Abstract
Background
Prior studies have shown a close link between exercise and development of arrhythmogenic right ventricular cardiomyopathy. How much exercise restriction reduces ventricular arrhythmia (VA), how genotype modifies its benefit, and whether it reduces risk sufficiently to defer implantable cardioverter‐defibrillator (ICD) placement in arrhythmogenic right ventricular cardiomyopathy are unknown.
Methods and Results
We interviewed 129 arrhythmogenic right ventricular cardiomyopathy patients (age: 34.0±14.8 years; male: 60%) with ICDs (36% primary prevention) about exercise participation. Exercise change was defined as annual exercise duration and dose in the 3 years before clinical presentation minus that after presentation. The primary outcome was appropriate ICD therapy for VA. During the 5.1 years (interquartile range: 2.7–10.8 years) after presentation, 74% (95/129) patients reduced exercise dose and 85 (66%) patients experienced the primary outcome. In multivariate analyses, top tertile reduction in exercise duration and dose were both associated with less VA (duration: hazard ratio: 0.23 [95% confidence interval, 0.07–0.81]; dose: hazard ratio: 0.14 [95% confidence interval, 0.04–0.44]). Greater reduction in exercise dose conferred greater reduction in VA (P=0.01 for trend). Patients without desmosomal mutations and those with primary‐prevention ICDs benefited more from exercise reduction (P=0.16 and P=0.06 for interaction); however, 58% (18/31) of athletes who reduced exercise dose by >80% still experienced VA.
Conclusions
Exercise restriction should be recommended to all arrhythmogenic right ventricular cardiomyopathy patients with ICDs. Patients who are “gene‐elusive” and those with primary‐prevention devices may particularly benefit. Exercise reduction is unlikely to reduce arrhythmia sufficiently in high‐risk patients to alter decision‐making regarding ICD implantation.
Keywords: arrhythmogenic right ventricular cardiomyopathy, exercise, implantable cardioverter‐defibrillator, ventricular tachycardia
Subject Categories: Arrhythmias, Exercise, Cardiomyopathy
Clinical Perspective
What Is New?
Exercise reduction after clinical presentation is independently associated with lower arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy patients with implantable cardioverter‐defibrillators.
Greater reduction in exercise confers greater reduction in arrhythmic risk.
Patients with negative genetic testing results and those with primary‐prevention implantable cardioverter‐defibrillators may particularly benefit from exercise reduction.
What Are the Clinical Implications?
These data support substantially reducing exercise in all high‐risk arrhythmogenic right ventricular cardiomyopathy patients.
Reducing exercise dose (intensity×duration) is likely a more important goal than just limiting exercise duration in managing arrhythmogenic right ventricular cardiomyopathy patients.
Exercise reduction does not reduce the arrhythmic risk sufficiently in high‐risk arrhythmogenic right ventricular cardiomyopathy patients to alter the decision regarding implantable cardioverter‐defibrillator implantation.
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is an inherited cardiomyopathy characterized by life‐threatening ventricular arrhythmias (VAs), predominant right ventricular dysfunction, and sudden cardiac death.1 Approximately half of ARVC patients have pathogenic variants in genes encoding the cardiac desmosome. Endurance exercise increases penetrance and risk of incident arrhythmias among carriers.2, 3 A history of very high‐intensity aerobic exercise is common in ARVC patients without desmosomal mutations, suggesting a disproportionate role of exercise in the pathogenesis of disease in these “gene‐elusive” patients.4, 5
Strenuous exercise is associated with VAs in ARVC patients.2, 3, 4, 6, 7 Consistent with this, guidelines recommend avoidance of competitive exercise following diagnosis.1 Previous attempts to quantify the effect of exercise restriction on arrhythmic risk in established ARVC patients were limited by small sample size2 and low precision in exercise measurement.6 Unanswered clinical questions include, (1) To what extent reducing exercise alters arrhythmic risk? (2) Do genotype or history of VA predict which patients benefit most from reducing exercise? (3) Can exercise reduction alter risk of VAs sufficiently to affect decision‐making regarding implantable cardioverter‐defibrillator (ICD) placement in some circumstances.
Using data from the Johns Hopkins ARVC registry, we address these questions in a cohort of definite ARVC patients with ICDs, with the overarching goal of providing evidence to help personalize exercise guidelines for ARVC patients.
Methods
The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure.
Patient Selection
Patients were recruited from the Johns Hopkins ARVC registry, which prospectively enrolls ARVC patients and their family members. Active registry participants are routinely invited to participate in a detailed interview about their exercise history. The population for this study included (1) definite ARVC patients, (2) who were implanted with an ICD, and (3) had participated in a structured exercise interview. Diagnosis of definite ARVC was based on the 2010 Task Force Criteria.8 The Johns Hopkins School of Medicine Institutional Review Board approved the study. Participants provided written informed consent.
