Abstract
Introduction
Patients with COPD have increased respiratory loads and altered blood gases, both of which affect vascular function and sympathetic activity. Sleep, particularly rapid eye movement (REM) sleep, is known to exacerbate hypoxia and respiratory loads. Therefore, we hypothesize that nasal high flow (NHF), which lowers ventilatory loads, reduces sympathetic activity during sleep and that this effect depends on COPD severity.
Methods
We performed full polysomnography in COPD patients (n=17; FEV1, 1.6±0.6 L) and in matched controls (n=8). Participants received room air (RA) at baseline and single night treatment with O2 (2 L/min) and NHF (20 L/min) in a random order. Finger pulse wave amplitude (PWA), a measure of vascular sympathetic tone, was assessed by photoplethysmography. Autonomic activation (AA) events were defined as PWA attenuation ≥30% and indexed per hour for sleep stages (AA index [AAI]) at RA, NHF, and O2).
Results
In COPD, sleep apnea improved following O2 (REM-apnea hypopnea index [AHI] with RA, O2, and NHF: 18.6±20.9, 12.7±18.1, and 14.4±19.8, respectively; P=0.04 for O2 and P=0.06 for NHF). REM-AAI was reduced only following NHF in COPD patients (AAI-RA, 21.5±18.4 n/h and AAI-NHF, 9.9±6.8 n/h, P=0.02) without changes following O2 (NHF-O2 difference, P=0.01). REM-AAI reduction was associated with lung function expressed as FEV1 and FVC (FEV1: r=−0.59, P=0.001; FEV1/FVC: r=−0.52 and P=0.007).
Conclusion
NHF but not elevated oxygenation reduces peripheral vascular sympathetic activity in COPD patients during REM sleep. Sympathetic off-loading by NHF, possibly related to improved breathing mechanics, showed a strong association with COPD severity.
Keywords: COPD, sleep, nasal high flow, oxygen therapy, sympathetic activity, pulse wave amplitude
Introduction
Although sleep in normal individuals provides a restful period for both the pulmonary and cardiovascular systems, COPD patients often exhibit marked worsening of breathing, arterial blood gases, and hemodynamic function during sleep, particularly during rapid eye movement (REM) sleep.1–4 These changes resemble those seen during acute exacerbation and worsening of hyperinflation and are associated with increased morbidity and mortality.2,5,6 Thus, the assessment of breathing and vascular function during sleep may provide an opportunity to identify patients at risk for exacerbation and to determine treatment effects targeting prevention of disease progression.
Long-term O2 therapy (LTOT) is the standard therapy for chronic respiratory failure in patients with advanced COPD.7 LTOT improves survival and health-related quality of life. However, O2 has not been proven successful in patients with mild to moderate COPD and isolated nocturnal hypoxia.8 Possible explanations include the inability of supplemental O2 to reduce sympathetic nerve activity in patients with stable heart failure9 or vascular stiffness in awake patients with stable COPD.10 In contrast, nasal high flow (NHF) therapy improves breathing mechanics by dead space clearance, resolution of mild upper airway obstruction, and reduction of dynamic hyperinflation.11 Outcome studies addressing the effects of NHF in COPD are sparse, but limited data indicate positive effects of NHF in patients with acute and chronic respiratory failure.12–14 In addition, studies evaluating possible mechanisms by which NHF may provide benefit in COPD patients, such as improvement in cardiovascular or autonomic factors, are lacking. The lack of data is partially explained by the methodological challenges in reliably assessing autonomic vascular parameters in this patient group.
Finger vascular tone assessed by pulse wave amplitude (PWA) changes has been shown to highly correlate with sympathetic activation of vascular sympathetic alpha receptors.15 Vascular tone is sensitive to autonomic changes induced by sleep stages,16 and both local and systemic changes pharmacologically induced by alpha receptor blockade.17,18 Assessment of vascular tone has been used to identify increased sympathetic activity in chronic cardiovascular diseases such as systemic hypertension and cardiac failure.19 Recently, finger vascular tone obtained during sleep has been shown to be an independent predictor for cardiovascular risk in patients with sleep disordered breathing.20 Therefore, we opted to use digital pulse wave analysis as a non-invasive, continuous measure for the assessment of autonomic vascular function during sleep in COPD patients.
In the current study, we examined vascular tone during sleep in response to O2 and NHF. We conducted a randomized controlled trial in mild to moderate COPD patients and controls matched for age, sex, and smoking status. We hypothesized that 1) NHF but not O2 reduces sympathetic activity and that 2) improvements in sympathetic activity are dependent on lung function and therefore 3) effects of NHF on sympathetic activity is more pronounced in patients with COPD than controls.
Methods
Study settings
This single-center study was conducted at the Johns Hopkins Clinical Research Unit in conjunction with the Division of Pulmonary and Critical Care Medicine. The study was performed according to the Declaration of Helsinki. Written and oral informed consent was obtained from each participant for this study, which was approved by the Johns Hopkins Medical Institution Human Investigations Review Board. The study was registered at ClinicalTrials.gov (NCT01764165).
