Abstract
Background
Reducing readmission after catheter ablation (CA) in atrial fibrillation (AF) is important.
Methods and Results
We utilized National Readmission Data (NRD) 2010–2014. AF was identified by International Classification of Diseases, Ninth Edition, Clinical Modification (ICD‐9‐CM) diagnostic code 427.31 in the primary field, while first CA of AF was identified via ICD‐9‐procedure code 37.34. Any admission within 30 or 90 days of index admission was considered a readmission. Cox proportional hazard regression was used to adjust for confounders. The primary outcomes were 30‐ and 90‐day readmissions and the secondary outcome was AF recurrence. In total, 1 128 372 patients with AF were identified from January 1, 2010 to September 30, 2014. Of which 37 360 (3.3%) underwent CA. Patients aged ≥65 years and female sex were less likely to receive CA for AF. Overall, 10.9% and 16.5% of CA patients were readmitted within 30 and 90 days post‐CA, respectively. Most common causes of readmissions were arrhythmia (AF, atrial flutter), heart failure, pulmonary causes (pneumonia, chronic obstructive pulmonary disease) and bleeding complications (gastrointestinal bleed, intracranial hemorrhage). Patients with diabetes mellitus, heart failure, coronary artery disease (CAD), chronic pulmonary and kidney disease, prior stroke/transient ischemic attack (TIA), female sex, length of stay ≥2 and disposition to the facility were prone to higher 30‐ and 90‐day readmissions post‐CA. Predictors of increase in AF recurrence post‐CA were female sex, diabetes mellitus, chronic pulmonary disease, and length of stay ≥2. Trends of 90‐day readmission and AF recurrence were found to improve over the study period.
Conclusions
We identified several demographic and clinical factors associated with the use of CA in AF, and short‐term outcomes of the same, which could potentially help in the patient selection and improve outcomes.
Keywords: atrial fibrillation, catheter ablation, causes, readmission
Subject Categories: Atrial Fibrillation, Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator
Clinical Perspective
What Is New?
We examined readmissions after atrial fibrillation ablations from the national readmissions database and noted that readmission rate of 10.9% and 16.5% within the first 30 and 90 days.
Female sex, increased length of stay during index hospitalization, and increased comorbidity burden were the principal predictors of increase in readmission rates.
Patients with private insurance and ablations performed in hospitals with high volume correlated to minimal readmission rates.
What Are the Clinical Implications?
Readmission may be an appropriate quality measure for atrial fibrillation ablation.
Targeted interventions that focus on the immediate postoperative period and high‐risk patients could result in further reduction in procedural morbidity and reduce readmissions.
Atrial fibrillation (AF) is the most common clinically significant cardiac arrhythmia and affects almost 2.3 million people in the US population.1 Catheter ablation (CA) of AF is one of the most effective rhythm control strategies for AF patients in anti‐arrhythmic drug (AAD) resistant AF.2 Despite the use of different ablation strategies and different sources of energy (cryoenergy versus radiofrequency), the success rate of single procedures has ranged between 20% and 60%. During the first 90 days, which is considered the “blanking period,” re‐admissions are common.3
However, after CA, apart from the recurrence of atrial arrhythmias, there are many other causes which are responsible for high readmission rates, but the data available on them are limited. Our study aims to evaluate 1) baseline characteristics of AF cohort undergoing CA, 2) specific admission diagnoses, predictors, and trends of 30‐ and 90‐day readmission in CA of AF patients, and 3) predictors and trends of AF recurrence in 90 days after CA of AF patients.
Methods
Data Source
The study was derived from the Healthcare Cost and Utilization Project's (HCUP) National Readmission Database (NRD) of 2010–2014, sponsored by the Agency for Healthcare Research and Quality. The NRD is one of the largest publicly available all‐payer inpatient care databases in the United States, which includes data on ≈15 million discharges in year 2014, estimating roughly 35 million discharges from 22 states with reliable, verified linkage numbers. NRD represents 49.3% of total US hospitalizations. Patients were tracked during the same year using the variable “NRD_visitlink,” and time between 2 admissions was calculated by subtracting variable “NRD_DaysToEvent”. Time to readmission was calculated by subtracting the length of stay (LOS) of index admissions to time between 2 admissions. Sampling weights provided by the sponsor were used to produce national estimates. The details regarding the NRD data are available online.4
Data Selection
We queried NRD database using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for AF (427.31) in the primary field. Catheter ablation was identified by ICD‐9 procedural code 37.34 in either primary or secondary field. Patients having a secondary diagnosis of atrial flutter, paroxysmal supraventricular tachycardia, atrioventricular (AV) nodal tachycardia, Wolff‐Parkinson‐White syndrome, paroxysmal ventricular tachycardia and ventricular premature complexes were excluded. Also, to avoid inclusions of patients undergoing AV junction ablation, we excluded patients with diagnostic or procedural codes indicating prior or current implantation of a pacemaker or implantable cardioverter defibrillator. Also, cases with open surgical ablations during the hospitalization were excluded. Furthermore, patients aged <18 years, with missing data for age, sex, or mortality were excluded. Patients with multiple CA for AF, only first admission of all was included as index admission. Index admissions from October, November, and December were excluded as NRD did not follow patients over the years, therefore, 90 days follow up would not be available for these index admissions. We identified in total 1 128 372 (AF) and 37 360 (CA in AF) index admissions (Table S1). Similar methods were used previously.5, 6 Patients who were readmitted to any hospital within 30 and 90 days within the same calendar year were further evaluated.
Outcomes
Primary outcomes of our study were 30‐ and 90‐day readmissions and the secondary outcome was AF recurrence which was defined as readmission with a primary diagnosis of AF or repeat AF ablation during readmission. Causes (admitting diagnosis) of readmission were identified by using ICD‐9‐CM codes in primary diagnosis filed during readmission observation. We identified 553 different ICD‐9 CM diagnosis codes and combined the ones with similar diagnoses to make clinically important groups (Table S2). Numbers were verified by M.D. and V.K. independently.
Definition of Variables
NRD variables were used to identify patients’ demographic characteristics including age, sex, hospital characteristics (bed size and teaching status), patient‐specific characteristics including median household income category for patient's zip code, primary payer, admission type, admission day, and discharge disposition.7 Discharge to skilled nursing facility and intermediate care facility were classified as the disposition to facility. Comorbidities such as obesity, hypertension, diabetes mellitus, chronic obstructive pulmonary disease (COPD), alcohol abuse, vascular disease history, and anemia were identified by variables provided in NRD, which uses ICD‐9‐CM diagnoses and the diagnosis‐related group in effect on the discharge date. These comorbidities were likely to have originated before the hospital stay and not directly related to the primary diagnosis or main reason for admission.8 Other comorbidities were identified by ICD‐9‐CM codes in secondary diagnosis field which included heart failure, coronary artery disease (CAD), chronic kidney disease, prior coronary artery bypass graft, hyperthyroidism, mitral valve disease, prior stroke/transient ischemic attack (TIA) etc (Table S3).
Deyo modification of Charlson Comorbidity Index, which contains 17 comorbid conditions with differential weights, was used to define the severity of comorbid conditions. This score ranges from 0 to 33, with higher scores corresponding to a larger burden of comorbid conditions (Table S4).9 Annual hospital volume of procedure was calculated by using unique hospital identification provided by NRD. We also evaluated LOS provided by NRD. Cost of hospitalization was calculated by merging cost to charge ratio provided by HCUP to the main data set and after adjusting for inflation.10
Statistical Analysis
SAS 9.4 (SAS Institute Inc, Cary, NC) was utilized for analyses. Wilcoxon rank sum test was used for differences between continuous variables, as data were nonparametric, while chi‐square test was used for the differences between categorical variables. We assessed each outcome using Kaplan–Meier curve before running multivariate model (Figures S1 through S3). Multivariate predictors of 30‐day readmission, 90‐day readmission and AF recurrence were evaluated using Cox proportional hazard regression with hospital identification as clustering effect. For 30 and 90‐day readmission, we first assessed all available known risk factors for AF, known risk factors for stroke/TIA in AF patients and other patient/hospital specific variables mentioned in Table 1. In multivariate analysis, we only included variables with a statistically significant difference in readmission using the univariate method. We also ran the hierarchical multivariate regression with hospital identification as clustering effect with all same variables mentioned above to assess predictors of CA amongst AF patients (Table S5).
