Abstract
The epithelial–mesenchymal transition (EMT) confers mesenchymal properties on epithelial cells and has been closely associated with the acquisition of aggressive traits by carcinoma cells. EMT programs are orchestrated by a set of pleiotropically acting transcription factors (TFs). The actions of these EMT-TFs enable the early steps of metastasis: local invasion and subsequent dissemination of carcinoma cells to distant sites. However, in most malignancies, the subsequent outgrowth of micrometastatic deposits into macroscopic metastases has the greatest impact on clinical progression. Such metastatic “colonization” reflects the ability of disseminated tumor cells to adapt to a foreign tissue microenvironment. The outgrowth of a metastasis is also thought to be associated with self-renewal, the defining cellular trait of cancer stem cells (CSCs), also termed tumor-initiating cells. Importantly, molecular links between EMTTFs and self-renewal have emerged, suggesting that EMT programs play critical roles both early and late in the metastatic cascade. The genetic and epigenetic mechanisms that regulate the activation of EMTTFs and the traits they induce are areas under intensive investigation. Such studies may provide new opportunities for therapeutic intervention and help to overcome tumor heterogeneity and therapeutic resistance.
Keywords: Epithelial–mesenchymal transition, Metastasis, Cancer stem cells, Plasticity, Self-renewal
1. Introduction
In many tumor types, the presence of distant metastases marks stage IV and indicates, almost invariably, incurable disease and relatively short overall survival [1]. On a biological level, our understanding of metastasis has been greatly advanced by viewing it as a series of distinct steps that together comprise the “invasion–metastasis cascade” [2,3]. As the first step, cancer cells in the primary tumor acquire the ability to invade into the surrounding tissue: in carcinomas, this requires breaching of the basement membrane that confines the epithelial compartment. Tumor cells then must gain access to lymphatic and blood vessels, enter into the lumina of these vessels (intravasation), survive transport through these vessels, and exit from the vasculature (extravasation). Finally, in a process often termed colonization, small cell clumps or singly disseminated tumor cells (micrometastases) must acquire the ability to survive and proliferate in the microenvironment of a foreign tissue in order to form macroscopic metastases.
The complexity of the metastatic process raises a major conceptual problem: how do tumor cells acquire all of the individual properties that together comprise the metastatic cascade? A mechanistic solution to this conundrum is provided by the existence of a multi-faceted cell-biological program that enables carcinoma cells to acquire a number of the traits required to accomplish the initial steps of metastatic cascade. Hence, rather than being pieced together one-by-one, many of the cell-biological traits needed to complete the metastatic cascade can be choreographed by small numbers of centrally acting, pleiotropic regulators; this greatly simplifies how we conceptualize this complex multi-step process. Thus, the epithelial–mesenchymal transition (EMT) represents a cellular program that confers on neoplastic epithelial cells the biological traits needed to accomplish most of the steps of the invasion–metastasis cascade [4–6].
In this review, we discuss the mechanisms through which EMT programs enable different steps of the metastatic cascade and the emerging connection between EMT programs and the traits displayed by CSCs. More specifically, we focus on the dual roles of certain transcription factors (TFs) that orchestrate EMT programs (EMT-TFs) and thereby impart traits required both for physical dissemination and entrance into the CSC state. Finally, we discuss the relevance of these connections between EMT and self-renewal for developing new strategies to overcome therapeutic resistance.
2. EMT programs and the early steps of metastasis
EMT programs were first observed in the context of embryonic development, where they function as transdifferentiation programs that effect critical morphogenetic steps, such as gastrulation and neural crest formation [7,8]. Specifically, EMTs generate mesenchymal cell types from epithelial and endothelial precursors. These epithelial–mesenchymal conversions are crucial for cell movements that take place during morphogenesis, such as neural crest migration. This explains why the EMTs described in carcinoma cells have been portrayed as opportunistic activations of normally latent, early embryogenic cell-biological programs [5,6].
A group of pleiotropic TFs have been found capable of orchestrating EMT programs [9,10]. EMT-TFs are usually expressed by a cell in response to certain contextual signals that it receives; alternatively, their expression may be forced experimentally. By either route, the expression of these EMT-TFs causes a profound re-arrangement of cell behavior and thus tissue organization with widespread functional ramifications [11].
The defining characteristic of epithelial cell sheets is the lateral cell–cell tethering of individual epithelial cells to their neighbors, which is achieved through multiple intercellular junctions, specifically, desmosomes as well as adherens, tight and gap junctions. These cell–cell junctions permit only cohesive cell movements of the epithelial cells, which are further restricted in normal tissues by the underlying basement membranes, which allow only lateral movements within the epithelial cell layer. Most EMT-TFs are transcriptional repressors and many, such as Snail [12], Slug [13], Zeb1 [14] and Twist [15], directly repress mediators of epithelial adhesion, the most important of which is E-Cadherin, an integral component of adherens junctions. Snail [16] and Slug [17] have also been shown to directly repress the expression of claudins, which are necessary for the assembly of tight junctions between adjacent cells.
