Abstract
Sleep–wake disturbances are common in liver cirrhosis and associated with impaired quality of life. The most common abnormalities are insomnia (difficulties falling asleep and maintaining sleep, or unrefreshing sleep), excessive daytime sleepiness, and sleep–wake inversion (disturbances of circadian rhythmicity). The underlying pathophysiological mechanisms for sleep disturbances in cirrhosis are complex and may include disturbed metabolism of melatonin and glucose, alterations in thermoregulation, and altered ghrelin secretion profiles. Sleep–wake abnormalities are related to the presence of hepatic encephalopathy (HE) and improvement in sleep parameters can be observed when HE is properly managed. A few non-specific treatments for sleep–wake abnormalities have been tried with encouraging results for hydroxyzine and modafinil. However, due to the potential for medication toxicity in these disabled patients, further studies are needed to address the potential role of non-drug therapies in this population (eg, cognitive behavioral therapy, mindfulness, yoga) that have demonstrated usefulness in insomnia disorders.
Keywords: sleep disorders, hepatic encephalopathy, liver cirrhosis, excessive daytime sleepiness, circadian rhythm, insomnia
Introduction
Liver cirrhosis is a common life-threatening hepatic disorder. The main causes of cirrhosis are related to harmful alcohol consumption, viral hepatitis B and C, metabolic disorders, and non-alcoholic fatty liver disease. These causal factors explain the growing prevalence of the disorder which has reached 0.27% of the population in the United States.1 Clinical manifestations of chronic cirrhosis are highly variable and include ascites, jaundice, gastrointestinal hemorrhage, and hepatic encephalopathy (HE). Fatigue and sleep disturbances have also been observed and this can lead to impairments in quality of life (QoL).2,3 Several sleep disturbances have been described in cirrhosis. However, the underlying pathophysiological mechanisms are complex and not yet fully understood.4 The purpose of this review was to describe these different disorders, their impact, and current treatment options.
Clinical manifestations of sleep disturbances in liver cirrhosis
Sleep disturbances in liver cirrhosis are closely related to the presence of HE. HE has a broad spectrum of clinical severity, ranging from subclinical disorders (covert) to overt HE with coma.5 Diagnosis of HE still remains difficult and is primarily based on clinical findings. In recent American and European guidelines, altered sleep rhythm is an essential factor in the diagnosis of HE.6 In a recent study by Singh et al,7 32% of patients with liver cirrhosis exhibited HE. In these patients, sleep disorders have serious implications for daytime functioning and are best described as a panel of sleep–wake abnormalities.4 This was first described in 1954, by Sherlock et al, as a phenomenon of “sleep–wake inversion.”8 The most common abnormalities include insomnia (difficulty falling asleep or maintaining sleep, or unrefreshing sleep), excessive daytime sleepiness (EDS), and sleep–wake inversion (disturbances of circadian rhythmicity).
If most sleep disturbances in cirrhotic patients are attributed to HE,9 sleep disturbances seem to be already present in compensated cirrhosis. Indeed, Bajaj et al have observed EDS and poor quality of sleep in compensated cirrhotics compared to healthy controls.10 Comparison of sleep in cirrhosis with prior HE vs without prior HE has also highlighted a further deterioration of sleep quality, with more non-restorative sleep.11 Etiology of cirrhosis does not influence the occurrence/the clinical picture of sleep disturbances that have been observed both in series of patients affected exclusively or mainly by hepatitis C2,10,11,12 and in series including a majority of alcoholic cirrhosis.13
Other common sleep disorders, such as obstructive sleep apnea syndrome or restless legs syndrome, are also more prevalent in patients with liver cirrhosis and will not be discussed in the present review.14,15
Characteristics and prevalence of sleep disturbances in liver cirrhosis
The reported prevalence of sleep–wake abnormalities in cirrhosis depends upon the diagnostic tools used to assess them. Subjective tools, such as questionnaires and sleep logs, can be used to collect sleep quality and daytime complaints. Objective methods classically applied in the sleep lab, such as polysomnography (PSG) and actigraphy, allow more precise assessment.
It should also be emphasized that correlations between subjective and objective sleep disturbances are not strong. This is due to the fact that patients, especially insomniacs, tend to underestimate their sleep quality.16–18 Therefore, it seems essential to rely on both subjective and objective methods to obtain a global picture of sleep in patients.
