Blood cryptococcal antigen (CrAg) titers are associated with concurrent subclinical cryptococcal meningitis in at least a third of CrAg-positive patients with advanced human immunodeficiency virus infection, which may increase mortality rates. Blood CrAg titers can guide management in this population.
Keywords: Meningitis, Cryptococcal, Cryptococcosis, Diagnosis, Mass screening
Abstract
Background
High mortality rates among asymptomatic cryptococcal antigen (CrAg)–positive patients identified through CrAg screening, despite preemptive fluconazole treatment, may be due to undiagnosed cryptococcal meningitis.
Methods
Symptoms were reviewed in CrAg-positive patients identified by screening 19233 individuals with human immunodeficiency virus infection and CD4 cell counts <100/µL at 17 clinics and 3 hospitals in Johannesburg from September 2012 until September 2015, and at 2 hospitals until June 2016. Cerebrospinal fluid samples from 90 of 254 asymptomatic patients (35%) and 78 of 173 (45%) with headache only were analyzed for cryptococcal meningitis, considered present if Cryptococcus was identified by means of India ink microscopy, culture, or CrAg test. CrAg titers were determined with stored blood samples from 62 of these patients. The associations between blood CrAg titer, concurrent cryptococcal meningitis, and mortality rate were assessed.
Results
Cryptococcal meningitis was confirmed in 34% (95% confidence interval, 25%–43%; 31 of 90) of asymptomatic CrAg-positive patients and 90% (81%–96%; 70 of 78) with headache only. Blood CrAg titer was significantly associated with concurrent cryptococcal meningitis in asymptomatic patients (P < .001) and patients with headache only (P = .003). The optimal titer for predicting cryptococcal meningitis was >160 (sensitivity, 88.2%; specificity, 82.1%); the odds ratio for concurrent cryptococcal meningitis was 34.5 (95% confidence interval, 8.3–143.1; P < .001).
Conclusions
About a third of asymptomatic CrAg-positive patients have concurrent cryptococcal meningitis. More effective clinical assessment strategies and antifungal regimens are required for CrAg-positive patients, including investigation for cryptococcal meningitis irrespective of symptoms. Where it is not possible to perform lumbar punctures in all CrAg-positive patients, blood CrAg titers should be used to target those most at risk of cryptococcal meningitis.
Cryptococcal antigenemia is strongly predictive of subsequent cryptococcal meningitis in human immunodeficiency virus (HIV)–infected adults with CD4+ T-lymphocyte (CD4 cell) counts <100/µL [1, 2]. This forms the basis of a “screen-and-treat” approach to early detection, whereby HIV-infected patients with CD4 cell counts <100/µL are tested for cryptococcal antigen (CrAg) in blood (whole blood, plasma, or serum) before commencing antiretroviral therapy (ART). If the CrAg test is positive and patients have no signs or symptoms of meningitis, they are treated with a preemptive course of fluconazole; 800 mg/d for 2 weeks, followed by 400 mg/d for 8 weeks and then 200 mg/d pending immune reconstitution with ART. This strategy is included in World Health Organization (WHO) management guidelines for patients with advanced HIV infection [3–5] and has been adopted as recommended practice in several countries [6, 7]. However, the optimal management of patients who have cryptococcal antigenemia and do not have overt clinical evidence of meningitis is yet to be determined.
Evidence in recent years suggests that the CrAg screen-and-treat approach reduces the incidence of subsequent cryptococcal meningitis and death [8–10]. However, most studies have found a persistent and independent association between cryptococcal antigenemia and mortality, despite preemptive fluconazole therapy [8–12], implying that CrAg-positive patients may not be adequately investigated and treated under current guidelines.
A proportion of CrAg-positive patients may have meningeal infection with Cryptococcus without exhibiting any signs or symptoms, or reporting headache only [10, 13]. These cases of “subclinical cryptococcal meningitis” are likely to be underrecognized, because WHO guidelines do not specifically recommend lumbar punctures (LPs) among asymptomatic CrAg-positive patients [4], and where the procedure is routinely offered, LP uptake is poor [9, 10, 14]. Furthermore, many resource-limited settings where CrAg screening is now being implemented do not have access to the required equipment or health workers with the ability to perform LPs at the screening site [15].
Headache is commonly and inconsistently reported among patients with advanced HIV infection [16–19], and prior studies have found having a headache to be a poor predictor of cryptococcal meningitis in CrAg-positive patients [10, 19]. Physicians therefore frequently omit to perform LPs if headache occurs without any other neurological signs or symptoms [9, 10, 14, 17].