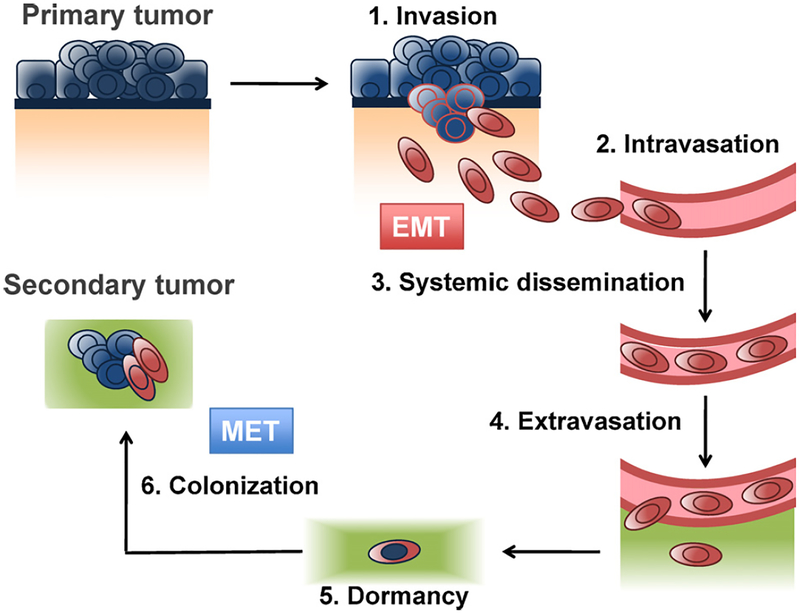
While the loss of epithelial traits during an EMT is reasonably well understood, the mechanisms allowing the concomitant acquisition of mesenchymal features remains poorly characterized. These involve the up-regulation of N-Cadherin, the mesenchymal intermediary filament Vimentin and extracellular matrix (ECM) components such as Fibronectin. These changes culminate in a front-to-back polarization of individual cells and an acquired ability to migrate and invade into the surrounding stroma as single cells [5]. Acting in concert, these alterations are critical to completing the earlier steps of the metastatic cascade, i.e., local invasion, intravasation, survival while transiting through the circulation, and extravasation (Fig. 1). Indeed, these EMT-associated traits may often suffice to enable the translocation of primary carcinoma cells to the parenchyma of distant tissues.
Fig. 1.
The metastatic cascade. EMT programs are involved in early steps of the metastatic cascade, where they enable the invasion into the stroma (depicted in orange, underdlying the basement membrane in blue) and translocation of carcinoma cells (depicted in blue, changing to red when undergoing an EMT) to distant parenchyma (green). EMT programs are dynamically regulated and, during the last step of the metastatic cascade, colonization, carcinoma cells are thought to switch back to an epithelial state (blue cells) through the reverse process, mesenchymal–epithelial transition (MET).
3. EMT programs and the late steps of metastasis
3.1. Cancer stem cells and the metastatic cascade
The last step of the invasion–metastasis cascade – colonization – is likely to require adaptation of a disseminated cancer cells to the microenvironment of a foreign tissue. This represents a complex topic in and on itself and will not be covered in this review [18]. It seems unlikely that these adapative steps are enabled by EMT programs and thus may require additional changes to cells.
In addition, the trait of self-renewal also seems to be essential. In the narrowest sense, self-renewal enables a cell to generate progeny that are copies of itself; in the context of stem-cell biology, a self-renewing stem cell is also depicted as being able to spawn more differentiated progeny. Such self-renewing cells have been described in various neoplasias, where they are termed cancer stem cells (CSCs). It seems plausible that the tumor-initiating trait (discussed below) associated with the CSC state is critical to the ability of disseminated cancer cells to serve as founders of new colonies of metastatic cells, i.e., micro- and macrometastases.
CSCs have been operationally defined through their ability to generate tumors with high efficiency when injected in limiting dilutions into immunocompromised host mice [19]. CSCs were prospectively isolated first from acute myeloid leukemias based on cell-surface marker expression [20], and later in solid malignancies such as breast [21], brain [22], colon [23,24] and pancreatic cancer[25].
In all of these cases, CSCs have been defined operationally by their ability to seed new tumors in appropriate host mice; hence, the term tumor-initiating cells (TICs) reflects the operational definition of CSCs. More specifically, cancer cell populations have been implanted at various limiting dilutions in order to quantify the representation of TICs in heterogeneous populations of cancer cells. In most cases, these have involved the implantation of human cancer cell populations into immunocompromised mouse hosts, generating tumor xenografts. However, more recently, implantation of murine tumors into syngeneic immunocompetent hosts has been reported as well [26]. The latter experiments have been important, in that some criticism of the CSC model has been directed at experiments in which functional host immune systems were not operative, raising questions about the involvement of immune mechanisms in the formation of CSCs [27]. Thus, tumor-initiating rates of human melanoma cells have been shown to vary greatly in mouse strains with differing degrees of immunosuppression [28].
Importantly, the existing model of CSCs does not dictate that they invariably comprise only rare subpopulations within tumors[29]. Instead, their representation within neoplastic cell populations is likely to be dictated by a variety of factors, including (i) the normal cell-of-origin from which the tumors arise, (ii) the genetic and epigenetic modifications that cancer cells have accumulated during the course of multi-step tumor progression, (iii) the contextual signals that such tumors experience in the tumor microenvironment, and (iv) the nature of the host in which the tumor is implanted [19]. In the last case, the immune competence of the host, as mentioned, as well as the anatomical site of implantation, may represent important determinants of the measured numbers of CSC.
These considerations dictate that the current methods of quantifying CSCs in larger populations of cancer cells can never yield absolute measurements of their representation. Instead, their output depends on the precise specifications of the tumor-initiating assay deployed to test for the presence of TICs/CSCs. Moreover, tumor-initiating assays used to quantify CSCs have also proven highly useful in gauging tumor aggressiveness and heterogeneity: recent evidence suggests that the aggressive clinical behavior of primary breast cancer samples can be correlated with their content of TICs [30].
Importantly, the ability of CSCs to initiate new tumors might be of critical importance for colonization, the last step of the metastatic cascade. As mentioned above, the ability to spawn an essentially unlimited number of progeny is a trait usually ascribed to stem cells, and thus involves the abilities of a cell to both self-renew and give rise to progeny that lack self-renewing ability [19,31–33]. In fact, the ability of a cancer cell to seed an entire tumor following experimental implantation and the ability to seed a macroscopic growth following metastatic dissemination appear to be very similar processes, leading to the notion that metastasis-forming ability is limited to CSCs [34]. Stated differently, cancer cells that complete the early steps of the metastatic cascade, but lack the ability to proliferate and self-renew at a distant site, may well persist as micrometastatic deposits that have no clinical consequences for the cancer patient (Fig. 1). While attractive in concept, these notions have not yet been proven by experimentation.
