Abstract
Background
The treatment of mycotic abdominal aortic aneurysm requires surgery and antimicrobial therapy. Since prosthetic reconstructions carry a considerable risk of reinfection, biological grafts are noteworthy alternatives. The current study evaluated the durability, infection resistance, and midterm outcome of biological grafts in treatment of mycotic abdominal aortic aneurysm.
Methods and Results
All patients treated with biological graft in 6 countries between 2006 and 2016 were included. Primary outcome measures were 30‐ and 90‐day survival, treatment‐related mortality, and reinfection rate. Secondary outcome measures were overall mortality and graft patency. Fifty‐six patients (46 males) with median age of 69 years (range 35–85) were included. Sixteen patients were immunocompromised (29%), 24 (43%) had concomitant infection, and 12 (21%) presented with rupture. Bacterial culture was isolated from 43 (77%). In‐situ aortic reconstruction was performed using autologous femoral veins in 30 patients (54%), xenopericardial tube‐grafts in 12 (21%), cryopreserved arterial/venous allografts in 9 (16%), and fresh arterial allografts in 5 (9%) patients. During a median follow‐up of 26 months (range 3 weeks–172 months) there were no reinfections and only 3 patients (5%) required assistance with graft patency. Thirty‐day survival was 95% (n=53) and 90‐day survival was 91% (n=51). Treatment‐related mortality was 9% (n=5). Kaplan–Meier estimation of survival at 1 year was 83% (95% confidence interval, 73%–94%) and at 5 years was 71% (52%–89%).
Conclusions
Mycotic abdominal aortic aneurysm repair with biological grafts is a durable option for patients fit for surgery presenting an excellent infection resistance and good overall survival.
Keywords: allograft, aneurysm, aorta, autologous vein, femoral vein, graft, in situ reconstruction, infection, vein
Subject Categories: Cardiovascular Surgery, Treatment, Aneurysm, Vascular Disease, Computerized Tomography (CT)
Clinical Perspective
What Is New?
High rate of reinfection and subsequent mortality are linked to prosthetic treatment of mycotic abdominal aortic aneurysms, but durability of biological reconstruction material in primary aortic infections lacks evidence.
Current study on largest cohort of patients treated with open repair and biological grafts shows no reinfections, good graft patency, and acceptable mortality.
What Are the Clinical Implications?
Because of low postoperative mortality and good long‐term graft durability, patients fit for open surgery should be considered for in‐line reconstruction with biological material.
Surgical treatment with biological material spares patients from lifelong antimicrobial therapy and related complications.
Introduction
Mycotic aortic aneurysms (MAA)—the term first described as a septic embolic complication of endocarditis—is currently being used for any aneurysm secondary to aortic infection.1 MAAs are a rare, but life‐threatening entity with rapid clinical course and devastating outcomes without surgical intervention.2 Even with adequate treatment, mortality remains as high as 18% to 43%.3, 4, 5, 6, 7
Bacterial infection inoculates the arterial wall through a damaged intimal layer, resulting in destroyed media or occluded vasa vasorum with secondary arterial wall ischemia. The weakened wall loses its elasticity and is predisposed to aneurysm formation.8 Rapid aneurysmal growth induces pain and infection, which may promptly evolve to sepsis. Patients are often immunosuppressed and/or have concomitant infections; therefore, early diagnosis, adequate antimicrobial therapy, and surgery are crucial for favorable outcomes.3, 9, 10
The feasibility of in‐situ prosthetic grafting or endovascular aortic repair (EVAR) of mycotic abdominal aortic aneurysms (MAAA) has been acknowledged by previous studies.10, 11, 12, 13, 14, 15 Minimally invasive EVAR provides a favorable early survival rate at 3 months, but the risk of reinfection and associated late mortality remains a problem.10, 11, 12, 13 Because of poor outcomes in graft patency, aortic stump blow‐out and numerous reinfections, extra‐anatomical bypass has been abandoned as a treatment of choice in many centers.16 In‐situ prosthetic alignment is the preferred alternative to EVAR, yet a substantial rate of mortality persists because of infectious complications.10, 11, 15
Earlier studies have shown biological vascular grafts to be more resistant to microbial infection than synthetic analogues.17, 18 Autologous femoral veins, allografts, and recently added self‐made xenopericardial tubes are the most frequently used biological materials in aorto‐iliac position. They have been applied successfully as a reconstruction material in case of aortic graft infection; however, their performance in treatment of MAAA lacks evidence.19, 20, 21, 22, 23
The purpose of this study was to ascertain the experience using biological grafts in the treatment of MAAA by assessing reinfection rate, graft‐related complications, and treatment‐related and midterm survival.
Methods
Because of regulations for the protection of privacy, the data, analytic methods, and study material will not be automatically made available to other researchers for purposes of reproducing the results or replicating the procedure. However, data could be made available within limits of legal regulations of patient data access upon reasonable request to the corresponding author.
All consecutive patients treated for MAAA with abdominal aorto‐iliac in‐situ reconstruction with biological material in collaborating centers from January 2006 to December 2016 were included (Table 1). Patient data were collected from vascular registries and hospital records by investigators. Identical data collection sheets were filled out in collaborating centers from 6 countries and eligibility was revised in Helsinki University Hospital. Two patients included were treated earlier than 2006, since data on MAAA patients were available from earlier years in 2 centers. Patients who had mycotic aneurysms, but were primarily operated on with EVAR as a bridging method or with prosthetic graft, were excluded.