Exercise Interviews
Structured telephone interviews were conducted, as described previously.2 Participants were prompted to list regular exercise done for leisure or recreation, work, and transportation. Participants were interviewed once after the ICD implant. Exercise interviews were conducted a median of 5.2 years (interquartile range [IQR]: 2.1–10.9 years) after the diagnosis and 4.5 years (IQR: 1.6–10.3 years) after the ICD implant. Intensity and duration of each regularly performed exercise were recorded. Intensity of each activity was rated as light, moderate, or vigorous by the participant using language and definitions from the Multi‐Ethnic Study of Atherosclerosis Typical Week Physical Activity Survey.9
Exercise History Analysis
We calculated change in duration and dose of regular exercise following clinical presentation for each patient based on interview responses. Clinical presentation was defined as the first medical visit for a cardiac indication related to ARVC. Exercise duration was calculated for each activity and summed to achieve the total hours spent exercising. These values were annualized. Exercise dose was calculated using metabolic equivalent of task hours (MET‐hours), as done in previous studies.4 The individual MET value for each exercise activity was assigned according to the 2011 Compendium of Physical Activity.10, 11 The MET value for each exercise activity was multiplied by its duration, which was annualized to obtain MET‐hours per year. Change in exercise duration was quantified as the annual exercise hours in the 3 years before clinical presentation minus the annual exercise hours after presentation but before censoring. Similarly, change in exercise dose was quantified as the annual exercise MET‐hours in the 3 years before clinical presentation minus those after presentation.
Patients were also categorized as athletes or nonathletes based on their athletic activities in the 3 years preceding presentation. As in previous literature,5, 12 athletes were defined as those performing at least 3 hours per week of sports with a moderate to intense dynamic component (at least 6 METs in intensity), translating to 936 MET‐hours per year.
End Points
The primary outcome was the first appropriate ICD therapy for a VA. Review of intracardiac ECGs or device interrogation interpretation by the referring electrophysiologists when the ECG was unavailable were used to adjudicate arrhythmias, as described previously.13 Decisions regarding implantation and programming of ICDs were made by individual managing electrophysiologists on a case‐by‐case basis.
Covariates
Demographic and clinical data were drawn from the Johns Hopkins ARVC registry. Baseline data included sex, date of birth, date and type of presentation, family history, and results of cardiac testing consisting of 12‐lead ECG, 24‐hour Holter monitoring, 2‐dimensional transthoracic echocardiography, and cardiac magnetic resonance imaging. These covariates were selected based on reported risk factors for VA in ARVC.1, 13, 14, 15, 16 Genotype included the results of sequencing of the desmosomal genes PKP2 (plakophilin 2), DSG2 (desmoglein 2), DSC2 (desmocollin‐2), DSP (desmoplakin), and JUP (plakoglobin) at a minimum. Family members with ARVC were screened only for mutations identified in the proband.
Each patient was also classified by whether they met class I, IIa, IIb, or III indications for ICD implantation per the International Task Force Consensus Statement.1 Briefly, class I indications (ICD indicated) included history of sustained VA, severe right ventricular dysfunction (fractional area change ≤17% or ejection fraction ≤35%), and/or severe left ventricle dysfunction (ejection fraction ≤35%). Class IIa indications (ICD should be considered) included ≥1 major risk factor: syncope; nonsustained ventricular tachycardia; and/or moderate dysfunction of right (fractional area change between 24% and 17% or ejection fraction 36–40%), left (ejection fraction 36–45%), or both ventricles. Class IIb indications (ICD may be considered) included ≥1 minor risk factor.1 Patients with class III indications (ICD not indicated) had no risk factors.
Statistical Analysis
Baseline characteristics of patients were compared using the χ2 test, Kruskal–Wallis test, t test, and Wilcoxon rank sum test, as appropriate.
Annual exercise duration (hours) and dose (MET‐hours) before and after clinical presentation were compared by the Wilcoxon signed rank test. Cox proportional hazards models were used to assess the association between tertiles of absolute exercise reduction (in both duration and dose) and the primary outcome. ICD implantation was the start of follow‐up in survival analyses. For patients without the primary outcome, the end of follow‐up was last clinical follow‐up or exercise interview, whichever came first. Model 1 was adjusted for age at presentation, sex, proband status, primary versus secondary prevention, and annual exercise duration or dose before presentation. Model 2 was additionally adjusted for genotype, family history of sudden cardiac death, syncope, presence of T‐wave inversion in at least 3 precordial leads, right and left ventricular function on echocardiography and/or cardiac magnetic resonance imaging, and history of ablation for ventricular tachycardia before presentation. The proportional hazards assumption was met in the Schoenfeld residuals test. Stratified analyses by ICD indications (primary versus secondary prevention) and genotype were performed, with interaction tested in the Cox models.
A subanalysis was performed for patients who had been athletes in the 3 years before presentation. Because these individuals had the greatest opportunity to restrict exercise, we assessed their extent of exercise restriction and the impact on survival from ICD therapy.
We also calculated incidence rates of the primary outcome by exercise reduction (upper half versus lower half) within each class of ICD indications (Ia, IIa, IIb, and III). The incidence rates within each class were compared by the Fisher exact test.
Statistical analyses were performed using Stata/IC 14.2 (StataCorp). A 2‐sided P<0.05 was considered statistically significant.
Results
Study Population
Baseline characteristics of the 129 ARVC patients (51 female [40%]) included in this study are shown in Table 1. Most (n=107, 83%) were probands. More than half (n=73, 59%) carried ≥1 pathogenic variant in genes associated with ARVC. Median age at clinical presentation was 31 years (IQR: 22–44 years). As per the study design, all had ICDs (47 for primary prevention [36%]). The median delay from clinical presentation to diagnosis was 0.02 year (IQR: 0.0–1.4 years). The median delay from clinical presentation to ICD implantation was 0.2 year (IQR: 0.04–2.2 years). At diagnosis, most (n=109, 85%) patients were prescribed β‐blockers. Less than a quarter (n=28, 23%) were taking antiarrhythmic medications (sotalol, 19; amiodarone, 3; dofetilide, 1; dronedarone, 1; flecainide, 2; verapamil, 1; and mexiletine and disopyramide, 1).