Prevalence estimates of sleep disturbances in liver cirrhosis based on questionnaires such as the Pittsburgh Sleep Quality Index (PSQI),3,13,19–24 hospital-specific questionnaires,25 the Basic Nordic Sleep Questionnaire,12 and the Sleep Timing and Sleep Quality Screening questionnaire20 range from 48% to 81%. This is much higher than the prevalence observed in the general population.26 Patient complaints include increased sleep latency (SL), reduced total sleep time (TST), and frequent awakenings.4,25 When compared to healthy subjects,2,13,25,27 the sleep of cirrhotic patients was worse in three out of four studies.
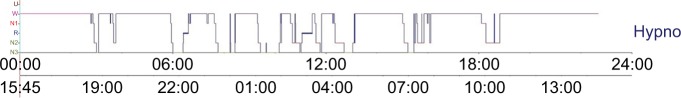
Objective measurements, by actigraphy, have confirmed the altered sleep quality in cirrhotic patients: reduced SL, and frequent awakenings.2,12,25,27–29 PSG has also been performed, confirming the presence of short TST, decreased sleep effi-ciency (SE), frequent awakenings, and lower amounts of slow wave sleep (SWS) and rapid eye movement (REM) sleep in cirrhotic patients.7,28,33 Animal studies in cirrhotic rat models have corroborated these findings.34 Interestingly, when we performed 24h-PSG, we were able to show that the amount of objective sleep over 24 hours is much more important than nighttime sleep (575 vs 471 minutes), since a number of naps are recorded in this population (Figure 1).28
Figure 1.
Typical 24h-hypno of a cirrhotic patient, showing four naps over day and a disturbed sleep with several wake periods after sleep onset.
Notes: Real time is showed on the lower timeline.
Abbreviations: N1, sleep stage 1; N2, sleep stage 2; N3, sleep stage 3; W, wake; hypno, hypnogram; U, unknown sleep stage; R, rapid eye movement sleep.
When compared to healthy subjects, deteriorated sleep quality was confirmed by actigraphy in cirrhotic patients in all studies.2,25,27
EDS, which is present in 21%–50% of cirrhotic patients, has also been well documented using the Epworth Sleepiness Score (ESS).2,12,13,21,24 Compared to healthy subjects, cirrhotic patients show a higher degree of EDS.2,13
Objective and subjective sleep–wake measurements are summarized in Tables 1 and 2.
Table 1.
Comparison of sleep–wake parameters between cirrhotic patients and healthy controls
| Study | Cirrhotic patients | Controls | ||||||
|---|---|---|---|---|---|---|---|---|
| PSQI | ||||||||
| Normal sleep: score >5 | Montagnese et al13 | 8* | 5 | |||||
| Marques et al24 | 5 | 6 | ||||||
| Heeren et al2 | 13* | 5 | ||||||
| ESS | ||||||||
| >10: excessive daytime sleepiness | Marques et al24 | 4 | 5 | |||||
| Heeren et al2 | 10* | 7 | ||||||
| SF-36 | ||||||||
| <50: impaired QoL | Physical component summary | Mental component summary | Physical component summary | Mental component summary | ||||
| Montagnese et al13 | 36* | 46* | 50 | 50 | ||||
| Marques et al24 | 41* | 51 | 50 | 51 | ||||
| Heeren et al2 (sum score, max value: 400) | 181* | 164* | 330 | 304 | ||||
| Actigraphy | ||||||||
| TIB (min) | SE (%) | WASO (min) | TIB (min) | SE (%) | WASO (min) | |||
| Córdoba et al25 | 434 | 80* | 59* | 434 | 94 | 16 | ||
| Marques et al24 | NA | 69* | NA | NA | 81 | NA | ||
| Heeren et al2 | 500* | 81* | 86* | 441 | 85 | 58 | ||
Note:
P<0.05.
Abbreviations: PSQI, Pittsburgh Sleep Quality Index; ESS, Epworth Sleepiness Score; SF-36, 36-question shortform instrument; QoL, quality of life; TIB, time in bed; SE, sleep efficiency; WASO, wake after sleep onset; NA, not assessed.
Table 2.
