cells in the tumor microenvironment, while there was no infiltration of both cells in the microenvironment of patients with benign nodular goiters, indicating that Th17 cells participate in PTC Immune response process.
Th17 cells are major contributors to IL-17 cytokines and play key roles in the progression of autoimmune diseases, tumors and inflammation [24]. Th17 cells play immune roles through the stimulating of IL-6 and transforming growth factor TGF-β [14]. There is evidence that Th17 cells act as indirect immune effectors in anti-tumor immunity [25]. This study found that Th17 cells with high expression of RORγt had infiltration in PTC tissues with or without HT, and there was a negative correlation between RORγt and lymph node metastasis. A correlation between Th17 cell infiltration and low lymph node metastasis was also found, proving that Th17 might promote antitumor responses in both PTC patients with and without HT.
It was found in our study that the nuclear transcription protein RORγt of Th17 was expressed in the tumor tissue of PTC patients with or without HT, and the ratio of lymph node metastasis in patients with high RORγt expression was lower than patients with low expression, and the results of RT-PCR also showed that the expression of RORγt mRNA in PTC patients with HT was higher than PTC patients. Correlation analysis also confirmed that the positive expression of RORγt was negatively correlated with lymph node metastasis in patients with PTC with or without HT, indicating that RORγt might inhibit the lymph node metastasis and exert anti-tumor activity in the tumor microenvironment of PTC patients with or without HT. It has been reported that PTC with HT is associated with low invasiveness and good prognosis, suggesting that autoimmune thyroiditis is a risk factor for the development of thyroid cancer but also protective against disease progression [3,4,26]. HT is a subtype of autoimmune thyroiditis with serum TGAb and TPOAb detection rate of 90% to 95%. It is suggested that the effects of TPOAb and TGAb are part of the relationship between HT and thyroid cancer. TGAb is more significant that recognize the common TG epitope of thyroid cancer and HT to promote the transition from Hashimoto’s thyroiditis into thyroid cancer [27]. Kryczek et al. [28] reported that Th17 cells might contribute to the protection of anti-tumor immunity and predict improvements in the survival of patients with ovarian cancer, which is consistent with our results. However, Tosolini et al. [29] reported that high density Th17 cells predict poor prognosis in metastatic colorectal cancer. So Th17 cells may have multiple effects in different tumors and in different stages of the same tumor.
HT may be a risk factor for thyroid cancer, but the prognosis of thyroid cancer with HT is good. JunSoo Jeong et al. found that HT with thyroid cancer is characterized by multiple lesions, younger age, smaller foci, less lymphatic and distant metastases, and less invasive outside the capsule [30]. Kwak HY also found that thyroid cancer patients with HT are predominantly female with a lower degree of malignancy, a lower rate of lymph node metastasis, and a lower recurrence rate [31]. The followings may be the reasons why PTC patients with HT may get better prognosis: (1) HT patients autoimmune react against thyroid-specific antigen to destroy the thyroid cancer cells expressing cell surface-specific thyroid antigen [32], preventing thyroid cancer cell migration and repetition. (2) There is more cytotoxic T lymphocyte-mediated immune response and humoral immune response in HT-combined thyroid cancer than in simple thyroid cancer or simple HT, and IL-1 produced by immune response can control the proliferation of tumor cells [33]. (3) Fas and Fas ligands in the thyroid follicular cells of HT patients stimulate the apoptosis of cells and participate in the destruction of tumor cells [34]. (4) Cunha study showed that the good prognosis of thyroid cancer with HT is correlated with the infiltration of lymphocytes, B cells, giant cells and Th17 cells [35]. This study shows that Th17 cells in the tumor microenvironment of PTC patients with HT in Yunnan Plateau region can inhibit the lymph node metastasis of PTC and exert anti-tumor effects, which may also be the reason that the prognosis of PTC patients with HT is better than that of PTC alone. Our results further confirm that RORγt+ Th17+ cellular immune responses play a protective role in the progression of PTC.