Table 1.
Baseline Characteristics of 129 ARVC Patients
| Overall (%) | Most Reduction (%)a | Less Reduction (%)a | P Values | |
|---|---|---|---|---|
| (N=129) | (n=43) | (n=86) | ||
| Female | 51 (40) | 19 (44) | 32 (37) | 0.44 |
| Age at presentation, y | 31 (22–44) | 28 (21–38) | 34 (24–48) | 0.67 |
| Age at defibrillator implant, y | 36 (24–48) | 33 (23–42) | 38 (25–50) | 0.12 |
| Follow‐up duration, years | 5 (3–11) | 4 (2–11) | 6 (3–11) | 0.22 |
| White | 125 (97) | 42 (98) | 83 (97) | 0.72 |
| Genes with pathogenic or likely pathogenic variants | 0.82 | |||
| Plakophilin 2 | 55 (45) | 19 (45) | 36 (44) | |
| Otherb | 18 (15) | 5 (12) | 13 (16) | |
| None | 50 (41) | 18 (43) | 32 (40) | |
| Type of presentation | 0.26 | |||
| Symptomatic but alive | 107 (83) | 38 (88) | 69 (80) | |
| Resuscitated after cardiac arrest | 10 (8) | 1 (2) | 9 (10) | |
| Asymptomatic | 12 (9) | 4 (9) | 8 (9) | |
| Proband | 107 (83) | 36 (81) | 72 (84) | 0.74 |
| Primary prevention | 47 (36) | 18 (42) | 29 (34) | 0.37 |
| Syncope | 32 (25) | 7 (16) | 25 (30) | 0.098 |
| Family history of sudden death | 11 (9) | 2 (5) | 9 (10) | 0.27 |
| Nonsustained VT | 45 (35) | 20 (47) | 25 (29) | 0.050 |
| T‐wave inversions ≥3 precordial leads | 86 (78) | 32 (82) | 54 (75) | 0.40 |
| Inducibility in electrophysiology study | 70 (76) | 23 (74) | 47 (77) | 0.76 |
| History of VT ablation | 37 (29) | 12 (28) | 25 (29) | 0.86 |
| Right ventricular function | 0.88 | |||
| FAC ≤17% or EF ≤35% | 26 (30) | 10 (33) | 16 (29) | |
| FAC 17%–24% or EF 36%–40% | 14 (16) | 5 (17) | 9 (16) | |
| Left ventricular function | 0.68 | |||
| EF ≤35% | 5 (5) | 1 (3) | 4 (6) | |
| EF 36%–45% | 13 (12) | 4 (10) | 9 (13) | |
Data presented are median and interquartile range for continuous variables and number of cases with percentage for categorical variables. Data were collected at the time of implantable cardioverter‐defibrillator implantation. ARVC indicates arrhythmogenic right ventricular cardiomyopathy; EF, ejection fraction; FAC, fractional area change; VT, ventricular tachycardia.
Most reduction: top tertile of reduction in annual exercise duration; less reduction: second and third tertiles.
Other genes with pathogenic variants included desmoplakin (6 patients), desmoglein 2 (4 patients), desmocollin‐2 (1 patient), plakoglobin (1 patient), phospholamban (1 patient), and digenic or compound heterozygous mutations (6 patients).
Exercise History
In the 3 years preceding clinical presentation, most patients (n=103, 80%) were athletes (at least 936 MET‐hours/y). Median annual exercise duration and dose during this period was 360 h/y (IQR: 184–653 h/y) and 2249 MET‐hours/y (IQR: 1015–4682 MET‐hours/y), respectively. In the 3 years after clinical presentation, patients significantly reduced their average annual exercise duration to 182 h/y (IQR: 49–448 h/y; P<0.001) and dose to 1111 MET‐hours/y (IQR: 336–3072 MET‐hours/y; P<0.001). Patients implanted for secondary and primary prevention alike significantly reduced exercise duration and dose (Figure 1). Among patients who had been athletes (at least 936 MET‐hours/y) during the 3 years before diagnosis, most reduced their exercise, with a third (31/103) reducing exercise dose by at least 80% after diagnosis. Significant heterogeneity was seen in exercise participation over time for each participant and between participants.
Figure 1.
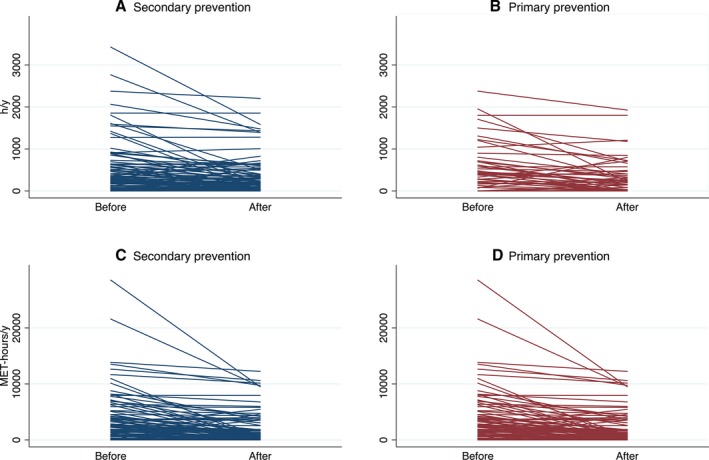
Exercise duration (A and B) and dose (C and D) before and after presentation in arrhythmogenic right ventricular cardiomyopathy patients with secondary‐ and primary‐prevention ICD. Each line represents 1 patient. Before indicates the average exercise duration (or dose) in the 3 years before presentation. After indicates the average from presentation to 3 years later or first appropriate ICD therapy (if present). The differences between before and after were all significant (P<0.001). ICD indicates implantable cardioverter‐defibrillator; MET, metabolic equivalent of task hours.
