Abstract
Background
Whether ventricular arrhythmias (VAs) represent a feature of the adaptive changes of the athlete's heart remains elusive. We aimed to assess the prevalence, determinants, and underlying substrates of VAs in young competitive athletes.
Method and Results
We studied 288 competitive athletes (age range, 16–35 years; median age, 21 years) and 144 sedentary individuals matched for age and sex who underwent 12‐lead 24‐hour ambulatory electrocardiographic monitoring. VAs were evaluated in terms of number, complexity (ie, couplet, triplet, or nonsustained ventricular tachycardia), exercise inducibility, and morphologic features. Twenty‐eight athletes (10%) and 13 sedentary individuals (11%) showed >10 isolated premature ventricular beats (PVBs) or ≥1 complex VA (P=0.81). Athletes with >10 isolated PVBs or ≥1 complex VA were older (median age, 26 versus 20 years; P=0.008) but did not differ with regard to type of sport, hours of training, and years of activity compared with the remaining athletes. All athletes with >10 isolated PVBs or ≥1 complex VA had a normal echocardiographic examination; 17 of them showing >500 isolated PVBs, exercise‐induced PVBs, and/or complex VA underwent additional cardiac magnetic resonance, which demonstrated nonischemic left ventricular late gadolinium enhancement in 3 athletes with right bundle branch block PVBs morphologic features.
Conclusions
The prevalence of >10 isolated PVBs or ≥1 complex VA at 24‐hour ambulatory electrocardiographic monitoring did not differ between young competitive athletes and sedentary individuals and was unrelated to type, intensity, and years of sports practice. An underlying myocardial substrate was uncommon and distinctively associated with right bundle branch block VA morphologic features.
Keywords: cardiac magnetic resonance, sports cardiology, sudden cardiac death, ventricular arrhythmias
Subject Categories: Sudden Cardiac Death, Arrhythmias, Exercise
Clinical Perspective
What Is New?
By comparing the burden of ventricular arrhythmias (VAs) at 24‐hour 12‐lead ambulatory electrocardiographic monitoring in young competitive athletes and sedentary individuals, we found that the proportion of subjects showing frequent or complex arrhythmias was low and similar in the 2 groups.
The presence of VAs was unrelated to the intensity of physical training, years of sports activity, type of sport, and degree of left ventricular remodeling, as evidenced by echocardiography.
A subset of apparently healthy athletes with right bundle branch block–like complex and/or exercise‐induced VA showed late enhancement at cardiac magnetic resonance with a nonischemic distribution.
What Are the Clinical Implications?
VAs in young athletes should not be simply dismissed as part of the adaptive changes of the heart to regular sport activity (“athlete's heart”) but, similar to the general population, they should be interpreted as a possible sign of an underlying disease.
Evaluation of VA morphologic features, complexity, and relation to exercise, rather than the count of premature ventricular beats, is useful for differentiating between benign and other potentially pathologic variants.
Introduction
The leading causes of malignant arrhythmic events and sudden cardiac death (SCD) in the athlete include cardiomyopathies that predispose to the abrupt onset of sustained ventricular tachycardia (VT) or ventricular fibrillation during exercise.1 As a consequence, to detect ventricular arrhythmias (VAs) on the athlete's ECG raises concern of an underlying cardiac disease at risk of SCD.2, 3 Interpretation of VA in athletes with no overt structural abnormalities is still a matter of debate.
A milestone study on elite athletes found that most VA occur in the absence of an underlying heart disease and tend to disappear with detraining, suggesting that they may be considered a feature of the adaptive changes of the athlete's heart.4, 5 However, these findings were limited to a single‐center experience and were not in keeping with the results of old studies performed in the 1980s and 1990s, which demonstrated no differences in the VA burden at 24‐hour ambulatory electrocardiographic monitoring between athletes and sedentary individuals.6, 7, 8, 9, 10, 11 Moreover, these previous investigations focused on the number and complexity of VAs without characterizing the morphologic features of premature ventricular beats (PVBs), a parameter that may help to identify the site of origin of the arrhythmia and the possible underlying myocardial substrate.12
The present study was designed to compare the burden of VAs recorded by 24‐hour 12‐lead ambulatory electrocardiographic monitoring in a large cohort of apparently healthy young athletes with that of sedentary individuals and to evaluate the association between VAs and the intensity, duration, and type of training. Athletes with >10 isolated PVBs/hour or ≥1 complex VA (ie, ≥1 couplet, triplet, or nonsustained VT) underwent an imaging study to assess the presence of an underlying myocardial substrate.
Methods
The data that support the findings of this study are available from the corresponding author on reasonable request.
Athletes
The study protocol was approved by the local Ethical Committee, and all subjects (or parents/guardians if subjects were <18 years old) gave written informed consent. Participants were not paid.
Athletes were recruited at the Center of Sports Medicine, Public Health System (Padova, Italy) from September 1, 2015 to December 23, 2016, which was the time period required to enroll the prespecified number of individuals. According to the Italian law, all athletes engaged in competitive sports activity must undergo preparticipation cardiovascular evaluation, including family and personal history, physical examination, resting 12‐lead ECG, and exercise testing. Further examinations are reserved to athletes showing abnormalities at first‐line investigations. Athletes who were considered eligible for competitive sports activity on the basis of preparticipation screening and fulfilled the inclusion criteria were offered participation in the study. Inclusion criteria were the following: (1) age range from 15 to 35 years; (2) engagement in all competitive sports, with the exception of disciplines at low static/low dynamic cardiovascular demand, according to the classification of Mitchell et al13; (3) ≥6 hours of exercise per week; (4) no known structural cardiovascular disease, excluding mild valvular disease or previous myocarditis/pericarditis that was considered clinically healed; (5) no contraindications to contrast‐enhanced cardiac magnetic resonance (CMR); and (6) availability to undergo imaging investigations in addition to electrocardiographic monitoring, if required.
At the time of enrollment, the following information was collected: the type of sport (in case of multiple disciplines, that accounting for the majority of training hours was considered), hours of training per week, cumulative years of practice of competitive sport activity, previous symptoms, and family history of premature sudden death (<50 years old in women and <40 years old in men), coronary artery disease, or cardiomyopathy in first‐degree relatives.
Sedentary Individuals
A group of sedentary subjects, who were engaged in ≤2 hours per week of leisure‐time physical exercise and matched to athletes for sex and age class (≤25 and >25 years old) with a 1:2 ratio, was recruited with advertisement in the local press and social media during the same time period. Except for sports activity, inclusion criteria were the same of athletes: in particular, sedentary individuals had to have a negative history of cardiac disease (including arrhythmias). Information about previous symptoms and family history was collected at the time of enrollment.
