Abstract
Objectives: Since large cell neuroendocrine carcinoma (LCNEC) is a relatively rare histologic type of primary lung cancer, little is known about the immunological status of patients with LCNEC. We aimed to clarify the expression and prognostic impact of programmed cell death ligand 1 (PD-L1), CD8, CD4, and Forkhead box protein P3 (Foxp3) in LCNEC. Methods: We retrospectively analyzed PD-L1, CD8, CD4, and Foxp3 expressions in 95 surgically resected LCNEC. PD-L1 positive staining was determined in tumors with more than 1% of tumor cells stained to any intensity, and CD8, CD4, and Foxp3 positivity was determined in tumors with more than 5% of lymphocytes stained. Results: Positive expression of PD-L1, CD8, CD4, and Foxp3 was observed in 70 (74%), 52 (55%), 76 (80%), and 43 (45%) tumors, respectively. The expression of PD-L1 was significantly correlated with positive lymphatic permeation. Positive correlations were mutually observed among tumor infiltrating immune cells. Univariate and multivariate analyses showed that positive pleural invasion and Foxp3 negative expression were independent unfavorable prognostic factors for overall survival (OS). Advanced pathological stage, positive pleural invasion, CD4 negative expression in cancer stroma, and Foxp3 negative expression were identified as independent unfavorable prognostic factors for recurrence free survival (RFS). Conclusions: Foxp3 positive tumor infiltrating lymphocytes (TILs) were an independent favorable prognostic factor for both OS and RFS, whereas CD4 positive TILs were an independent significant unfavorable prognostic factor for RFS. The high frequency of PD-L1 expression could support the use of anti-programmed cell death 1 antibody in the treatment of LCNEC.
Keywords: Large cell neuroendocrine carcinoma (LCNEC), lung cancer, PD-L1, Foxp3, tumor infiltrating lymphocyte (TIL)
Introduction
Large cell neuroendocrine carcinoma (LCNEC) is a relatively rare tumor, which accounts for approximately 2-4% of all lung cancers [1]. LCNEC has been initially classified as a subgroup of large cell carcinoma, but is now pathologically classified as a high grade neuroendocrine tumor (NET), all together with small cell lung cancer (SCLC) [2]. Furthermore, patients with LCNEC are clinically treated with the conventional chemotherapeutic regimen administered to those with SCLC [3]. Because of its rarity, there has been no established biomarker to predict the outcome after treatment.
Anti-programmed cell death-1 (PD-1) antibody has brought a transformative change in the treatment of advanced or metastatic non-small cell lung cancer (NSCLC), and has drastically changed the survival of patients with metastatic NSCLC [4-7]. Immune checkpoint inhibitors have been identified as one of the principal therapies in addition to cytotoxic chemotherapy and tyrosine kinase inhibitors in NSCLC. The expression of programmed cell death-ligand 1 (PD-L1) within cancer cells is thought as a significant biomarker to predict efficacy of anti-PD-1 treatment. Indeed, the chemotherapeutic strategy using anti-PD-1 antibody has been considered for metastatic or recurrent NSCLC according to the expression status of PD-L1. Several researchers have described the relationship between the prognostic impact and the expression levels of PD-L1 in patients with SCLC [3,8-10]. Three of these studies have reported that patients with positive expression of PD-L1 yielded significantly favorable survival than those with PD-L1 negative expression [3,8,10], whereas one study has demonstrated that the expression of PD-L1 was closely related to poor prognosis in SCLC [9]. This difference might be associated with the sample size, the various antibodies for immunohistochemistry, and the threshold for PD-L1 positivity. Nevertheless, the expression of PD-L1 seems to be a useful marker to predict a good outcome in SCLC. Recently, Tsuruoka et al. described that the overall survival (OS) time of LCNEC and SCLC tended to be longer in patients with positive expression of PD-L1 than in those with PD-L1 negative expression [11]. Inamura et al. also reported same tendency in LCNEC [12]. However, it remains unclear whether PD-L1 could predict a good outcome in patients with LCNEC. In their analysis, tissue microarray (TMA) blocks were used for immunohistochemistry [11,12]; however, surgically resected tumor specimens are actually suitable for the correct assessment of PD-L1 expression because of delicate cut-off values, such as 1% or 5%, and the heterogeneous staining pattern of PD-L1 expression in whole tumor tissues.
Tumor infiltrating lymphocytes (TILs) have been also used as a prognostic marker in NSCLC, and recent meta-analysis has demonstrated that high level of CD8 T cells infiltration in tumor stroma or tumor nest and high level of CD4 T cells infiltration in tumor stroma are linked to a good prognosis in lung cancer, whereas high level of Foxp3 T cells infiltration in tumor stroma is identified as a poor prognostic predictor [13]. Little is known about the relationship between TILs and prognostic significance in patients with NET of the lung, such as LCNEC.
Based on this background information, we conducted this study to evaluate the relationship between the clinicopathological significance and immunological expression of PD-L1, CD8, CD4, and Foxp3 in surgically resected LCNEC.
Methods
Patient selection and follow-up
This was a multi-institutional joint retrospective study conducted by researchers at Gunma University Hospital, Gunma Prefectural Cancer Center, Maebashi Red Cross Hospital, National Hospital Organization Takasaki General Medical Center, and National Hospital Organization Shibukawa Medical Center. The study protocol was approved by the Institutional Review Board of each participating institution according to the Helsinki Declaration.
Ninety-eight patients underwent surgical resection of LCNEC at the participating institutions between April 2000 and March 2016. After excluding 2 patients with inadequate clinicopathological data and 1 patient with poor quality specimen, we enrolled 95 patients with LCNEC into this study.