Table 1.
Baseline Characteristics of Atrial Fibrillation Patients With and Without Ablation
| Atrial Fibrillation | Overall | P Value | ||
|---|---|---|---|---|
| Without Ablation | With Ablation | |||
| Index admission | 1 091 012 (96.7%) | 37 360 (3.3%) | 1 128 372 | |
| Patient level variables | ||||
| Age (median, IQR) y | 73 (62–82) | 65 (57–72) | 72 (62–82) | <0.001 |
| Age groups, % | <0.001 | |||
| 18 to 49 y | 7.9 | 10.2 | 8.0 | |
| 50 to 64 y | 22.7 | 37.3 | 23.2 | |
| 65 to 79 y | 38.2 | 45.3 | 38.5 | |
| ≥80 y | 31.1 | 7.2 | 30.3 | |
| Sex, % | <0.001 | |||
| Male | 46.5 | 64.2 | 47.1 | |
| Female | 53.5 | 35.8 | 52.9 | |
| Charlson comorbidity index, %a | <0.001 | |||
| 0 | 36.4 | 57.9 | 37.1 | |
| 1 | 28.4 | 24.8 | 28.3 | |
| ≥2 | 35.2 | 17.4 | 34.6 | |
| Comorbidities, % | ||||
| Obesityb | 14.8 | 15.3 | 14.8 | 0.015 |
| Obstructive sleep apneaα | 7.9 | 14.2 | 8.2 | <0.001 |
| Hypertensionb | 69.5 | 62.8 | 69.3 | <0.001 |
| Diabetes mellitusb | 25.2 | 19.0 | 25.0 | <0.001 |
| Heart failurec | 29.3 | 15.9 | 28.8 | <0.001 |
| Coronary artery diseaseβ | 32.0 | 25.2 | 31.8 | <0.001 |
| Chronic pulmonary diseaseb | 21.7 | 14.2 | 21.5 | <0.001 |
| Chronic kidney disease∞ (stage 3 or more) | 7.9 | 3.3 | 7.8 | <0.001 |
| Prior coronary artery bypass graft® | 6.9 | 5.3 | 6.8 | <0.001 |
| HyperthyroidismΩ | 1.7 | 0.8 | 1.7 | <0.001 |
| Alcohol abuseb | 4.7 | 1.1 | 4.5 | <0.001 |
| Mitral valve diseaseμ | 8.8 | 7.9 | 8.7 | <0.001 |
| Prior stroke/transient ischemic attack© | 9.1 | 6.6 | 9.0 | <0.001 |
| Vascular disease historyb | 6.7 | 4.6 | 6.6 | <0.001 |
| Anemiab | 12.7 | 5.2 | 12.4 | <0.001 |
| Median household income category for patient's zip code, %d | <0.001 | |||
| 0 to 25th percentile | 27.6 | 18.4 | 27.3 | |
| 26 to 50th percentile | 26.0 | 22.4 | 25.8 | |
| 51 to 75th percentile | 23.7 | 26.5 | 23.8 | |
| 76 to 100th percentile | 21.3 | 30.9 | 21.6 | |
| Primary payer, % | <0.001 | |||
| Medicare | 68.1 | 52.0 | 67.6 | |
| Medicaid | 4.34 | 2.68 | 4.29 | |
| Private including health maintenance organization | 21.5 | 42.3 | 22.2 | |
| Self‐pay/no charge/other | 5.8 | 3.0 | 5.7 | |
| Hospital characteristics | ||||
| Hospital bed size, %e | <0.001 | |||
| Small | 14.5 | 3.5 | 14.1 | |
| Medium | 24.3 | 17.7 | 24.1 | |
| Large | 61.2 | 78.9 | 61.8 | |
| Hospital teaching status, %f | <0.001 | |||
| Non‐teaching | 56.5 | 25.1 | 55.4 | |
| Teaching | 43.5 | 74.9 | 44.6 | |
| Admission type, % | <0.001 | |||
| Non‐elective | 90.3 | 29.9 | 88.3 | |
| Elective | 9.6 | 70.1 | 11.6 | |
| Admission day, % | <0.001 | |||
| Weekdays | 78.7 | 96.2 | 79.2 | |
| Weekend | 21.3 | 3.8 | 20.8 | |
| Disposition, % | <0.001 | |||
| Home | 87.0 | 97.9 | 87.4 | |
| Facility | 11.0 | 1.8 | 10.7 | |
| In hospital mortality, % | 1.0 | 0.1 | 1.0 | <0.001 |
| Length of stay (median, IQR) days | 2 (1–4) | 1 (1–3) | 2 (1–4) | <0.001 |
| Cost of hospitalization (median, IQR)f | 5114 (3272–8404) | 6043 (3824–9874 | 5335 (3391–8792) | <0.001 |
α, β, ∞, ®, Ω, μ, ©: comorbidities were identified by appropriate ICD‐9‐CM diagnosis codes in secondary diagnosis field (Table S3).
ICD‐9‐CM indicates International Classification of Diseases, Ninth Edition, Clinical Modification.
Charlson/Deyo Comorbidity Index (CCI) was calculated as per Deyo classification.
Variables are AHRQ comorbidity measures.
Heart failure is identified by ICD‐9 codes in secondary diagnosis field and it includes systolic, diastolic and combined heart failure.
Represents a quartile classification of the estimated median household income of residents within the patients’ zip code, derived from zip code‐demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
The bed size cutoff points divided into small, medium, and large have been done so that approximately one third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
A hospital is considered to be a teaching hospital if it has an American Medical Association‐approved residency program, is a member of the Council of Teaching Hospitals or has a ratio of full‐time equivalent interns and residents to beds of 0.25 or higher. https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
For AF recurrence, we investigated risk factors for AF and patient/hospital specific variables mentioned in Table S6. For multivariate regression (Cox‐proportional regression), we only included variables with a statically significant difference in AF recurrence using the univariate method. Included variables were age, sex, Charlson/Deyo Comorbidity Index, hypertension, diabetes mellitus, chronic obstructive pulmonary disease, chronic kidney disease (CKD) stage 3 or more, hyperthyroidism, alcoholism, anemia, median household income, hospital volume, admission type, disposition, LOS for index admission stay (Table S6). 30‐day readmission, 90‐day readmission and AF recurrence models were run on patients who survived index admission. The patient who died during readmission was considered censored observations for AF recurrence model. The P‐value of <0.05 was considered significant. Cochrane Armitage test was used to assess P‐value for trends of categorical variables such as in‐hospital mortality and simple linear regression were used to assess P‐value for trends of continuous variables such as LOS and cost of care. The study was exempt from institutional review board approval, and the requirement for informed consent was waived because database uses previously collected deidentified data.
Results
Cohort Characteristics
As described in Table 1 and Table S5, our analysis included 1 128 372 index admissions for AF (68.8% aged ≥65 years and 52.9% females). Of which 37 360 (3.3%) underwent CA for AF. Overall, patients aged ≥65 years (52.5% versus 69.3%, P<0.001) (odds ratio [OR]: 0.82, 95% confidence interval [CI]: 0.76–0.89, P<0.001) and females (35.8% versus 53.5%, P<0.001) (OR: 0.76, 95% CI: 0.73–0.78, P<0.001) were less likely to receive CA for AF. Patients with higher median household income (75th–100th percentile) (30.9% versus 21.3%, P<0.001) (OR: 1.92, 95% CI: 1.82–2.02, P<0.001), private insurance (42.3% versus 21.5%, P<0.001) (OR: 1.11, 95% CI: 1.05–1.17, P<0.001) compared with Medicare were more likely to undergo CA for AF. Patients with comorbidities such as hypertension, diabetes mellitus, heart failure, CAD, chronic pulmonary disease, CKD stage 3 or more, hypothyroidism, mitral valve disease, prior stroke/TIA and anemia were less likely to receive CA for AF. Large hospitals and teaching hospitals were more likely to perform CA for AF (Table 1).