Study participants and procedures
Subjects were recruited from the community. A total of 26 subjects met inclusion criteria for the current study and accepted a 4-day (three nights) stay at the research unit. COPD patients (n=18) had spirometry confirmed airway obstruction according to the GOLD criteria (post-bronchodilator FEV1/FVC, <0.7). One COPD patient did not meet the signal quality criteria during all three experimental conditions and was excluded from the final analysis. Additional inclusion criteria were age >40 years, smoking >10 pack years, body mass index (BMI) <40 kg/m2, and an apnea hypopnea index (AHI) of <10 events/hour. Exclusion criteria included impaired renal function, acute illness including COPD exacerbation within the past 6 weeks, unstable cardiovascular disease, sleep efficacy <30%, respiratory failure with daytime O2 saturation (SaO2) level of ≤88%, and use of sedative/hypnotic medication. Controls were defined by normal spirometry data at rest (FEV1/FVC, >0.8) and were matched with a ratio of 2:1 for age, BMI, and smoking status with history of at least 10 pack years (n=8). Blood gases analysis were assessed at baseline in both COPD patients and controls.
During the study, patients and controls performed three polysomnographic (PSG) sleep recordings: one at room air (RA; baseline), one during single night treatment with NHF, and one with supplemental O2 (2L/min via nasal tube). Supplemental O2 was used as a control to NHF because we expect COPD patients to develop REM-related hypoxia. Treatment order was randomized using a computer-generated coding system performed by a unit within the hospital not linked to the study. NHF was applied with a commercially available device (MyAirvo2; F&P Healthcare, Auckland, New Zealand) using a flow rate of 20 L/min and air temperature of 34°C–37°C. Adaptation to NHF and supplemental O2 was performed during wakefulness in the evening prior to the study night.
Assessment of sleep
A standard PSG study was performed including the assessment of sleep (electrooculogram, electromyogram, and electroencephalogram), breathing (air flow by thermistors and nasal pressure, thorax, and abdominal efforts by respiratory plethysmography), and blood oxygenation/CO2 content (saturation, transcutaneous partial pressure of carbon dioxide [pCO2]). A minimum recording length of 8 hours including at least 6 hours of treatment (O2 or NHF) was obtained. Sleep stages and breathing events were scored according to the American Academy of Sleep Medicine criteria (AASM)21 including the alternative hypopnea criteria. Intermittent hypoxia was assessed as O2 desaturation index of 3% or more. In patients with borderline reductions in SaO2, periodic breathing and hypopneas often are associated with a desaturation of 3% or more even in the absence of an arousal. On RA, these events would be counted, while on O2, these events do not meet the criteria of the AASM and thus would not constitute the respiratory disturbance index (RDI). For consistency of scoring, we did not consider modifying the AASM criteria accordingly.
Analysis of the digital pulse wave
A standard pulse oximeter was applied during PSG (Embla N7000; Natus, Pleasanton, CA, USA). Overnight photoplethysmography (oximeter probe) was used for the assessment of the pulse wave signal (AC-signal component, sampling frequency 100 Hz). Comparable to the previously published methodology,22 PWA was calculated for each pulse curve, and amplitude attenuations of at least 30% from the preceding baseline (20 s) in wave amplitude were automatically calculated using default software (REMlogic; Natus). Amplitude attenuations were indexed per hour of actual sleep time as autonomic activation index (AAI) for different sleep stages: REM sleep; non-REM (NREM) stages 1, 2, and 3 (N1, N2, and N3, respectively); as well as during wakefulness (wake).
Statistics
Descriptive statistics are presented as mean±SD. Statistical significance was set at P<0.05, two tailed. Between-group differences were tested by Student’s t-test, Kruskal–Wallis test, or the chi-squared test (for equally/not equally distributed data and distributions). After the exclusion of significant carryover or sequence effects between the different experimental conditions (P>0.1, respectively), within-group differences were tested by means of the paired Student’s t-test. Pearson’s correlation analysis assessed associations between changes in AAI by treatment and lung function parameters at baseline. Analyses were performed using SPSS (version 22.0; IBM Corporation, Armonk, NY, USA).
Results
Baseline
The mean age of COPD patients was 55.5±6.4 years and of controls was 58.1±7.4 years, and participants were predominantly female. Spirometry tests and arterial blood gas analysis demonstrated mild to moderate COPD in the patient group and normal findings in the controls. Demographics and anthropometric data did not differ between COPD and control subjects according to the matching procedure (Table 1).
Table 1.
Anthropometric data and pulmonary function (patients and controls)
| Variable | Control (n=8), mean±SD | COPD (n=17), mean±SD | Statistics |
|---|---|---|---|
| Age (years) | 55.5±6.4 | 58.1±7.4 | ns |
| Female sex (%) | 87 | 65 | ns |
| Height (in) | 63.9±3.9 | 66.1±3.9 | ns |
| Weight (lbs) | 155.5±35.8 | 163.9±43.1 | ns |
| BMI (kg/m2) | 26.8±6.1 | 26.2±5.9 | ns |
| Systolic BP | 124.5±11.1 | 127.0±20.3 | ns |
| Diastolic BP | 73.2±4.5 | 74.8±13.7 | ns |
| FVC (L) | 2.9±0.7 | 2.8±0.7 | ns |
| FEV1 (L) | 2.2±0.4 | 1.6±0.6 | 0.009 |
| FEV1 %PRED | 86.7±18.2 | 56.8±18.1 | 0.001 |
| FEV1/FVC | 77.9±7.0 | 55.3±10.5 | <0.001 |
| DLCO (mL/min/mmHg) | 17.8±5.3 | 16.0±6.0 | ns |
| pCO2 (mmHg)a | 40.7±2.7 | 40.6±4.9 | ns |
| PO2 (mmHg)a | 80.5±15.6 | 80.4±23.4 | ns |
| HCO3 (mmol/L)a | 25.0±2.0 | 25.7±2.2 | ns |
| O2 saturation (%)a | 95.9±1.3 | 94.3±3.9 | ns |
| Base excess (mmol/L)a | 0.7±1.9 | 1.5±1.8 | ns |
Note:
Blood gases available in 7 controls and 13 COPD patients.