The histology of LCNEC was confirmed at Gunma University according to the World Health Organization criteria. The stages of pathological tumor-node-metastasis were established using the International System for Staging Lung Cancer adopted by the American Joint Committee on Cancer and the Union Internationale Centre le Cancer. The follow-up period for censored cases ranged from 16 days to 5131 days (median, 1113 days).
Immunohistochemical staining
For PD-L1, CD8, CD4, and Foxp3, immunohistochemical staining was performed according to the procedures described in a previous study [14]. All sections were deparaffinized in xylene, rehydrated, and then incubated with fresh 0.3% hydrogen peroxide in 100% methanol for 30 min at room temperature to block endogenous peroxidase activity. After rehydration through a graded series of ethanol treatments, PD-L1 was retrieved using the universal HIER antigen retrieval reagent (Abcam, ab208572) at 120°C for 20 min in autoclave, and then, sections were passively cooled to room temperature. Antigen retrieval was performed using Immunosaver (NJ15T, NEM) at 98-100°C for 30 min for CD4, CD8, and Foxp3 staining. After rinsing in 0.1 M phosphate buffered saline (PBS, pH 7.4) to block non-specific binding sites, sections were incubated with protein block serum-free reagent (DAKO, Carpinteria, CA, USA) for 30 min at room temperature. Rabbit monoclonal antibodies against PD-L1 (Cell Signaling, E1L3NR, 1:200 dilution), CD8 (Abcam ab4055, 1:600 dilution), CD4 (Abcam ab133616, 1:200 dilution), and Foxp3 (Abcam ab20034, 1:200 dilution) were used as primary antibodies. PD-L1 expression on each cell was considered positive when membrane staining was observed. A semi-quantitative scoring method was used for PD-L1 expression, as follows: 0 ≤ 1%, 1 = 1-5%, 2 = 6-10%, 3 = 11-25%, 4 = 26-50%, 5 ≥ 51% of cells were positive according to a previous report [14]. Tumors with score ≥ 1 were graded as PD-L1 positive according to previous studies [4,11]. CD8, CD4, and Foxp3 expressions were semi-quantitatively evaluated on the extent of positive lymphocytes infiltrating with tumor specimens. CD8 and CD4 expressions on lymphocytes were considered positive when membrane staining was observed. The CD8 and CD4 expressions were examined in a whole cancer tissue, as well as separately in cancer nest only and cancer stroma only in order to clarify the prognostic impact of their localization. Foxp3 expression on lymphocytes was considered positive when nuclear staining was observed. Foxp3 positive TILs were present only in cancer stroma. CD8, CD4, and Foxp3 positivity was defined in tumors with more than 5% of positive lymphocytes, according to a previous report [14]. The tissue sections were examined in a blinded fashion using light microscopy by at least two of the authors (Y.O and K.K). In case of any discrepancies, both investigators evaluated the slides simultaneously, until reaching a consensus on their final assessment. Neither of the investigators had any knowledge of the patient outcomes.
Statistical analysis
OS was defined as the time interval between the date of tumor resection and the date of death from any cause or censored date. Recurrence free survival (RFS) was defined as the time interval between the date of tumor resection and the date of any recurrence detected or death from other cause than cancer death or the last follow-up. For univariate analyses, survival rates were estimated by the Kaplan-Meier method, and differences in survival between subgroups were compared by the log-rank test. Multivariate analyses were performed using the Cox proportional hazard model. Forward and backward stepwise procedures were performed to determine the prognostic effect of combined factors. Chi-squared test was performed to evaluate the relationship between categorical valuables, and Student’s t-test was used to evaluate continuous variables. All of the reported P values were two-sided, and the significance level was set at less than 0.05. All statistical analyses were performed using SPSS Statistics 20 statistical software (Dr. SPSS II for Windows; standard version 20.0; SPSS Inc., Chicago, IL, USA).
Results
Patients’ characteristics
The patients’ characteristics are listed in Table 1. Of the 95 patients, 82 were men and 13 were women, with a median age of 74 years. Ninety-one patients (96%) had smoking history. Histologically, 77 patients (81%) had pure LCNEC and 18 patients (19%) had combined LCNEC. Lymphatic permeation, vascular invasion, and pleural invasion were observed in 67%, 73%, and 39% of patients, respectively.
Table 1.
Patient characteristics
| N (%) | |
|---|---|
| Age: median (range) | 74 (36-88) |
| Gender | |
| Male/Female | 82/13 (86/14) |
| Smoking history | |
| No/Yes | 4/91 (4/96) |
| Histology | |
| LCNEC only | 77 (81) |
| Combined LCNEC | 18 (19) |
| Pathological stage | |
| IA (IA1, IA2, IA3) | 29 (31) |
| IA1 | 3 (3) |
| IA2 | 17 (18) |
| IA3 | 9 (9) |
| IB | 25 (27) |
| IIA | 8 (8) |
| IIB | 16 (17) |
| IIIA | 14 (15) |
| IV | 3 (3) |
| Lymphatic permeation | |
| No/Yes | 31/62 (33/67) |
| Vascular invasion | |
| No/Yes | 25/67 (27/73) |
| Pleural invasion | |
| No | 57 (61) |
| Yes | 37 (39) |
| PL1 | 18 (19) |
| PL2 | 14 (15) |
| PL3 | 5 (5) |
| Adjuvant therapy | |
| No/Yes | 72/23 (76/24) |
PL: pleural invasion.