Studies have shown an association between blood CrAg titer and the development of subsequent cryptococcal meningitis and/or mortality in CrAg-positive patients [1, 11, 19, 20]. However, the relationship between blood CrAg titer and concurrent cryptococcal meningitis has not yet been systematically investigated. We performed a cross-sectional study (1) to establish the prevalence of subclinical and minimally symptomatic (patients with headache only) cryptococcal meningitis and (2) to determine whether blood CrAg titer was predictive of concurrent cryptococcal meningitis in CrAg-positive individuals identified during routine screening, who were asymptomatic or reported headache only, and who underwent LP. A subsequent prospective cohort study assessed the relationship between CrAg titer and concurrent cryptococcal meningitis, and between CrAg titer and death within 6 months.
METHODS
The studies were conducted at 17 primary care clinics and 3 hospitals in Johannesburg from September 2012 until September 2015, and at 2 hospitals (Helen Joseph and Tambo Memorial Hospital) until June 2016. Ethics approval was granted by the University of the Witwatersrand and the London School of Hygiene and Tropical Medicine; the study protocol was also cleared by the Centers for Disease Control and Prevention. All HIV-infected individuals presenting to these facilities during the study period with a CD4 cell count <100/µL had a qualitative CrAg test performed in the laboratory, performed using a lateral flow assay (ImmunoMycologics) on remnant ethylenediaminetetraacetic acid–containing blood from the CD4 cell count sample. If they were CrAg positive, individuals aged >16 years were invited to participate in the study.
If patients were enrolled, information (including the presence of headache or confusion) was collected from them, or from their medical records, by professional study nurses using standardized structured questionnaires. CrAg-positive patients were managed by their usual health providers, who received regular training on national guidelines for CrAg screening and treatment [6], delivered by study investigators. The recommended management was initial assessment for any symptoms or signs of meningitis and, if these were present, urgent referral for investigation of cryptococcal meningitis with LP and subsequent treatment as appropriate [6]. If symptoms and signs of meningitis were absent, the guidelines suggested that LP should be considered “if available.” If LP was not performed, or if it excluded a diagnosis of cryptococcal meningitis, a course of preemptive fluconazole for ≥12 months was recommended, following WHO guidance [4].
We carried out a cross-sectional study to establish the prevalence (using exact binomial confidence intervals [CIs]) of concurrent cryptococcal meningitis, and the relationship with blood CrAg titer, in participants with neither headache nor confusion, and those with headache only, who underwent LP within a month of review of their CrAg test result. Sample sizes of 88 and 62 participants were required to determine estimated prevalences of concurrent cryptococcal meningitis of 35% and 80% in the asymptomatic and headache-only groups, respectively, with 10% precision. Concurrent cryptococcal meningitis was defined as occurring in those who had Cryptococcus identified by means of cerebrospinal fluid (CSF) microscopy with India ink, fungal culture, and/or CSF CrAg testing.
CrAg-positive whole blood samples were sent from the diagnostic facilities to the reference laboratory during the study period, and were stored at −70°C. CrAg titers were determined using the CrAg lateral flow assay on serially diluted samples of thawed unspun whole blood, using manufacturer’s instructions. Titers were read manually by 3 investigators, who were blinded to the CSF results and to each others’ readings. Serially diluted blood samples were tested until the next reading was negative. If readings were discordant, the higher reading was used as long as there was agreement within a double dilution.
The association between blood CrAg titer and concurrent cryptococcal meningitis was tested using a Mann-Whitney U test for the asymptomatic, headache-only, and combined groups. A receiver operating characteristic curve was used to establish an optimal “cutoff” titer that could be used to screen for concurrent cryptococcal meningitis, and the sensitivity and specificity of this titer was determined. The cutoff titer was then used to estimate the odds ratio (OR) of concurrent cryptococcal meningitis with a high blood CrAg titer, and other variables were assessed for their association with cryptococcal meningitis.
Prospective data on ART, antifungal treatment and mortality were obtained from clinic and phone-call follow-up and/or review of clinical and laboratory records for up to 3 years. Progression to death within 6 months in those with or without concurrent cryptococcal meningitis and with high or low blood CrAg titer was examined using Kaplan-Meier estimates and a multivariate Cox proportional hazards model. The following variables were considered potential confounders: age, sex, baseline CD4 cell count, ART status, headache, and receipt of any antifungal therapy after LP. Assuming a 15% risk of death among those with a blood CrAg titer ≤160 (based on a previous study [20]), with a 2-sided significance of 95% and power of 80%, a sample size of ≥120 individuals was required in each group to detect a difference in mortality rate of ≥15% among those with blood CrAg titer >160.
