Abstract
Purpose
Population-based studies have revealed a high prevalence of cognitive impairment after stroke. We aimed to determine the impact of serum magnesium (Mg2+) levels on the occurrence of poststroke cognitive impairment (PSCI).
Patients and methods
Acute ischemic stroke patients (n = 327) were enrolled in our study and serum Mg2+ levels were assessed on admission. The cognitive performance of each patient was evaluated using the Mini–Mental State Examination (MMSE) at a 1-month follow-up visit.
Results
One hundred five (32.1%) patients were diagnosed with PSCI at 1-month poststroke. The serum Mg2+ levels in both the PSCI group and the non-PSCI group were significantly lower than those in normal control group (P<0.001). In addition, the PSCI group had lower levels of serum Mg2+ compared to the non-PSCI group (P=0.003). In the binary logistic regression analysis, a serum Mg2+ level of ≤0.82 mmol/L was significantly associated with an increased risk of developing PSCI by the 1-month follow-up (OR 2.236, 95% CI 1.232–4.058, P=0.008), as was age (OR 1.043, 95% CI 1.014–1.073, P=0.003).
Conclusion
Our results demonstrate the existence of a significant association between low levels of serum Mg2+ and the occurrence of PSCI 1-month poststroke, and these results suggest that low levels of serum Mg2+ on admission may serve as a risk factor for developing PSCI by 1-month poststroke.
Keywords: cognition, magnesium, stroke, risk factor
Introduction
Poststroke cognitive impairment (PSCI) is one of the frequent residual sequelae of stroke worldwide. The prevalence of PSCI reported in previous studies varied from 20 to 80% in various countries.1,2 Cognitive impairments in stroke survivors not only raise the risk of disability and mortality3 but also lead to the recurrence of vascular events.4 Recently, an increasing number of studies have focused on the role of risk factors in the development of dementia after stroke; although older age and sex have been reported to be risk factors for PSCI,5 these factors are not preventable. Thus, there is an urgent need to identify novel and preventable risk factors of PSCI.
In recent years, it has been found that there was a significant drop in serum magnesium (Mg2+) levels in acute ischemic stroke (AIS) patients.6,7 In addition, studies have indicated that low serum Mg2+ levels contributed to unsatisfactory short-term functional outcomes after stroke.8,9 Contrarily, Mg2+-enriched dietary intake has been proved to reduce the incidence of ischemic stroke,10 and moderate amount of Mg2+ dietary approaching the Dietary Reference Intake (DRI) even could improve functional outcomes after stroke.11,12
Evidence for a role of serum Mg2+ on cognitive function comes from both clinical and animal studies. Clinical studies have shown that low levels of serum Mg2+ exerted negative effects on cognitive function,13 while Mg2+ supplements attenuated cognitive impairment.14 In animal models, Mg2+ also was found to be involved in cognitive impairment; increasing Mg2+ in the brain was found to led to enhancements in learning and memory in rats via improved functional connectivity and synaptic plasticity,15 which had been demonstrated to be involved in cognitive impairment.16,17 Besides, Mg2+ was found to boost the memory restorative effect by reducing the synaptic loss and restoring the N-methyl-d-aspartic acid receptor (NMDAR) signaling pathway in Alzheimer’s disease mice.18
Considering the important role of Mg2+ in both stroke and cognition, it is plausible that serum Mg2+ levels may play an important role in the development of PSCI. However, no study to date has examined the association between Mg2+ and cognitive function in a stroke setting, and additional studies in this area are urgently needed. Therefore, the aim of our study was to explore the role of serum Mg2+ levels in PSCI and we hypothesized that low levels of serum Mg2+ are associated with an increased incidence of PSCI.
Patients and methods
Patients and study design
All first-ever or recurrent AIS patients hospitalized in the Department of Neurology, The First Affiliated Hospital of Wenzhou Medical University, from October 2013 to June 2015 were consecutively screened for this observational prospective cohort study. The inclusion criteria included the following: 1) patients were between the age of 18 and 80 years; 2) diagnosis of AIS was confirmed by computed tomography (CT) or magnetic resonance imaging (MRI) on admission; 3) onset of AIS events was less than 2 weeks on admission; and 4) patients had the ability and willingness to give informed consent. The exclusion criteria applied to patients included the following: 1) a history of dementia or cognitive impairment; 2) present or past psychiatric disorders, such as depression; 3) neurodegenerative diseases such as Parkinson’s disease; 4) a history of a malignant tumor; 5) a history of nootropic or antipsychotic drug use; 6) severe aphasia, visual or auditory impairment, or a chaotic conscious state that made them unable to complete neuropsychological assessments; 7) a fasting state or severe renal failure with acute oliguresis or diuresis; 8) severe metabolic abnormalities, such as calcium metabolism disorder and diabetes mellitus ketoacidosis; and 9) ongoing use of Mg2+ supplement drugs, diuretics, dehydrants, or insulin after the stroke onset. Meanwhile, 110 healthy control subjects were recruited from a health survey conducted at The First Affiliated Hospital of Wenzhou Medical University. Subjects with any personal or familial history of psychiatric illness were excluded. All subjects were free of severe physical diseases including AIS.