Polysomnographic parameters of cirrhotic patients
| Study | Patients | Polysomnographic parameters | ||||||
|---|---|---|---|---|---|---|---|---|
| TST (min) | SE (%) | SL (min) | N1 + N2 (%) | N3 (%) | REM (%) | WASO (min) | ||
| Teodoro et al33 | Controls | 357 | 84 | 14 | 62 | 17 | 21 | 52 |
| Teodoro et al33 | Cirrhotics | 330↓ | 74↓ | 28↑ | 68↑ | 19 | 14↓ | 115↑ |
| Bajaj et al31 | Covert HE | 0↓ | 19↓ | |||||
| Watanabe et al32 | Covert HE | 291↓ | 14↓ | 36 | ||||
| Singh et al7 | Covert HE | 231↓ | 59↓ | 48↑ | 51 | 35 | 13↓ | 160↑ |
| Bruyneel et al28 | Recurrent HE | 475 | 78↓ | 12 | 64↑ | 33 | 3↓ | 146↑ |
| Liu et al30 | Covert HE | 516 | 80↓ | 36↑ | 70↑ | 14↓ | ||
Notes: For information, values of control subjects are shown.
represents decreased and
represents increased values, when related to the control group of Teodoro et al.33 Bold values indicate normal values in controls, to be able to compare with patient values on the next lines.
Abbreviations: HE, hepatic encephalopathy; TST, total sleep time; SE, sleep efficiency; SL, sleep latency; REM, rapid eye movement sleep; WASO, wake after sleep onset; N1, sleep stage 1; N2, sleep stage 2; N3, sleep stage 3.
Chronotypology also seems to be disturbed in cirrhotic patients. Córdoba et al25 showed a marked eveningness chronotype in these patients. In contrast, in comparison with healthy subjects, Montagnese et al13 observed no significant difference. However, this team stressed that sleep quality was deteriorated in evening-type patients.
Impact of sleep disturbances in liver cirrhosis
Sleep disturbances have been shown to negatively impact QoL in cirrhotic patients and have been measured in the majority of studies by the SF-362,13,21,28 or the Chronic Liver Disease Questionnaire.3,22 For example, QoL in patients with liver cirrhosis is worse than QoL in healthy controls2,13 for both the Physical Component Summary and the Mental Component Summary. Samanta et al, in a study comparing 100 cirrhotic patients with minimal HE or no HE, showed that minimal HE was associated with poor sleep and EDS, but also that sleep disturbances aggravate neuropsychiatric impairment.21 Sleep disturbances have also been shown to negatively impact psychological distress and depression.35
Pathophysiological mechanisms of sleep disturbances in liver cirrhosis
Sleep disturbances have been associated with the presence of HE and also with disruptions of melatonin metabolism in cirrhotic patients. Some of the features of sleep–wake regulation disturbances may be attributable to melatonin metabolism impairments because melatonin is metabolized by the liver. In patients with cirrhosis, the daytime melatonin level is high,4 the clearance of melatonin is lower at night,36 and melatonin secretion patterns are modified so that the peak of secretion is delayed.36,37 The delayed peak of melatonin secretion is associated with the degree of hepatic failure.29,36 The clinical consequence of these changes is delayed sleep onset.29
Animal studies in rats with portacaval anastomosis (a reliable model of HE) have reported impairment of circadian loco-motor activity and also disturbances in the rhythm of pineal melatonin content.38 A dysfunction of the suprachiasmatic nuclei circadian clock has been also proposed to explain the alteration of melatonin secretion patterns.4
However, EDS does not seem to be related to melatonin metabolism disturbances36 but rather to the degree of HE.4 Indeed, researchers have shown an association between HE severity and EDS,13,21,22 and the absence of EDS had a 92% negative predictive value for HE-triggered hospitalizations during an 8-month follow-up period in 58 cirrhotics.39
The causal factors associated with deteriorated sleep in patients with liver cirrhosis are still under investigation, and the data are inconsistent. It has been shown, with PSQI, that sleep deterioration is not related to the presence/degree of HE.13 However, another study, which assessed sleep by PSG, reported parallel improvements in sleep quality and HE.28 In an animal model of HE in rats, Felipo et al showed that HE rats had more awakenings during sleep and more naps during active phase than controls,40 supporting the association between sleep disturbances and HE. In another study, high levels of interleukin-6, and not the presence of HE, were associated with poor sleep, measured by PSQI.14 Based on these contradictory observations, further studies are clearly needed.