Arrhythmic Outcomes
Over a median follow‐up of 5.1 years (IQR: 2.6–10.8 years) after ICD implantation, 85 of 129 patients (66%) experienced VA. Sixty‐six (78%) received appropriate therapy for ventricular tachycardia (median cycle length: 298 ms [IQR: 270–330 ms]) and 19 (22%) for rapid VT, ventricular fibrillation, or flutter (median cycle length: 220 ms [IQR: 207–230 ms]).
Table 2 (column 1) shows the univariate associations of demographic and clinical characteristics with the primary outcome. As expected, secondary‐prevention patients had worse survival from a VA requiring ICD therapy (hazard ratio [HR]: 2.01; 95% confidence interval [CI], 1.24–3.26). Male sex, being the family proband, and inducibility on electrophysiology study were also significantly associated with poorer survival from the primary outcome. There was no significant difference in survival from first ICD therapy by genotype or history of ablation for VAs.
Table 2.
HRs for ICD Therapy for VT/VF According to Exercise History and Clinical Characteristics
| Unadjusted | Model 1 | Model 2 | |
|---|---|---|---|
| HR (95% CI)a | HR (95% CI)a | HR (95% CI)a | |
| Exercise reduction (before presentation minus after presentation) | |||
| Durationb | 0.56 (0.34–0.92) | 0.41 (0.22–0.77) | 0.23 (0.07–0.81) |
| Doseb | 0.57 (0.35–0.93) | 0.37 (0.20–0.68) | 0.14 (0.04–0.44) |
| Age at presentation (y) | |||
| Q1: 17.4±2.5 | Reference | ||
| Q2: 26.4±2.7 | 0.85 (0.47–1.56) | ||
| Q3: 37.7±3.9 | 1.13 (0.64–2.00) | ||
| Q4: 54.9±7.0 | 0.63 (0.33–1.19) | ||
| Male | 1.73 (1.08–2.75) | ||
| Proband | 2.89 (1.47–5.67) | ||
| Pathogenic or likely pathogenic variant | 1.25 (0.79–1.98) | ||
| ICD for secondary prevention | 2.01 (1.24–3.26) | ||
| Syncope | 1.20 (0.73–1.96) | ||
| Family history of SCD | 0.69 (0.32–1.52) | ||
| Nonsustained VT | 0.99 (0.62–1.57) | ||
| T‐wave inversion on >3 leads | 1.39 (0.76–2.54) | ||
| Inducibility in electrophysiology study | 2.90 (1.43–5.91) | ||
| History of VT ablation | 1.34 (0.85–2.13) | ||
| Right ventricle FAC ≤24% or EF ≤40% | 1.38 (0.77–2.48) | ||
| Left ventricle EF ≤45% | 0.95 (0.50–1.83) | ||
Model 1: adjusted for sex, age at presentation (quartiles), primary or secondary prevention, proband status, and annual exercise duration and dose before clinical presentation (quartiles). Model 2: additionally adjusted for ablation before implant, desmosomal mutations, syncope, family history of SCD, T‐wave inversions (>3 leads or not), right ventricular function (FAC ≤17% or EF ≤35%, FAC 17%–24% or EF 36%–40%, or other), and left ventricular function (EF ≤35%, EF 36%–45%, or other). CI indicates confidence interval; EF, ejection fraction; FAC, fractional area change; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; Q, quartile; SCD, sudden cardiac death; VF, ventricular fibrillation; VT, ventricular tachycardia.
HRs were calculated comparing being in the top tertile of exercise reduction and the rest.
Exercise reduction was defined as annual exercise hours (or metabolic equivalent of task hours) in the 3 years before clinical presentation minus those after.
To What Extent Does Exercise Reduction Alter Arrhythmic Risk?
As shown in Table 2, patients who reduced annual exercise dose the most (top tertile) had significantly better survival from the primary outcome (HR: 0.57; 95% CI, 0.35–0.93). This inverse association between reduction in exercise dose and the primary outcome was strengthened after adjustment for demographic variables and exercise levels before presentation (model 1, HR: 0.37; 95% CI, 0.20–0.68). It was further strengthened by adjusting for established risk factors for VA in ARVC (model 2, HR: 0.14; 95% CI, 0.04–0.44). Thus, patients who reduced annual exercise dose the most were 86% less likely to experience appropriate ICD therapy than their counterparts after controlling for potential confounders. Reduction in exercise duration was similarly associated with the primary outcome (model 1, HR: 0.41; 95% CI, 0.22–0.77; model 2, HR: 0.23; 95% CI, 0.07–0.81; Table 2). We also performed sensitivity analyses in probands and the results were similar (Table S1).