This study protocol was performed in accordance with the ethical guidelines of the 2013 Declaration of Helsinki and was approved by the Medical Ethics Committee of The First Affiliated Hospital of Wenzhou Medical University, and written informed consent was provided by all participants or their relatives.
Clinical variables and assessments
Demographic and clinical characteristics were collected via standardized questionnaires through face-to-face interviews. Education levels were categorized as illiterate, primary school, and secondary school and above according to years of education.
Cognitive function assessment was performed at a 1-month follow-up by using the Mini–Mental State Examination (MMSE). The MMSE is a traditional and well-accepted scale for detecting current cognitive dysfunction. Based on the education level, an MMSE score of ≤19 points (illiterate), ≤22 points (primary school education level), and ≤26 points (secondary school and above education level) was adopted as the diagnostic cutoff value for cognitive impairment.19 A lower MMSE score means more severe cognitive dysfunction. The National Institutes of Health Stroke Scale (NIHSS) was used to evaluate the stroke severity on admission. The Hamilton Depression Rating Scale 17-item (HAMD-17) was used to screen depressive symptoms at the 1-month follow-up. The Hamilton Anxiety Rating Scale (HAMA) was used to screen anxiety symptoms at the 1-month follow-up. Besides, we assessed the poststroke functional outcomes at 1-month follow-up with the modified Barthel Index (BI) and modified Rankin Scale (mRS; a score of greater than 2 was defined as poor outcome). All the evaluations were carried out by trained neurological physicians who were blind to the other clinical data of the patients.
Definition of PSCI
PSCI is a series of syndromes that occurs within 6 months of a stroke event and meets the diagnostic criteria for cognitive impairment.20,21 PSCI includes not only the cognitive impairment resulting from various new stroke events but also the deterioration of cognitive function within 6 months of a new stroke event in Alzheimer’s disease patients. Currently, the diagnostic criteria for cognitive impairments mainly based on the results of neuropsychological assessment scales, such as the MMSE,22 Montreal Cognitive Assessment (MoCA),23 and mini-cognitive (Mini-Cog) assessment.24 In this study, the diagnosis of cognitive impairment was established when the MMSE scores were below the diagnostic cutoff value described earlier.25
Serum Mg2+ measurements
Peripheral blood samples were acquired within 12–18 hours after admission. The serum Mg2+ concentration was measured by dimethylaniline blue colorimetry using a Beckman Coulter automatic analyzer (AU5800) at our hospital’s laboratory. Patients were divided into the following three groups based on serum Mg2+ levels: ≤0.82, 0.83–0.88, and ≥0.89 mmol/L.
Statistical analyses
For continuous variables with a normal distribution or a skewed distribution, results were described as the mean ± SD or median (IQR), respectively, while for categorical variables, the results were described using proportions. For univariate comparisons between groups, proportions were compared by using Chi-squared tests and one-way ANOVA, and Student’s t-tests were used for variables normally distributed; for variables with an asymmetrical distribution, the Mann–Whitney U-test and Kruskal–Wallis test were used. When ANOVA showed significant differences among the groups, Bonferroni corrections were used for pairwise comparisons. When Kruskal–Wallis tests showed significant differences among the groups, pairwise multiple comparisons followed. Binary logistic regression analysis was performed to quantify the association between serum Mg2+ levels and the development of PSCI. All potential confounders with a P-value of <0.05 in the univariate analyses and variables such as NIHSS score and history of stroke were introduced into the regression models. These results are represented as adjusted ORs and their 95% CIs. All statistical analyses were performed using SPSS 21.0 (IBM Corporation, Armonk, NY, USA), and a P-value of <0.05 was considered statistically significant.