Interleukin-17 (IL-17) is a proinflammatory cytokine secreted by CD4+ and CD8+ T cells [36]. CD4+ T cells (Th17 cells) that form IL-17 have been identified as a new subset of helper T cells [37]. CD8+ T cells (Tc17 cells) that form IL-17 have also been identified in mice and human beings that can be produced by in vitro priming with Th17 polarized cytokines [38]. Th17 and Tc17 cells have been found in types of human cancer and murine models. IL-17A- deficient mice are more likely to suffer lung melanoma. Th17-polarized cells are found to be more potent anti-tumor than Th1 cells [39]. The adoptive transfer of tumor-specific Tc17 cells reduces the tumor volume in nude mice and produces IFN-γ persistently [40], which suggests that Th17 and Tc17 cells exert anti-tumor effects. Th17-type immune responses that produce IL-17 function occur in patients with autoimmune diseases, including HT [41]. In this study, it was found that RORγt was negatively correlated with lymph node metastasis in PTC/HT patients, and serum IL-17 concentration was negatively correlated with central group lymph node metastasis. It was similar to the above conclusion, indicating that Th17 cells in PTC patients with HT might have anti-tumor immune function. Therefore, the impact of IL-17+Th17 cells in the tumor microenvironment comes with two sides: promoting tumor growth and inhibiting tumor growth. The specific effect on the tumor may also depend on other cells, such as: natural killer cells, neutrophils, γδT cells, and other cytokines.
It has been shown in some studies that PTC patients and related HT patients have good clinical prognosis [33,42], while not in other studies [43]. Based on this and previous studies, it can be speculated that cytokines may play a protective role of tumor surveillance and anti-tumor immunity in Th2-type immune responses in tumor microenvironments [44]. Most cytokines may have many conflicting functions in tumor immune surveillance and tumor immunity, so it still requires additional studies to determine the significance of these findings.
Caveolae is a flask like cell membrane invagination structure that takes part in many cell life activities. Caveolae mainly exist in adipocytes, fibroblasts, type I alveolar cells, vascular smooth muscle cells, epithelial cells, endothelial cells and rhabdomyocytes. Caveolin (CAV1) is the main component of caveolae, which belongs to integrated membrane protein. It may be related to the concentration of specific lipids, the modification of signal transduction molecules and the scaffold proteins in caveolin. CAV1 is associated with cell proliferation, invasion, malignant transformation, metastasis, signal transduction and multidrug resistance [45]. CAV1 is the most important marker protein of pit membrane structure, located on human chromosome 7q31.1. This locus on human chromosome 7 is often broken or deleted in many malignant tumors, so it is speculated that CAV1 is a tumor suppressor gene [46].
As a candidate tumor suppressor gene, CAV1 interacts with many other signaling molecules via phosphorylation and/or dephosphorylation to inhibit tumor cell proliferation, apoptosis, adhesion and metastasis [47]. It was reported that CAV1 was down-regulated in breast cancer [48], lung cancer [49] and gastric cancer [50] and up-regulated in papillary thyroid carcinoma [51] due to mutation, loss of heterozygosity and hypermethylation. The expression of CAV1 in epithelial cells is more intense in classical PTC than in other histological types. There was a positive correlation between CAV1 expression and lymph node metastasis [52]. CAV1 plays an oncogene role in reported solid tumors, and the expression of CAV1 is negatively correlated with the degree of tumor invasion, we predict that CAV1 may also play an inhibitory role in thyroid cancer. Our study shows that RORγt is positively correlated with CAV1 and may interact with each other. Therefore, we speculate that RORγt may play a role in thyroid cancer which is involved in the lymph node metastasis of HT-PTC by regulating five genes, LTA, LTB, CXCL13, IKZF1 and CXCR5, together with CAV1. Further functional experiments are needed to verify this hypothesis.
Acknowledgements
This work was supported by The Research Projects Inside Research Institute of Medical and Health Institutions of Yunnan Province (2014N269).
Disclosure of conflict of interest
None.