PD-L1 expression and TILs
Positive expression of PD-L1, CD8, CD4, and Foxp3 was observed in 70 (74%), 52 (55%), 76 (80%), and 43 (45%) tumors, respectively (Table 2). Representative images for PD-L1, CD8, CD4, and Foxp3 expressions are shown in Figure 1. Although the rate of PD-L1 positivity was high, only 7 patients (7%) had tumors with more than 50% of tumor cells expressing PD-L1 (score 5+) (Table S1). CD8 positive TILs were abundant in cancer nest, whereas most of CD4 positive TILs were present in cancer stroma.
Table 2.
PD-L1, CD8, CD4 and Foxp3 expression rate
| Factors | Cut off value | N (%) |
|---|---|---|
| PD-L1 expression | ||
| Negative | < 1% | 25 (26) |
| Positive | ≥ 1% | 70 (74) |
| CD8 expression in whole tumor | ||
| Negative | < 5% | 43 (45) |
| Positive | ≥ 5% | 52 (55) |
| CD8 expression in cancer nest | ||
| Negative | < 5% | 24 (25) |
| Positive | ≥ 5% | 71 (75) |
| CD8 expression in cancer stroma | ||
| Negative | < 5% | 45 (47) |
| Positive | ≥ 5% | 50 (53) |
| CD4 expression in whole tumor | ||
| Negative | < 5% | 19 (20) |
| Positive | ≥ 5% | 76 (80) |
| CD4 expression in cancer nest | ||
| Negative | < 5% | 80 (84) |
| Positive | ≥ 5% | 15 (16) |
| CD4 expression in cancer stroma | ||
| Negative | < 5% | 17 (18) |
| Positive | ≥ 5% | 78 (82) |
| Foxp3 expression | ||
| Negative | < 5% | 52 (55) |
| Positive | ≥ 5% | 43 (45) |
Figure 1.
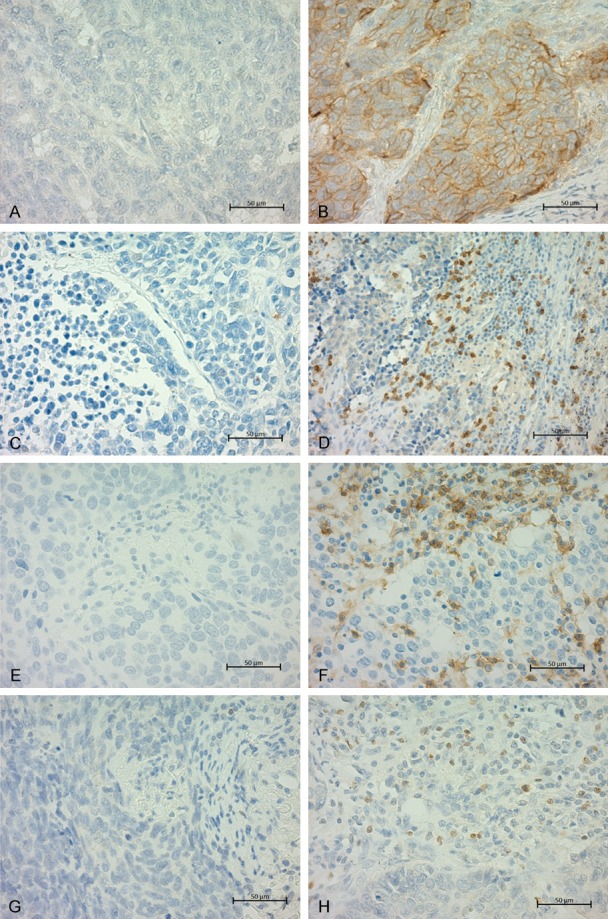
Representative immunohistochemical findings of LCNEC are shown. PD-L1 negative (A) and PD-L1 positive are shown (B). CD8 negative (C), CD8 positive (D), CD4 negative (E), CD4 positive (F), Foxp3 negative (G) and Foxp3 positive (H) are shown.
Clinicopathological characteristics according to PD-L1 expression and TILs
The clinicopathological features according to PD-L1 expression and TILs are listed in Table 3. The expression of PD-L1 was significantly correlated with lymphatic permeation. For correlation among immunological expressions, positive correlations were observed between PD-L1 and CD4, PD-L1 and Foxp3, CD8 and CD4, CD8 and Foxp3, and CD4 and Foxp3. Except PD-L1 and CD8 expressions, there were significant mutually positive correlations among tumor infiltrating immune cells.
Table 3.