Predictors of All Cause Readmission
Of the 37 360 patients who underwent CA, 3802 (10.9%) and 6164 (16.5%) patients required readmission in 30 and 90 days post‐CA, respectively. Patients with diabetes mellitus (25.6% versus 17.7%, P<0.001) (hazard ratio [HR]: 1.10, 95% CI: 1.03–1.19, P=0.01), heart failure (26.4% versus 13.8%, P<0.001) (HR: 1.17, 95% CI: 1.08–1.26, P<0.001), CAD (33.7% versus 23.5%, P<0.001) (HR: 1.18, 95% CI: 1.11–1.25, P<0.001), chronic pulmonary disease (21.3% versus 12.8%, P<0.001) (HR: 1.17, 95% CI: 1.09–1.26, P<0.001), CKD stage 3 or more (6.9% versus 2.6%, P<0.001) (HR: 1.26, 95% CI: 1.12–1.41, P<0.001), prior stroke/TIA (8.5% versus 6.3%, P<0.001) (HR: 1.10, 95% CI: 1.00–1.21, P=0.041), anemia (10% versus 4.3%, P<0.001) (HR: 1.27, 95% CI: 1.16–1.39, P<0.001), female sex (43.6% versus 34.3%, P<0.001) (HR: 1.20, 95% CI: 1.13–1.26, P<0.001), discharge to facility after index hospitalization (4.5% versus 1.3%, P<0.001) (HR: 1.25, 95% CI: 1.10–1.43, P<0.001), and index hospitalization LOS ≥2 days were associated with increased 90‐day readmissions. Patients with private insurance (29.6% versus 44.8%, P<0.001) (HR: 0.69, 95% CI: 0.64–0.76, P<0.001) compared with Medicare were associated with lower readmissions (Tables 2 and 3). Similar results were observed with 30‐day readmission (Tables 2 and 3). Higher hospital volume was associated with reduced 90‐day readmission (HR: 0.99, 95% CI: 0.99–0.99, P=0.046) but could not reach to statistical significance with 30 days follow up (Tables 2 and 3). In separate analysis of an entire population of AF, we found that CA was associated with decreased 90‐day readmission compared with no CA. Similar results were not observed at 30 days (Table 3, Table S7). Subgroup analysis of 90‐day readmission for CA patients (Table S8), showed similar results in most subgroups except in older patients (aged ≥80 years), patients with obesity, CKD stage 3 or more, hyperthyroidism, and alcoholism.
Table 2.
Baseline Characteristics of Catheter Ablation in Atrial Fibrillation Patients With or Without Readmission
| Catheter Ablation of Atrial Fibrillation Patients | P Value | P Value | ||||
|---|---|---|---|---|---|---|
| No Readmission | 30‐Day Readmission | 90‐Day Readmission | Overall | |||
| Index admission | 31 196 (89.1%) | 3802 (10.9%) | 6164 (16.5%) | 37 360 | 30‐day readmission vs no readmission | 90‐day readmission vs no readmission |
| Patient level variables | ||||||
| Age (median, IQR) y | 65 (57–71) | 68 (60–74) | 68 (60–74) | 65 (57–72) | <0.001 | <0.001 |
| Age groups (%) | <0.001 | <0.001 | ||||
| 18 to 49 y | 3363 (10.8%) | 286 (7.5%) | 449 (7.3%) | 3811 (10.2%) | ||
| 50 to 64 y | 12 082 (38.7%) | 1168 (30.7%) | 1837 (29.8%) | 13 917 (37.3%) | ||
| 65 to 79 y | 13 842 (44.4%) | 1843 (48.5%) | 3085 (50.1%) | 16 928 (45.3%) | ||
| ≥80 y | 1909 (6.1%) | 504 (13.3%) | 793 (12.9%) | 2705 (7.2%) | ||
| Sex (%) | <0.001 | <0.001 | ||||
| Male | 20 505 (65.7%) | 2107 (55.4%) | 3477 (56.4%) | 23 981 (64.2%) | ||
| Female | 10 691 (34.3%) | 1695 (44.6%) | 2687 (43.6%) | 13 379 (35.8%) | ||
| Charlson Comorbidity Index (%)a | <0.001 | <0.001 | ||||
| 0 | 18 986 (60.9%) | 1613 (42.4%) | 2626 (42.6%) | 21 613 (57.9%) | ||
| 1 | 7528 (24.1%) | 1109 (29.2%0 | 1724 (28.0%) | 9250 (24.8) | ||
| ≥2 | 4683 (15.0%) | 1081 (28.4%0 | 1813 (29.4%) | 6497 (17.4%) | ||
| Comorbidities (%) | ||||||
| Obesityb | 4636 (14.9%) | 667 (17.6%0 | 1071 (17.4%) | 5705 (15.3%) | <0.001 | <0.001 |
| Obstructive sleep apneaα | 4502 (14.4%) | 522 (13.7) | 814 (13.2) | 5316 (14.2%) | 0.254 | 0.012 |
| Hypertensionb | 19 357 (62.1%) | 2515 (66.2%) | 4099 (66.5%) | 23 458 (62.8%) | <0.001 | <0.001 |
| Diabetes mellitusb | 5512 (17.7%) | 969 (25.5%) | 1579 (25.6%) | 7091 (19.0%) | <0.001 | <0.001 |
| Heart failurec | 4293 (13.8%) | 1010 (26.6%) | 1626 (26.4%) | 5922 (15.9%) | <0.001 | <0.001 |
| Coronary artery diseaseβ | 7328 (23.5%) | 1255 (33.0%) | 2078 (33.7%) | 9404 (25.2%) | <0.001 | <0.001 |
| Chronic pulmonary diseaseb | 3996 (12.8%) | 823 (21.6%) | 1315 (21.3%) | 5313 (14.2%) | <0.001 | <0.001 |
| Chronic kidney disease∞ (stage 3 or more) | 802 (2.6%) | 249 (6.5%) | 425 (6.9%) | 1225 (3.3%) | <0.001 | <0.001 |
| Prior coronary artery bypass graft® | 1522 (4.9%) | 240 (6.3%) | 445 (7.2%) | 1969 (5.3%) | <0.001 | <0.001 |
| HyperthyroidismΩ | 237 (0.8%) | 31 (0.8%) | 53 (0.9%) | 291 (0.8%) | 0.759 | 0.439 |
| Alcohol abuseb | 346 (1.1%) | 35 (0.9%) | 63 (1.0%) | 407 (1.1%) | 0.310 | 0.531 |
| Mitral valve diseaseμ | 2343 (7.5%) | 387 (10.2%) | 591 (9.6%) | 2933 (7.9%) | <0.001 | <0.001 |
| Prior stroke/transient ischemic attack© | 1950 (6.3%) | 338 (8.9%) | 526 (8.5%) | 2473 (6.6%) | <0.001 | <0.001 |
| Vascular disease historyb | 1295 (4.2%) | 253 (6.7%) | 420 (6.8%) | 1715 (4.6%) | <0.001 | <0.001 |
| Anemiab | 1338 (4.3%) | 378 (10.0%) | 616 (10.0%) | 1954 (5.2%) | <0.001 | <0.001 |
| Median household income category for patient's zip code (%)d | <0.001 | <0.001 | ||||
| 0 to 25th percentile | 5553 (17.8%) | 858 (22.6%) | 1328 (21.6%) | 6882 (18.4%) | ||
| 26 to 50th percentile | 6888 (22.1%) | 921 (24.2%) | 1492 (24.2%) | 8380 (22.4%) | ||
| 51 to 75th percentile | 8307 (26.6%) | 990 (26.0%) | 1592 (25.8%) | 9900 (26.5%) | ||
| 76 to 100th percentile | 9889 (31.7%) | 971 (25.5%) | 1638 (26.6%) | 11 529 (30.9%) | ||
| Primary payer (%) | <0.001 | <0.001 | ||||
| Medicare | 15 439 (49.5%) | 2434 (64.0%) | 3978 (64.5%) | 19 416 (51.9%) | ||
| Medicaid | 795 (2.6%) | 126 (3.3%) | 205 (3.3%) | 1001 (2.7%) | ||
| Private including health maintenance organization | 13 979 (44.8%) | 1137 (29.9%) | 1823 (29.6%) | 15 800 (42.3%) | ||
| Self‐pay/no charge/other | 976 (3.1%) | 105 (2.8%) | 155 (2.5%) | 1132 (3.0%) | ||
| Hospital characteristics | ||||||
| Hospital bed size (%)e | 0.012 | 0.249 | ||||
| Small | 1061 (3.4%) | 122 (3.2%) | 235 (3.8%) | 1296 (3.5%) | ||
| Medium | 5522 (17.7%) | 603 (15.9%) | 1081 (17.5%) | 6605 (17.7%) | ||
| Large | 24 614 (78.9%) | 3077 (80.9%) | 4847 (78.6%) | 29 458 (78.9%) | ||
| Hospital teaching status (%)f | 0.009 | 0.003 | ||||
| Non‐teaching | 7740 (24.8%) | 10 108 (26.8%) | 1638 (26.6%) | 9377 (25.1%) | ||
| Teaching | 23 456 (75.2%) | 2784 (73.2%) | 4526 (73.4%) | 27 983 (74.9%) | ||
