Abstract
Chronic cerebral hypoperfusion (CCH) has become a crucial factor contributing to neurological disorders and cognitive deficits. Olfactory ensheathing cells (OECs) transplantation has been widely used to repair central nerve systems (CNS) injury, however, whether this intervention has therapeutic effects on CCH-induced cognitive dysfunction and brain damages is still unknown. In this study, we sought to determine the potential therapeutic effects of OECs transplantation on CCH. Two days after the establishment of 2VO rat model, OECs or its medium transplantation were performed via intrastriatal injection. In our study, OECs treatment significantly improved learning and memory in 2VO rats. Transplantation of OECs also significantly reduced brain cell death, neuroinflammation and oxidative stress. Mechanistically, transplantation of OECs increased the expression of nuclear factor-like 2 (Nrf2) and hemeoxygenase 1 (HO-1). Finally, treatment with Brusatol, a Nrf2 inhibitor, markedly abolished the neuroprotective effects of OECs on cognitive decline, oxidative stress, Nrf2 and HO-1 expression. These results demonstrated that OECs transplantation protected CCH-induced cognitive impairment and brain injury by suppressing neuroinflammation and oxidative stress. The activation of Nrf2/HO-1 signaling pathway may contribute to the neuroprotection of OECs transplantation in CCH.
Keywords: Chronic cerebral hypoperfusion, OECs transplantation, Nrf2/HO-1 pathway
Introduction
Vascular dementia (VD) has become a crucial factor contributing to neurological disorders and cognitive decline worldwide especially in ageing [1]. More than 20% of dementia are responsible for chronic cerebral hypoperfusion (CCH) [2]. CCH can injury learning and memory mainly through inducing oxidative stress, neuronal cell death/apoptosis, blood-brain barrier breakdown and neuroinflammation [3-5]. However, the pathogenesis of CCH is still not yet entirely clear and there is no effective treatment for CCH-induced cognitive deficits [6]. Thus, it is of great importance to develop novel therapeutic strategies for CCH.
Oxidative stress has been considered as one of the major risk factors in the pathogenesis of vascular dementia during CCH [7]. Oxidative stress induced by CCH can lead to neuronal cell death and brain damages [8]. Therefore, inhibiting of oxidative stress may be a potential therapeutic target for preventing cognitive decline induced by CCH. Nuclear factor erythroid 2-related factor 2 (Nrf2) as a well-known phage II detoxifying enzyme acts as an essential regulator to prevent oxidative stress. Activated Nrf2 can translocate into the nucleus, bind to the antioxidant response elements (AREs) and regulate the expression of antioxidant including quinone oxidoreductase 1 (NQO1) and hemeoxygenase 1 (HO-1) [9]. Previous studies have demonstrated that the Nrf2 pathway can be impaired in the brain during CCH progression [10]. Nrf2 acts as a promising target to alleviate oxidative damages and cognitive impairment caused by CCH [7]. Thus, targeting Nrf2 signaling pathway attracts great attentions for development of novel therapeutic strategies for CCH.
Olfactory ensheathing Cells (OECs) are unique glial cells existing in peripheral nerve systems (PNS) and central nerve systems (CNS). OECs can secrete many neurotrophins like brain-derived neurophic factor (BDNF), glial cell-line derived neurotrophic factor (GDNF) and nerve growth factor (NGF) [11]. Due to the capacity of regeneration, OECs transplantation has been widely used to repair neural injuries. Li et al. demonstrated that transplanted OECs repaired rat corticospinal tract [12]. Intracerebral transplantation of OECs improved neurological dysfunction induced by cerebral ischemia [13]. OECs transplantation also has been used in the treatment of spinal cord injury and neurodegenerative diseases [14,15]. However, the effects of OECs transplantation on CCH-induced cognitive deficits and brain damages have not yet been investigated.
In the present study, we conducted a straightforward study to determine whether OECs transplantation could improve cognitive function and brain damages in a 2VO rat model and to explore whether OECs transplantation can protect against CCH-induced brain damages via the Nrf2/HO-1 pathway.
Materials and methods
Animals
Male Sprague-Dawley rats (180-200 g) were purchased from the Experimental Animal Center of Lukang (Jining, China) and housed at a controlled temperature (21-23°C) and humidity (50-60%) with a 12 h light-dark cycle. All animal operative protocols were approved by the Institutional Animal Care and Use Committee of Taishan Medical University and conducted in accordance with the National Institutes of Health (NIH) guideline for the Care and Use of Laboratory Animals. After acclimatization for one week, rats were randomly divided into 6 groups: Sham, 2VO, 2VO+medium (2VO+Me), 2VO+OECs (2VO+OECs), 2VO+OECs+brusatol (2VO+OECs+Bru) and 2VO+brusatol (2VO+Bru). Chronic cerebral hypoperfusion model were generated as previously described [16]. Briefly, rats were anesthetized by 10% chloralhydrate (300 mg/kg, i.p.), the bilateral common carotid arteries were carefully separated from the cervical sympathetic and vagal nerves, and then were doubly ligated with silk sutures. The same procedure was conducted in the Sham group, but without carotid arteries occlusion. During the operation, rectal temperature of rats were maintained at 37°C using a heating lamp. Two days later, intrastriatal injection of the medium with or without OECs was performed. The Nrf2 inhibitor brusatolin 2VO+OECs+Bru and 2VO+Bru groups was administered at 3 µmol/kg/day by gavage from the day of transplantation until the day of euthanasia. After 3 weeks treatment, morris water maze (MWM) test was performed and the rats were sacrificed to obtain the brain tissues for further analysis.