RESULTS
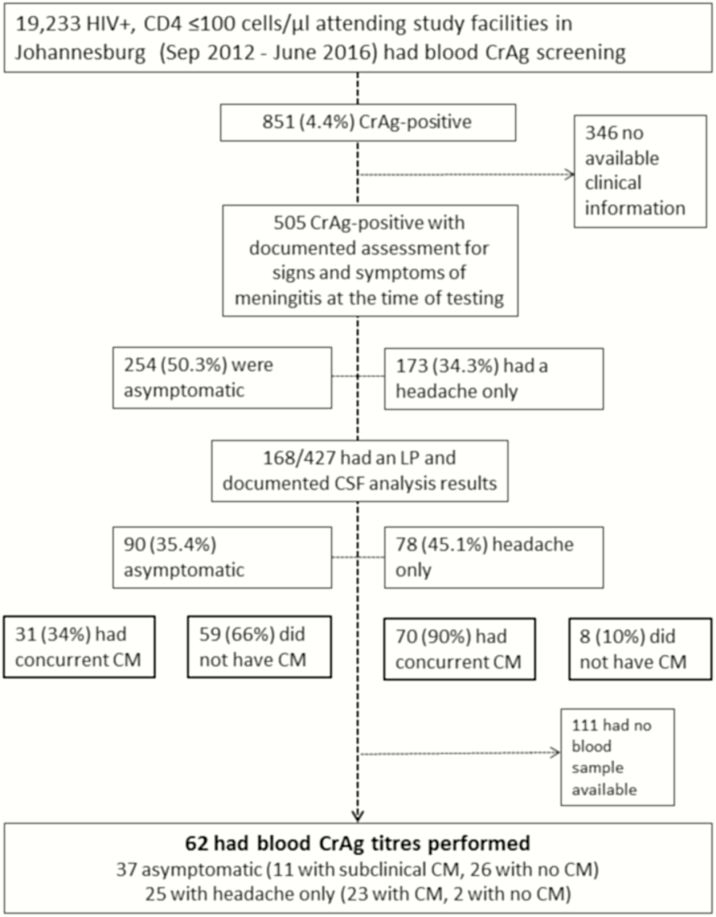
Of 19233 HIV-infected patients >16 years old with CD4 cell counts <100/µL who were screened during the study period, 851 were CrAg positive (4.4%; 95% CI, 4.1%–4.7%). Demographic and clinical data (Supplementary Table S1) including signs and symptoms of meningitis were available for 505 patients (59.3%). Of these, 254 (50.3%; 95% CI, 45.8%–54.7%) reported no headache or confusion at the time of the CrAg test, 173 (34.3%; 30.1%–38.6%) reported a headache without any confusion, and 78 (15.4%; 12.4%–18.9%) were confused (see Figure 1). There were no significant differences in age (P = .29), sex (P = .48) or CD4 cell count (P = .25) between those groups with no symptoms, headache only, or confusion (Supplementary Table S1).
Figure 1.
Flow-chart to show individuals included and excluded from studies. Abbreviations: CM, cryptococcal meningitis; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; LP, lumbar puncture.
CSF results from an LP performed within 30 days after receiving the CrAg result (median, 2.5 days; interquartile range [IQR], 1–7 days) were available for 90 of 254 asymptomatic CrAg-positive patients (35.4%) and 78 of 173 (45.1%) with headache only. Of note, no LP was recorded for 50 of 173 patients (28.9%) who reported a headache (and results were not available for the other 45 patients who were recorded to have undergone LP). There was no significant difference in age or sex; however, CD4 cell counts were lower in those who underwent LP than in those who did not (median, 22/µL [IQR, 7–39/µL] vs 27/µL [10–53/µL]; P = .01) (Supplementary Table S1).
On analysis of CSF, 31 of 90 asymptomatic patients (34%; 95% CI, 25%–45%) and 70 of 78 (90%; 81%–96%) with headache only had evidence of meningeal infection with Cryptococcus. Having a headache compared with no symptoms was strongly predictive of concurrent cryptococcal meningitis (OR, 16.7; 95% CI, 7.1–39.0; P < .001). There was no significant difference between patients with and those without concurrent cryptococcal meningitis in terms of age (median, 37 vs 39 years; P = .24) or sex (male, 53% vs 44%; P = .3), although CD4 cell counts were lower in those with concurrent cryptococcal meningitis (median, 19/µL [IQR, 5–35/µL] vs 25 [10–42/µL]; P = .06).