| Hospital volume (median, IQR) | 41 (24–109) | 52 (18–83) | 53 (18–90) | 47 (22–106) | <0.001 | <0.001 |
| Admission type (%) | <0.001 | <0.001 | ||||
| Non‐elective | 8813 (28.3%) | 1426 (37.5) | 2353 (38.2%) | 11 163 (29.9%) | ||
| Elective | 22 383 (71.8%) | 2376 (62.5) | 3805 (61.7%) | 26 182 (70.1%) | ||
| Admission day (%) | <0.001 | <0.001 | ||||
| Weekdays | 30 160 (96.7%) | 3566 (93.8%) | 5774 (93.7%) | 35 933 (96.2%) | ||
| Weekend | 1036 (3.3%) | 236 (6.2%) | 390 (6.3%) | 1427 (3.8%) | ||
| Disposition (%) | <0.001 | <0.001 | ||||
| Home | 30 706 (98.4%) | 3626 (95.4%) | 5882 (95.4%) | 36 587 (97.9%) | ||
| Facility | 412 (1.3%) | 173 (4.6%) | 278 (4.5%) | 687 (1.8%) | ||
| In‐hospital mortality (%) | 53 (0.2%) | 0 (0.0%) | 0 (0.0%) | 52 (0.1%) | ||
| Length of stay (median, IQR) days | 1 (1–2) | 2 (1–4) | 2 (1–4) | 1 (1–3) | <0.001 | <0.001 |
| Cost of hospitalization (median, IQR)f | 23 581 (17 282–30 209) | 22 799 (15 836–31 167) | 22 318 (15 235–30 315 | 23 414 (17 004–30 220) | <0.001 | <0.001 |
α, β, ∞, ®, Ω, μ, ©: comorbidities were identified by appropriate ICD‐9‐CM diagnosis codes in secondary diagnosis field (Table S3).
ICD‐9‐CM indicates International Classification of Diseases, Ninth Edition, Clinical Modification.
Charlson/Deyo Comorbidity Index (CCI) was calculated as per Deyo classification.
Variables are AHRQ comorbidity measures.
Heart failure is identified by ICD‐9 codes in secondary diagnosis field and it includes systolic, diastolic and combined heart failure.
Represents a quartile classification of the estimated median household income of residents within the patients’ zip code, derived from zip code‐demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
The bed size cutoff points divided into small, medium, and large have been done so that approximately one third of the hospitals in a given region, location, and teaching status combination would fall within each bed size category. https://www.hcup-us.ahrq.gov/db/vars/hosp_bedsize/nrdnote.jsp.
A hospital is considered to be a teaching hospital if it has an American Medical Association‐approved residency program is a member of the Council of Teaching Hospitals or has a ratio of full‐time equivalent interns and residents to beds of 0.25 or higher. https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
Table 3.
Predictors of 30 and 90‐Day (All Cause) Readmission in Catheter Ablation of AF Patients
| Patient Level Variables | 30‐Day Readmissiona | 90‐Day Readmission | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | LL | UL | P Value | HR | LL | UL | P Value | |
| Age (continuous variable)b | 1.01 | 1.01 | 1.01 | <0.001 | 1.01 | 1.01 | 1.01 | <0.001 |
| Age groups | ||||||||
| 18 to 49 y | Referent | Referent | Referent | Referent | Referent | Referent | ||
| 50 to 64 y | 1.00 | 0.88 | 1.14 | 0.996 | 1.00 | 0.90 | 1.12 | 0.922 |
| 65 to 79 y | 0.90 | 0.77 | 1.05 | 0.175 | 0.98 | 0.87 | 1.11 | 0.752 |
| ≥80 y | 1.14 | 0.95 | 1.37 | 0.166 | 1.17 | 1.01 | 1.35 | 0.034 |
| Female | 1.22 | 1.14 | 1.31 | <0.001 | 1.20 | 1.13 | 1.26 | <0.001 |
| Charlson Comorbidity Indexc | ||||||||
| 0 | Referent | Referent | Referent | Referent | Referent | Referent | ||
| 1 | 1.24 | 1.12 | 1.37 | <0.001 | 1.22 | 1.12 | 1.32 | <0.001 |
| ≥2 | 1.16 | 1.00 | 1.35 | 0.049 | 1.26 | 1.12 | 1.42 | <0.001 |
| Comorbidities | ||||||||
| Obesityd | 1.11 | 1.02 | 1.21 | 0.018 | 1.12 | 1.04 | 1.20 | 0.002 |
| Hypertensiond | 0.94 | 0.87 | 1.00 | 0.067 | 0.94 | 0.89 | 1.00 | 0.034 |
| Diabetes mellitusd | 1.13 | 1.03 | 1.25 | 0.010 | 1.10 | 1.03 | 1.19 | 0.009 |
| Heart failuree | 1.19 | 1.08 | 1.32 | <0.001 | 1.17 | 1.08 | 1.26 | <0.001 |
| Coronary artery diseaseβ | 1.17 | 1.08 | 1.27 | <0.001 | 1.18 | 1.11 | 1.25 | <0.001 |
| Chronic pulmonary diseased | 1.22 | 1.10 | 1.34 | <0.001 | 1.17 | 1.09 | 1.26 | <0.001 |
| Chronic kidney disease∞ (stage 3 or more) | 1.28 | 1.10 | 1.48 | 0.001 | 1.26 | 1.12 | 1.41 | <0.001 |
| Prior coronary artery bypass graft® | 0.86 | 0.75 | 0.99 | 0.037 | 0.95 | 0.85 | 1.05 | 0.327 |
| Mitral valve diseaseμ | 1.13 | 1.01 | 1.26 | 0.028 | 1.07 | 0.99 | 1.17 | 0.105 |
| Prior stroke/Transient ischemic attack© | 1.14 | 1.02 | 1.28 | 0.026 | 1.10 | 1.00 | 1.21 | 0.041 |
| Vascular disease historyd | 1.08 | 0.95 | 1.24 | 0.237 | 1.08 | 0.98 | 1.20 | 0.123 |
| Anemiad | 1.28 | 1.14 | 1.43 | <0.001 | 1.27 | 1.16 | 1.39 | <0.001 |
| Median household income category for patient's zip codef | ||||||||
| 0 to 25th percentile | Referent | Referent | Referent | Referent | Referent | Referent | ||
| 26 to 50th percentile | 0.93 | 0.85 | 1.03 | 0.149 | 0.97 | 0.90 | 1.04 | 0.357 |
| 51 to 75th percentile | 0.94 | 0.86 | 1.03 | 0.205 | 0.97 | 0.90 | 1.04 | 0.341 |
| 76 to 100th percentile | 0.88 | 0.80 | 0.97 | 0.009 | 0.94 | 0.87 | 1.01 | 0.092 |
| Primary payer | ||||||||
| Medicare | Referent | Referent | Referent | Referent | Referent | Referent | ||
| Medicaid | 0.96 | 0.78 | 1.17 | 0.655 | 1.02 | 0.88 | 1.20 | 0.748 |
| Private including health maintenance organization | 0.67 | 0.60 | 0.74 | <0.001 | 0.69 | 0.64 | 0.76 | <0.001 |
| Self‐pay/no charge/other | 0.73 | 0.59 | 0.91 | 0.004 | 0.74 | 0.62 | 0.88 | <0.001 |
| Hospital characteristics of index admission | ||||||||
| Teaching hospitalsg | 0.97 | 0.90 | 1.04 | 0.382 | 0.96 | 0.91 | 1.02 | 0.174 |
| Hospital volume (per 1 increase) | 1.00 | 0.99 | 1.00 | 0.131 | 0.99 | 0.99 | 0.99 | 0.046 |
| Admission characteristics of index admission | ||||||||
| Elective admission | 0.95 | 0.88 | 1.02 | 0.156 | 0.91 | 0.86 | 0.96 | <0.001 |
| Weekend admission | 1.06 | 0.92 | 1.21 | 0.435 | 1.08 | 0.97 | 1.21 | 0.157 |
| Discharge characteristics of index admission | ||||||||
| Disposition to facility | 1.30 | 1.10 | 1.53 | 0.002 | 1.25 | 1.10 | 1.43 | <0.001 |
| Length of stay, days | ||||||||
| 1 | Referent | Referent | Referent | Referent | Referent | Referent | ||
| 2 to 3 | 1.39 | 1.28 | 1.50 | <0.001 | 1.22 | 1.15 | 1.30 | <0.001 |
| >3 | 1.75 | 1.59 | 1.92 | <0.001 | 1.56 | 1.45 | 1.68 | <0.001 |
| In separate analysis with entire AF population | ||||||||
| Catheter ablation of AF | 1.02 | 0.97 | 1.08 | 0.424 | 0.89 | 0.86 | 0.92 | <0.001 |
Α, β, ∞, ®, Ω, μ, ©: comorbidities were identified by appropriate ICD‐9‐CM diagnosis codes in secondary diagnosis field (Table S3).