Abbreviations: BMI, body mass index; BP, blood pressure; DLCO, diffusing capacity of the lungs for carbon monoxide; ns, not significant; pCO2, partial pressure of carbon dioxide; PO2, partial pressure of O2; PRED, predicted.
Sleep quality and the degree of sleep disordered breathing did not differ between COPD patients and controls (Table 2). Sleep apnea indices during REM sleep increased slightly without significant differences between groups. Overnight saturation and transcutaneous pCO2 during NREM sleep did not differ.
Table 2.
Sleep quality and sleep disordered breathing at baseline
| Variable | Control (n=8), mean±SD | COPD (n=17), mean±SD | Statistics |
|---|---|---|---|
| Total sleep time (minutes) | 396.3±39.8 | 376.6±87.7 | n.s. |
| Sleep efficiency (%) | 84.6±4.3 | 79.6±17.9 | n.s. |
| Sleep latency (minutes) | 15.8±16.9 | 14.9±20.7 | n.s. |
| REM sleep latency (minutes) | 89.2±33.6 | 99.3±59.5 | n.s. |
| WASO (minutes) | 55.4±15.4 | 79.4±67.4 | n.s. |
| Sleep stage N1 (%) | 11.1±6.0 | 13.9±9.0 | n.s. |
| Sleep stage N2 (%) | 50.2±14.7 | 52.0±9.0 | n.s. |
| Sleep stage N3 (%) | 16.7±11.5 | 15.7±11.7 | n.s. |
| Sleep stage REM (%) | 21.9±8.5 | 18.4±6.2 | n.s. |
| Arousal index (n/h) | 10.3±6.6 | 11.0±5.3 | n.s. |
| Arousal index NREM (n/h) | 9.4±5.4 | 10.0±6.6 | n.s. |
| Arousal index REM (n/h) | 9.3±7.8 | 12.2±10.4 | n.s. |
| RDI (n/h) | 10.9±9.6 | 8.0±7.8 | n.s. |
| RDI NREM (n/h) | 9.5±8.2 | 5.9±7.1 | n.s. |
| RDI REM (n/h) | 17.0±19.4 | 18.6±20.9 | n.s. |
| Average SaO2 (%) | 95.2±1.8 | 93.9±2.9 | n.s. |
| tcCO2 mean awake (mmHg) | 39.6±6.3 | 42.4±6.1 | n.s. |
| tcCO2 mean NREM (mmHg) | 43.0±7.0 | 43.7±5.5 | n.s. |
| tcCO2 mean REM (mmHg) | 50.6±12.9 | 53.0±12.5 | n.s. |
Abbreviations: n/h, numbers/hour; n.s., not significant; N1–N3, non-rapid eye movement sleep stages 1–3; RDI, respiratory disturbance index; NREM, non-REM; REM, rapid eye movement sleep; SaO2, O2 saturation; tcCO2, transcutaneous carbon dioxide; WASO, wakefulness after sleep onset.
Effect of NHF and O2 on sleep quality and quantity
Sleep latency was significantly longer during the night on O2 compared to NHF and RA in the COPD group (20.4±28.9 minutes vs 14.3±24.0 vs 14.9±20.7 minutes, P=0.01; Table 3). There was no difference in other standard sleep quality indices, and % distribution of sleep stages did not differ within and between groups.
Table 3.
Treatment effects of NHF and supplemental O2 on sleep, oxygenation, and sleep disordered breathing
| Variable | Controls | COPD | ||||
|---|---|---|---|---|---|---|
| Condition | Room air | O2 | NHF | Room air | O2 | NHF |
| Sleep quality | ||||||
| TST (minutes) | 396.3±39.8 | 371.0±68.3 | 362.1±65.0 | 376.6±87.7 | 347.9±94.9 | 361.0±50.6 |
| Sleep efficiency (%) | 84.6±4.3 | 81.8±15.0 | 79.1±13.1 | 79.6±17.9 | 74.4±19.4 | 78.4±11.6 |
| S-latency (minutes) | 15.8±16.9 | 12.5±11.0 | 18.8±22.9 | 14.9±20.7 | 20.4±28.9a | 14.3±24.0 |
| REM latency (minutes) | 89.2±33.6 | 104.1±81.0 | 117.1±50.7 | 99.3±59.5 | 138.8±79.6 | 158.8±89.8 |
| WASO (minutes) | 55.4±15.4 | 71.2±62.8 | 77.6±50.2 | 79.4±67.4 | 97.5±77.8 | 87.0±52.6 |
| N1 (%) | 11.1±6.0 | 9.9±5.1 | 12.6±5.2 | 13.9±9.0 | 14.2±9.3 | 13.6±11.3 |
| N2 (%) | 50.2±14.7 | 56.7±15.9 | 56.7±12.2 | 52.0±9.0 | 51.7±14.4 | 57.6±14.4 |
| N3 (%) | 16.7±11.5 | 14.1±11.6 | 12.1±10.5 | 15.7±11.7 | 15.4±14.5 | 13.4±9.7 |
| REM (%) | 21.9±8.5 | 19.4±8.5 | 18.7±7.6 | 18.4±6.2 | 18.6±6.4 | 15.3±8.7 |