References
- 1.Baretic M. 100 years of Hashimoto thyroiditis, still an intriguing disease. Acta Med Croatica. 2011;65:453–457. [PubMed] [Google Scholar]
- 2.Dailey ME, Lindsay S, Skahen R. Relation of thyroid neoplasms to Hashimoto disease of the thyroid gland. AMA Arch Surg. 1955;70:291–297. doi: 10.1001/archsurg.1955.01270080137023. [DOI] [PubMed] [Google Scholar]
- 3.Lun Y, Wu X, Xia Q, Han Y, Zhang X, Liu Z, Wang F, Duan Z, Xin S, Zhang J. Hashimoto’s thyroiditis as a risk factor of papillary thyroid cancer may improve cancer prognosis. Otolaryngol Head Neck Surg. 2013;148:396–402. doi: 10.1177/0194599812472426. [DOI] [PubMed] [Google Scholar]
- 4.Huang BY, Hseuh C, Chao TC, Lin KJ, Lin JD. Well-differentiated thyroid carcinoma with concomitant Hashimoto’s thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol. 2011;22:144–149. doi: 10.1007/s12022-011-9164-9. [DOI] [PubMed] [Google Scholar]
- 5.Villagelin DG, Santos RB, Romaldini JH. Is diffuse and peritumoral lymphocyte infiltration in papillary thyroid cancer a marker of good prognosis? J Endocrinol Invest. 2011;34:e403–408. doi: 10.3275/7870. [DOI] [PubMed] [Google Scholar]
- 6.Kimura A, Kishimoto T. Th17 cells in inflammation. Int Immunopharmacol. 2011;11:319–322. doi: 10.1016/j.intimp.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Maddur MS, Miossec P, Kaveri SV, Bayry J. Th17 cells: biology, pathogenesis of autoimmune and inflammatory diseases, and therapeutic strategies. Am J Pathol. 2012;181:8–18. doi: 10.1016/j.ajpath.2012.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Ye J, Livergood RS, Peng G. The role and regulation of human Th17 cells in tumor immunity. Am J Pathol. 2013;182:10–20. doi: 10.1016/j.ajpath.2012.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tanaka J, Ogura T, Sato H, Hatano M. Establishment and biological characterization of an in vitro human cytomegalovirus latency model. Virology. 1987;161:62–72. doi: 10.1016/0042-6822(87)90171-1. [DOI] [PubMed] [Google Scholar]
- 10.Challeton C, Branea F, Schlumberger M, Gaillard N, de Vathaire F, Badie C, Antonini P, Parmentier C. Characterization and radiosensitivity at high or low dose rate of four cell lines derived from human thyroid tumors. Int J Radiat Oncol Biol Phys. 1997;37:163–169. doi: 10.1016/s0360-3016(96)00449-x. [DOI] [PubMed] [Google Scholar]
- 11.Liu H, Zeng Q, Cui Y, Zhao L, Zhang L, Fu G, Hou C, Zhang S, Yu L, Jiang C, Wang Z, Chen X, Wang A. The role of the IRE1 pathway in excessive iodide- and/or fluoride-induced apoptosis in Nthy-ori 3-1 cells in vitro. Toxicol Lett. 2014;224:341–348. doi: 10.1016/j.toxlet.2013.11.001. [DOI] [PubMed] [Google Scholar]
- 12.Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. doi: 10.1038/nmeth.2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swann JB, Smyth MJ. Immune surveillance of tumors. J Clin Invest. 2007;117:1137–1146. doi: 10.1172/JCI31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura A, Kishimoto T. IL-6: regulator of Treg/Th17 balance. Eur J Immunol. 2010;40:1830–1835. doi: 10.1002/eji.201040391. [DOI] [PubMed] [Google Scholar]
- 15.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, Matsuzuka F, Kakudoh K, Kuma K, Tamai H. The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab. 1995;80:3421–3424. doi: 10.1210/jcem.80.12.8530576. [DOI] [PubMed] [Google Scholar]