We also examined whether there was a dose‐response relationship between amount of reduction in exercise and appropriate ICD therapy. After controlling for clinical and demographic factors, compared with the bottom‐tertile reduction, middle‐ and top‐tertile reductions in dose were associated with successively lower hazards of VA (middle, HR: 0.64; 95% CI, 0.24–1.69; top, HR: 0.10; 95% CI, 0.02–0.43). The trend was statistically significant (P=0.01). A dose‐response relationship was not seen for reduction in exercise duration, with only those who reduced duration of exercise benefiting the most (Table S2).
Does Genotype or History of VA Predict Which Patients Benefit More From Exercise Reduction?
We next examined whether genotype or history of VA was associated with the extent of benefit derived from reduction in exercise. As shown in Table 1, patients with and without known pathogenic variants were equally likely to have substantially reduced (top tertile) exercise duration (P=0.82). Likewise, there was no difference in exercise duration reduction between patients implanted for primary and secondary prevention (P=0.37). There was also no association between reduction in exercise dose and either genotype (P=0.34) or ICD indication (P=0.20).
Figure 2 presents adjusted HRs for VA according to reduction in exercise dose for genotype‐positive versus gene‐elusive patients and for primary‐ versus secondary‐prevention patients. As can be appreciated, reduction in annual exercise dose appeared particularly beneficial in patients with primary‐prevention devices (P=0.06 for interaction). Similarly, only gene‐elusive patients had a statistically significant reduction in VA risk; however, the difference in adjusted HRs by genotype did not reach conventional statistical significance (P=0.16 for interaction). The adjusted HRs depicted in Figure 2 and both unadjusted and adjusted HRs associated with reduction in exercise duration and dose stratified by genotype and ICD indication can be found in Table S3.
Figure 2.
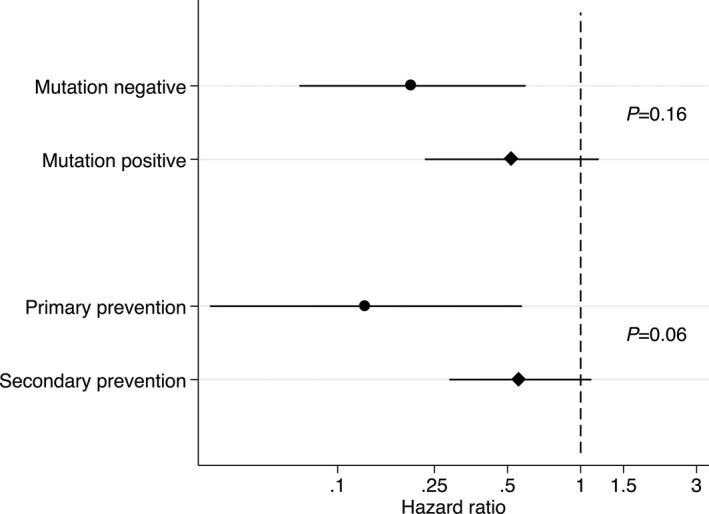
Adjusted hazard ratios for implantable cardiac defibrillator therapy for ventricular tachycardia or ventricular fibrillation according to reduction in exercise dose stratified by genotype and primary vs secondary prevention. Sex, age at presentation (quartiles), primary or secondary prevention, mutation, proband, and annual exercise dose before clinical presentation (quartiles) were adjusted. P values for interactions are listed.
Can Exercise Reduction Alter Risk of VAs Sufficiently to Affect Decision‐Making Regarding ICD Placement?
We next examined whether the magnitude of reduction in the risk of VA requiring ICD therapy associated with exercise restriction was sufficient to affect decision‐making for ICD placement. We examined this question in 2 ways. First, we stratified the population based on the International Task Force Consensus Statement algorithm for ICD placement in ARVC1 and examined the extent to which exercise reduction was associated with primary outcomes in each risk group. Most patients had class I indications for ICD implant (class I, 97; class IIa, 24; class IIb, 7; and class III, 1). Figure 3 shows the incidence rates of ICD therapy in patients with class I and IIa indications (too few patients with class IIb or III) stratified by extent of exercise reduction. As can be appreciated, among the highest risk patients (Class I), incidence rate was significantly lower in patients with greater (upper half) reduction in exercise dose (P=0.003). However, because absolute risk remained high (22% per year), it was unlikely that this reduction would significantly change clinical decision‐making.
Figure 3.
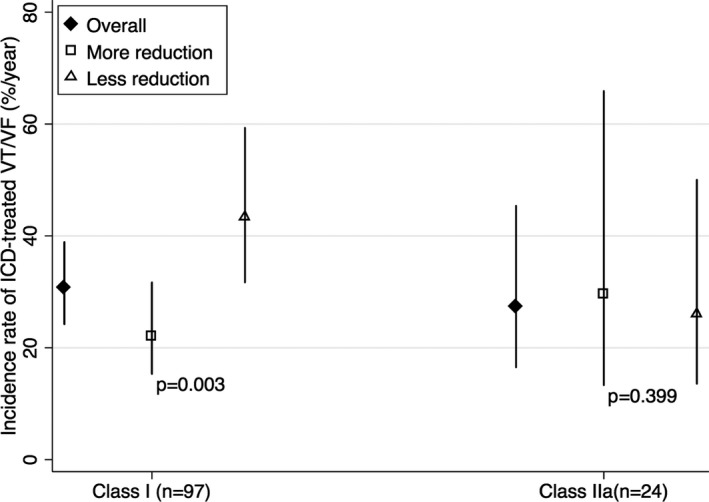
Incidence rates for appropriate ICD therapy for ventricular tachycardia or ventricular fibrillation according to reduction in exercise dose (upper vs lower half) in patients with class I and IIa ICD indications. Too few patients had class IIb (7 patients) or class III (1 patient) indications. P values by Fisher exact test for differences in incidence rates are listed. ICD indicates implantable cardioverter‐defibrillator; VT/VF, ventricular tachycardia/ventricular fibrillation.