| Arousal index | 10.3±6.6 | 9.2±6.1 | 11.1±12.2 | 11.0±5.3 | 10.5±6.4 | 14.6±10.5 |
| ARI NREM (n/h) | 9.4±5.4 | 10.8±8.6 | 11.4±13.0 | 10.0±6.6 | 11.6±12.0 | 15.1±12.1 |
| ARI REM (n/h) | 9.3±7.8 | 7.8±4.3 | 7.5±7.1 | 12.2±10.4 | 10.4±7.8 | 11.1±11.4 |
| Respiration during sleep | ||||||
| RDI (n/h) | 10.9±9.6 | 3.7±2.2b | 13.7±16.9 | 8.0±7.8 | 6.0±6.2b | 7.5±8.0 |
| RDI NREM (n/h) | 9.5±8.2 | 3.1±2.3 | 14.0±20.8 | 5.9±7.1 | 5.2±6.3 | 6.3±7.1 |
| RDI REM (n/h) | 17.0±19.4 | 8.3±9.2b | 15.4±18.0 | 18.6±20.9 | 12.7±18.1c | 14.4±19.8 |
| 3% desaturation index | 14.3±13.1 | 4.3±2.7b | 18.2±22.6 | 10.1±8.8 | 6.7±6.9c | 8.6±8.3 |
| Average SaO2 (%) | 95.2±1.8 | 97.8±1.0d | 95.3±1.9 | 93.9±2.9 | 97.2±1.8d | 93.7±3.7 |
| tcCO2 awake | 39.6±6.3 | 38.3±3.7 | 41.4±7.6 | 42.4±6.1 | 41.6±5.0 | 44.9±9.5 |
| tcCO2 NREM | 43.0±7.0 | 40.3±4.3 | 42.0±6.7 | 43.7±5.5 | 44.5±5.1 | 46.2±9.6 |
| CO2max NREM | 50.6±12.9 | 46.5±5.3 | 46.7±8.1 | 53.0±12.5 | 52.4±7.9 | 53.6±13.0 |
Note:
P<0.01,
P<0.05,
P<0.1,
P<0.001.
Abbreviations: ARI, arousal index; NHF, nasal high flow; NREM, non-REM; n.s., not significant; N1–N3, NREM sleep stages 1–3; RDI, respiratory disturbance index; REM, rapid eye movement; SaO2, O2 saturation; S-latency, sleep latency in minutes; tcCO2, transcutaneous carbon dioxide in mmHg; TST, total sleep time; WASO, wakefulness after sleep onset.
Effect of NHF and supplemental O2 on nocturnal hypoxia and sleep disordered breathing
Supplemental O2 increased mean overnight saturation compared to baseline and NHF in both COPD patients and controls (Table 3). No changes in transcutaneous CO2 were observed following O2 and NHF treatments. In both groups, the RDI was comparable between the baseline and NHF treatment nights in NREM and REM sleep. In contrast, the RDI was reduced during O2 treatment, which could be explained by the scoring rules as mentioned above in “Methods” section (Table 3).
Effect of NHF and O2 on sympathetic vascular tone
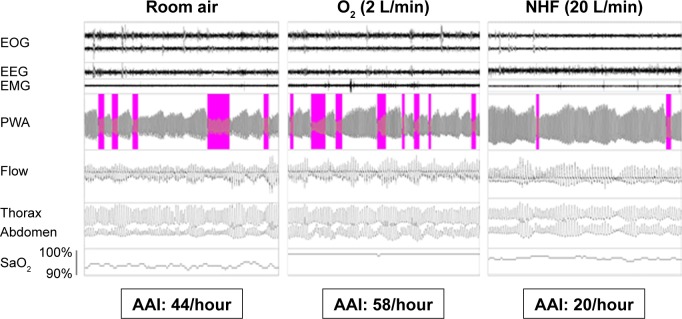
Overnight PWA variability at baseline did not differ between the COPD patients and the controls (Table 3). During NREM sleep, NHF and O2 therapy did not systematically change PWA attenuations. In addition, the duration of PWA events was similar in all three conditions (data not shown). In contrast, NHF treatment decreased AAI during REM sleep significantly in the COPD patients but not in controls (Table 4). NHF reduced AAI significantly better than O2 during REM sleep in COPD patients (Table 4; Figure 1 [example in a single patient] and Figure 2 [pooled data]).
Table 4.
AA indices during REM sleep, NREM sleep, and the NREM sleep stages N1, N2, and N3
| Variable | Controls | COPD | ||||
|---|---|---|---|---|---|---|
| Condition | Room air | O2 | NHF | Room Air | O2 | NHF |
| AAI-REM | 28.2±18.4 | 37.0±21.3 | 35.6±29.6 | 21.5±18.4 | 22.7±19.8 | 9.9±6.8a |
| AAI-NREM | 17.5±10.4 | 16.2±14.1 | 17.4±14.6 | 11.4±11.1 | 13.4±12.7 | 12.4±12.9 |
| AAI-N1 | 23.7±15.1 | 18.5±14.2 | 25.5±18.0 | 12.8±8.2 | 13.6±9.3 | 14.3±10.3 |
| AAI-N2 | 18.8±11.5 | 16.1±15.0 | 17.4±14.8 | 11.9±12.5 | 13.4±12.2 | 12.0±13.4 |
| AAI-N3 | 9.8±8.5 | 14.6±16.3 | 8.6±8.8 | 7.1±12.6 | 11.4±17.2 | 9.0±17.6 |
Notes:
P=0.01. The AAIs are given as means±SD for the different sleep stages. P-values are listed for between-group (COPD and controls) and within-group differences (three treatment conditions). REM sleep of at least 15 minutes duration was observed in 15 of 17 COPD patients and all eight controls.