Ambulatory Electrocardiographic Monitoring
Twelve‐lead 24‐hour ambulatory electrocardiographic monitoring (H12+; Mortara Instruments Inc) was performed in all study participants. Athletes were asked to perform a training session of at least 30 to 60 minutes during the ambulatory electrocardiographic recording. Sedentary individuals were instructed to perform their habitual daily life activities. Recordings were reviewed by 2 cardiologists (A.Z., A.C.): in particular, every single ectopic beat, pause, or artifact and all families of normal beats were confirmed manually. Recordings with >2 hours of artifacts or missing signals were considered inadequate and repeated.
The VA burden was graded as follows: 1 indicates no PVBs; 2, 1 to 10 isolated PVBs and no complex VAs; 3, 11 to 100 isolated PVBs and no complex VAs; 4, >100 isolated PVBs and no complex VAs; 5, ≥1 couplet or triplet; and 6, nonsustained VT. The PVB morphologic features were classified as left bundle branch block like (LBBB) if the ectopic QRS complex was predominantly negative in lead V1 or right bundle branch block like (RBBB) if the ectopic QRS complex was predominantly positive or isodiphasic in lead V1. PVBs with a LBBB/inferior axis (positive QRS complex in aVF and negative QRS complex in V1 and aVL) configuration were considered of ventricular outflow tract origin; PVBs with a QRS duration ≤130 ms resembling a typical RBBB/left or right axis deviation were considered of fascicular origin.12, 14 PVBs with ≥2 morphologic features that accounted for ≥10% of all PVBs were classified as polymorphic.
Imaging Evaluation
Echocardiography was performed in all athletes with >10 isolated PVBs/hour or ≥1 complex VA. According to previous studies indicating that cardiovascular diseases are more commonly observed in athletes with frequent (>500 PVBs/24 hours), exercise‐induced, or complex VAs, further imaging study by CMR was reserved to athletes fulfilling these arrhythmic criteria, in addition to those with abnormal echocardiographic findings.5, 15, 16, 17 Echocardiography and CMR were also performed in a control group of athletes with no or rare (≤10) isolated PVBs matched with a 1:1 ratio for sex, age class, and type of sport, who volunteered to participate.
Echocardiography
Using a standardized protocol, all examinations were performed by 3 cardiologists with experience in research echocardiography (L.P.B., D.M., and A.N.) using a commercially available Vivid E9 ultrasound machine (GE Vingmed Ultrasound AS, Horten, Norway) equipped with a M5S probe. All patients were examined in the left lateral position using grayscale second‐harmonic 2‐dimensional imaging, with the adjustment of image contrast, frequency, depth, and sector size for adequate frame rate and optimal left ventricular (LV) border visualization. All echocardiographic measurements were obtained and recorded according with current recommendations.18
Measurements of diastolic and systolic dimensions of the LV, right ventricle, left atrium, and right atrium and measure of the aorta were obtained from parasternal long‐axis view and standard apical views. Care was taken to avoid LV foreshortening in both apical views, and images were recorded during breath holding to minimize respiratory movements. The apical 4‐chamber view was used for the measurement of LV myocardial function with color tissue Doppler, and sample volumes were located at the lateral and septal mitral annulus for pulsed tissue Doppler measurements of the systolic and diastolic velocities. All tracings and recordings contained at least 3 cardiac cycles to allow averaging of measurements.
Cardiac magnetic resonance
CMR was performed with 1.5‐T systems (Magnetom Avanto [Siemens Medical Solutions, Germany]; Achieva [Philips North American Corporation]) using dedicated software, a phased‐array surface receiver coil, and vectocardiogram trigger. According to the protocols recommended by the Society for Cardiovascular Magnetic Resonance,19 we acquired steady‐state free precession sequence (true FISP) cine images in sequential short‐axis views (slice thickness, 6 mm; gap, 0 mm), standard 4‐, 3‐, and 2‐chamber views, a long‐axis view of the right ventricle, and a dedicated view for the right ventricular outflow tract. LV volume, wall thickness, and systolic function were calculated from the short‐axis cine images, excluding papillary muscles from the myocardium.
For detection of myocardial edema, T2 triple inversion recovery sequences were applied before contrast administration and acquired at the same location as cine images. The presence of myocardial edema on T2‐weighted images was diagnosed using a dark blood preparation on sequences to reduce artifacts and surface coil reception field inhomogeneity; moreover, to avoid misdiagnosis of subendocardial edema resulting from “slow‐flow artifacts,” T2‐weighted images were compared with the corresponding cine images, using a side‐by‐side approach on the same cardiac phase. Semiquantitative analysis was performed using regional T2‐ratio method, and myocardial edema was diagnosed in the presence of an increased signal intensity (ie, >2 SDs above skeletal muscle), as recommended.19 All edematous regions were confirmed in 2 perpendicular views. Ten minutes after IV administration of contrast agent (gadobenate dimeglumine; Multihance; Bracco; 0.2 mmol/kg of body weight), 2‐dimensional segmented fast low‐angle shot inversion recovery sequences were acquired in the same views of cine images, covering the entire ventricles. Inversion times were adjusted to null normal myocardium using the Look‐Locker sequence, and images were repeated in 2 separate phase‐encoding directions to exclude artifacts. Myocardial late gadolinium enhancement (LGE) was assessed with an automated thresholding and considered present if signal intensity was >3 SDs above remote myocardium in 2 orthogonal views.19 Isolated junctional LGE was not considered because it is a common and nonpathologic finding in athletes.20
The images of CMR were evaluated and analyzed independently by 2 observers (M.D.L., M.P.M.), blinded to clinical findings, ECG features, and coronary angiography findings, with the use of dedicated software (CMR42; Circle Cardiovascular Imaging Inc). In case of disagreement, a third observer was consulted.
Statistical Analysis
Continuous and categorical variables were expressed as median (with 25th–75th percentiles) and number (percentage), respectively. Categorical variables were compared using the χ2 or Fisher exact test, as appropriate. Continuous data were compared using the Mann‐Whitney U test because normality could not be assumed for any variable. Unweighted and weighted (for age class and sex) odds ratios (with 95% confidence intervals) were calculated using the Cochran‐Mantel‐Haenszel method for the primary end point analysis. Instead, matching factors were not accounted for in the statistical analysis when athletes with and without VA who underwent imaging investigation were compared, because of the small sample size. P<0.05 was considered statistically significant. Data were analyzed with SPSS, version 23 (IBM). Study data were collected and managed using REDCap electronic data capture tools, hosted at University of Padova (Padova, Italy).