Table 1.
Patients Treated for AAA and MAAA in the Collaborating Centers and Registries
| Center | Operated AAA/MAAA | Reconstruction Material for MAAA | ||||||
|---|---|---|---|---|---|---|---|---|
| EVAR | Prosthetic Graft | Autologous FV | Bovine Pericard | Cryopreserved Arterial/Venous Allografts | Fresh Allografts | |||
| Finland | Helsinki University Hospital | 1093/21 | 4 | 2 | 13 | ··· | 0/2 | ··· |
| Denmark | Aarhus University Hospital | 1236/10 | ··· | 2 | 8 | ··· | ··· | ··· |
| Italy | Siena University Hospital | 643/9 | 2 | 2 | ··· | ··· | ··· | 5 |
| France | Nancy University Hospital | 2100/7 | 2 | 1 | ··· | 2 | 2/0 | |
| Switzerlanda | Bern University Hospital | 2230/20 | 2 | 3 | ··· | 10 | 5/0 | ··· |
| Swedenb | Register data, incl. 28 hospitals | 15 130/120 | 70 | 41 | 9 | ··· | ··· | ··· |
| Total | 22 432/187 | 80 | 51 | 30 | 12 | 7/2 | 5 | |
AAA indicates abdominal aortic aneurysm; EVAR, endovascular aortic repair; FV, femoral vein; MAAA, mycotic abdominal aortic aneurysm.
Data‐collection period January 2002 to December 2016, includes patient treated with biological graft before 2006.
Data collection period January 2001 to December 2014, includes 1 patient treated with biological graft before 2006.
Diagnosis of mycotic aortic aneurysm was defined by the presence of 2 or more of the following criteria: clinical presentation (pain, fever, and sepsis), laboratory parameters (elevated leukocyte count and C‐reactive protein, positive cultures from blood or aortic tissue), and radiologic findings on computed tomography or magnetic resonance imaging (saccular/multilobular aneurysm, periaortic soft tissue mass, or gas). The diagnosis was confirmed with intraoperative infectious findings of MAAA. Reinfection was defined as graft infection, aorto‐enteric fistula, or recurrent MAA.
Type of biological graft used was decided by the collaborating center based on availability, previous experience, and patient's clinical condition. In Helsinki, Aarhus, and Sweden, autologous femoral veins are the preferred reconstruction material in case of vascular infection; in Bern and Nancy, self‐made pericardial tubes are used or cryopreserved arterial allografts if available. Siena prefers fresh arterial allografts and therefore availability is the main limiting factor for biological reconstruction. Autologous femoral veins were harvested simultaneously with aortic exposure by a second team. The valves were resected and veins were used as straight tubes or sewn together into pantaloon configuration with nonabsorbable polypropylene running suture. Upper anastomosis was reinforced with patients’ fascia lata.20 Preparation of the pericardial tube graft was performed by tailoring a large bovine pericardial patch according to the desired size for aortic reconstruction. Then it was sewn to construct a tube using a polypropylene 4–0 suture.23 Cryopreserved arterial allografts were commercial, thawed and handled as instructed by the providing cryobank. Fresh arterial allografts were rinsed in antibiotic solution for a minimum of 15 minutes after harvesting and were implanted immediately.
The collected data included demographic parameters (sex, age, smoking), comorbidities (hypertension, diabetes mellitus, ischemic heart disease, cerebrovascular disease, chronic obstructive pulmonary disease, renal insufficiency and dialysis treatment, steroid treatment or immunosuppressive condition), clinical signs (abdominal/back pain, fever, additional infection, sepsis), laboratory results (C‐reactive protein, white blood cell count, blood culture, tissue culture from operating field), radiological findings (aneurysm location, saccular/multilobular aneurysm, periaortic soft tissue mass or gas, aneurysm size and location, rupture), and whether 18F‐fluorodeoxyglucose positron emission tomography /computed tomography were performed to confirm the diagnosis. Operative data included type of aortic reconstruction and concomitant procedures, used graft material, renal protection if needed in case of suprarenal cross‐clamping, operation time, blood loss, and clamping time. Postoperative data included length of intensive care unit and hospital stay, postoperative complications, follow‐up time, graft‐related complications and interventions, major amputations, 30‐day, treatment‐related, and late mortality and cause of death. Data on antibiotics used were collected from the pre‐ and postoperative period; also the length of antibiotic treatment, from diagnosis to operation and postoperatively, was recorded.
Statistical analysis was performed using SPSS 22 software (IBM Corp, Armonk, NY). Continuous variables are expressed as median (range). All statistical tests were 2‐tailed, and a P<0.05 was considered significant. Univariable analysis was performed to analyze associated risk factors for mortality at 30 days, and treatment‐related and overall mortality were determined using Fisher exact test and binary logistic regression. The following variables were tested: sex, age, smoking, hypertension, diabetes mellitus, ischemic heart disease, cerebrovascular disease, chronic obstructive pulmonary disease, renal insufficiency and dialysis treatment, steroid treatment or immunosuppressive condition, concomitant infection, C‐reactive protein at the time of diagnosis and operation, aneurysm rupture, operation time, blood loss, suprarenal clamping requiring renal protection, clamping time, additional vascular reconstruction to aortic reconstruction, other concomitant procedure, Salmonella versus non‐Salmonella infection, and positive versus negative culture.