Results
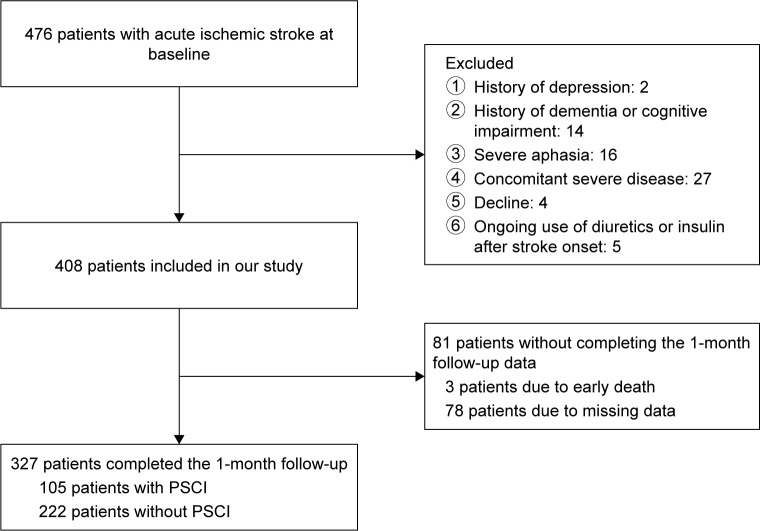
The flowchart of our study is shown in Figure 1. During the screening period, 476 consecutive patients with AIS were screened and 327 patients were enrolled in our study. No significant differences were found between patients who dropped out from our study and patients who enrolled in terms of age (62.12±10.43 vs 62.50±10.19; P=0.614), sex (male/female) (55/26 vs 207/120; P=0.191), NIHSS score (3 [1–6] vs 3 [1–4]; P=0.094), and serum Mg2+ levels (0.85 [0.80–0.90] vs 0.85 [0.81–0.90]; P=0.736).
Figure 1.
Study recruitment profile.
Abbreviation: PSCI, poststroke cognitive impairment.
Baseline characteristics of patients are shown in Table 1. The mean (± SD) age of the stroke patients who enrolled was 62.50±10.19 years. Their median (IQR) NIHSS score was 3 (1–4). Of the 327 patients who formed the study sample, 105 (54 men, 51 women) patients were diagnosed with PSCI at the 1-month follow-up and the incidence of PSCI at 1-month poststroke was 32.1%. The average serum Mg2+ concentrations in all stroke patients and the normal control group were 0.85±0.08 and 0.96±0.17 mmol/L, respectively (P<0.001), and the average serum Mg2+ concentrations in the non-PSCI and PSCI groups were 0.86±0.07 and 0.83±0.09 mmol/L, respectively. The overall serum Mg2+ concentration of all stroke patients was significantly lower than the concentration in normal controls (P<0.001). Furthermore, there was a significant intergroup difference in terms of the serum Mg2+ levels (P<0.001) among PSCI, non-PSCI, and normal control groups. The serum Mg2+ concentration in normal subjects was significantly higher than both non-PSCI patients (P<0.001) and PSCI patients (P<0.001), and the PSCI group showed a lower serum Mg2+ levels than the non-PSCI group (0.83±0.09 vs 0.86±0.07; P<0.05). Besides, there was no significant difference in terms of age and sex between normal control group and non-PSCI group. Meanwhile, there was also no difference in terms of age and sex between normal control group and PSCI group. Compared to non-PSCI group, PSCI group had significant lower levels of serum Mg2+ concentration (P=0.003, Figure 2), were significantly older (65.39±10.08 vs 61.14±9.97; P<0.001), had a higher proportion of female (54/51 vs 153/69; P=0.003), and were more likely to be less educated, female, and a current smoker. No significant differences were observed in the other variables, such as lesion location, vascular risk factors, poor functional outcome, and blood glucose levels on admission.
Table 1.