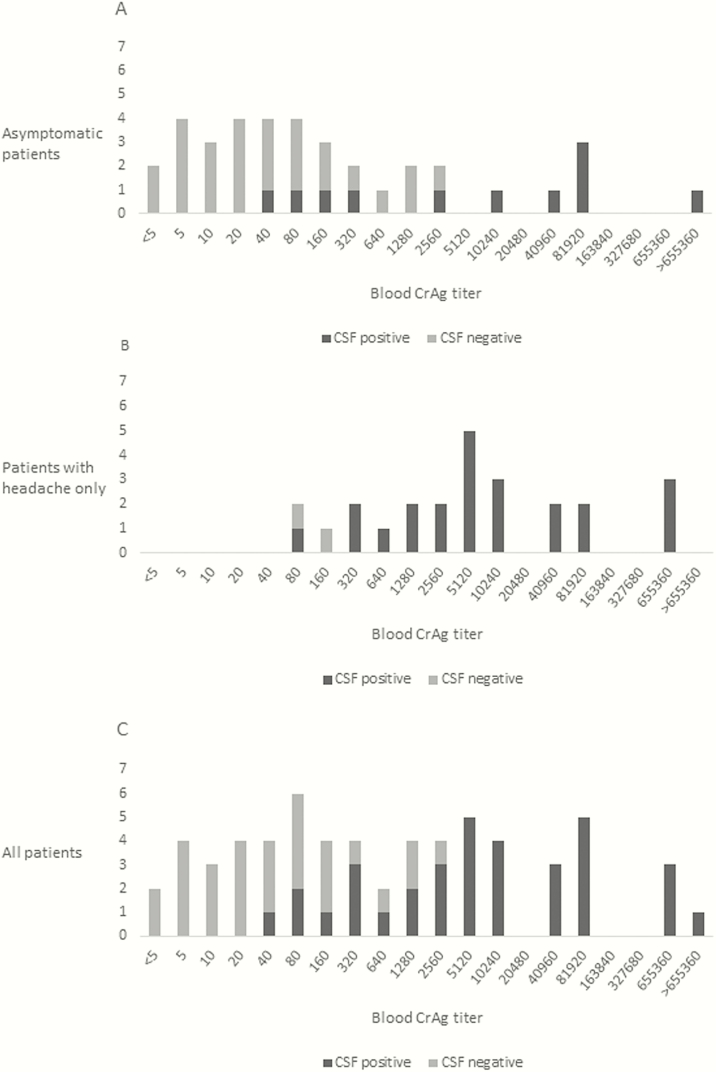
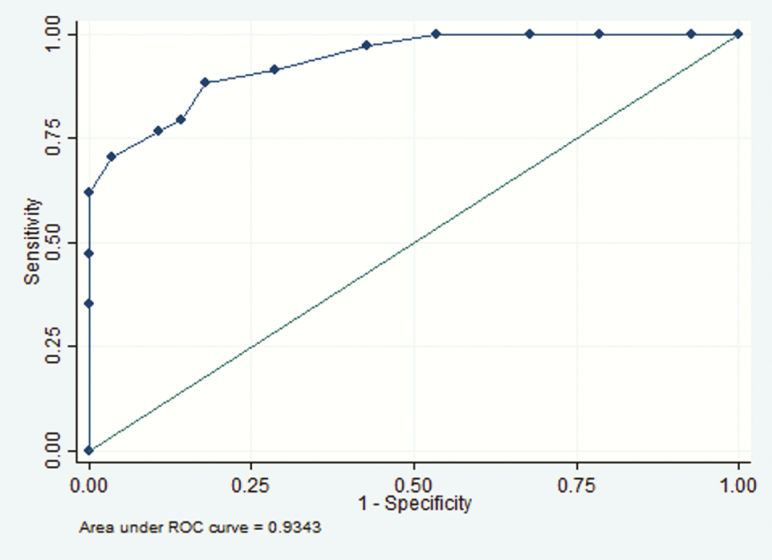
Thirty-seven (41%) of the 90 asymptomatic patients with CSF results, and 25 (32%) of the 78 with headache only had stored samples available for blood CrAg titer analysis. Patients from whom blood samples were available were not significantly different from those without samples in terms of age, sex, or CD4 cell count (P ≥ .05) (Supplementary Table S1). Titers ranged from <5 to 2560 (median, 40; IQR, 10–160) in those with no evidence of meningeal involvement, and from 40 to 6.7 × 107 (median, 5120; 1280–81 920) in those with cryptococcal meningitis (Figure 2). Blood CrAg titer was significantly associated with cryptococcal meningitis in both asymptomatic patients (P < .001) and those with headache only (P = .003), with area under the receiver operating characteristic curve of 0.93 (Figure 3). The optimal cutoff titer for predicting concurrent subclinical cryptococcal meningitis was >160, with sensitivity of 88.2% and specificity 82.1% (Table 1). A blood CrAg titer of >160 had an OR of 34.5 for concurrent cryptococcal meningitis (95% CI, 8.3–143.1; P < .001) in the combined group and 11.2 (2.3–54.6; P = .002) in the asymptomatic group. The association between a blood titer of >160 (or ≥320) and concurrent cryptococcal meningitis remained significant, even with adjustment for CD4 cell count (OR, 38.4; 95% CI, 8.0–185.0; P < .001).
Figure 2.
Blood cryptococcal antigen (CrAg) titers in asymptomatic CrAg-positive patients (n = 37) (A), minimally symptomatic CrAg-positive patients (n = 25) (B), and combined cohort of asymptomatic and minimally symptomatic patients (n = 62) (C), with or without concurrent cryptococcal meningitis. CSF, cerebrospinal fluid.