A recent finding that might explain delayed sleep onset in cirrhotic patients has implicated alterations in thermoregu-lation. Heat loss through vasodilation is essential for sleep onset,41 and it has been demonstrated, in cirrhotic patients compared to healthy controls, that the circadian variation of core body temperature is impaired, such that patients are unable to decrease their distal temperature at the end of the day.42 Similar results have been observed in rats, confirming that alterations in body temperature rhythms are associated with HE.40 The underlying pathophysiologic mechanism proposed by the authors is that this is provoked by the hyperdynamic circulatory syndrome caused by splanchnic and systemic vasodilation.42
Another recent observation focused on glucose fluctuations in cirrhotic patients, highlighting that greater fluctuations were associated with higher PSQI scores.43 Since 70% of cirrhotic patients suffer from glucose intolerance or diabetes,44 this could also partly explain the disturbed sleep patterns in these patients.
Finally, Bajaj et al have also observed, in five cirrhotic men with minimal HE compared to matched controls, that low values of ghrelin in cirrhotic patients were associated with SWS loss on PSG.31
Many lines of research remain to be explored in order to understand these complex pathophysiological mechanisms. A summary of current research in this area is shown in Table 3.
Table 3.
Possible pathophysiological mechanisms explaining sleep–wake abnormalities in cirrhotic patients
| Clinical features | Pathophysiological mechanisms |
|---|---|
| Delayed sleep onset | Decreased melatonin clearance Delayed melatonin peak Altered circadian variation of core body temperature |
| Excessive daytime sleepiness | Hepatic encephalopathy (HE) |
| Short total sleep time, low sleep efficiency, frequent awakenings | HE Increased interleukin-6 Glucose level fluctuations Low values of ghrelin |
Treatment for sleep disturbances in liver cirrhosis
As sleep disturbances and EDS are mainly related to HE, it has been hypothesized that HE treatment could positively influence these disorders. Other non-specific treatments for sleep disturbances have also been tried. They are detailed here.
Effectiveness of HE treatment on sleep disturbances
Non-absorbable disaccharides
Lactulose and lactitol aim to decrease ammonia absorption from the gut and are effective for improving HE.9,45,46 Treatment improves neurocognition and QoL.47 A recent work from Singh et al, comparison of cirrhotic patients without HE and those with minimal HE who were given lactulose for 3 months, showed that HE treatment led to improvements in EDS (measured by ESS), sleep quality (increases in TST, SE, SL, amount of REM sleep on PSG), and QoL (measured by the SF-36).7
Rifaximin
Rifaximin is a non-absorbable oral antibiotic currently recommended for the treatment of refractory HE and to maintain remission of these episodes.48 Its mechanism of action is complex and is thought to involve a decrease in the load of pathogenic microbial flora. Rifaximin improves neurocognitive function and QoL in HE.31,49 With regard to sleep, we have compared 15 recurrent HE patients before and after a rifaximin course. No changes in subjective nighttime sleep quality and daytime sleepiness were observed. REM sleep amounts were increased on PSG, but no changes were observed for EDS or the need for naps, despite improvements in HE scores.28
Probiotics and l-ornithine l-aspartate
These medications have been proved to improve HE and QoL.46,50 However, no specific studies are currently focused on sleep disturbances.
Liver transplant
One retrospective study has assessed sleep changes before and after liver transplant in 83 patients using questionnaires. Curiously, sleep quality improvement was excellent in patients with alcoholic liver disease but almost nonexistent in hepatitis C patients.51 It could be interesting to assess sleep disturbances and treatment effects according to the causal factors of cirrhosis.
Effectiveness of specific treatments on sleep disturbances
Light therapy
A single study on 12 decompensated cirrhotic patients assessed the effect of morning bright light therapy on sleep quality. Compared to controls, no effect was observed on sleep quality, EDS, or QoL.52
Sedatives
One randomized controlled study using the antihistamine hydroxyzine has shown, after a 10-day course, an improvement in SE.53
Stimulants
Modafinil, which is a usual treatment used to fight EDS in narcolepsy, has been tested in primary biliary cirrhosis in two studies. The medication was effective in 76% of 42 patients in terms of EDS, fatigue, and QoL.54 In the second study, on 21 patients, the effects were excellent on EDS and fatigue after 2 months of treatment, with a good toxicity profile.55
Conclusion
Sleep–wake abnormalities are common in liver cirrhosis and associated with impaired QoL. They should be properly addressed with objective measurements to rule out covert HE and to trigger specific adequate management.