Baseline clinical characteristics in non-PSCI and PSCI patients at 1 month
| Non-PSCI (n=222) | PSCI (n=105) | P-value | |
|---|---|---|---|
| Demographic characteristics | |||
| Age (years), mean ± SD | 61.14±9.97 | 65.39±10.08 | <0.001 |
| Male/female | 153/69 | 54/51 | 0.003 |
| Married status, n (%) | 202 (91.0) | 90 (85.7) | 0.180 |
| Education levels, n (%) | 0.029 | ||
| Illiterate | 55 (24.8) | 41 (39) | |
| Primary school | 103 (46.4) | 38 (36.2) | |
| Postsecondary school | 64 (28.8) | 26 (24.8) | |
| TOAST classification, n (%) | 0.741 | ||
| LA | 180 (81.1) | 84 (80) | |
| CE | 12 (5.4) | 6 (5.7) | |
| SA | 23 (10.8) | 11 (10.5) | |
| SOE | 4 (1.8) | 1 (1.0) | |
| SUE | 2 (0.9) | 3 (2.9) | |
| Lesion location, n (%) | 0.840 | ||
| Left hemisphere | 69 (31.1) | 37 (35.2) | |
| Right hemisphere | 79 (35.6) | 31 (29.5) | |
| Brainstem | 36 (16.2) | 18 (17.1) | |
| Cerebellum | 10 (4.5) | 4 (3.8) | |
| Others | 28 (12.6) | 15 (14.3) | |
| Multiple infarcts, n (%) | 85 (38.3) | 52 (49.5) | 0.056 |
| Vascular risk factors, n (%) | |||
| Hypertension | 157 (70.7) | 73 (69.5) | 0.897 |
| Diabetes mellitus | 63 (28.4) | 37 (35.2) | 0.247 |
| Hyperlipidemia | 21 (9.5) | 10 (9.5) | 1.000 |
| Coronary artery disease | 14 (6.3) | 8 (7.6) | 0.814 |
| History of stroke | 17 (7.7) | 12 (11.4) | 0.299 |
| Current smoking | 73 (32.9) | 20 (19.0) | 0.012 |
| Current drinking | 89 (40.1) | 38 (36.2) | 0.544 |
| NIHSS score, median (IQR) | 2.5 (1–4) | 3 (1.5–5) | 0.210 |
| HAMA score, median (IQR) | 4 (1–7) | 4 (1–8) | 0.800 |
| HAMD score, median (IQR) | 4 (2–7) | 5 (1–9) | 0.616 |
| Poor outcome, n (%) | 31 (14.2) | 22 (21.0) | 0.148 |
| BI score, median (IQR) | 100 (98–100) | 100 (91–100) | |
| Laboratory data | |||
| Mg2+ (mmol/L), mean ± SD | 0.86±0.07 | 0.83±0.09 | 0.003 |
| HbA1c (mmol/L), mean ± SD | 6.36±1.59 | 6.28±1.26 | 0.707 |
| FBG (mmol/L), mean ± SD | 5 (4.4–5.9) | 4 (4.6–6.4) | 0.257 |
| PBG (mmol/L), mean ± SD | 7.6 (6.0–10.6) | 8.1 (6.0–12.4) | 0.391 |
Abbreviations: BI, Barthel Index; CE, cardioembolism; FBG, fasting blood glucose; HAMA, Hamilton Anxiety Rating Scale; HAMD, Hamilton Depression Rating Scale; HbA1c, hemoglobin A1c; LA, large-artery atherosclerosis; Mg2+, magnesium; MMSE, Mini–Mental State Examination; NIHSS, National Institutes of Health Stroke Scale; PBG, postprandial blood glucose; PSCI, poststroke cognitive impairment; SA, small-artery occlusion lacunar; SOE, stroke of other determined etiology; SUE, stroke of other undetermined etiology; TOAST, Trial of Org 10172 in Acute Stroke Treatment.
Figure 2.
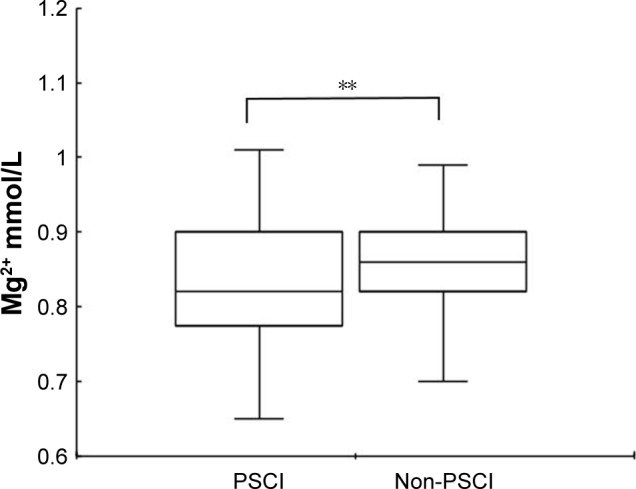
Comparisons of serum Mg2+ levels in patients with PSCI and non-PSCI.
Notes: In the box-and-whisker plots, the horizontal line in the middle of each box indicates the median value; the lower and upper ends of the box represent the 25th and 75th percentiles, and the peripheral lines extending to the outer fences represent 10th and 90th percentiles, respectively. **P<0.01 compared with the non-PSCI group via the Mann–Whitney U-test.
Abbreviations: Mg2+, magnesium; PSCI, poststroke cognitive impairment.
As shown in Table 2, further comparisons revealed that there were significant differences between the non-PSCI group and the PSCI group in serum Mg2+ levels across tertiles of patients (P<0.001). In addition, the proportion of patients in the low tertile (≤0.82 mmol/L) was significantly higher in the PSCI group than in the non-PSCI group (51.4 vs 29.3%, respectively, P<0.001) and the proportion of patients in the intermediate tertile (0.83–0.88 mmol/L) was lower in the PSCI group than in the non-PSCI group (P=0.011).