Results
Characteristics of the Study Cohorts
During the 18‐month study period, 1377 young athletes (aged 15 to 35 years) underwent preparticipation screening. Of those athletes, 125 did not meet the enrollment criteria because they were engaged in sports at low cardiovascular demand (N=90), had cognitive deficits (N=19), or were considered not eligible for competitive sports activity because of a cardiovascular disease (N=16). Of the remaining 1268 young athletes, 288 (median age, 21.00 [25th–75th percentile, 19.00–25.00] years; 71% males) agreed to participate in the study. Athletes who did and those who did not agree to participate in the study did not differ in terms of age (21 versus 20 years old; P=0.34), male sex (71% versus 69%; P=0.55), and type of sports activity (endurance versus nonendurance) (56% versus 52%; P=0.25). The athletic cohort practiced a variety of sports, including soccer (N=72 [25%]), volleyball (N=43 [15%]), running (N=36 [13%]), rugby (N=17 [6%]), basketball (N=14 [5%]), cycling (N=14 [5%]), triathlon (N=11 [4%]), swimming (N=11 [4%]), martial arts (N=10 [3%]), tennis (N=10 [3%]), and other sports (N=50 [18%]). The control group included 144 subjects (median age, 22.00 [25th–75th percentile, 20.00–24.00] years; 71% males). The family history was positive for coronary artery disease, premature sudden death, or cardiomyopathies in 75 athletes (26%) and 27 sedentary individuals (19%; P=0.09). Palpitations were reported by 7 athletes (2%) and 12 sedentary individuals (8%; P=0.005). A history of mitral valve prolapse with no or mild regurgitation was reported by 4 athletes (1%) and 2 sedentary individuals (1%; P=1.0), whereas no athlete or control subject reported previous myocarditis or pericarditis.
Ambulatory Electrocardiographic Monitoring (24 Hour and 12 Lead)
Ventricular arrhythmic burden in athletes and sedentary individuals
Table 1 shows the grading of VAs in young athletes and sedentary individuals. At least 1 PVB was recorded by 24‐hour 12‐lead ambulatory electrocardiographic monitoring in 170 athletes (59%) and 57 sedentary individuals (40%; P<0.001), and at least 1 complex VA (couplet, triplet, or nonsustained VT) was recorded in 16 athletes (6%) and 8 sedentary individuals (6%; P=1.0). Compared with sedentary individuals, athletes significantly more often showed rare (≤10) and isolated PVBs (142 [49%] versus 41 [28%]; P<0.001), whereas there was no difference in the prevalence of more frequent or complex VAs.
Table 1.
Comparison Between the Burden of VAs at 24‐Hour Ambulatory Electrocardiographic Monitoring in Athletes and Sedentary Individuals
| Variable | Athletes (N=288) | Sedentary Individuals (N=144) | P Value |
|---|---|---|---|
| No PVBs | 118 (41) | 88 (61) | <0.001 |
| 1–10 Isolated PVBs | 142 (49) | 41 (28) | <0.001 |
| 11–100 Isolated PVBs | 2 (1) | 3 (2) | 0.34 |
| >100 Isolated PVBs | 10 (3) | 5 (3) | 1.0 |
| ≥1 Couplet or triplet | 10 (3) | 5 (3) | 1.0 |
| ≥1 Run (≥4 beats) | 6 (2) | 3 (2) | 1.0 |
Data are given as number (percentage). PVB indicates premature ventricular beat; VA, ventricular arrhythmia.
The proportion of athletes and sedentary individuals with >10 isolated PVBs/hour or ≥1 complex VA was similar (28 [10%] versus 16 [11%] [P=0.62]; unweighted odds ratio, 0.85 [95% C.I. 0.44–1.62] [P=0.62]; weighted odds ratio, 0.86 [95% C.I. 0.44–1.64] [P=0.68]). The prevalence of athletes and sedentary individuals with >10 isolated PVBs/hour or ≥1 complex VA in each age and sex subgroup is provided in Table S1.
The burden of VAs did not differ between subjects with and without a history of palpitations, both in the athletic and sedentary control cohorts (Table S2).
Impact of age, sex, and type and intensity of physical exercise on the arrhythmic burden
Athletes with >10 isolated PVBs/hour or ≥1 complex VA were older (median age, 26 versus 20 years; P=0.008) but did not differ with regard to sex, type of sport, hours of training, and years of sports activity compared with other athletes (Table 2). Characteristics of VA according to sex in the 28 athletes with >10 isolated PVBs/hour or ≥1 complex VA are shown in Table S3.
Table 2.
Comparison Between Athletes With and Without >10 Isolated PVBs or ≥1 Complex VA at 24‐Hour Ambulatory Electrocardiographic Monitoring With Regard to Age, Sex, and Sports Activity
| Variable | Athletes With >10 Isolated PVBs or ≥1 Complex VA (N=28) | Athletes With ≤10 Isolated PVBs and No Complex VA (N=260) | P Value |
|---|---|---|---|
| Male sex | 21 (75) | 181 (70) | 0.55 |
| Age, y | 26 (19–31) | 20 (17–25) | 0.008 |
| Training time, h/wk | 7 (6–9) | 8 (6–10) | 0.12 |
| Training time, h/y | 330 (280–415) | 350 (310–455) | 0.16 |
| Sports practice, y | 7 (3–10) | 8 (4–11) | 0.63 |
| Highly dynamic (endurance) sporta | 19 (68) | 142 (55) | 0.18 |
| Mean heart rate, bpm | 70 (63–75) | 72 (67–76) | 0.20 |
| Minimum heart rate, bpm | 39 (35–43) | 40 (37–43) | 0.33 |
| Maximum heart rate, bpm | 176 (166–182) | 170 (158–184) | 0.53 |
| Positive family historyb | 3 (11) | 72 (28) | 0.07 |
| Previous palpitations | 1 (4) | 6 (2) | 0.51 |
Data are given as number (percentage) or median (25th–75th percentile). BPM indicates beats per minute; PVB, premature ventricular beat; VA, ventricular arrhythmia.
According to the classification of Mitchell et al.15
For premature (<40 years old in men and <50 years old in women) sudden death, inherited cardiomyopathies, or coronary artery disease.
Morphologic features of VAs
Among the 142 athletes with 1 to 10 isolated PVBs (median, 2 [25th–75th percentile, 1–3] PVBs), the following predominant PVB morphologic features were identified: outflow tract (LBBB/inferior axis) in 54 athletes (38%), fascicular (RBBB and QRS duration ≤130 ms) in 22 athletes (15%); LBBB/intermediate or superior axis in 31 athletes (22%); and RBBB and QRS duration >130 ms in 15 athletes (11%). The remaining 20 athletes (14%) had polymorphic PVBs.
