Abstract
Background
There is accumulating evidence that serum levels of non–high‐density lipoprotein cholesterol (non–HDL‐C) are a more accurate predictor of cardiovascular outcomes when compared with low‐density lipoprotein cholesterol. However, we recently found that higher serum concentrations of triglycerides are associated with better outcomes in patients undergoing hemodialysis. Therefore, we hypothesized that the association of serum levels of non–HDL‐C (which includes triglyceride‐rich lipoproteins) with outcomes may also be different in patients undergoing hemodialysis when compared with other patient populations.
Methods and Results
We studied the association of baseline and time‐dependent serum levels of non–HDL‐C with all‐cause and cardiovascular mortality using Cox proportional hazard regression models in a nationally representative cohort of 50 118 patients undergoing incident hemodialysis from January 1, 2007, to December 31, 2011. In time‐dependent models adjusted for case mix and surrogates of malnutrition and inflammation, a graded inverse association between non–HDL‐C level and mortality was demonstrated with hazard ratios (95% confidence intervals) of the lowest (<60 mg/dL) and highest (≥160 mg/dL) categories: 1.88 (1.72–2.06) and 0.73 (0.64–0.83) for all‐cause mortality and 2.07 (1.78–2.41) and 0.75 (0.60–0.93) for cardiovascular mortality, respectively (reference, 100–115 mg/dL). In analyses using baseline values, non–HDL‐C levels <100 mg/dL were also associated with significantly higher mortality risk across all levels of adjustment. Similar associations were found when evaluating non‐HDL/HDL cholesterol ratio and mortality, with the highest all‐cause and cardiovascular mortality being observed in patients with decreased non‐HDL/HDL‐C ratio (<2.5).
Conclusions
Contrary to the general population, decrements in non–HDL‐C and non‐HDL/HDL cholesterol ratio were paradoxically associated with increased all‐cause and cardiovascular mortality in patients undergoing incident hemodialysis. The underlying mechanisms responsible for these associations await further investigation.
Keywords: dyslipidemia, hemodialysis, high‐density lipoprotein, mortality, non–high‐density lipoprotein
Subject Categories: Lipids and Cholesterol, Nephrology and Kidney, Epidemiology, Mortality/Survival
Clinical Perspective
What Is New?
This study evaluated the association of serum non–high‐density lipoprotein cholesterol level with all‐cause and cardiovascular mortality in patients undergoing incident hemodialysis.
Contrary to the general population, reduced levels of non–high‐density lipoprotein cholesterol and non–high‐density lipoprotein/high‐density lipoprotein cholesterol ratio were paradoxically associated with poor overall survival and increased cardiovascular mortality in patients undergoing incident hemodialysis.
What Are the Clinical Implications?
These observations further highlight the unique nature of dyslipidemia and cardiovascular disease in patients undergoing hemodialysis and underscore the need for additional investigations aimed at deciphering the role of lipoproteins as an index of risk and target of therapy in the population undergoing dialysis.
Introduction
Serum non–high‐density lipoprotein cholesterol (non–HDL‐C), defined as the difference between serum total cholesterol and HDL‐C, accounts for all the cholesterol content of lipoprotein particles that have traditionally been viewed as proatherogenic, including low‐density lipoprotein (LDL), lipoprotein (a), and triglyceride‐rich lipoproteins (ie, intermediate‐density lipoprotein, very low‐density lipoprotein, and chylomicrons).1, 2, 3, 4 It has been postulated that in non‐HDLs, cholesterol is the main component that plays a major role in atherogenicity. Hence, the association of serum levels of these lipoproteins with worse outcomes is driven by their cholesterol content. Accordingly, it is the cholesterol component of the triglyceride‐rich lipoproteins that is viewed as the major contributor to their deleterious role in atherosclerosis and pathogenesis of cardiovascular disease. Therefore, non–HDL‐C, which encompasses all cholesterol content not associated with HDL, has received special attention as a more accurate predictor of cardiovascular risk and outcomes.5, 6, 7, 8, 9, 10 This notion has been further explained by the fact that non–HDL‐C is more representative of all apolipoprotein B (ApoB)–containing lipoproteins, whereas serum LDL cholesterol (LDL‐C) in isolation comprises only a subset of serum ApoB‐associated cholesterol content.1, 2, 3, 4
It is well known that the association of serum lipids with outcomes is significantly altered in patients with end‐stage renal disease (ESRD) when compared with the general population.11, 12, 13 In this regard, we recently found that elevated serum concentrations of triglycerides were paradoxically associated with a reduced risk of death in a cohort of patients undergoing hemodialysis.14 In addition, several studies have found that higher serum HDL cholesterol levels are associated with worse survival in patients with chronic kidney disease and those undergoing hemodialysis.15, 16, 17 However, the relationship between serum non–HDL‐C level and outcomes is less clear, given that most of the data available are based on small prospective studies with a limited number of patients and major limitations.18, 19 Meanwhile, the largest observational cohort study conducted in 45 390 patients undergoing prevalent hemodialysis showed a positive association between elevated serum non–HDL‐C levels and higher rates of incident myocardial and cerebral infarction; however, these findings were not associated with an increased risk of mortality.20 Furthermore, all the studies mentioned were conducted in Japanese patients and, therefore, their findings cannot be generalized to a wider population of patients undergoing hemodialysis. Given the paradoxical associations between serum triglyceride levels and outcomes in patients undergoing maintenance hemodialysis, we hypothesized that in patients with ESRD undergoing maintenance hemodialysis, the association of serum concentrations of non–HDL‐C with mortality is different than that observed in the general population. Hence, we evaluated the association of serum non–HDL‐C level with all‐cause and cardiovascular mortality in a diverse population of patients undergoing incident hemodialysis from a large US dialysis organization with uniform practice patterns and highly standardized laboratory values that were all measured in a single laboratory. Furthermore, we also examined the association between non‐HDL/HDL cholesterol (non‐HDL/HDL‐C) ratio and risk of mortality to account for any potential role that serum HDL cholesterol may play in these findings.
Methods
The data, analytic methods, and study materials cannot be made available to other researchers for purposes of reproducing the results or replicating the procedure, given that data are provided under contract with the large dialysis organization and are at its disposal. Hence, this center may not override the contractual agreements. Additional details about the analytical methods can be provided on request.
Study Population and Data Source
The study cohort was composed of all patients with ESRD who were initiated on hemodialysis between January 1, 2007, and December 31, 2011, within 1 of the outpatient facilities of a large dialysis organization and were followed up over a period of 5 years.21 Patients were included provided that they were ≥18 years, were treated with only in‐center hemodialysis for at least 60 days, and had serum non–HDL‐C measured during the first 91‐day period of hemodialysis (baseline quarter). Patients were further excluded if they had an outlier non–HDL‐C concentration <0.5th or >99.5th percentile of observed values. Accordingly, the final study population consisted of 51 185 patients (Figure S1).
All data were obtained from electronic records of the dialysis organization. To minimize measurement variability, all repeated measures of every relevant variable within each 3‐month period starting from the date of first dialysis were averaged to obtain 1 quarterly mean value. Blood samples were drawn using standardized techniques in all dialysis clinics and were transported to a central laboratory in Deland, FL, typically within 24 hours. All laboratory values were measured using automated and standardized methods. The study was approved by the University of California (Irvine, CA) institutional review board. Given the large sample size, anonymity of the patients studied, and nonintrusive nature of the research, the requirement for written consent was waived. The data, analytic methods, and study materials cannot be made available to other researchers for purposes of reproducing the results or replicating the procedures, given that data are provided under contract with the large dialysis organization and are at its disposal. Hence, this center may not override the contractual agreements. Additional details about the analytical methods can be provided on request.
Exposure and Outcome Ascertainment
The primary and secondary exposures of interest were serum non–HDL‐C and non‐HDL/HDL‐C ratio levels, which were calculated by subtracting HDL‐C level from total cholesterol (TC) level and by dividing non‐HDL by HDL values, respectively. Given a possible nonlinear relationship with mortality rates, non‐HDL level and non‐HDL/HDL‐C ratio were treated as categorical variables and divided into 8 a priori selected categories: <60, 60 to <85, 85 to <100, 100 to <115 (reference), 115 to <130, 130 to <145, 145 to 160, and ≥160 mg/dL for non‐HDL‐C; and <1.5, 1.5 to <2.0, 2.0 to <2.5, 2.5 to <3.0 (reference), 3.0 to <3.5, 3.5 to <4.0, 4.0 to 4.5, and ≥4.5 for non‐HDL/HDL‐C ratio. These increments and the reference categories were selected on the basis of clinically relevant guidelines (ie, <100 and >40 mg/dL for target levels in non‐HDL and HDL cholesterol, respectively, and corresponding 2.5 for non‐HDL/HDL‐C ratio) or because they were the modal category or adjacent to the modal category with similar sample size or event rates as the modal category to allow for the most powerful analyses. We also treated non–HDL‐C level and non‐HDL/HDL‐C ratio as a continuous variable and modeled a nonlinear effect by using a restricted cubic spine function.