Table 2.
Magnesium levels across tertiles of patients
| Non-PSCI patients (n=222) | PSCI patients (n=105) | P-value | |
|---|---|---|---|
| Magnesium, n (% of total population) | <0.001 | ||
| Tertile 1 (≤0.82 mmol/L) | 65 (29.3) | 54 (51.4) | <0.001 |
| Tertile 2 (0.83–0.88 mmol/L) | 81 (36.5) | 23 (21.9) | 0.011 |
| Tertile 3 (≥0.89 mmol/L) | 76 (34.2) | 28 (26.7) | 0.204 |
Abbreviation: PSCI, poststroke cognitive impairment.
In an analysis using all participants, with the high tertile (≥0.89 mmol/L) taken as a reference and PSCI occurrence as a dependent variable in the binary logistic regression analysis, after adjusting for potential confounders, there remained an independent association between the lowest tertile of serum Mg2+ level and the occurrence of PSCI (OR 2.236, 95% CI 1.232–4.058, P=0.008). In addition, age was significantly associated with the occurrence of PSCI at the 1-month follow-up (OR 1.043, 95% CI 1.014–1.073, P=0.003) (Table 3).
Table 3.
Multivariate logistic model of the clinical determinants of PSCI
| Variables | OR (95% CI) | P-value |
|---|---|---|
| Magnesium | 0.001 | |
| Tertile 1 | 2.236 (1.232–4.058) | 0.008 |
| Tertile 2 | 0.765 (0.395–1.480) | 0.426 |
| Age | 1.043 (1.014–1.073) | 0.003 |
| Sex | 1.614 (0.885–2.943) | 0.118 |
| Education levels | 0.527 | |
| Illiterate | 0.776 (0.412–1.460) | 0.431 |
| Primary school | 1.083 (0.515–2.276) | 0.833 |
| Current smoking | 0.747 (0.385–1.449) | 0.389 |
| NIHSS score | 1.094 (0.994–1.205) | 0.066 |
| History of stroke | 1.648 (0.709–3.827) | 0.246 |
Abbreviations: NIHSS, National Institutes of Health Stroke Scale; PSCI, poststroke cognitive impairment.
Discussion
In this study, we observed that a low level of serum Mg2+ was independently associated with the occurrence of PSCI at 1-month poststroke. To the best of our knowledge, this is the first report exploring the correlations between serum levels of Mg2+ on admission and PSCI in AIS patients.
In our study, 32.1% of AIS patients developed PSCI by a 1-month follow-up visit, which is in line with previous studies.1,2 Meanwhile, age was shown to be a risk factor for the development of PSCI, which is in accordance with the existing literature.5
There are two hypotheses regarding the role of Mg2+ in cognitive impairment. One hypothesis is the direct regulating effect of neuronal Mg2+ on the NMDAR,13 which is well-known to be the predominant molecule involved in synaptic plasticity and learning and memory.26–28 Neuronal Mg2+ is essential in the regulation of the excitability of NMDAR by modulating the open duration and coincidence detection ability of the NMDAR via pore blockade.29,30 Furthermore, experimental studies indicated that increased extracellular Mg2+ upregulated and enhanced activity-dependent NMDAR-dependent signaling and, thus, resulted in an enhancement of synaptic plasticity in cultured neurons.15,31 When Mg2+ is deficient, fewer NMDA channels are blocked by the extracellular Mg2+ and the NMDAR becomes hyperexcitable,29 which might impair synaptic plasticity and learning and memory function.31,32
Other hypothesized pathways are oxidative stress and chronic inflammation.33,34 Mg2+ deficiency has been found to increase the production of free oxygen radicals, stimulate the excessive production and release of proinflammatory molecules such as tumor necrosis factor-α (TNF-α), interleukins, and nitric oxide, all of which are responsible for triggering the development of a proinflammatory state both in experimental animals and in humans,33,35–37 and thereby, increase the risk of cognitive impairment. In addition, it has been reported that Mg2+ deficiency may impair cell membrane integrity and function and increase susceptibility of the body to oxidative stress.38 Similar to the results documented in previous studies,39,40 our previous study found that the concentrations of serum 8-hydroxydeoxyguanosine (8-OHdG) and malon-dialdehyde (MDA), both of which are widely perceived as markers of oxidative stress, were significantly higher in the PSCI group than in the non-PSCI group. Previous studies also suggested that increased levels of interleukin-6 and C-reactive protein (CRP) are associated with PSCI.41,42
In addition, serum concentrations of Mg2+ are remarkably constant in healthy subjects and correlate well with intracellular free Mg2+,43 a physiologically active form of the element Mg2+; therefore, serum Mg2+ could be regarded as the most applicable clinical indicator of Mg2+ metabolic disorders.