Abbreviations: AA, autonomic activation; AAI, autonomic activation index; NHF, nasal high flow; NREM, non-REM; N1–N3, NREM sleep stages 1–3; REM, rapid eye movement.
Figure 1.
Example of changes in PWA during REM sleep in one COPD patient on three different conditions: RA, O2 supplementation, and NHF treatment.
Notes: Autonomic activation events were defined as a reduction in PWA of 30% or more compared to a prior baseline period. Automatically scored autonomic activation events are highlighted in the pulse wave tracings.
Abbreviations: AAI, autonomic activation index; EEG, electroencephalogram; EMG, electromyogram; EOG, electrooculogram; NHF, nasal high flow; PWA, pulse wave amplitude; RA, room air; REM, rapid eye movement.
Figure 2.

Modification of vascular AAI with nasal high flow and supplemental O2 compared to the room air condition during REM sleep.
Note: AAI values (mean ± SD) are shown for COPD patients during REM sleep.
Abbreviations: AAI, autonomic activation index; RA, room air; REM, rapid eye movement.
In REM sleep the reduction in AAI was associated with the degree of lung function impairment captured as FEV1 and FEV1/FVC ratio (r=−0.59, P=0.002, and r=0.52, P=0.007, respectively; Figure 3) with those with greater lung function impairment showing a greater change in autonomic vascular activation in response to NHF. Individuals with a FEV1<1.65 L showed a significant reduction in AAI compared to those with intermediate and normal lung function (FEV1 >2 L; ANOVA: P=0.005; Figure 4).
Figure 3.

Correlation between the reduction of vascular AAI during REM sleep by NHF compared with the room air condition (y-axis) and impaired lung function assessed as FEV1 (x-axis).
Note: Note that a reduction of REM-AAI during NHF treatment translates to a positive value on the y-axis and vice versa.
Abbreviations: AAI, autonomic activation index; NHF, nasal high flow; REM, rapid eye movement.
Figure 4.
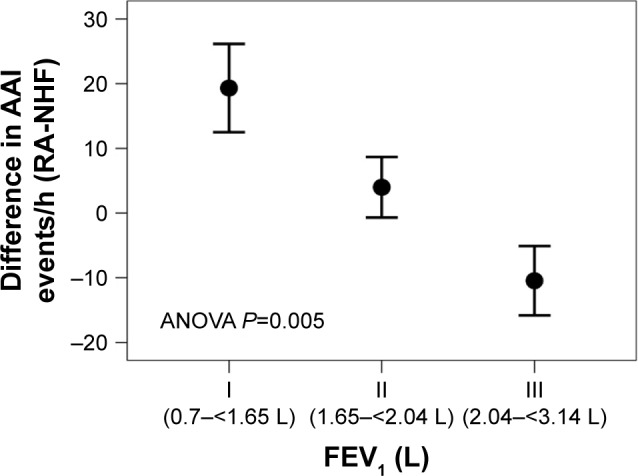
Relation between FEV1 (tertiles I–III) and the difference in sympathetic activity (AAI) between the room air condition and NHF during REM sleep.
Notes: All 25 subjects have been included in this analysis. FEV1 was grouped according to tertiles. Note that a reduction of REM-AAI during NHF treatment translates to a positive value on the y-axis. Individuals with the lowest lung function (0.7-<1.65L) showed a significant reduction in AAI compared to those with intermediate (1.65-<2.04L) and normal (2.04-<3.14L) lung function.
Abbreviations: AAI, autonomic activation index; NHF, nasal high flow; REM, rapid eye movement.
Discussion
In this study of representative patients with COPD, we provide several novel findings related to NHF, a novel treatment option for COPD patients. First, NHF, but not O2, reduced vascular sympathetic activity during sleep. Second, reductions in sympathetic activity were sleep state dependent, with greatest reductions seen in REM sleep. Third, reductions in sympathetic activity were seen in patients with COPD but not in smoking controls. Finally, the reductions in sympathetic activity correlate with decline in lung function, and a FEV1 of <1.65 L appears to be the threshold at which NHF reduces sympathetic load during REM sleep. All in all, our data indicate that COPD patients may have increased sympathetic activity during REM sleep that can be reduced by NHF.