Of the 28 athletes with >10 isolated PVBs or ≥1 complex VA (median, 63 [25th–75th percentile, 4–400] PVBs), 11 (39%) showed outflow tract PVBs and 4 (14%) showed fascicular PVBs; the remaining 13 athletes showed VAs with a RBBB configuration and QRS duration >130 ms (N=5 [19%]), LBBB configuration and intermediate or superior axis (N=6 [21%]) or polymorphic VAs (N=2 [7%]). Athletes with outflow tract or fascicular arrhythmias showed a higher number of PVBs (median, 200 [25th–75th percentile, 4–580] versus 12 [25th–75th percentile, 4–141]; P<0.001) but a lower prevalence of ≥1 complex VA (3/15 [20%] versus 13/13 [100%]; P<0.001) compared with athletes with other arrhythmia morphologic features. Six athletes showed >500 PVBs/24 hours, associated with ≥1 complex VA in 4: 5 with an outflow tract morphologic feature and 1 with a fascicular morphologic feature.
Short runs of nonsustained VT (range, 4–6 beats) occurred in 6 athletes; the morphologic features of the QRS during VT were RBBB in 3 (including 1 that occurred during sports activity) and LBBB in 3 (including 2 that occurred during sports activity).
Imaging Studies
Echocardiography was performed in all 28 athletes with >10 isolated PVBs or ≥1 complex VA and in 28 control athletes matched for age, sex, and sport discipline with no or ≤10 isolated PVBs (Table 3). No athletes (with or without >10 isolated PVBs or ≥1 complex VA) had moderate or severe dilatation, either atrial or ventricular, systolic ventricular dysfunction, abnormal LV filling pattern, or relevant valvular and aortic disease.
Table 3.
Echocardiographic Findings in Athletes With >10 Isolated PVBs or ≥1 Complex VA at 24‐Hour Ambulatory Electrocardiographic Monitoring and Control Athletes
| Variable | Athletes With >10 Isolated PVBs or ≥1 Complex VA (N=28) | Control Athletes With ≤10 Isolated PVBs (N=28) | P Value |
|---|---|---|---|
| LA volume, mL/m2 | 33 (28–37) | 30 (26–33) | 0.47 |
| LA volume ≥36 mL/m2 | 8 (28.6) | 5 (17.9) | 0.34 |
| RA volume, mL/m2 | 29 (22–38) | 25 (22–35) | 0.29 |
| RA volume ≥36 mL/m2 | 9 (32.1) | 6 (21.4) | 0.37 |
| LV septal thickness, mm | 9 (8–9) | 8 (8–9) | 0.47 |
| LV end‐diastolic diameter, mm | 49 (46–52) | 48 (41–54) | 0.73 |
| LV posterior wall thickness, mm | 9 (8–10) | 9 (8–9) | 0.62 |
| LV EF, % | 63 (59–65) | 62 (61–64) | 0.73 |
| LV EF <53% | 0 | 0 | ··· |
| LV EDV, mL/m2 | 67 (55–73) | 62 (56–74) | 0.36 |
| LV EDV >75 mL/m2 | 6 (21.4) | 7 (25.0) | 0.75 |
| Regional LV WMA | 0 | 0 | ··· |
| Abnormal LV filling pattern | 0 | 0 | ··· |
| RV FAC, % | 39 (38–46) | 42 (41–48) | 0.36 |
| RV FAC <35% | 0 | 0 | ··· |
| RV EDA, cm2 | 12.3 (11.3–13.2) | 11.7 (10.8–12.6) | 0.68 |
| RV EDA >12 cm2/m2 | 8 (28.6) | 6 (21.4) | 0.5 |
| Regional RV WMA | 0 | 0 | ··· |
| Mild ascending aorta dilation | 1 (3.6) | 0 | 1.0 |
Data are given as median (25th–75th percentile) or number (percentage). EDA indicates end‐diastolic area; EDV, end‐diastolic volume; EF, ejection fraction; FAC, fractional area change; LA, left atrium; LV, left ventricular; PVB, premature ventricular beat; RA, right atrium; RV, right ventricular; VA, ventricular arrhythmia; WMA, wall motion abnormality.
By study design, additional CMR was performed in 17 athletes because of frequent (>500 PVBs/24 hours), exercise‐induced, or complex VAs and in 17 control athletes with no or ≤10 isolated PVBs matched for age, sex, and type of sport discipline (Table 4). Among the 17 athletes with VA, 4 showed PVBs with a LBBB/inferior axis morphologic feature (suggesting outflow tract origin), 6 showed a LBBB/intermediate or superior axis configuration (suggesting right ventricular free wall origin), 5 showed an RBBB configuration and QRS duration >130 ms (suggesting LV free wall origin), and 2 showed polymorphic (both RBBB and LBBB) arrhythmias.
Table 4.
CMR Findings in Athletes With Frequent PVBs (>500/24 Hours), PVBs Induced by Exercise or Complex VA, at 24‐Hour Ambulatory Electrocardiographic Monitoring and Control Athletes
| Variable | Athletes With Frequent PVBs (>500/24 Hours), PVBs Induced by Exercise or Complex VA (N=17) | Control Athletes With <10 Isolated PVBs (N=17) | P Value |
|---|---|---|---|
| LV EDV, mL/mq | 85 (76–94) | 87 (78–97) | 0.73 |
| LV EF, % | 61 (56–68) | 58 (57–64) | 0.43 |
| Maximum LV wall thickness, mm | 9 (8–10) | 9 (8–9) | 0.84 |
| Regional LV WMA | 0 | 0 | ··· |
| RV EDV, mL/mq | 86 (74–93) | 82 (76–95) | 0.64 |
| RV EF, % | 55 (51–63) | 59 (57–67) | 0.38 |
| Regional RV WMA | 0 | 0 | ··· |
| LV myocardial edema | 0 | 0 | ··· |
| LV LGEa | 3 (18) | 0 | 0.23 |
| RV LGE | 0 | 0 | ··· |
| Pericardial LGE | 0 | 0 | ··· |
Data are given as median (25th–75th percentile) or number (percentage). CMR indicates cardiac magnetic resonance; EDV, end‐diastolic volume; EF, ejection fraction; LGE, late gadolinium enhancement; LV, left ventricular; PVB, premature ventricular beat; RV, right ventricular; VA, ventricular arrhythmia; WMA, wall motion abnormality.
Excluding isolated junctional LGE.
All athletes (with or without VAs) exhibited normal wall thickness, cavity size, and systolic ventricular function. Isolated LV LGE with a nonischemic distribution (ie, midmyocardial or subepicardial) was distinctively found in 3 athletes with VAs. The clinical characteristics of the 3 athletes with LV LGE are summarized in Table 5 and shown in Figures 1, 2 through 3. Of 7 athletes, 3 (43%) with RBBB or polymorphic VAs showed LV LGE compared with 0 of the 10 athletes with LBBB morphologic feature of VA (P=0.05). In all 3 athletes with LV LGE, the ectopic QRS morphologic feature and axis were concordant with the regional distribution of the myocardial lesion.
Table 5.