Correlation among immunological expression and clinicopathological factors
| N | PD-L1 expression | CD8 expression | CD4 expression | Foxp3 expression | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||
| Negative (N = 25) | Positive (N = 70) | p-valuea | Negative (N = 52) | Positive (N = 43) | p-valuea | Negative (N = 19) | Positive (N = 76) | p-valuea | Negative (N = 52) | Positive (N = 43) | p-valuea | ||
| Age | |||||||||||||
| < 74 | 46 | 11 | 35 | 0.61 | 27 | 19 | 0.45 | 12 | 34 | 0.15 | 26 | 20 | 0.74 |
| ≥ 74 | 49 | 14 | 35 | 25 | 24 | 7 | 42 | 26 | 23 | ||||
| Gender | |||||||||||||
| Male | 82 | 19 | 63 | 0.08 | 43 | 39 | 0.26 | 14 | 68 | 0.07 | 44 | 38 | 0.60 |
| Female | 13 | 6 | 7 | 9 | 4 | 5 | 8 | 8 | 5 | ||||
| Smoking history | |||||||||||||
| Yes | 91 | 23 | 68 | 0.27 | 50 | 41 | 0.85 | 18 | 73 | 0.80 | 50 | 41 | 0.85 |
| No | 4 | 2 | 2 | 2 | 2 | 1 | 3 | 2 | 2 | ||||
| Stage | |||||||||||||
| I | 54 | 14 | 40 | 0.60 | 29 | 25 | 0.82 | 8 | 46 | 0.15 | 27 | 27 | 0.29 |
| II-IV | 41 | 11 | 40 | 23 | 18 | 11 | 30 | 25 | 16 | ||||
| Lymphatic permeation | |||||||||||||
| No | 31 | 4 | 27 | 0.04b | 17 | 14 | 0.88 | 3 | 28 | 0.10 | 15 | 16 | 0.46 |
| Yes | 62 | 20 | 42 | 33 | 29 | 15 | 47 | 35 | 27 | ||||
| Vascular invasion | |||||||||||||
| No | 25 | 6 | 19 | 0.89 | 17 | 8 | 0.08 | 4 | 21 | 0.60 | 17 | 8 | 0.11 |
| Yes | 67 | 17 | 50 | 32 | 35 | 14 | 53 | 33 | 34 | ||||
| Pleural invasion | |||||||||||||
| No | 57 | 13 | 44 | 0.45 | 33 | 24 | 0.38 | 9 | 48 | 0.30 | 29 | 28 | 0.42 |
| Yes | 37 | 11 | 26 | 18 | 19 | 9 | 28 | 22 | 15 | ||||
| CD8 expression | |||||||||||||
| Negative | 52 | 16 | 36 | 0.28 | - | - | - | - | - | - | |||
| Positive | 43 | 9 | 34 | - | - | - | - | - | - | ||||
| CD4 expression | |||||||||||||
| Negative | 19 | 7 | 12 | < 0.01b | 6 | 1 | < 0.01b | - | - | - | - | ||
| Positive | 76 | 18 | 58 | 0 | 42 | - | - | - | - | ||||
| Foxp3 expression | |||||||||||||
| Negative | 52 | 18 | 34 | 0.04b | 39 | 13 | < 0.01b | 17 | 35 | < 0.01b | - | - | |
| Positive | 43 | 7 | 36 | 13 | 30 | 2 | 41 | - | - | ||||
Peason’s chi-square test;
denotes significance.
Univariate and multivariate survival analyses
The 5-year OS rate of all patients was 51.6%. Of 95 patients, 40 patients died and 36 patients developed recurrent diseases. Mean survival time is 1207 days (3.3 years). The OS and RFS curves according to the expression of PD-L1, CD8, CD4, and Foxp3 are shown in Figure 2. The RFS of the patients with PD-L1 positive expression tended to be higher than those with negative expression, without statistical significance (Figure 2A, 2B). There were no survival differences in the expression status of CD8 (Figure 2C, 2D), irrespective of the location (Figure S1), neither of CD4 in the whole tumor tissue (Figure 2E, 2F), nor of CD4 in cancer nest (Figure S2). When the evaluation of CD4 expression was limited to cancer stroma, the patients with CD4 positive TILs in cancer stroma had significantly worse RFS than those with CD4 negative TILs in cancer stroma (Figure 2G, 2H). The patients with tumors with Foxp3 positive TILs had significantly better prognosis than those with Foxp3 negative LCNEC, for both OS and RFS (Figure 2I, 2J).
Figure 2.

Overall survival (OS) and recurrence free survival (RFS) curves according to PD-L1 (A, B), CD8 (C, D), CD4 (E, F), CD4 in cancer stroma (G, H) and Foxp3 (I, J) are shown.
Table 4 shows univariate and multivariate analyses of OS and RFS. Univariate and multivariate analyses showed that positive pleural invasion and Foxp3 negative expression were independent unfavorable prognostic factors for OS. For RFS, advanced pathological stage, positive lymphatic permeation, positive pleural invasion, CD4 positive expression in cancer stroma, and Foxp3 negative expression were identified as significant unfavorable prognostic factors in univariate analysis. Of them, multivariate analysis showed that besides advanced pathological stage and positive pleural invasion, CD4 positive expression in cancer stroma, and Foxp3 negative expression were identified as independent unfavorable prognostic factors for RFS.
Table 4.