PWA as a marker for sympathetic activity during sleep
PWA of the finger arteries is a composite measure of temperature regulation and vascular and autonomic functions.23–27 Digital PWA is influenced by endothelial vascular properties and blood flow, proximal arteriovenous shunts, and local environmental factors (pH, metabolites, and circulating humoral factors). However, rapid changes of PWA are determined by the degree of sympathetic nerve activity24 or by hypoxic and arousal responses to sleep apnea.28,29 Indeed, increased sympathetic activity caused by arousals from sleep apnea markedly decreases PWA due to sympathetic activation of the vascular alpha receptors.17 In the same study, nocturnal supplemental O2 was not able to modify the PWA response similar to the findings in our study. Rapid changes in PWA during sleep in a population-based sample were also positively associated with elevations in daytime blood pressure and the diagnosis of hypertension, both known to be linked to increased sympathetic activity.19 Finally, the treatment of hypertension with systemic pharmacological sympathetic blockade of alpha receptors significantly reduced both PWA and blood pressure swings related to sleep apnea.18 Although PWA analysis was performed for the first time in the COPD patient population, there is no indication that physiology of finger pulse wave is different in this patients population. Indeed, previous studies studied vascular stiffness as an indirect measure of sympathetic activity in COPD patients. Pulse wave analysis showed a strong association with applanation tonometry as the gold standard,34 and increased vascular stiffness during REM sleep was documented in COPD patients but not in controls.35 In summary, experimental data during sleep strongly link PWA attenuations with sympathetic activity, suggesting that the reduction of AAI events after NHF treatment seen in the current study can be interpreted as a marker of modified sympathetic nervous system output.
Effect of O2 and NHF on sympathetic activity
COPD is frequently associated with alterations in gas exchange and ventilatory control during sleep.1,2 The prevalence of isolated nocturnal hypoxemia has been reported in up to 70% of COPD patients, even in patients with normal daytime SaO2.30,31 Although clinical trials did not demonstrate survival benefits of nocturnal O2 therapy, it is commonly prescribed to prevent the development of pulmonary hypertension and reduce hypoxic cardiovascular stress particularly during REM sleep.7,32 However, the effects of O2 on cardiovascular stress during sleep have not been examined.
As mentioned above, we measured the sympathetic activity via finger plethysmography, which has been demonstrated to be a sensitive marker for activation of the alpha sympathetic nervous system. Although supplemental O2 improved SaO2 during sleep, it was not associated with a reduction in alpha sympathetic activity during both NREM and REM sleep. Thus, restoration of arterial blood gases during NREM and REM sleep appears not to protect the arterial vascular system during sleep. In contrast, NHF was associated with a significant reduction in sympathetic activity during REM sleep compared to baseline and supplemental O2. The reductions in sympathetic activity compared to baseline suggest that COPD patients have a modifiable component of sympathetic activity during REM sleep. This is in line with the data reporting increased muscle sympathetic activity during wakefulness in COPD patients.33 The beneficial effects of NHF on sympathetic activity suggest that mechanical offloading of the respiratory system by this intervention rather than increase in oxygenation may also affect peripheral alpha sympathetic activity. Although we did not determine the precise mechanisms of NHF on respiratory mechanics, it is likely that NHF reduced ventilatory loads such as reducing dead space ventilation or ventilatory demand according to the previous data.11,13,36 Further evidence is given by our findings that sympathetic offloading during NHF treatment correlated strongly with the degree of lung function impairment. This finding supports the hypothesis that loading of respiratory mechanics in COPD patients is associated with adverse effects on autonomic and vascular functions.
Strength and limitations
Several strengths of our study need to be mentioned. First, to our knowledge, this is the first study reporting the effect of NHF on sympathetic activity during sleep. Our state-of-the art study protocol combined both a case–control and a randomized treatment design to evaluate both disease- and treatment-specific effects on nocturnal sympathetic activity. Furthermore, our PWA analysis method as a surrogate marker of sympathetic activity is well established and supported by a number of experimental and interventional data.15,17,18,25 Limitations of our study include the limited number of patients and controls investigated. Reduced sample size increases the possibility of a type II error, particularly in the control group. However, our findings on reduced sympathetic activity during REM sleep after NHF treatment are very robust, and the results can be verified when other thresholds for PWA-related autonomic activations are defined (eg, AA threshold defined as >10% or >50% PWA attenuation from baseline; data not shown). Treatment duration was limited to 1 night in a group of mild to moderate COPD, which clearly limits any conclusion to be drawn on the overall clinical significance of our findings. Indeed, our data clearly indicate that the use of NHF may have even stronger beneficial effects on sympathetic activity during sleep in patients with more compromised lung function seen in COPD patients stage III and IV. Finally, our results could be confounded by age, smoking status, and gender. The limited number of subjects did not allow to strengthen our correlation by doing some multivariate regression models. However, the current pilot study was designed to evaluate short-term effects to design long-term studies properly by addressing the effects of NHF and O2 on both ventilator and cardiovascular parameters. Those studies may include outcome parameters such as frequency of acute exacerbations and death. Moreover since a majority of death is due to cardiovascular morbidity, reductions in autonomic function may become a specific treatment target for NHF therapy to improve cardiovascular outcome of COPD patients.
Clinical implications
Our data suggest for the first time that NHF may not only have beneficial effects on respiratory stability during sleep but also reduces sympathetic activity during REM sleep in COPD patients. The observed association between reduction in sympathetic activity with NHF and the degree of impaired lung function suggests that worsening COPD disease severity is associated with increased sympathetic activity during sleep and that NHF may be used to mitigate cardiovascular burden of COPD patients. A FEV1 of <1.65 L appears to be the threshold for significant reductions in sympathetic activity. Thus, our data support the initiation of clinical trials addressing long-term effects of NHF in patients with the entire disease spectrum of COPD. Similarly, the findings of this study may be applicable to patients with other chronic disorders who may benefit from reductions in sympathetic activity.