Reagents
Brusatol (purity >98%) was purchased from Adamas Reagent Co., Ltd (Shanghai, China). Antibodies against p75 nerve growth factor receptor (NGFRp75), Nrf2, HO-1, GFAP and Phospho-NF-κB p65 were purchased from Abcam (Cambridge, MA, USA). Antibodies against Bax, Bcl-2, cleaved caspase-9, cleaved case-pase-PARP, and Lamin B were obtained from Cell Signaling Technology (Beverly, MA, USA). TNF-α and IL-6 Elisa kits were purchased from CusabioBiotech Co., Ltd. (Wuhan, China). Tunel staining apoptosis kits were obtained from Roche (Mannheim, Germany). The commercial kits to determine CuZu/Mn-superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA) and H2O2 were purchased from Beyotime Biotech Co., Ltd. (Haimen, China).
OECs culture
OECs were obtained as described previously [17]. Briefly, postnatal 3-5 days SD rats were sacrificed and the olfactory bulbs were dissected and minced. The tissue was treated with 0.125% trypsin at 37°C in a 5% CO2 incubator for 30 min and then stopped by Dulbecco’s modified Eagle medium (DMEM)/F12 medium supplemented with 10% fetal calfserum. After centrifugation, the cells were incubated with the same medium plus 2 mM glutamine. The OECs were identified by immunohistochemistry staining with NGFRp75 antibody to determine cell purity. After a third passage, the OECs were collected and adjusted to 1.0×106 cells/µl for transplantation.
OECs transplantation
For transplantation, the OECs of passage three were trypsinized and centrifuged at 1,500 rpm for 10 min. After washing with PBS three times, the cell density was adjusted to 1.0×106 cells/μl before transplantation. Two days after the establishment of 2VO model, rats were anaesthetized with chloralhydrate (300 mg/kg i.p.) and placed on a stereotaxic apparatus. The rats in the 2VO+OECs and 2VO+OECs+Brugroups received 2 µl cell suspension (containing 2.0×106 cells) via intrastriatal injection. The rats in 2VO+Me group were injected with a similar volume of culture medium only at the same site.
Behavioral tests
Morris water maze apparatus were used to evaluate rat spatial memory ability. Briefly, the circular pool was filled with water (22-23°C) to 40 cm deep and rendered opaque by the addition of white, non-toxic paint. A circular platform was placed 2 cm below the water surface in the center of a specific quadrant of the pool and remained unchanged for all trials. The day before the experiment, all rats were released with their faces into the water in order to adapt to the environment. For each trial, the rats were given 60 s to locate the escape platform. If the rats could search the platform and stand on it, the performance point was recorded as its escape latency. On the contrary, if not, its performance was noted as 60 s. The escape latency was recorded and compared as task performance. Meantime, a probe trial was performed 24 h after the last training trailin which the circular platform was removed and the animals were allowed to swim for 60 s. The number of target annulus crossovers and the time spent in the target quadrant were measured.
Neurological severity score
The levels of neurological deficits were evaluated using the neurological severity score (NSS) and recorded after the medium with or without OECs transplantation 1, 2 and 3 weeks. The lowest score is 0 points and the highest score is 18 points. Normal rats recorded 0 points, mild injury recorded 1-6 points, moderate injury recorded 7-12 points, and severe injury recorded 13-18 points. Each rat was observed and recorded by two observers who were blinded to the treatment of rats and the average value was taken.
Measurement of oxidative stress
Oxidative stress was evaluated by determination of CuZu/Mn-superoxide dismutase (SOD), glutathione (GSH), malondialdehyde (MDA) and H2O2 levels with commercial kits. All assays were measured according to the manufacturer’s instructions.
Tunel staining
Deparaffinized brain sections as described above were used for cell apoptosis analysis. TUNEL staining was performed using an apoptosis kit. Sections were counterstained with hematoxylin, mounted using Permount and observed under the fluorescence microscope (Leica, Microsystems, Berlin, Germany).
Immunohistochemistry
The brain tissues were fixed in 4% paraformaldehyde at 4°C overnight. For immunochemistry experiments, the tissues were dehydrated in 30% sucrose for 72 h. Frozen 40 µm thick sections were blocked by 8% normal goat serum for 4 h. Then, the sections were incubated in primary antibodies at 4°C overnight. After being washed by PBS, the sections were incubated with horseradish peroxidase-conjugated secondary antibody. Staining was visualized using the substrate DAB. Images were obtained by digital microscope (Leica, Microsystems, Berlin, Germany).
Immunoblotting
Proteins were extracted from brain tissues, briefly, the brain tissues were homogenized in ice-cold radio immunoprecipitation assay (RIPA) buffer for 15 min, followed by centrifugation at 12000×g at 4°C for 30 min. Protein concentrations of the extracts were measured by BCA assay. The protein was electrophoresed on 8-12% sodium dodecyl sulfate polyacrylamide gelelectrophoresis (SDS-PAGE), and then transferred to nitrocellulose membranes. The membrane was incubated at 4°C overnight in 5% non-fat milk in TBST and then incubated with primary antibody. The membranes were then probed with the appropriate secondary antibodies at room temperature for 2 h and washed by TBST for three times. The protein bands were detected using an odyssey Infrared Fluorescence Imaging System.