Univariate and Multivariate analyses
| OS | RFS | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Univariate analysis | Multivariate analysis | Univariate analysis | Multivariate analysis | |||||
|
| ||||||||
| N = 95 | p-valuea | HR | p-valueb | N = 92 | p-valuea | HR | p-valueb | |
| Age | ||||||||
| ≥ 74/< 74 | 49/46 | 0.12 | 48/44 | 0.13 | ||||
| Gender | ||||||||
| Male/Female | 82/13 | 0.32 | 79/13 | 0.22 | ||||
| Smoking history | ||||||||
| Yes/No | 4/91 | 0.50 | 3/89 | 0.81 | ||||
| Stage | ||||||||
| II-IV/I | 41/54 | 0.13 | 38/54 | 0.02c | 1.97 (1.05-3.71) | 0.03c | ||
| Lymphatic permeation | ||||||||
| Yes/No | 62/31 | 0.10 | 29/61 | < 0.01c | 1.93 (0.94-3.97) | 0.07 | ||
| Vascular invasion | ||||||||
| Yes/No | 67/25 | 0.72 | 66/23 | 0.09 | ||||
| Pleural invasion | ||||||||
| Yes/No | 37/57 | 0.02c | 2.82 (1.21-3.93) | < 0.01c | 35/56 | < 0.01c | 2.23 (1.23-4.04) | < 0.01c |
| Adjuvant therapy | ||||||||
| No/Yes | 72/23 | 0.08 | 70/22 | 0.71 | ||||
| PD-L1 expression | ||||||||
| Negative/Positive | 25/70 | 0.25 | 25/67 | 0.09 | ||||
| CD8 expression in whole tumor | ||||||||
| Negative/Positive | 52/43 | 0.53 | 50/42 | 0.95 | ||||
| CD8 expression in cancer nest | ||||||||
| Negative/Positive | 71/24 | 0.67 | 69/23 | 0.61 | ||||
| CD8 expression in cancer stroma | ||||||||
| Negative/Positive | 45/50 | 0.44 | 43/49 | 0.82 | ||||
| CD4 expression in whole tumor | ||||||||
| Negative/Positive | 19/76 | 0.43 | 19/73 | 0.38 | ||||
| CD4 expression in cancer nest | ||||||||
| Negative/Positive | 80/15 | 0.69 | 77/15 | 0.68 | ||||
| CD4 expression in cancer stroma | ||||||||
| Negative/Positive | 17/78 | 0.14 | 17/75 | 0.03c | 0.31 (0.11-0.82) | 0.02c | ||
| Foxp3 expression | ||||||||
| Negative/Positive | 52/43 | 0.01c | 1.97 (1.06-3.68) | 0.03c | 50/42 | < 0.01c | 1.97 (1.05-3.71) | 0.04c |
Log-rank test;
Cox proportional hazard model;
denotes significance.
Discussion
In this study, we revealed the prognostic impact of PD-L1 expression and TILs in LCNEC. Until now, there have been limited reports about the prognostic impact of immune cells infiltration in LCNEC. Of immune-related cells, infiltration of Foxp3 negative TILs was an independent unfavorable prognostic factor for OS and RFS. The presence of CD4 positive TILs in cancer stroma was also identified as an independent unfavorable prognostic factor for RFS in patients with LCNEC.
In terms of Foxp3 expression, our results were contradictory to a previous meta-analysis of lung cancer, reporting that low number of Foxp3 T cells infiltration in tumor stroma was identified as a good prognostic factors [13]. One of the reasons might be the difference of tumor histology among the population. In most of former studies, adenocarcinoma or NSCLC were examined. Even though these studies included patients with LCNEC, the total numbers of patients was small. The condition of immune cell infiltration might be different according to tumor histology between NET and the others, especially in LCNEC. Another reason is that a previous meta-analysis has included only a few studies on Foxp3 expression. The sample size seems to be too small to conclude the prognostic impact of Foxp3 in whole lung cancer.
Foxp3 positive regulatory T cells (Treg) are potent mediators of dominant self-tolerance in the periphery and abundant CD4 positive T cells expressing Foxp3 are responsible for suppressing the anti-tumor immune response. Abundant Foxp3 positive T cells have been thought to be a poor prognostic factor in various cancers; however, some studies have shown favorable prognostic impact of Foxp3 positive Treg infiltration in some neoplasms. In a meta-analysis on gastric cancer, Zheng et al. described that intra-tumoral Foxp3 positive T cells were associated with poor survival, whereas extra-tumoral Foxp3 positive T cells invasion was associated with better survival [15]. Authors suggested that Foxp3 T cells have opposite functions in the intra- and extra-tumoral environment; our result was consistent with the extra-tumoral (i.e. stromal) Foxp3 expression observed in their study. Some reports on colorectal cancer have also shown favorable impact of Foxp3 expression on patient outcome [16-18]. Saito et al. revealed that colorectal cancer, which is commonly infiltrated by suppression-competent Foxp3-positive Treg cells, can be classified into two types by the degree of non-suppressive T cells with low Foxp3 expression [19]. Colorectal cancer with abundant infiltration of Foxp3 positive but low expressing T cells showed significantly better prognosis than those with predominantly Foxp3 highly expressing Treg cell infiltration. Authors described that functionally distinct subpopulations of tumor-infiltrating Foxp3 positive T cells contribute in opposing ways to determining prognosis. We did not investigate the expressing levels and relationship between other markers, but many T cells expressing low Foxp3 levels might be included within the Foxp3 positive T cells.
Regarding CD4 positive TILs, one meta-analysis has shown that high number of CD4 positive T cells infiltration in tumor stroma was identified as a good prognostic factor. In contrast, another meta-analysis has shown that high number of CD4 positive T cells infiltration in the whole tumor tissue was associated with a good prognosis for OS of patients with lung cancer [20]. The discrepancy between their analysis and our result might be explained by the same reason as that for Foxp3, such as histology and small sample size. Generally, CD4 positive T cells were thought to suppress anti-tumor immune response [21], but the prognostic impact has not been clarified in a large patient size. In LCNEC, stromal CD4 positive T cells might suppress the anti-tumor immune response, as demonstrated in previous reports in other cancers [22,23].
Until now, many studies have investigated the frequency and prognostic impact of PD-L1 expression in lung cancer [9,24-35]. Several reports have shown that PD-L1 expression was an independent unfavorable factor for survival in lung adenocarcinoma [25,29,32,36], lung squamous cell carcinoma [27,28], SCLC [9], and NSCLC [34]. However, some reports have shown no significant differences between PD-L1 positive and negative tumors [26], while others have shown that PD-L1 was an independent favorable prognostic factor in NSCLC [10,35,37,38]. It is controversial whether high PD-L1 expression is prognostic or non-prognostic, and whether favorable or unfavorable. Because tumor immune microenvironment might be different depending on histologic variations, we examined the outcome limited to LCNEC. In our study, patients with PD-L1 positive tumor had better but not significant RFS, which was consistent with a previous report on LCNEC [11,12]. Despite similar prognosis and same cut-off value of PD-L1 used in Tsuruoka et al., PD-L1 expression positive rate was much lower than that of our study (10.4% vs 73.7%). Although Inamura et al. used 5% as a cut-off value of PD-L1 positivity, PD-L1 expression positive rate (26.8%) was also lower than that of ours. This differences might be caused by the different methodology; those authors used TMA for evaluation and they enrolled patients for a long-term period (1982-2010 [11], and 1990-2014 [12]). However, considering our data that showed high PD-L1 expression in LCNEC, anti-PD-1 antibody might be effective to LCNEC.