Conclusion
In this study, we could demonstrate that improved breathing mechanics with single night treatment of NHF but not elevated oxygenation through supplemental O2 reduces vascular sympathetic activity in COPD patients during REM sleep. Sympathetic off-loading by NHF showed a strong association with the degree of COPD severity. The results encourage further research on the long-term efficacy of NHF in patients with different COPD diseases severities.
Acknowledgments
This study was supported by the Swedish Heart and Lung Foundation (HLF 20130488, 20150313, and 20160584), by grants from the Swedish state under the agreement between the Swedish government and the county councils, the ALF-agreement (ALFGBG 725601), and the National Institute of Health (R01 HL105546). This article was submitted as an abstract for the American Thoracic Society 2016 International Conference; May 13–18, 2016; San Francisco, CA, USA.
Footnotes
Author contributions
HS and LG take responsibility for the content of the manuscript, including the data and data analysis. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Flenley DC. Sleep in chronic obstructive lung disease. Clin Chest Med. 1985;6(4):651–661. [PubMed] [Google Scholar]
- 2.Mcnicholas WT, Verbraecken J, Marin JM. Sleep disorders in COPD: the forgotten dimension. Eur Respir Rev. 2013;22(129):365–375. doi: 10.1183/09059180.00003213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Donoghue FJ, Catcheside PG, Eckert DJ, Mcevoy RD. Changes in respiration in NREM sleep in hypercapnic chronic obstructive pulmonary disease. J Physiol. 2004;559(Pt 2):663–673. doi: 10.1113/jphysiol.2004.066084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Donoghue FJ, Catcheside PG, Ellis EE, et al. Australian trial of Noninvasive Ventilation in Chronic Airflow Limitation investigators. Sleep hypoventilation in hypercapnicchronic obstructive pulmonary disease: prevalence and associated factors. Eur Respir J. 2003;21(6):977–984. doi: 10.1183/09031936.03.00066802. [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR. Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med. 2010;182(3):325–331. doi: 10.1164/rccm.200912-1869OC. [DOI] [PubMed] [Google Scholar]
- 6.Fletcher EC, Donner CF, Midgren B, et al. Survival in COPD patients with a daytime PaO2 greater than 60 mm Hg with and without nocturnal oxyhemoglobin desaturation. Chest. 1992;101(3):649–655. doi: 10.1378/chest.101.3.649. [DOI] [PubMed] [Google Scholar]
- 7.Global Initiative for Chronic Obstruc tive Lung Disease GOLD 2017 Global Strategy for the Diagnosis, Manage ment and Prevention of COPD. [Accessed October 2, 2018]. Available from: https://goldcopd.org/gold-2017-global-strategy-diagnosis-management-prevention-copd/
- 8.Chaouat A, Weitzenblum E, Kessler R, et al. A randomized trial of nocturnal oxygen therapy in chronic obstructive pulmonary disease patients. Eur Respir J. 1999;14(5):1002–1008. doi: 10.1183/09031936.99.14510029. [DOI] [PubMed] [Google Scholar]
- 9.Andreas S, Bingeli C, Mohacsi P, Lüscher TF, Noll G. Nasal oxygen and muscle sympathetic nerve activity in heart failure. Chest. 2003;123(2):366–371. doi: 10.1378/chest.123.2.366. [DOI] [PubMed] [Google Scholar]
- 10.Bartels MN, Jelic S, Basner RC, Ngai P, Gonzalez JM, de Meersman RE. Supplemental oxygen increases arterial stiffness in chronic obstructive pulmonary disease. Respir Med. 2004;98(1):84–89. doi: 10.1016/j.rmed.2002.09.001. [DOI] [PubMed] [Google Scholar]
- 11.Biselli P, Grossman PR, Kirkness JP, et al. The effect of increased lung volume in chronic obstructive pulmonary disease on upper airway obstruction during sleep. J Appl Physiol (1985) 2015;119(3):266–271. doi: 10.1152/japplphysiol.00455.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frat JP, Thille AW, Mercat A, et al. FLORALI Study Group. REVA Network High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372(23):2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 13.Nilius G, Franke KJ, Domanski U, Rühle KH, Kirkness JP, Schneider H. Effects of nasal insufflation on arterial gas exchange and breathing pattern in patients with chronic obstructive pulmonary disease and hypercapnic respiratory failure. Adv Exp Med Biol. 2013;755:27–34. doi: 10.1007/978-94-007-4546-9_4. [DOI] [PubMed] [Google Scholar]
- 14.Bräunlich J, Seyfarth HJ, Wirtz H. Nasal High-flow versus noninvasive ventilation in stable hypercapnic COPD: a preliminary report. Multidiscip Respir Med. 2015;10(1):27. doi: 10.1186/s40248-015-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grote L, Zou D, Kraiczi H, Hedner J. Finger plethysmography – a method for monitoring finger blood flow during sleep disordered breathing. Respir Physiol Neurobiol. 2003;136(2–3):141–152. doi: 10.1016/s1569-9048(03)00090-9. [DOI] [PubMed] [Google Scholar]
- 16.Hedner J, White DP, Malhotra A, et al. Sleep staging based on autonomic signals: a multi-center validation study. J Clin Sleep Med. 2011;7(3):301–306. doi: 10.5664/JCSM.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zou D, Grote L, Eder DN, Peker Y, Hedner J. Obstructive apneic events induce alpha-receptor mediated digital vasoconstriction. Sleep. 2004;27(3):485–489. doi: 10.1093/sleep/27.3.485. [DOI] [PubMed] [Google Scholar]