The multivariable Cox regression analysis for overall survival was performed using variables with P<0.2 in the univariable analysis. The survival analysis was performed according to the Kaplan–Meier method.
The study had clinical approval. Because of the retrospective nature of the research, permission from the ethical committee was not required.
Results
During the study period, 22 432 abdominal aortic aneurysms were treated in collaborating centers, of which 187 (0.8%) were MAAAs. Eighty of 187 patients were treated with EVAR (43%), 51 (27%) with open prosthetic repair, and 56 (30%) with biological material. Sweden was the only participating country where the preferred treatment for MAAA is prosthetic grafting or EVAR (n=111/120, 93%). In other collaborating centers, biological reconstruction is first line of treatment and prosthetic reconstruction was used only in case of patients in poor condition, misdiagnosis, emergency, lack of biological material, or as a bridging method. Only patients operated on with biological material as a primary treatment method were included in the study.
There were 46 (82%) men and 10 (18%) women with a median age of 69 years (range 35–85). Fifteen (27%) patients presented with sepsis at arrival to the center with vascular surgery service and 3 (5%) were septic at the time of operation. Patient characteristics, clinical presentation, and operative details are listed in Table 2.
Table 2.
Preoperative Characteristics and Operative Details
| Total Amount of Patients, n (%) | 56 (100) |
|---|---|
| Male, n (%) | 46 (82) |
| Age (y), median (range) | 69 (35–85) |
| Preoperative conditions, n (%) | |
| Hypertension | 44 (79) |
| Coronary heart disease | 17 (30) |
| COPD | 12 (21) |
| Renal insufficiency/dialysis | 9 (16) |
| Diabetes mellitus | 16 (29) |
| Cerebrovascular disease | 5 (9) |
| Smoking | 31 (60) |
| Immunocompromising condition | 16 (29) |
| Alcohol abuse | 8 (14) |
| Steroid treatment | 3 (5) |
| Renal failure | 3 (5) |
| HIV | 1 (2) |
| Other | 3 (5) |
| Clinical presentation, n (%) | |
| Fever | 42 (75) |
| Abdominal/back pain | 50 (89) |
| Concomitant infection | 24 (43) |
| Gastroenteritis | 7 (13) |
| Spondylodiscitis | 4 (7) |
| Pneumonia | 4 (7) |
| UTI | 3 (5) |
| Cellulitis | 2 (4) |
| Cholecystitis | 2 (4) |
| Septic arthritis | 1 (2) |
| Infected Charcot foot | 1 (2) |
| Parodontitis | 1 (2) |
| Endocarditis | 1 (2) |
| Herpes Zoster | 1 (2) |
| Median WBC count at the time of diagnosis, ×109/L (range) | 14 (3.6–31) |
| Median WBC count at the time of operation, ×109/L (range) | 8.8 (3.7–27.7) |
| Median CRP level at the time of diagnosis, mg/L (range) | 132 (6–407) |
| Median CRP level at the time of operation, mg/L (range) | 41 (3–407) |
| Aneurysm characteristics, n (%) | |
| Aneurysm location | |
| Suprarenal/visceral | 6 (11) |
| Para‐/juxtarenal | 6 (11) |
| Infrarenal | 41 (72) |
| Iliac | 2 (4) |
| Multiple | 1 (2) |
| Periaortic soft tissue edema | 45 (80) |
| Periaortic/intravascular gas | 7 (13) |
| Saccular, multilocular aneurysm | 42 (75) |
| Rupture | 12 (21) |
| Median aortic aneurysm size, mm (range) | 46 (30–80) |
| Perioperative details, n (%) | |
| Aortic reconstruction | |
| In‐situ aorto‐aortic/aorto‐iliac reconstruction | 55 (98) |
| Aortic patch‐plasty with venous visceral reconstruction | 1 (2) |
| Aortic reconstruction material | |
| Autologous femoral veins | 30 (54) |
| Bovine pericardial self‐made tube | 12 (21) |
| Fresh arterial allografts | 5 (9) |
| Cryopreserved arterial allografts | 7 (12) |
| Cryopreserved venous allografts | 2 (4) |
| Adjunctive procedure | 16 (29) |
| Visceral vascular reconstruction | 13 (23) |
| Gastrointestinal procedure | 3 (5) |
| Renal protection used | 15 (27) |
| Cold fluid infusion | 9 (16) |
| Temporary shunts | 3 (5) |
| Extracorporeal perfusion | 1 (2) |
| N/A | 2 (4) |
| Omental coverage of aortic reconstruction | 23 (41) |
| Open abdomen treatment postoperatively | 17 (30) |
| Median operation time, min (range) | 270 (150–590) |
| Median aortic clamping time, min (range) | 92 (25–257) |
| Median blood loss, mL (range) | 1700 (200–17 000) |
COPD indicates chronic obstructive pulmonary disease; CRP, C‐reactive protein; HIV, human immunodeficiency virus; N/A, not available; UTI, urinary tract infection; WBC, white blood cell count.