Characteristics of Athletes With VAs and Late Enhancement at CMR
| Age, y | Sex | Sport | Family and Personal History | 24‐h Ambulatory Electrocardiographic Monitoring | LGE Pattern and Distribution | Figures |
|---|---|---|---|---|---|---|
| 17 | M | Running | Negative | 49 Isolated PVBs and 2 couplets with a RBBB configuration predominantly during exercise | Subepicardial/midmyocardial stria involving the basal portion of the inferolateral left ventricular wall | 1 |
| 31 | M | Basketball | Negative | 1 Couplet with a LBBB/superior axis configuration at rest and 1 run of nonsustained ventricular tachycardia (6 beats) with a RBBB morphologic feature during exercise | Midmyocardial stria involving the basal portion of the inferolateral left ventricular wall | 2 |
| 24 | M | Rugby | Negative | 27 Isolated PVBs with a RBBB configuration during exercise | Midmyocardial stria involving the basal inferior wall | 3 |
CMR indicates cardiac magnetic resonance; LBBB, left bundle branch block; LGE, late gadolinium enhancement; M, male; PVB, premature ventricular beat; RBBB, right bundle branch block; VA, ventricular arrhythmia.
Figure 1.
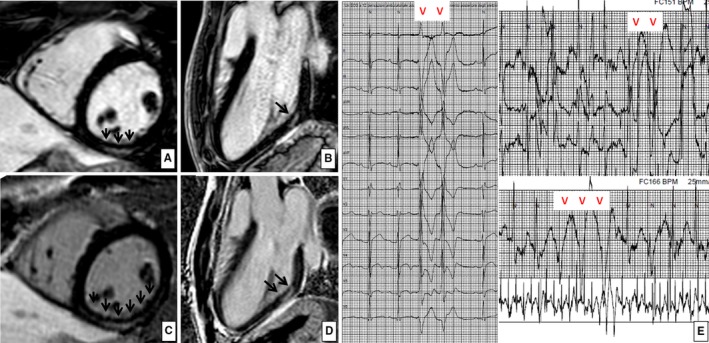
Nonischemic left ventricular late enhancement in a 17‐year‐old runner. Postcontrast cardiac magnetic resonance, short‐axis, and 3‐chamber long‐axis views at baseline (A and B) and after 6 months of follow‐up (C and D), demonstrating a “stria” of late gadolinium enhancement with a subepicardial/midmyocardial distribution (suggesting nonischemic origin) involving the basal portion of the inferolateral left ventricular wall. E, The ambulatory 24‐hour electrocardiographic monitoring demonstrated exercise‐induced complex ventricular arrhythmias with a right bundle branch block configuration, consistent with a left ventricular origin, both at baseline and at follow‐up.
Figure 2.
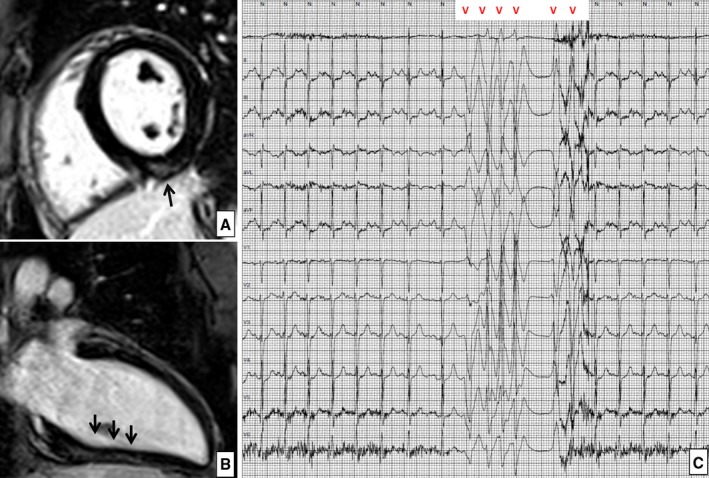
Left ventricular late enhancement in a 30‐year‐old basketball player. Postcontrast cardiac magnetic resonance short‐axis (A) and 2‐chamber long‐axis (B) views demonstrating an intramural “stria” of late gadolinium enhancement involving the basal and mid portion of the inferior left ventricular wall. C, The ambulatory 24‐hour electrocardiographic monitoring demonstrated an exercise‐induced run of nonsustained ventricular tachycardia with a left bundle branch block configuration, consistent with a left ventricular origin.
Figure 3.
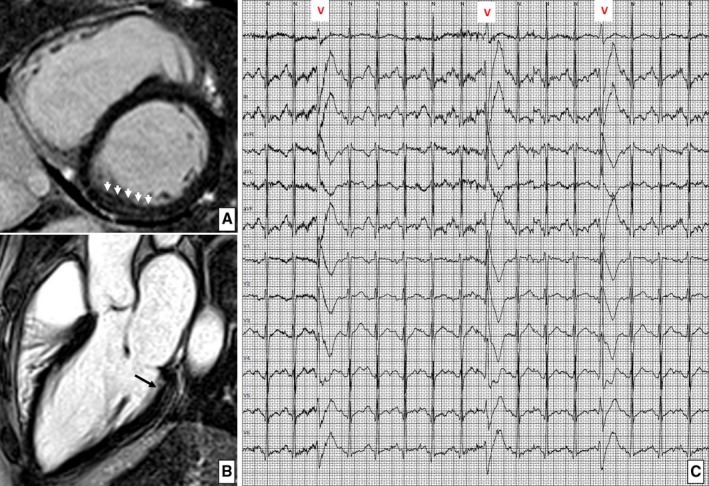
Left ventricular late enhancement in a 24‐year‐old rugby player. Postcontrast cardiac magnetic resonance short‐axis (A) and 3‐chamber long‐axis (B) views demonstrating an intramural “stria” of late gadolinium enhancement involving the basal portion of the inferior left ventricular wall. C, The ambulatory 24‐hour electrocardiographic monitoring demonstrated exercise‐induced isolated premature ventricular beats with a right bundle branch block configuration, consistent with a left ventricular origin.
The 3 athletes with complex and/or exercised‐induced VAs and underlying LV LGE were prescribed β blockers and advised to limit sports activity. Over a period of follow‐up of 18, 15, and 9 months, respectively, the outcome was uneventful in all.
Discussion
Our study evaluated, for the first time, the VA burden by 12‐lead 24‐hour ambulatory electrocardiographic monitoring (including a training session) in a large sample of young athletes who were considered eligible for competitive sports activity at preparticipation screening and in a group of sedentary individuals. The aim was to assess whether VAs were more prevalent in young competitive athletes than sedentary individuals; showed a relation to the intensity of physical training, type of sport, and years of activity; and associated with an underlying pathologic myocardial substrate. The main findings were as follows: (1) the proportion of athletes showing >10 isolated PVBs or ≥1 complex VA was low (10%) and similar to that of sedentary individuals; (2) VAs were unrelated to the intensity of physical training, years of sports activity, type of sport (endurance versus nonendurance), and degree of LV remodeling, as evidenced by echocardiography; (3) although echocardiographic examination did not show any pathologic substrate, contrast‐enhanced CMR unmasked an underlying nonischemic LV LGE, suggesting myocardial fibrosis in a subset of apparently healthy athletes with RBBB‐like complex and/or exercise‐induced VAs (Figure 4).