AF indicates atrial fibrillation; CI, confidence interval; HR, hazard ratio; ICD‐9‐CM, International Classification of Diseases, Ninth Edition, Clinical Modification; LL, lower limit; UL, upper limit.
Patients who were readmitted after 30 days were excluded from 30‐day analysis.
Two separate multivariate models were conducted, first one with age as continuous variables and second with age as group variable.
Charlson/Deyo Comorbidity Index (CCI) was calculated as per Deyo classification.
Variables are AHRQ comorbidity measures.
Heart failure is identified by ICD‐9 codes in secondary diagnosis field and it includes systolic, diastolic, and combined heart failure.
Represents a quartile classification of the estimated median household income of residents within the patients’ zip code, derived from zip code‐demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
A hospital is considered to be a teaching hospital if it has an American Medical Association‐approved residency program, is a member of the Council of Teaching Hospitals or has a ratio of full‐time equivalent interns and residents to beds of 0.25 or higher. https://www.hcup-us.ahrq.gov/db/vars/hosp_ur_teach/nrdnote.jsp.
Admitting Diagnoses of Readmission
Out of 3802 and 6164 readmissions (over 30 and 90 days, respectively) cardiac causes (58.4% and 59.1%, respectively) were the most prevalent cause of readmission, of which arrhythmia (32.6% and 35.2%) was the most common including (AF [22.7% and 24.8%] and atrial flutter [4.8% and 5.1%]) followed by heart failure (13.8% and 12.6%), pericardial complications (2.6% and 2.1%) and ischemic heart disease (1.9% and 2.3%). While pulmonary causes (primarily pneumonia [3.4% and 2.7%] followed by COPD [2.1% and 2.2%]) were responsible for 8.4% 30‐day readmission and 8.3% 90‐day readmission, whereas bleeding complications consisted of gastrointestinal bleed (1.5% and 1.7%), intracranial hemorrhage (0.6%) and other bleed (3.4% and 2.7%) made the third most prevalent cause of readmission. Other important causes were infections (5% and 4.9%) (including sepsis [2.6% and 2.3%]) and stroke/TIA (1.7% and 1.6%) (Table 4).
Table 4.
Admitting Diagnosis of Readmitted Patients Amongst CA of AF
| 30‐Day Readmission | 90‐Day Readmission | |
|---|---|---|
| Causes of readmission after CA of AF | ||
| Total readmissions | 3803 | 6164 |
| Cardiac causes | 58.4%a | 59.1%a |
| Heart failureb | 13.8% | 12.6% |
| Arrhythmia | 32.6% | 35.2% |
| Atrial fibrillation | 22.7% | 24.8% |
| Atrial flutter | 4.8% | 5.1% |
| Heart block excluding 1st degree block | 0.2% | 0.1% |
| Sinoatrial node dysfunction | 1.51% | 1.64% |
| Paroxysmal ventricular tachycardia | 0.67% | 0.68% |
| Paroxysmal supraventricular tachycardia | 0.47% | 0.52% |
| Othersc | 2.26% | 2.35% |
| Ischemic heart disease | 1.9% | 2.3% |
| Heart valve disease | 0.4% | 0.7% |
| Hypertension | 0.3% | 0.3% |
| Hypotension/dizziness/syncope | 1.7% | 1.4% |
| Pericardial complications | 2.6% | 2.1% |
| Other cardiac complication | 5.2% | 4.5% |
| Vascular causes/complications | 3.0%a | 2.2%a |
| Infections | 5.0%a | 4.9%a |
| Sepsis | 2.6% | 2.3% |
| Pulmonary causes/complications | 8.4%a | 8.3%a |
| Chronic obstructive pulmonary diseases | 2.1% | 2.2% |
| Pneumonia | 3.4% | 2.7% |
| Other respiratory causes/complications | 2.9% | 3.4% |
| Gastrointestinal causes/complication | 3.8%a | 4.3%a |
| Neurological complications | 2.7%a | 2.8%a |
| Ischemic stroke/Transient ischemic attack | 1.7% | 1.6% |
| Other neurological causes/complications | 1.0% | 1.2% |
| Kidney or urinary causes/complications | 4.5%a | 3.7%a |
| Electrolyte imbalance | 0.6% | 0.8% |
| Acute/acute on chronic kidney failure | 1.6% | 1.5% |
| Other kidney/urinary tract etiology/complication | 2.4% | 1.4% |
| Hematology | 1.0%a | 0.5%a |
| Anemia | 0.7% | 0.3% |
| Others | 0.3% | 0.3% |
| Trauma/fracture/poisoning | 1.0%a | 1.2%a |
| Endocrine causes | 0.5%a | 0.6%a |
| Diabetes mellitus | 0.3% | 0.4% |
| Others | 0.2% | 0.2% |
| Malignancy | 0.9%a | 1.2%a |
| Bleeding complications | 5.4%a | 5.0%a |
| Gastrointestinal bleed | 1.5% | 1.7% |
| Intracranial bleed | 0.6% | 0.6% |
| Intracranial bleed without trauma | 0.4% | 0.4% |
| Intracranial bleed with trauma | 0.1% | 0.2% |
| Other bleeding complications | 3.4% | 2.7% |
| Psychiatry causes | 0.6%a | 0.7%a |
| Pulmonary embolism (PE)/deep venous thrombosis (DVT) | 1.3%a | 1.1%a |
| Othersd | 3.6%a | 4.5%a |
AF indicates atrial fibrillation; CA, catheter ablation.
Overall percentage for respective system.
Heart failure includes systolic, diastolic, and combined heart failure.
Other arrhythmia includes: anomalous atrioventricular excitation, long QT syndrome, other specified conduction disorders, conduction disorder, unspecified, paroxysmal tachycardia, unspecified, supraventricular premature beats, other premature beats, other specified cardiac dysrhythmias, cardiac dysrhythmia, unspecified, first degree atrioventricular block, ventricular fibrillation, cardiac arrest.
Skin, subcutaneous, joints, non‐specific laboratory findings, non‐specific symptoms.