Statistical analysis
All data were expressed as mean ± SEM and analyzed using the SPSS software version 19.0. Statistical analysis was performed by the One-way analysis of variance (ANOVA) followed by Tukey’s post-hoc test. P<0.05 was considered as statistically significant.
Results
Characterization of OECs in vitro culture
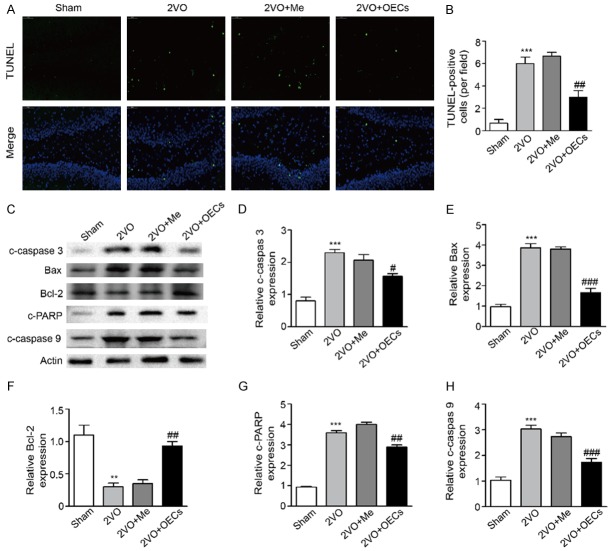
Before transplantation, the purity of olfactory ensheathing cells obtained from the rats’ olfactory bulbs used in this study was determined by assessing the proportion of NGFRp75-positive cells. As shown in Figure 1, after a third passage, the cell cultures consisted of nearly 90% NGFRp75-positive cells. Consistent with OEC morphology, the NGFRp75-positive OECs were characterized by primarily fusiform or spindle forms.
Figure 1.
The purity of olfactory ensheathing cells used in our study was determined by assessing the proportion of NGFRp75-positive cells. A. The immunocytochemical staining for NGFRp75 was used to identify the purity of OECs before transplantation. B. Quantitation of NGFRp75-positive cells of passage three. Data were expressed as mean ± SEM.
OECs transplantation improved CCH-induced cognitive dysfunction
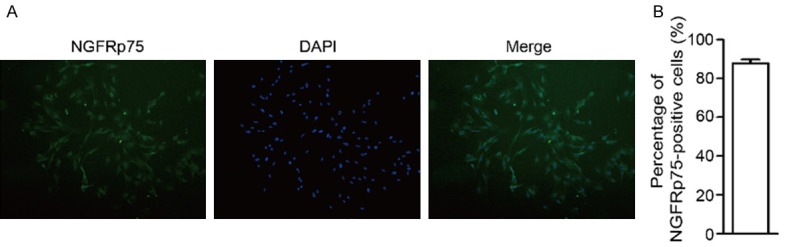
To examine whether OECs transplantation attenuated CCH-induced cognitive decline, we conducted the OECs treatment for 3 weeks followed by morris water maze test. As shown in Figure 2A and 2C, the escape latencies were decreased progressively in all groups during the five training days without affecting swimming speed. However, compared with 2VO group, the escape latencies in 2VO+OECs group were significantly restored. Meanwhile, the spatial memory of four groups were assessed by the probe trial performed at 24 h after the last training session. The number of crossing the platform in 2VO group obviously decreased as compared to the Sham group, while OECs transplantation markedly increased the number of crossing compared with 2VO group (Figure 2D). The probe trial test also showed that the rats spent more time in the target quadrant under OECs treatment (Figure 2E). Furthermore, the NSS of the rats in 2VO group was significantly higher than that in the sham group. At 2 and 3 weeks after transplantation, the scores of the OECs treatment group was lower than 2VO and 2VO+Me groups (Figure 2F). Collectively, these results suggested that OECs transplantation significantly ameliorated CCH-induced cognitive dysfunction.
Figure 2.
Effects of OECs transplantation on the performance of rats in morris water maze after OECs treatment. A. Latency to reach platform of four groups on the first 5 training days. B. Representative swim traces of the rats in morris water maze test on the fifth day. C. Swimming speeds were measured in the four groups. D. The number of crossing the platform of four groups. E. Time spent in the target quadrant of four groups. F. The neurological severity score of the rats after transplantation with or without OECs in four groups. Data were expressed as mean ± SEM (n = 5 per group). **P<0.01 vs. the Sham group, ***P<0.001 vs. the Sham group, ##P<0.01 vs. the 2VO group, ###P<0.001 vs. the 2VO group.