Figure 4.
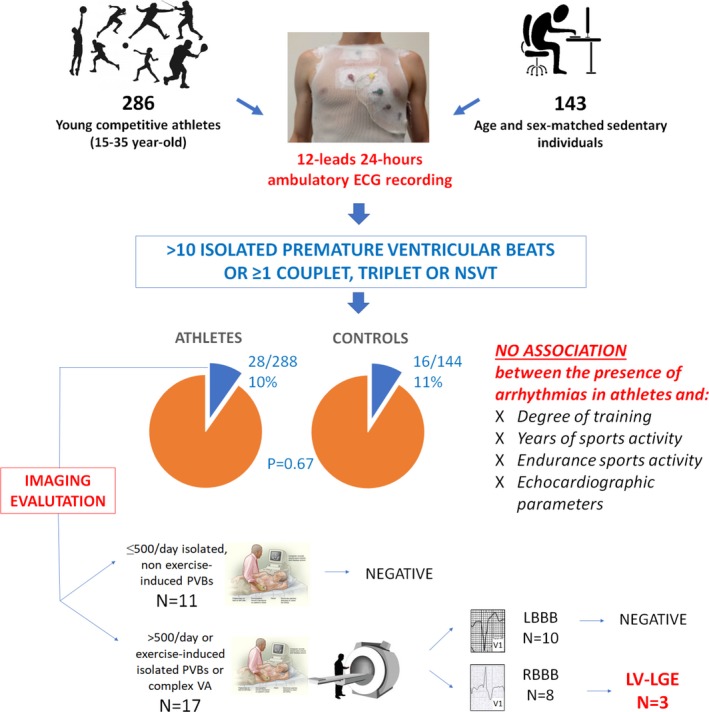
Summary of the study methods and main findings. LBBB indicates left bundle branch block; LGE, late gadolinium enhancement; LV, left ventricular; NSVT, nonsustained ventricular tachycardia; PVB, premature ventricular beat; RBBB, right bundle branch block; VA, ventricular arrhythmia.
Prevalence and Determinants of VAs in Athletes
There are conflicting results of previous studies addressing the proarrhythmic effect of sports activity with regular exercise and competition. Biffi et al reported on a group of 355 elite athletes who underwent 24‐hour ambulatory electrocardiographic monitoring because of PVBs at baseline ECG or palpitations.5 This group represented 2.3% of all athletes who were evaluated at the Institute of Sports Science of the Italian National Olympic Committee. Among them, 71 athletes (corresponding to 0.4% of all screened athletes) exhibited >2000 PVBs or nonsustained VT. In this study, most athletes with VAs had no evidence of underlying structural abnormalities, the arrhythmias tended to decrease after detraining, and the follow‐up was uneventful: all these findings are consistent with the concept that VAs may be considered a feature of the “athlete's heart.”4, 5 Moreover, Palatini et al compared 40 endurance athletes with 40 sedentary individuals and found that the prevalence of complex VAs at ambulatory electrocardiographic monitoring was higher in trained individuals.10 Other studies comparing the prevalence of VA at 24‐hour ambulatory electrocardiographic monitoring in healthy athletes versus sedentary individuals disproved the concept of a proarrhythmic effect of sports activity by demonstrating that only a minority of athletes exhibited frequent or complex VAs with a prevalence that did not differ with that of their sedentary counterpart6, 7, 8, 9, 11 (Table S4). In addition, Delise et al reported that the proportion of athletes with VAs who showed a reduction in the arrhythmic burden during follow‐up was similar in a group undergoing detraining and in a group who continued training.21
At variance with the present study, previous investigations performed in the 1980s and 1990s enrolled smaller cohort of athletes, lacked imaging evaluation (with the exception of one study, which reported echocardiography findings), and did not assess the determinants of VAs. Most important, our study was the first to use a 12‐lead ambulatory electrocardiographic monitoring system. The results of our study confirmed and extended previous observations against the proarrhythmic effect of regular training in young athletes by showing that the proportion of athletes showing >10 isolated PVBs or ≥1 complex VA (ie, couplet, triplet, or nonsustained VT) was low and similar to sedentary individuals (10% versus 11%) and that the VA burden in the athletes group was unrelated to the duration and intensity of sports activity as well as to the degree of training‐induced LV remodeling at echocardiography. This latter finding is an agreement with a previous study on 175 top‐level athletes without cardiovascular abnormalities showing that the VA burden was not associated with the degree of adaptive LV hypertrophy.22
Interestingly, we found that the prevalence of rare PVBs (<10/day) was significantly higher in athletes than in sedentary individuals. Whether this finding represents an early sign of cardiac adaption to the strains of athletic workouts needs to be confirmed by further studies focused on older athletic population, given the relationship between increasing age and worsening arrhythmic burden observed in the present investigation.
Morphologic Features of VAs in Athletes
ECG is an important screening test for identification of leading causes of SCD in young competitive athletes. However, some at‐risk arrhythmogenic diseases, such as focal myocarditis, early/minor cardiomyopathy, and segmental nonischemic LV scar, may be associated with a normal ECG.1, 20, 23, 24 Our study is unique in that ambulatory electrocardiographic monitoring used a 12‐lead configuration system: that offers the potential to increase the screening sensitivity for identification of these ECG‐undetectable concealed arrhythmic substrates by showing not only the presence but also the morphologic features of VAs. This latter characteristic provides important information about their site of origin, mechanism, and possible underlying substrate.12
Our results are in keeping with those of previous studies suggesting that frequent isolated PVBs in young athletes most commonly show infundibular or fascicular morphologic features, which denote benign arrhythmias caused by automatic foci in the absence of a pathologic myocardial substrate that are usually suppressed by exercise.12, 14 Indeed, using 12‐lead electrocardiographic monitoring, we found that all athletes with >500 PVBs/24 hours had either an infundibular or fascicular morphologic feature. All athletes with these VA morphologic features had normal imaging evaluation, including echocardiography and CMR. On the other hand, 13 of our 16 athletes with complex VA (couplets, triplets, or nonsustained VT) exhibited an ectopic QRS morphologic feature other than infundibular or fascicular. These VA variants are less common and possibly associated with an underlying disease.12
Arrhythmic Substrates
CMR is increasingly used in the evaluation of athletes with apparently idiopathic VAs because it may increase the sensitivity of identifying concealed arrhythmogenic substrates, thanks to its tissue characterization ability.14 According to a prespecified study protocol, we reserved CMR in case of frequent, complex, or exercise‐induced arrhythmias, which are a recognized subgroup with high risk of underlying cardiovascular disease.5, 15, 16, 17 In 3 of these athletes, postcontrast CMR sequences allowed identification of LV LGE with a subepicardial/midmyocardial (ie, nonischemic) pattern, which was not associated with wall motion abnormalities detectable by either echocardiography or cine‐CMR because of its segmental distribution confined to the outer layer of LV musculature. All 3 athletes exhibited arrhythmias with a RBBB configuration, which was concordant with the LV location of LGE, whereas CMR was negative in all athletes with LBBB arrhythmias and control athletes. These findings are in agreement with previous studies showing that a RBBB‐like morphologic feature of VA, but not LBBB, predicts the presence of an underlying LV scar.20, 25 Moreover, in athletes showing VA with a LBBB morphologic feature, no abnormalities of the right ventricle were evidenced by both echocardiography and CMR using dedicated morphofunctional and postcontrast views.