Figure 3.
Receiver operating characteristic (ROC) curve for blood cryptococcal antigen and cryptococcal meningitis among patients with no symptoms or signs of meningitis and those reporting headache only (n = 62).
Table 1.
Sensitivity and Specificity for Concurrent Cryptococcal Meningitis by Blood Cryptococcal Antigen Titer Cutoff Level in Patients With No Symptoms or Signs of Meningitis or With Headache Only (n = 62)a
| Blood CrAg Titer Cutoff | Sensitivity (95% CI), % | Specificity (95% CI), % |
|---|---|---|
| >5 | 100 (89.7–100) | 21.4 (8.3–41.0) |
| >10 | 100 (89.7–100) | 32.1 (15.8–52.4) |
| >20 | 100 (89.7–100) | 46.4 (27.5–66.1) |
| >40 | 97.1 (84.7–99.9) | 57.1 (37.2–75.5) |
| >80 | 91.2 (76.3–98.1) | 71.4 (51.3–86.8) |
| >160 | 88.2 (72.5–96.7) | 82.1 (63.1–93.9) |
| >320 | 79.4 (62.1–91.3) | 85.7 (67.3–96.0) |
| >640 | 76.5 (58.8–89.3) | 89.2 (71.8–97.7) |
| >1280 | 70.6 (52.5–84.9) | 96.4 (81.7–99.9) |
| >2560 | 61.8 (43.6–77.8) | 100 (87.7–100) |
Abbreviations: CI, confidence interval; CrAg, cryptococcal antigen.
aCrAg titers were determined using the serial dilution method.
All participants with symptom review and LP results available (n = 168) were followed up for a median of 37 days (range, 1–180 days). Of those with available data, appropriate antifungal therapy was started in 89 of 107 (83%); 44 of 45 patients (98%) with cryptococcal meningitis received intravenous amphotericin B and oral fluconazole, and 45 of 62 of those without (73%) received oral fluconazole.
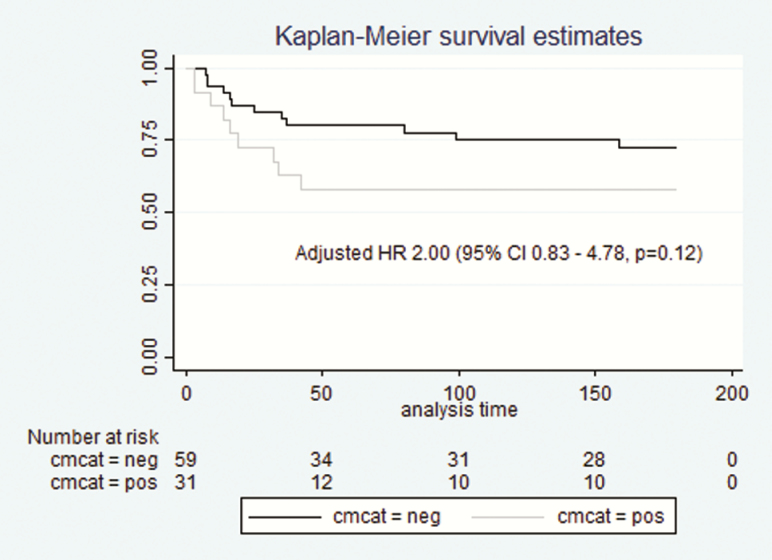
Of 101 patients who had concurrent cryptococcal meningitis, 22 (22%) died, and 6 (6%) were lost to follow-up during the first 6 months, compared with 12 (18%) and 8 (12%) of 67 patients without cryptococcal meningitis. No association was found between age, sex, CD4 cell count, CrAg titer (categorical analysis of high or low CrAg titer using a cut-off of >160 or >320), headache, or ART status, and risk of death. However, receipt of any antifungal agent (amphotericin B, fluconazole, or a combination) was found to be protective (hazard ratio [HR], 0.20; 95% CI, .08–.49; P < .001). With adjustment for receipt of antifungal therapy, the HR for death in those with concurrent cryptococcal meningitis was 2.00 (95% CI, .83–4.78; P = .12) in asymptomatic patients (Figure 4) and 1.82 (.88–3.79; P = .11) in the combined cohort. There remained no significant association between having a high or low (cut-off >160) CrAg titer above 160 and mortality risk (HR, 1.58; 95% CI, .57–4.36; P = .38).
Figure 4.
Kaplan-Meier survival estimates in 90 asymptomatic cryptococcal antigen (CrAg)–positive patients with or without subclinical cryptococcal meningitis (CM). CI, confidence interval; HR, hazard ratio.