Underlying pathophysiological mechanisms are complex and include disturbed metabolism of melatonin and glucose, alterations of thermoregulation, and altered ghrelin secretion. As sleep–wake abnormalities occur when HE is present, the cornerstone of treatment is based on HE treatment. A few non-specific treatments for sleep–wake abnormalities have been tried, with encouraging results for hydroxyzine and modafinil. However, due to the increased potential for medication toxicity in these disabled patients, further studies are needed to address the potential role of non-drug therapies in this population (eg, cognitive behavioral therapy, mindfulness, yoga) that have demonstrated usefulness in insomnia disorders.
Acknowledgments
The authors acknowledge the contribution of a medical writer, Sandy Field, PhD, to the preparation of this manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Scaglione S, Kliethermes S, Cao G, et al. The Epidemiology of Cirrhosis in the United States: A Population-based Study. J Clin Gastroenterol. 2015;49(8):690–696. doi: 10.1097/MCG.0000000000000208. [DOI] [PubMed] [Google Scholar]
- 2.Heeren M, Sojref F, Schuppner R, et al. Active at night, sleepy all day--sleep disturbances in patients with hepatitis C virus infection. J Hepatol. 2014;60(4):732–740. doi: 10.1016/j.jhep.2013.11.030. [DOI] [PubMed] [Google Scholar]
- 3.Ghabril M, Jackson M, Gotur R, et al. Most Individuals With Advanced Cirrhosis Have Sleep Disturbances, Which Are Associated With Poor Quality of Life. Clin Gastroenterol Hepatol. 2017;15(8):1271–1278.e6. doi: 10.1016/j.cgh.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montagnese S, De Pittà C, De Rui M, et al. Sleep-wake abnormalities in patients with cirrhosis. Hepatology. 2014;59(2):705–712. doi: 10.1002/hep.26555. [DOI] [PubMed] [Google Scholar]
- 5.Acharya C, Bajaj JS. Current Management of Hepatic Encephalopathy. Am J Gastroenterol. 2018 Jul 13; doi: 10.1038/s41395-018-0179-4. Epub. [DOI] [PubMed] [Google Scholar]
- 6.American Association for the Study of Liver Disease; European Association for the Study of the Liver Hepatic encephalopathy in chronic liver disease: 2014 practice guideline by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases. J Hepatol. 2014;61(3):642–659. doi: 10.1016/j.jhep.2014.05.042. [DOI] [PubMed] [Google Scholar]
- 7.Singh J, Sharma BC, Puri V, Sachdeva S, Srivastava S. Sleep disturbances in patients of liver cirrhosis with minimal hepatic encephalopathy before and after lactulose therapy. Metab Brain Dis. 2017;32(2):595–605. doi: 10.1007/s11011-016-9944-5. [DOI] [PubMed] [Google Scholar]
- 8.Sherlock S, Summerskill WH, White LP, Phear EA. Portal-systemic encephalopathy; neurological complications of liver disease. Lancet. 1954;267(6836):454–457. [PubMed] [Google Scholar]
- 9.Blei AT, Córdoba J, Practice Parameters Committee of the American College of Gastroenterology Hepatic Encephalopathy. Am J Gastro-enterol. 2001;96(7):1968–1976. doi: 10.1111/j.1572-0241.2001.03964.x. [DOI] [PubMed] [Google Scholar]
- 10.Bajaj JS, Thacker LR, Leszczyszyn D, et al. Effects of obstructive sleep apnea on sleep quality, cognition, and driving performance in patients with cirrhosis. Clin Gastroenterol Hepatol. 2015;13(2):390–397.e1. doi: 10.1016/j.cgh.2014.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kappus MR, Leszczyszyn DJ, Moses L, Raman S, Heuman DM, Bajaj JS. Effect of obstructive sleep apnea on the sleep architecture in cirrhosis. J Clin Sleep Med. 2013;9(3):247–251. doi: 10.5664/jcsm.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mostacci B, Ferlisi M, Baldi Antognini A, et al. Sleep disturbance and daytime sleepiness in patients with cirrhosis: a case control study. Neurol Sci. 2008;29(4):237–240. doi: 10.1007/s10072-008-0973-7. [DOI] [PubMed] [Google Scholar]