The clinical relevance of this finding is that nonischemic LV scar is an increasingly reported cause of life‐threatening VAs and SCD in the athletes, often undetected by routine electrocardiographic and echocardiographic screening.1, 20 However, this perspective is based on previous observations on selected athletes who were referred to tertiary center or experienced cardiac arrest. The risk of SCD during sports activity and recommendations for sports eligibility of the low (but not negligible, ≈1%) proportion of our asymptomatic athletes with VA and LV LGE remain to be established.
Study Limitations
The main limitation is that we enrolled a cohort of volunteers rather than a consecutive series of athletes undergoing preparticipation screening. Although baseline characteristics of those who did and did not agree to participate were similar, we cannot exclude a selection bias. Because sedentary individuals were also recruited on a voluntary basis, they may not be representative of the general sedentary population. Moreover, the study included Italian white athletes and, thus, the study results may not be generalized to athletes of other countries and ethnicities. Recruitment of a larger group of athletes than sedentary individuals, which was determined by logistical reasons, may have influenced the matching efficiency. Although echocardiography and CMR studies were not performed systematically in the athletic population, the threshold for imaging investigation in athletes with VAs was low, and a comparable group of control athletes with no VAs underwent imaging study. On the other hand, the correlation between VA characteristics and underlying myocardial substrate in the population of sedentary individuals was beyond the scope of the study.
Conclusions
At variance with a traditional perspective that VAs are a consequence of the structural and neuroautonomic remodeling of the athlete's heart, our study showed that the prevalence of VAs in young competitive athletes is low, similar to that of sedentary individuals and unrelated to the type and intensity of sports activity. Similar to the nonathletic general population, VAs in the athletes may be associated with an underlying myocardial substrate potentially at risk of SCD. Current guidelines recommend that athletes with VAs should undergo further clinical and imaging workup to exclude an underlying pathologic substrate on the basis of a high number and complexity of PVBs.2, 3 Our results suggest that a multiparametric approach considering VA morphologic features, complexity, and relation to exercise, rather than the PVBs count alone, is useful for differentiating between benign and other potentially pathologic variants. In particular, demonstration of complex or exercise‐induced PVBs with a RBBB morphologic feature should enable accurate clinical evaluation, including CMR, to exclude a concealed LV myocardial scar. Whether the ambulatory electrocardiographic monitoring should become systematically part of preparticipation evaluation of athletes goes beyond the scope of our pathophysiological study; however, our findings suggest that when ambulatory electrocardiographic monitoring is performed in athletes with VAs, it should have a 12‐lead configuration and include an exercise session.
Sources of Funding
The study was funded by the research grant budget for integrated departmental research 2016 (University of Padova, Padova, Italy) and by the Target Projects RF‐2013‐02356762 grant of the Italian Ministry of Health.
Disclosures
None.
Supporting information
Table S1. Prevalence of Athletes and Sedentary Controls Showing >10 Isolated Premature Ventricular Beats or ≥1 Complex Ventricular Arrhythmias According to Sex and Age Class
Table S2. Comparison Between the Burden of Ventricular Arrhythmias at 24‐Hours 12‐Leads Ambulatory ECG Monitoring in Athletes and Controls With and Without a History of Palpitations
Table S3. Ventricular Arrhythmias Grading and Prevalent Morphology in the Subgroup of 28 Athletes With >10 Isolated PVBs/Hour or ≥1 Complex VA According to Sex
Table S4. Summary of Previous Studies Comparing the Burden of Ventricular Arrhythmias in Athletes and Sedentary Controls
Acknowledgments
We thank the Policlinico Abano Terme (Padova, Italy) for unrestricted support to the study and the staff of the Center for Sports Medicine, Ospedale Dei Colli, AULSS 6 (Padova, Italy) for help in recruiting the athletes.
(J Am Heart Assoc. 2018;7:e009171 DOI: 10.1161/JAHA.118.009171.)