The primary and secondary outcomes of interest were time to all‐cause and cardiovascular death, respectively. Data on cardiovascular death were obtained by identifying primary cause of death from the electronic medical records. For mortality analyses, patients remained at risk until death, censoring for unavailability for follow‐up, discontinuation of dialysis therapy, kidney transplantation, transfer to a nonaffiliated dialysis clinic, or end of the study period (December 31, 2011).
Statistical Analyses
Cox proportional hazard regression models were separately performed to study the associations of non–HDL‐C level and non‐HDL/HDL‐C ratio with subsequent mortality using 2 approaches: (1) fixed models with baseline values were examined to ascertain long‐term exposure‐mortality associations; and (2) time‐dependent models were assessed to account for changes in exposure over time and to ascertain their short‐term associations.22 In time‐dependent models, non–HDL‐C level, non‐HDL/HDL‐C ratio, and all other continuous variables were calculated and updated at each quarter (91‐day interval) over the entire follow‐up. For each analysis, 3 hierarchical levels of adjustment models were constructed on the basis of a priori considerations: (1) unadjusted models; (2) case‐mix–adjusted models that included age, sex, race/ethnicity (white, black, Hispanic, Asian, or other), primary insurance (Medicare, Medicaid, and others), initial vascular access type (central venous catheter, arteriovenous fistula, arteriovenous graft, or other), 9 comorbid conditions (diabetes mellitus, hypertension, atherosclerotic heart disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, dyslipidemia, chronic obstructive pulmonary disease, and malignancy), and dialysis dose, as indicated by single‐pool Kt/V; (3) fully adjusted models, which included all covariates in the case‐mix model plus malnutrition‐inflammation‐cachexia syndrome variables, including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron‐binding capacity, ferritin, LDL‐C, and body mass index (body weight in kilograms divided by height in meters squared). Additional adjustment for statin therapy ever used at any time during the follow‐up was conducted on the fully adjusted model to determine whether statin treatment would affect the association between non–HDL‐C or non‐HDL/HDL‐C ratio and mortality. Because cardiovascular and noncardiovascular death are competing events, their association with 2 outcomes was assessed by means of semiparametric competing risk regression in sensitivity analysis.23 We additionally explored the continuous, potentially nonlinear, relationship between non–HDL‐C and non‐HDL/HDL‐C ratio and mortality by using fully adjusted restricted cubic spline models with 4 knots. All mortality associations were expressed as a hazard ratio and 95% confidence interval. Proportional hazards assumptions were checked by graphical and formal testing, including Schoenfeld residuals, and the assumption was not violated.
To test the robustness of our findings, we also performed subgroup analyses on the basis of a priori defined variables (eg, age, sex, race/ethnicity, diabetes mellitus, hypertension, atherosclerotic heart disease, congestive heart failure, statin therapy, and serum TC, LDL‐C, HDL‐C, albumin, and ferritin levels). Subgroups of the continuous variables were created by dichotomization of these variables at the median value. The frequency of missing data was low (≤0.5%, ascertained at baseline) for most covariates in multivariate‐adjusted models, except for statin therapy (10.2%), for which patients were excluded from an analysis without imputation. In time‐varying analyses, for patients with data for serum non–HDL‐C at baseline but missing for subsequent follow‐up, the last available non–HDL‐C level was assumed to be unchanged until the next measurement or occurrence of the event (death or censor). Information about cause of death was also missing in 1393 of 12 859 deaths (10.8%) in this cohort. Data were summarized using proportions, means (±SD), or median (interquartile range), as appropriate. All analyses were implemented using Stata, version 13.1 (Stata Corporation, College Station, TX).
Results
Study Population
Among a total of 208 820 patients undergoing dialysis, 51 185 who met eligibility criteria were included in the final analyses for all‐cause mortality (Figure S1). The mean±SD age of the patients included was 62.8±14.9 years, 44% were women, and 63% were diabetic. The median values of baseline non–HDL‐C and non‐HDL/HDL‐C ratio were 105 (interquartile range, 82–133) mg/dL and 2.7 (interquartile range, 2.0–3.8), respectively. The baseline characteristics of the overall as well as the subset of patients according to non–HDL‐C and non‐HDL/HDL‐C ratio categories are summarized in Table 1 and Table S1. Patients with higher non–HDL‐C level and non‐HDL/HDL‐C ratio tended to be younger, were less likely to be diabetic, and were more likely to have higher TC, LDL‐C, triglyceride, and phosphorus levels.
Table 1.
Baseline Characteristics of 51 185 Patients According to Baseline Serum Non–HDL‐C Level
| Characteristics | Overall (N=51 185) | Serum Non‐HDL‐C Concentrations, mg/dL | |||||||
|---|---|---|---|---|---|---|---|---|---|
| <60 (n=3048) | 60–<85 (n=11 015) | 85–<100 (n=8701) | 100–<115 (n=7919) | 115–<130 (n=6575) | 130–<145 (n=4759) | 145–<160 (n=3321) | ≥160 (n=5847) | ||
| Age, y | 62.8±14.9 | 67.3±12.9 | 65.7±14.1 | 64.2±14.6 | 63.0±14.9 | 61.6±15.1 | 60.2±14.9 | 59.4±15.1 | 58.0±14.9 |
| Sex, % women | 43.6 | 33.7 | 36.6 | 40.1 | 43.8 | 46.0 | 47.8 | 52.2 | 55.3 |
| Race, % | |||||||||
| White | 46.4 | 58.3 | 50.9 | 48.0 | 45.3 | 43.7 | 42.1 | 40.6 | 41.1 |
| Black | 31.9 | 24.1 | 28.1 | 30.8 | 32.5 | 34.5 | 35.0 | 36.7 | 35.9 |
| Hispanic | 15.2 | 12.4 | 14.6 | 15.1 | 15.6 | 15.5 | 15.6 | 16.7 | 16.2 |
| Asian | 3.0 | 2.3 | 2.8 | 2.8 | 3.2 | 2.9 | 3.4 | 2.8 | 3.3 |
| Others | 3.4 | 3.0 | 3.6 | 3.3 | 3.4 | 3.4 | 3.9 | 3.2 | 3.4 |