Predictors of AF Recurrence
One thousand nine hundred sixty‐four patients (5.3%) out of 37 360 patients came back to hospital with primary diagnosis of AF or for the repeat AF ablation procedure (Table S6). Trends of AF recurrence suggest that half of overall AF recurrence takes place in the first 21 to 30 days post discharge (Figure 1). Predictors of increase in AF recurrence post‐AF ablation were diabetes mellitus (HR: 1.30, 95% CI: 1.06–1.61, P=0.013), chronic pulmonary disease (HR: 1.29, 95% CI: 1.04–1.61, P=0.021), female sex (HR: 1.28, 95% CI: 1.11–1.46, P<0.001) and index hospitalization LOS ≥2 days (LOS 2–3 days: HR: 1.34, 95% CI: 1.15–1.56, P<0.001) (LOS >3 days: HR: 1.60, 95% CI: 1.32–1.95, P<0.001). While, a predictor of decreased AF recurrence was higher hospital volume (HR: 0.998, 95% CI: 0.997–0.999, P<0.001) (Table 5).
Figure 1.
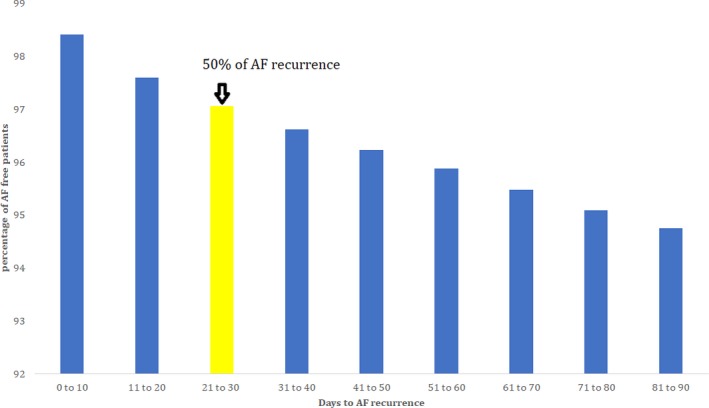
Trends of atrial fibrillation (AF) recurrence.
Table 5.
Predictors of Atrial Fibrillation Recurrence Over 90 Days Post‐Catheter Ablation of Atrial Fibrillation
| Patient Level Variables | Atrial Fibrillation Recurrence Post‐Catheter Ablation | |||
|---|---|---|---|---|
| HR | LL | UL | P Value | |
| Age (continuous variable)a | 1.00 | 0.99 | 1.01 | 0.493 |
| Age groups | ||||
| 18 to 49 y | Referent | Referent | Referent | |
| 50 to 64 y | 1.14 | 0.87 | 1.48 | 0.351 |
| 65 to 79 y | 1.23 | 0.90 | 1.69 | 0.194 |
| ≥80 y | 1.08 | 0.72 | 1.62 | 0.707 |
| Female | 1.28 | 1.11 | 1.46 | <0.001 |
| Charlson Comorbidity Indexb | ||||
| 0 | Referent | Referent | Referent | |
| 1 | 1.01 | 0.83 | 1.24 | 0.901 |
| ≥2 | 0.96 | 0.73 | 1.27 | 0.787 |
| Comorbidities | ||||
| Obesityc | 1.16 | 0.97 | 1.39 | 0.102 |
| Hypertensionc | 1.01 | 0.87 | 1.16 | 0.964 |
| Diabetes mellitusc | 1.30 | 1.06 | 1.61 | 0.013 |
| Chronic pulmonary diseasec | 1.29 | 1.04 | 1.61 | 0.021 |
| Chronic kidney disease∞ (stage 3 or more) | 0.90 | 0.58 | 1.39 | 0.645 |
| HyperthyroidismΩ | 0.45 | 0.15 | 1.40 | 0.169 |
| Alcohol abusec | 0.31 | 0.10 | 0.96 | 0.042 |
| Anemiac | 1.23 | 0.94 | 1.60 | 0.134 |
| Median household income category for patient's zip coded | ||||
| 1. 0 to 25th percentile | Referent | Referent | Referent | |
| 2. 26 to 50th percentile | 1.11 | 0.91 | 1.35 | 0.320 |
| 3. 51 to 75th percentile | 0.99 | 0.81 | 1.20 | 0.906 |
| 4. 76 to 100th percentile | 0.90 | 0.74 | 1.10 | 0.292 |
| Primary payer | ||||
| Medicare | Referent | Referent | Referent | |
| Medicaid | 1.39 | 0.94 | 2.04 | 0.098 |
| Private including health maintenance organization | 0.85 | 0.68 | 1.05 | 0.132 |
| Self‐pay/no charge/other | 0.86 | 0.55 | 1.34 | 0.502 |
| Hospital characteristics of index admission | ||||
| Hospital volume (per 1 increase) | 0.998 | 0.997 | 0.999 | <0.001 |
| Admission characteristics of index admission | ||||
| Electives admission | 0.91 | 0.78 | 1.05 | 0.190 |
| Discharge characteristics of index admission | ||||
| Disposition to facility | 0.69 | 0.38 | 1.28 | 0.241 |
| Length of stay, days | ||||
| 1 | Referent | Referent | Referent | |
| 2 to 3 | 1.34 | 1.15 | 1.56 | <0.001 |
| >3 | 1.60 | 1.32 | 1.95 | <0.001 |
CI indicates confidence interval; HR, hazard ratio; LL, lower limit; UL, upper limit. α, β, ∞, ®, Ω, μ, ©: comorbidities were identified by appropriate ICD‐9‐CM diagnosis codes in secondary diagnosis field (Table S3).
ICD‐9‐CM indicates International Classification of Diseases, Ninth Edition, Clinical Modification.
Two separate multivariate models were conducted, first one with age as continuous variables and second with age as group variable.
Charlson/Deyo Comorbidity Index (CCI) was calculated as per Deyo classification.
Variables are AHRQ comorbidity measures.
Represents a quartile classification of the estimated median household income of residents within the patients’ zip code, derived from zip code‐demographic data obtained from Claritas. The quartiles are identified by values of 1 to 4, indicating the poorest to wealthiest populations. Because these estimates are updated annually, the value ranges vary by year. https://www.hcup-us.ahrq.gov/db/vars/zipinc_qrtl/nrdnote.jsp.
Trends of Short‐Term Outcomes
Rate of 30‐day readmission and in‐hospital mortality (data not shown in table) have not changed from year 2010 to 2014; while 90‐day readmission rate (from 17% in 2010 to 15% in 2014, 0.55% reduction per year, P<0.001) and AF recurrence rate (from 6.5% in 2010 to 3.7%, 0.66% reduction per year, P<0.001) were improving over the years (Figure 2). Resource utilization including LOS (median, interquartile range) (from 1 [1–2] days in 2010 to 2 [1–3] days in 2014, 0.11‐day increments per year, P<0.001) and cost of care (from $22 561 [16 129–28 141] in 2010 to $23 779 [16 861–30 845] in 2014, $621 increment per year, P<0.001) were increasing over the study period (data not shown in table).
Figure 2.
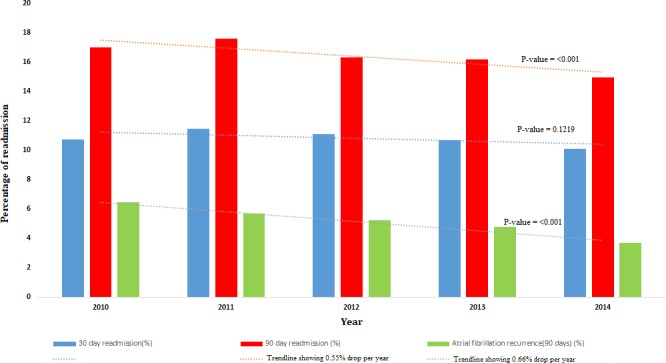
Trends of short‐term outcomes.