Second, we examined the absolute risk of primary outcomes in patients who reduced exercise the most—31 athletes who reduced exercise dose by ≥80%. In this group, more than half (18/31, 58%) nonetheless had appropriate ICD therapy. Of the 26 patients who were nonathletes, 19 (73%) had appropriate ICD therapy. They were not active before presentation (median exercise dose: 225 MET‐hours/y) and did not have much room to reduce their exercise (194 MET‐hours/y after presentation).
Discussion
Main Findings
In this study of 129 ARVC patients with ICDs, absolute reduction in exercise duration and dose after clinical presentation was associated with less VA treated by ICD. Furthermore, greater reduction in exercise dose conferred greater reduction in arrhythmic risk. Two distinct groups of patients appeared to benefit more from exercise reduction: gene‐elusive patients and patients whose ICDs were implanted for primary prevention. However, even among previously athletic individuals who decreased their exercise substantially, the risk of arrhythmias remained high, suggesting that, in most cases, plans to reduce exercise are likely to be insufficient to influence decision‐making for ICD implant.
Prior Literature
Current guidelines advise against participation in competitive exercise for definite ARVC patients.1, 17 These recommendations are based on the evidence that exercise is associated with the development and increased severity of the ARVC phenotype both in animal18, 19 and human studies.2, 3, 4 However, whether reduction in exercise after diagnosis affects the disease trajectory is largely unknown. By comparing 8 patients who remained at top exercise duration after presentation with 8 patients who did not, James et al2 first observed that exercise reduction significantly reduced incident VA in ARVC patients with desmosomal variants. Ruwald et al6 later reported that ARVC patients who continued competitive sports after diagnosis experienced more VA than those who stopped, but the result was not statistically significant. Because of small sample sizes, neither study could adjust for confounders, examine the extent of benefit from changing exercise, or perform stratified analyses to explore which patient subsets might particularly benefit from limiting exercise.
Toward Personalized Exercise Recommendations
To answer these important questions, we performed a detailed assessment of absolute exercise reduction in a larger study population. After accounting for potential confounders, exercise reduction was associated with 86% less hazard of ICD‐treated VA in these high‐risk ARVC patients. The impressive effect size provides direct evidence supporting substantial reduction of exercise after diagnosis in these patients.
The extent of benefit was larger for reducing exercise dose than for reducing exercise duration. In addition, a dose response was seen for reduction of exercise dose, whereas there was no difference in outcomes of patients in the bottom and middle tertiles with reduction of exercise duration. These results are unsurprising because exercise dose captured more information than exercise duration (eg, 1 hour of leisure walking and 1 hour of playing a basketball game have the same duration but very different doses). Together, these results suggest that reducing exercise dose is likely a more important treatment goal than limiting duration in managing ARVC patients. It is interesting to consider these results in light of the findings of Ruwald et al that recreational sports conferred no greater risk of VA than being inactive.6
No studies have previously explored the interaction between genotype and the influence of exercise in follow‐up in ARVC patients. We found that exercise reduction appeared to particularly benefit gene‐elusive patients. This is in line with the hypothesis that ARVC‐like phenotype may be acquired through intense exercise without established genetic predisposition.20 After the removal of the insult from mechanical stress imposed by exercise, gene‐elusive patients may have a better prognosis than those born with desmosomal mutations that no therapy can modify at this moment. Larger studies are needed to confirm this relationship.
Interestingly, patients with primary prevention devices also benefited more from exercise reduction. This could be related to timing; exercise reduction may be too late to alter the arrhythmic course in patients who have demonstrated full‐blown disease by experiencing VA.
In the clinic, the question often arises whether athletic patients can sufficiently reduce their arrhythmic risk by exercise reduction to consider deferring ICD implantation. Our data showed that, despite remarkable exercise reduction, more than half of previously athletic patients still experienced VA. Furthermore, incorporating exercise restriction into the current risk‐stratification algorithm showed that although risk was reduced, it meant only that those patients with a class I indication had risks more typical of class IIa patients. This suggests that an ICD should still be recommended if high‐risk features exist in most cases, regardless of exercise plans. Nonetheless, limiting VA is important, given the possibility of increased mortality21 and definite psychological stress22 associated with ICD shocks.
We also observed that ARVC patients who reduced exercise the most had the least ICD‐treated VA. Although it is intuitive that greater reduction results in greater benefits in ARVC, prior studies suggested that recreational sports conferred no greater risk of VA than being inactive.6 Our patients had ICDs and were at high arrhythmic risk, which might explain the difference.
Although current guidelines list recreational low‐intensity sports as a possible exception for definite ARVC patients despite the recommendation against competitive or endurance sports,1 this finding supports our current clinical approach of recommending that high‐risk ARVC patients dramatically reduce participation in exercise after diagnosis.