| Primary insurance, % | |||||||||
| Medicare | 53.3 | 58.9 | 56.6 | 54.0 | 53.0 | 51.1 | 50.8 | 49.8 | 50.0 |
| Medicaid | 6.4 | 5.0 | 5.7 | 6.2 | 6.5 | 6.9 | 7.1 | 7.4 | 7.4 |
| Others | 40.3 | 36.1 | 37.7 | 39.8 | 40.5 | 42.1 | 42.1 | 42.8 | 42.6 |
| Initial vascular access type, % | |||||||||
| Central venous catheter | 74.2 | 72.5 | 72.3 | 73.1 | 73.8 | 74.7 | 76.4 | 76.3 | 77.4 |
| Arteriovenous fistula | 15.7 | 17.6 | 17.1 | 16.6 | 16.1 | 14.6 | 14.2 | 14.7 | 12.9 |
| Arteriovenous graft | 4.3 | 4.4 | 4.4 | 4.3 | 4.4 | 4.8 | 3.7 | 3.8 | 4.2 |
| Others and unknown | 5.8 | 5.5 | 6.2 | 6.0 | 5.7 | 5.9 | 5.7 | 5.2 | 5.5 |
| Comorbidities, % | |||||||||
| Diabetes mellitus | 62.8 | 66.2 | 65.2 | 63.5 | 61.9 | 61.8 | 59.8 | 59.6 | 62.4 |
| Hypertension | 51.8 | 46.1 | 50.8 | 51.8 | 52.3 | 52.7 | 54.0 | 53.9 | 52.1 |
| Congestive heart failure | 36.6 | 41.2 | 37.9 | 35.3 | 35.7 | 35.8 | 36.3 | 35.8 | 36.9 |
| Atherosclerotic heart disease | 18.2 | 19.0 | 19.3 | 18.7 | 18.3 | 17.4 | 16.5 | 18.0 | 17.3 |
| Other cardiovascular disease | 16.7 | 19.6 | 17.6 | 17.2 | 16.8 | 15.9 | 14.9 | 16.2 | 15.1 |
| Cerebrovascular disease | 1.7 | 1.9 | 1.7 | 1.6 | 1.8 | 1.7 | 1.6 | 2.0 | 1.9 |
| Dyslipidemia | 34.6 | 35.0 | 35.7 | 34.9 | 34.1 | 34.2 | 33.9 | 33.6 | 34.3 |
| COPD | 5.2 | 6.2 | 5.8 | 5.3 | 4.7 | 5.1 | 4.8 | 4.9 | 4.5 |
| History of malignancy | 2.3 | 2.2 | 2.4 | 2.4 | 2.3 | 2.2 | 2.3 | 1.9 | 2.1 |
| Statin therapy, % | 41.2 | 47.0 | 47.7 | 44.3 | 40.2 | 36.8 | 36.2 | 34.1 | 35.7 |
| Dialysis dose: single‐pool Kt/V | 1.5±0.3 | 1.4±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 | 1.5±0.3 |
| Body mass index, kg/m2 | 28.3±7.4 | 28.0±7.3 | 27.9±7.1 | 28.1±7.3 | 28.0±7.3 | 28.4±7.5 | 28.7±7.5 | 28.7±7.6 | 29.1±7.7 |
| Lipid parameters | |||||||||
| Total cholesterol, mg/dL | 151.1±42.6 | 88.6±14.6 | 112.7±15.5 | 132.5±14.6 | 147.5±14.5 | 162.8±14.9 | 177.6±14.6 | 193.0±15.1 | 230.0±29.3 |
| LDL, mg/dL | 79.5±33.7 | 33.8±8.1 | 50.5±11.0 | 64.8±12.0 | 76.7±13.2 | 88.5±14.2 | 100.4±15.4 | 112.9±16.2 | 142.7±28.3 |
| Triglyceride, mg/dL | 158.6±89.4 | 91.4±35.8 | 116.0±49.4 | 137.4±59.9 | 153.4±71.7 | 169.6±75.9 | 190.3±91.0 | 202.6±97.5 | 249.1±130.5 |
| HDL, mg/dL | 40.2±14.0 | 36.6±13.2 | 39.2±13.6 | 40.4±13.9 | 40.6±13.8 | 41.0±14.3 | 40.8±14.0 | 41.3±14.4 | 41.3±14.4 |
| Non‐HDL, mg/dL | 110.8±39.3 | 51.9±5.5 | 73.5±7.0 | 92.1±4.3 | 106.9±4.3 | 121.7±4.3 | 136.7±4.3 | 151.6±4.3 | 188.7±25.3 |
| Non‐HDL/HDL ratio | 3.1±1.6 | 1.6±0.8 | 2.1±0.9 | 2.6±1.0 | 2.9±1.3 | 3.3±1.3 | 3.7±1.4 | 4.1±1.4 | 5.1±2.2 |
| Other laboratory parameters | |||||||||
| Hemoglobin, g/dL | 11.1±1.2 | 10.8±1.2 | 11.0±1.2 | 11.1±1.2 | 11.1±1.2 | 11.2±1.2 | 11.2±1.2 | 11.1±1.2 | 11.2±1.2 |
| White blood cells, ×103/μL | 7.8±2.6 | 7.6±2.6 | 7.7±2.8 | 7.8±2.6 | 7.8±2.6 | 7.8±2.5 | 7.8±2.4 | 7.9±2.5 | 8.0±2.5 |
| Albumin, g/dL | 3.5±0.5 | 3.4±0.5 | 3.5±0.5 | 3.5±0.4 | 3.5±0.4 | 3.6±0.5 | 3.6±0.5 | 3.5±0.5 | 3.5±0.5 |
| Calcium, mg/dL | 9.1±0.6 | 9.1±0.6 | 9.1±0.5 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 | 9.1±0.6 |
| Phosphorus, mg/dL | 4.9±1.1 | 4.6±1.0 | 4.8±1.1 | 4.9±1.1 | 4.9±1.1 | 5.0±1.1 | 5.0±1.1 | 5.1±1.1 | 5.1±1.1 |
| Intact PTH, pg/mL | 316 (199–488) | 261 (166–397) | 287 (183–445) | 311 (198–478) | 322 (201–495) | 334 (211–511) | 341 (219–522) | 341 (221–524) | 350 (214–538) |
| Bicarbonate, mEq/L | 23.6±2.7 | 24.0±2.8 | 23.9±2.8 | 23.7±2.7 | 23.6±2.7 | 23.5±2.7 | 23.5±2.7 | 23.4±2.7 | 23.3±2.6 |
| TIBC, mg/dL | 226.0±48.4 | 220.5±55.7 | 223.6±50.9 | 225.8±47.7 | 227.3±47.2 | 227.4±46.4 | 228.5±46.0 | 227.8±46.3 | 227.8±47.3 |
| Ferritin, ng/mL | 286 (168–486) | 280 (163–469) | 283 (166–477) | 282 (169–478) | 285 (168–487) | 294 (171–492) | 294 (172–490) | 298 (172–503) | 289 (166–501) |
Data are presented as means±SDs, medians (interquartile ranges), or percentages. COPD indicates chronic obstructive pulmonary disease; HDL, high‐density lipoprotein; HDL‐C, HDL cholesterol; LDL, low‐density lipoprotein; PTH, parathyroid hormone; TIBC, total iron‐binding capacity.
During a median follow‐up of 1.6 years (interquartile range, 0.9–2.7 years; total time at risk, 96 999 patient‐years), a total of 12 859 all‐cause deaths occurred (mortality rate, 133 per 1000 patient‐years; 95% confidence interval, 130–135 per 1000 patient‐years). Of these, 11 466 individuals (89.2%) had data available on primary cause of death, in which 4578 (39.9%) were attributed to cardiovascular mortality (mortality rate, 48 per 1000 patient‐years; 95% confidence interval, 47–50 per 1000 patient‐years). Sudden cardiac death (62.0%) was the most common cause of death in this study, followed by congestive heart failure (12.7%) (Table S2). The demographic, comorbidity, and laboratory characteristics of patients whose cause of death was known were mostly similar to those who did not have a known cause of death (Table S3).
Association of Non–HDL‐C With Outcomes
In analyses using baseline values, incrementally lower non–HDL‐C levels at baseline (<100 mg/dL) were associated with significantly higher all‐cause and cardiovascular mortality at all 4 levels of adjustment (Table 2 and Figure S2). In time‐dependent models, there was a graded inverse and linear association between serum non–HDL‐C levels and all‐cause and cardiovascular mortality. Fully adjusted hazard ratios (95% confidence intervals) from the lowest to highest categories were 1.88 (1.72–2.06), 1.35 (1.26–1.45), 1.14 (1.07–1.22), 0.86 (0.79–0.93), 0.87 (0.79–0.95), 0.79 (0.71–0.89), and 0.73 (0.64–0.83) for all‐cause mortality and 2.07 (1.78–2.41), 1.43 (1.27–1.60), 1.15 (1.03–1.29), 0.87 (0.76–0.99), 0.78 (0.66–0.92), 0.74 (0.61–0.90), and 0.75 (0.60–0.93) for cardiovascular mortality. These associations remained largely unchanged, despite additional adjustment for statin therapy (Table 3 and Figure 1). Moreover, in sensitivity analyses of competing risk regression models in which noncardiovascular death was assigned as a competing event, similar findings were observed (Table S4).
Table 2.