3.2. EMT and the acquisition of stem-cell traits
Of fundamental importance biologically, the activation of EMT programs has been associated with the acquisition of stem cell (SC) traits by normal and neoplastic cells [35,36]. The connection between EMT and SCs was most unexpected, indeed counterintuitive, as it indicated that epithelial SCs express a wide array of mesenchymal markers. However, currently available evidence indicates that the EMT-SC connection holds for both normal epithelial cell populations as well as populations of carcinoma cells [35,37]. Among other implications, this finding suggests that neo-plastic cell populations need not invent novel SC programs in order to acquire SC subpopulations; instead, EMT programs would seem to provide a ready source of CSCs by enabling the dedifferentiation of the more epithelial cells within carcinomas. Moreover, the SC programs that have been found, for example, in mammary carcinoma cells appear to be very similar to those operating within the normal mammary tissue. Hence, carcinoma cells can appropriate SC programs that were already operative in normal antecedent cell populations.
This connection between EMT and epithelial SCs indicates that the EMT process is doubly dangerous for the cancer patient: by imparting mesenchymal traits to carcinoma cells, an EMT can generate cellular traits associated with high-grade malignancy, including motility, invasiveness and a resistance to apoptosis; these can lead in turn to metastatic dissemination [4]. In addition, by imparting the trait of self-renewal to carcinoma cells, the EMT creates cancer cells that are qualified to seed the large colonies of cancer cells that form macroscopic metastases.
This apparent connection between EMT programs and the generation of metastatic CSCs should drive the development of therapeutic strategies designed to interfere with EMT programs operating in tumors. Accumulating evidence suggests that EMT programs generate cells with many of the traits of CSCs, in various malignancies, such as breast [35,36], pancreatic [38] colorectal [39] and hepatocellular carcinoma [40], However, it remains unclear whether passage through an EMT directly generates CSCs or simply cells that are poised to become CSCs.
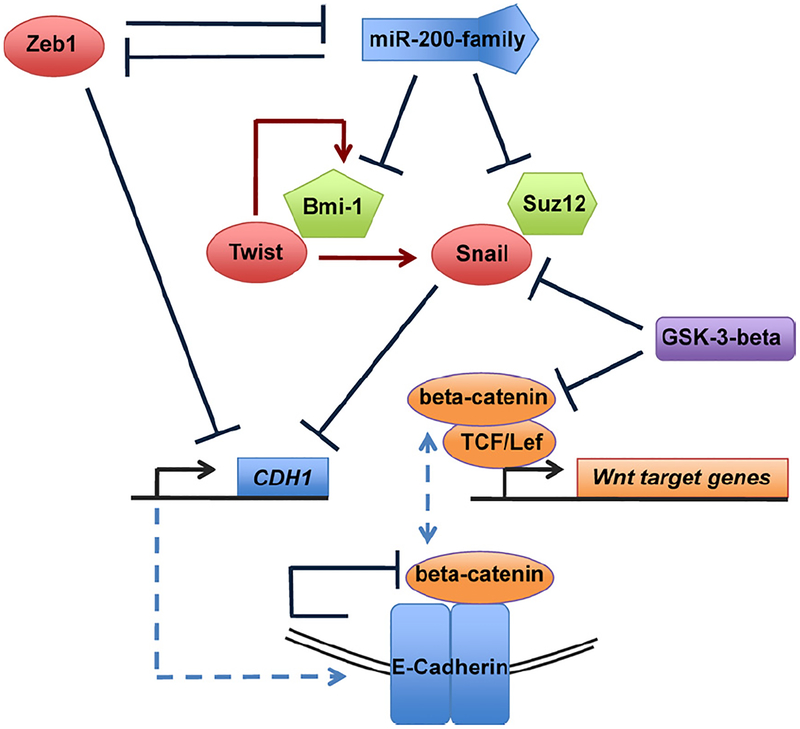
In fact, molecular connections between the EMT program and the stem-cell state are beginning to emerge and thus substantiate the above-described connection between passage through an EMT and acquisition of SC traits (Fig. 2). First, the Zeb1 EMT-TF has been shown to negatively regulate expression of the microRNAs that function to suppress stemness, specifically members of the miR-200 (miR-)200 family [41–43]. These miR-200 family members have been shown, in turn, to suppress expression of the polycomb protein Bmi-1, which is known to be critical to support the stem-cell state in both cancer cells and embryonic stem cells [37,44]. Hence, by suppressing miR-200 expression, the Zeb1 TF is able to enhance expression of Bmi-1 (Fig. 2, molecular links between EMT and stemness). Interestingly, Zeb1 and miR-200 family members have been shown to repress one another in a double negative-feedback loop, indicating that they act as homeostatic switches in the regulation of epithelial plasticity [41,42,45,46].
Fig. 2.
Molecular links between proteins implicated in self-renewal of normal and cancer stem cells (polycomb proteins Bmi-1, histone modifying protein Suz12 and Wnt/beta-catenin signaling) and EMT-TFs (Zeb1, Twist, Snail). Blue arrows indicate repressive/inhibitory actions, red arrows an upregulation. In the absence of active Wnt/beta-catenin signaling, GSK-3-beta phosphorylates both Snail and beta-catenin, thereby targeting them for ubiquitination and destruction in the proteasome. Both Snail and Zeb1 repress the CDH1 gene whose product, E-Cadherin (indicated through dashed blue line) binds and inactivates beta-catenin, thereby preventing it to associated with TCF/LEF-TFs (dashed blue line) and activate Wnt target genes in the nucleus.