- 17.Gupta S, Patel A, Folstad A, Fenton C, Dinauer CA, Tuttle RM, Conran R, Francis GL. Infiltration of differentiated thyroid carcinoma by proliferating lymphocytes is associated with improved disease-free survival for children and young adults. J Clin Endocrinol Metab. 2001;86:1346–1354. doi: 10.1210/jcem.86.3.7310. [DOI] [PubMed] [Google Scholar]
- 18.Melillo RM, Guarino V, Avilla E, Galdiero MR, Liotti F, Prevete N, Rossi FW, Basolo F, Ugolini C, de Paulis A, Santoro M, Marone G. Mast cells have a protumorigenic role in human thyroid cancer. Oncogene. 2010;29:6203–6215. doi: 10.1038/onc.2010.348. [DOI] [PubMed] [Google Scholar]
- 19.Proietti A, Ugolini C, Melillo RM, Crisman G, Elisei R, Santoro M, Minuto M, Vitti P, Miccoli P, Basolo F. Higher intratumoral expression of CD1a, tryptase, and CD68 in a follicular variant of papillary thyroid carcinoma compared to adenomas: correlation with clinical and pathological parameters. Thyroid. 2011;21:1209–1215. doi: 10.1089/thy.2011.0059. [DOI] [PubMed] [Google Scholar]
- 20.Schlumberger M, Lacroix L, Russo D, Filetti S, Bidart JM. Defects in iodide metabolism in thyroid cancer and implications for the followup and treatment of patients. Nat Clin Pract Endocrinol Metab. 2007;3:260–269. doi: 10.1038/ncpendmet0449. [DOI] [PubMed] [Google Scholar]
- 21.Kamma H, Fujii K, Ogata T. Lymphocytic infiltration in juvenile thyroid carcinoma. Cancer. 1988;62:1988–1993. doi: 10.1002/1097-0142(19881101)62:9<1988::aid-cncr2820620919>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 22.French JD, Kotnis GR, Said S, Raeburn CD, McIntyre RC Jr, Klopper JP, Haugen BR. Programmed death-1+ T cells and regulatory T cells are enriched in tumor-involved lymph nodes and associated with aggressive features in papillary thyroid cancer. J Clin Endocrinol Metab. 2012;97:E934–943. doi: 10.1210/jc.2011-3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Souza SL, Montalli Da Assumpcao LV, Ward LS. Impact of previous thyroid autoimmune diseases on prognosis of patients with well-differentiated thyroid cancer. Thyroid. 2003;13:491–495. doi: 10.1089/105072503322021160. [DOI] [PubMed] [Google Scholar]
- 24.Chung AS, Wu X, Zhuang G, Ngu H, Kasman I, Zhang J, Vernes JM, Jiang Z, Meng YG, Peale FV, Ouyang W, Ferrara N. An interleukin-17-mediated paracrine network promotes tumor resistance to anti-angiogenic therapy. Nat Med. 2013;19:1114–1123. doi: 10.1038/nm.3291. [DOI] [PubMed] [Google Scholar]
- 25.Zou W, Restifo NP. T(H)17 cells in tumour immunity and immunotherapy. Nat Rev Immunol. 2010;10:248–256. doi: 10.1038/nri2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dvorkin S, Robenshtok E, Hirsch D, Strenov Y, Shimon I, Benbassat CA. Differentiated thyroid cancer is associated with less aggressive disease and better outcome in patients with coexisting Hashimotos thyroiditis. J Clin Endocrinol Metab. 2013;98:2409–2414. doi: 10.1210/jc.2013-1309. [DOI] [PubMed] [Google Scholar]
- 27.Fiore E, Rago T, Latrofa F, Provenzale MA, Piaggi P, Delitala A, Scutari M, Basolo F, Di Coscio G, Grasso L, Pinchera A, Vitti P. Hashimoto’s thyroiditis is associated with papillary thyroid carcinoma: role of TSH and of treatment with L-thyroxine. Endocr Relat Cancer. 2011;18:429–437. doi: 10.1530/ERC-11-0028. [DOI] [PubMed] [Google Scholar]