Discussion
Herein, we report the largest all‐player data in the United States from a nationwide readmission database on the causes and predictors of all‐cause readmission, and several short‐term outcomes in patients with AF undergoing catheter ablation. The important findings of our study were: 1) readmission rate of 10.9% and 16.5% was noted within first 30 and 90 days of discharge from index admission, respectively for CA of AF; 2) female sex, increased LOS of index hospitalization, disposition to facility, and comorbidities (diabetes mellitus, heart failure, CAD, chronic pulmonary disease, CKD, prior stroke/TIA and anemia) were the predictors of increased readmissions whereas private insurance coverage, high hospital volume for the catheter ablation, and non‐urgent admission correlated to improved 90‐day readmission; 3) most common causes of readmission were cardiac, where AF recurrence was the most common followed by heart failure; and 4) female sex, diabetes mellitus, chronic pulmonary disease, and higher LOS during index hospitalization were the predictors of increased AF recurrence requiring hospitalization.
The strength of our study is the large sample size representing real‐world data. This is in contrast to a large number of previous studies on post‐CA of AF readmission, which have been restricted by their comparatively small sample size11 or selective population cohorts.5, 6, 12 We noted a higher 90‐day readmission rate (16.5%) in our study compared with data published by Noseworthy et al13 (13.4%). This difference in readmission rate is likely explained by the inclusion of only privately insured patients from Optum Labs Data Warehouse database by Noseworthy et al.13 The key observation in our study was a significantly lower 30‐day readmission rate (10.9%) in ablated AF patients compared with data published by Freeman et al12 who reported a 30‐day readmission rate ranging between 15.3% to 15.5% in patients admitted with principal diagnosis for AF during years 1999–2011. Similarly, Munir et al14 noted a 30‐day readmission rate of 15.1% using a patient database similar to the database in our study. A lower readmission rate observed in our study reflects the salutary effect of catheter ablation in AF patients. Moreover, our study also noted catheter ablation to be a significant predictor of reduced 90‐day readmission among AF patients (Table 3 and Table S6) thus supporting the same observation. Furthermore, females were found to be less likely to receive CA for AF than men despite higher number of AF hospitalizations. The causality of the same is unknown but our results were similar to previous studies on cardiac intervention such as use of Cardiac Resynchronization Therapy15 and treatment of non‐ST‐segment elevation acute coronary syndromes.16
Our study noted several predictors of increased readmission after CA in AF patients thus identifying the vulnerable subgroup of patients who can benefit from individualized in‐hospital as well as post‐discharge care. Similar to a prior study,13 we identified older age, female sex, and certain comorbidities (diabetes mellitus, heart failure, CAD, CKD, chronic pulmonary disease) to be associated with high 90‐day readmission rates in AF patients undergoing CA. Similar characteristics except age predicted increased 30‐day readmission related to CA in AF patients. High hospital volume for CA was noted to be associated with reduced readmission as noted by Shah et al5 thus reaffirming the notion that increased procedure experience is associated with better outcomes. Additionally, prior stroke/TIA was a significant predictor of increase readmission in our study converse to the findings published in the previous studies.5, 13
AF recurrence was the most common etiology (22.7% at 30 days and 24.8% at 90 days follow‐up) for readmission followed by heart failure in the study population. Various studies have reported post‐ablation AF recurrence rate ranging between 13% and 44% with a long‐term follow up.17, 18 Observation by Shah et al5 that 26.9% of readmissions after 30‐day follow‐up in catheter ablated AF patients were attributable to AF and atrial flutter coincide with our study. Readmission because of heart failure partly related to the high prevalence of the same in the study population but heart failure has been a well‐recognized complication after extensive catheter ablation.19 High heart failure rate in readmitted subjects in our study compared with Noseworthy et al13 is partly explained by the greater proportion of subjects aged ≥65 years in our study. Readmission rate because of stroke/TIA and bleeding noted in our study is similar to previously published studies.17, 20, 21, 22
Our study reported an AF recurrence rate of 5.3% which is in line with the observation made by Noseworthy et al13 who reported an AF recurrence of 5.4% during the 90‐day follow‐up after CA in AF patients. We identified patient subgroups at high risk of AF recurrence after catheter ablation. Females were noted to have higher association to AF recurrence compared with males which again reaffirms the sex‐related variation in outcomes after catheter ablation for AF. Contrary to Berruezo et al23 hypertension was not found to be a predictor of AF recurrence in our study. Additionally, we noted diabetes mellitus and chronic pulmonary disease to be associated with a high likelihood of AF recurrence. Our findings support the previously noted observation by Goudis24 that concurrent COPD in AF patients leads to worse outcomes in terms of AF progression, AF recurrence after catheter ablation, and overall mortality. Increased hospital volume for the catheter ablation predicted lower AF recurrence in our study, again highlighting the salubrious importance of increased institutional experience. This information is of high value in patient selection for catheter ablation.
We noted a significant decline in AF recurrence and 90‐day readmission rate in post‐ablated AF patients during the study period which endorses the findings published by Noseworthy et al.13 This gradual betterment in outcomes reflects the irrefutable effects of improvement in catheter technology, institutional experience, and post‐ablation anticoagulation techniques. However, a rise in AF ablation related cost of care and length of hospitalization over the study period was noted as well. Our observation that those subgroups of AF patients with higher use of catheter ablation had better outcomes as well (ie, male patients, subjects with higher household income or private insurance coverage, patients admitted in medium/large hospitals, hospitals with academic affiliation or those having non‐urgent admission) supports the current inclusion model for catheter ablation.
Limitations
Though our study originates from the well‐designed National Readmission Database from the HCUP, there remain few limitations. The major limitation of our study holds true for most large administrative database analyses, which includes errors in coding primary diagnoses, and under‐reporting of secondary diagnoses. Also, clinically insignificant differences can sometimes be presented as statistically significant difference because of large sample size. Furthermore, our data lack some clinical information such as duration of atrial fibrillation, anti‐arrhythmic drugs, anti‐coagulation use, and cardiac biomarkers. And NRD does not carry information about the race and results from invasive and non‐invasive diagnostic modalities which could have predicted adverse outcomes in a much better way. Nevertheless, the NRD database is clinically sound with its near population‐based case ascertainment, a large sample size, and valid information on readmissions, with the representation of patients from all regions, not limited to Medicare/Medicaid beneficiaries. The largest publicly available and nationally representative sample allowed us to evaluate some meaningful outcome results for increasingly relevant quality parameters.
Conclusions
We reported increased hospitalization post AF ablation in women, patients with multiple higher comorbidities whereas lower rates of hospitalization in high volume hospitals and ablations were performed in patients with private insurance. Identifying high‐risk group for readmissions and potential reasons and interventions to mitigate them could reduce resource utilization.
Disclosures
None.
Supporting information
Table S1. International Classification of Disease, Version 9 (ICD‐9) Codes Used for Atrial Fibrillation and AF ablation Case Identification
Table S2. ICD 9 Codes for Admitting Diagnosis of Readmission
Table S3. ICD 9 Codes Used to Identify Comorbidities
Table S4. Deyo's Modification of Charlson's Comorbidity Index (CCI)
Table S5. Multivariate Analysis of Utilization of Catheter Ablation for Atrial Fibrillation
Table S6. Baseline Characteristics of Patients of Catheter Ablation in Atrial Fibrillation With and Without Atrial Fibrillation Recurrence
Table S7. Multivariate Analysis of 30‐ and 90‐Day Readmission in Atrial Fibrillation Patients
Table S8. Impact of Catheter Ablation on 90‐Day Readmission in Different Subgroups of Atrial Fibrillations
Figure S1. Kaplan–Meier survival curve for 30‐day readmission.
Figure S2. Kaplan–Meier survival cure for 90‐day readmission.
Figure S3. Kaplan–Meier survival of atrial fibrillation recurrence over 90 days.
(J Am Heart Assoc. 2018;7:e009294 DOI: 10.1161/JAHA.118.009294.)