References
- 1. Corrado D, Zorzi A. Sudden death in athletes. Int J Cardiol. 2017;237:67–70. [DOI] [PubMed] [Google Scholar]
- 2. Corrado D, Pelliccia A, Heidbuchel H, Sharma S, Link M, Basso C, Biffi A, Buja G, Delise P, Gussac I, Anastasakis A, Borjesson M, Bjornstad HH, Carre F, Deligiannis A, Dugmore D, Fagard R, Hoogsteen J, Mellwig KP, Panhuyzen‐Goedkoop N, Solberg E, Vanhees L, Drezner J, Estes NA III, Iliceto S, Maron BJ, Peidro R, Schwartz PJ, Stein R, Thiene G, Zeppilli P, McKenna WJ. Recommendations for interpretation of 12‐lead electrocardiogram in the athlete. Eur Heart J. 2010;31:243–259. [DOI] [PubMed] [Google Scholar]
- 3. Sharma S, Drezner JA, Baggish A, Papadakis M, Wilson MG, Prutkin JM, La Gerche A, Ackerman MJ, Borjesson M, Salerno JC, Asif IM, Owens DS, Chung EH, Emery MS, Froelicher VF, Heidbuchel H, Adamuz C, Asplund CA, Cohen G, Harmon KG, Marek JC, Molossi S, Niebauer J, Pelto HF, Perez MV, Riding NR, Saarel T, Schmied CM, Shipon DM, Stein R, Vetter VL, Pelliccia A, Corrado D. International recommendations for electrocardiographic interpretation in athletes. J Am Coll Cardiol. 2017;69:1057–1075. [DOI] [PubMed] [Google Scholar]
- 4. Biffi A, Maron BJ, Verdile L, Fernando F, Spataro A, Marcello G, Ciardo R, Ammirati F, Colivicchi F, Pelliccia A. Impact of physical deconditioning on ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2004;44:1053–1058. [DOI] [PubMed] [Google Scholar]
- 5. Biffi A, Pelliccia A, Verdile L, Fernando F, Spataro A, Caselli S, Santini M, Maron BJ. Long‐term clinical significance of frequent and complex ventricular tachyarrhythmias in trained athletes. J Am Coll Cardiol. 2002;40:446–452. [DOI] [PubMed] [Google Scholar]
- 6. Viitasalo MT, Kala R, Eisalo A. Ambulatory electrocardiographic recording in endurance athletes. Br Heart J. 1982;47:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Viitasalo MT, Kala R, Eisalo A. Ambulatory electrocardiographic findings in young athletes between 14 and 16 years of age. Eur Heart J. 1984;5:2–6. [DOI] [PubMed] [Google Scholar]
- 8. Talan DA, Bauernfeind RA, Ashley WW, Kanakis C Jr, Rosen KM. Twenty‐four hour continuous ECG recordings in long‐distance runners. Chest. 1982;82:19–24. [DOI] [PubMed] [Google Scholar]
- 9. Pilcher GF, Cook AJ, Johnston BL, Fletcher GF. Twenty‐four‐hour continuous electrocardiography during exercise and free activity in 80 apparently healthy runners. Am J Cardiol. 1983;52:859–861. [DOI] [PubMed] [Google Scholar]
- 10. Palatini P, Maraglino G, Sperti G, Calzavara A, Libardoni M, Pessina AC, Dal Palu C. Prevalence and possible mechanisms of ventricular arrhythmias in athletes. Am Heart J. 1985;110:560–567. [DOI] [PubMed] [Google Scholar]
- 11. Bjornstad H, Storstein L, Meen HD, Hals O. Ambulatory electrocardiographic findings in top athletes, athletic students and control subjects. Cardiology. 1994;84:42–50. [DOI] [PubMed] [Google Scholar]
- 12. D'Ascenzi F, Zorzi A, Alvino F, Bonifazi M, Corrado D, Mondillo S. The prevalence and clinical significance of premature ventricular beats in the athlete. Scand J Med Sci Sports. 2017;27:140–151. [DOI] [PubMed] [Google Scholar]
- 13. Mitchell JH, Haskell WL, Raven PB. Classification of sports. J Am Coll Cardiol. 1994;24:864–866. [DOI] [PubMed] [Google Scholar]
- 14. Luebbert J, Auberson D, Marchlinski F. Premature ventricular complexes in apparently normal hearts. Card Electrophysiol Clin. 2016;8:503–514. [DOI] [PubMed] [Google Scholar]
- 15. Verdile L, Maron BJ, Pelliccia A, Spataro A, Santini M, Biffi A. Clinical significance of exercise‐induced ventricular tachyarrhythmias in trained athletes without cardiovascular abnormalities. Heart Rhythm. 2015;12:78–85. [DOI] [PubMed] [Google Scholar]
- 16. Heidbuchel H, Corrado D, Biffi A, Hoffmann E, Panhuyzen‐Goedkoop N, Hoogsteen J, Delise P, Hoff PI, Pelliccia A. Recommendations for participation in leisure‐time physical activity and competitive sports of patients with arrhythmias and potentially arrhythmogenic conditions, part II: ventricular arrhythmias, channelopathies and implantable defibrillators. Eur J Cardiovasc Prev Rehabil. 2006;13:676–686. [DOI] [PubMed] [Google Scholar]
- 17. Zipes DP, Link MS, Ackerman MJ, Kovacs RJ, Myerburg RJ, Estes NAM III. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 9: arrhythmias and conduction defects: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e315–e325. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 19. Schulz‐Menger J, Bluemke DA, Bremerich J, Flamm SD, Fogel MA, Friedrich MG, Kim RJ, von Knobelsdorff‐Brenkenhoff F, Kramer CM, Pennell DJ, Plein S, Nagel E. Standardized image interpretation and post processing in cardiovascular magnetic resonance: Society for Cardiovascular Magnetic Resonance (SCMR) board of trustees task force on standardized post processing. J Cardiovasc Magn Reson. 2013;15:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zorzi A, Perazzolo Marra M, Rigato I, De Lazzari M, Susana A, Niero A, Pilichou K, Migliore F, Rizzo S, Giorgi B, De Conti G, Sarto P, Serratosa L, Patrizi G, De Maria E, Pelliccia A, Basso C, Schiavon M, Bauce B, Iliceto S, Thiene G, Corrado D. Nonischemic left ventricular scar as a substrate of life‐threatening ventricular arrhythmias and sudden cardiac death in competitive athletes. Circ Arrhythm Electrophysiol. 2016;9:e004229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Delise P, Lanari E, Sitta N, Centa M, Allocca G, Biffi A. Influence of training on the number and complexity of frequent VPBs in healthy athletes. J Cardiovasc Med (Hagerstown). 2011;12:157–161. [DOI] [PubMed] [Google Scholar]
- 22. Biffi A, Maron BJ, Di Giacinto B, Porcacchia P, Verdile L, Fernando F, Spataro A, Culasso F, Casasco M, Pelliccia A. Relation between training‐induced left ventricular hypertrophy and risk for ventricular tachyarrhythmias in elite athletes. Am J Cardiol. 2008;101:1792–1795. [DOI] [PubMed] [Google Scholar]
- 23. Moon JC, Fisher NG, McKenna WJ, Pennell DJ. Detection of apical hypertrophic cardiomyopathy by cardiovascular magnetic resonance in patients with non‐diagnostic echocardiography. Heart. 2004;90:645–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sen‐Chowdhry S, Syrris P, Prasad SK, Hughes SE, Merrifield R, Ward D, Pennell DJ, McKenna WJ. Left‐dominant arrhythmogenic cardiomyopathy: an under‐recognized clinical entity. J Am Coll Cardiol. 2008;52:2175–2187. [DOI] [PubMed] [Google Scholar]
- 25. Oebel S, Dinov B, Arya A, Hilbert S, Sommer P, Bollmann A, Hindricks G, Paetsch I, Jahnke C. ECG morphology of premature ventricular contractions predicts the presence of myocardial fibrotic substrate on cardiac magnetic resonance imaging in patients undergoing ablation. J Cardiovasc Electrophysiol. 2017;28:1316–1323. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Prevalence of Athletes and Sedentary Controls Showing >10 Isolated Premature Ventricular Beats or ≥1 Complex Ventricular Arrhythmias According to Sex and Age Class
Table S2. Comparison Between the Burden of Ventricular Arrhythmias at 24‐Hours 12‐Leads Ambulatory ECG Monitoring in Athletes and Controls With and Without a History of Palpitations
Table S3. Ventricular Arrhythmias Grading and Prevalent Morphology in the Subgroup of 28 Athletes With >10 Isolated PVBs/Hour or ≥1 Complex VA According to Sex
Table S4. Summary of Previous Studies Comparing the Burden of Ventricular Arrhythmias in Athletes and Sedentary Controls