Associations of Baseline Serum Non–HDL‐C With All‐Cause and Cardiovascular Mortality
| Non‐HDL‐C, mg/dL | Unadjusted | Case Mix | Case Mix and MICS | Case Mix, MICS, and Statin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| All‐cause mortality | ||||||||||||
| <60 | 1.98 | 1.70–1.96 | <0.001 | 1.73 | 1.61–1.87 | <0.001 | 1.63 | 1.49–1.79 | <0.001 | 1.64 | 1.48–1.82 | <0.001 |
| 60–<85 | 1.42 | 1.36–1.58 | <0.001 | 1.33 | 1.25–1.41 | <0.001 | 1.31 | 1.22–1.40 | <0.001 | 1.32 | 1.23–1.42 | <0.001 |
| 85–<100 | 1.11 | 1.25–1.45 | 0.001 | 1.06 | 1.00–1.13 | 0.050 | 1.07 | 1.01–1.14 | 0.035 | 1.09 | 1.01–1.17 | 0.022 |
| 100–<115 | 1.00 | … | … | 1.00 | … | … | 1.00 | … | … | 1.00 | … | … |
| 115– <130 | 0.96 | 0.94–1.10 | 0.292 | 1.01 | 0.94–1.08 | 0.881 | 0.99 | 0.92–1.05 | 0.704 | 1.01 | 0.94–1.09 | 0.794 |
| 130–<145 | 0.87 | 0.81–0.96 | 0.001 | 0.96 | 0.88–1.03 | 0.255 | 0.93 | 0.85–1.01 | 0.093 | 0.95 | 0.86–1.04 | 0.257 |
| 145–<160 | 0.90 | 0.84–0.99 | 0.013 | 1.01 | 0.92–1.10 | 0.874 | 0.95 | 0.86–1.06 | 0.360 | 0.99 | 0.89–1.11 | 0.890 |
| ≥160 | 0.86 | 0.81–0.95 | <0.001 | 1.01 | 0.94–1.09 | 0.700 | 0.89 | 0.80–1.00 | 0.049 | 0.98 | 0.86–1.10 | 0.698 |
| Cardiovascular mortality | ||||||||||||
| <60 | 2.06 | 1.82–2.33 | <0.001 | 1.75 | 1.54–1.99 | <0.001 | 1.65 | 1.41–1.93 | <0.001 | 1.73 | 1.46–2.06 | <0.001 |
| 60–<85 | 1.52 | 1.38–1.68 | <0.001 | 1.40 | 1.27–1.54 | <0.001 | 1.37 | 1.22–1.53 | <0.001 | 1.41 | 1.25–1.60 | <0.001 |
| 85–<100 | 1.18 | 1.06–1.31 | 0.002 | 1.12 | 1.01–1.25 | 0.029 | 1.13 | 1.01–1.26 | 0.030 | 1.16 | 1.03–1.31 | 0.013 |
| 100–<115 | 1.00 | … | … | 1.00 | … | … | 1.00 | … | … | 1.00 | … | … |
| 115–<130 | 0.93 | 0.83–1.05 | 0.224 | 0.96 | 0.86–1.08 | 0.538 | 0.95 | 0.84–1.08 | 0.440 | 0.97 | 0.85–1.11 | 0.670 |
| 130–<145 | 0.91 | 0.80–1.04 | 0.179 | 1.00 | 0.88–1.14 | 0.986 | 0.98 | 0.85–1.13 | 0.804 | 1.01 | 0.87–1.18 | 0.882 |
| 145–<160 | 0.91 | 0.79–1.06 | 0.229 | 1.03 | 0.89–1.20 | 0.673 | 1.00 | 0.84–1.18 | 0.996 | 1.09 | 0.91–1.31 | 0.326 |
| ≥160 | 0.88 | 0.78–1.00 | 0.048 | 1.03 | 0.91–1.17 | 0.607 | 0.97 | 0.80–1.17 | 0.728 | 1.06 | 0.86–1.29 | 0.587 |
Adjustments in case‐mix model: age, sex, race/ethnicity, primary insurance, vascular access type, comorbid conditions, and single‐pool Kt/V; case‐mix plus MICS models: case‐mix–adjusted model plus body mass index and laboratory parameters, including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron‐binding capacity, ferritin, and low‐density lipoprotein cholesterol; case‐mix plus MICS plus statin models: statin therapy in addition to all covariates in case‐mix and MICS models. CI indicates confidence interval; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; MICS, malnutrition‐inflammation‐cachexia syndrome.
Table 3.
Associations of Time‐Varying Serum Non–HDL‐C With All‐Cause and Cardiovascular Mortality
| Non‐HDL‐C, mg/dL | Unadjusted | Case Mix | Case Mix and MICS | Case Mix, MICS, and Statin | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | HR | 95% CI | P Value | |
| All‐cause mortality | ||||||||||||
| <60 | 2.40 | 2.25–2.56 | <0.001 | 2.17 | 2.03–2.32 | <0.001 | 1.88 | 1.72–2.06 | <0.001 | 1.96 | 1.78–2.17 | <0.001 |
| 60–<85 | 1.51 | 1.43–1.60 | <0.001 | 1.43 | 1.35–1.51 | <0.001 | 1.35 | 1.26–1.45 | <0.001 | 1.39 | 1.29–1.50 | <0.001 |
| 85–<100 | 1.18 | 1.11–1.26 | <0.001 | 1.15 | 1.08–1.22 | <0.001 | 1.14 | 1.07–1.22 | <0.001 | 1.16 | 1.08–1.25 | <0.001 |
| 100–<115 | 1.00 | … | … | 1.00 | … | … | 1.00 | … | … | 1.00 | … | … |
| 115–<130 | 0.87 | 0.80–0.94 | <0.001 | 0.90 | 0.83–0.97 | 0.006 | 0.86 | 0.79–0.93 | <0.001 | 0.88 | 0.81–0.96 | 0.003 |
| 130–<145 | 0.83 | 0.76–0.91 | <0.001 | 0.90 | 0.82–0.98 | 0.012 | 0.87 | 0.79–0.95 | 0.003 | 0.88 | 0.80–0.98 | 0.018 |
| 145–<160 | 0.77 | 0.70–0.85 | <0.001 | 0.83 | 0.75–0.91 | <0.001 | 0.79 | 0.71–0.89 | <0.001 | 0.81 | 0.71–0.91 | 0.001 |
| ≥160 | 0.78 | 0.72–0.85 | <0.001 | 0.87 | 0.80–0.95 | 0.001 | 0.73 | 0.64–0.83 | <0.001 | 0.78 | 0.68–0.90 | 0.001 |
| Cardiovascular mortality | ||||||||||||
| <60 | 2.51 | 2.25–2.80 | <0.001 | 2.21 | 1.98–2.47 | <0.001 | 2.07 | 1.78–2.41 | <0.001 | 2.19 | 1.86–2.59 | <0.001 |
| 60–<85 | 1.57 | 1.42–1.73 | <0.001 | 1.46 | 1.33–1.61 | <0.001 | 1.43 | 1.27–1.60 | <0.001 | 1.49 | 1.31–1.69 | <0.001 |
| 85–<100 | 1.20 | 1.08–1.33 | 0.001 | 1.15 | 1.03–1.28 | 0.011 | 1.15 | 1.03–1.29 | 0.015 | 1.18 | 1.04–1.34 | 0.008 |
| 100–<115 | 1.00 | … | … | 1.00 | … | … | 1.00 | … | … | 1.00 | … | … |
| 115–<130 | 0.89 | 0.78–1.01 | 0.068 | 0.92 | 0.81–1.04 | 0.191 | 0.87 | 0.76–0.99 | 0.035 | 0.89 | 0.77–1.02 | 0.104 |
| 130–<145 | 0.78 | 0.67–0.90 | 0.001 | 0.84 | 0.73–0.98 | 0.023 | 0.78 | 0.66–0.92 | 0.003 | 0.80 | 0.67–0.95 | 0.012 |
| 145–<160 | 0.75 | 0.63–0.89 | 0.001 | 0.80 | 0.68–0.96 | 0.013 | 0.74 | 0.61–0.90 | 0.002 | 0.80 | 0.65–0.99 | 0.037 |
| ≥160 | 0.81 | 0.70–0.93 | 0.003 | 0.90 | 0.78–1.04 | 0.139 | 0.75 | 0.60–0.93 | 0.008 | 0.82 | 0.65–1.03 | 0.084 |
Adjustments in case‐mix model: age, sex, race/ethnicity, primary insurance, vascular access type, comorbid conditions, and single‐pool Kt/V; case‐mix plus MICS models: case‐mix–adjusted model plus body mass index and laboratory parameters, including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron‐binding capacity, ferritin, and low‐density lipoprotein cholesterol; case‐mix plus MICS plus statin models: statin therapy in addition to all covariates in case‐mix and MICS models. CI indicates confidence interval; HDL‐C, high‐density lipoprotein cholesterol; HR, hazard ratio; MICS, malnutrition‐inflammation‐cachexia syndrome.
Figure 1.
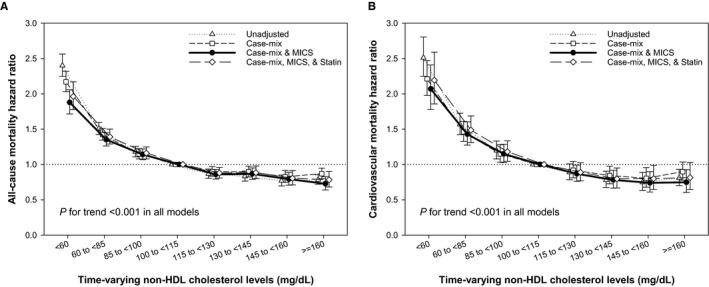
Time‐varying all‐cause (A) and cardiovascular (B) mortality hazard ratios (and 95% confidence interval error bars) by serum non–high‐density lipoprotein (non‐HDL) cholesterol levels. Adjustments in case‐mix model: age, sex, race/ethnicity, primary insurance, vascular access type, comorbid conditions, and single‐pool Kt/V; case‐mix plus malnutrition‐inflammation‐cachexia syndrome (MICS) models: case‐mix–adjusted model plus body mass index and laboratory parameters, including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron‐binding capacity, ferritin, and low‐density lipoprotein cholesterol; case‐mix plus MICS plus statin models: statin therapy in addition to all covariates in case‐mix and MICS models.
When we examined baseline (Figure S3) and time‐varying (Figure 2) non–HDL‐C as a continuous variable in fully adjusted cubic spine models, similar inverse relationships between serum non–HDL‐C levels and mortality were found. Further subgroup analyses again confirmed that time‐varying non–HDL‐C level <100 mg/dL (versus ≥100 mg/dL as reference) was associated with significantly increased risk of all‐cause and cardiovascular mortality across all prespecified subgroups (Figure 3).