Limitations
Exercise participation was assessed retrospectively, so recall bias and social desirability bias (patients may feel less guilty reporting less exercise after diagnosis) were possible. Because both would bias the result to null, they were unlikely to affect study validity. Because patients had to survive to the exercise interview, mortality was not available as an outcome; however, the mortality rate is very low in ARVC patients with an ICD.23 Next, the study design does not allow the comparison between aerobic and anaerobic activities at the same intensity. Most patients were participating in aerobic activities, so we could not characterize the risk of anaerobic activities. Because this study is registry based, ICD programming was at the discretion of the treating electrophysiologists. In addition, ICD‐treated VA may overestimate the incidence of life‐threatening arrhythmias. As in every observational study, residual confounding is possible. Finally, this study assesses only the association of exercise restriction with appropriate ICD therapy for VA. Experiencing VA is not necessarily a sign of structural disease progression in ARVC, although prior research suggests structural progression is also associated with exercise in ARVC patients.3, 24
Conclusions
Exercise reduction after clinical presentation in ARVC is independently associated with a decrease in appropriate ICD therapy for VA. Restriction of exercise should be recommended to ARVC patients regardless of mutation status and original indication for ICD, although patients who are gene‐elusive and those with primary‐prevention ICDs may particularly benefit. Exercise reduction is unlikely to reduce the risk of arrhythmia sufficiently in high‐risk patients to alter clinical decision‐making regarding ICD implant.
Sources of Funding
This work was supported by the 2017 Clinical Research Award in Honor of Mark Josephson and Hein Wellens Scholarship from the Heart Rhythm Society (to Wang). The authors wish to acknowledge funding from the Dr. Francis P. Chiaramonte Private Foundation, Boston Scientific Corp., and Foundation Leducq (16 CVD 02; all to Calkins). The Johns Hopkins ARVC program is supported by the Leyla Erkan Family Fund for ARVD Research, the Dr. Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella Family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments.
Disclosures
Dr Calkins is a consultant for Medtronic Inc. and St. Jude Medical. Dr Calkins receives research support from Boston Scientific Corp and Ms Tichnell and Dr James receive salary support from this grant. Dr Tandri receives research support from Abbott. The remaining authors have no disclosures to report.
Supporting information
Table S1. Hazard Ratios for Implantable Cardioverter‐Defibrillator Therapy for Ventricular Tachycardia/Ventricular Fibrillation According to Exercise History and Clinical Characteristics in Probands
Table S2. Dose‐Response Relationship Between Reduction in Exercise (Duration and Dose) and Defibrillator Therapy for Ventricular Tachycardia/Ventricular Fibrillation
Table S3. Hazard Ratios for Implantable Cardioverter‐Defibrillator (ICD) Therapy for Ventricular Tachycardia/Ventricular Fibrillation According to Reduction in Exercise (Duration and Dose) Stratified by Mutation Status and Indication for ICD
Acknowledgments
The authors are grateful to the patients and families who made this work possible.
(J Am Heart Assoc. 2018;7:e008843 DOI: 10.1161/JAHA.118.008843.)
References
- 1. Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, Tandri H, Paul M, Schmied C, Pelliccia A, Duru F, Protonotarios N, Estes NA III, McKenna WJ, Thiene G, Marcus FI, Calkins H. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J. 2015;36:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. James CA, Bhonsale A, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise increases age‐related penetrance and arrhythmic risk in arrhythmogenic right ventricular dysplasia/cardiomyopathy–associated desmosomal mutation carriers. J Am Coll Cardiol. 2013;62:1290–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Saberniak J, Hasselberg NE, Borgquist R, Platonov PG, Sarvari SI, Smith H‐J, Ribe M, Holst AG, Edvardsen T, Haugaa KH. Vigorous physical activity impairs myocardial function in patients with arrhythmogenic right ventricular cardiomyopathy and in mutation positive family members. Eur J Heart Fail. 2014;16:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sawant AC, Bhonsale A, te Riele AS, Tichnell C, Murray B, Russell SD, Tandri H, Tedford RJ, Judge DP, Calkins H. Exercise has a disproportionate role in the pathogenesis of arrhythmogenic right ventricular dysplasia/cardiomyopathy in patients without desmosomal mutations. J Am Heart Assoc. 2014;3:e001471 DOI: 10.1161/JAHA.114.001471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. La Gerche A, Robberecht C, Kuiperi C, Nuyens D, Willems R, de Ravel T, Matthijs G, Heidbüchel H. Lower than expected desmosomal gene mutation prevalence in endurance athletes with complex ventricular arrhythmias of right ventricular origin. Heart. 2010;96:1268–1274. [DOI] [PubMed] [Google Scholar]
- 6. Ruwald A‐C, Marcus F, Estes NM III, Link M, McNitt S, Polonsky B, Calkins H, Towbin JA, Moss AJ, Zareba W. Association of competitive and recreational sport participation with cardiac events in patients with arrhythmogenic right ventricular cardiomyopathy: results from the North American multidisciplinary study of arrhythmogenic right ventricular cardiomyopathy. Eur Heart J. 2015;36:1735–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mazzanti A, Ng K, Faragli A, Maragna R, Chiodaroli E, Orphanou N, Monteforte N, Memmi M, Gambelli P, Novelli V, Bloise R, Catalano O, Moro G, Tibollo V, Morini M, Bellazzi R, Napolitano C, Bagnardi V, Priori SG. Arrhythmogenic right ventricular cardiomyopathy: clinical course and predictors of arrhythmic risk. J Am Coll Cardiol. 2016;68:2540–2550. [DOI] [PubMed] [Google Scholar]
- 8. Marcus FI, McKenna WJ, Sherrill D. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Turkbey EB, Jorgensen NW, Johnson C, Bertoni AG, Polak JF, Roux AVD, Tracy RP, Lima JA, Bluemke DA. Physical activity and physiologic cardiac remodeling in a community setting: the Multi‐Ethnic Study of Atherosclerosis (MESA). Heart. 2010;96:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR Jr, Tudor‐Locke C, Greer JL, Vezina J, Whitt‐Glover MC, Leon AS. 2011 Compendium of Physical Activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43:1575–1581. [DOI] [PubMed] [Google Scholar]
- 11. Compendium of physical activities [Internet]. Available at: https://sites.google.com/site/compendiumofphysicalactivities/. Accessed December 24, 2017.