OECs transplantation alleviated CCH-induced apoptosis
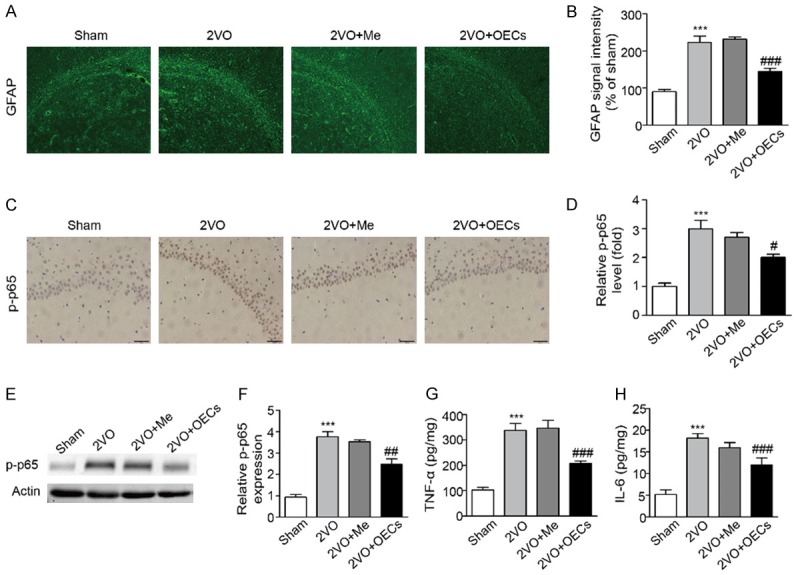
To ascertain the protective role of OECs transplantation on CCH-induced brain damages. We studied the influence of OECs transplantation on brain cell death. Tunel assay showed that there was a significant Tunel-positive staining in the brain in 2VO and 2VO+Me groups while the number of Tunel-positive cells in the 2VO+OECs group were significantly lower (Figure 3A and 3B). Meanwhile, the apoptosis related proteins Bax, Bcl-2, cleaved caspase-3, cleaved casepase-9 and cleaved caspase-PARP in the brain were detected by western blot. Compared with the sham groups, 2VO treatment significantly decreased Bcl-2 as well as increased Bax, cleaved caspase-3, cleaved casepase-9 and cleaved caspase-PARP expression, whereas OECs transplantation effectively reversed these changes in 2VO+OECs group (Figure 3). These results manifested that OECs transplantation effectively reduced cell death in rat 2VO model.
Figure 3.
Effects of OECs transplantation on apoptosis in the brain. Tunel-positive staining was performed in 2VO-treated rats. Apoptosis associated proteins cleaved caspase-3, Bax, Bcl-2, cleaved caspase-PARP and cleaved casepase-9 were measured by western blot analysis. A. Representative images of tunel-positive cells to measure apoptotic cells. B. Quantitative analysis of tunel-positive cells. C. Apoptosis related proteins cleaved caspase-3, Bax, Bcl-2, cleaved caspase-PARP and cleaved casepase-9 in the brain were detected by western blot. D. Densitometric analysis of relative cleaved caspase-3 protein expression. E. Densitometric analysis of relative Bax protein expression. F. Densitometric analysis of relative Bcl-2 protein expression. G. Densitometric analysis of relative cleaved caspase-PARP protein expression. H. Densitometric analysis of relative cleaved casepase-9 protein expression. Data were expressed as mean ± SEM (n = 5 per group). **P<0.01 vs. the Sham group, ***P<0.001 vs. the Sham group, ##P<0.01 vs. the 2VO group, ###P<0.001 vs. the 2VO group.
OECs transplantation alleviated CCH-induced neuroinflammation
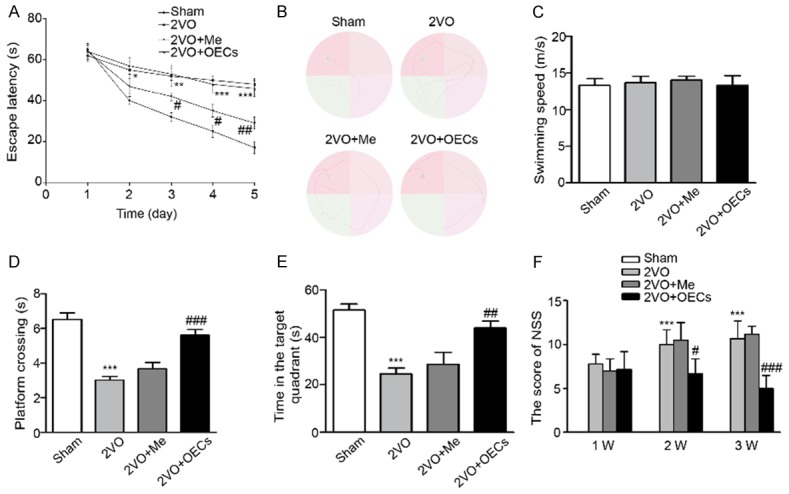
To assess the effects of OECs transplantation on neuroinflammation caused by CCH, we detected astroglial activation and inflammatory factors in brain tissues. As shown in Figure 4, compared with the 2VO group, the expressions of GFAP and p-p65 in the 2VO+OECs group significantly decreased in 2VO+OECs group. p-p65 immunoblot confirmed this result (Figure 4E). Meanwhile, the expression of inflammatory-associated factors TNF-α and IL-6 triggered by CCH were significantly decreased in 2VO+OECs group (Figure 4G and 4H). These results manifested that OECs transplantation alleviated neuroinflammation in rat 2VO model.
Figure 4.
Effects of OECs transplantation on neuroinflammation in 2VO-treated rats. Neuroinflammation related proteins GFAP, p-p65, TNF-α and IL-6 were detected in four groups. A. Representative images of GFAP expression to measure astroglial activation. B. Quantitative analysis of GFAP expression to measure astroglial activation. C. Representative images of p-p65 expression in four groups. D. Quantitative analysis of p-p65 expression in four groups. E. Neuroinflammation related proteins p-p65 in the brain were detected by western blot. F. Densitometric analysis of relative p-p65 protein expression. G. Inflammatory cytokine of TNF-α in the brain were measured by Elisa kit. H. Inflammatory cytokine of IL-6 in the brain were measured by Elisa kit. Data were expressed as mean ± SEM (n = 5 per group). **P<0.01 vs. the Sham group, ***P<0.001 vs. the Sham group, ##P<0.01 vs. the 2VO group, ###P<0.001 vs. the 2VO group.