This study had several limitations. First of all, we did not observe the effect of anti-PD-1 antibody treatment and whether PD-L1 expression is predictive for the therapeutic outcome. In this study, we only examined the prognostic impact of immune-related markers including PD-L1. Another limitation is that we collected surgically resected tumor samples for a relatively long period. PD-L1 expression might be different between new and old samples, as it has been previously noted [39].
Conclusions
In conclusion, we showed the prognostic impact of PD-L1 expression and TILs in LCNEC. Foxp3 positive TILs were an independent significant good prognostic factor for both OS and RFS. CD4 positive TILs were conversely an independent significant poor prognostic factor for RFS. The high frequency of PD-L1 positive expression could further support the use of anti-PD-1 antibody in the treatment of LCNEC and a good tumor response following treatment, same as in other NSCLC subtypes.
Acknowledgements
The authors thank Dr. Bolag Altan, Dr. Bilguun Erkhem-Ochir, Ms. Mai Yagame, Ms. Harumi Kanai for their technical support, and Ms. Kaori Takeshita for their support in data collection.
Disclosure of conflict of interest
None.
Abbreviations
- CI
Confidence interval
- HR
Hazard ratio
- LCNEC
Large cell neuroendocrine carcinoma
- ND
Nodal dissection
- NSCLC
Non-small cell lung cancer
- OS
Overall survival
- PD-L1
Programmed death-ligand 1
- RFS
Recurrence free survival
- TIL
Tumor infiltrating lymphocyte
Supporting Information
References
- 1.Fasano M, Della Corte CM, Papaccio F, Ciardiello F, Morgillo F. Pulmonary large-cell neuroendocrine carcinoma: from epidemiology to therapy. J Thorac Oncol. 2015;10:1133–1141. doi: 10.1097/JTO.0000000000000589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Travis WD, Brambilla E, Nicholson AG, Yatabe Y, Austin JH, Beasley MB, Chirieac LR, Dacic S, Duhig E, Flieder DB, Geisinger K, Hirsch FR, Ishikawa Y, Kerr KM, Noguchi M, Pelosi G, Powell CA, Tsao MS, Wistuba I WHO Panel. The 2015 world health organization classification of lung tumors: impact of genetic, clinical and radiologic advances since the 2004 classification. J Thorac Oncol. 2015;10:1243–1260. doi: 10.1097/JTO.0000000000000630. [DOI] [PubMed] [Google Scholar]
- 3.Ishii H, Azuma K, Kawahara A, Yamada K, Imamura Y, Tokito T, Kinoshita T, Kage M, Hoshino T. Significance of programmed cell death-ligand 1 expression and its association with survival in patients with small cell lung cancer. J Thorac Oncol. 2015;10:426–430. doi: 10.1097/JTO.0000000000000414. [DOI] [PubMed] [Google Scholar]
- 4.Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, Barlesi F, Kohlhaufl M, Arrieta O, Burgio MA, Fayette J, Lena H, Poddubskaya E, Gerber DE, Gettinger SN, Rudin CM, Rizvi N, Crino L, Blumenschein GR Jr, Antonia SJ, Dorange C, Harbison CT, Graf Finckenstein F, Brahmer JR. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brahmer J, Reckamp KL, Baas P, Crino L, Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE, Holgado E, Waterhouse D, Ready N, Gainor J, Aren Frontera O, Havel L, Steins M, Garassino MC, Aerts JG, Domine M, Paz-Ares L, Reck M, Baudelet C, Harbison CT, Lestini B, Spigel DR. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Herbst RS, Baas P, Kim DW, Felip E, Perez-Gracia JL, Han JY, Molina J, Kim JH, Arvis CD, Ahn MJ, Majem M, Fidler MJ, de Castro G Jr, Garrido M, Lubiniecki GM, Shentu Y, Im E, Dolled-Filhart M, Garon EB. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi: 10.1016/S0140-6736(15)01281-7. [DOI] [PubMed] [Google Scholar]
- 7.Reck M, Rodriguez-Abreu D, Robinson AG, Hui R, Csoszi T, Fulop A, Gottfried M, Peled N, Tafreshi A, Cuffe S, O’Brien M, Rao S, Hotta K, Leiby MA, Lubiniecki GM, Shentu Y, Rangwala R, Brahmer JR KEYNOTE-024 Investigators. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375:1823–1833. doi: 10.1056/NEJMoa1606774. [DOI] [PubMed] [Google Scholar]
- 8.Yu H, Batenchuk C, Badzio A, Boyle TA, Czapiewski P, Chan DC, Lu X, Gao D, Ellison K, Kowalewski AA, Rivard CJ, Dziadziuszko R, Zhou C, Hussein M, Richards D, Wilks S, Monte M, Edenfield W, Goldschmidt J, Page R, Ulrich B, Waterhouse D, Close S, Jassem J, Kulig K, Hirsch FR. PD-L1 expression by two complementary diagnostic assays and mrna in situ hybridization in small cell lung cancer. J Thorac Oncol. 2017;12:110–120. doi: 10.1016/j.jtho.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang YL, Yang CY, Huang YL, Wu CT, Yang PC. High PD-L1 expression is associated with stage IV disease and poorer overall survival in 186 cases of small cell lung cancers. Oncotarget. 2017;8:18021–18030. doi: 10.18632/oncotarget.14935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Toyokawa G, Takada K, Haratake N, Takamori S, Akamine T, Katsura M, Fujishita T, Shoji F, Okamoto T, Oda Y, Maehara Y. Favorable disease-free survival associated with programmed death ligand 1 expression in patients with surgically resected small-cell lung cancer. Anticancer Res. 2016;36:4329–4336. [PubMed] [Google Scholar]