DISCUSSION
More than a third of CrAg-positive patients with no signs or symptoms of meningitis, and 90% of those reporting headache only, had evidence of meningeal involvement at CSF analysis. In both groups, higher blood CrAg titers were associated with an increased risk of concurrent cryptococcal meningitis. A cutoff of >160 had moderate sensitivity (88.2%) and specificity (82.1%) for predicting CNS disease; however, no single titer cutoff could distinguish with 100% accuracy between those with and those without concurrent cryptococcal meningitis.
Previous studies have found higher CrAg titers to be associated with subsequent cryptococcal meningitis and death, with titers above a cutoff of about 160 consistently indicating increased risk (Tables 2 and 3). Apart from our study, limited evidence exists for an association between CrAg titer and concurrent cryptococcal meningitis in patients with no symptoms or signs of meningitis, or reporting headache only. Where CrAg-positive patients have undergone LP after screening, the prevalence of concurrent cryptococcal meningitis is 25%–78%, with increased risk in those with higher blood CrAg titers [10, 13, 19, 21]. However, many of these studies include symptomatic as well as asymptomatic patients. Our study established a significant risk of subclinical concurrent cryptococcal meningitis, which was associated with higher blood CrAg titers. This finding is important for informing management of CrAg-positive patients identified through the expansion of screening programs worldwide.
Table 2.
Studies Analyzing Association of Blood Cryptococcal Antigen Titer and Subsequent Cryptococcal Meningitis and/or Death
| Country | Year | Study design | Association between blood CrAg titer and CM and/or mortality | Notes | Ref |
|---|---|---|---|---|---|
| Association between blood CrAg titer and subsequent CM and/or mortality | |||||
| South Africa | 2009 | Retrospective analysis of CrAg titers (using Latex Agglutination, LA) on pre-ART blood samples from 46 patients. | Higher baseline titer associated with increased risk of mortality (p=0.02), subsequent cryptococcal meningitis (p=0.03) and relapse (all >512) within 1 y. | No baseline LP performed. Symptoms and fluconazole use unknown. |
[1] |
| Tanzania | 2011 | CrAg screening of all HIV positive hospital admissions. Serum CrAg titers (LFA) on 17/333 CrAg-positive patients. | Higher titer associated with mortality (p=0.004). | All symptomatic, 15/17 had cryptococcal meningitis. | [17] |
| Tanzania | 2015 | Retrospective analysis of blood CrAg titers (using LFA) on pre-ART blood samples from 21 asymptomatic patients. | Titer of >160 associated with subsequent cryptococcal meningitis (adjusted OR, 4.83; 95% CI, 1.24–8.41; P = .008) within 1 y. | 3 patients with titers ≤160 died of unknown causes. | [11] |
| Uganda | 2016 | Cluster randomized trial of CrAg screen and treat strategy. CrAg titers (LFA) on 151 asymptomatic patients. | Titer of ≥160 associated with subsequent cryptococcal meningitis (HR 9.2, 95% CI 2.14-39.58, p<0.01) (unpublished data) | Increased risk of death or subsequent cryptococcal meningitis if titer ≥160 and CD4 ≤50. | [20] |
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; CM, cryptococcal meningitis; CrAg, cryptococcal antigen; HIV, human immunodeficiency virus; HR, hazard ratio; LA, latex agglutination; LFA, lateral flow assay; LP, lumbar puncture; OR, odds ratio.
Table 3.
Studies Analyzing Association Between Blood Cryptococcal Antigen Titer and Concurrent Cryptococcal Meningitis and/or Death
| Country | Year | Study design | Association between blood CrAg titer and concurrent CM | Notes | Ref |
|---|---|---|---|---|---|
| Association between blood CrAg titer and concurrent CM | |||||
| Democratic Republic of Congo (DRC) | 1989 | Cross-sectional study of 44 newly diagnosed HIV infected adults performing baseline blood and CSF CrAg titers (LA). | Higher titers associated with increasing risk of concurrent cryptococcal meningitis (PPV 92% for titer >128). | Concurrent CM present in 29/44 (66%) CrAg-positive patients. Symptoms unknown. |
[21] |
| Cambodia | 2007 | Cross-sectional study screening patients with CD4 ≤200 cells/µL and performing LPs in all CrAg-positive patients (n=53). | Median titer (LA) higher in those with than those without concurrent CM (2048 vs. 16, p<0.0001). | Concurrent CM present in 41/53 (78%). Most symptomatic. |
[19] |
| Thailand | 2010 | Retrospective study performed blood CrAg titers (LA) on 12 asymptomatic CrAg-positive patients who had baseline LPs. | Higher CrAg titers in those with CM than those without (128-1024 vs. 8-128). | Concurrent subclinical CM in 3/12 (25%). | [13] |
| South Africa | 2016 | Prospective study implementing CrAg screen and treat. Blood CrAg titers (LFA) on 10 patients who had a baseline LP. | Higher titers associated with concurrent CM No CM in patients with titers of <160. |
Concurrent CM present in 4/10 (40%). Symptoms unknown. |
[10] |
| Ethiopia | 2017 | Cross-sectional screening of all HIV- infected adults admitted to hospital. Serum CrAg titers (LFA) and LP for all CrAg positive patients. | Serum titers of ≥160 in 12/16 (75%) patients with concurrent CM. Two patients without concurrent CM had serum titers of 40. | Concurrent CM in 16/18 (89%). All symptomatic. | [22] |
Abbreviations: CM, cryptococcal meningitis; CrAg, cryptococcal antigen; CSF, cerebrospinal fluid; HIV, human immunodeficiency virus; LA, latex agglutination; LFA, lateral flow assay; LP, lumbar puncture; PPV, positive predictive value.