Yet other links between these molecules and the SC program have been discovered. Thus, miR-200b has been reported to suppress expression of Suz12 [47], a histone-modifying enzyme that has been shown to be necessary for transcriptional repression of the CDH1 gene (E-Cadherin) by the Zeb1 and Snail EMT-TFs [48]. And as mentioned above, E-Cadherin, in turn, is the keystone of the epithelial state, functioning as the fundamental structural component of adherens junctions (reviewed by [49,50]). Hence, repression of epithelial differentiation and acquisition of stemness appear intimately interconnected (Fig. 2).
While EMT-TFs may regulate proteins involved in self-renewal, the opposite may also occur, i.e., proteins or pathways implicated in self-renewal directly regulate EMT-TF. For example, nuclear beta-catenin, an indication of active Wnt-beta-catenin cascade signaling, has been specifically observed at the invasion front of colorectal carcinoma, where an EMT occurs [51]. These and a multitude of other observations suggested that Wnt-beta-catenin signaling is involved in the induction of EMTs in carcinomas and, thereby, in the generation of CSCs [49].
At the same time, Wnt-beta-catenin signaling has been shown to be crucial for the maintenance of various somatic stem-cell populations [52–54] as well as being active in CSCs in a variety of cancers, particularly in breast [55], colon [56], liver carcinomas [57] and other malignancies, such as multiple myelomas [58] and myeloid leukemias [59–62]. This explains why the Wnt-beta-catenin signaling has been proposed as a major therapeutic target for the eradication of CSCs [63,64].
Importantly, Wnt signaling is also critically involved in regulating turnover and activity of the EMT-TF Snail in the context of breast cancer [65] as well as normal hepatocyte regeneration [66]. These studies provide a mechanistic link between Wnt signaling and regulation of EMT programs through Snail. Thus, the CDH1 gene encoding E-Cadherin, one of the transcriptional targets repressed by Snail, has been proposed to be a direct negative regulator of Wnt signaling: its cytoplasmic tail binds beta-catenin and sequesters it within the adherens junction complex, thereby preventing it from translocating to the nucleus where it can act as a transcriptional co-factor of TCF–LEF complexes [67]. Therefore, Snail, in turn, through its ability to repress E-Cadherin expression might thereby not only contribute to the suppression of epithelial characteristics, but also reinforce Wnt-beta-catenin signaling (Fig. 2).
3.3. EMT-TFs and apoptosis
CSCs exhibit a number of properties that would not seem to be directly connected to the trait of self-renewal, but might nonetheless be positively regulated by EMT-TFs. For example, Twist has been shown to directly suppress apoptosis through various mechanisms: by suppressing the pro-apoptotic effects of the Myc oncogene [68], through activation of NF-kappaB signaling [69], and by repression of p53-induced proapoptotic genes [70]. The EMTTF Slug has also been shown to antagonize p53-induced apoptosis, by repressing the Bcl-2 antagonist PUMA in normal hematopoietic progenitor cells [71], and in the neoplastic cells of chronic myeloid leukemia cells [72]. The resulting elevated resistance to apoptosis might well contribute to a crucial property of metastasizing CSCs by promoting carcinoma cell survival during early steps of metastasis and, following dissemination, during their attempts at gaining a foothold in distant, potentially inhospitable tissue microenvironments.
The discovery that EMT programs generate carcinoma cells with heightened resistance to apoptosis helps to explain a widely observed phenomenon in clinical oncology: populations of carcinoma cells that emerge following various cytotoxic treatments express, relative to the initially treated cells, elevated levels of certain mesenchymal markers. These observations have been made in a variety of carcinomas [38,73,74]. Given these observations and the known properties of EMT programs, it becomes highly likely that these treatment-resistant subpopulations of carcinoma cells have passed through EMTs. In light of the emerging links between EMTs and the stem-cell state, this holds the further implication that these therapy-resistant survivors are greatly enriched for CSCs, which can then proceed to regenerate entirely new tumor populations and thereby drive clinical relapse.
3.4. EMT programs are regulated in a dynamic fashion
A critical question concerns the mechanisms that are responsible for the expression of the central coordinators of EMT programs, the EMT-TFs. This question becomes especially acute during the latter stages of tumor progression, when these programs are major determinants of carcinoma cell behavior. One key insight has come from the realization that EMT programs are dynamically maintained and reversible during both normal development and tumor pathogenesis. Thus, EMTs that are activated in the context of the primary tumor may empower carcinoma cells to translocate to anatomically distant sites, while the reverse process, termed the mesenchymal–epithelial transition (MET) may operate in these sites, enabling the disseminated cells to regain an epithelial phenotype (Fig. 1). This reversion to an epithelial state may be critical to their successful colonization of distant organs [75,76].
Pioneering work in this area on the regulation of Wnt signaling in colon cancer revealed striking analogies between the expression of nuclear beta-catenin in invading colon carcinoma cells and morphogenetic patterning during embryonic gastrulation [77]. This led subsequently to the speculation that colon carcinoma cells undergo an EMT specifically at the invasion front. Among other consequences, these observations provided striking evidence that the context of carcinoma cells – in this case their close apposition to the nearby tumor-associated stroma – was an important determinant of their behavior, specifically their activation of EMT programs[34].
Indeed, such reversible regulation of epithelial–mesenchymal interconversion is highly conserved evolutionarily and is indispensable for organ morphogenesis. To cite an example, during renal organogenesis the mesenchyme surrounding the ureteric bud, itself the product of an EMT, gives rise to kidney epithelium through an MET [78]. This developmental origin of the kidney epithelium has been utilized to develop therapeutic strategies for renal fibrosis, a pathology driven by EMT processes occurring in kidney epithelial cells [79,80].
These and other observations provide evidence that EMTs are provoked in epithelial cells by signals that they receive from their neighbors in the adjacent stroma. More specifically, such contextual signals induce the expression of EMT-TFs in the epithelial cells, which respond by executing the multiple changes associated with an EMT program [5]. Consequently, when these mesenchymal cells subsequently migrate and no longer experience these inciting contextual signals, they may revert via an MET to the epithelial state of their ancestors.