OECs transplantation inhibited oxidativestress and activated Nrf2/HO-1 pathway in 2VO rats
Considering the crucial role of oxidativestress in the progress of CCH [7]. The effects of OECs transplantation on SOD activity, MDA, H2O2 and GSH contents in the brain were measured. Compared with the Sham group, the SOD activity and GSH concentration in 2VO group were significantly decreased, whereas the levels of MDA and H2O2 were obviously increased. However, OECs transplantation restored activities of SOD and GSH and significantly decreased MDA and H2O2 levels while the medium transplantation has little effects on these oxidative indicators (Figure 5). To investigate the molecular mechanisms behind the neuroprotection of OECs transplantation against CCH-induced brain injury, we then investigated the protein expression of HO-1 and Nrf2 in the brain. As shown in Figure 5E, HO-1 and Nrf2 expression were significantly decreased in 2VO group, whereas OECs transplantation increased the HO-1 and Nrf2 expression. These findings indicated that OECs transplantation reduced CCH-induced brain injury partly by activating Nrf2/HO-1 pathway.
Figure 5.
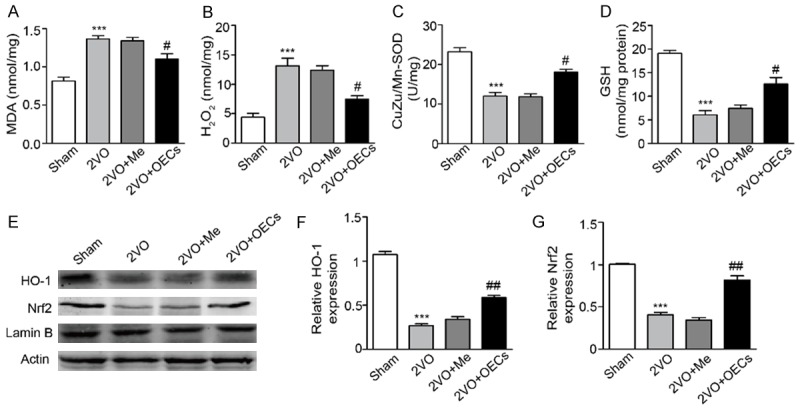
Effects of OECs transplantation on brain oxidative stress and Nrf2/HO-1 signaling pathway in 2VO-treated rats. A-D. Oxidative parameters including MDA, H2O2, CuZn/Mn-SOD and GSH were determined in brain tissues. E. Protein expression of HO-1 and Nrf-2 were determined by western blot. F. Densitometric analysis of relative HO-1 protein expression. G. Densitometric analysis of relative Nrf-2 protein expression. Data were expressed as mean ± SEM (n = 5 per group). ***P<0.001 vs. the Sham group, #P<0.05 vs. the 2VO group, ##P<0.01 vs. the 2VO group.
Brusatol treatment reversed neuroprotective of OECs transplantation in 2VO rats
To further study the mechanism of the neuroprotective role of OECs transplantation, rats in 2VO+OECs+Bru and 2VO+Bru groups were treated with the Nrf2 inhibitor Brufrom the day of OECs transplantation until the day of sacrifice. As shown in Figure 6A and 6C, in morris water maze test, we found that compared with 2VO group, OECs transplantation significantly decreased the escape latencies and increased the time in the target quadrant, whereas the OECs-mediated protection was partly blocked by Bru in 2VO+OECs+Bru group. As oxidative stress has been considered as one of the major risk factor of CCH-induced brain injury, we examined oxidative stress indicators. As shown in Figure 6D and 6E, compared with 2VO+OECs group, the brain SOD activity and MDA concentration were both abolished in 2VO+OECs+Bru group. Meanwhile, Bru treatment also reversed increasing expression of Nrf2 and HO-1 in the brain induced by OECs transplantation (Figure 6F). Taken together, these results indicated that Nrf2/HO-1 pathway is involved in the neuroprotective effects of OECs transplantation on CCH-induced cognitive deficits and brain damages.
Figure 6.