Blood and/or tissue culture were positive in 43 (77%) cases, 10 (18%) were positive for Salmonella spp. and 33 (59%) for non‐Salmonella infection. Identified microorganisms are listed in Table 3. Antimicrobial therapy was assigned based on the susceptibility of the isolated strains. Duration was designated individually based on isolated strains, concurrent infections, clinical severity of the infection, and postoperative course of events. Median time of antibiotic therapy preoperatively was 1 week (ranging from a few hours to 13 weeks), and postoperatively 8.5 weeks (range 1–35). At the end of follow‐up, in 3 cases the full duration of antimicrobial therapy remained unknown because of short follow‐up time. Lifelong therapy was not necessary in any case.
Table 3.
Isolated Microorganisms
| Total Amount of Patients With Positive Culture, n (%) | 43 (77) |
|---|---|
| Salmonella spp. | 10 (18) |
| Non‐Salmonella strains | 33 (59) |
| Staphylococcus aureus/MRSA | 15/3 (27/5) |
| Streptococcus spp. | 7 (13) |
| Echerichia coli | 5 (9) |
| Candida spp. | 2 (4) |
| Campylobacterium spp. | 2 (4) |
| Brevibacterium casei | 1 (2) |
| Eikenella corrodens | 1 (2) |
| Bacteroides fragilis | 1 (2) |
| Fusiobacterium necrophorum | 1 (2) |
| Yersinia enterocolitica | 1 (2) |
MRSA indicates methicillin‐resistant Staphylococcus aureus.
Postoperative complications necessitating reoperation occurred in 16 (29%) of the patients, but none of them were graft related. Medical complications occurred in 14 (25%), of which severe renal dysfunction was most prevalent. Recorded postoperative complications are listed in Table 4.
Table 4.
Postoperative Complications
| Total Amount of Patients, n (%) | 56 (100) |
|---|---|
| Surgical complications, n (%) | 16 (29) |
| Reoperation because of bleeding | 6 (11) |
| Leg hematoma | 2 (4) |
| Diffuse abdominal bleeding | 2 (4) |
| GI bleeding | 2 (4) |
| Spleen | 1 (2) |
| Reoperation because of ischemia | 3 (5) |
| Lower limb ischemia/amputation | 2/1 (4/2) |
| Renal ischemia | 1 (2) |
| GI and urologic complications | 5 (9) |
| Ileus | 2 (4) |
| Duodenal lesion | 1 (2) |
| Cholecystitis | 1 (2) |
| Ureteral lesion | 1 (2) |
| Other | |
| Wound complications | 3 (5) |
| Occlusion of av‐fistula | 1 (2) |
| Medical complications, n (%) | 14 (25) |
| Severe renal dysfunction | 9 (16) |
| New‐onset dialysis, temporary/permanent | 7/2 (13/4) |
| Cardiac complications | 2 (4) |
| Respiratory complications | 1 (2) |
| Acute stroke | 1 (2) |
| Other | |
| Delirium | 4 (7) |
| Wound infection | 2 (4) |
| Paraparesis | 1 (2) |
| HIT | 1 (2) |
| Hyperalgesia | 1 (2) |
av‐fistula indicates arteriovenous fistula; GI, gastrointestinal; HIT, heparin‐induced thrombocytopenia.
Median stay in the intensive care unit was 1 day (range 0–30) and in the vascular surgery department was 18 days (range 7–60). The vast majority of the patients were transferred to other medical facilities to continue intravenous antimicrobial therapy or rehabilitation, but detailed information on this is lacking.
Outcomes
The median follow‐up time was 26 months (range 3 weeks–172 months). The Kaplan–Meier survival estimates at 30 days was 95% (95% confidence interval [CI], 89%–100%), 91% at 90 days (CI, 82–99%), 83% at 1 year (CI, 73%–94%), and 71% at 5 years (CI, 52%–89%). For details see Figure. Overall treatment‐related mortality was 9% (n=5) (Table 5).
Figure 1.
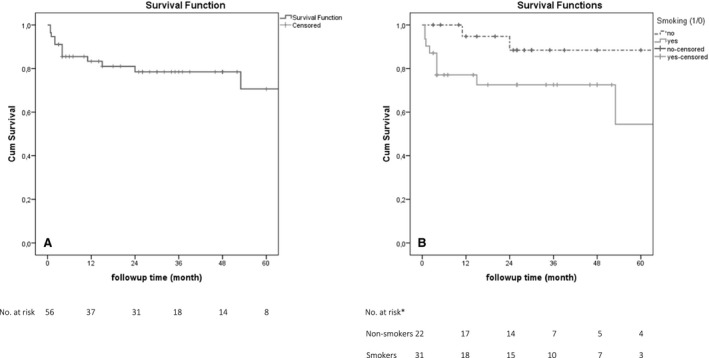
Kaplan–Meier curves demonstrating overall 5‐year survival (A) and among smokers vs nonsmokers (B). *No data available about smoking status in 3 patients.
Table 5.