Given the above findings, we put forward the view that low levels of serum Mg2+ are associated with the occurrence of PSCI and may predict its development at 1-month poststroke.
Several limitations of our study should be mentioned. First, according to our inclusion/exclusion criteria, patients recruited in our study cohort were limited to those without severe aphasia and other serious conditions, which may result in a bias in the estimates of PSCI incidence. Second, serum Mg2+ levels were measured only on admission in our study; it would be better to measure serum Mg2+ at the time of the cognitive screening to further explore how serum Mg2+ levels change over time following the stroke. Third, as Mg2+ can be found in many common foods, diet may have an impact on Mg2+ levels in the human body. Information on the dietary intake of patients was not collected, and thus, there may have been an influence of differing diets on the results. Fourth, 1-month follow-up was chosen for the assessment of PSCI rather than 3- or 6-month follow-up, which might be more appropriate to assess the cognitive impairment after stroke. Fifth, cognition function may not be fully reflected with MMSE; other neuropsychological tests should be evaluated together with MMSE to better assess the cognition function of patients.
Conclusion
Despite these limitations, our study demonstrated that low levels of serum Mg2+ on admission were independently associated with the development of PSCI at 1-month poststroke. The result suggested that low levels of serum Mg2+ were predictive of the subsequent PSCI by 1-month poststroke, and for patients with AIS, the determination of serum Mg2+ levels after admission is necessary. Further multicenter and randomized controlled trials are critical to confirm the causal relationship between serum Mg2+ levels and PSCI.
Acknowledgments
This study was supported by Wenzhou Municipal Sci-Tech Bureau Program (Y20160002), National Key Technology Research and Development Program of the Ministry of Science and Technology of China (grant number: 2015BAI13B01), as well as the Projects of National Science Foundation of China (no 81873799). We are greatly indebted to the staff and to the patients for their contributions to this study.
Footnotes
Author contributions
Xinjie Tu and Jincai He designed the study and wrote the protocol. Xinjie Tu, Huihua Qiu, Shasha Lin, Weilei He, Guiqian Huang, Xingru Zhang, and Yuemin Wu collected the data of the study. Xinjie Tu conducted literature searches and provided summaries of previous research studies. Xinjie Tu conducted the statistical analysis. Xinjie Tu and Huihua Qiu wrote the first draft of the manuscript. All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Jacquin A, Binquet C, Rouaud O, et al. Post-stroke cognitive impairment: high prevalence and determining factors in a cohort of mild stroke. J Alzheimers Dis. 2014;40(4):1029–1038. doi: 10.3233/JAD-131580. [DOI] [PubMed] [Google Scholar]
- 2.Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2014;2(8):80. doi: 10.3978/j.issn.2305-5839.2014.08.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Levine DA, Davydow DS, Hough CL, Langa KM, Rogers MA, Iwashyna TJ. Functional disability and cognitive impairment after hospitalization for myocardial infarction and stroke. Circ Cardiovasc Qual Outcomes. 2014;7(6):863–871. doi: 10.1161/HCQ.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee M, Saver JL, Hong KS, et al. Cognitive impairment and risk of future stroke: a systematic review and meta-analysis. CMAJ. 2014;186(14):E536–E546. doi: 10.1503/cmaj.140147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gottesman RF, Hillis AE. Predictors and assessment of cognitive dysfunction resulting from ischaemic stroke. Lancet Neurol. 2010;9(9):895–905. doi: 10.1016/S1474-4422(10)70164-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cojocaru IM, Cojocaru M, Tănăsescu R, Iacob SA, Iliescu I. Changes of magnesium serum levels in patients with acute ischemic stroke and acute infections. Rom J Intern Med. 2009;47(2):169–171. [PubMed] [Google Scholar]
- 7.Altura BT, Memon ZI, Zhang A, et al. Low levels of serum ionized magnesium are found in patients early after stroke which result in rapid elevation in cytosolic free calcium and spasm in cerebral vascular muscle cells. Neurosci Lett. 1997;230(1):37–40. doi: 10.1016/s0304-3940(97)00471-0. [DOI] [PubMed] [Google Scholar]