In the context of carcinoma development, the EMT-inducing signals appear to originate in the adjacent stroma. For example, the TGF-beta growth factor has emerged as a major regulator of EMT in development [81] and disease [82]. In fact, a diverse set of extracellular signals has been reported to induce EMTs in various epithelial cell types, including, besides TGF-beta ligands, Wnt, Notch, Sonic Hedgehog and growth factors that activate tyrosine kinase receptors, such as EGF [5]. Moreover, other stimuli that originate within the tumor microenvironment have been implicated in the induction of EMT, notably hypoxia. Specifically, hypoxia-inducible factor alpha (HIF1-alpha) has been shown to directly activate Twist1 in head and neck squamous carcinomas [83].
While certain carcinoma cells can be induced to readily undergo an EMT in response to treatment with TGF-beta, the great majority of epithelial cell lines fail to do so [84]. This indicates that in many biological contexts, exposure to TGF-beta, while necessary, may not be sufficient to induce an EMT and may therefore require the collaborative actions of yet other signaling agents. This is illustrated during the process of gastrulation, the formation of the mesendoderm during early embryonic development in chordates, and other bilateria. During this process, TGF-beta induces the primitive streak, the initial site of mesoderm formation, doing so in cooperation with signals from the Wnt pathway and possibly other pathways that remain to be uncovered [85]. Indeed, it has been recently shown that both beta-catenin dependent, canonical as well as non-canonical Wnt signaling pathway collaborate with TGF-beta to induce and then maintain mesenchymal and stem-cell traits in both normal and neoplastic breast epithelial cells [86]. Accordingly, future studies investigating the regulation of EMT–MET transitions in cancer cells will need to take into account the signaling contexts that trigger EMTs and METs.
4. Conclusion and perspectives
One key implication for the development of therapies directed against high-grade malignancies is the necessity to identify agents and therapeutic strategies that specifically target CSCs, since these appear to be major sources of therapeutic resistance and tumor regrowth. Some initial steps have been made in this direction [87], and indeed many others are likely to follow. This strategy, attractive as it is in concept, may be complicated by one critical problem implicit in the earlier discussions: contextual signals in the microenvironment induce the activation of EMT programs in carcinoma cells, and EMT programs lead these cells, in turn, into the stem-cell state. Because of this cellular plasticity, the initial elimination of CSCs by targeted agents may be followed closely by the generation of new CSCs arising from the non-SCs within the treated population [88].
This consideration makes it apparent that durable clinical responses can only be achieved by the elimination of both subpopulations of cells within tumors [27]. This might be accomplished in the future by the use of CSC-targeted therapies applied together with more conventional agents that are already known to target the non-SCs within carcinomas. Alternatively, certain agents may be found capable of simultaneously eliminating both cell populations.
In principle, the mechanisms of EMT induction, as described above, should present drug developers with multiple molecular targets. In practice, this may be quite challenging. The most desirable targets are surely the EMT-TFs that act pleiotropically to induce the EMT and, we propose, maintain the stem-cell state. By shutting down their function, CSCs may exit the stem-cell state and enter into compartments of the more differentiated epithelial cells within carcinomas. Accordingly, EMT-TFs like Twist, Snail and ZEB1 might be targeted by future generations of low molecular weight drugs. Unfortunately, the discovery and development of drugs that target various TFs has proven most challenging over the past decade, in part because these proteins do not bear the catalytic clefts that are useful for the docking of drug molecules (Box 1).
BOX 1.
A more attractive prospect comes from the discoveries that EMTs are induced by contextual signals, such as TGF-beta, EGF, FGFs, Wnt and Notch ligands. In our own work, we find that signals of this sort are critically involved in both the initiation of EMT programs and the subsequent maintenance of cells in the resulting stem-cell state [87]. These signaling pathways have already been the objects of intensive drug development, largely because they play roles in the pathogenesis of a wide variety of diseases. In fact, the history of drug development to interrupt signaling pathways has been far more encouraging. This raises the question of whether combinations of drugs targeting these signaling pathways will one day be deployed to induce the exit of CSCs from the stem-cell state, rendering them more susceptible to conventional therapeutics that target the more differentiated epithelial cells in carcinomas.