30‐Day and Treatment‐Related Mortality
| Age (y) | Presentation | Immunosuppressive Condition | Rupture | Urgent Operation | MAAA Location and Reconstruction | Microbiology | Postoperative Complications | Cause of Death | Postoperative Day of Death |
|---|---|---|---|---|---|---|---|---|---|
| 67 | Abdominal pain, fever, enteritis | No | No | No | Infrarenal, in‐situ reconstruction with FV | Salmonella | Myocardial infarction | Acute myocardial infarction | 3 |
| 74 | Abdominal pain | No | No | No | Juxtarenal, in‐situ with cryopreserved allograft and renal bypass | Staph. aureus | Left limb amputation because of gangrene, stroke, hyperalgesia | Acute stroke | 5 |
| 73 | Abdominal pain, fever, spondylodiscitis | No | No | Yes | Juxtarenal, in‐situ reconstruction with bovine pericard and bilateral renal artery reimplantation | MRSA | Bilateral renal ischemia, renal failure, paraplegia, sepsis | MODS | 23 |
| 56 | Abdominal pain, septic, infected gangrene | Alcohol abuse | Yes | Yes | Suprarenal, in‐situ reconstruction with bovine pericard and renal arteries reimplantation | Staph. aureus | GI bleeding, myocardial infarction, ARDS | Acute myocardial infarction, sepsis | 88 |
| 54 | Abdominal pain, sepsis, septic arthritis | No | Yes | Yes | Visceral, aortic patch plasty and renal artery reconstruction with FV | Staph. aureus | Renal failure, sepsis | MODS | 120 |
ARDS indicates acute respiratory distress syndrome; FV, femoral vein; GI, gastrointestinal; MAAA, mycotic abdominal aortic aneurysm; MODS, multiorgan dysfunction syndrome; MRSA, methicillin‐resistant Staphylococcus aureus.
In the univariable analysis, none of the variables was significantly associated with 30‐day mortality. Need for renal protection during suprarenal clamping (P=0.015), operation time (P=0.046), and C‐reactive protein level at the time of diagnosis (P=0.049) were associated with treatment‐related mortality. Smoking was the only factor associated with higher overall mortality (P=0.034) (Figure), while negative bacterial culture was associated with lower mortality (P=0.026). In a Cox regression model using variables with P<0.2, smoking (hazard ratio 10.1; 95% CI, 1.09–92.96; P=0.042) and level of C‐reactive protein at the time of diagnosis (hazard ratio 1.0; 95% CI, 1.00–1.01; P=0.013) were associated with all‐cause mortality.
There were no reinfections diagnosed, and the overall freedom from graft interventions was 95% (n=53). One patient had graft limb thrombosis treated with thrombectomy, and 2 patients needed percutaneous transluminal angioplasty for graft limb stenosis. No grafts were lost during the follow‐up.
Discussion
This study is the result of an international collaboration between 6 different countries and is to date the largest cohort of patients treated with open repair and biological grafting for the MAA with an abdominal location. The study demonstrates a survival of 95% at 30 days and 71% at 5 years, which is comparable to the treatment of elective degenerative abdominal aortic aneurysm (30‐day and 5‐year survival for EVAR 98% and 67%, for open repair 95% and 75%, respectively).19 During the follow‐up time, there were no reinfections and only 3 patients required interventions to maintain graft patency. Despite the variety of biological grafts used, there were no graft‐related fatal complications.
The infection resistance and long‐term outcome of biological grafts in treatment of mycotic aneurysms has been reported earlier in small case series or mixed with data on aortic graft infection.20, 22, 23, 24 Patients with aortic graft infection are challenging; in addition to highly prevalent cardiovascular risk factors, operating in scar tissue complicates the treatment even further. Concentrating exclusively on treatment of MAAA makes the current cohort more homogeneous and strengthens the study. Although consecutive patients were included, only 12 (21%) of them presented with rupture and 6 (11%) of MAAAs encompassed suprarenal aorta. Rupture and suprarenal aneurysm location are known to be associated with increased risk of aneurysm‐related death.3, 4, 11, 12 Suprarenal location in combination with systemic inflammatory response system diminishes survival even further. Patients presenting with systemic inflammatory response system are also at higher risk for reinfection and subsequent mortality; therefore, prosthetic reconstruction should be used with caution in these patients.25
Cryopreservation has improved the permanence of allografts, but degeneration, possible aneurysmatic dilatation, and subsequent rupture remain challenging.22, 26, 27, 28 Also, availability and costs limit more widespread use. Furthermore, 5 patients in the present study were treated with fresh allografts, which are known to have even higher risk for complications.26 Despite the lack of signs of degeneration in the current cohort, we call for caution and suggest using autologous femoral veins or cryopreserved allografts if possible. Femoral veins as autologous material have shown excellent performance related to long‐term infectious complications, but time‐consuming harvesting is a limitation in a center unable to recruit a second team of surgeons.20, 21 Graft durability is acceptable, balloon angioplasty is occasionally needed, and overall long‐term patency is >90%.20, 21 In the current study, autologous femoral veins were used in 30 patients with primary patency of 90% and primary assisted/secondary patency of 100%.
Xenopericardial tubes in the current material were self‐made from xenopericardial patch, but presently commercial options are available as well. Made of bovine, porcine, or equine pericardium, they are easy to handle, readily available, and additionally are resistant to several endospores, microorganisms, and viruses, because of glutaraldehyde solution.29 Earlier reports on self‐made xenopericardial tubes have been encouraging with no graft‐related late mortality or reinfections.23, 24, 30 The present study yielded correlating results, but the considerably small number of patients in all of them calls for further investigations.