- 18.Zou D, Grote L, Eder DN, Radlinski J, Hedner J, A Double-Blind HJ. A double-blind, crossover study of Doxazosin and Enalapril on peripheral vascular tone and nocturnal blood pressure in sleep apnea patients. Sleep Med. 2010;11(3):325–328. doi: 10.1016/j.sleep.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 19.Zou D, Grote L, Radlinski J, Eder DN, Lindblad U, Hedner J. Nocturnal pulse wave attenuation is associated with office blood pressure in a population based cohort. Sleep Med. 2009;10(8):836–843. doi: 10.1016/j.sleep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Grote L, Sommermeyer D, Zou D, Eder DN, Hedner J. Oximeter-based autonomic state indicator algorithm for cardiovascular risk assessment. Chest. 2011;139(2):253–259. doi: 10.1378/chest.09-3029. [DOI] [PubMed] [Google Scholar]
- 21.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications 1st Westchester. IL: American Academy of Sleep Medicine; 2007. For the American Academy of Sleep Medicine. editor. [Google Scholar]
- 22.Sommermeyer D, Zou D, Ficker JH, et al. Detection of cardiovascular risk from a photoplethysmographic signal using a matching pursuit algorithm. Med Biol Eng Comput. 2016;54(7):1111–1121. doi: 10.1007/s11517-015-1410-8. [DOI] [PubMed] [Google Scholar]
- 23.Burton AC. The range and variability of the blood flow in the human fingers and the vasomotor regulation of body temperature. American Journal of Physiology-Legacy Content. 1939;127(3):437–453. [Google Scholar]
- 24.Bini G, Hagbarth KE, Hynninen P, Wallin BG. Thermoregulatory and rhythm-generating mechanisms governing the sudomotor and vaso-constrictor outflow in human cutaneous nerves. J Physiol. 1980;306:537–552. doi: 10.1113/jphysiol.1980.sp013413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen J. Photoplethysmography and its application in clinical physiological measurement. Physiol Meas. 2007;28(3):R1–R39. doi: 10.1088/0967-3334/28/3/R01. [DOI] [PubMed] [Google Scholar]
- 26.Elgendi M. On the analysis of fingertip photoplethysmogram signals. Curr Cardiol Rev. 2012;8(1):14–25. doi: 10.2174/157340312801215782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chan GS, Fazalbhoy A, Birznieks I, Macefield VG, Middleton PM, Lovell NH. Spontaneous fluctuations in the peripheral photoplethysmographic waveform: roles of arterial pressure and muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2012;302(3):H826–H836. doi: 10.1152/ajpheart.00970.2011. [DOI] [PubMed] [Google Scholar]
- 28.Catcheside PG, Chiong SC, Mercer J, Saunders NA, Mcevoy RD. Noninvasive cardiovascular markers of acoustically induced arousal from non-rapid-eye-movement sleep. Sleep. 2002;25(7):797–804. doi: 10.1093/sleep/25.7.797. [DOI] [PubMed] [Google Scholar]
- 29.O’Donnell CP, Allan L, Atkinson P, Schwartz AR. The effect of upper airway obstruction and arousal on peripheral arterial tonometry in obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166(7):965–971. doi: 10.1164/rccm.2110072. [DOI] [PubMed] [Google Scholar]
- 30.Lewis CA, Fergusson W, Eaton T, Zeng I, Kolbe J. Isolated nocturnal desaturation in COPD: prevalence and impact on quality of life and sleep. Thorax. 2009;64(2):133–138. doi: 10.1136/thx.2007.088930. [DOI] [PubMed] [Google Scholar]
- 31.Sanders MH, Newman AB, Haggerty CL, et al. Sleep Heart Health Study. Sleep and sleep-disordered breathing in adults with predominantly mild obstructive airway disease. Am J Respir Crit Care Med. 2003;167(1):7–14. doi: 10.1164/rccm.2203046. [DOI] [PubMed] [Google Scholar]
- 32.Gottlieb DJ, Punjabi NM, Mehra R, et al. CPAP versus oxygen in obstructive sleep apnea. N Engl J Med. 2014;370(24):2276–2285. doi: 10.1056/NEJMoa1306766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ashley C, Burton D, Sverrisdottir YB, Sander M, Mckenzie DK, Macefield VG. Firing probability and mean firing rates of human muscle vasoconstrictor neurones are elevated during chronic asphyxia. J Physiol. 2010;588(Pt 4):701–712. doi: 10.1113/jphysiol.2009.185348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Clarenbach CF, Stoewhas AC, van Gestel AJ, et al. Comparison of photoplethysmographic and arterial tonometry-derived indices of arterial stiffness. Hypertens Res. 2012;35(2):228–233. doi: 10.1038/hr.2011.168. [DOI] [PubMed] [Google Scholar]
- 35.Grote L, Sommermeyer D, Ficker J, et al. REM Sleep Imposes a Vascular Load in COPD Patients Independent of Sleep Apnea. COPD. 2017;14(6):565–572. doi: 10.1080/15412555.2017.1365119. [DOI] [PubMed] [Google Scholar]
- 36.Biselli P, Fricke K, Grote L, et al. Reductions in dead space ventilation with Nasal High Flow depend on physiologic dead space volume – Metabolic hood measurements during sleep in patients with COPD and controls. Eur Respir J. 2018;51(5):pii: 1702251. doi: 10.1183/13993003.02251-2017. [DOI] [PubMed] [Google Scholar]