- 28.Kryczek I, Wu K, Zhao E, Wei S, Vatan L, Szeliga W, Huang E, Greenson J, Chang A, Rolinski J, Radwan P, Fang J, Wang G, Zou W. IL-17+regulatory T cells in the microenvironments of chronic inflammation and cancer. J Immunol. 2011;186:4388–4395. doi: 10.4049/jimmunol.1003251. [DOI] [PubMed] [Google Scholar]
- 29.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pages F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 30.Jeong JS, Kim HK, Lee CR, Park S, Park JH, Kang SW, Jeong JJ, Nam KH, Chung WY, Park CS. Coexistence of chronic lymphocytic thyroiditis with papillary thyroid carcinoma: clinical manifestation and prognostic outcome. J Korean Med Sci. 2012;27:883–889. doi: 10.3346/jkms.2012.27.8.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kwak HY, Chae BJ, Eom YH, Hong YR, Seo JB, Lee SH, Song BJ, Jung SS, Bae JS. Does papillary thyroid carcinoma have a better prognosis with or without Hashimoto thyroiditis? Int J Clin Oncol. 2015;20:463–473. doi: 10.1007/s10147-014-0754-7. [DOI] [PubMed] [Google Scholar]
- 32.Del Rio P, Cataldo S, Sommaruga L, Concione L, Arcuri MF, Sianesi M. The association between papillary carcinoma and chronic lymphocytic thyroiditis: does it modify the prognosis of cancer? Minerva Endocrinol. 2008;33:1–5. [PubMed] [Google Scholar]
- 33.Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, Hong SJ, Gong G, Shong YK. Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 2009;71:581–586. doi: 10.1111/j.1365-2265.2009.03537.x. [DOI] [PubMed] [Google Scholar]
- 34.Ramaswamy M, Cleland SY, Cruz AC, Siegel RM. Many checkpoints on the road to cell death: regulation of Fas-FasL interactions and Fas signaling in peripheral immune responses. Results Probl Cell Differ. 2009;49:17–47. doi: 10.1007/400_2008_24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cunha LL, Morari EC, Guihen AC, Razolli D, Gerhard R, Nonogaki S, Soares FA, Vassallo J, Ward LS. Infiltration of a mixture of immune cells may be related to good prognosis in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2012;77:918–925. doi: 10.1111/j.1365-2265.2012.04482.x. [DOI] [PubMed] [Google Scholar]
- 36.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 37.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 38.Kondo T, Takata H, Matsuki F, Takiguchi M. Cutting edge: phenotypic characterization and differentiation of human CD8+ T cells producing IL-17. J Immunol. 2009;182:1794–1798. doi: 10.4049/jimmunol.0801347. [DOI] [PubMed] [Google Scholar]
- 39.Muranski P, Boni A, Antony PA, Cassard L, Irvine KR, Kaiser A, Paulos CM, Palmer DC, Touloukian CE, Ptak K, Gattinoni L, Wrzesinski C, Hinrichs CS, Kerstann KW, Feigenbaum L, Chan CC, Restifo NP. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood. 2008;112:362–373. doi: 10.1182/blood-2007-11-120998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hinrichs CS, Kaiser A, Paulos CM, Cassard L, Sanchez-Perez L, Heemskerk B, Wrzesinski C, Borman ZA, Muranski P, Restifo NP. Type 17 CD8+ T cells display enhanced antitumor immunity. Blood. 2009;114:596–599. doi: 10.1182/blood-2009-02-203935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Figueroa-Vega N, Alfonso-Perez M, Benedicto I, Sanchez-Madrid F, Gonzalez-Amaro R, Marazuela M. Increased circulating pro-inflammatory cytokines and Th17 lymphocytes in Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2010;95:953–962. doi: 10.1210/jc.2009-1719. [DOI] [PubMed] [Google Scholar]