References
- 1. Feinberg WM, Blackshear JL, Laupacis A, Kronmal R, Hart RG. Prevalence, age distribution, and gender of patients with atrial fibrillation: analysis and implications. Arch Intern Med. 1995;155:469–473. [PubMed] [Google Scholar]
- 2. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 3. Clarnette JA, Brooks AG, Mahajan R, Elliott AD, Twomey DJ, Pathak RK, Kumar S, Munawar DA, Young GD, Kalman JM, Lau DH, Sanders P. Outcomes of persistent and long‐standing persistent atrial fibrillation ablation: a systematic review and meta‐analysis. Europace. 2017;00:1–11. [DOI] [PubMed] [Google Scholar]
- 4. HCUP . Health Care and Utilization Project, NRD data overview. 2017.
- 5. Shah RU, Freeman JV, Shilane D, Wang PJ, Go AS, Hlatky MA. Procedural complications, rehospitalizations, and repeat procedures after catheter ablation for atrial fibrillation. J Am Coll Cardiol. 2012;59:143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piccini JP, Sinner MF, Greiner MA, Hammill BG, Fontes JD, Daubert JP, Ellinor PT, Hernandez AF, Walkey AJ, Heckbert SR, Benjamin EJ, Curtis LH. Outcomes of Medicare beneficiaries undergoing catheter ablation for atrial fibrillation. Circulation. 2012;126:2200–2207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. HCUP . Healthcare and Utilization Project, NRD data description. 2014.
- 8. Healthcare Cost and Utilization Project (HCUP) Comorbidity Software V. Available at: https://www.hcup-us.ahrq.gov/toolssoftware/comorbidity/comorbidity.jsp. Accessed June 1, 2016.
- 9. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases. J Clin Epidemiol. 1992;45:613–619. [DOI] [PubMed] [Google Scholar]
- 10. The US inflation calculator. Available at: http://www.Usinflationcalculator.Com/. Accessed May 8, 2018.
- 11. Weerasooriya R, Khairy P, Litalien J, Macle L, Hocini M, Sacher F, Lellouche N, Knecht S, Wright M, Nault I, Miyazaki S, Scavee C, Clementy J, Haissaguerre M, Jais P. Catheter ablation for atrial fibrillation: are results maintained at 5 years of follow‐up? J Am Coll Cardiol. 2011;57:160–166. [DOI] [PubMed] [Google Scholar]
- 12. Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National trends in atrial fibrillation hospitalization, readmission, and mortality for Medicare beneficiaries, 1999–2013. Circulation. 2017;135:1227–1239. [DOI] [PubMed] [Google Scholar]
- 13. Noseworthy PA, Kapa S, Haas LR, Van Houten H, Deshmuk AJ, Mulpuru SK, McLeod CJ, Asirvatham SJ, Friedman PA, Shah ND, Packer DL. Trends and predictors of readmission after catheter ablation for atrial fibrillation, 2009–2013. Am Heart J. 2015;170:483–489. [DOI] [PubMed] [Google Scholar]
- 14. Munir MB, Sharbaugh MS, Ahmad S, Patil S, Mehta K, Althouse AD, Saba S. Causes and predictors of 30‐day readmissions in atrial fibrillation (from the nationwide readmissions database). Am J Cardiol. 2017;120:399–403. [DOI] [PubMed] [Google Scholar]
- 15. Alaeddini J, Wood MA, Amin MS, Ellenbogen KA. Gender disparity in the use of cardiac resynchronization therapy in the United States. Pacing Clin Electrophysiol. 2008;31:468–472. [DOI] [PubMed] [Google Scholar]
- 16. Blomkalns AL, Chen AY, Hochman JS, Peterson ED, Trynosky K, Diercks DB, Brogan GX, Boden WE, Roe MT, Ohman EM. Gender disparities in the diagnosis and treatment of non‐ST‐segment elevation acute coronary syndromes: large‐scale observations from the CRUSADE (can rapid risk stratification of unstable angina patients suppress adverse outcomes with early implementation of the American College of Cardiology/American Heart Association Guidelines) National Quality Improvement Initiative. J Am Coll Cardiol. 2005;45:832–837. [DOI] [PubMed] [Google Scholar]
- 17. D'Ascenzo F, Corleto A, Biondi‐Zoccai G, Anselmino M, Ferraris F, di Biase L, Natale A, Hunter RJ, Schilling RJ, Miyazaki S, Tada H, Aonuma K, Yenn‐Jiang L, Tao H, Ma C, Packer D, Hammill S, Gaita F. Which are the most reliable predictors of recurrence of atrial fibrillation after transcatheter ablation? A meta‐analysis. Int J Cardiol. 2013;167:1984–1989. [DOI] [PubMed] [Google Scholar]
- 18. Stabile G, Bertaglia E, Senatore G, De Simone A, Zoppo F, Donnici G, Turco P, Pascotto P, Fazzari M, Vitale DF. Catheter ablation treatment in patients with drug‐refractory atrial fibrillation: a prospective, multi‐centre, randomized, controlled study (Catheter Ablation for the Cure of Atrial Fibrillation Study). Eur Heart J. 2006;27:216–221. [DOI] [PubMed] [Google Scholar]
- 19. Tan HW, Wang XH, Shi HF, Sun YM, Zhou L, Gu JN, Han B, Jiang WF, Yang GS, Liu X. Congestive heart failure after extensive catheter ablation for atrial fibrillation: prevalence, characterization, and outcome. J Cardiovasc Electrophysiol. 2011;22:632–637. [DOI] [PubMed] [Google Scholar]
- 20. Pappone C, Rosanio S, Augello G, Gallus G, Vicedomini G, Mazzone P, Gulletta S, Gugliotta F, Pappone A, Santinelli V, Tortoriello V, Sala S, Zangrillo A, Crescenzi G, Benussi S, Alfieri O. Mortality, morbidity, and quality of life after circumferential pulmonary vein ablation for atrial fibrillation: outcomes from a controlled nonrandomized long‐term study. J Am Coll Cardiol. 2003;42:185–197. [DOI] [PubMed] [Google Scholar]
- 21. Oral H, Chugh A, Ozaydin M, Good E, Fortino J, Sankaran S, Reich S, Igic P, Elmouchi D, Tschopp D, Wimmer A, Dey S, Crawford T, Pelosi F Jr, Jongnarangsin K, Bogun F, Morady F. Risk of thromboembolic events after percutaneous left atrial radiofrequency ablation of atrial fibrillation. Circulation. 2006;114:759–765. [DOI] [PubMed] [Google Scholar]
- 22. Freeman JV, Tabada GH, Reynolds K, Sung SH, Liu TI, Gupta N, Go AS. Contemporary procedural complications, hospitalizations, and emergency visits after catheter ablation for atrial fibrillation. Am J Cardiol. 2018;121:602–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Berruezo A, Tamborero D, Mont L, Benito B, Tolosana JM, Sitges M, Vidal B, Arriagada G, Méndez F, Matiello M, Molina I, Brugada J. Pre‐procedural predictors of atrial fibrillation recurrence after circumferential pulmonary vein ablation. Eur Heart J. 2007;28:836–841. [DOI] [PubMed] [Google Scholar]
- 24. Goudis CA. Chronic obstructive pulmonary disease and atrial fibrillation: an unknown relationship. J Cardiol. 2017;69:699–705. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. International Classification of Disease, Version 9 (ICD‐9) Codes Used for Atrial Fibrillation and AF ablation Case Identification
Table S2. ICD 9 Codes for Admitting Diagnosis of Readmission
Table S3. ICD 9 Codes Used to Identify Comorbidities
Table S4. Deyo's Modification of Charlson's Comorbidity Index (CCI)
Table S5. Multivariate Analysis of Utilization of Catheter Ablation for Atrial Fibrillation
Table S6. Baseline Characteristics of Patients of Catheter Ablation in Atrial Fibrillation With and Without Atrial Fibrillation Recurrence
Table S7. Multivariate Analysis of 30‐ and 90‐Day Readmission in Atrial Fibrillation Patients
Table S8. Impact of Catheter Ablation on 90‐Day Readmission in Different Subgroups of Atrial Fibrillations
Figure S1. Kaplan–Meier survival curve for 30‐day readmission.
Figure S2. Kaplan–Meier survival cure for 90‐day readmission.
Figure S3. Kaplan–Meier survival of atrial fibrillation recurrence over 90 days.