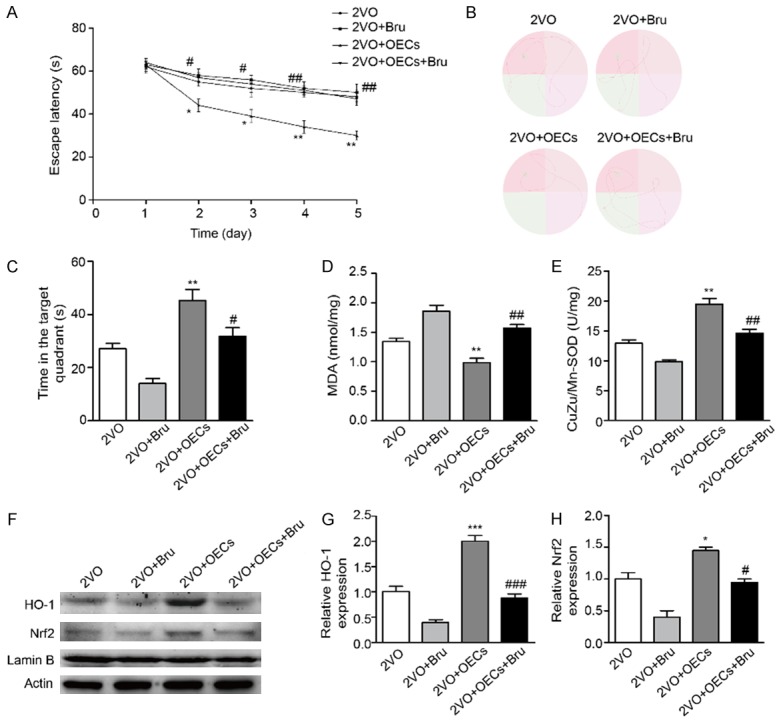
Treatment with Bru abolished OECs transplantation induced increase of Nrf2/HO-1 in 2VO-treated rats. A. Latency to reach platform of four groups on the first 5 training days. B. Representative swim traces of the rats in morris water maze test on the fifth day. C. Time spent in the target quadrant of four groups. D, E. Oxidative parameters including MDA and CuZn/Mn-SOD were determined in brain tissues. F. Protein expression of HO-1 and Nrf-2 were determined by western blot. G. Densitometric analysis of relative HO-1 protein expression. H. Densitometric analysis of relative Nrf-2 protein expression. Data were expressed as mean ± SEM (n = 5 per group). *P<0.05 vs. the 2VO group, **P<0.01 vs. the 2VO group, #P<0.05 vs. the 2VO+OECs group, ##P<0.01 vs. the 2VO+OECs group.
Discussion
This study is the first time to test the therapeutic potential of OECs transplantation against CCH-induced cognitive deficits and brain damages. We found that OECs transplantation significantly decreased escape latencies, increased time spent in the target quadrant and crossing the platform location. Moreover, transplantation of OECs could decrease neural cell death, neuroinflammation and oxidative stress. Importantly, we demonstrated that OECs transplantation upregulated Nrf2 and HO-1 protein expression, however, this protection was partly abolished by Nrf2 inhibitor. Based on these results, we proposed that OECs transplantation exerted neuroprotective effects on CCH-induced brain injury and cognitive deficits through Nrf2/HO-1 pathway (Figure 7).
Figure 7.
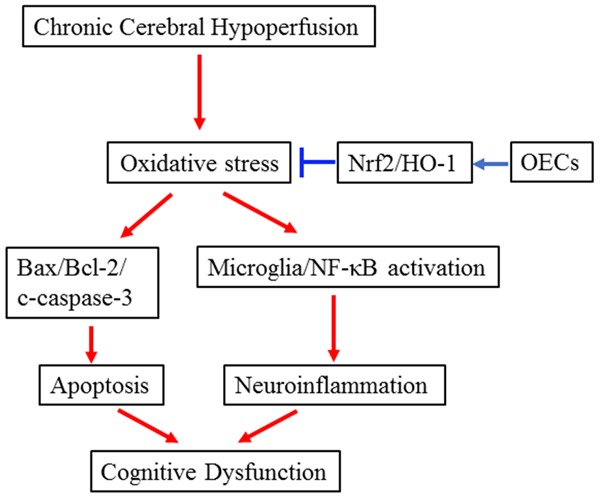
The schematic diagram of OECs transplantation on CCH-induced cognitive dysfunction and brain damages.
Olfactory ensheathing cells (OECs), a unique type of macroglial cells, have the ability to promote remyelination, secrete multiple neurotrophins and promote regeneration [18]. Several studies have demonstrated that OECs transplantation was an effective therapy protecting against spinal cord injury [19]. OECs treatment significantly improved neurological dysfunction in murine models of stroke [13] and promoted the recovery of neurological functions in rats with traumatic brain injury [20]. However, the protective effects of OECs transplantation on CCH-induced cognitive impairment was little demonstrated. To investigated the effects of OECs transplantation on CCH-induced brain injury, rat 2VO model in this study was established. In the present study, we found that OECs transplantation significantly restored escape latencies, increased time spent in the target quadrant and crossing the platform location. These results demonstrated that OECs transplantation effectively alleviated CCH-induced spatial learning and memory impairment.
Many studies have demonstrated that CCH could induce neuronal cell death and neuroinflammation [3,5]. Apoptosis and neuroinflammation are also closely related to CCH-induced cognitive decline [21,22]. Previous study has found that transplantation of OECs downregulated the apoptotic molecules in rats with traumatic brain injury [20]. OECs-conditioned medium ameliorated Aβ25-35-induced neuronal damages by inhibiting the mitochondria-mediated apoptotic pathway [23]. Transplantation of OECs significantly decreased the expression of GFAP and pro-inflammatory factors in the spinal cord [24]. Liu et al. also demonstrated that OECs transplantation exerted anti-inflammatory mechanism in traumatic brain injury [25]. In the present study, we found that OECs transplantation significantly decreased CCH-induced apoptosis as well as inhibited the activation of astrocytes and NF-κB followed by the decreased TNF-α and IL-6 expression in the brain, suggesting that OECs transplantation effectively improved CCH-induced brain damages.