Acknowledgements
The authors thank members of the Weinberg lab for insightful discussion. Research in the Weinberg lab is supported by the NIH/NCI (R.A.W.: CA12515 and DE020817), MIT Ludwig Center for Molecular Oncology (R.A.W.), Ludwig Fellowship for Metastasis Research (C.S.), Breast Cancer Research Foundation (R.A.W.), Harvard Breast Cancer SPORE (R.A.W.) and DoD BCRP Idea Award (R.A.W.).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- [1].Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. European Journal of Cancer 2010;46(7):1177–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Geiger TR, Peeper DS. Metastasis mechanisms. Biochimica et Biophysica Acta 2009;1796(2):293–308. [DOI] [PubMed] [Google Scholar]
- [3].Fidler IJ. Critical determinants of metastasis. Seminars in Cancer Biology 2002;12(2):89–96. [DOI] [PubMed] [Google Scholar]
- [4].Singh A, Settleman J. EMT, cancer stem cells and drug resistance: an emerging axis of evil in the war on cancer. Oncogene 2010;29(34):4741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial–mesenchymal transitions in development and disease. Cell 2009;139(5):871–90. [DOI] [PubMed] [Google Scholar]
- [6].Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA. Epithelial–mesenchymal transitions: the importance of changing cell state in development and disease. Journal of Clinical Investigation 2009;119(6):1438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Shook D, Keller R. Mechanisms, mechanics and function of epithelial–mesenchymal transitions in early development. Mechanisms of Development 2003;120(11):1351–83. [DOI] [PubMed] [Google Scholar]
- [8].Nieto MA. The ins and outs of the epithelial to mesenchymal transition in health and disease. Annual Review of Cell and Developmental Biology 2011. [DOI] [PubMed] [Google Scholar]
- [9].Ouyang G, Wang Z, Fang X, Liu J, Yang CJ. Molecular signaling of the epithelial to mesenchymal transition in generating and maintaining cancer stem cells. Cellular and Molecular Life Sciences 2010;67(15):2605–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Moreno-Bueno G, Peinado H, Molina P, Olmeda D, Cubillo E, Santos V, et al. The morphological and molecular features of the epithelial-to-mesenchymal transition. Nature Protocols 2009;4(11):1591–613. [DOI] [PubMed] [Google Scholar]
- [11].Yilmaz M, Christofori G. Mechanisms of motility in metastasizing cells. Molecular Cancer Research 2010;8(5):629–42. [DOI] [PubMed] [Google Scholar]
- [12].Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, et al. The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nature Cell Biology 2000;2(2):76–83. [DOI] [PubMed] [Google Scholar]
- [13].Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A. The transcription factor Slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with Snail and E47 repressors. Journal of Cell Science 2003;116(Pt 3):499–511. [DOI] [PubMed] [Google Scholar]
- [14].Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, et al. DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 2005;24(14):2375–85. [DOI] [PubMed] [Google Scholar]
- [15].Yang MH, Hsu DS, Wang HW, Wang HJ, Lan HY, Yang WH, et al. Bmi1 is essential in Twist1-induced epithelial–mesenchymal transition. Nature Cell Biology 2010;12(10):982–92. [DOI] [PubMed] [Google Scholar]
- [16].Ikenouchi J, Matsuda M, Furuse M, Tsukita S. Regulation of tight junctions during the epithelium–mesenchyme transition: direct repression of the gene expression of claudins/occludin by Snail. Journal of Cell Science 2003;116(Pt10):1959–67. [DOI] [PubMed] [Google Scholar]
- [17].Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, et al. The transcription factors Slug and Snail act as repressors of Claudin-1 expression in epithelial cells. The Biochemical Journal 2006;394(Pt 2):449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shibue T, Weinberg RA. Metastatic colonization: settlement, adaptation and propagation of tumor cells in a foreign tissue environment. Seminars in Cancer Biology 2011;21(2):99–106. [DOI] [PubMed] [Google Scholar]
- [19].Alison MR, Lim SM, Nicholson LJ. Cancer stem cells: problems for therapy? The Journal of Pathology 2010. [DOI] [PubMed] [Google Scholar]
- [20].Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 1994;367(6464):645–8. [DOI] [PubMed] [Google Scholar]
- [21].Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proceedings of the National Academy of Sciences of the United States of America 2003;100(7):3983–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature 2004;432(7015):396–401. [DOI] [PubMed] [Google Scholar]
- [23].O’Brien CA, Pollett A, Gallinger S, Dick JE. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 2007;445(7123):106–10. [DOI] [PubMed] [Google Scholar]
- [24].Ricci-Vitiani L, Lombardi DG, Pilozzi E, Biffoni M, Todaro M, Peschle C, et al. Identification and expansion of human colon-cancer-initiating cells. Nature 2007;445(7123):111–5. [DOI] [PubMed] [Google Scholar]
- [25].Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, et al. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell 2007;1(3):313–23. [DOI] [PubMed] [Google Scholar]
- [26].Naka K, Hoshii T, Muraguchi T, Tadokoro Y, Ooshio T, Kondo Y, et al. TGF-beta-FOXO signalling maintains leukaemia-initiating cells in chronic myeloid leukaemia. Nature 2010;463(7281):676–80. [DOI] [PubMed] [Google Scholar]
- [27].Shackleton M Moving targets that drive cancer progression. The New England Journal of Medicine 2010;363(9):885–6. [DOI] [PubMed] [Google Scholar]
- [28].Quintana E, Shackleton M, Sabel MS, Fullen DR, Johnson TM, Morrison SJ. Efficient tumour formation by single human melanoma cells. Nature 2008;456(7222):593–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kennedy JA, Barabe F, Poeppl AG, Wang JC, Dick JE. Comment on “Tumor growth need not be driven by rare cancer stem cells”. Science 2007;318(5857):1722, author reply 1722. [DOI] [PubMed] [Google Scholar]
- [30].Pece S, Tosoni D, Confalonieri S, Mazzarol G, Vecchi M, Ronzoni S, et al. Biological and molecular heterogeneity of breast cancers correlates with their cancer stem cell content. Cell 2010;140(1):62–73. [DOI] [PubMed] [Google Scholar]