Figure 2.
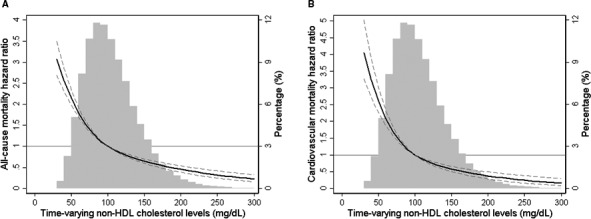
Multivariate adjusted hazard ratios of all‐cause (A) and cardiovascular (B) mortality associated with time‐varying non–high‐density lipoprotein (non‐HDL) cholesterol levels in Cox model using restricted cubic spines, adjusted for age, sex, race/ethnicity, primary insurance, vascular access type, comorbidities, single‐pool Kt/V, body mass index, hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron‐binding capacity, ferritin, and low‐density lipoprotein cholesterol. A histogram of observed time‐varying non‐HDL cholesterol values and hazard reference ratio of 1 (solid line) is overlaid.
Figure 3.
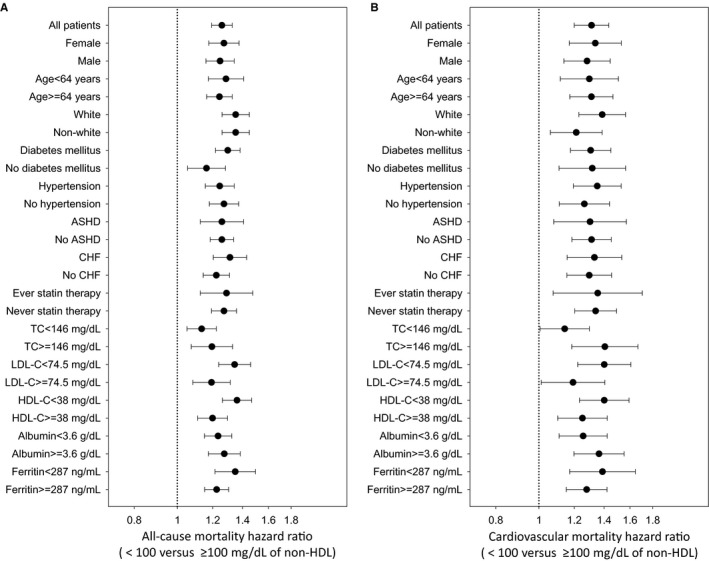
Overall and subgroup analyses of association between low time‐varying non–high‐density lipoprotein (non‐HDL) cholesterol (HDL‐C) levels <100 mg/dL and all‐cause (A) and cardiovascular (B) mortality, compared with those with ≥100 mg/dL as reference. Models were adjusted for age, sex, race/ethnicity, primary insurance, vascular access type, comorbid conditions, single‐pool Kt/V, body mass index, serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron‐binding capacity, ferritin, and low‐density lipoprotein cholesterol (LDL‐C). Points and bars represent hazard ratio estimates and 95% confidence intervals, respectively. ASHD indicates atherosclerotic heart disease; CHF, congestive heart failure; TC, total cholesterol.
Association of Non–HDL‐C/HDL‐C Ratio With Outcomes
To further address the combined outcome predictability of non‐HDL and HDL‐C in the population undergoing hemodialysis, we also examined the association between non‐HDL/HDL‐C ratio and mortality (Tables S5 and S6). We found that incremental lower value of non‐HDL/HDL‐C ratio was associated with higher risk of cardiovascular and all‐cause mortality, with the highest death risk observed in the lowest baseline (Figures S4 and S5) and time‐varying (Figures 4 and 5) non‐HDL/HDL‐C ratio categories. These paradoxical associations were again confirmed in various subgroup analyses, with significantly higher all‐cause and cardiovascular mortality in non‐HDL/HDL‐C ratio <2.5 compared with ≥2.5 as reference (Figure S6).
Figure 4.
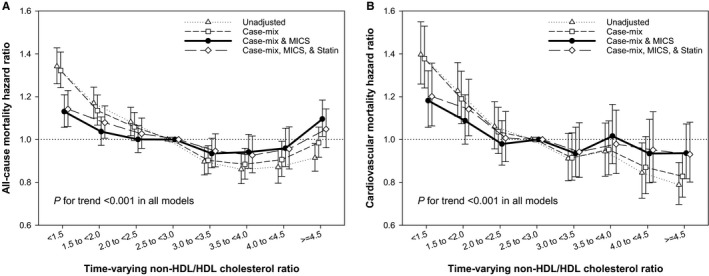
Time‐varying all‐cause (A) and cardiovascular (B) mortality hazard ratios (and 95% confidence interval error bars) by serum non–high‐density lipoprotein (non‐HDL)/HDL cholesterol ratios. Adjustments in case‐mix model: age, sex, race/ethnicity, primary insurance, vascular access type, comorbid conditions, and single‐pool Kt/V; case‐mix plus malnutrition‐inflammation‐cachexia syndrome (MICS) models: case‐mix–adjusted model plus body mass index and laboratory parameters, including serum hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron‐binding capacity, ferritin, and low‐density lipoprotein cholesterol; case‐mix plus MICS plus statin models: statin therapy in addition to all covariates in case‐mix and MICS models.
Figure 5.
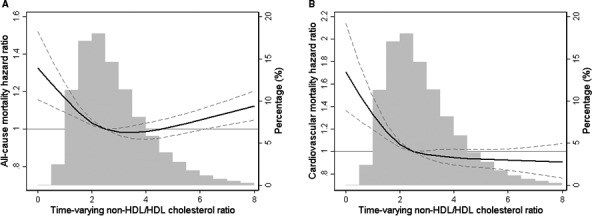
Multivariate adjusted hazard ratios of all‐cause (A) and cardiovascular (B) mortality associated with time‐varying non–high‐density lipoprotein (non‐HDL)/HDL cholesterol ratios in Cox model using restricted cubic spines, adjusted for age, sex, race/ethnicity, primary insurance, vascular access type, comorbidities, single‐pool Kt/V, body mass index, hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron‐binding capacity, ferritin, and low‐density lipoprotein cholesterol. A histogram of observed time‐varying non‐HDL/HDL cholesterol ratios and hazard reference ratio of 1 (solid line) is overlaid.
Discussion
In the largest study of serum non–HDL‐C in patients with ESRD conducted to date, we found that not only low non–HDL‐C level (<100 mg/dL) but also reduced non‐HDL/HDL‐C ratio (<2.5) show a paradoxical association with poor survival and increased cardiovascular mortality in patients undergoing hemodialysis. To the best of our knowledge, this is the first published study to demonstrate the inverse association between non–HDL‐C or non‐HDL/HDL‐C ratio and mortality. These findings are in contrast to the associations seen in the general population in whom increments in non‐HDL‐C or non‐HDL/HDL‐C ratio are associated with worse outcomes.
Although current clinical guidelines on the management of patients with dyslipidemia acknowledge the central role of LDL‐C in atherosclerosis, there is also accumulating evidence that other lipid measures, such as non–HDL‐C, may be more accurate predictors of cardiovascular risk than LDL.24, 25 In this regard, numerous observational studies have found serum levels of non–HDL‐C to be positively and strongly associated with a higher risk of cardiovascular mortality and poor outcomes.5, 6, 7, 8, 9, 10 Therefore, the potential use of non–HDL‐C as an index of risk and a target for therapy has been considered a viable option for addressing residual risk in cardiovascular disease. However, extrapolation of findings from studies in the general population to patients with ESRD undergoing hemodialysis is unlikely to be accurate because it is increasingly becoming clear that the dyslipidemia of patients with ESRD and advanced chronic kidney disease is unique and the association of serum lipoprotein concentrations with outcomes is different in these patients when compared with other patient populations. In this regard, dyslipidemia in patients undergoing hemodialysis is typically characterized by normal serum total and LDL‐C levels, reduced HDL‐C levels, and increased concentrations of triglycerides and triglyceride‐rich lipoproteins, including chylomicrons, intermediate‐density lipoprotein, and very low‐density lipoprotein.13, 26 In regard to outcomes, higher concentrations of LDL‐C have not been found to be associated with worse survival in large observational studies of patients undergoing hemodialysis.14 Moreover, hydroxymethylglutaryl–CoA reductase inhibitor (statin) therapy targeting serum LDL‐C levels in patients undergoing hemodialysis has not been shown to improve cardiovascular or overall survival.27, 28, 29 Accordingly, current guidelines in patients undergoing dialysis do not call for treatment of patients undergoing hemodialysis with statins. If a patient is receiving statin therapy at the time of transition, the patient is recommended to continue; however, starting statins de novo is not recommended.30 This is consistent with our findings indicating that patients with high LDL‐C were less likely to be taking statins in this cohort (Table 1).