- 12. Mitchell JH, Haskell W, Snell P, Van Camp SP. Task Force 8: classification of sports. J Am Coll Cardiol. 2005;45:1364–1367. [DOI] [PubMed] [Google Scholar]
- 13. Orgeron GM, James CA, Te Riele A, Tichnell C, Murray B, Bhonsale A, Kamel IR, Zimmerman SL, Judge DP, Crosson J, Tandri H, Calkins H. Implantable cardioverter‐defibrillator therapy in arrhythmogenic right ventricular dysplasia/cardiomyopathy: predictors of appropriate therapy, outcomes, and complications. J Am Heart Assoc. 2017;6:e006242 DOI: 10.1161/JAHA.117.006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bhonsale A, James CA, Tichnell C. Incidence and predictors of implantable cardioverter‐defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter‐defibrillator implantation for primary prevention. J Am Coll Cardiol. 2011;58:1485–1496. [DOI] [PubMed] [Google Scholar]
- 15. Corrado D, Calkins H, Link MS. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144–1152. [DOI] [PubMed] [Google Scholar]
- 16. Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K, Marcus F, Estes NM. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol. 2014;64:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Priori SG, Blomström‐Lundqvist C, Mazzanti A. 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the Task Force for the Management of Patients with Ventricular Arrhythmias and the Prevention of Sudden Cardiac Death of the European Society of Cardiology (ESC) Endorsed by: association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J. 2015;36:2793–2867. [DOI] [PubMed] [Google Scholar]
- 18. Kirchhof P, Fabritz L, Zwiener M. Age‐and training‐dependent development of arrhythmogenic right ventricular cardiomyopathy in heterozygous plakoglobin‐deficient mice. Circulation. 2006;114:1799–1806. [DOI] [PubMed] [Google Scholar]
- 19. Chelko SP, Asimaki A, Andersen P, Bedja D, Amat‐Alarcon N, DeMazumder D, Jasti R, MacRae CA, Leber R, Kleber AG, Saffitz JE, Judge DP. Central role for GSK3β in the pathogenesis of arrhythmogenic cardiomyopathy. JCI Insight. 2016;1:e85923 Available at: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4861310/. Accessed September 29, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. La Gerche A, HeidbüChel H, Burns AT, Mooney DJ, Taylor AJ, Pfluger HB, Inder WJ, Macisaac AI, Prior DL. Disproportionate exercise load and remodeling of the athlete's right ventricle. Med Sci Sports Exerc. 2011;43:974–981. [DOI] [PubMed] [Google Scholar]
- 21. Sweeney MO, Sherfesee L, DeGroot PJ, Wathen MS, Wilkoff BL. Differences in effects of electrical therapy type for ventricular arrhythmias on mortality in implantable cardioverter‐defibrillator patients. Heart Rhythm. 2010;7:353–360. [DOI] [PubMed] [Google Scholar]
- 22. Sears SF, Hauf JD, Kirian K, Hazelton G, Conti JB. Posttraumatic stress and the implantable cardioverter‐defibrillator patient. Circ Arrhythm Electrophysiol. 2011;4:242–250. [DOI] [PubMed] [Google Scholar]
- 23. Groeneweg JA, Bhonsale A, James CA. Clinical presentation, long‐term follow‐up, and outcomes of 1001 arrhythmogenic right ventricular dysplasia/cardiomyopathy patients and family members. Circ Cardiovasc Genet. 2015;8:437–446. [DOI] [PubMed] [Google Scholar]
- 24. Sen‐Chowdhry S, Syrris P, Ward D, Asimaki A, Sevdalis E, McKenna WJ. Clinical and genetic characterization of families with arrhythmogenic right ventricular dysplasia/cardiomyopathy provides novel insights into patterns of disease expression. Circulation. 2007;115:1710–1720. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Hazard Ratios for Implantable Cardioverter‐Defibrillator Therapy for Ventricular Tachycardia/Ventricular Fibrillation According to Exercise History and Clinical Characteristics in Probands
Table S2. Dose‐Response Relationship Between Reduction in Exercise (Duration and Dose) and Defibrillator Therapy for Ventricular Tachycardia/Ventricular Fibrillation
Table S3. Hazard Ratios for Implantable Cardioverter‐Defibrillator (ICD) Therapy for Ventricular Tachycardia/Ventricular Fibrillation According to Reduction in Exercise (Duration and Dose) Stratified by Mutation Status and Indication for ICD