- [31].Shackleton M Normal stem cells and cancer stem cells: similar and different. Seminars in Cancer Biology 2010;20(2):85–92. [DOI] [PubMed] [Google Scholar]
- [32].Bomken S, Fiser K, Heidenreich O, Vormoor J. Understanding the cancer stem cell. British Journal of Cancer 2010;103(4):439–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Greaves M Cancer stem cells: back to Darwin? Seminars in Cancer Biology 2010;20(2):65–70. [DOI] [PubMed] [Google Scholar]
- [34].Brabletz T, Jung A, Spaderna S, Hlubek F, Kirchner T. Opinion: migrating cancer stem cells—an integrated concept of malignant tumour progression. Nature Reviews Cancer 2005;5(9):744–9. [DOI] [PubMed] [Google Scholar]
- [35].Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 2008;133(4):704–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Morel AP, Lievre M, Thomas C, Hinkal G, Ansieau S, Puisieux A. Generation of breast cancer stem cells through epithelial–mesenchymal transition. PLoS One 2008;3(8):e2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, et al. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell 2009;138(3):592–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, et al. Acquisition of epithelial–mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Research 2009;69(6):2400–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Brabletz T, Hlubek F, Spaderna S, Schmalhofer O, Hiendlmeyer E, Jung A, et al. Invasion and metastasis in colorectal cancer: epithelial–mesenchymal transition, mesenchymal–epithelial transition, stem cells and beta-catenin. Cells, Tissues, Organs 2005;179(1–2):56–65. [DOI] [PubMed] [Google Scholar]
- [40].Niu RF, Zhang L, Xi GM, Wei XY, Yang Y, Shi YR, et al. Up-regulation of Twist induces angiogenesis and correlates with metastasis in hepatocellular carcinoma. Journal of Experimental & Clinical Cancer Research 2007;26(3): 385–94. [PubMed] [Google Scholar]
- [41].Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, et al. A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Reports 2008;9(6): 582–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial–mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. The Journal of Biological Chemistry 2008;283(22):14910–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes & Development 2008;22(7):894–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, et al. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nature Cell Biology 2009;11(12):1487–95. [DOI] [PubMed] [Google Scholar]
- [45].Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, et al. A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Research 2008;68(19):7846–54. [DOI] [PubMed] [Google Scholar]
- [46].Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, et al. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nature Cell Biology 2008;10(5):593–601. [DOI] [PubMed] [Google Scholar]
- [47].Iliopoulos D, Lindahl-Allen M, Polytarchou C, Hirsch HA, Tsichlis PN, Struhl K. Loss of miR-200 inhibition of Suz12 leads to polycomb-mediated repression required for the formation and maintenance of cancer stem cells. Molecular Cell 2010;39(5):761–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Herranz N, Pasini D, Diaz VM, Franci C, Gutierrez A, Dave N, et al. Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Molecular and Cellular Biology 2008;28(15):4772–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Schmalhofer O, Brabletz S, Brabletz T. E-cadherin, beta-catenin, and ZEB1 in malignant progression of cancer. Cancer and Metastasis Reviews 2009;28(1–2):151–66. [DOI] [PubMed] [Google Scholar]
- [50].Yang J, Weinberg RA. Epithelial–mesenchymal transition: at the crossroads of development and tumor metastasis. Developmental Cell 2008;14(6): 818–29. [DOI] [PubMed] [Google Scholar]
- [51].Brabletz T, Jung A, Hermann K, Gunther K, Hohenberger W, Kirchner T. Nuclear overexpression of the oncoprotein beta-catenin in colorectal cancer is localized predominantly at the invasion front. Pathology, Research and Practice 1998;194(10):701–4. [DOI] [PubMed] [Google Scholar]
- [52].Chien AJ, Conrad WH, Moon RT. A Wnt survival guide: from flies to human disease. The Journal of Investigative Dermatology 2009;129(7):1614–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Brabletz S, Schmalhofer O, Brabletz T. Gastrointestinal stem cells in development and cancer. The Journal of Pathology 2009;217(2):307–17. [DOI] [PubMed] [Google Scholar]
- [54].Goessling W, North TE, Loewer S, Lord AM, Lee S, Stoick-Cooper CL, et al. Genetic interaction of PGE2 and Wnt signaling regulates developmental specification of stem cells and regeneration. Cell 2009;136(6):1136–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Charafe-Jauffret E, Monville F, Ginestier C, Dontu G, Birnbaum D, Wicha MS. Cancer stem cells in breast: current opinion and future challenges. Pathobiology 2008;75(2):75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Vermeulen L, De Sousa EMF, van der Heijden M, Cameron K, de Jong JH, Borovski T, et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nature Cell Biology 2010;12(5):468–76. [DOI] [PubMed] [Google Scholar]
- [57].Nejak-Bowen KN, Monga SP. Beta-catenin signaling, liver regeneration and hepatocellular cancer: sorting the good from the bad. Seminars in Cancer Biology 2010;21(1):44–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Agarwal JR, Matsui W. Multiple myeloma: a paradigm for translation of the cancer stem cell hypothesis. Anticancer Agents in Medicinal Chemistry 2010;10(2):116–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Wang R, Liang J, Yu HM, Liang H, Shi YJ, Yang HT. Retinoic acid maintains self-renewal of murine embryonic stem cells via a feedback mechanism. Differentiation 2008;76(9):931–45. [DOI] [PubMed] [Google Scholar]
- [60].Groen RW, Oud ME, Schilder-Tol EJ, Overdijk MB, ten Berge D, Nusse R, et al. Illegitimate WNT pathway activation by beta-catenin mutation or autocrine stimulation in T-cell malignancies. Cancer Research 2008;68(17): 6969–77. [DOI] [PubMed] [Google Scholar]
- [61].Jost E, Schmid J, Wilop S, Schubert C, Suzuki H, Herman JG, et al. Epigenetic inactivation of secreted Frizzled-related proteins in acute myeloid leukaemia. British Journal of Haematology 2008;142(5):745–53. [DOI] [PubMed] [Google Scholar]