Furthermore, ESRD is not only associated with HDL deficiency but also with altered HDL function and properties, such that subgroups of HDL particles become proinflammatory in patients with advanced kidney disease being treated with dialysis.31, 32 These findings may partly explain the association of elevated HDL‐C levels with worse outcomes in some subgroups of patients with chronic kidney disease/hemodialysis.15, 16, 17 In addition, recently, we reported that higher serum concentrations of triglycerides are paradoxically associated with decreased risk of cardiovascular and all‐cause mortality in patients undergoing hemodialysis.14 In light of these findings, it is not surprising that, in the current study, we find a graded inverse association between serum levels of non–HDL‐C, which include the major triglyceride‐rich lipoproteins, and mortality in patients undergoing incident hemodialysis. Furthermore, evaluation of non–HDL‐C in the context of serum HDL‐C level does not change the observation made in this study given that lower non‐HDL/HDL‐C ratio is also associated with worse outcomes.
The underlying mechanisms responsible for these paradoxical associations are not clear at this time, but several potential explanations can be considered. First, because non–HDL‐C is calculated on the basis of serum TC and HDL‐C levels, low non–HDL‐C may be a surrogate of low TC or high HDL‐C levels. In such cases, increased risk of mortality may be attributable to factors that can cause low TC, such as malnutrition, cachexia, and high burden of systemic inflammation.11, 12, 33 In addition, elevated serum HDL‐C levels in patients with ESRD with an inflammatory milieu may lead to generation of proinflammatory HDL, which can play a proatherogenic role and be associated with worse survival.31, 34 Therefore, a combination of these 2 factors can lead to the findings being reported in this study. Second, there are data indicating that lipids and lipoproteins may have some protective properties and, therefore, their deficiency is not only not going to have a positive impact on survival in all settings, but it may in fact have detrimental effects in a particular group of patients. For instance, Rauchhaus et al35, 36 demonstrated that by binding to bacterial endotoxins, lipoproteins may play a role in preventing chronic inflammation and, hence, low levels of lipoproteins may contribute to the chronic proinflammatory state observed in some patients, such as those undergoing hemodialysis. Third, as indicated earlier, non–HDL‐C represents all ApoB‐containing lipoproteins, a significant portion of which are triglyceride rich and play a crucial role in the delivery and metabolism of triglycerides and fatty acids. Although the direct role of triglycerides in the pathogenesis of cardiovascular disease is an area of ongoing investigation, triglycerides are a rich source of energy and play a critical role in energy delivery/homeostasis.37 It is well known that nontraditional risk factors, such as oxidative stress, inflammation, and the resultant cachexia and protein energy wasting, play a major role in the mortality associated with ESRD and hemodialysis.38, 39 Therefore, although factors that improve energy delivery and metabolism in populations not at risk for protein energy wasting may be associated with obesity and worse outcomes, in the population undergoing hemodialysis, these features may play a more complex role, leading to their association with improved survival. Nevertheless, it is also critical to note that these associations are not necessarily indicative of a causal relationship and may be surrogates for unidentified mechanisms that can link energy metabolism, lipoproteins, and outcomes in ESRD.40 Therefore, our findings may provide important clues for future investigations aimed at deciphering the role of triglycerides and triglyceride‐rich lipoproteins in ESRD‐related cardiovascular and overall outcomes. Finally, another important potential explanation for the seemingly paradoxical associations between serum non–HDL‐C levels and mortality in patients undergoing hemodialysis is the time dependency of the deleterious effects imparted by these lipoproteins. Given that the atherogenic impact of ApoB and triglyceride‐rich lipoproteins typically takes effect over an extended period of time, the possibility that serum non–HDL‐C levels in the short‐term are more reflective of other factors, such as energy metabolism and nutrition, cannot be discounted.41 Hence, improved outcomes can be observed in the short‐term, whereas during long‐term follow‐up, elevated non–HDL‐C levels may be associated with worse cardiovascular and all‐cause mortality.22 However, given the significant increase in the short‐term risk of death in patients with ESRD and the fact that long‐term survival by definition is dependent on short‐term survival, the value of these observations cannot be overlooked.
Several strengths of our study, when compared with previous investigations in this patient population, include examination of a large nationally representative cohort of patients undergoing incident hemodialysis with a relatively long follow‐up of up to 5 years, availability of baseline and time‐varying measures, laboratory measurements based on uniform protocols conducted in one single laboratory, and detailed data on comorbidities and laboratory variables. However, there are several limitations that should also be mentioned. First, given the observational nature of our study design, the findings will need to be interpreted accordingly, and conclusions about causality cannot be drawn on the basis of these results. Second, information about the fasting state of the patients before collection of serum in our cohort is not available. On the basis of common clinical practices in hemodialysis care, it is conceivable that most patients in this cohort may be in a nonfasting state. However, a study published by Desmeules et al4 demonstrated that fasting does not appear to affect serum non–HDL‐C levels and that nonfasting samples are adequate for evaluation of this index in patients undergoing hemodialysis. Third, clinical evaluation of serum lipids at this time does not routinely include measurement and analysis of lipoprotein subfractions (including apolipoprotein A and ApoB levels). Lack of these measurements is a limitation of this study because they may provide additional clues about the underlying mechanism/s responsible for the observed associations between serum non–HDL‐C levels and outcomes. Fourth, given that we did not have information on all traditional and nontraditional cardiovascular disease risk factors, including smoking status, serum levels of high‐sensitivity C‐reactive protein, and other potent inflammatory cytokines, we cannot rule out residual confounding. However, we did try to address this shortcoming by extensive adjustment for measured covariates, including clinical and laboratory parameters. Finally, 10% of patients in this study did not have data available on cardiovascular mortality, raising concerns for selection bias. However, we did compare all baseline characteristics between the subjects with and without data on cause of death and found no meaningful differences between the 2 groups (Table S3).
In conclusion, reduced levels of non–HDL‐C and non‐HDL/HDL‐C ratio were paradoxically associated with poor overall survival and increased cardiovascular mortality in patients with incident hemodialysis. This relationship remained significant even after extensive adjustment for relevant clinical and laboratory covariates and subgroup analyses. These observations further highlight the unique nature of dyslipidemia and cardiovascular disease in patients undergoing hemodialysis and underscore the need for additional investigations aimed at deciphering the role of lipoproteins as an index of risk and target of therapy in the population with ESRD.
Sources of Funding
Kalantar‐Zadeh is supported by National Institutes of Health (NIH; National Institute of Diabetes and Digestive and Kidney Diseases [NIDDK]) grants K24‐DK091419 and R01‐DK078106 and philanthropic grants from Harold Simmons, Louis Change, Joseph Lee, and AVEO, Inc. Kovesdy is supported by NIH (NIDDK) grants R01‐DK096920 and U01‐DK102163. Rhee is supported by NIH (NIDDK) grant K23‐DK102903. Moradi is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (1 IK CX 001043‐01A2). Streja is supported by a career development award from the Office of Research and Development of the Department of Veterans Affairs (IK2‐CX001266‐01).
Disclosures
Kalantar‐Zadeh has received honoraria and/or support from Abbott, Abbvie, Alexion, Amgen, American Society of Nephrology, Astra‐Zeneca, AVEO, Chugai, DaVita, Fresenius, Genetech, Haymarket Media, Hospira, Kabi, Keryx, National Institutes of Health, National Kidney Foundation, Relypsa, Resverlogix, Sanofi, Shire, Vifor, and ZS‐Pharma. Moradi has received funding from the National Institutes of Health, Department of Veterans Affairs and Novartis. The remaining authors have no disclosures to report.
Supporting information
Table S1. Baseline Characteristics of 51 185 Patients According to Baseline Serum Non‐High‐Density Lipoprotein/High‐Density Lipoprotein Cholesterol Level
Table S2. Detailed Causes of Death Among 4578 Patients Who Died From Cardiovascular Disease
Table S3. Baseline Characteristics in Patients With or Without Data on Cause of Death Among a Total of 12 859 Patients Who Died During the Follow‐Up Period (January 1, 2007 to December 31, 2011)
Table S4. Competing Risk Regression Analyses of Association of Serum Non‐High‐Density Lipoprotein Cholesterol Levels and Cardiovascular Mortality
Table S5. Associations of Baseline Non‐High‐Density Lipoprotein to High‐Density Lipoprotein Cholesterol Ratio With All‐Cause and Cardiovascular Mortality
Table S6. Associations of Time‐Varying Non‐High‐Density Lipoprotein to High‐Density Lipoprotein Cholesterol Ratio With All‐Cause and Cardiovascular Mortality
Figure S1. Flow chart of patient selection for the cohort. non‐HDL indicates non‐high‐density lipoprotein.