Oxidative stress has been regarded as an important factor in the progression of CCH. The levels of malondialdehyde (MDA) and H2O2 were increased obviously while glutathione (GSH) and superoxide dismutase (SOD) were decreased significantly during CCH [7]. In our study, OECs treatment significantly reduced MDA and H2O2 levels as well as increased GSH and SOD activities in rat 2VO models. Our results suggested that oxidative stress in the brain induced by CCH may be alleviated by OECs treatment. Consistent with our study, Liu et al. also found that OECs transplantation enhanced the antioxidant capability in the hippocampus of COpoisoned rats [15]. HO-1, a rate-limiting enzyme in heme catabolism, plays a critical role in regulating oxidative stress. In our study, we investigated the effects of OECs transplantation on HO-1 and Nrf2 expression. The upregulation of HO-1 and Nrf2 protein expression in the brain by OECs transplantation is apparently in line with the inhibitory action of OECs transplantation on brain oxidative stress. To further investigate the role of Nrf2/HO-1 signaling pathway in CCH, we used Bru, a specific Nrf2 inhibitor, to elucidate the underlying mechanisms. As expected, enhanced expression of Nrf2 and HO-1 in the brain caused by OECs transplantation was partly abolished by Bru. In addition, Bru treatment diminished the neuroprotective effects of OECs transplantation, as evidenced by increased escape latencies, upregulated MDA levels and decreased SOD activities. Therefore, our results indicated that the activation of Nrf2/HO-1 signaling pathway is involved in the protective effects of OECs transplantation on CCH-induced brain damages.
In summary, the present study was first time to demonstrate that OECs transplantation protected against CCH-induced cognitive impairment. OECs transplantation inhibited apoptosis, decreased neuroinflammation and increased antioxidant capability in 2VO rat models. The underlying molecular mechanism may relate to the activated Nrf2/HO-1 signaling pathway.
Acknowledgements
This work was supported by the Natural Science Foundation of China (Grant No. 81471212 and No. 81271275), the Natural Science Foundation of Shandong (Grant No. ZR2013FL031), and the Institutes of Higher Education Science and Technique Foundation of Shandong Province (Grant No. J15LE12).
Disclosure of conflict of interest
None.
References
- 1.Mascalchi M, Ginestroni A, Toschi N, Poggesi A, Cecchi P, Salvadori E, Tessa C, Cosottini M, De Stefano N, Pracucci G, Pantoni L, Inzitari D, Diciotti S. The burden of microstructural damage modulates cortical activation in elderly subjects with MCI and leuko-araiosis. A DTI and fMRI study. Hum Brain Mapp. 2014;35:819–830. doi: 10.1002/hbm.22216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Qi Q, Xu J, Lv P, Dong Y, Liu Z, Hu M, Xiao Y, Jia Y, Jin W, Fan M, Zhang D, Meng N. DL-3-nbutylphthalide alleviates vascular cognitive impairment induced by chronic cerebral hypoperfusion by activating the Akt/Nrf2 signaling pathway in the hippocampus of rats. Neurosci Lett. 2017;672:59–64. doi: 10.1016/j.neulet.2017.11.051. [DOI] [PubMed] [Google Scholar]
- 3.Jin W, Jia Y, Huang L, Wang T, Wang H, Dong Y, Zhang H, Fan M, Lv P. Lipoxin A4 methyl ester ameliorates cognitive deficits induced by chronic cerebral hypoperfusion through activating ERK/Nrf2 signaling pathway in rats. Pharmacol Biochem Behav. 2014;124:145–152. doi: 10.1016/j.pbb.2014.05.023. [DOI] [PubMed] [Google Scholar]
- 4.Roman GC. Vascular dementia prevention: a risk factor analysis. Cerebrovasc Dis. 2005;20(Suppl 2):91–100. doi: 10.1159/000089361. [DOI] [PubMed] [Google Scholar]
- 5.Farkas E, Luiten PG, Bari F. Permanent, bilateral common carotid artery occlusion in the rat: a model for chronic cerebral hypoperfusion-related neurodegenerative diseases. Brain Res Rev. 2007;54:162–180. doi: 10.1016/j.brainresrev.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 6.Scherr M, Trinka E, Mc Coy M, Krenn Y, Staffen W, Kirschner M, Bergmann HJ, Mutzenbach JS. Cerebral hypoperfusion during carotid artery stenosis can lead to cognitive deficits that may be independent of white matter lesion load. Curr Neurovasc Res. 2012;9:193–199. doi: 10.2174/156720212801619009. [DOI] [PubMed] [Google Scholar]
- 7.Zhao RR, Xu F, Xu XC, Tan GJ, Liu LM, Wu N, Zhang WZ, Liu JX. Effects of alpha-lipoic acid on spatial learning and memory, oxidative stress, and central cholinergic system in a rat model of vascular dementia. Neurosci Lett. 2015;587:113–119. doi: 10.1016/j.neulet.2014.12.037. [DOI] [PubMed] [Google Scholar]
- 8.Choi DH, Lee KH, Kim JH, Seo JH, Kim HY, Shin CY, Han JS, Han SH, Kim YS, Lee J. NADPH oxidase 1, a novel molecular source of ROS in hippocampal neuronal death in vascular dementia. Antioxid Redox Signal. 2014;21:533–550. doi: 10.1089/ars.2012.5129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y. Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett. 2005;579:3029–3036. doi: 10.1016/j.febslet.2005.04.058. [DOI] [PubMed] [Google Scholar]