EVAR have been enthusiastically adopted to treat MAAA. Significantly lower postoperative morbidity and mortality are the benefits of EVAR, allowing treatment of older and sicker patients who would otherwise be given palliative treatments.31 However, long‐term survival seems inferior to open treatment in case of degenerative aortic aneurysm repair, so that conventional surgery may be advantageous to younger populations in case of MAAA as well.32 In a nationwide study by Sörelius et al, excellent early survival of 96% at 3 months was presented after endovascular treatment of MAAA. Still, a reinfection rate of 24%, strongly linked to late mortality, indicates the hidden danger of prosthetic graft implantation among infected tissue.11 Similar results were seen in an international multicenter study with 3‐month survival of 86% and reinfection rate of 27%.10 Biological grafts showed no reinfections, nor graft‐related fatal complications; 5‐year survival of 71% was superior to previous studies with prosthetic grafts, but graft durability and overall superiority need longer follow‐up and further studies.10, 11, 15
Prosthetic in‐situ reconstruction is a conventional alternative to biological grafts. Infected tissue can be debrided and copious saline irrigation performed. Some centers prefer rifampicin‐soaked Dacron grafts, possibly providing extra protection against gram‐positive cocci.3, 14, 33, 34 Omental‐pedicle cover is as important as in biological reconstructions.3, 14, 20, 21, 22, 34 The postoperative mortality has no advantage to biological grafts and reinfection rate ranges 12% to 18%.11, 15 Hsu et al reported a reinfection rate and total graft‐related mortality of 5% during 2‐year follow‐up.35 However, the number of patients was small, the vast majority had Salmonella infection, known as a predictive factor for superior outcome, and numerous patients were in good general condition receiving antimicrobial treatment preoperatively for 4 to 6 weeks.2, 10, 35
Antimicrobial therapy is a crucial part of complex treatment of MAAA. It should be started intravenously as early as possible with broad‐spectrum antibiotics or based on susceptibility of the available strains. Articles published on prosthetic reconstructions emphasize preoperative antimicrobial therapy at least 1 week for stable patients to eliminate bacteremia and impregnate surrounding tissue with antibiotics, yet the evidence for less postoperative infectious complications is lacking.7, 12, 35 The duration of antimicrobial therapy remains unclear, depending on the surgical method and postoperative course, varying from 6 weeks to lifelong.10, 11, 12, 35, 36 With close surveillance, antibiotics may be discontinued in selected cases, provided there is no clinical, hematological, or radiological prove of ongoing infection. In the present study, the median time for antibiotic treatment was 8 weeks postoperatively, and no in‐hospital survivor needed lifelong treatment.
Salmonella infections cause more rapid aneurysm growth and rupture, strongly affecting the natural course of the disease. Yet non‐Salmonella infections are known to be associated with higher aneurysm‐related complications and death after treatment.2, 3, 10, 36 In the current study, none of them was significantly associated with death or reinfections. However, negative culture was associated with overall survival, suggesting the benefit of the elimination of active bacteria before surgery to produce a more favorable outcome.10, 35
The main limitation of the study was lack of comparison between in‐situ reconstruction with biological grafts and other treatment methods. However, the vast majority of patients treated with EVAR (n=70/80) or open prosthetic reconstruction (n=41/51) were from the Swedish national registry (Table 1), and patients treated from 2006 to 2014 were included in the nationwide study published by Sörelius et al.11 Therefore, the results of Sörelius’ study and the current study are being considered as somewhat comparable. None of the centers that participated have reliable data on patients treated with medical treatment only; therefore, the possibility of case selections exist. However, since aneurysm‐related mortality is >60% with sole medical treatment in case of mycotic aortic aneurysms, conservative treatment was decided only in rare cases of palliative care or unfitness of any intervention.15 MAAAs are extremely rare; therefore, organizing a randomized controlled multicenter trial would be particularly challenging with so few patients per institution. The retrospective nature of the study causes a possibility of selection bias, and makes it impossible to standardize follow‐up protocols in different centers and acquire missing data from collaborators. The small number of patients sets limitations for statistical analyses.
Conclusion
There is no consensus on surgical treatment of MAAA because of the complexity of the disease and lack of obvious superiority of any single treatment method. Open repair with biological grafting for MAAAs demonstrated a midterm survival that was higher compared with other methods, most likely because of no reinfections. The surgical approach also carries acceptable morbidity for patients who are fit for open surgery.
Disclosures
None.
(J Am Heart Assoc. 2018;7:e008104 DOI: 10.1161/JAHA.117.008104.)
These results were presented at the Charing Cross Symposium Global Stars and Rising Stars Session, April 24 to 27, 2018, in London, UK.