- 13.Montagnese S, Middleton B, Skene DJ, Morgan MY. Night-time sleep disturbance does not correlate with neuropsychiatric impairment in patients with cirrhosis. Liver Int. 2009;29(9):1372–1382. doi: 10.1111/j.1478-3231.2009.02089.x. [DOI] [PubMed] [Google Scholar]
- 14.Chou TC, Liang WM, Wang CB, Wu TN, Hang LW. Obstructive sleep apnea is associated with liver disease: a population-based cohort study. Sleep Med. 2015;16(8):955–960. doi: 10.1016/j.sleep.2015.02.542. [DOI] [PubMed] [Google Scholar]
- 15.Franco RA, Ashwathnarayan R, Deshpandee A, et al. The high prevalence of restless legs syndrome symptoms in liver disease in an academic-based hepatology practice. J Clin Sleep Med. 2008;4(1):45–49. [PMC free article] [PubMed] [Google Scholar]
- 16.Neu D, Mairesse O, Hoffmann G, et al. Sleep quality perception in the chronic fatigue syndrome: correlations with sleep efficiency, affective symptoms and intensity of fatigue. Neuropsychobiology. 2007;56(1):40–46. doi: 10.1159/000110727. [DOI] [PubMed] [Google Scholar]
- 17.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53(3):737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 18.Sato M, Yamadera W, Matsushima M, Itoh H, Nakayama K. Clinical efficacy of individual cognitive behavior therapy for psycho-physiological insomnia in 20 outpatients. Psychiatry Clin Neurosci. 2010;64(2):187–195. doi: 10.1111/j.1440-1819.2009.02060.x. [DOI] [PubMed] [Google Scholar]
- 19.Tsai CF, Chu CJ, Wang YP, et al. Increased serum interleukin-6, not minimal hepatic encephalopathy, predicts poor sleep quality in nonalcoholic cirrhotic patients. Aliment Pharmacol Ther. 2016;44(8):836–845. doi: 10.1111/apt.13765. [DOI] [PubMed] [Google Scholar]
- 20.Gencdal G, Gunsar F, Meral CE, et al. Sleep disorders in cirrhotics; how can we detect? Liver Int. 2014;34(8):1192–1197. doi: 10.1111/liv.12485. [DOI] [PubMed] [Google Scholar]
- 21.Samanta J, Dhiman RK, Khatri A, et al. Correlation between degree and quality of sleep disturbance and the level of neuropsychiatric impairment in patients with liver cirrhosis. Metab Brain Dis. 2013;28(2):249–259. doi: 10.1007/s11011-013-9393-3. [DOI] [PubMed] [Google Scholar]
- 22.Labenz C, Baron JS, Toenges G, et al. Prospective evaluation of the impact of covert hepatic encephalopathy on quality of life and sleep in cirrhotic patients. Aliment Pharmacol Ther. 2018;48(3):313–321. doi: 10.1111/apt.14824. [DOI] [PubMed] [Google Scholar]
- 23.De Rui M, Middleton B, Sticca A, et al. Sleep and circadian rhythms in hospitalized patients with decompensated cirrhosis: effect of light therapy. Neurochem Res. 2015;40(2):284–292. doi: 10.1007/s11064-014-1414-z. [DOI] [PubMed] [Google Scholar]
- 24.Marques DM, Teixeira HR, Lopes AR, et al. Sleep Quality Assessment and Daytime Sleepiness of Liver Transplantation Candidates. Transplant Proc. 2016;48(7):2356–2360. doi: 10.1016/j.transproceed.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 25.Córdoba J, Cabrera J, Lataif L, Penev P, Zee P, Blei AT. High prevalence of sleep disturbance in cirrhosis. Hepatology. 1998;27(2):339–345. doi: 10.1002/hep.510270204. [DOI] [PubMed] [Google Scholar]
- 26.Morphy H, Dunn KM, Lewis M, Boardman HF, Croft PR. Epidemiology of insomnia: a longitudinal study in a UK population. Sleep. 2007;30(3):274–280. [PubMed] [Google Scholar]
- 27.Bersagliere A, Raduazzo ID, Nardi M, et al. Induced hyperammonemia may compromise the ability to generate restful sleep in patients with cirrhosis. Hepatology. 2012;55(3):869–878. doi: 10.1002/hep.24741. [DOI] [PubMed] [Google Scholar]