In our study, LPs were offered at the discretion of patients’ health practitioners, who may have elicited different signs and symptoms from patients than those recorded by study nurses. This may have led to an overestimation of the burden of concurrent cryptococcal meningitis and a stronger association with headache than has been previously observed, because LPs would have been more likely offered to patients for whom there was greater suspicion of cryptococcal meningitis, and for patients admitted to health facilities where LP was readily available. Patients who underwent LPs had lower CD4 cell counts than those who did not (22/µL vs 27/µL). Of note, the proportion of patients with subclinical cryptococcal meningitis observed was similar to proportions reported in previous studies [10, 13].
Our study was also limited by the number of patients with available data, which did not reach the required sample size for survival analysis and may explain the lack of significant association between concurrent cryptococcal meningitis or high CrAg titer and mortality rate, as described elsewhere [1, 17, 20]. In addition, almost all patients who had cryptococcal meningitis diagnosed and had available antifungal data received some amphotericin B, which may have reduced the mortality rate in this group and in those with a high blood CrAg titer. Other variables that are known to affect prognosis but were not available for analysis in this study include the presence of cryptococcal immune reconstitution inflammatory syndrome, adherence to antifungal therapy, concomitant diseases, HIV load, CSF cell counts, and LP opening pressure, as well as whether or not repeated LP was performed.
Despite these limitations, our results highlight a substantial risk of concurrent cryptococcal meningitis among patients who are CrAg positive at screening but lack symptoms and signs that typically lead clinicians to investigate for meningitis with an LP. Because patients with cryptococcal antigenemia, treated preemptively with fluconazole therapy, have a 2.5-fold increased risk of death compared with individuals with similar CD4 cell counts without cryptococcal antigenemia [8–11], a more aggressive approach to management should be considered. Where possible, LP should be used routinely to investigate for cryptococcal meningitis in all CrAg-positive patients, even those without symptoms. However, experience suggests that only a minority of asymptomatic or mildly symptomatic patients are willing to undergo LP, even if carefully counseled. In this study, more than a quarter of CrAg-positive patients with a headache did not undergo LP, although concurrent cryptococcal meningitis was diagnosed in 90% of those who did. Alternatively, blood CrAg titer could be used to tailor patient management. Those with higher CrAg titers, perhaps identified with a semiquantitative CrAg test, could be targeted for more intensive, but still feasible and sustainable, antifungal therapy. Further work is required to determine whether such an approach could improve the outcomes in patients with cryptococcal antigenemia.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Notes
Acknowledgments. Phelly Matlapeng, Saneliswe Nkabinde, Matshediso Mkhwanazi, Siphiwe Kuta, and Neo Legare (National Institute for Communicable Diseases) provided assistance with data retrieval.
Disclaimer. The findings and conclusions in this article are those of the authors and do not necessarily represent the official positions of their sponsoring agencies.
Financial support. This work was supported by the South African Medical Research Council (self-initiated research grant to T. O), the Meningitis Research Foundation (grant 1604.0 to R. M. W.) and the US Centers for Disease Control and Prevention (grant CDC-RFA-GH15-1575 awarded to the National Health Laboratory Service, principal investigator N. P. G.) C.S and N.P.G are supported by an NIH R01 grant (grant 1R01AI118511-01A1, sub-recipient, co-principal investigator N. P. G.)