Figure S2. A fixed all‐cause (A) and cardiovascular (B) mortality hazard ratios (and 95% confidence interval error bars) by baseline serum non‐high‐density lipoprotein (non‐HDL) cholesterol levels.
Figure S3. Multivariate adjusted hazard ratios of all‐cause (A) and cardiovascular (B) mortality associated with baseline non‐high‐density lipoprotein (non‐HDL) cholesterol levels in Cox model using restricted cubic spines, adjusted age, sex, race/ethnicity, primary insurance, vascular access type, comorbidities, single‐pool Kt/V, body mass index, hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, and low‐density lipoprotein cholesterol.
Figure S4. A fixed all‐cause (A) and cardiovascular (B) mortality hazard ratios (and 95% confidence interval error bars) by baseline non‐high‐density lipoprotein to high‐density lipoprotein (non‐HDL/HDL) cholesterol ratios.
Figure S5. Multivariate adjusted hazard ratios of all‐cause (A) and cardiovascular (B) mortality associated with baseline non‐high‐density lipoprotein to high‐density lipoprotein (non‐HDL/HDL) cholesterol ratios in Cox model using restricted cubic spines, adjusted age, sex, race/ethnicity, primary insurance, vascular access type, comorbidities, single‐pool Kt/V, body mass index, hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, and low‐density lipoprotein cholesterol.
Figure S6. Overall and subgroup analyses of association between low time‐varying non‐high‐density lipoprotein to high‐density lipoprotein (non‐HDL/HDL) cholesterol ratios <2.5 and all‐cause (A) and cardiovascular (B) mortality, compared to those with ≥2.5 as reference.
Acknowledgments
We thank DaVita Clinical Research for providing the clinical data for this research.
(J Am Heart Assoc. 2018;7:e009096 DOI: 10.1161/JAHA.118.009096.)
References
- 1. Wanner C, Krane V. Non‐high‐density lipoprotein cholesterol: a target of lipid‐lowering in dialysis patients. Am J Kidney Dis. 2003;41:S72–S75. [DOI] [PubMed] [Google Scholar]
- 2. Shoji T. Serum lipids and prevention of atherosclerotic cardiovascular events in hemodialysis patients. Clin Exp Nephrol. 2014;18:257–260. [DOI] [PubMed] [Google Scholar]
- 3. Verbeek R, Hovingh GK, Boekholdt SM. Non‐high‐density lipoprotein cholesterol: current status as cardiovascular marker. Curr Opin Lipidol. 2015;26:502–510. [DOI] [PubMed] [Google Scholar]
- 4. Desmeules S, Arcand‐Bossé JF, Bergeron J, Douville P, Agharazii M. Nonfasting non‐high‐density lipoprotein cholesterol is adequate for lipid management in hemodialysis patients. Am J Kidney Dis. 2005;45:1067–1072. [DOI] [PubMed] [Google Scholar]
- 5. Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non‐HDL cholesterol, apolipoproteins A‐I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. [DOI] [PubMed] [Google Scholar]
- 6. Pischon T, Girman CJ, Sacks FM, Rifai N, Stampfer MJ, Rimm EB. Non‐high‐density lipoprotein cholesterol and apolipoprotein B in the prediction of coronary heart disease in men. Circulation. 2005;112:3375–3383. [DOI] [PubMed] [Google Scholar]
- 7. Di Angelantonio E, Sarwar N, Perry P, Kaptoge S, Ray KK, Thompson A, Wood AM, Lewington S, Sattar N, Packard CJ, Collins R, Thompson SG, Danesh J. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302:1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu J, Sempos C, Donahue RP, Dorn J, Trevisan M, Grundy SM. Joint distribution of non‐HDL and LDL cholesterol and coronary heart disease risk prediction among individuals with and without diabetes. Diabetes Care. 2005;28:1916–1921. [DOI] [PubMed] [Google Scholar]
- 9. Lu W, Resnick HE, Jablonski KA, Jones KL, Jain AK, Howard WJ, Robbins DC, Howard BV. Non‐HDL cholesterol as a predictor of cardiovascular disease in type 2 diabetes: the strong heart study. Diabetes Care. 2003;26:16–23. [DOI] [PubMed] [Google Scholar]
- 10. Eliasson B, Gudbjörnsdottir S, Zethelius B, Eeg‐Olofsson K, Cederholm J; National Diabetes Register (NDR) . LDL‐cholesterol versus non‐HDL‐to‐HDL‐cholesterol ratio and risk for coronary heart disease in type 2 diabetes. Eur J Prev Cardiol. 2014;21:1420–1428. [DOI] [PubMed] [Google Scholar]
- 11. Liu Y, Coresh J, Eustace JA, Longenecker JC, Jaar B, Fink NE, Tracy RP, Powe NR, Klag MJ. Association between cholesterol level and mortality in dialysis patients: role of inflammation and malnutrition. JAMA. 2004;291:451–459. [DOI] [PubMed] [Google Scholar]
- 12. Iseki K, Yamazato M, Tozawa M, Takishita S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int. 2002;61:1887–1893. [DOI] [PubMed] [Google Scholar]
- 13. Kilpatrick RD, McAllister CJ, Kovesdy CP, Derose SF, Kopple JD, Kalantar‐Zadeh K. Association between serum lipids and survival in hemodialysis patients and impact of race. J Am Soc Nephrol. 2007;18:293–303. [DOI] [PubMed] [Google Scholar]
- 14. Chang TI, Streja E, Soohoo M, Kim TW, Rhee CM, Kovesdy CP, Kashyap ML, Vaziri ND, Kalantar‐Zadeh K, Moradi H. Association of serum triglyceride to HDL cholesterol ratio with all‐cause and cardiovascular mortality in incident hemodialysis patients. Clin J Am Soc Nephrol. 2017;12:591–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bowe B, Xie Y, Xian H, Balasubramanian S, Zayed MA, Al‐Aly Z. High density lipoprotein cholesterol and the risk of all‐cause mortality among U.S. veterans. Clin J Am Soc Nephrol. 2016;11:1784–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Moradi H, Abhari P, Streja E, Kashyap ML, Shah G, Gillen D, Pahl MV, Vaziri ND, Kalantar‐Zadeh K. Association of serum lipids with outcomes in Hispanic hemodialysis patients of the West versus East Coasts of the United States. Am J Nephrol. 2015;41:284–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Moradi H, Streja E, Kashyap ML, Vaziri ND, Fonarow GC, Kalantar‐Zadeh K. Elevated high‐density lipoprotein cholesterol and cardiovascular mortality in maintenance hemodialysis patients. Nephrol Dial Transplant. 2014;29:1554–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Nishizawa Y, Shoji T, Kakiya R, Tsujimoto Y, Tabata T, Ishimura E, Nakatani T, Miki T, Inaba M. Non‐high‐density lipoprotein cholesterol (non‐HDL‐C) as a predictor of cardiovascular mortality in patients with end‐stage renal disease. Kidney Int Suppl. 2003;84:S117–S120. [DOI] [PubMed] [Google Scholar]
- 19. Echida Y, Ogawa T, Otsuka K, Ando Y, Nitta K. Serum non‐high‐density lipoprotein cholesterol (non‐HDL‐C) levels and cardiovascular mortality in chronic hemodialysis patients. Clin Exp Nephrol. 2012;16:767–772. [DOI] [PubMed] [Google Scholar]
- 20. Shoji T, Masakane I, Watanabe Y, Iseki K, Tsubakihara Y. Elevated non‐high‐density lipoprotein cholesterol (non‐HDL‐C) predicts atherosclerotic cardiovascular events in hemodialysis patients. Clin J Am Soc Nephrol. 2011;6:1112–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuttykrishnan S, Kalantar‐Zadeh K, Arah OA, Cheung AK, Brunelli S, Heagerty PJ, Katz R, Molnar MZ, Nissenson A, Ravel V, Streja E, Himmelfarb J, Mehrotra R. Predictors of treatment with dialysis modalities in observational studies for comparative effectiveness research. Nephrol Dial Transplant. 2015;3:1208–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dekker FW, de Mutsert R, van Dijk PC, Zoccali C, Jager KJ. Survival analysis: time‐dependent effects and time‐varying risk factors. Kidney Int. 2008;74:994–997. [DOI] [PubMed] [Google Scholar]
- 23. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 24. Stone NJ, Robinson JG, Lichtenstein AH, Bairey Merz CN, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd‐Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC Jr, Watson K, Wilson PW, Eddleman KM, Jarrett NM, LaBresh K, Nevo L, Wnek J, Anderson JL, Halperin JL, Albert NM, Bozkurt B, Brindis RG, Curtis LH, DeMets D, Hochman JS, Kovacs RJ, Ohman EM, Pressler SJ, Sellke FW, Shen WK, Smith SC Jr, Tomaselli GF; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(suppl 2):S1–S45. [DOI] [PubMed] [Google Scholar]