- 28.Bruyneel M, Sersté T, Libert W, et al. Improvement of sleep architecture parameters in cirrhotic patients with recurrent hepatic encephalopathy with the use of rifaximin. Eur J Gastroenterol Hepatol. 2017;29(3):302–308. doi: 10.1097/MEG.0000000000000786. [DOI] [PubMed] [Google Scholar]
- 29.Montagnese S, Middleton B, Mani AR, Skene DJ, Morgan MY. Sleep and circadian abnormalities in patients with cirrhosis: features of delayed sleep phase syndrome? Metab Brain Dis. 2009;24(3):427–439. doi: 10.1007/s11011-009-9146-5. [DOI] [PubMed] [Google Scholar]
- 30.Liu C, Zhou J, Yang X, Lv J, Shi Y, Zeng X. Changes in sleep architecture and quality in minimal hepatic encephalopathy patients and relationship to psychological dysfunction. Int J Clin Exp Med. 2015;8(11):21541–21548. [PMC free article] [PubMed] [Google Scholar]
- 31.Bajaj JS, Saeian K, Schubert CM, Franco R, Franco J, Heuman DM. Disruption of sleep architecture in minimal hepatic encephalopathy and ghrelin secretion. Aliment Pharmacol Ther. 2011;34(1):103–105. doi: 10.1111/j.1365-2036.2011.04681.x. [DOI] [PubMed] [Google Scholar]
- 32.Watanabe A. Cerebral changes in hepatic encephalopathy. J Gastroen-terol Hepatol. 1998;13(7):752–760. doi: 10.1111/j.1440-1746.1998.tb00728.x. [DOI] [PubMed] [Google Scholar]
- 33.Teodoro VV, Bragagnolo MA, Jr, Lucchesi LM, et al. Polysomnographic sleep aspects in liver cirrhosis: a case control study. World J Gastroen-terol. 2013;19(22):3433–3438. doi: 10.3748/wjg.v19.i22.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llansola M, Cantero JL, Hita-Yañez E, et al. Progressive reduction of sleep time and quality in rats with hepatic encephalopathy caused by portacaval shunts. Neuroscience. 2012;201:199–208. doi: 10.1016/j.neuroscience.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Bianchi G, Marchesini G, Nicolino F, et al. Psychological status and depression in patients with liver cirrhosis. Dig Liver Dis. 2005;37(8):593–600. doi: 10.1016/j.dld.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 36.Montagnese S, Middleton B, Mani AR, Skene DJ, Morgan MY. On the origin and the consequences of circadian abnormalities in patients with cirrhosis. Am J Gastroenterol. 2010;105(8):1773–1781. doi: 10.1038/ajg.2010.86. [DOI] [PubMed] [Google Scholar]
- 37.Steindl PE, Finn B, Bendok B, Rothke S, Zee PC, Blei AT. Disruption of the diurnal rhythm of plasma melatonin in cirrhosis. Ann Intern Med. 1995;123(4):274–277. doi: 10.7326/0003-4819-123-4-199508150-00005. [DOI] [PubMed] [Google Scholar]
- 38.Zee PC, Mehta R, Turek FW, Blei AT. Portacaval anastomosis disrupts circadian locomotor activity and pineal melatonin rhythms in rats. Brain Res. 1991;560(1–2):17–22. doi: 10.1016/0006-8993(91)91209-j. [DOI] [PubMed] [Google Scholar]
- 39.De Rui M, Schiff S, Aprile D, et al. Excessive daytime sleepiness and hepatic encephalopathy: it is worth asking. Metab Brain Dis. 2013;28(2):245–248. doi: 10.1007/s11011-012-9360-4. [DOI] [PubMed] [Google Scholar]
- 40.Felipo V, Piedrafita B, Barios JA, et al. Rats with minimal hepatic encephalopathy show reduced cGMP-dependent protein kinase activity in hypothalamus correlating with circadian rhythms alterations. Chronobiol Int. 2015;32(7):966–979. doi: 10.3109/07420528.2015.1057640. [DOI] [PubMed] [Google Scholar]
- 41.Weiss N, Attali V, Bouzbib C, Thabut D. Altered distal-proximal temperature gradient as a possible explanation for sleep-wake disturbances in cirrhotic patients. Liver Int. 2017;37(12):1776–1779. doi: 10.1111/liv.13590. [DOI] [PubMed] [Google Scholar]