- 10.Yang Y, Zhang J, Liu H, Zhang L. Change of Nrf2 expression in rat hippocampus in a model of chronic cerebral hypoperfusion. Int J Neurosci. 2014;124:577–584. doi: 10.3109/00207454.2013.863196. [DOI] [PubMed] [Google Scholar]
- 11.Wewetzer K, Verdu E, Angelov DN, Navarro X. Olfactory ensheathing glia and Schwann cells: two of a kind? Cell Tissue Res. 2002;309:337–345. doi: 10.1007/s00441-002-0607-y. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Field PM, Raisman G. Repair of adult rat corticospinal tract by transplants of olfactory ensheathing cells. Science. 1997;277:2000–2002. doi: 10.1126/science.277.5334.2000. [DOI] [PubMed] [Google Scholar]
- 13.Shyu WC, Liu DD, Lin SZ, Li WW, Su CY, Chang YC, Wang HJ, Wang HW, Tsai CH, Li H. Implantation of olfactory ensheathing cells promotes neuroplasticity in murine models of stroke. J Clin Invest. 2008;118:2482–2495. doi: 10.1172/JCI34363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu Q, Qin Q, Sun H, Zhong D, An R, Tian Y, Chen H, Jin J, Wang H, Li G. Neuroprotective effect of olfactory ensheathing cells cotransfected with Nurr1 and Ngn2 in both in vitro and in vivo models of Parkinson’s disease. Life Sci. 2018;194:168–176. doi: 10.1016/j.lfs.2017.12.038. [DOI] [PubMed] [Google Scholar]
- 15.Liu W, Zheng Q, Wang Y, Han X, Yuan L, Zhao M. Transplantation of olfactory ensheathing cells attenuates acute carbon monoxide poisoning-induced brain damages in rats. Neurochem Res. 2015;40:70–80. doi: 10.1007/s11064-014-1467-z. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Wu J, Gu J, Xiong Z, Wang F, Wang J, Wang W, Chen J. Baicalein improves cognitive deficits induced by chronic cerebral hypoperfusion in rats. Pharmacol Biochem Behav. 2007;86:423–430. doi: 10.1016/j.pbb.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal AK, Shukla S, Chaturvedi RK, Seth K, Srivastava N, Ahmad A, Seth PK. Olfactory ensheathing cell transplantation restores functional deficits in rat model of Parkinson’s disease: a cotransplantation approach with fetal ventral mesencephalic cells. Neurobiol Dis. 2004;16:516–526. doi: 10.1016/j.nbd.2004.04.014. [DOI] [PubMed] [Google Scholar]
- 18.Radtke C, Aizer AA, Agulian SK, Lankford KL, Vogt PM, Kocsis JD. Transplantation of olfactory ensheathing cells enhances peripheral nerve regeneration after microsurgical nerve repair. Brain Res. 2009;1254:10–17. doi: 10.1016/j.brainres.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 19.Ramon-Cueto A, Nieto-Sampedro M. Regeneration into the spinal cord of transected dorsal root axons is promoted by ensheathing glia transplants. Exp Neurol. 1994;127:232–244. doi: 10.1006/exnr.1994.1099. [DOI] [PubMed] [Google Scholar]
- 20.Wang YC, Xia QJ, Ba YC, Wang TY, Li NN, Zou Y, Shang FF, Zhou XF, Wang TH, Fu XM, Qi JG. Transplantation of olfactory ensheathing cells promotes the recovery of neurological functions in rats with traumatic brain injury associated with downregulation of bad. Cytotherapy. 2014;16:1000–1010. doi: 10.1016/j.jcyt.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Zhao W, Xu S, Peng Y, Ji X, Cao D, Li J, Liu B, Shi Q, Wang L, Wang X. Potassium 2-(1-hydroxypentyl)-benzoate improves learning and memory deficits in chronic cerebral hypoperfused rats. Neurosci Lett. 2013;541:155–160. doi: 10.1016/j.neulet.2013.01.053. [DOI] [PubMed] [Google Scholar]
- 22.Tsai TH, Sun CK, Su CH, Sung PH, Chua S, Zhen YY, Leu S, Chang HW, Yang JL, Yip HK. Sitagliptin attenuated brain damage and cognitive impairment in mice with chronic cerebral hypo-perfusion through suppressing oxidative stress and inflammatory reaction. J Hypertens. 2015;33:1001–1013. doi: 10.1097/HJH.0000000000000529. [DOI] [PubMed] [Google Scholar]
- 23.Fu QQ, Wei L, Sierra J, Cheng JZ, Moreno-Flores MT, You H. Olfactory ensheathing cell-conditioned medium reverts abeta25-35-induced oxidative damage in SH-SY5Y cells by modulating the mitochondria-mediated apoptotic pathway. Cell Mol Neurobiol. 2017;37:1043–1054. doi: 10.1007/s10571-016-0437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang J, Chen H, Duan Z, Chen K, Liu Z, Zhang L, Yao D, Li B. The effects of co-transplantation of olfactory ensheathing cells and schwann cells on local inflammation environment in the contused spinal cord of rats. Mol Neurobiol. 2017;54:943–953. doi: 10.1007/s12035-016-9709-5. [DOI] [PubMed] [Google Scholar]
- 25.Liu SJ, Zou Y, Belegu V, Lv LY, Lin N, Wang TY, McDonald JW, Zhou X, Xia QJ, Wang TH. Co-grafting of neural stem cells with olfactory en sheathing cells promotes neuronal restoration in traumatic brain injury with an anti-inflammatory mechanism. J Neuroinflammation. 2014;11:66. doi: 10.1186/1742-2094-11-66. [DOI] [PMC free article] [PubMed] [Google Scholar]