References
- 1. Osler W. The gulstonian lectures, on malignant endocarditis. BMJ. 1885;1:467–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hsu RB, Chang CI, Wu IH, Lin FY. Selective medical treatment of infected aneurysms of the aorta in high risk patients. J Vasc Surg. 2009;49:66–70. [DOI] [PubMed] [Google Scholar]
- 3. Oderich GS, Panneton JM, Bower TC, Cherry KJ Jr, Rowland CM, Noel AA, Hallett JW Jr, Gloviczki P. Infected aortic aneurysms: aggressive presentation, complicated early outcome, but durable results. J Vasc Surg. 2001;34:900–908. [DOI] [PubMed] [Google Scholar]
- 4. Yu SY, Hsieh HC, Ko PJ, Huang YK, Chu JJ, Lee CH. Surgical outcome for mycotic aortic and iliac aneurysm. World J Surg. 2011;35:1671–1678. [DOI] [PubMed] [Google Scholar]
- 5. Kyriakides C, Kan Y, Kerle M, Cheshire NJ, Mansfield AO, Wolfe JH. 11‐year experience with anatomical and extra‐anatomical repair of mycotic aortic aneurysms. Eur J Vasc Endovasc Surg. 2004;27:585–589. [DOI] [PubMed] [Google Scholar]
- 6. Touma J, Cochennec F, Parisot J, Fialaire Legendre A, Becquemin JP, Desgranges P. In situ reconstruction in native and prosthetic aortic infections using cryopreserved arterial allografts. Eur J Vasc Endovasc Surg. 2014;48:292–299. [DOI] [PubMed] [Google Scholar]
- 7. Jia X, Dong YF, Liu XP, Xiong J, Zhang HP, Guo W. Open and endovascular repair of primary mycotic aortic aneurysms: a 10‐year single‐center experience. J Endovasc Ther. 2013;20:305–310. [DOI] [PubMed] [Google Scholar]
- 8. Brown SL, Busuttil RW, Baker JD, Machleder HI, Moore WS, Barker WF. Bacteriologic and surgical determinants of survival in patients with mycotic aneurysms. J Vasc Surg. 1984;1:541–547. [PubMed] [Google Scholar]
- 9. Johansen K, Devin J. Mycotic aortic aneurysms. A reappraisal. Arch Surg. 1983;118:583–588. [DOI] [PubMed] [Google Scholar]
- 10. Sörelius K, Mani K, Björck M, Sedivy P, Wahlgren CM, Taylor P, Clough RE, Lyons O, Thompson M, Brownrigg J, Ivancev K, Davis M, Jenkins MP, Jaffer U, Bown M, Rancic Z, Mayer D, Brunkwall J, Gawenda M, Kölbel T, Jean‐Baptiste E, Moll F, Berger P, Liapis CD, Moulakakis KG, Langenskiöld M, Roos H, Larzon T, Pirouzram A, Wanhainen A; European MAA collaborators . Endovascular treatment of mycotic aortic aneurysms: a European multicenter study. Circulation. 2014;130:2136–2142. [DOI] [PubMed] [Google Scholar]
- 11. Sörelius K, Wanhainen A, Furebring M, Björck M, Gillgren P, Mani K. Nationwide study of the treatment of mycotic abdominal aortic aneurysms comparing open and endovascular repair. Circulation. 2016;134:1822–1832. [DOI] [PubMed] [Google Scholar]
- 12. Kan CD, Lee HL, Yang YJ. Outcome after endovascular stent graft treatment for mycotic aortic aneurysm: a systematic review. J Vasc Surg. 2007;46:906–912. [DOI] [PubMed] [Google Scholar]
- 13. Kan CD, Lee HL, Luo CY, Yang YJ. The efficacy of aortic stent grafts in the management of mycotic abdominal aortic aneurysm‐institute case management with systemic literature comparison. Ann Vasc Surg. 2010;24:433–440. [DOI] [PubMed] [Google Scholar]
- 14. Uchida N, Katayama A, Tamura K, Miwa S, Masatsugu K, Sueda T. In situ replacement for mycotic aneurysms on the thoracic and abdominal aorta using rifampicin‐bonded grafting and omental pedicle grafting. Ann Thorac Surg. 2012;93:438–442. [DOI] [PubMed] [Google Scholar]
- 15. Lin CH, Hsu RB. Primary infected aortic aneurysm: clinical presentation, pathogen, and outcome. Acta Cardiol Sin. 2014;30:514–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Berger P, Moll FL. Aortic graft infections: is there still a role for axillobifemoral reconstruction? Semin Vasc Surg. 2011;24:205–210. [DOI] [PubMed] [Google Scholar]
- 17. Bisdas T, Wilhelmi M, Haverich A, Teebken OE. Cryopreserved arterial homografts vs silver‐coated Dacron grafts for abdominal aortic infections with intraoperative evidence of microorganisms. J Vasc Surg. 2011;53:1274–1281. [DOI] [PubMed] [Google Scholar]
- 18. Owen K, Wilshaw SP, Homer‐Vanniasinkam S, Bojar RA, Berry H, Ingham E. Assessment of the antimicrobial activity of acellular vascular grafts. Eur J Vasc Endovasc Surg. 2012;43:573–581. [DOI] [PubMed] [Google Scholar]
- 19. Laine MT, Laukontaus SJ, Sund R, Aho PS, Kantonen I, Albäck A, Venermo M. A population‐based study of abdominal aortic aneurysm treatment in Finland 2000 to 2014. Circulation. 2017;136:1726–1734. [DOI] [PubMed] [Google Scholar]