- 42.Garrido M, Saccardo D, De Rui M, et al. Abnormalities in the 24-hour rhythm of skin temperature in cirrhosis: Sleep-wake and general clinical implications. Liver Int. 2017;37(12):1833–1842. doi: 10.1111/liv.13525. [DOI] [PubMed] [Google Scholar]
- 43.Haraguchi M, Miyaaki H, Ichikawa T, et al. Glucose fluctuations reduce quality of sleep and of life in patients with liver cirrhosis. Hepatol Int. 2017;11(1):125–131. doi: 10.1007/s12072-016-9762-1. [DOI] [PubMed] [Google Scholar]
- 44.Kruszynska YT, Home PD, McIntyre N. Relationship between insulin sensitivity, insulin secretion and glucose tolerance in cirrhosis. Hepatol-ogy. 1991;14(1):103–111. doi: 10.1002/hep.1840140117. [DOI] [PubMed] [Google Scholar]
- 45.Watanabe A, Sakai T, Sato S, et al. Clinical efficacy of lactulose in cirrhotic patients with and without subclinical hepatic encephalopathy. Hepatology. 1997;26(6):1410–1414. doi: 10.1053/jhep.1997.v26.pm0009397979. [DOI] [PubMed] [Google Scholar]
- 46.Mittal VV, Sharma BC, Sharma P, Sarin SK. A randomized controlled trial comparing lactulose, probiotics, and L-ornithine L-aspartate in treatment of minimal hepatic encephalopathy. Eur J Gastroenterol Hepatol. 2011;23(8):725–732. doi: 10.1097/MEG.0b013e32834696f5. [DOI] [PubMed] [Google Scholar]
- 47.Prasad S, Dhiman RK, Duseja A, Chawla YK, Sharma A, Agarwal R. Lactulose improves cognitive functions and health-related quality of life in patients with cirrhosis who have minimal hepatic encephalopathy. Hepatology. 2007;45(3):549–559. doi: 10.1002/hep.21533. [DOI] [PubMed] [Google Scholar]
- 48.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med. 2010;362(12):1071–1081. doi: 10.1056/NEJMoa0907893. [DOI] [PubMed] [Google Scholar]
- 49.Sidhu SS, Goyal O, Mishra BP, Sood A, Chhina RS, Soni RK. Rifaximin improves psychometric performance and health-related quality of life in patients with minimal hepatic encephalopathy (the RIME Trial) Am J Gastroenterol. 2011;106(2):307–316. doi: 10.1038/ajg.2010.455. [DOI] [PubMed] [Google Scholar]
- 50.Ong JP, Oehler G, Krüger-Jansen C, Lambert-Baumann J, Younossi ZM. Oral L-ornithine-L-aspartate improves health-related quality of life in cirrhotic patients with hepatic encephalopathy: an open-label, prospective, multicentre observational study. Clin Drug Investig. 2011;31(4):213–220. doi: 10.2165/11586700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 51.Bhat M, Wyse JM, Moodie E, et al. Prevalence and predictors of sleep disturbance among liver diseases in long-term transplant survivors. Can J Gastroenterol Hepatol. 2015;29(8):440–444. doi: 10.1155/2015/359640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.De Rui M, Middleton B, Sticca A, et al. Sleep and circadian rhythms in hospitalized patients with decompensated cirrhosis: effect of light therapy. Neurochem Res. 2015;40(2):284–292. doi: 10.1007/s11064-014-1414-z. [DOI] [PubMed] [Google Scholar]
- 53.Spahr L, Coeytaux A, Giostra E, Hadengue A, Annoni JM. Histamine H1 blocker hydroxyzine improves sleep in patients with cirrhosis and minimal hepatic encephalopathy: a randomized controlled pilot trial. Am J Gastroenterol. 2007;102(4):744–753. doi: 10.1111/j.1572-0241.2006.01028.x. [DOI] [PubMed] [Google Scholar]
- 54.Ian Gan S, de Jongh M, Kaplan MM. Modafinil in the treatment of debilitating fatigue in primary biliary cirrhosis: a clinical experience. Dig Dis Sci. 2009;54(10):2242–2246. doi: 10.1007/s10620-008-0613-3. [DOI] [PubMed] [Google Scholar]
- 55.Jones DE, Newton JL. An open study of modafinil for the treatment of daytime somnolence and fatigue in primary biliary cirrhosis. Aliment Pharmacol Ther. 2007;25(4):471–476. doi: 10.1111/j.1365-2036.2006.03223.x. [DOI] [PubMed] [Google Scholar]