- 11.Tsuruoka K, Horinouchi H, Goto Y, Kanda S, Fujiwara Y, Nokihara H, Yamamoto N, Asakura K, Nakagawa K, Sakurai H, Watanabe SI, Tsuta K, Ohe Y. PD-L1 expression in neuroendocrine tumors of the lung. Lung Cancer. 2017;108:115–120. doi: 10.1016/j.lungcan.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Inamura K, Yokouchi Y, Kobayashi M, Ninomiya H, Sakakibara R, Nishio M, Okumura S, Ishikawa Y. Relationship of tumor PD-L1 (CD274) expression with lower mortality in lung high-grade neuroendocrine tumor. Cancer Med. 2017;6:2347–2356. doi: 10.1002/cam4.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Geng Y, Shao Y, He W, Hu W, Xu Y, Chen J, Wu C, Jiang J. Prognostic role of tumor-infiltrating lymphocytes in lung cancer: a meta-analysis. Cell Physiol Biochem. 2015;37:1560–1571. doi: 10.1159/000438523. [DOI] [PubMed] [Google Scholar]
- 14.Kaira K, Higuchi T, Naruse I, Arisaka Y, Tokue A, Altan B, Suda S, Mogi A, Shimizu K, Sunaga N, Hisada T, Kitano S, Obinata H, Yokobori T, Mori K, Nishiyama M, Tsushima Y, Asao T. Metabolic activity by 18F-FDG-PET/CT is predictive of early response after nivolumab in previously treated NSCLC. Eur J Nucl Med Mol Imaging. 2018;45:56–66. doi: 10.1007/s00259-017-3806-1. [DOI] [PubMed] [Google Scholar]
- 15.Zheng X, Song X, Shao Y, Xu B, Chen L, Zhou Q, Hu W, Zhang D, Wu C, Tao M, Zhu Y, Jiang J. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget. 2017;8:57386–57398. doi: 10.18632/oncotarget.18065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.deLeeuw RJ, Kost SE, Kakal JA, Nelson BH. The prognostic value of FoxP3+ tumor-infiltrating lymphocytes in cancer: a critical review of the literature. Clin Cancer Res. 2012;18:3022–3029. doi: 10.1158/1078-0432.CCR-11-3216. [DOI] [PubMed] [Google Scholar]
- 17.Frey DM, Droeser RA, Viehl CT, Zlobec I, Lugli A, Zingg U, Oertli D, Kettelhack C, Terracciano L, Tornillo L. High frequency of tumor-infiltrating FOXP3(+) regulatory T cells predicts improved survival in mismatch repair-proficient colorectal cancer patients. Int J Cancer. 2010;126:2635–2643. doi: 10.1002/ijc.24989. [DOI] [PubMed] [Google Scholar]
- 18.Salama P, Phillips M, Grieu F, Morris M, Zeps N, Joseph D, Platell C, Iacopetta B. Tumor-infiltrating FOXP3+ T regulatory cells show strong prognostic significance in colorectal cancer. J. Clin. Oncol. 2009;27:186–192. doi: 10.1200/JCO.2008.18.7229. [DOI] [PubMed] [Google Scholar]
- 19.Saito T, Nishikawa H, Wada H, Nagano Y, Sugiyama D, Atarashi K, Maeda Y, Hamaguchi M, Ohkura N, Sato E, Nagase H, Nishimura J, Yamamoto H, Takiguchi S, Tanoue T, Suda W, Morita H, Hattori M, Honda K, Mori M, Doki Y, Sakaguchi S. Two FOXP3(+)CD4(+) T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med. 2016;22:679–684. doi: 10.1038/nm.4086. [DOI] [PubMed] [Google Scholar]
- 20.Zeng DQ, Yu YF, Ou QY, Li XY, Zhong RZ, Xie CM, Hu QG. Prognostic and predictive value of tumor-infiltrating lymphocytes for clinical therapeutic research in patients with non-small cell lung cancer. Oncotarget. 2016;7:13765–13781. doi: 10.18632/oncotarget.7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Correa LH, Correa R, Farinasso CM, de Sant’Ana Dourado LP, Magalhaes KG. Adipocytes and macrophages interplay in the orchestration of tumor microenvironment: new implications in cancer progression. Front Immunol. 2017;8:1129. doi: 10.3389/fimmu.2017.01129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, Jiang T, Wu A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer. 2014;110:2560–2568. doi: 10.1038/bjc.2014.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Q, Hao C, Cheng G, Wang L, Wang X, Li C, Qiu J, Ding K. High CD4(+) T cell density is associated with poor prognosis in patients with non-muscle-invasive bladder cancer. Int J Clin Exp Pathol. 2015;8:11510–11516. [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang M, Li G, Wang Y, Wang Y, Zhao S, Haihong P, Zhao H, Wang Y. PD-L1 expression in lung cancer and its correlation with driver mutations: a meta-analysis. Sci Rep. 2017;7:10255. doi: 10.1038/s41598-017-10925-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirai A, Yoneda K, Shimajiri S, Kuroda K, Hanagiri T, Fujino Y, Tanaka F. Prognostic impact of programmed death-ligand 1 expression in correlation with human leukocyte antigen class I expression status in stage I adenocarcinoma of the lung. J Thorac Cardiovasc Surg. 2018;155:382–392. e1. doi: 10.1016/j.jtcvs.2017.05.106. [DOI] [PubMed] [Google Scholar]