- 25. Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, Hoes AW, Jennings CS, Landmesser U, Pedersen TR, Reiner Ž, Riccardi G, Taskinen MR, Tokgozoglu L, Verschuren WM, Vlachopoulos C, Wood DA, Zamorano JL; Authors/Task Force Members . 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias. Eur Heart J. 2016;37:2999–3058. [DOI] [PubMed] [Google Scholar]
- 26. Moradi H, Vaziri ND. Molecular mechanisms of disorders of lipid metabolism in chronic kidney disease. Front Biosci (Landmark Ed). 2018;23:146–161. [DOI] [PubMed] [Google Scholar]
- 27. Wanner C, Krane V, März W, Olschewski M, Mann JF, Ruf G, Ritz E; German Diabetes and Dialysis Study Investigators . Atorvastatin in patients with type 2 diabetes mellitus undergoing hemodialysis. N Engl J Med. 2005;353:238–248. [DOI] [PubMed] [Google Scholar]
- 28. Fellström BC, Jardine AG, Schmieder RE, Holdaas H, Bannister K, Beutler J, Chae DW, Chevaile A, Cobbe SM, Grönhagen‐Riska C, De Lima JJ, Lins R, Mayer G, McMahon AW, Parving HH, Remuzzi G, Samuelsson O, Sonkodi S, Sci D, Süleymanlar G, Tsakiris D, Tesar V, Todorov V, Wiecek A, Wüthrich RP, Gottlow M, Johnsson E, Zannad F; AURORA Study Group . Rosuvastatin and cardiovascular events in patients undergoing hemodialysis. N Engl J Med. 2009;360:1395–1407. [DOI] [PubMed] [Google Scholar]
- 29. Baigent C, Landray MJ, Reith C, Emberson J, Wheeler DC, Tomson C, Wanner C, Krane V, Cass A, Craig J, Neal B, Jiang L, Hooi LS, Levin A, Agodoa L, Gaziano M, Kasiske B, Walker R, Massy ZA, Feldt‐Rasmussen B, Krairittichai U, Ophascharoensuk V, Fellström B, Holdaas H, Tesar V, Wiecek A, Grobbee D, de Zeeuw D, Grönhagen‐Riska C, Dasgupta T, Lewis D, Herrington W, Mafham M, Majoni W, Wallendszus K, Grimm R, Pedersen T, Tobert J, Armitage J, Baxter A, Bray C, Chen Y, Chen Z, Hill M, Knott C, Parish S, Simpson D, Sleight P, Young A, Collins R; SHARP Investigators . The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomised placebo‐controlled trial. Lancet. 2011;377:2181–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Wanner C, Tonelli M; Kidney Disease: Improving Global Outcomes Lipid Guideline Development Work Group Members . KDIGO Clinical Practice Guideline for Lipid Management in CKD: summary of recommendation statements and clinical approach to the patient. Kidney Int. 2014;85:1303–1309. [DOI] [PubMed] [Google Scholar]
- 31. Chang TI, Streja E, Moradi H. Could high‐density lipoprotein cholesterol predict increased cardiovascular risk? Curr Opin Endocrinol Diabetes Obes. 2017;24:140–147. [DOI] [PubMed] [Google Scholar]
- 32. Moradi H, Vaziri ND, Kashyap ML, Said HM, Kalantar‐Zadeh K. Role of HDL dysfunction in end‐stage renal disease: a double‐edged sword. J Ren Nutr. 2013;23:203–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bologa RM, Levine DM, Parker TS, Cheigh JS, Serur D, Stenzel KH, Rubin AL. Interleukin‐6 predicts hypoalbuminemia, hypocholesterolemia, and mortality in hemodialysis patients. Am J Kidney Dis. 1998;32:107–114. [DOI] [PubMed] [Google Scholar]
- 34. Yamamoto S, Yancey PG, Ikizler TA, Jerome WG, Kaseda R, Cox B, Bian A, Shintani A, Fogo AB, Linton MF, Fazio S, Kon V. Dysfunctional high‐density lipoprotein in patients on chronic hemodialysis. J Am Coll Cardiol. 2012;60:2372–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rauchhaus M, Coats AJ, Anker SD. The endotoxin‐lipoprotein hypothesis. Lancet. 2000;356:930–933. [DOI] [PubMed] [Google Scholar]
- 36. Sharma R, von Haehling S, Rauchhaus M, Bolger AP, Genth‐Zotz S, Doehner W, Oliver B, Poole‐Wilson PA, Volk HD, Coats AJ, Adcock IM, Anker SD. Whole blood endotoxin responsiveness in patients with chronic heart failure: the importance of serum lipoproteins. Eur J Heart Fail. 2005;7:479–484. [DOI] [PubMed] [Google Scholar]
- 37. Watt MJ, Steinberg GR. Regulation and function of triacylglycerol lipases in cellular metabolism. Biochem J. 2008;414:313–325. [DOI] [PubMed] [Google Scholar]
- 38. Obi Y, Qader H, Kovesdy CP, Kalantar‐Zadeh K. Latest consensus and update on protein‐energy wasting in chronic kidney disease. Curr Opin Clin Nutr Metab Care. 2015;18:254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tonelli M, Karumanchi SA, Thadhani R. Epidemiology and mechanisms of uremia‐related cardiovascular disease. Circulation. 2016;133:518–536. [DOI] [PubMed] [Google Scholar]
- 40. Vaziri ND. Role of dyslipidemia in impairment of energy metabolism, oxidative stress, inflammation and cardiovascular disease in chronic kidney disease. Clin Exp Nephrol. 2014;18:265–268. [DOI] [PubMed] [Google Scholar]
- 41. Ahmadi SF, Streja E, Zahmatkesh G, Streja D, Kashyap M, Moradi H, Molnar MZ, Reddy U, Amin AN, Kovesdy CP, Kalantar‐Zadeh K. Reverse epidemiology of traditional cardiovascular risk factors in the geriatric population. J Am Med Dir Assoc. 2015;16:933–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Characteristics of 51 185 Patients According to Baseline Serum Non‐High‐Density Lipoprotein/High‐Density Lipoprotein Cholesterol Level
Table S2. Detailed Causes of Death Among 4578 Patients Who Died From Cardiovascular Disease
Table S3. Baseline Characteristics in Patients With or Without Data on Cause of Death Among a Total of 12 859 Patients Who Died During the Follow‐Up Period (January 1, 2007 to December 31, 2011)
Table S4. Competing Risk Regression Analyses of Association of Serum Non‐High‐Density Lipoprotein Cholesterol Levels and Cardiovascular Mortality
Table S5. Associations of Baseline Non‐High‐Density Lipoprotein to High‐Density Lipoprotein Cholesterol Ratio With All‐Cause and Cardiovascular Mortality
Table S6. Associations of Time‐Varying Non‐High‐Density Lipoprotein to High‐Density Lipoprotein Cholesterol Ratio With All‐Cause and Cardiovascular Mortality
Figure S1. Flow chart of patient selection for the cohort. non‐HDL indicates non‐high‐density lipoprotein.
Figure S2. A fixed all‐cause (A) and cardiovascular (B) mortality hazard ratios (and 95% confidence interval error bars) by baseline serum non‐high‐density lipoprotein (non‐HDL) cholesterol levels.
Figure S3. Multivariate adjusted hazard ratios of all‐cause (A) and cardiovascular (B) mortality associated with baseline non‐high‐density lipoprotein (non‐HDL) cholesterol levels in Cox model using restricted cubic spines, adjusted age, sex, race/ethnicity, primary insurance, vascular access type, comorbidities, single‐pool Kt/V, body mass index, hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, and low‐density lipoprotein cholesterol.
Figure S4. A fixed all‐cause (A) and cardiovascular (B) mortality hazard ratios (and 95% confidence interval error bars) by baseline non‐high‐density lipoprotein to high‐density lipoprotein (non‐HDL/HDL) cholesterol ratios.
Figure S5. Multivariate adjusted hazard ratios of all‐cause (A) and cardiovascular (B) mortality associated with baseline non‐high‐density lipoprotein to high‐density lipoprotein (non‐HDL/HDL) cholesterol ratios in Cox model using restricted cubic spines, adjusted age, sex, race/ethnicity, primary insurance, vascular access type, comorbidities, single‐pool Kt/V, body mass index, hemoglobin, white blood cell count, albumin, calcium, phosphorus, intact parathyroid hormone, bicarbonate, total iron binding capacity, ferritin, and low‐density lipoprotein cholesterol.
Figure S6. Overall and subgroup analyses of association between low time‐varying non‐high‐density lipoprotein to high‐density lipoprotein (non‐HDL/HDL) cholesterol ratios <2.5 and all‐cause (A) and cardiovascular (B) mortality, compared to those with ≥2.5 as reference.