- 8.Cojocaru IM, Cojocaru M, Burcin C, Atanasiu NA. Serum magnesium in patients with acute ischemic stroke. Rom J Intern Med. 2007;45(3):269–273. [PubMed] [Google Scholar]
- 9.You S, Zhong C, Du H, et al. Admission Low Magnesium Level Is Associated with In-Hospital Mortality in Acute Ischemic Stroke Patients. Cerebrovasc Dis. 2017;44(1–2):35–42. doi: 10.1159/000471858. [DOI] [PubMed] [Google Scholar]
- 10.Adebamowo SN, Spiegelman D, Flint AJ, Willett WC, Rexrode KM, Magnesium Iof Intakes of magnesium, potassium, and calcium and the risk of stroke among men. Int J Stroke. 2015;10(7):1093–1100. doi: 10.1111/ijs.12516. [DOI] [PubMed] [Google Scholar]
- 11.Pan WH, Lai YH, Yeh WT, et al. Intake of potassium- and magnesium-enriched salt improves functional outcome after stroke: a randomized, multicenter, double-blind controlled trial. Am J Clin Nutr. 2017;106(5):1267–1273. doi: 10.3945/ajcn.116.148536. [DOI] [PubMed] [Google Scholar]
- 12.Afshari D, Moradian N, Rezaei M. Evaluation of the intravenous magnesium sulfate effect in clinical improvement of patients with acute ischemic stroke. Clin Neurol Neurosurg. 2013;115(4):400–404. doi: 10.1016/j.clineuro.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Kieboom BCT, Licher S, Wolters FJ, et al. Serum magnesium is associated with the risk of dementia. Neurology. 2017;89(16):1716–1722. doi: 10.1212/WNL.0000000000004517. [DOI] [PubMed] [Google Scholar]
- 14.Mack WJ, Kellner CP, Sahlein DH, et al. Intraoperative magnesium infusion during carotid endarterectomy: a double-blind placebo-controlled trial. J Neurosurg. 2009;110(5):961–967. doi: 10.3171/2008.9.17671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Slutsky I, Abumaria N, Wu LJ, et al. Enhancement of learning and memory by elevating brain magnesium. Neuron. 2010;65(2):165–177. doi: 10.1016/j.neuron.2009.12.026. [DOI] [PubMed] [Google Scholar]
- 16.Hottman DA, Li L. Protein prenylation and synaptic plasticity: implications for Alzheimer’s disease. Mol Neurobiol. 2014;50(1):177–185. doi: 10.1007/s12035-013-8627-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Billard JM. Ageing, hippocampal synaptic activity and magnesium. Magnes Res. 2006;19(3):199–215. [PubMed] [Google Scholar]
- 18.Huang Y, Huang X, Zhang L, et al. Magnesium boosts the memory restorative effect of environmental enrichment in Alzheimer’s disease mice. CNS Neurosci Ther. 2018;24(1):70–79. doi: 10.1111/cns.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang ZX, Hong X, Li H. The mini-mental state examination in the Chinese residents population aged 55 years and over in the urban and rural areas of Beijing. Chin J Neurol. 1999;32(03):149–153. [Google Scholar]
- 20.Skrobot OA, O’Brien J, Black S, et al. The Vascular Impairment of Cognition Classification Consensus Study. Alzheimers Dement. 2017;13(6):624–633. doi: 10.1016/j.jalz.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Mijajlović MD, Pavlović A, Brainin M, et al. Post-stroke dementia – a comprehensive review. BMC Med. 2017;15(1):11. doi: 10.1186/s12916-017-0779-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang A, Liu J, Meng X, et al. Association between oxidized low-density lipoprotein and cognitive impairment in patients with ischemic stroke. Eur J Neurol. 2018;25(1):185–191. doi: 10.1111/ene.13497. [DOI] [PubMed] [Google Scholar]
- 23.Delavaran H, Jönsson AC, Lövkvist H, et al. Cognitive function in stroke survivors: A 10-year follow-up study. Acta Neurol Scand. 2017;136(3):187–194. doi: 10.1111/ane.12709. [DOI] [PubMed] [Google Scholar]
- 24.McCarten JR, Anderson P, Kuskowski MA, McPherson SE, Borson S. Screening for cognitive impairment in an elderly veteran population: acceptability and results using different versions of the Mini-Cog. J Am Geriatr Soc. 2011;59(2):309–313. doi: 10.1111/j.1532-5415.2010.03249.x. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q, Zhao K, Cai Y, Tu X, Liu Y, He J. Prediabetes is associated with post-stroke cognitive impairment in ischaemic stroke patients. Brain Res. 2018;1687:137–143. doi: 10.1016/j.brainres.2017.12.034. [DOI] [PubMed] [Google Scholar]