- 26.Shi X, Wu S, Sun J, Liu Y, Zeng X, Liang Z. PD-L1 expression in lung adenosquamous carcinomas compared with the more common variants of non-small cell lung cancer. Sci Rep. 2017;7:46209. doi: 10.1038/srep46209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Igawa S, Sato Y, Ryuge S, Ichinoe M, Katono K, Hiyoshi Y, Otani S, Nagashio R, Nakashima H, Katagiri M, Sasaki J, Murakumo Y, Satoh Y, Masuda N. Impact of PD-L1 expression in patients with surgically resected non-small-cell lung cancer. Oncology. 2017;92:283–290. doi: 10.1159/000458412. [DOI] [PubMed] [Google Scholar]
- 28.Takada K, Okamoto T, Toyokawa G, Kozuma Y, Matsubara T, Haratake N, Akamine T, Takamori S, Katsura M, Shoji F, Oda Y, Maehara Y. The expression of PD-L1 protein as a prognostic factor in lung squamous cell carcinoma. Lung Cancer. 2017;104:7–15. doi: 10.1016/j.lungcan.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Wu S, Shi X, Sun J, Liu Y, Luo Y, Liang Z, Wang J, Zeng X. The significance of programmed cell death ligand 1 expression in resected lung adenocarcinoma. Oncotarget. 2017;8:16421–16429. doi: 10.18632/oncotarget.14851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsao MS, Le Teuff G, Shepherd FA, Landais C, Hainaut P, Filipits M, Pirker R, Le Chevalier T, Graziano S, Kratze R, Soria JC, Pignon JP, Seymour L, Brambilla E. PD-L1 protein expression assessed by immunohistochemistry is neither prognostic nor predictive of benefit from adjuvant chemotherapy in resected nonsmall cell lung cancer. Ann Oncol. 2017;28:882–889. doi: 10.1093/annonc/mdx003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.He Y, Yu H, Rozeboom L, Rivard CJ, Ellison K, Dziadziuszko R, Suda K, Ren S, Wu C, Hou L, Zhou C, Hirsch FR. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017;12:814–823. doi: 10.1016/j.jtho.2017.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Shimoji M, Shimizu S, Sato K, Suda K, Kobayashi Y, Tomizawa K, Takemoto T, Mitsudomi T. Clinical and pathologic features of lung cancer expressing programmed cell death ligand 1 (PD-L1) Lung Cancer. 2016;98:69–75. doi: 10.1016/j.lungcan.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 33.Vieira T, Antoine M, Hamard C, Fallet V, Duruisseaux M, Rabbe N, Rodenas A, Cadranel J, Wislez M. Sarcomatoid lung carcinomas show high levels of programmed death ligand-1 (PDL1) and strong immune-cell infiltration by TCD3 cells and macrophages. Lung Cancer. 2016;98:51–58. doi: 10.1016/j.lungcan.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 34.Sun JM, Zhou W, Choi YL, Choi SJ, Kim SE, Wang Z, Dolled-Filhart M, Emancipator K, Wu D, Weiner R, Frisman D, Kim HK, Choi YS, Shim YM, Kim J. Prognostic significance of PD-L1 in patients with non-small cell lung cancer: a large cohort study of surgically resected cases. J Thorac Oncol. 2016;11:1003–1011. doi: 10.1016/j.jtho.2016.04.007. [DOI] [PubMed] [Google Scholar]
- 35.Yang CY, Lin MW, Chang YL, Wu CT, Yang PC. Programmed cell death-ligand 1 expression is associated with a favourable immune microenvironment and better overall survival in stage I pulmonary squamous cell carcinoma. Eur J Cancer. 2016;57:91–103. doi: 10.1016/j.ejca.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 36.Azuma K, Ota K, Kawahara A, Hattori S, Iwama E, Harada T, Matsumoto K, Takayama K, Takamori S, Kage M, Hoshino T, Nakanishi Y, Okamoto I. Association of PD-L1 overexpression with activating EGFR mutations in surgically resected nonsmall-cell lung cancer. Ann Oncol. 2014;25:1935–1940. doi: 10.1093/annonc/mdu242. [DOI] [PubMed] [Google Scholar]
- 37.Velcheti V, Schalper KA, Carvajal DE, Anagnostou VK, Syrigos KN, Sznol M, Herbst RS, Gettinger SN, Chen L, Rimm DL. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi: 10.1038/labinvest.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cooper WA, Tran T, Vilain RE, Madore J, Selinger CI, Kohonen-Corish M, Yip P, Yu B, O’Toole SA, McCaughan BC, Yearley JH, Horvath LG, Kao S, Boyer M, Scolyer RA. PD-L1 expression is a favorable prognostic factor in early stage non-small cell carcinoma. Lung Cancer. 2015;89:181–188. doi: 10.1016/j.lungcan.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Patel SP, Kurzrock R. PD-L1 Expression as a predictive biomarker in cancer immunotherapy. Mol Cancer Ther. 2015;14:847–856. doi: 10.1158/1535-7163.MCT-14-0983. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