Potential conflicts of interest. J. S. N. received a speaker’s fee for lecture on disseminated cryptococcosis from Mylan. T. S. H. received a consultancy fee from Viamet Pharmaceuticals, honoraria from Pfizer, and tests for research purposes from Immuno-Mycologics. All other authors report no potential conflicts. The authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Jarvis JN, Lawn SD, Vogt M, Bangani N, Wood R, Harrison TS. Screening for cryptococcal antigenemia in patients accessing an antiretroviral treatment program in South Africa. Clin Infect Dis 2009; 48:856–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. French N, Gray K, Watera C et al. Cryptococcal infection in a cohort of HIV-1-infected Ugandan adults. AIDS 2002; 16:1031–8. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization. Rapid advice: diagnosis, prevention and management of cryptococcal disease in HIV-infected adults, adolescents and children. Geneva, Switzerland: World Health Organization, 2011. [PubMed] [Google Scholar]
- 4. World Health Organization. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection: recommendations for a public health approach. 2nd ed Geneva, Switzerland: World Health Organization, 2016. [PubMed] [Google Scholar]
- 5. World Health Organization. Guidelines for managing advanced HIV disease and rapid initiation of antiretroviral therapy. Geneva, Switzerland: World Health Organization, 2017. [PubMed] [Google Scholar]
- 6. Department of Health, South Africa. National consolidated guidelines for the prevention of mother-to-child transmission of HIV (PMTCT) and the management of HIV in children, adolescents and adults. Pretoria, South Africa: Department of Health; 2015. [Google Scholar]
- 7. Govender N, Meintjes G, Bicanic T et al. Guideline for the prevention, diagnosis and management of cryptococcal meningitis among HIV-infected persons: 2013 update. Southern African J HIV Med 2013;14:76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pac L, Horwitz MM, Namutebi AM et al. Implementation and operational research: integrated pre-antiretroviral therapy screening and treatment for tuberculosis and cryptococcal antigenemia. J Acquir Immune Defic Syndr 2015; 68:e69–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mfinanga S, Chanda D, Kivuyo SL et al. ; REMSTART trial team Cryptococcal meningitis screening and community-based early adherence support in people with advanced HIV infection starting antiretroviral therapy in Tanzania and Zambia: an open-label, randomised controlled trial. Lancet 2015; 385:2173–82. [DOI] [PubMed] [Google Scholar]
- 10. Longley N, Jarvis JN, Meintjes G et al. Cryptococcal antigen screening in patients initiating ART in South Africa: a prospective cohort study. Clin Infect Dis 2016; 62:581–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Letang E, Müller MC, Ntamatungiro AJ et al. Cryptococcal antigenemia in immunocompromised human immunodeficiency virus patients in rural Tanzania: a preventable cause of early mortality. Open Forum Infect Dis 2015; 2:ofv046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kapoor SW, Magambo KA, Kalluvya SE, Fitzgerald DW, Peck RN, Downs JA. Six-month outcomes of HIV-infected patients given short-course fluconazole therapy for asymptomatic cryptococcal antigenemia. AIDS 2015; 29:2473–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pongsai P, Atamasirikul K, Sungkanuparph S. The role of serum cryptococcal antigen screening for the early diagnosis of cryptococcosis in HIV-infected patients with different ranges of CD4 cell counts. J Infect 2010; 60:474–7. [DOI] [PubMed] [Google Scholar]
- 14. Thakur KT, Mateyo K, Hachaambwa L et al. Lumbar puncture refusal in sub-Saharan Africa: a call for further understanding and intervention. Neurology 2015; 84:1988–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petti CA, Polage CR, Quinn TC, Ronald AR, Sande MA. Laboratory medicine in Africa: a barrier to effective health care. Clin Infect Dis 2006; 42:377–82. [DOI] [PubMed] [Google Scholar]
- 16. Kirkland KE, Kirkland K, Many WJ Jr, Smitherman TA. Headache among patients with HIV disease: prevalence, characteristics, and associations. Headache 2012; 52:455–66. [DOI] [PubMed] [Google Scholar]
- 17. Wajanga BM, Kalluvya S, Downs JA, Johnson WD, Fitzgerald DW, Peck RN. Universal screening of Tanzanian HIV-infected adult inpatients with the serum cryptococcal antigen to improve diagnosis and reduce mortality: an operational study. J Int AIDS Soc 2011; 14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kisenge PR, Hawkins AT, Maro VP et al. Low CD4 count plus coma predicts cryptococcal meningitis in Tanzania. BMC Infect Dis 2007; 7:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Micol R, Lortholary O, Sar B et al. Prevalence, determinants of positivity, and clinical utility of cryptococcal antigenemia in Cambodian HIV-infected patients. J Acquir Immune Defic Syndr 2007; 45:555–9. [DOI] [PubMed] [Google Scholar]
- 20. Morawski B, Boulware DR, Nalintya E et al. Pre-ART cryptococcal antigen titer associated with preemptive fluconazole failure. In: Abstracts and posters presented at Conference on Retroviruses and Opportunistic Infections (CROI). Boston, MA, 2016. Abstract 159. [Google Scholar]
- 21. Desmet P, Kayembe KD, De Vroey C. The value of cryptococcal serum antigen screening among HIV-positive/AIDS patients in Kinshasa, Zaire. AIDS 1989; 3:77–8. [DOI] [PubMed] [Google Scholar]
- 22. Kempker RR, Blumberg HM, Temesgen O, Bornstein E, Mamuye AT. Point-of-care testing for cryptococcal disease among hospitalized human immunodeficiency virus–infected adults in Ethiopia. Am J Trop Med Hyg 2016; 95:786–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.