- [62].Zhao C, Blum J, Chen A, Kwon HY, Jung SH, Cook JM, et al. Loss of beta-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell 2007;12(6):528–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Takahashi-Yanaga F, Kahn M. Targeting Wnt signaling: can we safely eradicate cancer stem cells? Clinical Cancer Research 2010;16(12):3153–62. [DOI] [PubMed] [Google Scholar]
- [64].Wend P, Holland JD, Ziebold U, Birchmeier W. Wnt signaling in stem and cancer stem cells. Seminars in Cell & Developmental Biology 2010;21(8): 855–63. [DOI] [PubMed] [Google Scholar]
- [65].Yook JI, Li XY, Ota I, Hu C, Kim HS, Kim NH, et al. A Wnt-Axin2-GSK3beta cascade regulates Snail1 activity in breast cancer cells. Nature Cell Biology 2006;8(12):1398–406. [DOI] [PubMed] [Google Scholar]
- [66].Sekiya S, Suzuki A. Glycogen synthase kinase 3 beta-dependent Snail degradation directs hepatocyte proliferation in normal liver regeneration. Proceedings of the National Academy of Sciences of the United States of America 2011;108(27):11175–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Heuberger J, Birchmeier W. Interplay of cadherin-mediated cell adhesion and canonical Wnt signaling. Cold Spring Harbor Symposia on Quantitative Biology 2010;2(2):a002915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Valsesia-Wittmann S, Magdeleine M, Dupasquier S, Garin E, Jallas AC, Combaret V, et al. Oncogenic cooperation between H-Twist and N-Myc overrides failsafe programs in cancer cells. Cancer Cell 2004;6(6):625–30. [DOI] [PubMed] [Google Scholar]
- [69].Pham CG, Bubici C, Zazzeroni F, Knabb JR, Papa S, Kuntzen C, et al. Upregulation of Twist-1 by NF-kappaB blocks cytotoxicity induced by chemotherapeutic drugs. Molecular and Cellular Biology 2007;27(11):3920–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Maestro R, Dei Tos AP, Hamamori Y, Krasnokutsky S, Sartorelli V, Kedes L, et al. Twist is a potential oncogene that inhibits apoptosis. Genes & Development 1999;13(17):2207–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Wu WS, Heinrichs S, Xu D, Garrison SP, Zambetti GP, Adams JM, et al. Slug antagonizes p53-mediated apoptosis of hematopoietic progenitors by repressing puma. Cell 2005;123(4):641–53. [DOI] [PubMed] [Google Scholar]
- [72].Mancini M, Petta S, Iacobucci I, Salvestrini V, Barbieri E, Santucci MA. Zinc-finger transcription factor slug contributes to the survival advantage of chronic myeloid leukemia cells. Cellular Signalling 2010;22(8):1247–53. [DOI] [PubMed] [Google Scholar]
- [73].Rho JK, Choi YJ, Lee JK, Ryoo BY, Na II, Yang SH, et al. Epithelial to mesenchymal transition derived from repeated exposure to gefitinib determines the sensitivity to EGFR inhibitors in A549, a non-small cell lung cancer cell line. Lung Cancer 2009;63(2):219–26. [DOI] [PubMed] [Google Scholar]
- [74].Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proceedings of the National Academy of Sciences of the United States of America 2009;106(33):13820–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Chaffer CL, Brennan JP, Slavin JL, Blick T, Thompson EW, Williams ED. Mesenchymal-to-epithelial transition facilitates bladder cancer metastasis: role of fibroblast growth factor receptor-2. Cancer Research 2006;66(23):11271–8. [DOI] [PubMed] [Google Scholar]
- [76].Spaderna S, Schmalhofer O, Hlubek F, Berx G, Eger A, Merkel S, et al. A transient, EMT-linked loss of basement membranes indicates metastasis and poor survival in colorectal cancer. Gastroenterology 2006;131(3):830–40. [DOI] [PubMed] [Google Scholar]
- [77].Kirchner T, Brabletz T. Tumor patterning: analogies of neoplastic morphogenesis with embryogenesis. Verhandlungen der Deutschen Gesellschaft fur Pathologie 2000;84:22–7. [PubMed] [Google Scholar]
- [78].Hay ED, Zuk A. Transformations between epithelium and mesenchyme: normal, pathological, and experimentally induced. American Journal of Kidney Diseases 1995;26(4):678–90. [DOI] [PubMed] [Google Scholar]
- [79].Boutet A, De Frutos CA, Maxwell PH, Mayol MJ, Romero J, Nieto MA. Snail activation disrupts tissue homeostasis and induces fibrosis in the adult kidney. EMBO Journal 2006;25(23):5603–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Zeisberg M, Hanai J, Sugimoto H, Mammoto T, Charytan D, Strutz F, et al. BMP-7 counteracts TGF-beta1-induced epithelial-to-mesenchymal transition and reverses chronic renal injury. Nature Medicine 2003;9(7):964–8. [DOI] [PubMed] [Google Scholar]
- [81].Kimelman D, Christian JL, Moon RT. Synergistic principles of development: overlapping patterning systems in Xenopus mesoderm induction. Development 1992;116(1):1–9. [DOI] [PubMed] [Google Scholar]
- [82].Oft M, Peli J, Rudaz C, Schwarz H, Beug H, Reichmann E. TGF-beta 1 and Ha-Ras collaborate in modulating the phenotypic plasticity and invasiveness of epithelial tumor cells. Genes & Development 1996;10(19):2462–77. [DOI] [PubMed] [Google Scholar]
- [83].Yang MH, Wu MZ, Chiou SH, Chen PM, Chang SY, Liu CJ, et al. Direct regulation of TWIST by HIF-1alpha promotes metastasis. Nature Cell Biology 2008;10(3):295–305. [DOI] [PubMed] [Google Scholar]
- [84].Brown KA, Aakre ME, Gorska AE, Price JO, Eltom SE, Pietenpol JA, et al. Induction by transforming growth factor-beta1 of epithelial to mesenchymal transition is a rare event in vitro. Breast Cancer Research 2004;6(3):R215–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Downs KM. The enigmatic primitive streak: prevailing notions and challenges concerning the body axis of mammals. Bioessays 2009;31(8): 892–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Scheel C, Eaton EN, Li SH, Chaffer CL, Reinhardt F, Kah KJ, et al. Paracrine and autocrine signals induce and maintain mesenchymal and stem cell States in the breast. Cell 2011;145(6):926–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, et al. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Chaffer CL, Brueckmann I, Scheel C, Kaestli AJ, Wiggins PA, Rodrigues LO, et al. Normal and neoplastic nonstem cells can spontaneously convert to a stem-like state. Proceedings of the National Academy of Sciences of the United States of America 2011;108(19):7950–5. [DOI] [PMC free article] [PubMed] [Google Scholar]