- 20. Daenens K, Fourneau I, Nevelsteen A. Ten‐year experience in autogenous reconstruction with the femoral vein in the treatment of aortofemoral prosthetic infection. Eur J Vasc Endovasc Surg. 2003;25:240–245. [DOI] [PubMed] [Google Scholar]
- 21. Heinola I, Kantonen I, Jaroma M, Albäck A, Vikatmaa P, Aho P, Venermo M. Editor's choice—treatment of aortic prosthesis infections by graft removal and in situ replacement with autologous femoral veins and fascial strengthening. Eur J Vasc Endovasc Surg. 2016;51:232–239. [DOI] [PubMed] [Google Scholar]
- 22. Bisdas T, Bredt M, Pichlmaier M, Aper T, Wilhelmi M, Bisdas S, Haverich A, Teebken OE. Eight‐year experience with cryopreserved arterial homografts for the in situ reconstruction of abdominal aortic infections. J Vasc Surg. 2010;52:323–330. [DOI] [PubMed] [Google Scholar]
- 23. Czerny M, von Allmen R, Opfermann P, Sodeck G, Dick F, Stellmes A, Makaloski V, Bühlmann R, Derungs U, Widmer MK, Carrel T, Schmidli J. Self‐made pericardial tube graft: a new surgical concept for treatment of graft infections after thoracic and abdominal aortic procedures. Ann Thorac Surg. 2011;92:1657–1662. [DOI] [PubMed] [Google Scholar]
- 24. Weiss S, Tobler EL, von Tengg‐Kobligk H, Makaloski V, Becker D, Carrel TP, Schmidli J, Wyss TR. Self made xeno‐pericardial aortic tubes to treat native and aortic graft infections. Eur J Vasc Endovasc Surg. 2017;54:646–652. [DOI] [PubMed] [Google Scholar]
- 25. Fillmore AJ, Valentine RJ. Surgical mortality in patients with infected aortic aneurysms. J Am Coll Surg. 2003;196:435–441. [DOI] [PubMed] [Google Scholar]
- 26. Kieffer E, Gomes D, Chiche L, Fléron MH, Koskas F, Bahnini A. Allograft replacement for infrarenal aortic graft infection: early and late results in 179 patients. J Vasc Surg. 2004;39:1009–1017. [DOI] [PubMed] [Google Scholar]
- 27. Lesèche G, Castier Y, Petit MD, Bertrand P, Kitzis M, Mussot S, Besnard M, Cerceau O. Long‐term results of cryopreserved arterial allograft reconstruction in infected prosthetic grafts and mycotic aneurysms of the abdominal aorta. J Vasc Surg. 2001;34:616–622. [DOI] [PubMed] [Google Scholar]
- 28. Vogt PR, Brunner‐LaRocca HP, Lachat M, Ruef C, Turina MI. Technical details with the use of cryopreserved arterial allografts for aortic infection: influence on early and midterm mortality. J Vasc Surg. 2002;35:80–86. [DOI] [PubMed] [Google Scholar]
- 29. McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin Microbiol Rev. 1999;12:147–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kubota H, Endo H, Noma M, Ishii H, Tsuchiya H, Yoshimoto A, Takahashi Y, Inaba Y, Nishino Y, Nunokawa M, Hosoi Y, Ikezoe T, Nemoto M, Makino Y, Nemoto Y, Matsukura M, Sugiyama M, Abe N, Takeuchi H, Nagao G, Kondo E, Yanagida O, Yoshino H, Sudo K. Xenopericardial roll graft replacement for infectious pseudoaneurysms and graft infections of the aorta. J Cardiothorac Surg. 2015;10:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. EVAR trial participants . Endovascular aneurysm repair versus open repair in patients with abdominal aortic aneurysm (EVAR trial 1): randomised controlled trial. Lancet. 2005;365:2179–2186. [DOI] [PubMed] [Google Scholar]
- 32. Patel R, Sweeting MJ, Powell JT, Greenhalgh RM; EVAR trial investigators . Endovascular versus open repair of abdominal aortic aneurysm in 15‐years’ follow‐up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366–2374. [DOI] [PubMed] [Google Scholar]
- 33. Dubois M, Daenens K, Houthoofd S, Peetermans WE, Fourneau I. Treatment of mycotic aneurysms with involvement of the abdominal aorta: single‐centre experience in 44 consecutive cases. Eur J Vasc Endovasc Surg. 2010;40:450–456. [DOI] [PubMed] [Google Scholar]
- 34. Müller BT, Wegener OR, Grabitz K, Pillny M, Thomas L, Sandmann W. Mycotic aneurysms of the thoracic and abdominal aorta and iliac arteries: experience with anatomic and extra‐anatomic repair in 33 cases. J Vasc Surg. 2001;33:106–113. [DOI] [PubMed] [Google Scholar]
- 35. Hsu RB, Tsay YG, Wang SS, Chu SH. Surgical treatment for primary infected aneurysm of the descending thoracic aorta, abdominal aorta, and iliac arteries. J Vasc Surg. 2002;36:746–750. [DOI] [PubMed] [Google Scholar]
- 36. Sedivy P, Spacek M, El Samman K, Belohlavek O, Mach T, Jindrak V, Rohn V, Stadler P. Endovascular treatment of infected aortic aneurysms. Eur J Vasc Endovasc Surg. 2012;44:385–394. [DOI] [PubMed] [Google Scholar]