- 26.Corlew R, Wang Y, Ghermazien H, Erisir A, Philpot BD. Developmental switch in the contribution of presynaptic and postsynaptic NMDA receptors to long-term depression. J Neurosci. 2007;27(37):9835–9845. doi: 10.1523/JNEUROSCI.5494-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodríguez-Moreno A, Kohl MM, Reeve JE, et al. Presynaptic induction and expression of timing-dependent long-term depression demonstrated by compartment-specific photorelease of a use-dependent NMDA receptor antagonist. J Neurosci. 2011;31(23):8564–8569. doi: 10.1523/JNEUROSCI.0274-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Volianskis A, France G, Jensen MS, Bortolotto ZA, Jane DE, Collingridge GL. Long-term potentiation and the role of N-methyl-D-aspartate receptors. Brain Res. 2015;1621:5–16. doi: 10.1016/j.brainres.2015.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307(5950):462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 30.Mayer ML, Westbrook GL, Guthrie PB. Voltage-dependent block by Mg2+ of NMDA responses in spinal cord neurones. Nature. 1984;309(5965):261–263. doi: 10.1038/309261a0. [DOI] [PubMed] [Google Scholar]
- 31.Slutsky I, Sadeghpour S, Li B, Liu G. Enhancement of synaptic plasticity through chronically reduced Ca2+ flux during uncorrelated activity. Neuron. 2004;44(5):835–849. doi: 10.1016/j.neuron.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 32.Weed MR, Bookbinder M, Polino J, et al. Negative Allosteric Modulators Selective for The NR2B Subtype of The NMDA Receptor Impair Cognition in Multiple Domains. Neuropsychopharmacology. 2016;41(2):568–577. doi: 10.1038/npp.2015.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hans CP, Chaudhary DP, Bansal DD. Effect of magnesium supplementation on oxidative stress in alloxanic diabetic rats. Magnes Res. 2003;16(1):13–19. [PubMed] [Google Scholar]
- 34.Yang Y, Wu Z, Chen Y, et al. Magnesium deficiency enhances hydrogen peroxide production and oxidative damage in chick embryo hepatocyte in vitro. Biometals. 2006;19(1):71–81. doi: 10.1007/s10534-005-6898-1. [DOI] [PubMed] [Google Scholar]
- 35.Kramer JH, Mak IT, Phillips TM, Weglicki WB. Dietary magnesium intake influences circulating pro-inflammatory neuropeptide levels and loss of myocardial tolerance to postischemic stress. Exp Biol Med (Maywood) 2003;228(6):665–673. doi: 10.1177/153537020322800604. [DOI] [PubMed] [Google Scholar]
- 36.Regan RF, Guo Y. Magnesium deprivation decreases cellular reduced glutathione and causes oxidative neuronal death in murine cortical cultures. Brain Res. 2001;890(1):177–183. doi: 10.1016/s0006-8993(00)03156-5. [DOI] [PubMed] [Google Scholar]
- 37.Ondrus P, Alberty R, Vassanyiova Z. Importance of lipid peroxidation, protective enzymes and trace elements in chronic leg ischaemia. Eur J Clin Chem Clin Biochem. 1996;34(6):471–475. doi: 10.1515/cclm.1996.34.6.471. [DOI] [PubMed] [Google Scholar]
- 38.Barbagallo M, Belvedere M, Dominguez LJ. Magnesium homeostasis and aging. Magnes Res. 2009;22(4):235–246. doi: 10.1684/mrh.2009.0187. [DOI] [PubMed] [Google Scholar]
- 39.Baierle M, Nascimento SN, Moro AM, et al. Relationship between inflammation and oxidative stress and cognitive decline in the institutionalized elderly. Oxid Med Cell Longev. 2015;2015:804198. doi: 10.1155/2015/804198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schmidt R, Schmidt H, Curb JD, Masaki K, White LR, Launer LJ. Early inflammation and dementia: a 25-year follow-up of the Honolulu-Asia Aging Study. Ann Neurol. 2002;52(2):168–174. doi: 10.1002/ana.10265. [DOI] [PubMed] [Google Scholar]
- 41.Kulesh AA, Drobakha VE, Nekrasova IV, Kuklina EM, Shestakov VV. Neuroinflammatory, Neurodegenerative and Structural Brain Bio-markers of the Main Types of Post-Stroke Cognitive Impairment in Acute Period of Ischemic Stroke. Vestn Ross Akad Med Nauk. 2016;71(4):304–312. doi: 10.15690/vramn685. Russian. [DOI] [PubMed] [Google Scholar]
- 42.Rothenburg LS, Herrmann N, Swardfager W, et al. The relationship between inflammatory markers and post stroke cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23(3):199–205. doi: 10.1177/0891988710373598. [DOI] [PubMed] [Google Scholar]
- 43.Amighi J, Sabeti S, Schlager O, et al. Low serum magnesium predicts neurological events in patients with advanced atherosclerosis. Stroke. 2004;35(1):22–27. doi: 10.1161/01.STR.0000105928.95124.1F. [DOI] [PubMed] [Google Scholar]