- 42.Singh B, Shaha AR, Trivedi H, Carew JF, Poluri A, Shah JP. Coexistent Hashimoto’s thyroiditis with papillary thyroid carcinoma: impact on presentation, management, and outcome. Surgery. 1999;126:1070–1076. doi: 10.1067/msy.2099.101431. discussion 1076-1077. [DOI] [PubMed] [Google Scholar]
- 43.Lee JH, Kim Y, Choi JW, Kim YS. The association between papillary thyroid carcinoma and histologically proven Hashimoto’s thyroiditis: a meta-analysis. Eur J Endocrinol. 2013;168:343–349. doi: 10.1530/EJE-12-0903. [DOI] [PubMed] [Google Scholar]
- 44.Ellyard JI, Simson L, Parish CR. Th2-mediated anti-tumour immunity: friend or foe? Tissue Antigens. 2007;70:1–11. doi: 10.1111/j.1399-0039.2007.00869.x. [DOI] [PubMed] [Google Scholar]
- 45.Patel H, Abduljabbar R, Lai CF, Periyasamy M, Harrod A, Gemma C, Steel JH, Patel N, Busonero C, Jerjees D, Remenyi J, Smith S, Gomm JJ, Magnani L, Gyorffy B, Jones LJ, Fuller-Pace F, Shousha S, Buluwela L, Rakha EA, Ellis IO, Coombes RC, Ali S. Expression of CDK7, Cyclin H, and MAT1 is elevated in breast cancer and is prognostic in estrogen receptorpositive breast cancer. Clin Cancer Res. 2016;22:5929–5938. doi: 10.1158/1078-0432.CCR-15-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Engelman JA, Zhang XL, Galbiati F, Lisanti MP. Chromosomal localization, genomic organization, and developmental expression of the murine caveolin gene family (Cav-1, -2, and -3). Cav-1 and Cav-2 genes map to a known tumor suppressor locus (6-A2/7q31) FEBS Lett. 1998;429:330–336. doi: 10.1016/s0014-5793(98)00619-x. [DOI] [PubMed] [Google Scholar]
- 47.Razani B, Schlegel A, Liu J, Lisanti MP. Caveolin-1, a putative tumour suppressor gene. Biochem Soc Trans. 2001;29:494–499. doi: 10.1042/bst0290494. [DOI] [PubMed] [Google Scholar]
- 48.Joglekar M, Elbazanti WO, Weitzman MD, Lehman HL, van Golen KL. Caveolin-1 mediates inflammatory breast cancer cell invasion via the Akt1 pathway and RhoC GTPase. J Cell Biochem. 2015;116:923–933. doi: 10.1002/jcb.25025. [DOI] [PubMed] [Google Scholar]
- 49.Chen D, Shen C, Du H, Zhou Y, Che G. Duplex value of caveolin-1 in non-small cell lung cancer: a meta analysis. Fam Cancer. 2014;13:449–457. doi: 10.1007/s10689-014-9707-6. [DOI] [PubMed] [Google Scholar]
- 50.Gao X, Sun Y, Huang L, Chen XY, Zhang KL, Kong QY, Liu J, Li H. [Down-regulation of caveolin-1 in gastric carcinoma and its clinical biological significance] . Ai Zheng. 2005;24:311–316. [PubMed] [Google Scholar]
- 51.Shankar J, Wiseman SM, Meng F, Kasaian K, Strugnell S, Mofid A, Gown A, Jones SJ, Nabi IR. Coordinated expression of galectin-3 and caveolin-1 in thyroid cancer. J Pathol. 2012;228:56–66. doi: 10.1002/path.4041. [DOI] [PubMed] [Google Scholar]
- 52.Paskas S, Jankovic J, Marecko I, Isic Dencic T, Tatic S, Cvejic D, Savin S. Caveolin-1 expression in papillary thyroid carcinoma: correlation with clinicopathological parameters and BRAF mutation status. Otolaryngol Head Neck Surg. 2014;150:201–209. doi: 10.1177/0194599813512781. [DOI] [PubMed] [Google Scholar]